Abstract
The granulomagenic properties of serologically active glycolipids A1, B2, B3, and C isolated from Mycobacterium bovis BCG were studied. Glycolipid A1, dissolved in olive and injected intradermally in guinea pigs, was able to elicit a granulomatous response that seemed to be of the nonallergic type. This granulomagenic activity was quite striking since only 2 mug was necessary to elicit the reaction. The B and C glycolipids were milder granulomagenic agents. Glycolipid A1, dissolved in olive oil and injected intraperitoneally, was toxic for mice. Mice lost weight after the injection of as little as 10 mug of A1, although not even a dose of 100 mug was lethal. Glycolipid A1 failed to immunize mice against aerogenic infection with virulent tubercle bacilli.
Full text
PDF

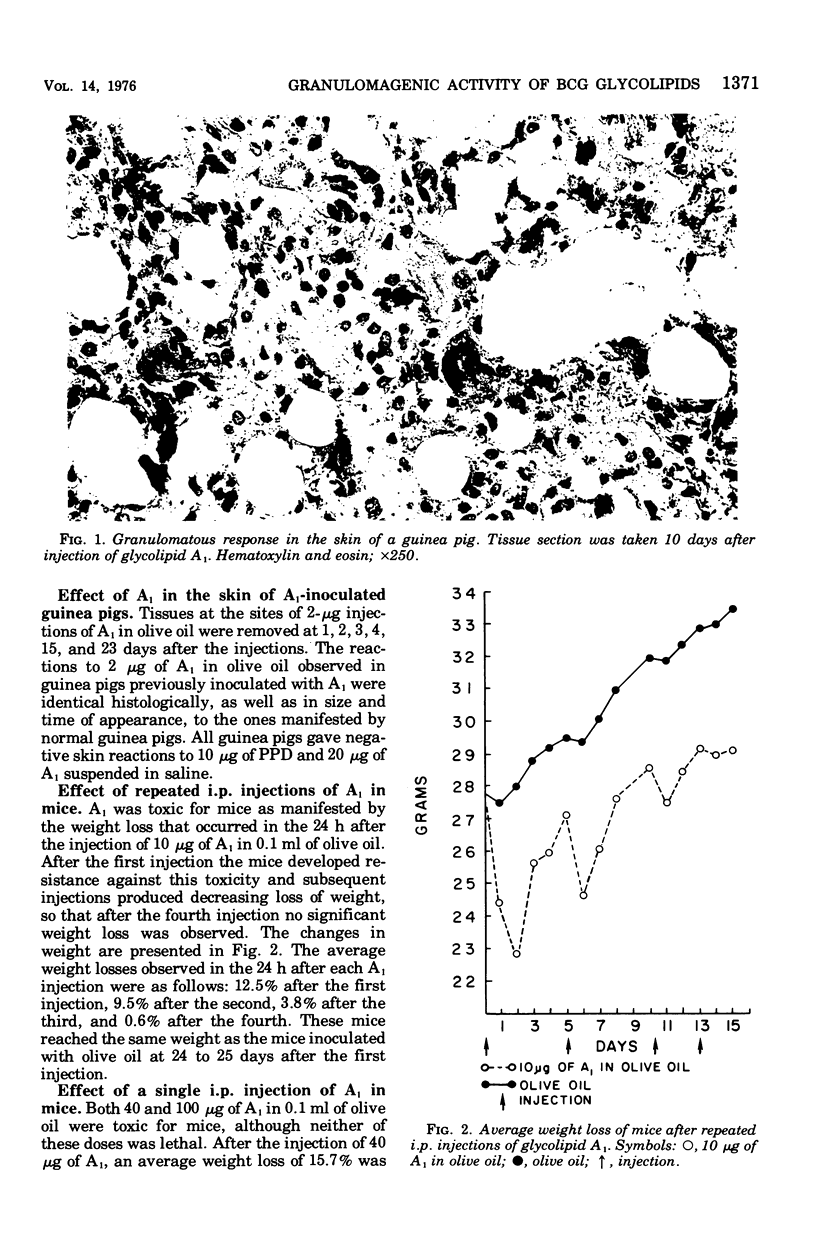


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma I., Ribi E. E., Meyer T. J., Zbar B. Biologically active components from mycobacterial cell walls. I. Isolation and composition of cell wall skeleton and component P3. J Natl Cancer Inst. 1974 Jan;52(1):95–101. doi: 10.1093/jnci/52.1.95. [DOI] [PubMed] [Google Scholar]
- BLOCH H., NOLL H. Studies on the virulence of Tubercle bacilli; the effect of cord factor on murine tuberculosis. Br J Exp Pathol. 1955 Feb;36(1):8–17. [PMC free article] [PubMed] [Google Scholar]
- Bekierkunst A. Acute granulomatous response produced in mice by trehalose-6,6-dimycolate. J Bacteriol. 1968 Oct;96(4):958–961. doi: 10.1128/jb.96.4.958-961.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekierkunst A., Levij I. S., Yarkoni E., Vilkas E., Adam A., Lederer E. Granuloma formation induced in mice by chemically defined mycobacterial fractions. J Bacteriol. 1969 Oct;100(1):95–102. doi: 10.1128/jb.100.1.95-102.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekierkunst A., Levij I. S., Yarkoni E., Vilkas E., Lederer E. Cellular reaction in the footpad and draining lymph nodes of mice induced by mycobacterial fractions and BCG bacilli. Infect Immun. 1971 Sep;4(3):245–255. doi: 10.1128/iai.4.3.245-255.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekierkunst A., Wang L., Toubiana R., Lederer E. Immunotherapy of Cancer with Nonliving BCG and Fractions Derived from Mycobacteria: Role of Cord Factor (Trehalose-6, 6'-Dimycolate) in Tumor Regression. Infect Immun. 1974 Nov;10(5):1044–1050. doi: 10.1128/iai.10.5.1044-1050.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Brehmer W., Brehmer W., Yamamoto K., Ribi E. Cutaneous granulomatous response to BCG cell walls with reference to cancer immunotherapy. Infect Immun. 1976 Feb;13(2):543–553. doi: 10.1128/iai.13.2.543-553.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWATA H., MYRVIK Q. N., LEAKE E. S. DISSOCIATION OF TUBERCULIN HYPERSENSITIVITY AS MEDIATOR FOR AN ACCELERATED PULMONARY GRANULOMATOUS RESPONSE IN RABBITS. J Immunol. 1964 Sep;93:433–438. [PubMed] [Google Scholar]
- Kato M. Antibody formation to trehalose-6,6'-dimycolate (cord factor) of Mycobacterium tuberculosis. Infect Immun. 1972 Feb;5(2):203–212. doi: 10.1128/iai.5.2.203-212.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M. Effect of anti-cord factor antibody on experimental tuberculosis in mice. Infect Immun. 1973 Jan;7(1):14–21. doi: 10.1128/iai.7.1.14-21.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M. Immunochemical properties of anti-cord factor antibody. Infect Immun. 1973 Jan;7(1):9–13. doi: 10.1128/iai.7.1.9-13.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariano M., Spector W. G. The formation and properties of macrophage polykaryons (inflammatory giant cells). J Pathol. 1974 May;113(1):1–19. doi: 10.1002/path.1711130102. [DOI] [PubMed] [Google Scholar]
- Meyer T. J., Ribi E. E., Azuma I., Zbar B. Biologically active components from mycobacterial cell walls. II. Suppression and regression of strain-2 guinea pig hepatoma. J Natl Cancer Inst. 1974 Jan;52(1):103–111. doi: 10.1093/jnci/52.1.103. [DOI] [PubMed] [Google Scholar]
- Meyer T. J., Ribi E., Azuma I. Biologically active components from mycobacterial cell walls. V. Granuloma formation in mouse lungs and guinea pig skin. Cell Immunol. 1975 Mar;16(1):11–24. doi: 10.1016/0008-8749(75)90181-1. [DOI] [PubMed] [Google Scholar]
- Moore V. L., Myrvik Q. N. Effect of antimacrophage serum on dermal tuberculin sensitivity and allergic pulmonary granuloma formation in rabbits. Infect Immun. 1970 Dec;2(6):810–814. doi: 10.1128/iai.2.6.810-814.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore V. L., Myrvik Q. N., Kato M. Role of cord factor (trehalose-6,6'-dimycolate) in allergic granuloma formation in rabbits. Infect Immun. 1972 Jul;6(1):5–8. doi: 10.1128/iai.6.1.5-8.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiardo Z., Middlebrook G. Delayed-type hypersensitivity and immunity against aerogenic tuberculosis in guinea pigs. Infect Immun. 1974 May;9(5):815–820. doi: 10.1128/iai.9.5.815-820.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiardo Z., Middlebrook G. Serologically active glycolipid families from Mycobacterium bovis BCG. I. Extraction, purification and immunologic studies. Am J Epidemiol. 1974 Dec;100(6):469–476. doi: 10.1093/oxfordjournals.aje.a112059. [DOI] [PubMed] [Google Scholar]
- Reggiardo Z., Middlebrook G. Serologically active glycolipid families from Mycobacterium bovis BCG. II. Serologic studies on human sera. Am J Epidemiol. 1974 Dec;100(6):477–486. doi: 10.1093/oxfordjournals.aje.a112060. [DOI] [PubMed] [Google Scholar]
- Ribi E., Meyer T. J., Azuma I., Parker R., Brehmer W. Biologically active components from mycobacterial cell walls. IV. Protection of mice against aerosol infection with virulent mycobacterium tuberculosis. Cell Immunol. 1975 Mar;16(1):1–10. doi: 10.1016/0008-8749(75)90180-x. [DOI] [PubMed] [Google Scholar]
- Saito R., Tanaka A., Sugiyama K., Azuma I., Yamamura Y. Adjuvant effect of cord factor, a mycobacterial lipid. Infect Immun. 1976 Mar;13(3):776–781. doi: 10.1128/iai.13.3.776-781.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R. G., MARSHALL A. H. The role of various chemical fractions of M. tuberculosis and other Mycobacteria in the production of allergic encephalomyelitis. Immunology. 1958 Apr;1(2):111–122. [PMC free article] [PubMed] [Google Scholar]




