Abstract
Chronic ingestion of water containing inorganic arsenic (iAs) has been linked to a variety of adverse health effects, including cancer, hypertension and diabetes. Current evidence suggests that the toxic methylated trivalent metabolites of iAs, methylarsonous acid (MAs) and dimethylarsinous acid (DMAsIII) play a key role in the etiology of these diseases. Both MAs and DMAsIII have been detected in urine of subjects exposed to iAs. However, the rapid oxidation of DMAsIII and, to a lesser extent, MAsIII in oxygen-rich environments leads to difficulties in the analysis of these metabolites in samples of urine collected in population studies. Results of our previous work indicate that MAsIII and DMAsIII are relatively stable in a reducing cellular environment and can be quantified in cells and tissues. In the present study, we used the oxidation state-specific hydride generation-cryotrapping-atomic absorption spectroscopy (HG-CT-AAS) to examine the presence and stability of these trivalent metabolites in the liver of mice and in UROtsa/F35 cells exposed to iAs. Tri- and pentavalent metabolites of iAs were analyzed directly (without chemical extraction or digestion). Liver homogenates prepared in cold deionized water and cell culture medium and lysates were stored at either 0 °C or −80 °C for up to 22 days. Both MAsIII and DMAsIII were stable in homogenates stored at −80 °C. In contrast, DMAsIII in homogenates stored at 0 °C began to oxidize to its pentavalent counterpart after 1 day; MAsIII remained stable for at least 3 weeks under these conditions. MAsIII and DMAsIII generated in UROtsa/F35 cultures were stable for 3 weeks when culture media and cell lysates were stored at −80 °C. These results suggest that samples of cells and tissues represent suitable material for the quantitative, oxidation state-specific analysis of As in laboratory and population studies examining the metabolism or toxic effects of this metalloid.
Introduction
Inorganic arsenic (iAs) is a natural, carcinogenic metalloid found in water sources worldwide, most commonly as arsenite (iAsIII) and arsenate (iAsV).1 The ingestion of drinking water containing high levels of iAs is associated with an array of adverse health effects, including cancer of the skin, lungs, liver and urinary bladder.2 Non-neoplastic effects of iAs exposure include vascular disease, hypertension, skin lesions and diabetes. 3–5 Chronic toxicity due to drinking water with high levels of iAs can lead to a collection of these symptoms, known as arsenicosis.6 The United States Environmental Protection Agency and World Health Organization lowered the safe level for As in drinking water from 50 to 10 ppb in response to evidence of iAs toxicity even at low exposure levels.7,8 For the tens of millions of people chronically exposed to iAs, outcomes vary widely and depend not only on the level of exposure but also on the inter-individual differences in As metabolism. Exposure levels and the pattern of iAs metabolism are commonly assessed through analysis of iAs metabolites in urine; however, concentrations of these metabolites in human tissues are not well researched.
The metabolism of iAs in humans is mediated by arsenic (+3 oxidation state) methyltransferase (AS3MT).9 The AS3MT-catalyzed and S-adenosylmethionine-dependent methylation of iAs yields both trivalent and pentavalent methylated arsenicals, including methylarsonic acid (MAsV), methylarsonous acid (MAsIII), dimethylarsinic acid (DMAsV) and dimethylarsinous acid (DMAsIII).10,11 Several intronic and exonic polymorphisms have been described for the human AS3MT gene, including Met287The (T → C), potentially altering the rates and yields of iAs methylation and contributing to the inter-individual differences in susceptibility to iAs toxicity.12,13
While the methylation of iAs is critical for its detoxification, recent evidence suggests that methylated trivalent As metabolites (MAsIII and DMAsIII) generated in the course of iAs metabolism in human cells and tissues are more cytotoxic and genotoxic than their pentavalent counterparts or iAs species.14 MAsIII and DMAsIII are also potent enzyme inhibitors, alter cell signalling pathways, and induce oxidative damage.15–17 Moreover, these As metabolites have been shown to inhibit insulin signalling and insulin-stimulated glucose uptake in cultured murine adipocytes, providing a potential mechanism for the diabetogenic effects of iAs exposure.18,19
The analysis of iAs metabolites in human population studies has been typically limited to urine. The half life of iAs in the human body measures in days. Thus, although urine is an important source of information about exposure to iAs, the urinary metabolites reflect only recent exposures.20 Furthermore, analysis of As species in urine of chronically exposed individuals produces a wide range of responses, making it difficult to elucidate the mechanisms responsible for iAs toxicity and the direct effects on target human tissues, such as, skin, urinary bladder, lungs, and liver. Interestingly, in vivo studies indicate that urinary excretion of iAs and its metabolites does not correspond to tissue distribution of these As species.21,22 For example, in mice exposed to iAsV in drinking water the predominant accumulation of MAs in the kidney and DMAs in the lungs has been reported.21,22 Furthermore, a study comparing human urinary metabolites of iAs to As species retained in bladder exfoliated cells found no correlation, suggesting that monitoring urine may not give a clear idea of As species stored in specific tissues.23 Thus, identification and quantification of iAs metabolites in human tissues, including the target organs, or in cells originating from these tissues could provide important information about the risk associated with iAs exposure and about the mechanism underlying the adverse effects of this exposure.
The oxidation state-specific speciation analysis of As in urine is complicated mainly by the low stability of methylated trivalent arsenicals, particularly DMAsIII. DMAsIII can be completely converted to DMAsV in several hours even in frozen urine; MAsIII can oxidize in several months.24 However, the rate of oxidation varies and depends, in part, on individual urine composition, further confusing the interpretation of the analytical data. To date, no attempt has been made to characterize the stability of the methylated trivalent As species in tissues and cells. Mammalian cells contain millimolar concentrations of a potent low-molecular weight reductant, glutathione (GSH), which is known to bind trivalent arsenicals, producing AsIII–thiol complexes.25 Thus, the binding to GSH or to protein thiols is likely to protect the methylated trivalent arsenicals from oxidation and extend their lifetime inside the cells.
The main objective of this study was to optimize the hydride generation-cryotrapping-atomic absorption spectroscopy (HG-CT-AAS) system for analysis of tissues and cells and to characterize the stability of trivalent arsenicals, particularly DMAsIII in these complex biological matrices. Here we report, for the first time, that DMAsIII is relatively stable in tissue homo-genates and cell lysates under conditions that are typically used for storage and transport of biological samples collected in laboratory and population studies. We, therefore, suggest that samples of the tissues targeted by iAs exposure or cells originated from these tissues are used to obtain more complete information about the metabolism of iAs or about the mechanisms of iAs toxicity in animals and humans.
Methods
Arsenicals
The following arsenicals were used for calibration and in cell culture experiments: sodium arsenite (NaAsIIIO2) and sodium arsenate (Na2HAsVO4) (>99% pure) were purchased from Sigma-Aldrich (St. Louis, MO). Methylarsonic acid, disodium salt (CH3AsVO(ONa)2), and dimethylarsinic acid ((CH3)2AsVO(OH)) both better than 98% pure were purchased from Chem Service (West Chester, PA). Oxomethyl-arsine (CH3AsO)4 which forms MAsIII in aqueous solution was provided by Dr William Cullen (University of British Columbia, Vancouver, Canada). The As content in each of the standards was determined by graphite furnace-AAS.26
Mice and treatments
Twelve week-old C57BL/6 mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA) and housed in polycarbonate cages (2 per cage) with corn cob bedding at the University of North Carolina Animal Facility (12 h light/dark cycle, 22 ± 1 °C and humidity 50 ± 10%), accredited by the American Association for Accreditation of Laboratory Animal Care. Mice were allowed free access to food (Lab Diet 5058, Nutrition International, Brentwood, MO) and pure deionized water (DIW) or DIW containing iAsIII (50 mg As per L, i.e. 50 ppm). Mice were euthanized by cervical dislocation. Freshly dissected mice livers were processed as previously described.27 Liver homogenates were prepared in ice cold DIW (10% w/v). All procedures involving mice were approved by the UNC Institutional Animal Care and Use Committee.
Cultured cells and treatments
The SV-40 transformed human urothelial cell line, UROtsa, was obtained from Dr Unimye (Department of Urology, West Virginia University). The UROtsa/F35 clonal cell line expressing rat As3mt and capable of As methylation was previously generated from the parental UROtsa cell line using a retroviral pLEGFP-Nl gene delivery system.28 In the present study, UROtsa/F35 cells were cultured in MEM (Mediatech, Manassas, VA) supplemented with 10% heat inactivated FBS (Gemini Bio-Products, Sacramento, CA), 50 U penicillin (Sigma) per mL and 50 µg streptomycin (Sigma) per mL. Cells were cultured in 6-well plates (Corning, Inc., Corning, NY) at 37 °C in a humidified incubator with a 95% air/5% CO2 atmosphere. To generate DMAsIII, near confluent cells in 6-well plates were incubated with MAsIII in 2 mL of MEM/well for varying lengths of time. After incubation, media were aspirated into capped tubes, cells were lysed on ice in pre-chilled DIW for 20 minutes and harvested by scraping with a pipette tip to reduce transfer losses. In some experiments, cells were lysed in 0.5% Triton X-100. Control, unexposed UROtsa/F35 cells were used to establish the background levels of As species in the cell culture.
Depletion of intracellular GSH
In some experiments, the cultured UROtsa/F35 cells were treated with butathione sulfoximine (BSO), the inhibitor of GSH synthesis. BSO was added to the culture medium at a final concentration of 250 µM BSO for 24 hours. The GSH concentrations were measured before and after BSO treatment in cell lysates prepared in 2% sulfosalicylic acid, using a previously described enzymatic recycling assay.29
Speciation analysis of As by HG-CT-AAS
Arsenic species were analyzed by a semi-automated HG-CT-AAS using a AAnalyst 800 spectrometer (Perkin-Elmer, Norwalk, CT, USA) equipped with a multiple microflame quartz tube atomizer (multiatomizer) and coupled to a cryotrap. The HG and CT steps were controlled by a FIAS 400 flow injection accessory (Perkin-Elmer).26,30 Using this system, arsines from trivalent arsenicals (iAsIII, MAsIII and DMAsIII) are generated directly at pH 6; to generate arsines from both tri- and pentavalent arsenicals (iAsIII + V, MAsIII + V and DMAsIII + V), the As V-standards and samples are pre-reduced with L-cysteine (EMD Chemicals Inc., Gibbstown, NJ).26,30 The concentrations of iAsV, MAsV, and DMAsV are then determined as a difference between AAS signals obtained for cysteine-treated and untreated sample aliquots.
We have previously shown that the slopes of the calibration curves for the cysteine-treated AsV-standards prepared in DIW are identical with the slopes of curves generated for the trivalent arsenicals in the absence of cysteine.30 We have also shown that the slopes of the calibration curves for the cysteine-treated aqueous solutions of As standards do not significantly differ from the slopes of curves prepared for liver homogenates spiked with these standards and treated with cysteine.27 Therefore, calibration curves for quantification of tri- and pentavalent As species in this study were generated using aqueous solutions of the pentavalent standards (iAsV, MAsV, and DMAsV) treated with cysteine.
Acid digestion
To determine the recovery of As species during the direct analyses of mouse liver homogenates, aliquots of the homogenates were acid digested using a MARS Microwave Reaction System equipped with MarsXpress Temperature Control (CAM, Matthews, NC). Here, 100 µL of 10% homogenate was mixed with 3 mL of 2 M ultrapure phosphoric acid (EMD Chemicals, Inc.) and microwaved for 10 hours at 90 ± 4 °C. One millilitre of the digestate was neutralized with 0.25 mL of 10 N NaOH to a final pH of ~6. Because this digestion oxidizes all trivalent arsenicals to their pentavalent counterparts, the neutralized digestates were treated with 2% cysteine for 1 hour prior to analysis.27
Statistical analysis
All statistical analyses were performed using GraphPad Instat software package (GraphPad Software Inc., San Diego, CA). Linear regression and correlation analyses were employed to characterize the calibration curves. ANOVA followed by Bonferroni’s multiple comparison posttest was used to determine significant differences in As concentration between fresh, immediately analyzed samples and each time point during the storage of samples at either 0 °C or −80 °C This test was also used to determine differences in DMAsIII present in cell lysates after lysis with either DIW or 0.5% Triton X-100. Statistical significance was considered at the level of p < 0.01.
Results and discussion
Speciation analysis of As in biological matrices
The growing number of reports on widespread exposures to iAs in drinking water has triggered an intensive search for biomarkers that could serve as indicators of these exposures or could predict the susceptibility of individuals to diseases associated with chronic toxicity of iAs. For many years, the urinary concentrations of As species that originate from iAs metabolism in human tissues have been used as the exposure indicators. In addition, the composition of iAs metabolites in human urine (e.g., DMAs/MAs ratio) is believed to provide information about the capacity of individuals to methylate and thus to detoxify iAs.31,32 The attempts to determine concentrations of iAs metabolites, especially the toxic MAsIII and DMAsIII in tissues, have been rare and suffered problems associated with the lack of suitable analytical techniques and with low stability of these methylated trivalent arsenicals.
We have shown that in rat liver cytosol large portions of iAs and its methylated metabolites are bound to proteins.33 The binding of As species to proteins that has also been reported by others34,35 makes it necessary to perform digestions or extraction when chromatographic methods are used for speciation analysis of As in cells, tissues, or tissue fractions. However, both digestion and extraction can lead to oxidation or losses of the unstable and highly reactive methylated AsIII species.36 Our previous work has provided strong evidence that, in spite of their limited specificity, HG-based techniques coupled with a sensitive detector may provide a unique tool for the detection and quantification of MAsIII and DMAsIII in the biological matrices as complex as cultured cells or tissue.26,27,30,37 The present study continues this work by examining stability of the methylated trivalent arsenicals in liver homogenates and cell lysates using a HG-CT-AAS technique developed by our laboratories.
Optimization of HG-CT-AAS for analysis of slurries
The previously described HG-CT-AAS system was developed for analysis of solutions and relatively simple liquid biological matrices.26,30 This system was optimized in the present study for analysis of complex biological slurries, including tissue homogenates and crude cell lysates (Fig. SI, ESI†). To avoid plugging of the FIAS 400 injection port with tissue and cell fragments, the homogenates and cell lysates were introduced directly to the manifold. The sample (typically 500 µL) was pipetted into a 1 mL pipette tip connected to the FIAS 400 peristaltic pump. From the pipette tip, the sample was pumped into the system followed by 500 µL of DIW. The sample was then mixed with equal flows (2 mL min−1) of Tris-HCl buffer (pH 6) and NaBH4 solutions prior to entering the reaction coil and the gas–liquid separator. For drying of the gaseous phase prior to the cryotrapping stage, a cartridge filled with NaOH pellets was included.38 The cryotrapping step and AAS detection were performed without modifications as previously described.26
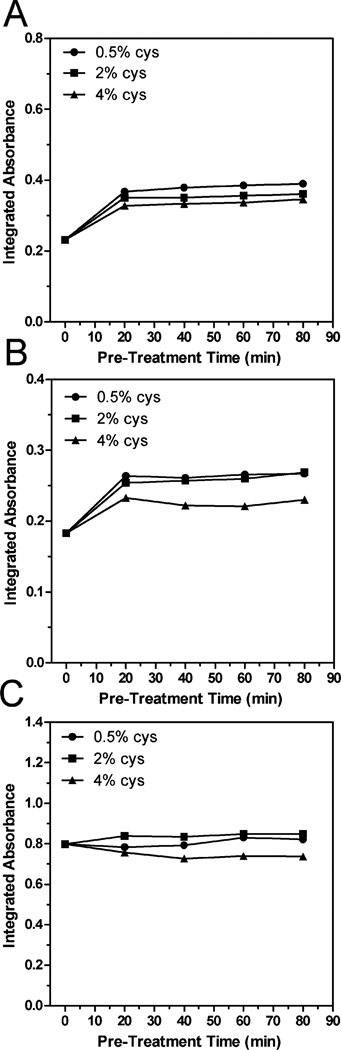
Further optimization focused on the pre-reduction with cysteine and on the components of the HG mixture. Here, liver homogenate from a single mouse exposed to iAsIII (50 ppm As) in drinking water was aliquoted, pre-treated with cysteine (0.5, 2 or 4%) for up to 80 minutes and analyzed directly by HG-CT-AAS. Results of the direct analysis suggest that treatment with 0.5–2% cysteine for at least 20 minutes is optimal for generation of arsines from all As species present in the homogenate (Fig. 1). It is unclear why the AAS signals were lower for homogenate aliquots treated with 4% cysteine. It is possible that portions of arsenicals in homogenates were reduced by high cysteine concentration to form volatile arsines, thus decreasing the amounts of arsines generated during the HG step.
Fig. 1.
Peak areas recorded for iAsIII + V (A), MAsIII + V(B), and DMAsIII + V (C) during the direct HG-CT-AAS analysis of aliquots of a mouse liver homogenate pre-treated with 0.5, 2, or 4% cysteine for up to 80 minutes. Time 0 minutes shows results of the direct analysis of liver homogenates prior to the cysteine treatment. Each point represents a single measurement.
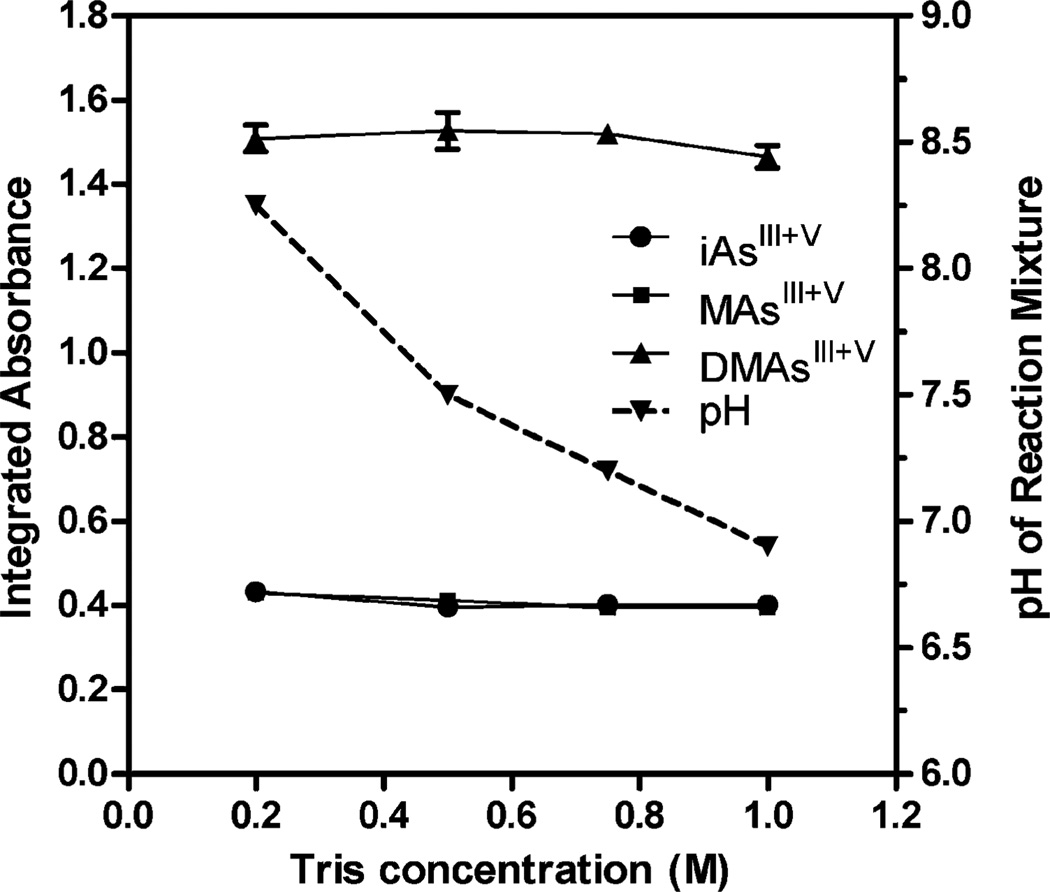
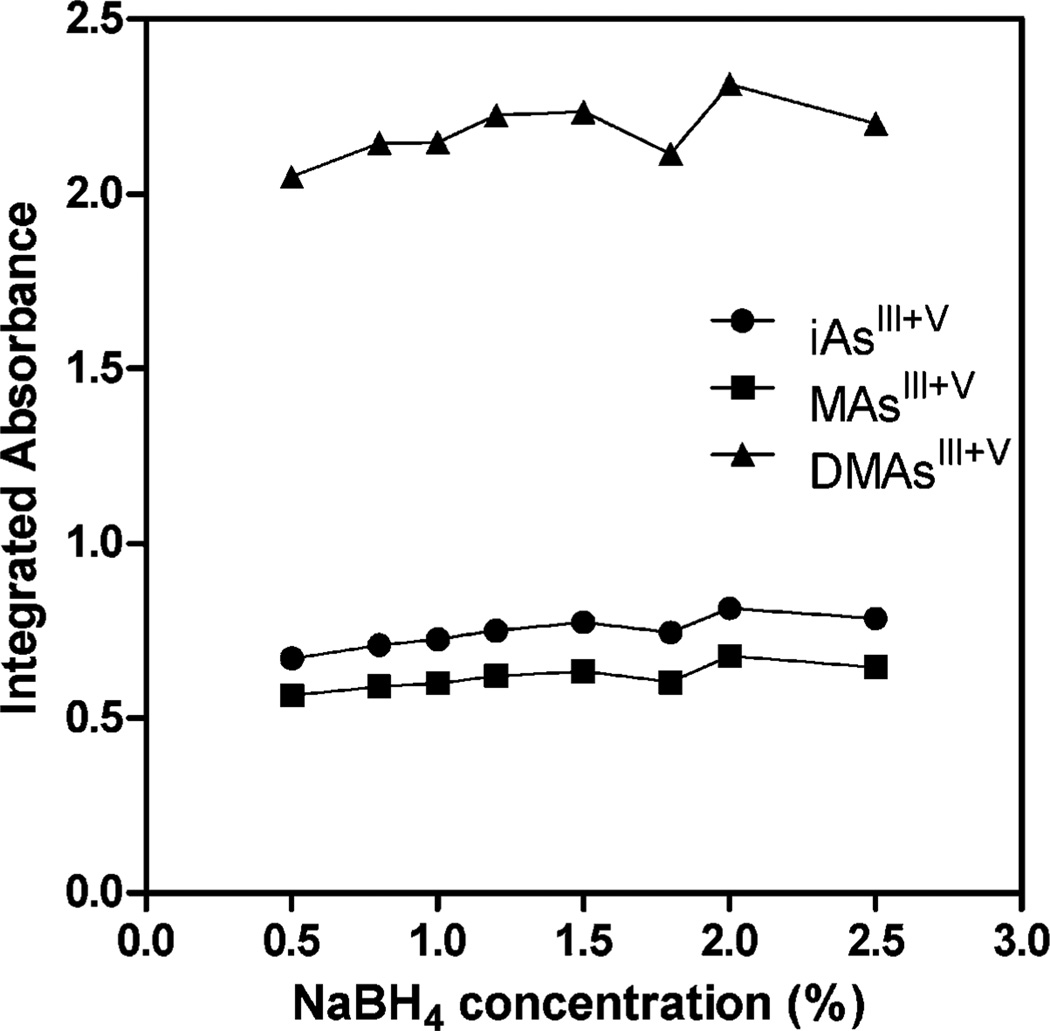
In the next step, AAS signals were tested in the reaction mixtures containing liver homogenates treated for 1 hour with 2% cysteine and varied concentrations of Tris-HCl (pH 6) or NaBH4. For this test we pooled liver homogenates from several mice exposed to 50 ppm As in drinking water. No significant differences in AAS signals were measured in mixtures containing 1% NaBH4 and 0.2 M to 1 M Tris, even though the pH measured after the HG reaction decreased from 8.3 to 6.9 (Fig. 2). Similarly, changing NaBH4 concentration (0.5% to 2.5%) in a reaction mixture containing 0.75 M Tris had little effect on the HG efficiency (Fig. 3).
Fig. 2.
HG-CT-AAS analysis of pooled mouse liver homogenates pre-treated with 2% cysteine for 1 hour: effect of Tris buffer concentration in the HG reaction mixture on AAS signals for iAsIII + V MAsIII + V, and DMAsIII + V. Each point represents mean ± SD for n = 3.
Fig. 3.
HG-CT-AAS analysis of pooled mouse liver homogenates pre-treated with 2% cysteine for 1 hour: effect of NaBH4 concentration in the HG reaction mixture on AAS signals for iAsIII + V, MAsIII + V,, and DMAsIII + V,. Each point represents mean ± SD for n = 3.
Thus, each of the experimental parameters we examined exhibits a relatively broad optimum, which is important for achieving robust and reliable analytical conditions. Based on these results, we concluded that the 1 hour treatment with 2% cysteine and the HG mixture containing 0.75 M Tris and 1% NaBH4 is optimal for analysis of liver homogenates. Notably, the same reaction conditions were previously used in our laboratories for analyses of liquid samples.26,30 To examine As recoveries under these conditions, additional aliquots of the homogenates were analyzed after digestion in phosphoric acid. The analysis of acid-digested homogenates showed that the direct analysis of the homogenates treated with 2% cysteine recovered ~100% of MAsIIIIII + V and DMAsIII + V. Consistent with our previous report,27 the average recovery of iAsIII + V was lower, reaching only about 85%.
The 1 hour treatment with 2% cysteine and the HG mixture containing 0.75 M Tris and 1% NaBH4 was used in all following experiments examining the stability of trivalent arsenicals in mouse liver homogenates and cell lysates.
Stability of trivalent arsenicals in mouse liver homogenates
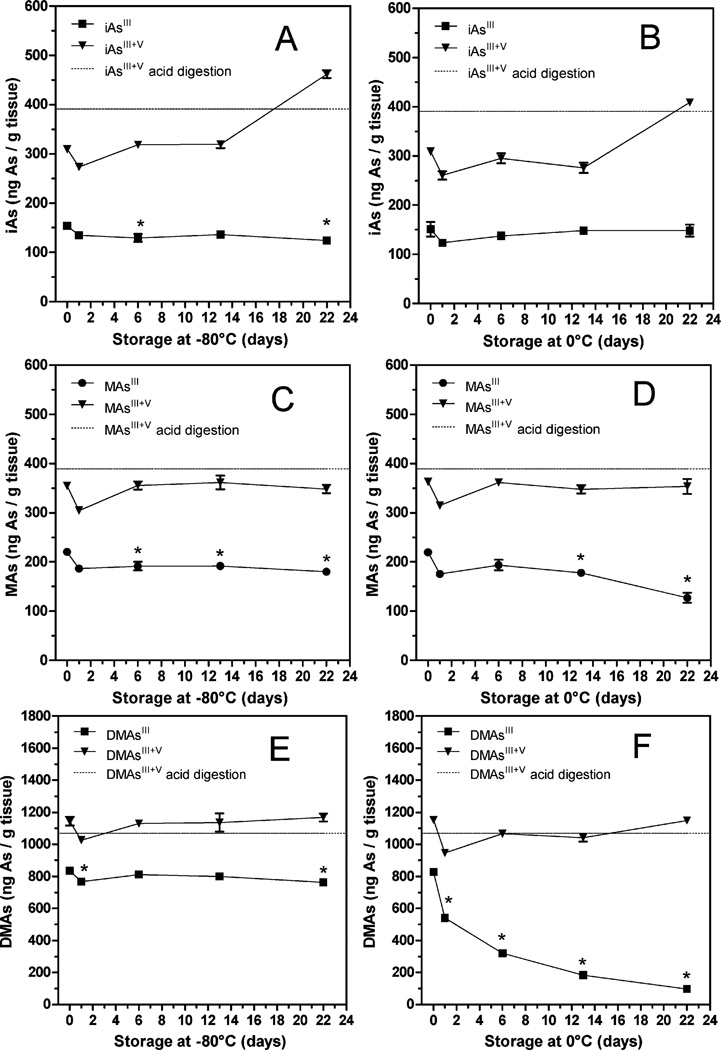
The stability of AsIII species was examined in a 10% homogenate prepared from a liver of a single mouse exposed to iAsIII in drinking water (50 ppm As) for 9 days. The homogenate was prepared in ice-cold DIW and immediately aliquoted. Three freshly prepared aliquots were analyzed directly for AsIII species; another 3 aliquots were pretreated with 2% cysteine and analyzed for AsIII + V species. The AsIII + V species were also analyzed in acid digested aliquots of the homogenate. To determine the stability of trivalent arsenicals, remaining aliquots were stored at 0 °C (on ice) or −80 °C and analyzed for AsIII and AsIII + V species after 1, 6, 13, and 22 days (Fig. 4).
Fig. 4.
Stability of trivalent arsenicals in a liver homogenate from a mouse exposed to iAsIII in drinking water (50 ppm As) for 9 days: liver homogenate was prepared in DIW. The direct HG-CT-AAS analysis was used to determine the concentrations of iAs (A, B), MAs (C, D) and DMAs (E, F) species in aliquots of the fresh homogenate and in aliquots stored at −80 °C (A, C, E) or 0 °C (B, D, F) for up to 22 days (mean ± SD, n = 3). To control for As recoveries during the direct analyses, iAsIII + V, MAsIII + V, and DMAsIII + V were determined in aliquots of the fresh homogenate digested in phosphoric acid (mean, n = 3). *The concentration is significantly different from that found in the fresh homogenate (p < 0.01).
In the fresh homogenate, trivalent arsenicals accounted for 65% of the sum of As species: iAsIII (8%), MAsIII (12%), and DMAsIII (45%). However, the recovery of iAsIII + V was only about 82% (Fig. 4A and B), suggesting that a part of iAs, possibly protein-bound iAsIII, is not available for the HG reaction under these conditions. The recoveries of iAs remained low for up to 13 days, but increased at day 22 for aliquots of the homogenate stored either at 0 °C (105%) or at −80 °C (113%) due to increased levels of iAsV. These data suggest that a prolonged storage may result in the release of iAsIII from high-affinity binding sites, followed by oxidation of iAsIII to iAsV. Notably, both MAsIII and DMAsIII were relatively stable in aliquots of the homogenate stored at −80 °C for up to 22 days. Only about 18% of MAsIII and 9% of DMAsIII oxidized under these conditions (Fig. 4C and E). In contrast, 42% of MAsIII and 88% of DMAsIII oxidized in aliquots of the homogenate stored at 0 °C (Fig. 4D and F).
Generation and stability of DMAsIII in UROtsa/F35 cells
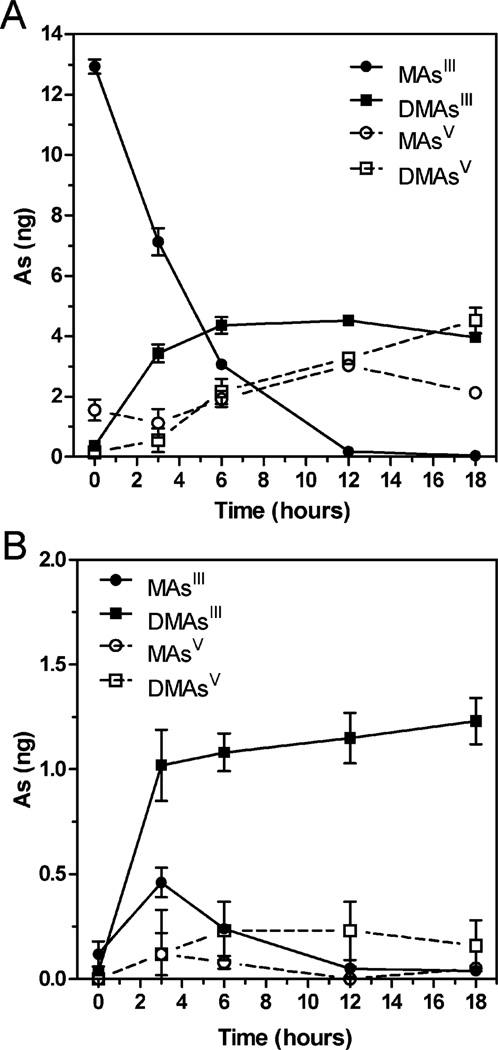
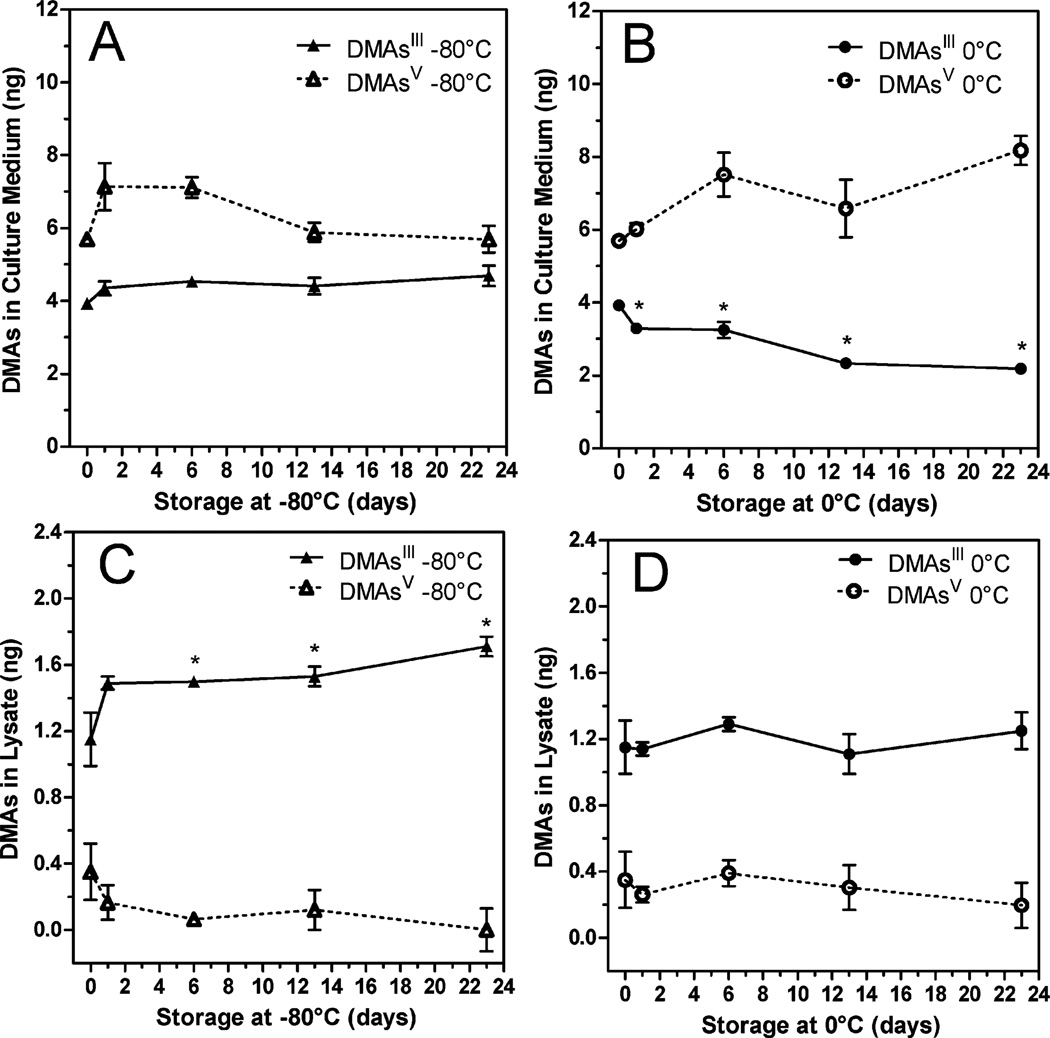
We have previously shown that cultured UROtsa/F35 cells treated with MAsIII produce DMAsIII and DMAsV.28 In the present study, DMAsIII was generated in UROtsa/F35 culture exposed to 0.1 µM MAsIII (15 ng As per well) for 18 hours. DMAsIII levels were monitored in the culture medium and in cell lysates that were prepared in ice-cold DIW. Fig. 5 shows that DMAsIII was the major product of MAsIII methylation and that a substantial portion of DMAsIII was retained by cells. DMAsIII represented almost 50% and 90% of all As species associated with the culture medium and cells, respectively. The stability of DMAsIII was examined in both the medium and cell lysates prepared in DIW and stored at either 0 °C or −80 °C for up to 23 days. DMAsIII in cell lysates was stable regardless of the storage temperature; DMAsIII in culture medium was stable only at −80 °C (Fig. 6). In contrast, almost 50% of DMAsIII oxidized in the medium stored at 0 °C.
Fig. 5.
Generation of DMAsIII in UROtsa/F35 culture exposed to 0.1 µM MAsIII (15 ng As per well) for up to 18 hours. Tri- and pentavalent As species were measured by HG-CT-AAS in culture medium (A) and cell lysates (B) before and after pretreatment with 2% cysteine (mean ± SD, n = 3).
Fig. 6.
Stability of DMAsIII in medium and cell lysates from UROtsa/F35 culture exposed to 0.1 µM MAsIII (15 ng As per well) for 18 hours: DMAsIII and DMAsV were analyzed in culture medium (A, B) and cell lysates (C, D) immediately after the exposure and after storage at −80 °C or 0 °C for up to 23 days (mean ± SD, n = 3). *The concentration is significantly different from that found in fresh medium or cell lysate (p < 0.01).
We also tested the stability of DMAsIII in cell lysates prepared in an ice-cold solution of 0.5% Triton X-100, a non-ionic laboratory detergent that is commonly used in biomedical studies for sample processing. We found that fresh cell lysates prepared in Triton X-100 contained significantly less DMAsIII than the lysates prepared in DIW. The oxidation state specific analysis showed that 54% of DMAsIII in these lysates oxidized to DMAsV (Fig. S2, ESI†).
To assess the role of GSH in the stability of DMAsIII, UROtsa/F35 cells were incubated with BSO for 24 hours and then exposed for 18 hours to MAsIII. The treatment with BSO decreased the intracellular GSH concentration to 15% of the original level, but had no effect on the stability of DMAsIII in cell lysates prepared in DIW and stored at either −80 °C or 0 °C (data not shown). Thus, it is possible that the remaining GSH provided sufficient protection for DMAsIII in cell lysates or that DMAsIII was primarily bound to protein thiols and could not be affected by BSO treatment.
Finally, we examined the stability of DMAsIII under conditions that are consistent with a shipment of samples from field studies to analytical laboratories. Here, UROtsa/F35 cells were exposed to 0.5 µM MAsIII (75 ng As per well) for 18 hours; aliquots of cell lysates prepared in DIW and aliquots of culture medium were placed in two polystyrene shipping containers filled with dry ice and two additional containers filled with ice packs that were pre-frozen at −80 °C. One of the containers with dry ice and one with ice packs were stored at UNC Chapel Hill and DMAsIII was analyzed in aliquots of the stored samples for up to 7 days. The other containers were shipped by an express postal service to Prague, Czech Republic for analysis in the Institute of Analytical Chemistry. The HG-CT-AAS analysis at UNC found that DMAsIII is stable in both cell lysates and media stored in dry ice for 2 days; however, significant loses of DMAsIII occurred at day 5 and 7 (Fig. S3, ESI†). DMAsIII oxidized faster in samples stored with ice packs. The shipment to Prague was delayed in customs for 4 days, resulting in a significant oxidation of DMAsIII in culture medium and cell lysates shipped in either dry ice or ice packs (Fig. S4, ESI†).
Conclusions
Previous work has shown that methylated trivalent metabolites of iAs, MAsIII and DMAsIII are unstable in human urine.24 We have recently reported that up to 50% of DMAsIII can oxidize during 24 hours even in urine stored in dry ice,39 making it difficult to detect and quantify this metabolite in urine samples collected in population studies. The work presented here confirms that the trivalent metabolites of iAs, iAsIII, MAsIII, and DMAsIII can be detected and quantified in fresh cell lysates and tissue homogenates by direct HG-CT-AAS analysis. The optimum conditions for analysis of the pentavalent arsenicals include the pre-treatment with 2% cysteine for 60 minutes and generation of arsines in the reaction mixture containing 1% NaBH4 and 0.75M Tris buffer (pH 6). Notably, the methylated trivalent metabolites of iAs, including DMAsIII, are stable for at least 3 weeks in cell lysates and tissue homogenates prepared in cold DIW and stored at −80 °C. When packed in dry ice, these types of samples can be shipped without major losses of DMAsIII, as long as the shipping time does not exceed 2 days and dry ice is not depleted during the shipment.
In summary, results of the present study suggest that, unlike urine, samples of tissues or cells collected in human population studies provide suitable material for the quantitative, oxidation state specific analysis of As species, assuming that these samples are properly handled and stored prior to the analysis. Thus, while urine analysis helps to estimate the levels of exposure to iAs and to evaluate the efficiency of iAs methylation, the analysis of tissues or cells may provide important information about the internal dose and chemical species of As, including highly toxic but unstable MAsIII and DMAsIII, in target tissues.
Supplementary Material
Acknowledgements
Funding was provided by GIL grant 200710.0028, NIH grant DK056350, Czech Science Foundation grant 203/09/1783, and AS CR project No. AV0Z40310501. The investigation by JC was supported by a predoctoral traineeship (National Research Service Award T32 ES007126) from the National Institute of Environmental Health Sciences, National Institute of Health. We thank Dr William Cullen (University of British Columbia) for providing oxomethylarsine for this study.
Footnotes
Electronic supplementary information (ESI) available: A scheme of the optimized HG-CT-AAS instrumentation and additional data on stability of DMAsIII in cell lysates. See DOI: 10.1039/c1mt00095k
Notes and references
- 1.Smedley PL, Kinniburgh DG. Appl. Geochem. 2002;17:517–568. [Google Scholar]
- 2.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monogr. Eval. Carcinog. Risks Hum. 2004;84:1–477. [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng CH, Chong CK, Tseng CP, Centeno JA. Ambio. 2007;36:82–84. doi: 10.1579/0044-7447(2007)36[82:bditil]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Wang CH, Hsiao CK, Chen CL, Hsu LI, Chiou HY, Chen SY, Hsueh YM, Wu MM, Chen CJ. Toxicol. Appl. Pharmacol. 2007;222:315–326. doi: 10.1016/j.taap.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Navas-Acien A, Silbergeld EK, Streeter RA, Clark JM, Burke TA, Guallar E. Environ. Health Perspect. 2006;114:641–648. doi: 10.1289/ehp.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saha KC. J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng. 2003;38:255–272. doi: 10.1081/ese-120016893. [DOI] [PubMed] [Google Scholar]
- 7.USEPA, National Drinking Water Regulations; Arsenic and Clarification to Compliance and New Source Contaminants Monitoring: Final Rule, 66. 2001. [Google Scholar]
- 8.The World Bank, Towards a more effective operational response. Arsenic contamination of groundwater in South and East Asian Countries, Volume 1, Policy Report, No. 31303. 2005. [Google Scholar]
- 9.Lin S, Shi Q, Nix FB, Styblo M, Beck MA, Herbin-Davis KM, Hall LL, Simeonsson JB, Thomas DJ. J. Biol. Chem. 2002;277:10795–10803. doi: 10.1074/jbc.M110246200. [DOI] [PubMed] [Google Scholar]
- 10.Waters SB, Devesa V, Del Razo LM, Styblo M, Thomas DJ. Chem. Res. Toxicol. 2004;17:404–109. doi: 10.1021/tx0342161. [DOI] [PubMed] [Google Scholar]
- 11.Thomas DJ, Li J, Waters SB, Xing W, Adair BM, Drobna Z, Devesa V, Styblo M. Exp. Biol. Med. (Maywood) 2007;232:3–13. [PMC free article] [PubMed] [Google Scholar]
- 12.Agusa T, Iwata H, Fujihara J, Kunito T, Takeshita H, Minh TB, Trang PT, Viet PH, Tanabe S. Toxicol. Appl. Pharmacol. 2009;236:131–141. doi: 10.1016/j.taap.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Drobna Z, Waters SB, Walton FS, LeCluyse EL, Thomas DJ, Styblo M. Toxicol. Appl. Pharmacol. 2004;201:166–177. doi: 10.1016/j.taap.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Thomas DJ, Styblo M, Lin S. Toxicol. Appl. Pharmacol. 2001;176:127–144. doi: 10.1006/taap.2001.9258. [DOI] [PubMed] [Google Scholar]
- 15.Lin S, Del Razo LM, Styblo M, Wang C, Cullen WR, Thomas DJ. Chem. Res. Toxicol. 2001;14:305–311. doi: 10.1021/tx0001878. [DOI] [PubMed] [Google Scholar]
- 16.Drobna Z, Jaspers I, Thomas DJ, Styblo M. FASEB J. 2003;17:67–69. doi: 10.1096/fj.02-0287fje. [DOI] [PubMed] [Google Scholar]
- 17.Wang TC, Jan KY, Wang AS, Gurr JR. Mutat. Res. 2007;615:75–86. doi: 10.1016/j.mrfmmm.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Paul DS, Hernandez-Zavala A, Walton FS, Adair BM, Dedina J, Matousek T, Styblo M. Toxicol. Appl. Pharmacol. 2007;222:305–314. doi: 10.1016/j.taap.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walton FS, Harmon AW, Paul DS, Drobna Z, Patel YM, Styblo M. Toxicol. Appl. Pharmacol. 2004;198:424–433. doi: 10.1016/j.taap.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Buchet JP, Lauwerys R, Roels H. Int. Arch. Occup. Environ. Health. 1981;48:71–79. doi: 10.1007/BF00405933. [DOI] [PubMed] [Google Scholar]
- 21.Kenyon EM, Del Razo LM, Hughes MF. Toxicol. Sci. 2005;85:468–175. doi: 10.1093/toxsci/kfi107. [DOI] [PubMed] [Google Scholar]
- 22.Kenyon EM, Del Razo LM, Hughes MF, Kitchin KT. Toxicology. 2005;206:389–401. doi: 10.1016/j.tox.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Zavala A, Valenzuela OL, Matousek T, Drobna Z, Dedina J, G Garcia-Vargas G, Thomas DJ, Del Razo LM, Styblo M. Environ. Health Perspect. 2008;116:1656–1660. doi: 10.1289/ehp.11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong ZL, Lu XF, Cullen WR, Le XC. J. Anal. At. Spectrom. 2001;16:1409–1413. [Google Scholar]
- 25.Delnomdedieu M, Basti MM, Styblo M, Otvos JD, Thomas DJ. Chem. Res. Toxicol. 1994;7:621–627. doi: 10.1021/tx00041a006. [DOI] [PubMed] [Google Scholar]
- 26.Matousek T, Hernandez-Zavala A, Svoboda M, Langrova L, Adair BM, Drobna Z, Thomas DJ, Styblo M, Dedina J. Spectrochim. Acta, Part B. 2008;63:396–106. doi: 10.1016/j.sab.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Currier JM, Svoboda M, de Moraes DP, Matousek T, Dedina J, Styblo M. Chem. Res. Toxicol. 2011;24:478–480. doi: 10.1021/tx200060c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drobna Z, Waters SB, Devesa V, Harmon AW, Thomas DJ, Styblo M. Toxicol. Appl. Pharmacol. 2005;207:147–159. doi: 10.1016/j.taap.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman I, Kode A, Biswas SK. Nat. Protocols. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez-Zavala A, Matousek T, Drobna Z, Paul DS, Walton F, Adair BM, Dedina J, Thomas DJ, Styblo M. J. Anal. At. Spectrom. 2008;23:342–351. doi: 10.1039/b706144g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hopenhayn-Rich C, Biggs ML, Smith AH, Kalman DA, Moore LE. Environ. Health Perspect. 1996;104:620–628. doi: 10.1289/ehp.96104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vahter M. Toxicology. 2002;181–182:211–217. doi: 10.1016/s0300-483x(02)00285-8. [DOI] [PubMed] [Google Scholar]
- 33.Styblo M, Thomas DJ. Toxicol. Appl. Pharmacol. 1997;147:1–8. doi: 10.1006/taap.1997.8256. [DOI] [PubMed] [Google Scholar]
- 34.Naranmandura H, Suzuki N, Suzuki KT. Chem. Res. Toxicol. 2006;19:1010–1018. doi: 10.1021/tx060053f. [DOI] [PubMed] [Google Scholar]
- 35.Yan H, Wang N, Weinfeld M, Cullen WR, Le XC. Anal. Chem. 2009;81:4144–4152. doi: 10.1021/ac900352k. [DOI] [PubMed] [Google Scholar]
- 36.Xie R, Johnson W, Spayd S, Hall GS, Buckley B. Anal. Chim. Acta. 2006;578:186–194. doi: 10.1016/j.aca.2006.06.076. [DOI] [PubMed] [Google Scholar]
- 37.Devesa V, Maria DR, Adair B, Drobna Z, Waters SB, Hughes MF, Styblo M, Thomas DJ. J. Anal. At. Spectrom. 2004;19:1460–1467. [Google Scholar]
- 38.Taurkova P, Svoboda M, Musil S, Matousek T. J. Anal. At. Spectrom. 2011;26:220–223. [Google Scholar]
- 39.Del Razo LM, García-Vargas GG, Valenzuela OL, Hernandez-Castellanos E, Sánchez-Peña LC, Drobná Z, Loomis D, Stýblo M. Environ. Health. 2011;10:73. doi: 10.1186/1476-069X-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.