Abstract
Objectives
Older adults with major depressive disorder (MDD) have the highest population-rate of suicide. White matter brain lesions (WML) are a potential biological marker for suicidality in young and middle-age adults and are correlated with cognitive impairment (CI) in older adults. In the current study of older patients with MDD, we examined 1) if a history of suicide attempts was associated with a more severe course of MDD; 2) if WML are a biological marker for suicide; and 3) if suicide attempt history is associated with CI mediated by WML.
Setting
Data from the Neurocognitive Outcomes of Depression in the Elderly.
Participants
Depressed patients (60+) who had ever attempted suicide (n=23) were compared to depressed patients (60+) who had not attempted suicide (n=223).
Measurements
Baseline and follow-up assessments were obtained for depressive symptoms (every 3 months) and cognitive functioning (every six months) over two years. Three MRI scans were conducted.
Results
At baseline, suicide attempters reported more severe past and present symptoms (e.g., depressive symptoms, current suicidal thoughts, psychotic symptoms, earlier age of onset, and more lifetime episodes) than non-attempters. Suicide attempters had more left WML at baseline, and suicide attempt history predicted a greater growth in both left and right WML. WML predicted cognitive decline; nonetheless, history of suicide attempt was unrelated to cognitive functioning.
Conclusions
Severity of depressive symptoms and WML are associated with suicide attempts in geriatric depressed patients. Suicide attempts predicted neurological changes, which may contribute to poorer long-term outcomes in elder attempters.
Keywords: Geriatric, major depression, white matter lesions
Older adults are disproportionately likely to die by suicide (1). Major Depressive Disorder (MDD) is one of the conditions most commonly associated with suicide in older adults (2). The severity of the disorder has been identified by some as a risk factor (3–5) particularly among the depressed elderly (6), though not all agree (7). Although suicide attempts do not need to be present to meet criteria for MDD (8), the presence of attempts may indicate a more severe course of the disorder. Indeed, long-term outcomes of older adults who have attempted suicide are poor (9).
Studies of predominately younger and middle-aged individuals have identified white matter lesions (WML) in the brain as a potential biological marker for suicidality (10, 11). A meta-analysis of four studies (12) found that suicide attempters had a significantly higher number of WML than did non-attempters. Jollant and colleagues noted that neurocognitive dysfunctions (including WML) in suicidal patients may facilitate the development of a suicidal crisis during stressful circumstances (13). It is possible that the presence of WML may result in decreased cognitive ability to cope with life stressors, which may lead to increased risk for suicidal behavior.
In older depressed patients, WML have been found to be associated with cognitive impairment (CI) (14). Previous research has provided evidence that older depressed patients with prior suicide attempts had a greater burden of subcortical grey matter hyperintensities compared with patients without such a history (15). This neurobiological finding reinforces the idea that suicide in later life may be associated with the destruction of neural pathways critical to the regulation of mood, cognition, and behavior. However, knowledge of WML associated with suicidality in older depressed adults is limited, and to our knowledge no study has examined if suicide attempts in the elderly predict growth in WML.
Given the association between WML and CI in older adults (16), if WML are also associated with suicide attempts, we might expect to see differences in cognitive functioning between elder attempters and non-attempters. Researchers (17) have demonstrated that older suicide attempters exhibited more impaired reward/punishment-based learning than did older non-attempters. Dombrovski et al. (17) suggested that their findings are consistent with lesions in the ventral prefrontal cortex.
The present analyses used data from the Neurocognitive Outcomes of Depression in the Elderly (NCODE) study (18). We had three primary hypotheses. First, we predicted that compared with non-attempters, older depressed patients with a history of suicide attempts would report more severe symptoms of depression at baseline (e.g., more depressive symptoms and more psychotic symptoms), a more severe lifetime course of MDD (e.g., earlier age of onset and more lifetime episodes), and more residual symptoms of depression over time (including more suicidal ideation). Second, we predicted that attempters would have more WML at baseline than non-attempters and show more growth in WML over time. Finally, we hypothesized that attempters would have poorer baseline cognitive functioning and would have an increase in cognitive decline over time. Specifically, we predicted that WML might mediate the association between past suicide attempts and cognitive decline.
Method
We analyzed data from the NCODE study, a longitudinal study of geriatric depression at Duke University Medical Center (DUMC) supported by the National Institute of Mental Health.
Participants
Informed Consent
Participants provided written informed consent. The research protocol is reviewed and approved annually by DUMC Institutional Review Board (IRB). Data analyses were conducted at Florida State University and were approved by the university’s IRB.
Recruitment
Participant recruitment began in 1994 and continues to the present.
Depressed participants
Participants seeking treatment for depression were recruited for the NCODE study if they were non-demented adults over age 60, met DSM-IV criteria for current MDD, and presented for inpatient or outpatient psychiatry services at DUMC or the Duke General Internal Medicine Clinic. Exclusion criteria included meeting criteria for another major psychiatric illness (schizophrenia, schizoaffective disorder, bipolar disorder, lifetime alcohol or substance dependence, and dementia) or having indicators of dementia or pre-clinical dementia. Such other disorders could contribute to cognitive decline or neurological changes. It should be noted that the patient sample included those with early- and late-onset depression. Indeed, approximately 25% had an onset by age 30, and approximately 25% had onset after the age of 60. Others had an onset in midlife.
Measures
Baseline demographic and depression assessment
Trained interviewers administered the Duke Depression Evaluation Schedule (DDES) to determine whether participants met diagnostic criteria for MDD. The DDES is a structured interview that assesses demographic information and DSM–IV current and lifetime MDD using the National Institute of Mental Health Diagnostic Interview Schedule (DIS), which has been found to have good validity and reliability (19).
Baseline number of DIS depressive symptoms
We computed the total number of depressive symptoms reported at baseline (experienced in the last six months), excluding symptoms related to suicide (Cronbach’s α= .89).
DIS total number of past and present psychotic experiences
Participants were asked if they currently or previously experienced a number of unusual experiences (Cronbach’s α= .70).
DIS suicide attempters and non-attempters
Participants were asked if they had ever attempted suicide (No/Yes).
Suicidal thoughts
A current suicidal thoughts scale (e.g., symptoms present in the last six months) was derived from the DIS suicide-related items (specifically excluding the suicide attempts item). Participants were asked if they thought about death and dying a lot, if they thought about wanting to die, and if they thought about committing suicide (Cronbach’s α=.70).
Montgomery Åsberg Depression Rating Scale (MADRS)
To determine the severity of depression at baseline and follow-up, a geriatric psychiatrist completed the MADRS for each participant. The MADRS assesses apparent sadness, reported sadness, inner tension, reduced sleep, reduced appetite, concentration difficulties, lassitude, inability to feel, pessimistic thoughts, and suicidal thoughts. Each item had a 7-point response scale, ranging from 0 to 6. All ten items had good toexcellent inter-rater reliability (20), with an intraclass correlation (ICC) of r=.93, p<.0001. The MADRS was administered every three months over a two-year period (Chronbach’s α=.92 across administrations).
MADRS suicidal ideation
One of the MADRS items assessed suicidal ideation using a 7-point scale from 0 (no ideation) to 6 (explicit plans for suicide when there is an opportunity; active preparations for suicide).
Mini Mental State Examination (MMSE)
The MMSE (21) assesses cognitive functioning in five areas: orientation, registration, attention and calculation, recall, and language. It also yields an objective measure of global cognitive functioning. The MMSE has been used extensively in epidemiological research of older adults. Scores were obtained at baseline and every six months over two years. Reliability across assessments was acceptable (α=.71).
Magnetic Resonance Imaging (MRI)
Participants underwent structural MRIs of the brain at baseline, year 2, and year 4. Baseline differences in volume between the attempters and non-attempters and changes in volume over time were examined for white matter lesions (WML; measured in ml) for both hemispheres.
The methodology of MRI measurement has been reported previously by Steffens and colleagues (22), Chen and colleagues (23) and Taylor and colleagues (24). Subjects were imaged with a 1.5 T whole-body MRI system (GE Medical Systems, Waukesha, WI, U.S.A.) using the standard head (volumetric) radiofrequency coil. A dual-echo fast-spin echo acquisition was obtained in the axial plane for morphometry. The pulse sequence parameters were: TR = 4000 ms, TE = 30, 135 ms, 32 kHz imaging bandwidth, echo train length = 16, with 3-mm section thickness and one excitation per phase-encoding increment, 20-cm FOV. The images were acquired in two separate acquisitions with a 3-mm gap between sections for each acquisition. The segmentation for gray and white matter hyperintensities in geriatric subjects was administrated at the Duke Neuropsychiatric Imaging Research Laboratory (NIRL). The NIRL-modified version of MrX software was used for the whole brain and cerebral hemispheres and included GML and WML volume measurements. Reliability was established by repeated measurements on multiple MRI scans by two raters. Inter-rater intraclass correlation coefficients were greater than 0.9 for all lesion measurements. The ICC for the total cerebrum volume was 0.998. Total cerebrum volume was included in all analyses of WML as a control variable.
Data Analytic Plan
Missing Data
Table 1 describes the sample by attempt status for those with complete baseline data and for those with missing data (at Time 2) on the MADRS, MMSE, or MRI. Table 1 also shows analyses comparing participants with complete and missing data. There were no significant differences on MMSE and MADRS scores. Similarly there were no apparent differences for baseline WML (left and right). Thus, it did not appear that attrition was selective. Thus, attrition did not appear to systematically influence the results. However, it is typically the case that the more severely ill patients are more likely to drop out.
Table 1.
Attrition of suicidal and non-suicidal participants: Comparison of participants with baseline data to participants missing data at Year 2.
| Time 1 Baseline |
Baseline MADRS |
DF | Mean | Standard deviation | F | p-value |
|---|---|---|---|---|---|---|
| Non Attempter have data. | N=195 | Df=1,212 | 27.17 | 7.646 | 2.398 | .069 |
| Attempter have data. | N=18 | 31.61 | 10.268 | |||
| Time 2 | MADRS | |||||
| Non-attempter missing data | N=30 | Df=1,24 | 25.67 | 8.691 | ||
| Attempter missing data | N=5 | 30.00 | 3.082 | |||
| Time 1 Baseline |
White matter lesions (left) | |||||
| Non attempter have data | N=96 | Df=1,106 | 2.5163 | 96 | 2.266 | .082 |
| Attempter have data | N=11 | 2.6817 | 11 | |||
| Time 2 | WML (left) | |||||
| Non-attempter missing data | N=121 | Df=1,131 | 3.9807 | 121 | ||
| Attempter missing data | N=11 | 4.1631 | 11 | |||
| Time 1 | MMSE | |||||
| Non attempter have data | N=215 | Df=1,234 | 27.78 | 3.044 | 1.721 | .163 |
| Attempter have data | N=20 | 28.65 | 1.268 | |||
| Time 2 | MMSE | |||||
| Non-attempter missing data | N=10 | Df=1,12 | 26.10 | 3.573 | ||
| Attempter missing data | N=3 | 27.00 | 1.732 |
Data Analytical Procedures
First, we determined the demographic characteristics of the attempters and non-attempters. Next, we conducted latent growth curve modeling. In addition, for those models that did not provide an ideal fit for the data, we also conducted analyses of variance (ANOVA) to provide further support for the findings. Finally, hierarchical regression analyses were conducted to determine if WML mediated the relation between suicide attempt history and cognitive decline.
Examination of the data suggested that some variables exhibited positive skew. To address this concern, all skewed variables were transformed following the procedures outlined by McClelland and Judd (25) (e.g., square root transformation was applied). Doing so attenuated the skew to within acceptable limits (skew statistic below 2.0 in SPSS). However, the subsequent results did not significantly change with the inclusion of the skew-corrected variables. Regarding the distribution of lesion volume prior to transformation, there were no participants who had zero lesions, and the average right lesion volume was 3.368 (SD=4.69). The right lesion volumes ranged from 0.23 to 31.40. The left lesion volumes demonstrated a similar distribution.
Latent growth curve modeling was performed using MPlus version 5.2 (26). Each assessment was used as an indicator of a latent intercept (baseline level of the variable) and latent slope (change in the variable over time), as well as a latent quadratic slope (acceleration of change). Standard fit criteria were used to evaluate the overall model fit, with non-significant χ2 value, CFI > .95, TLI > .90, and RMSEA < .06 indicating good fit (27). In large samples, χ2 is often significant regardless of fit; thus, one needs to rely on the other fit indicators (e.g., CFI, TLI, RMSEA) to examine model fit (28). Of note, our growth curve models accounted for time invariance by identifying each growth curve for each variable as having a specified time-ratio relative to the other assessments. For example, a measure assessed monthly can be stated to have a relative time ratio to another measure assessed in 3-month intervals, accounting for this time incongruence. A second consideration is that when there is a significant discrepancy in the size of the groups being compared (attempters versus non-attempters), model fit may be less ideal than when groups are roughly equivalent. Our growth curve models accounted for these missing data points with full information maximum likelihood estimation (FIML (29). This is a procedure for handling missing data that is less biased than ad hoc procedures such as listwise or pairwise deletion, or imputation of means 30,31).
Results
Table 2 presents the demographics by attempt status and associated statistics. Attempters and non-attempters did not differ significantly in age or gender; however, attempters reported significantly fewer years of education compared to non-attempters. Consistent with our prediction that suicide attempts would be associated with a more severe course of depressive disorder, attempters reported a younger age of onset (on average 18 years earlier), twice as many lifetime depressive episodes, more non-suicide related symptoms on the DIS, more current and past psychotic symptoms, and more suicidal ideation (excluding attempts) compared to non-attempters. There were no significant differences by race. Race distribution was as follows: African American 8.9%, Asian 9.3%, Caucasian 86.7%. There were six participants (2.4%) who identified themselves as being of mixed race. Among the participants, one respondent (0.4%) identified him- or herself as Indian, another respondent identified him- or herself as Iranian, one participant (0.4%) identified him- or herself as Lebanese and one participant (0.4%) identified him- or herself as Native American.
Table 2.
Demographic differences between attempters and non-attempters
| Attempters (N=23) Mean (SD); or% |
Non-Attempters (N=223) Mean (SD); or % |
F/χ2 | p-value | |
|---|---|---|---|---|
| Age | 66.74 (6.6) | 69.8 (7.5) | F=3.7 | p =.057 |
| Percent female | 66.7% | 78.3% | p=.350 | |
| Highest grade | 11.87 (4.15) | 13.55 (3.02) | F=8.02 | p = .005 |
| Age of onset | 28.50 (8.4) | 46.64 (20.2) | F=16.2 | P < .001 |
| Lifetime number of episodes | 9.50 (11.37) | 4.07 (3.8) | F=24.03 | P < .001 |
| Number of current DIS symptoms | 11.65 (3.4) | 9.23 (4.0) | F=12.1 | P < .01 |
| Number of psychotic symptoms | 1.48 (2.5) | 0.8 (1.4) | F=4.1 | P = .04 |
Note: df for F-values are 1,245
Based on initial examination of variable distributions, we examined the variables for potential skew. Although the MADRS total scores were not skewed, the suicide items from these questions were skewed. Also demonstrating skew were the MMSE variables and the right and left WML. Transformation of these positively skewed variables was conducted following the procedures outlined in McClelland and Judd (25), for which we applied square root transformations to these variables. Doing so attenuated the skew and brought it to within acceptable limits with all skew statistics reported in SPSS being below 2.0. These transformed data were then used in examination of the original analyses; however, the transformation of the data had trivial impact on results.
Latent Growth Curve Models
MADRS
The latent growth curve model examining attempters and non-attempters’ MADRS scores over the two-year period provided a good fit to the data (χ2=8.96, df=6, p =.17, CFI = 0.99, TLI = 0.96, RMSEA = .047). There were differences between groups on their baseline MADRS scores (intercept; β =.156, B=4.288, SE=1.734, Z=2.473, p= .014), indicating that attempters had a more severe baseline episode than did non-attempters. There were no differences in change of slope over the two-year follow-up period. Thus, residual symptoms did not differentiate attempters from non-attempters.
MADRS Suicidal Ideation
In a latent growth curve model, we examined differences between the two attempt groups on the single rating of the MADRS suicidal ideation item. The latent growth curve model only revealed differences between the groups at baseline (intercept; β = 0.346, B=.141, SE=.040, Z=3.57, p <.001), with attempters reporting higher levels of ideation than non-attempters. No significant differences were found over the two-year follow-up (slope). Because the model was a poor fit for the data, (χ2=219.2, df=25, p <.01, CFI =0.93, TLI =0.90, RMSEA=.102), we conducted ANOVAs to lend further support to the findings. Consistent with the findings, MADRS ratings of suicidal ideation at baseline were higher for the attempters (M=2.35, SD=1.7) than for non-attempters (M=1.4, SD=1.2; F(1,246)= 11.6, p <.01). Analyses for the other seven time points showed only one significant difference over time. At year 2, the attempters (M=1.13, SD=1.8) were rated as having higher suicidal ideation than were the non-attempters (M=0.30, SD=0.68, F(1,246)= 11.6, p <.01). Thus, in general, attempt status did not predict long-term suicidal ideation outcomes.
MMSE
The latent growth curve model examining differences between attempters and non-attempters on the MMSE over a two-year period revealed no differences between the groups at baseline (intercept) or over the two-year follow-up (slope). The model, however, was a poor fit to the data (χ2=136.2, df=38, p <01, CFI =0.91, TLI =0.90, RMSEA=.102), Therefore, we also examined attempters and non-attempters over the two-year period using simple ANOVAs, but still found no differences on the five time points (baseline, six months, 1-year, 1.5-years, 2-years) between attempters and non-attempters.
Neurological Data
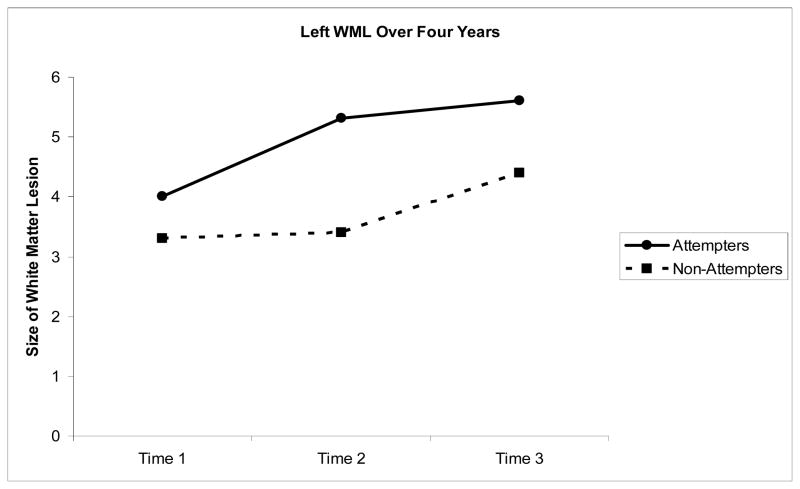
Left Hemisphere White Matter Lesions (Figure 1)
Figure 1.
Volume (ml) in Left matter lesions over four years
The latent growth model provided a good fit to the data (χ2=16.35, df=3, p =.001, CFI =0.98, TLI =0.95, RMSEA=.047). Attempt status predicted WML at baseline (intercept), with attempters having more left WML than non-attempters (β = −.344, B= −.84, SE=.23, Z= −3.64, p <.001). Attempters also had more growth in left WML over time (β =−.059, B= −.344, SE= .132, Z= −2.60, p < .01). To examine whether these findings were due in part to depression severity, we reran the analyses including age, age of onset, cerebrum size, MADRS baseline score, and lifetime number of episodes. The SEM model provided a good fit to the data (χ2=23.178,df= 7, p =.001, CFI =0.98, TLI =0.95, RMSEA=.03). Lifetime number of depressive episodes predicted growth in WML (β = .215, B=10.43, SE=4.00, Z=2.61, p <.01). Attempt status continued to predict baseline intercept (β = −.082, B= −.47, SE=.19, Z= −2.47, p <.05), and the attempters still had more growth in left WML over time (β = −.08, B= −.564, SE=.12, Z = −4.77, p < .001).
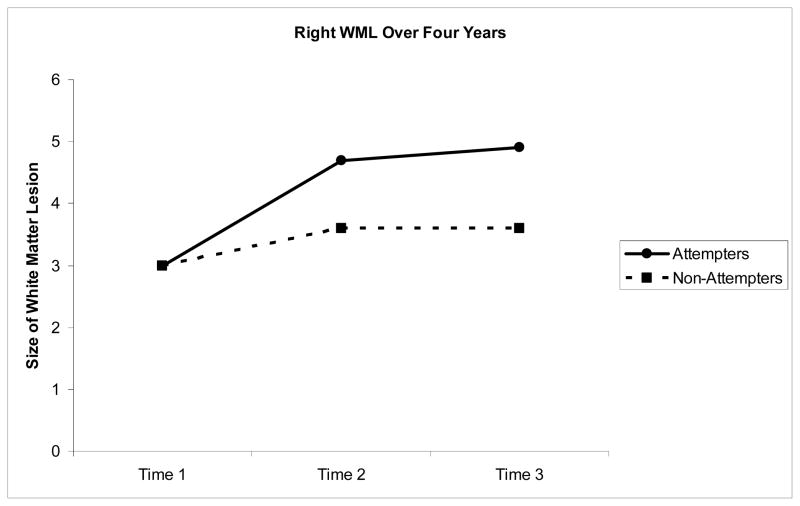
Right Hemisphere White Matter Lesions (Figure 2)
Figure 2.
Volume (ml) in Right matter lesions over four years
The latent growth model provided a good fit to the data (χ2=14.19, df=3, p =.003, CFI =0.99, TLI =0.98, RMSEA=.047). There were no differences in the groups on intercept (B=.045, SE=.028, Z=1.63, p=.10). Consistent with predictions, after controlling for age and baseline cerebrum size, attempters had a greater growth in right WML than did non-attempters (β = −0.36, B=−.70, SE=.20, Z=−3.52, p < .001). We repeated these analyses including age, age of onset, MADRS baseline score, and number of lifetime episodes. The latent growth model provided a good fit to the data (χ2=19.82, df=5, p =.001, CFI =0.99, TLI =0.96, RMSEA=.03). Again, the number of lifetime episodes of depression predicted growth in right-hemisphere WML (β = .224, B= 8.74, SE=3.48, Z=2.51, p =.012). Thus, severity of depression predicted growth in WML. After controlling for depression severity, there were no significant baseline intercept differences in attempt status on WML volume (B=.045, SE=.028, Z=1.63, p=.10). However, we still found attempters to have a greater growth in right WML than non-attempters (β = −.054, B= −.398, SE= −.098, Z= −4.07, p < .01).
Suicide attempt history, WML and the prediction cognitive decline
Although attempt status did not predict change in cognitive functioning in growth curve analyses, we examined whether attempt status predicted change in cognitive functioning from baseline to year two and whether cognitive decline was mediated by WML. These analyses were conducted using the indirect model command in Mplus. As described in Table 3, after controlling for baseline MMSE, age, and education, we found that past suicide attempts did not predict cognitive decline. However, we found that baseline right (but not left) WML predicted cognitive decline (β = −.101, SE =.04, Z= −4.6, p = .005). However, the indirect effects of attempt status to later cognitive decline via changes in WML were not significant. Given that the attempters had more left (but not right) WML at baseline, it appears that both attempt status and left baseline WML are unrelated to cognitive decline, contrary to our hypothesized meditational relationship.
Table 3.
Hierarchical regression analyses: Prediction of cognitive decline from Attempt Status
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Adjusted R2 | .212 | .208 | .283 |
| Degrees of Freedom Model | 3,132 | 5,130 | 6,129 |
| Degrees of Freedom for each variables | 1,132 | 1,130 | 1,129 |
| MMSE (Time 1) | F=4.16 (p=.03) | F=3.85 (p=.05) | F=4.13 (p =.04) |
| Sex | F=.33 (p=.57) | F=.42 (p =.52) | F=5.1 (p =.48) |
| Education | F=9.94 (p=.002) | F=10.23 (p =.002) | F=13.15 (p <.001) |
| Attempted Suicide | F=.181 (p=.671) | F=.214. (p=.644) | |
| Baseline White Matter Lesions (Right) | F=8.1 (p =.005) |
Note. This analysis demonstrates that a history of attempting suicide was unrelated to cognitive decline from baseline to year 2. However, right (but not left) white matter lesions predicted cognitive decline.
Discussion
While previous research has provided evidence for an association between WML and suicidality in young and middle-aged samples (3, 11, 32, 33), knowledge regarding geriatric depressed patients in this regard is limited; moreover, the current study was the first to our knowledge to investigate whether suicide attempt status predicted growth in WML over time. Further, the literature has been equivocal regarding the relationship of suicidality to the severity of the depressive disorder, though the majority of authors have acknowledged a likely relationship (7, 34–36).
The current study compared currently depressed patients (age 60+) who had ever attempted suicide (n=23) to depressed patients who had never attempted suicide (n=223). At baseline, suicide attempters reported more severe past and present symptoms of major depressive disorder (MDD) than non-attempters. Suicide attempters were also found to have more left WML at baseline and a greater growth in both left and right WML over time. Whereas WML at baseline predicted subsequent cognitive decline for the sample as a whole, importantly, history of suicide attempt was unrelated to cognitive functioning.
Our results are consistent with findings of previous research among younger and middle-aged adults identifying WML as possible biological markers for suicidality (12). These findings extend past results by indicating that elder attempters are at a greater risk for subsequent increase in neurological deficits. This could account, in part, for poorer outcomes among those with previous suicide attempts. Regardless of attempt status, growth in WML may place individuals at a greater risk for difficulties in cognitive processing when faced with difficult life circumstances, which may increase risk for suicide. While attempt status did not predict cognitive decline, WML did predict cognitive decline.
As described above, whereas WML at baseline predicted subsequent cognitive decline for the sample as a whole, history of suicide attempt was unrelated to cognitive functioning. Investigators generally agree that the etiology of WML in older adults is related to vascular events in older age (37, 38). It is not known, however, if WML associated with suicidality are also related to vascular events. It may be the case that WML growth is driven by a more severe course of the depressive disorder—which was consistent with one of our analyses. Specifically, in two separate growth curve analyses, we found that lifetime number of depressive episodes predicted growth in left and right WML. Nonetheless, even when controlling for severity of depression, suicide attempts predicted growth in WML. It may also be the case that the areas of the brain that are related to suicide and WML differ from the areas of the brain affected by vascular events. It may be that the areas of the brain affected by vascular events, but not WML associated with attempt status, contribute to general cognitive decline. Clearly, understanding of the similarities and differences associated with WML, associated with vascular events, and by attempt status needs to be better understood. One important line of research to pursue is to determine if the pattern of WML in the brain that resulting from vascular events differs from the pattern associated with attempt status. Future research should examine the etiology of WML and the mechanisms related to WML growth in older adults with a history of suicidality.
It is hypothesized that patients with WML are at higher risk for suicide attempts because of possible disruption of neuroanatomic pathways involved in mood regulation (39, 40). A recent study provided evidence of abnormal connectivity patterns in the prefrontal branch of the default-mode network (DMN; an organized functional network of several brain regions that are active during resting states) in acutely depressed elderly patients (41). Researchers (41,42) found that compared with non-depressed participants, depressed participants had decreased connectivity in the subgenual anterior cingulate cortex and increased connectivity in the dorsomedial prefrontal cortex and orbito-frontal cortex. Moreover, this abnormal connectivity was significantly correlated with white-matter hyperintensity burden. Although Wu et al. (41) did not examine WML in suicide attempters and non-attempters, the results are consistent with the hypothesis that WML contribute to disruptions in neuroanatomical pathways, which may confer a biological vulnerability to future suicidal behavior.
Because WML have been associated with suicide, and WML in the elderly have been associated with cognitive impairment, we proposed a link between suicide attempts and neurological dysfunction (13). We did not, however, find that attempters and non-attempters differed on baseline or follow-up cognitive assessments. However, the MMSE is a general assessment of cognitive functioning, so it is possible that subtle cognitive differences were not detected. Additionally, the MMSE is not an adequate test of executive functioning. Future research should use more sensitive and diverse measures of cognitive functioning. In the current study, while we found that attempts were associated with WML, attempts did not predict cognitive decline. Nonetheless, we found that regardless of participants’ attempt status, WML at baseline predicted a change in cognitive functioning over time. It is possible that our measure was not sensitive enough to find an association between attempts and cognitive decline, mediated by WML. It is also possible that there were some unknown differences in the etiology and consequences of the WML associated with cognitive impairment in depressed elderly and the WML associated with suicide. This is clearly an important area for future investigation.
Another direction for future investigation is a more detailed examination of the associations between suicide attempt status and both baseline and growth in WML volume to determine which association is stronger. In our examination of this association, the strength between baseline WML volume and attempts status was weakened (for the left hemisphere at least) after accounting for number of depressive episodes in the model. On the other hand, when the model accounted for number of depressive episodes, the association between attempt status and WML volume growth increased for both hemispheres. This finding suggests that although depressive episodes may impact WML growth, attempt status may have an influence on WML growth that is independent of depression.
There were several limitations of the present study. First, data on when participants attempted suicide were not available. Therefore, we cannot infer the direction of causality between suicide attempts and WML at baseline, although we did show a history of attempts did predict growth in WML over time. It will be important for future research to examine whether the WML developed before or after participants’ first suicide attempt to determine whether such lesions are risk factors for or consequences of suicide attempts. Second, only a small number of suicide attempters (n=23) were included, limiting statistical power. Despite this, we still found evidence of a significant association between suicide attempt history and the presence of both right and left WML.
Moreover, we did not have data on several risk factors for cognitive decline. Attempters and non-attempters may have had differences on some of these variables. Specifically, we did not have a measure of health functioning, smoking status or functional disability. We cannot rule out the possibility that a third variable may have accounted for the differences we found.
It is also important to note that we had considerable attrition, which is an important limitation. One way it is possible that this limitation may have influenced the results was that for the MRI data we had only 11 suicide attempters at Time 3. If we had a larger sample with more power, we may have found other differences (e.g., right WML) in the data. Furthermore, the attrition in this sample may limit generalizability of the findings to all depressed older adults who have attempted suicide. Another limitation was that handedness of participants in this study was not noted, so we were unable to account for potential handedness effects.
It should be noted that we do not have knowledge as to the rate of change associated with each variable. Some changes may occur at a more rapid rate than other variables. If we had a longer period of follow-up we may have found other variables to be associated to attempt status. Nonetheless, there was a clear organization of the data collected. Patients were uniformly assessed by time in the study. However, whereas the MADRS, MMSE and MRI were all collected at baseline, Year 1 and at Year 2, it is the case that we had more frequent assessments of the MADRS. The availability of more assessments on the MADRS (every 3 months) compared to the MMSE (Baseline, 6 months, Year 1, 18 months, Year 2) may have led to having more power in the growth curve model of the MADRS and relatively less power in the growth of errors on the MMSE. This limitation should be considered.
We should note that a few factors differed by suicide status at baseline that could influence the changes observed. To account for these issues we included baseline education and psychosis experiences as well as other indicators of severity of depression as covariates in the growth curve models. However, addition of these covariates did not change the results. Finally, although attempt status predicted the baseline and growth of WML volume, this association may be a marker for WML growth rather than a contributing factor.
The study also had a number of strengths. First, this is the first study of depressed older adults to test whether suicide attempt is associated with growth in WML. Second, participants were only included in the study if they met criteria for a current major depressive episode at baseline and did not meet criteria for any other disorders (e.g., dementia or preclinical dementia) that could cause neurological problems or account for the increase in WML. Given the exclusion criteria, the present study provides compelling evidence that the identified WML were associated specifically with participants’ history of depression and suicide attempt.
In conclusion, elderly depressed suicide attempters reported more severe past and present symptoms of MDD than non-attempters at the index episode but not at follow-up. Suicide attempters were also found to have more left WML at baseline and a greater growth in both left and right WML over time. As described above, whereas WML at baseline predicted subsequent cognitive decline for the sample as a whole, history of suicide attempts was unrelated to cognitive functioning. Volume changes in the size and number of white (as well as grey matter) lesions (for both hemispheres) have been associated with cognitive functioning. Investigators generally agree that the etiology of WML in older adults is related to vascular events in older age. It is not known, however, if WML associated with suicidality are also related to vascular events. It may be the case that WML growth is impacted by a more severe course of the depressive disorder rather than suicide status—which was consistent with one of our analyses. Specifically, in two separate growth curve analyses, we found that lifetime number of depressive episodes predicted growth in left and right WML. Nonetheless, even after controlling for severity of depression, suicide attempts predicted growth in WML, and, in fact, the association increased after accounting for depressive episodes. It may also be the case that the areas of the brain that are related to suicide and WML differ from the areas of the brain affected by vascular events that contribute to general cognitive decline. Clearly, understanding of the similarities and differences associated with WML associated with vascular events and by attempt needs to be better understood. One important line of research to pursue is to determine if the pattern of WML in the brain that result from vascular events differs from the pattern associated with attempt status. Future research should examine the etiology of WML and the mechanisms related to WML growth in older adults with a history of suicidality.
Acknowledgments
Support: This research was supported by grants R01 MH054846 and K24 MH70027.
Contributor Information
Natalie Sachs-Ericsson, Department of Psychology, Florida State University
Jennifer L. Hames, Department of Psychology, Florida State University
Thomas E. Joiner, Department of Psychology, Florida State University
Elizabeth Corsentino, Department of Psychology, Florida State University
Nicole C. Rushing, Department of Psychology, Florida State University
Emily Palmer, Department of Psychology, Florida State University.
Ian H. Gotlib, Department of Psychology, Stanford University
Edward A. Selby, Department of Psychology, Rutgers University
Steven Zarit, Department of Human Development and Family Studies, Penn State University
David C. Steffens, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center
References
- 1.Manton KG, Blazer DG, Woodbury MA. Suicide in middle age and later life: sex and race specific life table and cohort analyses. Journal Gerontol. 1987;42(2):219–27. doi: 10.1093/geronj/42.2.219. [DOI] [PubMed] [Google Scholar]
- 2.Waern M, Runeson BS, Allebeck P, Beskow J, Rubenowitz E, Skoog I, et al. Mental disorder in elderly suicides: a case-control study. Am J Psychiatry. 2002;159(3):450–5. doi: 10.1176/appi.ajp.159.3.450. [DOI] [PubMed] [Google Scholar]
- 3.Brådvik L, Mattisson C, Bogren M, Nettelbladt P. Long-term suicide risk of depression in the Lundby cohort 1947–1997 – severity and gender. Acta Psychiatr Scand. 2008;117(3):185. doi: 10.1111/j.1600-0447.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- 4.Yeates C, Duberstein P. In: Suicide in older adults: determinants of risk and opportunities for prevention. Hawton K, editor. Oxford, UK: Oxford University Press; 2005. [Google Scholar]
- 5.Uebelacker LA, Strong D, Weinstock LM, Miller IW. Likelihood of suicidality at varying levels of depression severity: a re-analysis of NESARC data. Suicide LifeThreat Behav. 2011;40(6):620. doi: 10.1521/suli.2010.40.6.620. [DOI] [PubMed] [Google Scholar]
- 6.Alexopoulos GS, Bruce ML, Hull J, Sirey JA, Kakuma T. Clinical determinants of suicidal ideation and behavior in geriatric depression. Arch Gen Psychiatry. 1999;56(11):1048–53. doi: 10.1001/archpsyc.56.11.1048. [DOI] [PubMed] [Google Scholar]
- 7.Tan LL, Wong HB. Severity of depression and suicidal ideations among elderly people in Singapore. Int Psychogeriatr. 2008;20(02):338–46. doi: 10.1017/S1041610207005789. [DOI] [PubMed] [Google Scholar]
- 8.APA. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. Washington, D.C: APA; 1994. [Google Scholar]
- 9.Hepple J, Quinton C. One hundred cases of attempted suicide in the elderly. Br J Psychiatry. 1997;171(1):42–6. doi: 10.1192/bjp.171.1.42. [DOI] [PubMed] [Google Scholar]
- 10.Ehrlich S, Noam GG, Lyoo IK, Kwon BJ, Clark MA, Renshaw PF. White matter hyperintensities and their associations with suicidality in psychiatrically hospitalized children and adolescents. J Am Acad Child Adoles Psychiatry. 2004;43(6):770. doi: 10.1097/01.chi.0000120020.48166.93. [DOI] [PubMed] [Google Scholar]
- 11.Ehrlich S, Breeze JL, Hesdorffer DC, Noam GG, Hong X, Alban RL, et al. White matter hyperintensities and their association with suicidality in depressed young adults. J AffectDisord. 2005;86(2–3):281. doi: 10.1016/j.jad.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Grangeon MC, Seixas C, Quarantini LC, Miranda-Scippa A, Pompili M, Steffens DC, et al. White matter hyperintensities and their association with suicidality in major affective disorders: a meta-analysis of magnetic resonance imaging studies. CNS Spectr. 2010;15(6):375–81. doi: 10.1017/s1092852900029242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jollant F, Lawrence NL, Olià E, Guillaume Sb, Courtet P. The suicidal mind and brain: a review of neuropsychological and neuroimaging studies. World J Biol Psychiatry. 2011;12(5):319–39. doi: 10.3109/15622975.2011.556200. [DOI] [PubMed] [Google Scholar]
- 14.Steffens DC, Potter GG, McQuoid DR, MacFall JR, Payne ME, Burke JR, et al. Longitudinal magnetic resonance imaging vascular changes, apolipoprotein E genotype, and development of dementia in the neurocognitive outcomes of depression in the elderly study. Am J Geriatr Psychiatry. 2007;15(10):839–49. doi: 10.1097/JGP.0b013e318048a1a0. [DOI] [PubMed] [Google Scholar]
- 15.Ahearn EP, Jamison KR, Steffens DC, Cassidy F, Provenzale JM, Lehman A, et al. MRI correlates of suicide attempt history in unipolar depression. Biol Psychiatry. 2001;50(4):266. doi: 10.1016/s0006-3223(01)01098-8. [DOI] [PubMed] [Google Scholar]
- 16.de Leeuw FE, Barkhof F, Scheltens P. Progression of cerebral white matter lesions in Alzheimer’s disease: a new window for therapy? J Neurol Neurosurg Psychiatry. 2005;76(9):1286–8. doi: 10.1136/jnnp.2004.053686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dombrovski AY, Clark L, Siegle GJ, Butters MA, Ichikawa N, Sahakian BJ, et al. Reward/punishment reversal learning in older suicide attempters. Am J Psychiatry. 2011;167(6):699–707. doi: 10.1176/appi.ajp.2009.09030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steffens DC, Welsh-Bohmer KA, Burke JR, Plassman BL, Beyer JL, Gersing KR, et al. Methodology and preliminary results from the Neurocognitive Outcomes of Depression in the Elderly study. J Geriatr Psychiatry Neurol. 2004;17(4):202–11. doi: 10.1177/0891988704269819. [DOI] [PubMed] [Google Scholar]
- 19.Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule: its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38(4):381–9. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 20.Williams JBW, Kobak KA. Development and reliability of a structured interview guide for the Montgomery-Asberg Depression Rating Scale (SIGMA) Br J Psychiatry. 2008;192(1):52–8. doi: 10.1192/bjp.bp.106.032532. [DOI] [PubMed] [Google Scholar]
- 21.Folstein M, Folstein S, McHugh P. Mini-Mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Chen PS, McQuoid DR, Payne ME, Steffens DC. White matter and subcortical gray matter lesion volume changes and late-life depression outcome: a 4-year magnetic resonance imaging study. Int Psychogeriatr. 2006;18(3):445–56. doi: 10.1017/S1041610205002796. [DOI] [PubMed] [Google Scholar]
- 23.Steffens D, Payne M, Greenberg D, Byrum C, Welsh-Bohmer K, Wagner H, et al. Hippocampal volume and incident dementia in geriatric depression. Am J Geriatr Psychiatry. 2002;10:62–71. [PubMed] [Google Scholar]
- 24.Taylor WD, Macfall JR, Payne ME, McQuoid DR, Steffens DC, Provenzale JM, et al. Orbitofrontal cortex volume in late life depression: influence of hyperintense lesions and genetic polymorphisms. Psychol Med. 2007;37(12):1763–73. doi: 10.1017/S0033291707000128. [DOI] [PubMed] [Google Scholar]
- 25.Judd CM, McClelland GH. Data analysis: A model comparison approach. New York: Harcourt Brace Jovanovich; 1989. [Google Scholar]
- 26.Muthén LK, Muthén BO. Mplus User’s Guide. 5. Los Angeles, CA: Muthén & Muthén; 2007. (1998–2007) [Google Scholar]
- 27.Hu L-T, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6(1):1–55. [Google Scholar]
- 28.Kline RB. Structural Equation Modeling. 2. New York: Guildford Press; 2005. Details of path analysis; pp. 133–5. [Google Scholar]
- 29.Anderson TW. Maximum likelihood estimates for a multivariate normal distribution when some observations are missing. J Am Stat Assoc. 1957;52:200–3. [Google Scholar]
- 30.Little RJ, Rubin DB. Statistical analysis with missing data. New York: Wiley; 1987. [Google Scholar]
- 31.Schafer JL. Analysis of incomplete multivariate data. London: Chapman & Hall; 1997. [Google Scholar]
- 32.Pompili M, Ehrlich S, De Pisa E, Mann J, Innamorati M, Cittadini A, et al. White matter hyperintensities and their associations with suicidality in patients with major affective disorders. Eur Arch Psychiatry Clin Neurosci. 2007;257(8):494. doi: 10.1007/s00406-007-0755-x. [DOI] [PubMed] [Google Scholar]
- 33.Pompili M, Innamorati M, Mann JJ, Oquendo MA, Lester D, Del Casale A, et al. Periventricular white matter hyperintensities as predictors of suicide attempts in bipolar disordersand unipolar depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1501. doi: 10.1016/j.pnpbp.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Kessing LV. Severity of depressive episodes according to ICD-10: prediction of risk of relapse and suicide. Br J Psychiatry. 2004;184(2):153–6. doi: 10.1192/bjp.184.2.153. [DOI] [PubMed] [Google Scholar]
- 35.Kessing LV. Subtypes of depressive episodes according to ICD-10: prediction of risk of relapse and suicide. Psychopathology. 2003;36(6):285–91. doi: 10.1159/000075186. [DOI] [PubMed] [Google Scholar]
- 36.Spijker J, de Graaf R, ten Have M, Nolen W, Speckens A. Predictors of suicidality in depressive spectrum disorders in the general population: results of the Netherlands Mental Health Survey and Incidence Study. Soc Psychiatry Psychiatr Epidemiol. 2010;45(5):513–21. doi: 10.1007/s00127-009-0093-6. [DOI] [PubMed] [Google Scholar]
- 37.Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular Depression’ hypothesis. Arch Gen Psychiatry. 1997;54(10):915–22. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 38.Baldwin RC, O’Brien J. Vascular basis of late-onset depressive disorder. Br J Psychiatry. 2002;180(2):157–60. doi: 10.1192/bjp.180.2.157. [DOI] [PubMed] [Google Scholar]
- 39.Soares JC, Mann JJ. The anatomy of mood disorders-review of structural neuroimaging studies. Biol Psychiatry. 1997;41:86– 106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 40.Taylor WD, Payne ME, Krishnan KRR, Wagner HR, Provenzale JM, Steffens DC, et al. Evidence of white matter tract disruption in MRI hyperintensities. Biol Psychiatry. 2001;50(3):179. doi: 10.1016/s0006-3223(01)01160-x. [DOI] [PubMed] [Google Scholar]
- 41.Wu M, Andreescu C, Butters MA, Tamburo R, Reynolds CF, Aizenstein H. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Res. 2011;194(1):39–46. doi: 10.1016/j.pscychresns.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]