Abstract
Introduction:
Previous exercise intervention studies for smoking cessation have been challenged by a number of methodological limitations that confound the potential efficacy of aerobic exercise for smoking cessation.
Methods:
The preliminary efficacy of a behavioral exercise intervention that incorporated features designed to address prior limitations was tested in a randomized controlled trial (RCT). Sixty-one smokers (65.6% female, mean age = 47.3 years, smoked a mean of 19.7 cigarettes/day) were randomized to receive either a 12-week exercise intervention or a 12-week health education contact control. Participants in both conditions received an 8-week telephone-delivered, standard smoking cessation protocol (with the transdermal nicotine patch). Follow-ups were conducted at the end of treatment (EOT), 6- and 12-month timepoints.
Results:
There were no differences between conditions with respect to the number of weekly exercise or health education sessions attended (9.3±2.8 vs. 9.3±3.0, respectively). While not statistically significant, participants in the exercise condition demonstrated higher verified abstinence rates (EOT: 40% vs. 22.6%, odds ratio [OR] = 2.28; 6- and 12-month follow-ups: 26.7% vs. 12.9%, OR = 2.46). Irrespective of treatment condition, higher levels of moderate-to-vigorous exercise were associated with lower levels of depressive symptoms during the intervention.
Conclusions:
The results of this small RCT point toward the benefit of a behavioral exercise intervention designed to address previous methodological limitations for smoking cessation. Given the potential public health impact of the demonstrated efficacy of exercise for smoking cessation, the continued development and optimization of exercise interventions for smokers through larger RCTs merits pursuit.
INTRODUCTION
The role of regular aerobic exercise (AE) as a potential aid to smoking cessation and reducing relapse risk is receiving increasingly greater attention (Ussher, Taylor, & Faulkner, 2012). In practice, exercise is now routinely recommended as a cessation aid by smoking professionals (Everson, Taylor, & Ussher, 2010) and is also endorsed by the current Clinical Practice Guideline for Treating Tobacco Use and Dependence (Fiore et al., 2008). Many smokers themselves identify exercise as a potentially useful quitting strategy (Everson-Hock, Taylor, & Ussher, 2010). Also, although smokers engage in regular exercise at lower levels than the national population (Emmons, Marcus, Linnan, Rossi, & Abrams, 1994; French, Hennrikus, & Jeffery, 1996), those that are exercising regularly upon making a quit attempt appear to be more likely to achieve long-term abstinence (Abrantes et al., 2009).
Nevertheless, in a recently updated Cochrane review, Ussher and colleagues (2012) concluded that there is currently insufficient evidence to determine whether regular exercise is an effective intervention strategy to promote smoking cessation. They also noted that most existing randomized controlled trials (RCTs) had significant methodological limitations, including small samples, interventions of insufficient intensity, lack of efforts to increase adherence, lack of contact control conditions, lack of objective measures of physical activity and fitness, and insufficient attention to temporal sequencing of exercise and quitting.
Regarding the important issue of adherence, rates have varied but have been generally and consistently quite low across studies. It can be argued that, without actual engagement in the exercise, the efficacy of exercise cannot be fairly determined. Findings from Marcus et al. (2005) found that, while there was no overall treatment effect between exercise and a health education control condition, when smokers engaged in higher levels of moderate-intensity AE per week, smoking abstinence was significantly higher. In an effort to address the issue of adherence, Williams and colleagues (2010) conducted a small, randomized study of an 8-week exercise program for female smokers with design features to ensure compliance such as enrolling only highly motivated women who completed a 2-week run-in, requiring all exercise to be supervised, and financially incentivizing attendance. Compared to controls, participants in the exercise condition were three times more likely to be abstinent at the end of treatment (EOT) and twice as likely to be abstinent at the 1-month follow-up. However, this study employed features that limited potential translation to a real-world context (e.g., 2-week run-in and all exercise was supervised at the study facility).
In addition to these limitations, up until recent years many exercise intervention studies incorporated physical activity of vigorous intensity. However, vigorous intensity exercise may not be appropriate for current smokers due to their compromised respiratory capacity. Indeed, Ussher, Nunziata, Cropley, & West (2001) found that smokers exercising at moderate intensity (as measured by heart rate) perceived their exertion to be in the high-intensity range (as measured by rate of perceived exertion [RPE]). As such, vigorous intensity exercise may be too difficult for sedentary smokers and ultimately affect exercise adherence. In addition, moderate-intensity (as opposed to vigorous intensity) AE has been consistently associated with higher levels of exercise adherence across varied populations (Perri et al., 2002).
Furthermore, the studies with the fewest methodological limitations (Kinnunen et al., 2008; Marcus et al., 1999; Marcus et al., 2005; Prapavessis et al., 2007) were only conducted with female smokers with exercise introduced as a means of decreasing post-cessation weight gain. However, weight gain can also be a relapse risk for male smokers (Borrelli, Spring, Niaura, Hitsman, & Papandonatos, 2001) and given the higher rates of smoking among males (CDC, 2011), well-designed studies examining the effect of exercise interventions for male smokers are also necessary. In addition, in both men and women, interventions that introduce exercise in a manner that extends beyond weight management to include benefits related to fitness and mental health functioning (e.g., improved mood and coping) are important to examine.
In the current study, we tested the preliminary efficacy of an AE intervention for smokers that was specifically designed to address these identified methodological limitations and allow for a more generalizable and disseminable intervention. The intervention included the following features: (a) moderate-intensity exercise, (b) a sequential approach through adoption of exercise for 1 month prior to quit date, (c) combined supervised plus home-based exercise, (d) inclusion of both female and male smokers, (e) a contingency-based, financial incentive component to increase exercise adherence, and (f) inclusion of a cognitive–behavioral group intervention to promote exercise adoption and adherence. Another notable aspect was the use of telephone counseling for smoking cessation, rather than face to face counseling, in considering future dissemination of the AE intervention as an adjunct to tobacco quitlines (e.g., Segan et al., 2011). In this initial RCT, AE was compared to a health education contact control (HEC) intervention that was designed to include the same level of contact between treatment providers. Both the AE and HEC groups received the same telephone counseling intervention for smoking cessation, as well as the transdermal nicotine patch (TNP). We hypothesized that abstinence rates would be significantly higher in the AE group relative to HEC, and that the AE group would show evidence of decreased cravings, withdrawal symptoms, and negative affect relative to HEC.
METHODS
Procedure
Participants were recruited from newspaper and radio advertisements. Participants were screened via telephone for current smoking level (at least 10 cigarettes/day) and extent of physical inactivity (had not participated regularly in AE for at least 20min per day, 3 days per week for the past 6 months). Participants appearing to meet study criteria were scheduled for a more comprehensive baseline assessment. Exclusion criteria included: current DSM-IV Axis I criteria for alcohol or drug abuse/dependence, bipolar disorder, eating disorder, psychotic disorder, current suicidality or homicidality, physical disabilities or medical problems prohibitive of engaging in AE (i.e., denied medical clearance by primary care physician), current pregnancy or intention to become pregnant during the following 3 months (i.e., during treatment), and current use of pharmacotherapy for smoking cessation (including nicotine replacement therapy).
Research staff obtained informed consent and then participants were evaluated using the diagnostic and screening measures detailed below to confirm eligibility. Upon receiving medical clearance to exercise, participants were assigned to one of two conditions using urn randomization (Stout, 1988), with body mass index (BMI), level of nicotine dependence, gender, and age included as blocking variables: (a) a 12-session, group AE intervention, or (b) HEC, equated for therapist and participant contact time. Participants in both conditions received an 8-session telephone counseling smoking cessation intervention that included TNP. Assessments occurred at baseline, 3(EOT)-, 6-, and 12-month follow-ups, with 85%, 80%, and 74% completion rates, respectively. Participants were compensated $50 for each follow-up assessment.
Participants
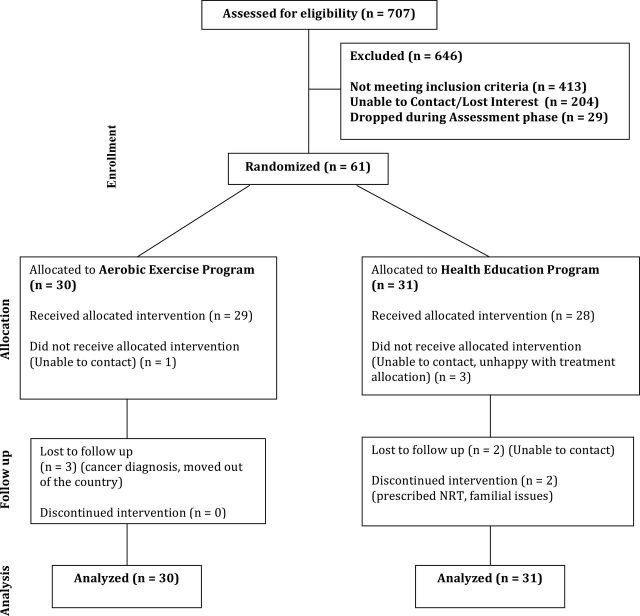
A total of 707 smokers expressed interest in participating in the study. Participants were excluded for the following reasons: too physically active (22%), an Axis I psychiatric disorder (15.4%), not able to attend group AE or HEC sessions (8.2%), a medical condition (5.7%), smoking less than 10 cigarettes/day (4.1%), and other reasons (3.1%; e.g., age, taking medications for cessation, no phone, already in another smoking study, and non-English speaker). An additional 32.7% of participants lost interest in the study after initial contact (n = 204) or at some point during the baseline assessment procedures (n = 29). Sixty-one participants (65.6% female; mean age of 47.3 years) were recruited and randomized to AE (n = 30) and HEC (n = 31). See Figure 1 for consort figure and Table 1 for baseline demographic characteristics.
Figure 1.
Consort figure.
Table 1.
Baseline Demographic, Smoking, and Exercise/Fitness Characteristics
| AE (n = 30) | HEC (n = 31) | |
|---|---|---|
| Demographic | ||
| Gender (% female) | 63.3% | 67.7% |
| Age (mean years [SD]) | 47.1 (8.5) | 47.5 (10.7) |
| Non-Hispanic White | 93.3% | 83.9% |
| Married or cohabitating | 51.7% | 37.9% |
| College graduate | 34.6% | 31.0% |
| Household income less than $30,000/year | 21.4% | 44.4% |
| Employed | 72.4% | 58.6% |
| Smoking | ||
| Smoking age of onset | 15.1 (3.5) | 15.9 (5.6) |
| Years of regular smoking | 27.4 (9.1) | 27.5 (11.1) |
| Number of lifetime quit attempts | 3.2 (2.8) | 4.4 (3.1) |
| Cigarettes/day | 20.3 (9.9) | 19.4 (8.1) |
| Nicotine dependence (FTND) | 5.9 (2.1) | 5.6 (1.6) |
| Exercise/fitness | ||
| Body mass index | 28.5 (4.2) | 28.9 (7.0) |
| Peak VO2 (ml/kg/min) | 27.8 (5.8) | 26.2 (9.6) |
| Percent body fat | 33.0 (6.9) | 32.1 (8.5) |
Note. AE = aerobic exercise; FTND = Fagerstrom Test for Nicotine Dependence; HEC = health education control.
AE Intervention
AE Component
Participants in the AE condition attended AE sessions, supervised by an exercise physiologist, once a week at the research fitness facility. Additionally, participants were given exercise prescriptions to engage in exercise a minimum of two to four (depending on the week of the intervention) additional times a week in the context of their own environment (e.g., in their home or through community resources) with a goal of progressing to 100 min of moderate-intensity exercise per week midway through the intervention and 150min per week by the last several weeks of the 12-week intervention. Participants were instructed to self-monitor their exercise by completing a weekly exercise log that included the type, duration, and RPE for each activity. Exercise sessions began at 20min per session with weekly gradual increases, with heart rate monitoring by the exercise physiologist to ensure training in the moderate-intensity range of 55%–69% of age-predicted maximal heart rate. Several types of exercise equipment were available to participants, including treadmills, recumbent bicycles, and elliptical machines.
Cognitive–Behavioral Counseling for Exercise Promotion Component
Given the demonstrated efficacy of behavioral modification strategies in increasing physical activity (see meta-analysis by Dishman & Buckworth, 1996), behavioral techniques were incorporated into the exercise intervention condition through 20-min weekly group sessions held on the same day and just prior to exercise sessions. Through these weekly groups, participants were guided on how to increase overall physical activity through behavioral changes in their daily lives. Each group session was focused on a certain topic designed to increase motivation to improve exercise adoption and maintenance (e.g., psychological and physical health benefits of exercise, barriers, time management, maintaining motivation, goal setting, social support).
Financial Incentive Component
In order to maximize adherence, participants were entitled to incentives for various levels of compliance to the exercise program. Participants received $5 for attending each combined exercise/cognitive–behavioral group session and $5 for returning their completed exercise self-monitoring form from the prior week (irrespective of actual exercise completed). In addition, they drew prizes from a fish bowl (value ranging from $10 to $50) for attending the weekly exercise counseling + exercise sessions on consecutive weeks.
HEC Intervention
Participants in the HEC condition were asked to attend weekly hour-long health education sessions on topics such as oral health, heart disease, cancer, sleep hygiene, and secondhand smoke, as they related to the effects of smoking. Health information was conveyed through lectures, handouts, in-group exercises, and Internet resources. Participants were not encouraged to make any changes in their behavior nor was physical activity discussed. Nevertheless, it was possible participants in the HEC condition would decide to begin exercising on their own. Participants in the HEC condition were also provided with the same incentives on the same “delivery schedule” as those in the AE condition, including the fish bowl drawings (see above), except they were paid the full $10 for attending each session as they had no self-monitoring forms to return.
Telephone Counseling Smoking Cessation Treatment
Participants in both the AE and HEC intervention received identical smoking cessation treatment consistent with the most recent Clinical Practice Guideline for Treating Tobacco Use and Dependence (Fiore et al., 2008). Treatment was delivered in 8, 20-min weekly telephone counseling sessions beginning in week 1 of the intervention. Four sessions were conducted prior to quit day (weeks 1–4) and focused on identifying high-risk situations and developing behavioral and cognitive strategies for coping with high-risk situations. The remaining four sessions were conducted on and after quit day (weeks 5–8) and focused on discussing quitting experiences, providing support, and developing relapse prevention strategies. All participants were given a standard 8-week course of Nicoderm CQ, 24-hr TNPs (21-mg strength for weeks 5–8, 14-mg strength for weeks 9–10, and 7-mg strength for weeks 11–12) and were educated about the use of the patch prior to quit date. Smokers who lapsed during treatment were encouraged to set a new quit date and continue to attempt to quit. Further, given that wearing the nicotine patch during exercise may increase the absorption of nicotine into the bloodstream, participants were instructed to remove the patch 1hr prior to engaging in exercise both during supervised and home-based sessions.
Measures
Phone Screen for Enrollment: In addition to screening about smoking and exercise behavior to determine preliminary eligibility, demographic information such as date of birth, gender, ethnicity/race, marital status, and education were collected.
The Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Version (First, Spitzer, Gibbon, & Williams, 1995): Relevant sections (mood, anxiety, psychotic screen, substance use disorders) were administered to confirm diagnostic eligibility.
Smoking History Interview: This measure was designed by the researchers and gathered smoking-related information including but not limited to: age of smoking initiation, number of years smoking, and previous quit attempts.
Timeline Followback—Smoking (Brown et al., 1998): Self-reports of smoking status were gathered at baseline and at 3-, 6-, and 12-month follow-up assessments using the timeline followback (TLFB) method adapted for smoking behavior. We have validated this procedure in a previous study (Brown et al., 1998).
Smoking Status: Self-reports of smoking status (7-day point prevalence abstinence) were collected from participants on weeks 5 through 12 during treatment, and at the 3-, 6-, and 12-month follow-ups. Self-reports of abstinence were verified by expired carbon monoxide (CO; utilizing an 10 ppm cutoff; SRNT Subcommittee on Biochemical Verification, 2002). In cases where CO was not available, significant other (SO) reports were used—a common, valid approach toward corroborating self-report in the absence of biochemical verification (Emont, Collins, & Zywiak, 1991; Ossip-Klein et al., 1991).
The Fagerstrom Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991) was used as a continuous measure of nicotine dependence.
The Center for Epidemiological Studies Depression Scale (CES-D, Radloff, 1977) was used for assessing depressive symptoms. This 20-item instrument has demonstrated good reliability and validity (Radloff, 1977).
The Positive and Negative Affect Scale (PANAS, Watson, Clark, & Tellegen, 1988), a 20-item self-report scale that has demonstrated good reliability and validity, was used to assess changes in mood.
Body mass index (BMI): Consistent with American College of Sports Medicine guidelines, BMI was calculated by dividing body weight in kilograms by height in meters squared. Height and weight was obtained by utilizing a Detecto medical scale.
Nicotine Withdrawal Symptoms: Withdrawal severity (categorized into craving, negative affect, somatic, and sleep symptoms) at baseline, weekly throughout treatment, and at 12-week follow-up (the point of patch discontinuation) was monitored using the Minnesota Withdrawal Scale, a reliable and sensitive scale (Hughes & Hatsukami, 1986).
Levels of Physical Activity: During each of the smoking TLFB interviews participants, reported an estimate of their daily physical activity including duration and intensity (by reporting RPE; (Borg, 1970). Levels of moderate-to-vigorous physical activity (MVPA) were calculated by summing all activity that was done at RPE levels of 11 or higher (Borg, 1970). The TLFB approach has shown good reliability and validity when utilized as an assessment of exercise behavior (Panza, Weinstock, Ash, & Pescatello, 2012).
Cardiorespiratory Fitness: The Rockport 1-Mile Walk Test is a measure of cardiorespiratory fitness for adults and has been validated for use on a treadmill (Pober, Freedson, Kline, McInnis, & Rippe, 2002). Duration of the walk test and heart rate upon completion were utilized to calculate an estimate of VO2peak-a measure of peak aerobic capacity and indication of cardiorespiratory fitness.
Data Analysis
Given the developmental nature of this project as well as the difficulty in utilizing effect sizes obtained from small pilot studies to power for larger trials (Kraemer & Kupfer, 2006; Loscalzo, 2009), the goal of this study was to examine the initial effects of the AE intervention with an understanding that our confidence intervals could potentially be large. However, important information for evaluating a new intervention and informing the design of future large-scale trials could nonetheless be obtained from the current study. Therefore, while a priori power analysis was not conducted, the obtained sample size of 61 (n = 30 for AE and n = 31 for HEC) provides relatively stable group means for the dependent measures of interest.
The main outcome analyses were based upon 7-day point prevalence abstinence (i.e., reported abstinence of at least 7 days prior to each scheduled follow-up) and used an intent to treat sample for primary outcomes. Self-report was always overridden by objective verification in the conservative direction; that is, smoking (Shumaker, 1986). Missing self-reports also were counted as smoking. Tests of the effects of treatment on the primary outcome variable (biochemically verified 7-day point prevalence abstinence) at the EOT (i.e., 3-month follow-up), 6- and 12-month follow-ups were conducted using generalized estimating equations (Liang & Zeger, 1986; Zeger & Liang, 1986). To compare levels of positive affect, negative affect, depressive symptoms, craving, withdrawal symptoms, levels of MVPA, and cardiorespiratory fitness assessed weekly from quit day (week 5) through the end of the 12-week treatment, we used linear mixed models that adjusted for gender, level of nicotine dependence, and corresponding baseline measures. Similar models evaluating changes in positive affect, negative affect, depressive symptoms, and withdrawal symptoms during the intervention were also conducted to examine the time-varying effects of MVPA irrespective of treatment condition. All analyses assumed alpha = 0.05.
RESULTS
Program Compliance
Thirty-one participants were randomized to HEC; three did not start the intervention for a total of n = 28 who attended at least one HEC session. Thirty participants were randomized to AE; one did not initiate the intervention for a total of n = 29 who attended at least one AE session. Among those initiating either intervention (n = 57), there was no difference between AE and HEC on the extent of program compliance, with participants in each group attending a similar number of group sessions (9.3±2.8 vs. 9.3±3.0 out of 12 sessions, for AE and HEC, respectively) Males and females did not significantly differ on the number of sessions attended. Abstinence at the end of the intervention was significantly associated with greater number of group sessions attended during the intervention in both conditions (10.8 vs. 7.8 sessions in AE; t = 3.18, df = 22, p < .01 and 11.6 vs. 7.5 sessions in HEC; t = 4.84, df = 25, p < .001). In addition to the weekly, supervised exercise session, participants engaged in various types of physical activity at home, during the remainder of the week. Activities included: walking (79.3%), treadmill (51.7%), bicycling (41.4%), sports (31.0%), housework (31.0%), elliptical trainer (31.0%), resistance training (20.7%), calisthenics (20.7%), aerobics (17.2%), yoga (17.2%), and running (3.2%).
Program compliance was also high for the telephone-delivered smoking cessation sessions and use of the nicotine patch. Out of a total of eight phone calls, participants completed an average of 6.95 sessions (6.9±1.5 vs. 7.0±1.8, for AE and HEC, respectively) and men and women did not differ on number of completed phone calls. As expected, compliance with use of the nicotine patch was higher in the earlier weeks of cessation and decreased over the course of the 8 weeks of use as participants resumed smoking—percentage of sample compliant with the nicotine patch: week 5 (quit week; 82.3%), week 6 (85.7%), week 7 (77.2%), week 8 (76.3%), week 9 (65.8%), week 10 (65.1%), week 11 (59.6%), and week 12 (25.9%).
As stated in the Methods section above, participants were instructed to remove the nicotine patch 1hr prior to engaging in any AE. Overall, this instruction was well received and there were no reports of any adverse events for utilizing the nicotine patch in the context of an exercise program.
Cessation Outcomes
See Table 2 for abstinence rates and odds ratios at each timepoint. Verified 7-day point prevalence abstinence at the end of the 12-week treatment, at 6-month follow-up, and at 12 month follow-up, respectively, was 40.0%, 26.7%, and 26.7% in AE compared to 22.6%, 12.9%, and 12.9% in HEC. Continuous abstinence at the end of the 12-week treatment, at 6-month follow-up, and at 12-month follow-up, respectively, was 30.0%, 23.3%, and 13.3% in AE compared to 25.8%, 9.7%, and 3.2% in HEC. Abstinence was primarily CO-verified with SO verification occurring for only one participant at the 6-month follow-up and one participant at the 12-month follow-up.
Table 2.
Verified 7-Day Point Prevalence and Continuous Abstinence Rates
| Assessment timepoint | 7-day point prevalence | Continuous abstinence | ||||
|---|---|---|---|---|---|---|
| AE (n = 30) | HEC (n = 31) | OR (95% CI) | AE (n = 30) | HEC (n = 31) | OR (95% CI) | |
| End of treatment (8 weeks postquit date) | 40.0% | 22.6% | 2.28 (0.75, 6.96) | 30.0% | 25.8% | 1.23 (0.4, 3.78) |
| 6-month follow-up (21 weeks postquit date) | 26.7% | 12.9% | 2.46 (0.65, 9.26) | 23.3% | 9.7% | 2.83 (0.66, 12.18) |
| 12-month follow-up (47 weeks postquit date) | 26.7% | 12.9% | 2.46 (0.65, 9.26) | 13.3% | 3.2% | 4.64 (0.48, 44.5) |
Note. AE = aerobic exercise; HEC = health education control; OR = odds ratio; CI = confidence interval.
The generalized estimating equation model evaluating EOT, 6- and 12-month verified abstinence included gender and level of dependence as planned covariates along with the linear effect of time. While participants in AE were on average 2.02 (95% CI = 0.71–6.04) times as likely to be abstinent relative to HEC throughout the entire year, this overall effect was not statistically significant (b = 0.72, SE = 0.55, p = .18). The effect of AE was reduced when examining continuous abstinence (b = −0.59, SE = 0.60, p = .33). We examined the potential moderating effect of gender and the interaction term with treatment was not statistically significant for point prevalence (b = 0.74, SE = 1.23, p = .55) or continuous abstinence (b = −1.24, SE = 1.48, p = .40).
Changes in Moods and Depressive Symptoms After Quitting
See Table 3 for means and SD of depressive symptoms, affect, and withdrawal symptoms for each condition across weeks 5–12 of the intervention. CES-D values were square root transformed due to positive skewness and beneficial normalizing effect on residuals. Prior to quit day, there were no significant differences in levels of positive affect (b = −0.60, SE = 2.40, p = .80), negative affect (b = −0.09, SE = 1.08, p = .93), or depressive symptoms (b = −1.93, SE = 1.97, p = .33). There was a significant decrease in negative affect (b = −0.23, SE = 0.09, p = .01) and depressive symptoms (b = −0.06, SE = 0.03, p = .02) following quit day. There was no detectable change in average levels of positive affect following quit day and considerable variability was observed (b = 0.03, SE = 0.14, p = .80). There were no significant overall differences in level of positive affect (b = −0.65, SE = 1.65, p = .69), negative affect (b = −0.45, SE = 1.58, p = .78), or depressive symptoms (b = −0.13, SE = 0.35, p = .72) or changes in these indices between AE and HEC participants.
Table 3.
Mean (SD) of Depressive Symptomatology, Affect, and Withdrawal Symptoms Between Treatment Conditions
| AE (n = 30) | HEC (n = 31) | Effect size (Cohen’s d) | |
|---|---|---|---|
| CES-D scores | |||
| Baseline | 6.3 (6.3) | 8.2 (7.2) | |
| Week 5 (quit date) | 7.3 (7.2) | 8.0 (6.2) | 0.09 |
| Week 7 (2 weeks postquit date) | 6.7 (9.7) | 10.9 (8.9) | 0.45 |
| Week 12 (last week of treatment) | 1.5 (9.1) | 9.4 (7.4) | 0.95 |
| PANAS positive affect | |||
| Baseline | 3.6 (0.9) | 3.5 (0.8) | |
| Week 5 (quit date) | 3.6 (0.9) | 3.7 (0.7) | 0.11 |
| Week 7 (2 weeks postquit date) | 3.9 (0.9) | 3.3 (1.0) | 0.39 |
| Week 12 (last week of treatment) | 4.1 (0.8) | 3.7 (0.8) | 0.53 |
| PANAS negative affect | |||
| Baseline | 1.2 (0.3) | 1.3 (0.5) | |
| Week 5 (quit date) | 1.4 (0.7) | 1.4 (0.3) | 0.00 |
| Week 7 (2 weeks postquit date) | 1.4 (0.8) | 1.4 (0.5) | 0.09 |
| Week 12 (last week of treatment) | 1.1 (0.2) | 1.2 (0.3) | 0.59 |
| Withdrawal symptoms—negative affect | |||
| Week 5 (quit date) | 5.1 (2.5) | 4.5 (1.5) | 0.29 |
| Week 7 (2 weeks postquit date) | 4.2 (2.0) | 5.6 (2.0) | 0.70 |
| Week 12 (last week of treatment) | 3.1 (0.3) | 4.6 (2.0) | 1.05 |
| Withdrawal symptoms—craving | |||
| Week 5 (quit date) | 4.9 (1.7) | 4.8 (1.3) | 0.07 |
| Week 7 (2 weeks postquit date) | 4.0 (1.6) | 4.4 (1.4) | 0.27 |
| Week 12 (last week of treatment) | 3.5 (1.2) | 3.9 (1.7) | 0.27 |
| Withdrawal symptoms—sleep | |||
| Week 5 (quit date) | 4.9 (1.5) | 5.5 (1.6) | 0.35 |
| Week 7 (2 weeks postquit date) | 5.8 (2.0) | 6.9 (2.5) | 0.46 |
| Week 12 (last week of treatment) | 4.7 (1.2) | 5.7 (1.7) | 0.68 |
| Withdrawal symptoms—somatic | |||
| Week 5 (quit date) | 4.8 (1.6) | 4.8 (1.3) | 0.00 |
| Week 7 (2 weeks postquit date) | 4.3 (1.8) | 5.4 (1.7) | 0.63 |
| Week 12 (last week of treatment) | 3.7 (0.8) | 4.4 (1.3) | 0.65 |
| Minutes of MVPA per week | |||
| Baseline | 77.3 (112.5) | 60.3 (99.5) | |
| Week 5 (quit date) | 138.3 (77.5) | 62.7 (104.8) | 0.82 |
| Week 7 (2 weeks postquit date) | 146.2 (96.5) | 78.2 (115.0) | 0.64 |
| Week 12 (last week of treatment) | 134.0 (99.4) | 70.1 (105.2) | 0.62 |
Note. AE = aerobic exercise; CES-D = Center for Epidemiological Studies Depression Scale; HEC = health education control; MVPA = moderate-to-vigorous physical activity; PANAS = Positive and Negative Affect Scale.
Changes in Craving and Nicotine Withdrawal Symptoms After Quitting
There was a significant decrease in craving (b = −0.16, SE = 0.03, p = .00), affective (b = −0.04, SE = 0.01, p = .00), somatic (b = −0.03, SE = 0.01, p = .00), and sleep disturbance withdrawal symptoms (b = −0.12, SE = 0.05, p = .02) during the 8 weeks following quit day (weeks 5–12). Participants in AE relative to HEC had similar levels of craving (b = −0.15, SE = 0.30, p = .62), and affective withdrawal (b = −0.10, SE = 0.09, p = .27), but significantly lower somatic (b = −0.14, SE = 0.07, p = .049) and sleep disturbance withdrawal symptoms (b = −1.01, SE = 0.49, p = .04) during treatment.
Changes in Levels of Physical Activity and Fitness
When data were missing, baseline minutes of MVPA and VO2peak were used. With adjustment for baseline levels of MVPA, participants in AE had significantly higher levels of physical activity than HEC both in the 4 weeks prior to (b = 1.18, SE = 0.40, p < .01) and 8 weeks following quit day (b = 1.37, SE = 0.43, p < .01), a difference that did not decrease significantly throughout treatment (treatment × time: b = −0.04, SE = 0.04, p = .31). During treatment, 60% of those in the AE condition (compared to 25.8% in HEC) achieved an average of ≥100min of MVPA throughout the course of the 12-week intervention—the target level of exercise midway through the AE intervention. There was a trend toward higher fitness levels (estimated VO2peak) at the EOT for participants in AE, relative to those in HEC (b = 1.22, SE = 0.76, p = .12; VO2peak for AE = 30.0ml/kg/min and VO2peak for HEC = 27.3ml/kg/min).
Levels of MVPA on Affect, Depressive and Withdrawal Symptoms, and Cessation Outcomes
We evaluated whether time-varying effects of MVPA, irrespective of treatment condition, may have changed over the course of the intervention. Although patterns of higher positive affect and lower negative affect were observed among exercisers (those achieving an average of ≥100min of MVPA throughout the course of the 12-week intervention), there were no statistically significant relationships between levels of exercise and levels of positive (b = 0.28, SE = 0.24, p = .24) or negative affect (b = −0.14, SE = 0.19, p = .46), craving (b = −0.10, SE = 0.06, p = .08), or affective withdrawal (b = −0.09, SE = 0.07, p = .24) during treatment. The interaction term of MVPA by time was not significant when evaluating relationship with positive (p = .99), negative affect (p = .19), craving (p = .23), or affective withdrawal (p = 0.26). Higher levels of MVPA during treatment were associated with significantly lower levels of depressive symptoms (b = −0.12, SE = 0.06, p < .04). For example, exercisers reported a range of 3.1–7.2 depressive symptoms in the weeks following quit date compared to an average of 8.0–12.0 among those exercising <100min per week. We did not observe a significant interaction of MVPA by time when evaluating levels of depressive symptoms during treatment.
We evaluated whether time-varying effects of MVPA, irrespective of treatment condition, may have been related to cessation outcomes. Those with increasing levels of MVPA did not have significantly higher odds of abstinence (b = 0.001, SE = 0.002, p = .361).
DISCUSSION
In the current study, we developed and conducted a preliminary RCT of an AE intervention for adult smokers, which was designed specifically to address methodological limitations of previous studies in this area and to improve generalizability for future dissemination to community settings. Notable features of our AE intervention were the inclusion of male smokers, moderate-intensity exercise engaged in at home and in a supervised setting that began a month prior to quit date, components to increase adherence (e.g., contingency-based financial incentive and cognitive–behavioral group sessions for exercise adoption), telephone-based smoking cessation counseling, and the TNP.
The results of this study demonstrate the feasibility and acceptability of the various intervention components. Very good adherence was observed for both the AE and HEC intervention sessions. Similarly, compliance with the telephone-delivered cessation session and the use of the nicotine patch was high. Also, with safety instructions to remove the nicotine patch prior to engaging in exercise bouts, no adverse events were reported with the use of the nicotine patch while engaging in an AE program. However, approximately a third of interested participants lost interest and declined further study involvement prior to completion of study enrollment and baseline assessments. Strategies for increasing engagement in the early stages of study recruitment are necessary to reach a larger number of individuals whose motivations for quitting may rapidly fluctuate.
Despite the preliminary nature of this project, the cessation outcomes were quite promising, indicating that participants assigned to AE were on average twice as likely to achieve abstinence (7-day point prevalence) as those in HEC. However, this difference was not statistically significant. It is possible that the health information provided in the HEC condition was sufficiently motivating to produce change. Anecdotally, participants often reported enjoying the HEC sessions and stated an appreciation for the novelty and usefulness of the information they were receiving. As such, the HEC condition was likely an active intervention that may have contributed to both cessation outcomes and increases in physical activity for some participants.
As with other smoking cessation trials, relapse to smoking in the year following treatment was high in this study. It is possible that extending intervention components beyond the initial 12-week intervention period may help to mitigate the decline in abstinence posttreatment. For example, incorporating features during the intervention (e.g., an activity monitor in AE to facilitate self-monitoring of MVPA or a health-related Web site for HEC) that could easily translate to independent, continued use upon completion of the 12-week intervention may help decrease smoking relapse. In addition, increasing the number of telephone-delivered cessation sessions from the current eight phone calls to equal the 12 AE or HEC sessions could have potentially afforded participants additional relapse-prevention planning. Also, the addition of booster telephone sessions has been found to improve long-term cessation rates in other studies (e.g., Rabius, Pike, Hunter, Wiatrek, & McAlister, 2007) and should be considered as a strategy to decrease relapse postintervention in future studies.
Previous studies have demonstrated consistent beneficial effects of AE on craving, withdrawal symptoms, and affect in smokers (Haasova et al., 2012; Roberts, Maddison, Simpson, Bullen, & Prapavessis, 2012; Taylor, Ussher, & Faulkner, 2007; Ussher et al., 2012). Therefore, these variables were examined as potential mediators of the relationship between group assignment (AE and HEC) and smoking cessation outcomes. Analyses indicated no group differences in trajectories of craving, affective withdrawal symptoms, negative affect, positive affect, or depressive symptoms from the quit date through the EOT. However, effect sizes appear promising such that with a larger sample, significance may be achieved.
Compared to participants in the HEC condition, AE participants reported lower somatic and fewer sleep disturbance withdrawal symptoms. Sleep has not been previously examined in the context of an exercise intervention for smokers. However, it is possible that sleep may potentially be a restorative factor that could improve resilience under the stress of early cessation. There is considerable support for the benefits of exercise as a nonpharmacological intervention to improve sleep (Driver & Taylor, 2000). Future studies should consider examining the role of AE in improving sleep among smokers undergoing a quit attempt.
In the current study, about one quarter of HEC participants also increased their physical activity; therefore, we conducted additional analyses that examined the effects of exercise on affect, depressive and withdrawal symptoms, and cessation outcomes independent of group assignment. Findings revealed that participants who exercised more frequently reported fewer depressive symptoms during the intervention period. This effect on depressive symptoms is consistent with systematic and meta-analytic reviews that demonstrate a consistent positive impact of exercise on clinical depressive symptomology (e.g., Mead et al., 2009). Increasingly, attention has been paid to the important roles negative affect and depression play in impeding efforts at smoking cessation (e.g., Pratt & Brody, 2010). Therefore, exercise may be particularly helpful for this at-risk subpopulation of smokers in their efforts to quit smoking.
A potentially key aspect of the AE intervention that has not been similarly implemented in prior exercise intervention for smoking cessation studies was the inclusion of the cognitive–behavioral component for promoting exercise engagement (Ussher et al., 2012). The few studies that have included exercise counseling have included brief 5-min psychoeducational content, whereas this study incorporated structured cognitive–behavioral strategies such as challenging negative thinking about exercise, goal setting with associated rewards, identifying and problem-solving barriers, and developing exercise-focused coping strategies for managing mood and affect. These cognitive–behavioral approaches may impact the experience of depressive symptoms during cessation.
The smoking cessation counseling was provided via telephone rather than in-person in an effort to increase disseminability. The counseling was similar to that typically provided by tobacco quitlines, which have been available in every U.S. state since 2004 and are also prevalent worldwide (Anderson & Zhu, 2007). Receipt of multiple sessions of proactive telephone counseling for smokers who initiate contact with a quitline has demonstrated efficacy (Fiore et al., 2008; Stead, Perera, & Lancaster, 2007). Ultimately, quitlines may play a key role in improving systems integration (Abrams, Graham, Levy, Mabry, & Orleans, 2010). Future studies may explore the integration of exercise counseling, such as the cognitive–behavioral session content utilized in this study, within the context of telephone-delivered smoking cessation treatment.
As this was a preliminary trial, the sample size was small and our power to detect statistically significant effects on smoking cessation outcomes was limited. Our sample underwent a rigorous medical and psychiatric screening, was mostly Caucasian, and had a higher education level than the general population of smokers (Hughes & Callas, 2010). In future trials, targeted multimedia campaigns may be necessary to boost recruitment of minority and low-education populations. These strategies may include: advertisements in newspapers serving minority communities, announcements in church bulletins, and placement of informational materials at community centers and ambulatory care clinics in predominantly minority neighborhoods with all materials tailored to low-literacy populations. In addition, cigarette smoking is highly comorbid with medical, psychiatric, and substance disorders (Grant, Hasin, Chou, Stinson, & Dawson, 2004; Lasser et al., 2000; USDHHS, 2004). Therefore, additional research is needed to determine the efficacy of exercise interventions for more diverse populations as well as for the significant proportion of smokers with psychiatric disorders, who are less physically active than the general population (Daumit et al., 2005).
This study tested the preliminary efficacy of an AE intervention for smoking cessation that attempted to address previously identified methodological limitations. The promising results suggest that a larger trial of this AE intervention is warranted. Future research can also explore the potential for adapting the AE intervention for use in other supervised exercise (e.g., local health club/gym) and nonsupervised (e.g., primary care) venues to allow for future dissemination of this approach so as to have high public health significance in decreasing the overall prevalence of cigarette smoking, thereby reducing smoking-related morbidity and mortality.
FUNDING
This work was supported by a National Institute on Drug Abuse-funded grant (K23 DA019950) awarded to Dr. AMA.
DECLARATION OF INTERESTS
None declared.
REFERENCES
- Abrams D. B., Graham A. L., Levy D. T., Mabry P. L., Orleans C. T. (2010). Boosting population quits through evidence-based cessation treatment and policy. American Journal of Preventive Medicine, 38(Suppl. 3), S351–S363. 10.1016/j.amepre.2009.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrantes A. M., Strong D. R., Lloyd-Richardson E. E., Niaura R., Kahler C. W., Brown R. A. (2009). Regular exercise as a protective factor in relapse following smoking cessation treatment. The American journal on addictions/American Academy of Psychiatrists in Alcoholism and Addictions, 18, 100–101. 10.1080/10550490802545182 [DOI] [PubMed] [Google Scholar]
- Anderson C. M., Zhu S. H. (2007). Tobacco quitlines: Looking back and looking ahead. Tobacco Control, 16(Suppl. 1), i81–i86. 10.1136/tc.2007.020701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G. (1970). Perceived exertion as an indicator of somatic stress. Scandinavian journal of rehabilitation medicine, 2, 92–98 [PubMed] [Google Scholar]
- Borrelli B., Spring B., Niaura R., Hitsman B., Papandonatos G. (2001). Influences of gender and weight gain on short-term relapse to smoking in a cessation trial. Journal of Consulting and Clinical Psychology, 69, 511–515 doi:10.1037/0022-006X.69.3.511 [DOI] [PubMed] [Google Scholar]
- Brown R. A., Burgess E. S., Sales S. D., Whiteley J. A., Evans D. M., Miller I. W. (1998). Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors, 12, 101–112 doi:10.1037/0893-164X.12.2.101 [Google Scholar]
- CDC. (2011). Vital signs: Current cigarette smoking among adults aged >/=18 years--United States, 2005–2010. Morbidity and Mortality Weekly Report, 60, 1207–1212 [PubMed] [Google Scholar]
- Daumit G. L., Goldberg R. W., Anthony C., Dickerson F., Brown C. H., Kreyenbuhl J., … Dixon L. B. (2005). Physical activity patterns in adults with severe mental illness. The Journal of nervous and mental disease, 193, 641–646 doi:10.1097/01.nmd.0000180737.85895.60 [DOI] [PubMed] [Google Scholar]
- Dishman R. K., Buckworth J. (1996). Increasing physical activity: A quantitative synthesis. Medicine and Science in Sports and Exercise, 28, 706–719 doi:10.1097/00005768-199606000-00010 [DOI] [PubMed] [Google Scholar]
- Driver H. S., Taylor S. R. (2000). Exercise and sleep. Sleep medicine reviews, 4, 387–402. 10.1053/smrv.2000.0110 [DOI] [PubMed] [Google Scholar]
- Emmons K. M., Marcus B. H., Linnan L., Rossi J. S., Abrams D. B. (1994). Mechanisms in multiple risk factor interventions: Smoking, physical activity, and dietary fat intake among manufacturing workers. Working Well Research Group. Preventive Medicine, 23, 481–489 doi:10.1006/pmed.1994.1066 [DOI] [PubMed] [Google Scholar]
- Emont S. L., Collins R. L., Zywiak W. H. (1991). Methodological note: Corroboration of self-reported smoking status using significant other reports. Addictive behaviors, 16, 329–333 doi:10.1016/0306-4603(91)90025-D [DOI] [PubMed] [Google Scholar]
- Everson E. S., Taylor A. H., Ussher M. (2010). Determinants of physical activity promotion by smoking cessation advisors as an aid for quitting: Support for the Transtheoretical Model. Patient Education and Counseling, 78, 53–56. 10.1016/j.pec.2009.05.004 [DOI] [PubMed] [Google Scholar]
- Everson-Hock E. S., Taylor A. H., Ussher M. (2010). Readiness to use physical activity as a smoking cessation aid: A multiple behaviour change application of the Transtheoretical Model among quitters attending Stop Smoking Clinics. Patient Education and Counseling, 79, 156–159. 10.1016/j.pec.2009.09.016 [DOI] [PubMed] [Google Scholar]
- Fiore M. C., Jaen C. R., Baker T. B., Bailey W. C., Benowitz N. L., Curry S. J., Dorfman S. F. (2008). Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; May 2008. [Google Scholar]
- First M. B., Spitzer R. L., Gibbon M., Williams J. B. W. (1995). Structured Clinical Interview for DSM-IV Axis I Disorders. [DOI] [PubMed]
- French S. A., Hennrikus D. J., Jeffery R. W. (1996). Smoking status, dietary intake, and physical activity in a sample of working adults. Health psychology: official journal of the Division of Health Psychology, American Psychological Association, 15, 448–454 doi:10.1037/0278-6133.15.6.448 [DOI] [PubMed] [Google Scholar]
- Grant B. F., Hasin D. S., Chou S. P., Stinson F. S., Dawson D. A. (2004). Nicotine dependence and psychiatric disorders in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry, 61, 1107–1115. 10.1001/archpsyc.61.11.1107 [DOI] [PubMed] [Google Scholar]
- Haasova M., Warren F. C., Ussher M., Janse Van Rensburg K., Faulkner G., Cropley M., … Taylor A. H. (2012). The acute effects of physical activity on cigarette cravings: systematic review and meta-analysis with individual participant data. Addiction (Abingdon, England), 108, 26–37. 10.1111/j.1360-0443.2012.04034.x [DOI] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127 doi:10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Hughes J. R., Callas P. W. (2010). Data to assess the generalizability of samples from studies of adult smokers. Nicotine & Tobacco Research: official journal of the Society for Research on Nicotine and Tobacco, 12, 73–76. ntp168 [pii]10.1093/ntr/ntp168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. R., Hatsukami D. (1986). Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry, 43, 289–294 doi:10.1001/archpsyc.1986.01800030107013 [DOI] [PubMed] [Google Scholar]
- Kinnunen T., Leeman R. F., Korhonen T., Quiles Z. N., Terwal D. M., Garvey A. J., Hartley H. L. (2008). Exercise as an adjunct to nicotine gum in treating tobacco dependence among women. Nicotine & Tobacco Research: official journal of the Society for Research on Nicotine and Tobacco, 10, 689–703. 10.1080/14622200801979043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer H. C., Kupfer D. J. (2006). Size of treatment effects and their importance to clinical research and practice. Biological psychiatry, 59, 990–996 doi:10.1016/j.biopsych.2005.09.014 [DOI] [PubMed] [Google Scholar]
- Lasser K., Boyd J. W., Woolhandler S., Himmelstein D. U., McCormick D., Bor D. H. (2000). Smoking and mental illness: A population-based prevalence study. JAMA: the journal of the American Medical Association, 284, 2606–2610 doi:10.1001/jama.284.20.2606 [DOI] [PubMed] [Google Scholar]
- Liang K.-Y., Zeger S. L. (1986). Longitudinal data analysis using generalized linear models. Biometrika, 73, 13–22 [Google Scholar]
- Loscalzo J. (2009). Pilot trials in clinical research: of what value are they? Circulation, 119, 1694–1696. 10.1161/CIRCULATIONAHA.109.861625 [DOI] [PubMed] [Google Scholar]
- Marcus B. H., Albrecht A. E., King T. K., Parisi A. F., Pinto B. M., Roberts M., … Abrams D. B. (1999). The efficacy of exercise as an aid for smoking cessation in women: A randomized controlled trial. Archives of Internal Medicine, 159, 1229–1234 doi:10.1001/archinte.159.11.1229 [DOI] [PubMed] [Google Scholar]
- Marcus B. H., Lewis B. A., Hogan J., King T. K., Albrecht A. E., Bock B., … Abrams D. B. (2005). The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: A randomized controlled trial. Nicotine & Tobacco Research: official journal of the Society for Research on Nicotine and Tobacco, 7, 871–880. 10.1080/14622200500266056 [DOI] [PubMed] [Google Scholar]
- Mead G. E., Morley W., Campbell P., Greig C. A., McMurdo M., Lawlor D. A. (2009). Exercise for depression. Cochrane Database of Systematic Reviews, CD004366. 10.1002/14651858.CD004366.pub4 [DOI] [PubMed] [Google Scholar]
- Ossip-Klein D. J., Giovino G. A., Megahed N., Black P. M., Emont S. L., Stiggins J., … Moore L. (1991). Effects of a smoker’s hotline: results of a 10-county self-help trial. Journal of consulting and clinical psychology, 59, 325–332 doi:10.1037/0022-006X.59.2.325 [DOI] [PubMed] [Google Scholar]
- Panza G. A., Weinstock J., Ash G. I., Pescatello L. S. (2012). Psychometric evaluation of the timeline followback for exercise among college students. Psychology of Sport and Exercise, 13, 779–788. 10.1016/j.psychsport.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri M. G., Anton S. D., Durning P. E., Ketterson T. U., Sydeman S. J., Berlant N. E., … Martin A. D. (2002). Adherence to exercise prescriptions: Effects of prescribing moderate versus higher levels of intensity and frequency. Health psychology: official journal of the Division of Health Psychology, American Psychological Association, 21, 452–458 doi:10.1037/0278-6133.21.5.452 [PubMed] [Google Scholar]
- Pober D. M., Freedson P. S., Kline G. M., McInnis K. J., Rippe J. M. (2002). Development and validation of a one-mile treadmill walk test to predict peak oxygen uptake in healthy adults ages 40 to 79 years. Canadian journal of applied physiology = Revue canadienne de physiologie appliquée, 27, 575–589 doi:10.1139/h02-033 [DOI] [PubMed] [Google Scholar]
- Prapavessis H., Cameron L., Baldi J. C., Robinson S., Borrie K., Harper T, Grove J. R. (2007). The effects of exercise and nicotine replacement therapy on smoking rates in women. Addictive Behaviors, 32, 1416–1432. 10.1016/j.addbeh.2006.10.005 [DOI] [PubMed] [Google Scholar]
- Pratt L. A., Brody D. J. (2010). Depression and smoking in the U.S. household population aged 20 and over, 2005–2008. NCHS Data Brief, 1–8 [PubMed] [Google Scholar]
- Rabius V., Pike K. J., Hunter J., Wiatrek D., McAlister A. L. (2007). Effects of frequency and duration in telephone counselling for smoking cessation. Tobacco Control, 16(Suppl. 1), i71–i74. 10.1136/tc.2007.019950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L. S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(Summer), 385–401 doi:10.1177/014662167700100306 [Google Scholar]
- Roberts V., Maddison R., Simpson C., Bullen C., Prapavessis H. (2012). The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect, and smoking behaviour: Systematic review update and meta-analysis. Psychopharmacology, 222, 1–15. 10.1007/s00213-012-2731-z [DOI] [PubMed] [Google Scholar]
- Segan C. J., Borland R., Wilhelm K. A., Bhar S. S., Hannan A. T., Dunt D. R., Ferretter I. T. (2011). Helping smokers with depression to quit smoking: Collaborative care with Quitline. The Medical journal of Australia, 195, S7–11 [DOI] [PubMed] [Google Scholar]
- Shumaker S. A. (1986). Proceedings of the National Working Conference on Smoking Relapse (Vol. 5) Hillsdale, NJ: L. Earlbaum Associates [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. (2002). Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research, 4, 149–159 [DOI] [PubMed] [Google Scholar]
- Stead L. F., Perera R., Lancaster T. (2007). A systematic review of interventions for smokers who contact quitlines. Tobacco Control, 16(Suppl. 1), i3–i8. 10.1136/tc.2006.019737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout R. L. (1988). Urn randomization: Description and rationale. Brown University School of Medicine.
- Taylor A. H., Ussher M. H., Faulkner G. (2007). The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review. Addiction (Abingdon, England), 102, 534–543. 10.1111/j.1360-0443.2006.01739.x [DOI] [PubMed] [Google Scholar]
- USDHHS. (2004). The Health Consequences of Smoking: A Report of the Surgeon General: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
- Ussher M., Nunziata P., Cropley M., West R. (2001). Effect of a short bout of exercise on tobacco withdrawal symptoms and desire to smoke. Psychopharmacology, 158, 66–72. 10.1007/s002130100846 [DOI] [PubMed] [Google Scholar]
- Ussher M. H., Taylor A., Faulkner G. (2012). Exercise interventions for smoking cessation. The Cochrane database of systematic reviews, 1, CD002295. 10.1002/14651858.CD002295.pub4 [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L. A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070 doi:10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Williams D. M., Whiteley J. A., Dunsiger S., Jennings E. G., Albrecht A. E., Ussher M. H., … Marcus B. H. (2010). Moderate intensity exercise as an adjunct to standard smoking cessation treatment for women: a pilot study. Psychology of Addictive Behaviors: journal of the Society of Psychologists in Addictive Behaviors, 24, 349–354. 10.1037/a0018332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger S. L., Liang K. Y. (1986). Longitudinal data analysis for discrete and continuous outcomes. Biometrics, 42, 121–130 doi:10.2307/2531248 [PubMed] [Google Scholar]