Abstract
Pressure measured with a cuff and sphygmomanometer in the brachial artery is accepted as an important predictor of future cardiovascular risk. However, systolic pressure varies throughout the arterial tree, such that aortic (central) systolic pressure is actually lower than corresponding brachial values, although this difference is highly variable between individuals. Emerging evidence now suggests that central pressure is better related to future cardiovascular events than is brachial pressure. Moreover, anti-hypertensive drugs can exert differential effects on brachial and central pressure. Therefore, basing treatment decisions on central, rather than brachial pressure, is likely to have important implications for the future diagnosis and management of hypertension. Such a paradigm shift will, however, require further, direct evidence that selectively targeting central pressure, brings added benefit, over and above that already provided by brachial artery pressure.
Keywords: Central pressure, Blood pressure, Anti-hypertensive treatment, Cardiovascular risk
Introduction
The brachial cuff sphygmomanometer was introduced into medical practice well over 100 years ago, enabling the routine, non-invasive, measurement of arterial blood pressure. Life insurance companies were among the first to capitalize on the information provided by cuff sphygmomanometry, by observing that blood pressure in largely asymptomatic individuals relates to future cardiovascular risk—observations that are now supported by a wealth of epidemiological data.1 The most recent Global Burden of Disease report2 identified hypertension as the leading cause of death and disability worldwide. Moreover, data from over 50 years of randomized controlled trials clearly demonstrate that lowering brachial pressure, in hypertensive individuals, substantially reduces cardiovascular events.1,3 For these reasons, measurement of brachial blood pressure has become embedded in routine clinical assessment throughout the developed world, and is one of the most widely accepted ‘surrogate measures’ for regulatory bodies.
The major driving force for the continued use of brachial blood pressure has been its ease of measurement, and the wide variety of devices available for clinical use. However, we have known for over half a century that brachial pressure is a poor surrogate for aortic pressure, which is invariably lower than corresponding brachial values. Recent evidence suggests that central pressure is also more strongly related to future cardiovascular events4–7 than brachial pressure, and responds differently to certain drugs.8,9 Appreciating this provides an ideal framework for understanding the much publicized inferiority of atenolol and some other beta-blockers,10 compared with other drug classes, in the management of essential hypertension. Although central pressure can now be assessed non-invasively with the same ease as brachial pressure, clinicians are unlikely to discard the brachial cuff sphygmomanometer without robust evidence that cardiovascular risk stratification, and monitoring response to therapy, are better when based on central rather than peripheral pressure. Central pressure assessment and accuracy will also have to be standardized, as it has been for brachial pressure assessment with oscillometric devices. This review will discuss our current understanding about central pressure and the evidence required to bring blood pressure measurement, and cardiovascular risk assessment into the modern era.
Physiological concepts
Arterial pressure varies continuously over the cardiac cycle, but in clinical practice only systolic and diastolic pressures are routinely reported. These are invariably measured in the brachial artery using cuff sphygmomanometry—a practice that has changed little over the last century. However, the shape of the pressure waveform changes continuously throughout the arterial tree. Although diastolic and mean arterial pressures are relatively constant, systolic pressure may be up to 40 mmHg higher in the brachial artery than in the aorta.11–13 This phenomenon of systolic pressure amplification arises principally because of an increase in arterial stiffness moving away from the heart. As the pressure wave travels from the highly elastic central arteries to the stiffer brachial artery, the upper portion of the wave becomes narrower, the systolic peak becomes more prominent, and systolic pressure increases (Figure 1).
Figure 1.
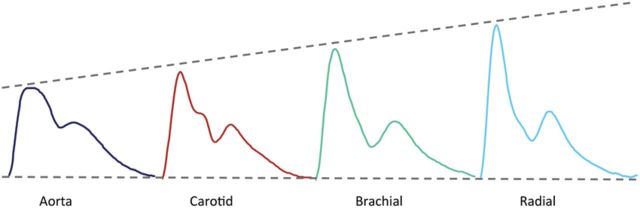
Amplification of the pressure waveform moving from the aorta to the radial artery.
Historically, two major paradigms have been used in an attempt to understand the changes in waveform morphology observed throughout the arterial system and in response to ageing, vasoactive mediators and drugs. The first, arterial waveform analysis, assumes that the arterial pressure waveform is a composite of a forward travelling wave, generated by left ventricular ejection, and a backward-travelling reflected wave arising from sites of impedance mismatch—i.e. arterial taper and differences in vessel stiffness, which often occur at bifurcations.14,15 This change in impedance is thought to generate numerous reflected ‘wavelets’ that sum together to produce a single ‘effective’ reflected wave, which is thought to augment, or increase systolic pressure in the central arteries. The augmentation index, which quantifies the extent of augmented pressure relative to the central pulse pressure, provides information about the amplitude and timing of backward-travelling waves within the central arteries. With an increase in augmented pressure (and augmentation index), the absolute aortic systolic pressure increases, and amplification, defined as the ratio of brachial and aortic pulse pressures, decreases.14
The second major paradigm initially viewed the arterial system as a two-element windkessel model (resistance and compliance), where a central reservoir fills during systole and empties during diastole. Although this model is useful for explaining haemodynamic mechanisms during diastole, it predicts the relationship between pressure and flow in systole relatively poorly, and any influence of wave propagation and reflection is effectively ignored.16 The addition of aortic characteristic impedance (three-element windkessel model) improves the prediction of pressure and flow throughout the entire cardiac cycle, but still does not permit the investigation of wave transmission characteristics. More recently, however, a variation on the windkessel model has been proposed,17 which incorporates both reservoir- and wave-based approaches into a single model, involving a central reservoir pressure and an excess pressure. The excess pressure is calculated as the difference between the measured pressure and the calculated reservoir component, and relates to wave propagation and reflection. Recent data based on this model dispute the more commonly held view that wave reflections contribute to systolic pressure augmentation, arguing instead that the magnitude of the augmentation pressure is principally determined by the arterial reservoir.18 Although controversial,19,20 this new paradigm has generated considerable research interest in recent months.
Irrespective of the precise mechanisms underlying the observed changes in wave shape or the models used to describe them, substantial pressure amplification does exist within the arterial tree. Importantly, the degree of systolic pressure amplification, both within- and between-individuals, is not fixed, and depends on a number of variables including age, gender, height and heart rate,21–25 as well as systemic diseases affecting the vasculature. Amplification is high in young people, especially men, in whom aortic systolic pressure measured invasively,11,26 or with the SphygmoCor device27 can be some 20–30 mmHg lower than that in the brachial artery. Individuals of shorter stature tend to have less amplification, i.e. for a given brachial pressure, central pressure is relatively higher. This is also true for those with lower heart rates, due to the inverse relationship between heart rate and central pressure augmentation. However, only ∼70% of the variability in pulse pressure amplification can be explained in multivariable regression models.21,28 This suggests that central pressure cannot be predicted with sufficient accuracy from brachial pressure by a statistical model but, rather, needs to be assessed directly, using appropriate methods.
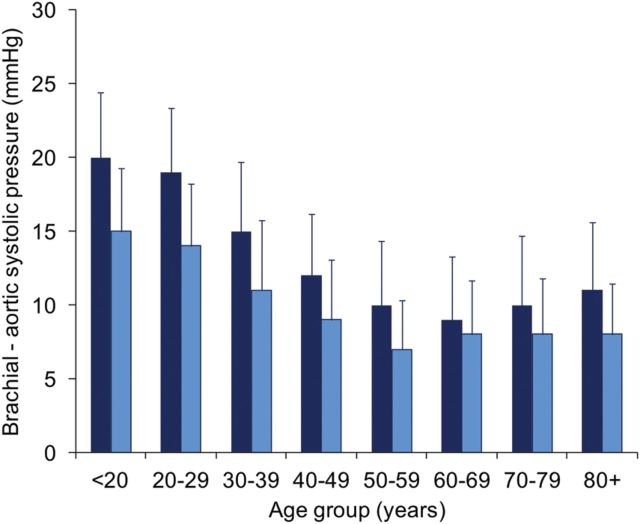
The potential clinical relevance of this variability in amplification became evident when we evaluated aortic and brachial pressure in a cohort of 10 000 volunteers.28 Even in those deemed to be healthy, there was a significant, and highly variable, difference between aortic and brachial systolic pressure at all ages (Figure 2). Moreover, when we stratified individuals by brachial artery blood pressure we observed a considerable overlap in aortic systolic pressure, such that over 70% of individuals categorized as having ‘high-normal’ brachial systolic pressure based on Joint European Cardiology and Hypertension Society guidelines29 had similar aortic pressures to those with stage 1 hypertension (Figure 3). Moreover, >30% of males and 10% of females with normal brachial blood pressure had aortic pressures in common with individuals with stage 1 hypertension. This will have important clinical implications if central pressure turns out to be a better predictor of cardiovascular risk, because it suggests that, currently, we may be treating some subjects with relatively low central pressures, and not treating individuals with elevated central pressures, because they have brachial systolic pressures under current treatment thresholds.
Figure 2.
Difference between brachial and aortic systolic blood pressure (SphygmoCor) in healthy men (dark blue bars; n = 2779) and women (light blue bars; n = 2869). The data represent means ± SD.
Figure 3.

Overlap in aortic systolic blood pressure despite no overlap in brachial systolic pressure, in healthy men and women (n = 5648). Over 70% of individuals with high-normal blood pressure had aortic systolic pressures in common with individuals with stage 1 hypertension.28
How to measure central pressure
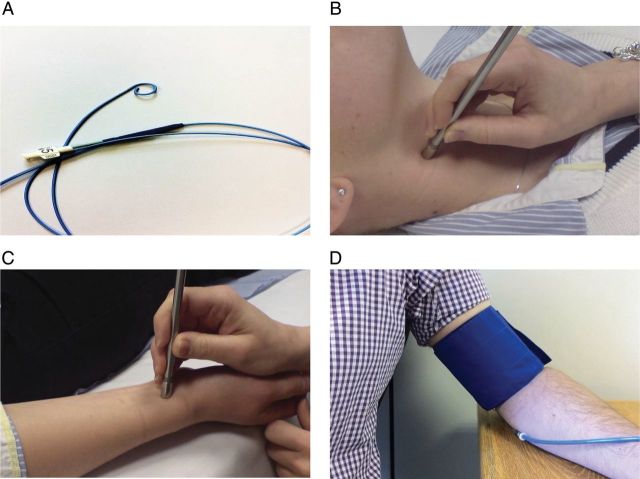
A number of methods are now available for assessing central pressure. The most direct method involves cardiac catheterization and recording of the blood pressure in the ascending aorta using a pressure-sensing catheter (Figure 4A). However, this is highly invasive, technically demanding and clearly unsuitable for use in routine screening of large populations. More recently, a number of non-invasive methods have been developed, where pressure waveforms are recorded from sites distal to the aorta, such as the carotid (Figure 4B), radial (Figure 4C) or brachial (Figure 4D) arteries, and calibrated to blood pressure recorded by cuff sphygmomanometry. Each of these approaches has their own strengths and limitations.
Figure 4.
Techniques for assessing central blood pressure. (A) Invasive cardiac catheterization; (B) direct applanation tonometry of the carotid artery; (C) applanation tonometry of the radial artery; (D) cuff-based oscillometry at the brachial artery.
Carotid artery pressure is often used as a surrogate for aortic pressure because of the close proximity of these arterial sites. Carotid pressure waveforms are recorded by applanation tonometry and then scaled to the brachial mean and diastolic pressures on the principle that unlike systolic pressure, mean, and diastolic pressure do not vary markedly throughout the arterial tree11 and are thus suitable for calibrating pressure waveforms recorded from other arterial sites. However, carotid waveforms of sufficient quality can be difficult to obtain in all individuals, especially in obese patients. The technique is highly operator-dependent, making it somewhat unreliable for routine high-throughput screening of central pressure in a non-specialist setting. Moreover, there is also likely to be a small degree of amplification between the carotid artery and aorta,14,22,30 which may lead to an over-estimation of aortic pressure.
Pulse wave analysis is an alternative method, where pressure waveforms are recorded from peripheral arteries (typically brachial or radial) and corresponding central aortic pressure derived either using a generalized transfer function, identification of the late systolic shoulder of the peripheral pressure waveform, or a proprietary algorithm. A variety of devices are now available which follow one or more of these principles, as summarized in Table 1.
Table 1.
Indirect, non-invasive methods for estimating central pressure
| Method of waveform recording | Device | Company | Method of calibration | Method of estimation | Clinical applicability† |
|---|---|---|---|---|---|
| Radial tonometry | BPro86,87 | HealthSTATS | Brachial–radial cuff BP | GTF (radial-aortic) | ++ |
| SphygmoCor12,88 | AtCor Medical | Brachial–radial cuff BP | (i) GTF (radial-aortic) | + | |
| (ii) Late systolic shoulder | + | ||||
| HEM9000AI39,77 | Omron | Brachial cuff BP | (i) Algorithm | ++ | |
| (ii) Late systolic shoulder | ++ | ||||
| Brachial cuff PVP | *ARCsolver89,90 | Brachial cuff BP | GTF (brachial-aortic) | +++ | |
| Centron cBP30135,91 | Centron Diagnostics | Brachial cuff BP | GTF (brachial-aortic) | ++++ | |
| Vicorder92 | Skidmore Medical | Brachial cuff BP | GTF (brachial-aortic) | +++ | |
| XCEL | AtCor Medical | Brachial cuff BP | GTF (brachial-aortic) | +++ | |
| Method of Sung et al.42 | Brachial cuff BP | Algorithm | ++ | ||
| Suprasystolic brachial cuff PVP | Arteriograph37,93 | TensioMed | Brachial cuff BP | Late systolic wave amplitude | +++ |
| Cardioscope II36,94 | Pulsecor | Brachial cuff BP | Algorithm | ++++ |
PVP, pulse volume plethysmography; GTF, generalized transfer function.
*Incorporated in Mobil-O-Graph PWA device (IEM GmbH).
†Personal view based on experience, operator-dependency, need for computer/software interface, with + indicating limited applicability to routine clinical practice and ++++ indicating high applicability.
A major criticism of these non-invasive devices is that peripheral waveforms are typically calibrated to brachial systolic and diastolic cuff pressures. These tend to under-estimate the ‘true’ (invasive) brachial artery pressure, leading to falsely low estimates of central pressure. However, brachial cuff pressure is used in the routine diagnosis and treatment of hypertension, and accepted by regulatory authorities as being better validated as a surrogate of outcome than intra-arterial (brachial) pressure.31 Moreover, recent data demonstrate that errors in the estimation of central pressure are equivalent to errors in brachial cuff sphygmomanometry.31,32 A second problem arises when radial waveforms are calibrated to brachial cuff pressure, because the presence of any brachial-to-radial amplification adds further to the under-estimation of central pressure. Recent data33,34 indicate that calibration with brachial mean and diastolic pressures may be preferable here. Newer cuff-based devices which scale brachial waveforms obtained with pulse volume plethysmography to the measured brachial cuff pressure, negate any potential influence of brachial-radial amplification. However, they do still tend to be lower than ‘true’ aortic pressure, due to the inaccurate brachial cuff pressure. Nevertheless, although further validation data are still emerging,35–37 collectively, these newer devices offer potential advantages in being less operator-dependent than hand-held tonometry methods and are potentially well-suited to use in the primary-care setting.
Central systolic pressure may also be estimated directly from the peripheral pressure waveform without use of a transfer function, as invasive data show that the late systolic shoulder of the peripheral pressure waveform approximates to aortic systolic pressure.38–40 However, further validation of this approximation of central pressure is required, since it may be inaccurate in younger individuals (with early, non-augmented peak systolic pressures), or those with low blood pressure.41 Algorithms may also be applied to the brachial or radial pressure waveforms to obtain estimates of the ‘true’ aortic pressure, i.e. as would be obtained with invasive measurement.39,42
Current evidence regarding the importance of central pressure
The heart, kidneys, and major arteries supplying the brain are exposed to aortic rather than brachial pressure. Therefore, there is a strong rationale to believe that cardiovascular events may ultimately be more closely related to central rather than brachial pressure. Evidence published over the last 12 years concerning the relationship between central pressure and both surrogate markers of risk and hard endpoints strongly support this concept.43
Central pressure is more closely correlated with widely accepted surrogate measures of cardiovascular risk such as carotid intima-media thickness (CIMT)4,44,45 and left ventricular mass (LVM),45–47 than brachial pressure in cross-sectional studies (see Supplementary material online, Table S1). Longitudinal observations provide greater support for the potential value of central pressure measurement. In the REASON Study,48 regression of LVM was more strongly related to change in central compared with brachial pressure and, after adjustment, only central pressure remained predictive. Similar observations were made in a substudy of ASCOT.49 Moreover, with anti-hypertensive therapy, the reduction in CIMT relates better to the fall in central pressure.50
The predictive value of central pressure has been investigated in a variety of patient cohorts (see Supplementary material online, Table S2). Out of 11 published studies, one was based on invasive measurements of central pressure7 with the rest using tonometry-based techniques. Nine studies reported that central pressure was independently related to future cardiovascular events. Surprisingly, the ANBP251 and Framingham Heart Study52 did not detect any systolic pressure amplification between the carotid and ‘brachial’ arteries and concluded that there was no advantage in assessing central in addition to brachial pressure. Four of the 11 studies also demonstrate incremental value of central over brachial pressure. Safar et al.5 found that after adjustment for confounders, only central pressure remained predictive in patients with renal failure. In the larger Strong Heart Study, central pressure was more strongly related to future cardiovascular events than brachial pressure, in disease-free individuals.4 Moreover, after mutual adjustment, brachial pressure ceased to be predictive. Further analyses in this cohort show that individuals with central pulse pressure ≥50 mmHg are greatest risk of future cardiovascular events.53 The Dicomano Study in Italy6 and a community-based Taiwanese study45 also observed a stronger association between cardiovascular events and central, rather than brachial pressure. In contrast, Mitchell et al.52 failed to show any additional value of carotid blood pressure in the Framingham Heart Study.
The main issue with the existing studies is that they are relatively underpowered to show convincingly that central pressure is meaningfully superior to brachial values in predicting events, especially given the correlation between the two (r = 0.6–0.9). A recent meta-analysis did confirm the independent predictive value of central pressure, and suggested that central pulse pressure may be a better predictor (P = 0.057).54 Unfortunately, not all of the larger studies were included and the findings were based on published summary statistics rather than individual patient data. Clearly, a full evidence synthesis with an individual patient meta-analysis of all existing studies (currently in progress) is required, together with a definitive outcome study, preferably using one of the newer, operator-independent devices which are more suited to the primary-care setting. Only then will we know the true value of central pressure, and whether it adds meaningfully to brachial pressure-based risk prediction.
Pharmacological reduction of central systolic pressure
Until relatively recently, it was widely believed that blood pressure reduction per se, matters more than the choice of anti-hypertensive agent.55 However, the results of two comprehensive meta-analyses,56,57 together with large comparison studies including the MRC-Elderly,58 LIFE,59 and ASCOT60 trials, all demonstrate that the beta-blocker, atenolol, is inferior to other major anti-hypertensive drug classes in preventing cardiovascular events. Interestingly, there is now convincing evidence that beta-blockers exert differential effects on brachial vs. central pressure. Such evidence may help to explain the adverse findings with atenolol in outcome studies and provides support for the hypothesis that drugs which lower central pressure the most will be more effective.
Numerous studies have now examined the influence of different anti-hypertensive drugs and novel/repurposed agents such as nitrates on brachial vs. central pressure (Table 2). However, these studies have typically included small numbers of patients, and have varied in the duration of treatment and methods used to assess central pressure. Moreover, only three studies have directly compared the effect of each of the major anti-hypertensive drug classes on brachial and central pressure.61–63 Nevertheless, monotherapy studies have universally demonstrated that conventional beta-blockers lower central pressure to a lesser extent than brachial pressure. Moreover, the REASON trial,9 which compared the effects of atenolol with the fixed-dose combination of the ACE inhibitor perindopril and the diuretic, indapamide, showed that, in a subset of patients, combination treatment led to similar reductions in brachial and central pressure, whereas the fall in central pressure with atenolol was only approximately half of that in brachial pressure. The CAFE substudy8 of the ASCOT trial60 subsequently reported that individuals randomized to atenolol had a 4.3 mmHg higher central systolic pressure than those given amlodipine, despite identical brachial pressures. Although modest, this differential effect observed with atenolol could explain most of the observed difference in outcome in the ASCOT study.
Table 2.
Comparative effect of anti-hypertensive drugs and nitrates on central systolic pressure
An important issue is whether atenolol is inferior to all other anti-hypertensive drugs, or whether the comparator agents are more efficacious than other drugs, including beta-blockers. In the EXPLOR study,64 the fall in aortic systolic pressure was ∼4 mmHg greater in individuals randomized to a valsartan/amlodipine combination vs. atenolol/amlodipine, indicating that even when combined with a calcium channel blocker, atenolol may not effectively protect against cardiovascular events. Moreover, it is important to recognize that not all beta-blockers are identical. The majority of studies has used atenolol, although Deary et al.62 used bisoprolol, with similar results. In contrast, newer, more selective vasodilating agents such as nebivolol65,66 and celiprolol50 may have a greater capacity to reduce central systolic pressure, by reducing wave reflections, although further studies are clearly required.67
The results of the studies described above lend support to the hypothesis that an inadequate reduction in central pressure may be associated with an adverse outcome. However, further large, randomized and properly powered clinical trials are required in order to provide definitive evidence that for equal reductions in brachial pressure, drugs which lower central pressure most will be better. While non-vasodilating beta-blockers are clearly inappropriate in this regard, nitric oxide donor drugs such as glyceryl trinitrate have the opposite effect of non-vasodilating beta-blockers by substantially reducing wave reflections.68 Although not a ‘classical’ or accepted anti-hypertensive drug, outside of the acute emergency setting, high doses of nitrates do appear to produce sustained reductions in brachial pressure during chronic dosing, despite concerns over tolerance.69–73 Limited data also suggest that nitrates may reduce central pressure more than brachial pressure; and low doses may reduce central pressure with almost no effect on brachial pressure,68,74 although concerns regarding development of tolerance and endothelial dysfunction75,76 remain to be resolved. If these concerns can be resolved, then nitrovasodilators offer a potential strategy with which to preferentially lower central pressure, and thus test the clinical value of assessing central pressure and targeting it therapeutically. However, whether this translates into favourable effects on outcome is yet to be examined.
Outstanding issues
Several methodological issues remain to be addressed before measurement of central pressure is fully integrated into clinical decision-making and of practical benefit for patients. Firstly, although a number of simple-to-use, reliable devices are now on the market, a standard approach to validation of new devices is required: should this be against the current market leaders, or against invasively determined aortic pressure? An analogy can be drawn from oscillometric sphygmomanometers, which are validated against mercury, rather than ‘true’ invasive brachial pressure. Also, should these devices provide estimates of ‘true’ central pressure irrespective of brachial cuff pressure? This approach can sometimes produce higher central pressure estimates than the measured brachial cuff pressure,39,77,78 which may seem unphysiological, but is due to the brachial cuff giving a falsely low estimate of brachial systolic pressure. The alternative approach calculates central pressure relative to the measured brachial cuff pressure, which tends to under-estimate the ‘true’ aortic pressure, but may be more intuitive. Finally, we need to adopt a standard method for calibrating peripheral waveforms, using either systolic/diastolic or mean arterial pressure/diastolic pressure, and to better understand the impact of brachial-radial and aortic-carotid amplification.
Another important issue is defining ‘cut-off’ values for central pressure. Although definitions and thresholds for brachial pressure are now well established, no such data exist for central pressure. Clearly, defining a ‘normal’ central pressure is impossible as blood pressure is normally distributed. Although the provision of age- and gender-specific reference ranges might seem attractive, these are never applied to brachial pressure. Moreover, such values are likely to be misleading because they imply that the progressive, age-related increase in blood pressure is without increased risk of cardiovascular disease and is thus physiological, rather than pathological. Nevertheless, it is important to recognize that because of the phenomenon of pressure amplification, one cannot simply apply the current brachial thresholds for diagnosing and treating hypertension to central pressure. An alternative approach might be to determine the usual amount of amplification, and then to ‘translate’ the current 140/90 brachial cut-off into a corresponding aortic value. Our previous data28 suggest that this value is ∼125/90, although clearly, further data are required. However, there are obvious limitations of this approach, not least the marked effect that age has on amplification. Nevertheless, the Strong Heart Study investigators observed that in over 2400 participants without overt cardiovascular disease, a central pulse pressure of ≥50 mmHg predicted an adverse cardiovascular outcome.53 Thus, for the first time, a clinically useful target for diagnosis and intervention, based on central pressure, has been identified, although these data require confirmation.
As discussed earlier, a full synthesis of the available evidence concerning central pressure and the risk of future cardiovascular events is now required. However, it will also be necessary to determine the clinical relevance of differences between brachial and central pressure for the individual patient, especially given the relatively high correlation between the two. Emerging data support the prognostic superiority of both 24-h ambulatory blood pressure monitoring (ABPM)79–81 and home monitoring81 in comparison with office measurements. Interestingly, a recent study82 demonstrated that 24-h ambulatory cuff pressures were comparable with office central pressure measurements in the prediction of risk, although the significance of this study awaits confirmation.83 As yet, there are no data comparing the predictive value of home monitoring vs. central pressure in the prediction of risk. Ultimately, it will be necessary to evaluate the prognostic value of 24-h ambulatory central pressure. With the recent development of ambulatory central pressure systems,84,85 this is now possible and it may be reasonable to hypothesize that 24-h central, rather than brachial ABPM would be superior in terms of risk prediction.
Finally, there is now a substantial body of evidence that anti-hypertensive drugs, and particularly beta-blockers, exert differential effects on brachial and central pressure. As a result, the pharmaceutical industry is becoming increasingly convinced that basing treatment decisions on central, rather than brachial pressure, is likely to have important implications for the future diagnosis and management of hypertension. However, cuff measurements of brachial systolic and diastolic pressure continue to remain the accepted surrogates by drug regulatory authorities. This means that new therapies will continue to be assessed on the basis of brachial measurements, which may ultimately serve as a potential barrier to novel drug development. Therefore, appropriately powered clinical trials demonstrating that preferential lowering of central pressure improves outcome, will ultimately be required before central pressure becomes an accepted surrogate of cardiovascular risk. Nitrovasodilating drugs may be particularly useful in this respect. Before such trials are completed, smaller studies based on established surrogates for cardiovascular disease, such as carotid IMT and LVM will be important in providing proof of principle that reduction in central rather than brachial pressure is a more effective therapeutic strategy.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
C.M.M. and I.B.W. are supported by the National Institute for Health Research Cambridge Biomedical Research Centre. M.J.R. is supported by the National Institute of Health and I.B.W. is a British Heart Foundation Senior Clinical Fellow.
Conflict of interest: C.M.M., J.R.C., and I.B.W. have received unrestricted educational donations and/or equipment from AtCor Medical, Centron Diagnostics (CMM) and IEM GmbH. MJR and SSF report no conflict of interest.
Supplementary Material
References
- 1.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 2.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, III, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 4.Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- 5.Safar ME, Blacher J, Pannier B, Guerin AP, Marchais SJ, Guyonvarc'h PM, London GM. Central pulse pressure and mortality in end-stage renal disease. Hypertension. 2002;39:735–738. doi: 10.1161/hy0202.098325. [DOI] [PubMed] [Google Scholar]
- 6.Pini R, Cavallini MC, Palmieri V, Marchionni N, Di Bari M, Devereux RB, Masotti G, Roman MJ. Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: the ICARe Dicomano Study. J Am Coll Cardiol. 2008;51:2432–2439. doi: 10.1016/j.jacc.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 7.Jankowski P, Kawecka-Jaszcz K, Czarnecka D, Brzozowska-Kiszka M, Styczkiewicz K, Loster M, Kloch-Badelek M, Wilinski J, Curylo AM, Dudek D. Pulsatile but not steady component of blood pressure predicts cardiovascular events in coronary patients. Hypertension. 2008;51:848–855. doi: 10.1161/HYPERTENSIONAHA.107.101725. [DOI] [PubMed] [Google Scholar]
- 8.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 9.Asmar RG, London GM, O'Rourke ME, Safar ME. Improvement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patient: a comparison with atenolol. Hypertension. 2001;38:922–926. doi: 10.1161/hy1001.095774. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson IB, McEniery CM, Cockcroft JR. Atenolol and cardiovascular risk: an issue close to the heart. Lancet. 2006;367:627–629. doi: 10.1016/S0140-6736(06)68238-X. [DOI] [PubMed] [Google Scholar]
- 11.Kroeker EJ, Wood EH. Comparison of simultaneously recorded central and peripheral arterial pressure pulses during rest, exercise and tilted position in man. Circ Res. 1955;3:623–632. doi: 10.1161/01.res.3.6.623. [DOI] [PubMed] [Google Scholar]
- 12.Pauca AL, O'Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 13.Ohte N, Saeki T, Miyabe H, Sakata S, Mukai S, Hayano J, Niki K, Sugawara M, Kimura G. Relationship between blood pressure obtained from the upper arm with a cuff-type sphygmomanometer and central blood pressure measured with a catheter-tipped micromanometer. Heart Vessels. 2007;22:410–415. doi: 10.1007/s00380-007-0998-5. [DOI] [PubMed] [Google Scholar]
- 14.Nichols WW, O'Rourke MF. McDonald's Blood Flow in Arteries: Theoretic, Experimental and Clinical Principles. 5th ed. London: Edward Arnold; 2005. [Google Scholar]
- 15.Westerhof N, Sipkema P, van den Bos GC, Elzinga G. Forward and backward waves in the arterial system. Cardiovasc Res. 1972;6:648–656. doi: 10.1093/cvr/6.6.648. [DOI] [PubMed] [Google Scholar]
- 16.Westerhof N, Lankhaar JW, Westerhof BE. The arterial Windkessel. Med Biol Eng Comput. 2009;47:131–141. doi: 10.1007/s11517-008-0359-2. [DOI] [PubMed] [Google Scholar]
- 17.Wang JJ, O'Brien AB, Shrive NG, Parker KH, Tyberg JV. Time-domain representation of ventricular-arterial coupling as a windkessel and wave system. Am J Physiol Heart Circ Physiol. 2003;284:H1358–H1368. doi: 10.1152/ajpheart.00175.2002. [DOI] [PubMed] [Google Scholar]
- 18.Davies JE, Baksi J, Francis DP, Hadjiloizou N, Whinnett ZI, Manisty CH, Aguado-Sierra J, Foale RA, Malik IS, Tyberg JV, Parker KH, Mayet J, Hughes AD. The arterial reservoir pressure increases with aging and is the major determinant of the aortic augmentation index. Am J Physiol Heart Circ Physiol. 2010;298:H580–H586. doi: 10.1152/ajpheart.00875.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mynard JP, Penny DJ, Davidson MR, Smolich JJ. The reservoir-wave paradigm introduces error into arterial wave analysis: a computer modelling and in vivo study. J Hypertens. 2012;30:734–743. doi: 10.1097/HJH.0b013e32834f9793. [DOI] [PubMed] [Google Scholar]
- 20.Segers P, Swillens A, Vermeersch S. Reservations on the reservoir. J Hypertens. 2012;30:676–678. doi: 10.1097/HJH.0b013e32835077be. [DOI] [PubMed] [Google Scholar]
- 21.Camacho F, Avolio A, Lovell NH. Estimation of pressure pulse amplification between aorta and brachial artery using stepwise multiple regression models. Physiol Meas. 2004;25:879–889. doi: 10.1088/0967-3334/25/4/008. [DOI] [PubMed] [Google Scholar]
- 22.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity the anglo-cardiff collaborative trial (ACCT) J Am Coll Cardiol. 2005;46:1753–1760. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson IB, Mohammad NH, Tyrrell S, Hall IR, Webb DJ, Paul VE, Levy T, Cockcroft JR. Heart rate dependency of pulse pressure amplification and arterial stiffness. Am J Hypertens. 2002;15:24–30. doi: 10.1016/s0895-7061(01)02252-x. [DOI] [PubMed] [Google Scholar]
- 24.Albaladejo P, Copie X, Boutouyrie P, Laloux B, Declere AD, Smulyan H, Benetos A. Heart rate, arterial stiffness, and wave reflections in paced patients. Hypertension. 2001;38:949–952. doi: 10.1161/hy1001.096210. [DOI] [PubMed] [Google Scholar]
- 25.London GM, Guerin AP, Pannier BM, Marchais SJ, Metivier F. Body height as a determinant of carotid pulse contour in humans. J Hypertens Suppl. 1992;10:S93–S95. [PubMed] [Google Scholar]
- 26.Kroeker EJ, Wood EH. Beat-to-beat alterations in relationship of simultaneously recorded central and peripheral arterial pressure pulses during Valsalva maneuver and prolonged expiration in man. J Appl Physiol. 1956;8:483–494. doi: 10.1152/jappl.1956.8.5.483. [DOI] [PubMed] [Google Scholar]
- 27.McEniery CM, Yasmin, Wallace S, Maki-Petaja K, McDonnell B, Sharman JE, Retallick C, Franklin SS, Brown MJ, Lloyd RC, Cockcroft JR, Wilkinson IB. Increased stroke volume and aortic stiffness contribute to isolated systolic hypertension in young adults. Hypertension. 2005;46:221–226. doi: 10.1161/01.HYP.0000165310.84801.e0. [DOI] [PubMed] [Google Scholar]
- 28.McEniery CM, Yasmin, McDonnell B, Munnery M, Wallace SM, Rowe CV, Cockcroft JR, Wilkinson IB. Central pressure: variability and impact of cardiovascular risk factors: the Anglo-Cardiff Collaborative Trial II. Hypertension. 2008;51:1476–1482. doi: 10.1161/HYPERTENSIONAHA.107.105445. [DOI] [PubMed] [Google Scholar]
- 29.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A. 2007 ESH-ESC Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Blood Press. 2007;16:135–232. doi: 10.1080/08037050701461084. [DOI] [PubMed] [Google Scholar]
- 30.Van Bortel LM, Balkestein EJ, van der Heijden-Spek JJ, Vanmolkot FH, Staessen JA, Kragten JA, Vredeveld JW, Safar ME, Struijker Boudier HA, Hoeks AP. Non-invasive assessment of local arterial pulse pressure: comparison of applanation tonometry and echo-tracking. J Hypertens. 2001;19:1037–1044. doi: 10.1097/00004872-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 31.O'Rourke MF, Adji A. Noninvasive studies of central aortic pressure. Curr Hypertens Rep. 2012;14:8–20. doi: 10.1007/s11906-011-0236-5. [DOI] [PubMed] [Google Scholar]
- 32.Shih YT, Cheng HM, Sung SH, Hu WC, Chen CH. Quantification of the calibration error in the transfer function-derived central aortic blood pressures. Am J Hypertens. 2011;24:1312–1317. doi: 10.1038/ajh.2011.146. [DOI] [PubMed] [Google Scholar]
- 33.Verbeke F, Segers P, Heireman S, Vanholder R, Verdonck P, Van Bortel LM. Noninvasive assessment of local pulse pressure: importance of brachial-to-radial pressure amplification. Hypertension. 2005;46:244–248. doi: 10.1161/01.HYP.0000166723.07809.7e. [DOI] [PubMed] [Google Scholar]
- 34.Mahieu D, Kips J, Rietzschel ER, De Buyzere ML, Verbeke F, Gillebert TC, De Backer GG, De Bacquer D, Verdonck P, Van Bortel LM, Segers P. Noninvasive assessment of central and peripheral arterial pressure (waveforms): implications of calibration methods. J Hypertens. 2010;28:300–305. doi: 10.1097/HJH.0b013e3283340a1a. [DOI] [PubMed] [Google Scholar]
- 35.Mekhail AM, Day LM, Goodhart AK, Wilkinson IB, McEniery CM. Non-invasive estimates of central systolic blood pressure: Comparison of the Centron cBP301 and SphygmoCor devices. Artery Res. 2012;6:109–113. [Google Scholar]
- 36.Climie RE, Schultz MG, Nikolic SB, Ahuja KD, Fell JW, Sharman JE. Validity and reliability of central blood pressure estimated by upper arm oscillometric cuff pressure. Am J Hypertens. 2012;25:414–420. doi: 10.1038/ajh.2011.238. [DOI] [PubMed] [Google Scholar]
- 37.Rezai MR, Goudot G, Winters C, Finn JD, Wu FC, Cruickshank JK. Calibration mode influences central blood pressure differences between SphygmoCor and two newer devices, the Arteriograph and Omron HEM-9000. Hypertens Res. 2011;34:1046–1051. doi: 10.1038/hr.2011.75. [DOI] [PubMed] [Google Scholar]
- 38.Pauca AL, Kon ND, O'Rourke MF. The second peak of the radial artery pressure wave represents aortic systolic pressure in hypertensive and elderly patients. Br J Anaesth. 2004;92:651–657. doi: 10.1093/bja/aeh121. [DOI] [PubMed] [Google Scholar]
- 39.Takazawa K, Kobayashi H, Shindo N, Tanaka N, Yamashina A. Relationship between radial and central arterial pulse wave and evaluation of central aortic pressure using the radial arterial pulse wave. Hypertens Res. 2007;30:219–228. doi: 10.1291/hypres.30.219. [DOI] [PubMed] [Google Scholar]
- 40.Guilcher A, Brett S, Munir S, Clapp B, Chowienczyk PJ. Estimating central SBP from the peripheral pulse: influence of waveform analysis and calibration error. J Hypertens. 2011;29:1357–1366. doi: 10.1097/HJH.0b013e3283479070. [DOI] [PubMed] [Google Scholar]
- 41.Hickson SS, Butlin M, Mir FA, Graggaber J, Cheriyan J, Khan F, Grace AA, Yasmin, Cockcroft JR, Wilkinson IB, McEniery CM. The accuracy of central SBP determined from the second systolic peak of the peripheral pressure waveform. J Hypertens. 2009;27:1784–1788. doi: 10.1097/hjh.0b013e32832e0b58. [DOI] [PubMed] [Google Scholar]
- 42.Sung SH, Cheng HM, Chuang SY, Shih YT, Wang KL, Chen YH, Lin SJ, Yu WC, Chen CH. Measurement of central systolic blood pressure by pulse volume plethysmography with a noninvasive blood pressure monitor. Am J Hypertens. 2012;25:542–548. doi: 10.1038/ajh.2011.259. [DOI] [PubMed] [Google Scholar]
- 43.Laurent S, Cockcroft JR, van Bortel LM, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson IB, Struijker Boudier HA. Abridged version of the expert consensus document. Artery Res. 2007;1:2–12. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 44.Boutouyrie P, Bussy C, Lacolley P, Girerd X, Laloux B, Laurent S. Association between local pulse pressure, mean blood pressure, and large-artery remodeling. Circulation. 1999;100:1387–1393. doi: 10.1161/01.cir.100.13.1387. [DOI] [PubMed] [Google Scholar]
- 45.Wang KL, Cheng HM, Chuang SY, Spurgeon HA, Ting CT, Lakatta EG, Yin FC, Chou P, Chen CH. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens. 2009;27:461–467. doi: 10.1097/hjh.0b013e3283220ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roman MJ, Okin PM, Kizer JR, Lee ET, Howard BV, Devereux RB. Relations of central and brachial blood pressure to left ventricular hypertrophy and geometry: the Strong Heart Study. J Hypertens. 2010;28:384–388. doi: 10.1097/HJH.0b013e328333d228. [DOI] [PubMed] [Google Scholar]
- 47.Covic A, Goldsmith DJ, Panaghiu L, Covic M, Sedor J. Analysis of the effect of hemodialysis on peripheral and central arterial pressure waveforms. Kidney Int. 2000;57:2634–2643. doi: 10.1046/j.1523-1755.2000.00124.x. [DOI] [PubMed] [Google Scholar]
- 48.de Luca N, Asmar RG, London GM, O'Rourke MF, Safar ME. Selective reduction of cardiac mass and central blood pressure on low-dose combination perindopril/indapamide in hypertensive subjects. J Hypertens. 2004;22:1623–1630. doi: 10.1097/01.hjh.0000125448.28861.fc. [DOI] [PubMed] [Google Scholar]
- 49.Manisty CH, Zambanini A, Parker KH, Davies JE, Francis DP, Mayet J, McG Thom SA, Hughes AD. Differences in the magnitude of wave reflection account for differential effects of amlodipine- versus atenolol-based regimens on central blood pressure: an Anglo-Scandinavian Cardiac Outcome Trial substudy. Hypertension. 2009;54:724–730. doi: 10.1161/HYPERTENSIONAHA.108.125740. [DOI] [PubMed] [Google Scholar]
- 50.Boutouyrie P, Bussy C, Hayoz D, Hengstler J, Dartois N, Laloux B, Brunner H, Laurent S. Local pulse pressure and regression of arterial wall hypertrophy during long-term antihypertensive treatment. Circulation. 2000;101:2601–2606. doi: 10.1161/01.cir.101.22.2601. [DOI] [PubMed] [Google Scholar]
- 51.Dart AM, Gatzka CD, Kingwell BA, Willson K, Cameron JD, Liang YL, Berry KL, Wing LM, Reid CM, Ryan P, Beilin LJ, Jennings GL, Johnston CI, McNeil JJ, Macdonald GJ, Morgan TO, West MJ. Brachial blood pressure but not carotid arterial waveforms predict cardiovascular events in elderly female hypertensives. Hypertension. 2006;47:785–790. doi: 10.1161/01.HYP.0000209340.33592.50. [DOI] [PubMed] [Google Scholar]
- 52.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roman MJ, Devereux RB, Kizer JR, Okin PM, Lee ET, Wang W, Umans JG, Calhoun D, Howard BV. High central pulse pressure is independently associated with adverse cardiovascular outcome the strong heart study. J Am Coll Cardiol. 2009;54:1730–1734. doi: 10.1016/j.jacc.2009.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31:1865–1871. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- 55.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 56.Carlberg B, Samuelsson O, Lindholm LH. Atenolol in hypertension: is it a wise choice? Lancet. 2004;364:1684–1689. doi: 10.1016/S0140-6736(04)17355-8. [DOI] [PubMed] [Google Scholar]
- 57.Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension?. A Meta-Analysis. Lancet. 2005;366:1545–1553. doi: 10.1016/S0140-6736(05)67573-3. [DOI] [PubMed] [Google Scholar]
- 58.Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ. 1992;304:405–412. doi: 10.1136/bmj.304.6824.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 60.Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 61.Morgan T, Lauri J, Bertram D, Anderson A. Effect of different antihypertensive drug classes on central aortic pressure. Am J Hypertens. 2004;17:118–123. doi: 10.1016/j.amjhyper.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 62.Deary AJ, Schumann AL, Murfet H, Haydock S, Foo RS, Brown MJ. Influence of drugs and gender on the arterial pulse wave and natriuretic peptide secretion in untreated patients with essential hypertension. Clin Sci. 2002;103:493–499. doi: 10.1042/cs1030493. [DOI] [PubMed] [Google Scholar]
- 63.Mackenzie IS, McEniery CM, Dhakam Z, Brown MJ, Cockcroft JR, Wilkinson IB. Comparison of the effects of antihypertensive agents on central blood pressure and arterial stiffness in isolated systolic hypertension. Hypertension. 2009;54:409–413. doi: 10.1161/HYPERTENSIONAHA.109.133801. [DOI] [PubMed] [Google Scholar]
- 64.Boutouyrie P, Achouba A, Trunet P, Laurent S. Amlodipine-valsartan combination decreases central systolic blood pressure more effectively than the amlodipine-atenolol combination: the EXPLOR study. Hypertension. 2010;55:1314–1322. doi: 10.1161/HYPERTENSIONAHA.109.148999. [DOI] [PubMed] [Google Scholar]
- 65.Dhakam Z, Yasmin, McEniery CM, Burton T, Brown MJ, Wilkinson IB. A comparison of atenolol and nebivolol in isolated systolic hypertension. J Hypertens. 2008;26:351–356. doi: 10.1097/HJH.0b013e3282f283c9. [DOI] [PubMed] [Google Scholar]
- 66.Kampus P, Serg M, Kals J, Zagura M, Muda P, Karu K, Zilmer M, Eha J. Differential effects of nebivolol and metoprolol on central aortic pressure and left ventricular wall thickness. Hypertension. 2011;57:1122–1128. doi: 10.1161/HYPERTENSIONAHA.110.155507. [DOI] [PubMed] [Google Scholar]
- 67.Elliott WJ, Childers WK. Should beta blockers no longer be considered first-line therapy for the treatment of essential hypertension without comorbidities? Curr Cardiol Rep. 2011;13:507–516. doi: 10.1007/s11886-011-0216-z. [DOI] [PubMed] [Google Scholar]
- 68.Kelly RP, Gibbs HH, O'Rourke MF, Daley JE, Mang K, Morgan JJ, Avolio AP. Nitroglycerin has more favourable effects on left ventricular afterload than apparent from measurement of pressure in a peripheral artery. Eur Heart J. 1990;11:138–144. doi: 10.1093/oxfordjournals.eurheartj.a059669. [DOI] [PubMed] [Google Scholar]
- 69.Stokes GS, Ryan M. Can Extended-Release Isosorbide Mononitrate be Used as Adjunctive Therapy for Systolic Hypertension? An Open Study Employing Pulse-Wave Analysis to Determine Effects of Antihypertensive Therapy. Am J Geriatr Cardiol. 1997;6:11–19. [PubMed] [Google Scholar]
- 70.Stokes GS, Bune AJ, Huon N, Barin ES. Long-term effectiveness of extended-release nitrate for the treatment of systolic hypertension. Hypertension. 2005;45:380–384. doi: 10.1161/01.HYP.0000156746.25300.1c. [DOI] [PubMed] [Google Scholar]
- 71.Stokes GS, Ryan M, Brnabic A, Nyberg G. A controlled study of the effects of isosorbide mononitrate on arterial blood pressure and pulse wave form in systolic hypertension. J Hypertens. 1999;17:1767–1773. doi: 10.1097/00004872-199917120-00015. [DOI] [PubMed] [Google Scholar]
- 72.Duchier J, Iannascoli F, Safar M. Antihypertensive effect of sustained-release isosorbide dinitrate for isolated systolic systemic hypertension in the elderly. Am J Cardiol. 1987;60:99–102. doi: 10.1016/0002-9149(87)90993-3. [DOI] [PubMed] [Google Scholar]
- 73.Starmans-Kool MJ, Kleinjans HA, Lustermans FA, Kragten JA, Breed JG, Van Bortel LM. Treatment of elderly patients with isolated systolic hypertension with isosorbide dinitrate in an asymmetric dosing schedule. J Hum Hypertens. 1998;12:557–561. doi: 10.1038/sj.jhh.1000664. [DOI] [PubMed] [Google Scholar]
- 74.Jiang XJ, O'Rourke MF, Jin WQ, Liu LS, Li CW, Tai PC, Zhang XC, Liu SZ. Quantification of glyceryl trinitrate effect through analysis of the synthesised ascending aortic pressure waveform. Heart. 2002;88:143–148. doi: 10.1136/heart.88.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomas GR, DiFabio JM, Gori T, Parker JD. Once daily therapy with isosorbide-5-mononitrate causes endothelial dysfunction in humans: evidence of a free-radical-mediated mechanism. J Am Coll Cardiol. 2007;49:1289–1295. doi: 10.1016/j.jacc.2006.10.074. [DOI] [PubMed] [Google Scholar]
- 76.Oelze M, Knorr M, Kroller-Schon S, Kossmann S, Gottschlich A, Rummler R, Schuff A, Daub S, Doppler C, Kleinert H, Gori T, Daiber A, Munzel T. Chronic therapy with isosorbide-5-mononitrate causes endothelial dysfunction, oxidative stress, and a marked increase in vascular endothelin-1 expression. Eur Heart J. 2013;34:3206–3216. doi: 10.1093/eurheartj/ehs100. [DOI] [PubMed] [Google Scholar]
- 77.Richardson CE, Maki-Pataja KM, McDonnell BJ, Hickson SS, Wilkinson IB, McEniery CM. Comparison of estimates of central systolic blood pressure and peripheral augmentation index obtained from the Omron HEM-9000AI and SphygmoCor systems. Artery Res. 2009;3:24–32. [Google Scholar]
- 78.Kips JG, Schutte AE, Vermeersch SJ, Huisman HW, Van Rooyen JM, Glyn MC, Fourie CM, Malan L, Schutte R, Van Bortel LM, Segers P. Comparison of central pressure estimates obtained from SphygmoCor, Omron HEM-9000AI and carotid applanation tonometry. J Hypertens. 2011;29:1115–1120. doi: 10.1097/HJH.0b013e328346a3bc. [DOI] [PubMed] [Google Scholar]
- 79.Staessen JA, Thijs L, Fagard R, O'Brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, Tuomilehto J, Webster J. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA. 1999;282:539–546. doi: 10.1001/jama.282.6.539. [DOI] [PubMed] [Google Scholar]
- 80.Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun JJ, Six RO, Van Der Niepen P, O'Brien E. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–2415. doi: 10.1056/NEJMoa022273. [DOI] [PubMed] [Google Scholar]
- 81.Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–1783. doi: 10.1161/01.CIR.0000160923.04524.5B. [DOI] [PubMed] [Google Scholar]
- 82.Huang CM, Wang KL, Cheng HM, Chuang SY, Sung SH, Yu WC, Ting CT, Lakatta EG, Yin FC, Chou P, Chen CH. Central versus ambulatory blood pressure in the prediction of all-cause and cardiovascular mortalities. J Hypertens. 2011;29:454–459. doi: 10.1097/HJH.0b013e3283424b4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schillaci G, Pucci G. Central and 24-h blood pressure: dwarfs standing upon the shoulders of giants? J Hypertens. 2011;29:430–433. doi: 10.1097/HJH.0b013e3283448222. [DOI] [PubMed] [Google Scholar]
- 84.Luzardo L, Lujambio I, Sottolano M, da Rosa A, Thijs L, Noboa O, Staessen JA, Boggia J. 24-h ambulatory recording of aortic pulse wave velocity and central systolic augmentation: a feasibility study. Hypertens Res. 2012;35:980–987. doi: 10.1038/hr.2012.78. [DOI] [PubMed] [Google Scholar]
- 85.Williams B, Lacy PS, Baschiera F, Brunel P, Dusing R. Novel description of the 24-hour circadian rhythms of brachial versus central aortic blood pressure and the impact of blood pressure treatment in a randomized controlled clinical trial: The Ambulatory Central Aortic Pressure (AmCAP) Study. Hypertension. 2013;61:1168–1176. doi: 10.1161/HYPERTENSIONAHA.111.00763. [DOI] [PubMed] [Google Scholar]
- 86.Ott C, Haetinger S, Schneider MP, Pauschinger M, Schmieder RE. Comparison of two noninvasive devices for measurement of central systolic blood pressure with invasive measurement during cardiac catheterization. J Clin Hypertens (Greenwich) 2012;14:575–579. doi: 10.1111/j.1751-7176.2012.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Theilade S, Hansen TW, Joergensen C, Lajer M, Rossing P. Tonometric devices for central aortic systolic pressure measurements in patients with type 1 diabetes: comparison of the BPro and SphygmoCor devices. Blood Press Monit. 2013;18:156–160. doi: 10.1097/MBP.0b013e328360fb19. [DOI] [PubMed] [Google Scholar]
- 88.Sharman JE, Lim R, Qasem AM, Coombes JS, Burgess MI, Franco J, Garrahy P, Wilkinson IB, Marwick TH. Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension. 2006;47:1203–1208. doi: 10.1161/01.HYP.0000223013.60612.72. [DOI] [PubMed] [Google Scholar]
- 89.Wassertheurer S, Kropf J, Weber T, van der Giet M, Baulmann J, Ammer M, Hametner B, Mayer CC, Eber B, Magometschnigg D. A new oscillometric method for pulse wave analysis: comparison with a common tonometric method. J Hum Hypertens. 2010;24:498–504. doi: 10.1038/jhh.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weber T, Wassertheurer S, Rammer M, Maurer E, Hametner B, Mayer CC, Kropf J, Eber B. Validation of a brachial cuff-based method for estimating central systolic blood pressure. Hypertension. 2011;58:825–832. doi: 10.1161/HYPERTENSIONAHA.111.176313. [DOI] [PubMed] [Google Scholar]
- 91.Brett SE, Guilcher A, Clapp B, Chowienczyk P. Estimating central systolic blood pressure during oscillometric determination of blood pressure: proof of concept and validation by comparison with intra-aortic pressure recording and arterial tonometry. Blood Press Monit. 2012;17:132–136. doi: 10.1097/MBP.0b013e328352ae5b. [DOI] [PubMed] [Google Scholar]
- 92.Pucci G, Cheriyan J, Hubsch A, Hickson SS, Gajendragadkar PR, Watson T, O'Sullivan M, Woodcock-Smith J, Schillaci G, Wilkinson IB, McEniery CM. Evaluation of the Vicorder, a novel cuff-based device for the noninvasive estimation of central blood pressure. J Hypertens. 2013;31:77–85. doi: 10.1097/HJH.0b013e32835a8eb1. [DOI] [PubMed] [Google Scholar]
- 93.Horvath IG, Nemeth A, Lenkey Z, Alessandri N, Tufano F, Kis P, Gaszner B, Cziraki A. Invasive validation of a new oscillometric device (Arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. J Hypertens. 2010;28:2068–2075. doi: 10.1097/HJH.0b013e32833c8a1a. [DOI] [PubMed] [Google Scholar]
- 94.Lowe A, Harrison W, El-Aklouk E, Ruygrok P, Al-Jumaily AM. Non-invasive model-based estimation of aortic pulse pressure using suprasystolic brachial pressure waveforms. J Biomech. 2009;42:2111–2115. doi: 10.1016/j.jbiomech.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 95.Hirata K, Vlachopoulos C, Adji A, O'Rourke MF. Benefits from angiotensin-converting enzyme inhibitor ‘beyond blood pressure lowering’: beyond blood pressure or beyond the brachial artery? J Hypertens. 2005;23:551–556. doi: 10.1097/01.hjh.0000160211.56103.48. [DOI] [PubMed] [Google Scholar]
- 96.London GM, Pannier B, Guerin AP, Marchais SJ, Safar ME, Cuche JL. Cardiac hypertrophy, aortic compliance, peripheral resistance, and wave reflection in end-stage renal disease. Comparative effects of ACE inhibition and calcium channel blockade. Circulation. 1994;90:2786–2796. doi: 10.1161/01.cir.90.6.2786. [DOI] [PubMed] [Google Scholar]
- 97.London GM, Pannier B, Vicaut E, Guerin AP, Marchais SJ, Safar ME, Cuche JL. Antihypertensive effects and arterial haemodynamic alterations during angiotensin converting enzyme inhibition. J Hypertens. 1996;14:1139–1146. doi: 10.1097/00004872-199609000-00015. [DOI] [PubMed] [Google Scholar]
- 98.Mitchell GF, Izzo JL, Jr, Lacourciere Y, Ouellet JP, Neutel J, Qian C, Kerwin LJ, Block AJ, Pfeffer MA. Omapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension: results of the conduit hemodynamics of omapatrilat international research study. Circulation. 2002;105:2955–2961. doi: 10.1161/01.cir.0000020500.77568.3c. [DOI] [PubMed] [Google Scholar]
- 99.Ahimastos AA, Natoli AK, Lawler A, Blombery PA, Kingwell BA. Ramipril reduces large-artery stiffness in peripheral arterial disease and promotes elastogenic remodeling in cell culture. Hypertension. 2005;45:1194–1199. doi: 10.1161/01.HYP.0000168945.44069.aa. [DOI] [PubMed] [Google Scholar]
- 100.Dart AM, Cameron JD, Gatzka CD, Willson K, Liang YL, Berry KL, Wing LM, Reid CM, Ryan P, Beilin LJ, Jennings GL, Johnston CI, McNeil JJ, Macdonald GJ, Morgan TO, West MJ, Kingwell BA. Similar effects of treatment on central and brachial blood pressures in older hypertensive subjects in the Second Australian National Blood Pressure Trial. Hypertension. 2007;49:1242–1247. doi: 10.1161/HYPERTENSIONAHA.106.085803. [DOI] [PubMed] [Google Scholar]
- 101.Aznaouridis KA, Stamatelopoulos KS, Karatzis EN, Protogerou AD, Papamichael CM, Lekakis JP. Acute effects of renin-angiotensin system blockade on arterial function in hypertensive patients. J Hum Hypertens. 2007;21:654–663. doi: 10.1038/sj.jhh.1002211. [DOI] [PubMed] [Google Scholar]
- 102.Jiang XJ, O'Rourke MF, Zhang YQ, He XY, Liu LS. Superior effect of an angiotensin-converting enzyme inhibitor over a diuretic for reducing aortic systolic pressure. J Hypertens. 2007;25:1095–1099. doi: 10.1097/HJH.0b013e3280ac1533. [DOI] [PubMed] [Google Scholar]
- 103.Dhakam Z, McEniery CM, Yasmin, Cockcroft JR, Brown MJ, Wilkinson IB. Atenolol and eprosartan: differential effects on central blood pressure and aortic pulse wave velocity. Am J Hypertens. 2006;19:214–219. doi: 10.1016/j.amjhyper.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 104.Stokes GS, Barin ES, Gilfillan KL. Effects of isosorbide mononitrate and AII inhibition on pulse wave reflection in hypertension. Hypertension. 2003;41:297–301. doi: 10.1161/01.hyp.0000049622.07021.4f. [DOI] [PubMed] [Google Scholar]
- 105.Mahmud A, Feely J. Favourable effects on arterial wave reflection and pulse pressure amplification of adding angiotensin II receptor blockade in resistant hypertension. J Hum Hypertens. 2000;14:541–546. doi: 10.1038/sj.jhh.1001053. [DOI] [PubMed] [Google Scholar]
- 106.Polonia J, Barbosa L, Silva JA, Bertoquini S. Different patterns of peripheral versus central blood pressure in hypertensive patients treated with beta-blockers either with or without vasodilator properties or with angiotensin receptor blockers. Blood Press Monit. 2010;15:235–239. doi: 10.1097/MBP.0b013e32833c8a64. [DOI] [PubMed] [Google Scholar]
- 107.Shah NK, Smith SM, Nichols WW, Lo MC, Ashfaq U, Satish P, Johnson JA, Epstein BJ. Carvedilol reduces aortic wave reflection and improves left ventricular/vascular coupling: a comparison with atenolol (CENTRAL Study) J Clin Hypertens (Greenwich) 2011;13:917–924. doi: 10.1111/j.1751-7176.2011.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.