Abstract
Survivors of childhood cancer frequently experience cancer-related cognitive dysfunction, commonly months to years after treatment for pediatric brain tumors, acute lymphoblastic leukemia (ALL), or tumors involving the head and neck. Risk factors for cancer-related cognitive dysfunction include young age at diagnosis, treatment with cranial irradiation, use of parenteral or intrathecal methotrexate, female sex, and pre-existing comorbidities. Limiting use and reducing doses and volume of cranial irradiation while intensifying chemotherapy have improved survival and reduced the severity of cognitive dysfunction, especially in leukemia. Nonetheless, problems in core functional domains of attention, processing speed, working memory and visual-motor integration continue to compromise quality of life and performance. We review the epidemiology, pathophysiology and assessment of cancer-related cognitive dysfunction, the impact of treatment changes for prevention, and the broad strategies for educational and pharmacological interventions to remediate established cognitive dysfunction following childhood cancer. The increased years of life saved after childhood cancer warrants continued study toward the prevention and remediation of cancer-related cognitive dysfunction, using uniform assessments anchored in functional outcomes.
Cancer-related cognitive dysfunction (referred to hereafter as “cognitive dysfunction”) affects one third or more of the estimated 350000 childhood cancer survivors in the US (1–9). Cognitive dysfunction is a symptom complex characterized by decline in full scale intelligence quotient (FSIQ) and/or impairment in core functional domains of attention, vigilance, working memory, executive function, processing speed, or visual motor integration (9–14). These core deficits can compromise social and academic performance and quality of life (HRQOL), even when FSIQ falls within average range (4,9,15–22). Patient, parent, and teacher reports describe children spending excessive time on homework yet having poor acquisition and retention (17,23–27), especially in reading, spelling and mathematics (28,29). Self-monitoring skills and peer relationships can be compromised (4,17,18,27,30–37), and post-traumatic stress is common (38,39).
Cognitive dysfunction varies in severity. It is most common in survivors of brain tumors or acute lymphoblastic leukemia (ALL). However, it can affect any child treated with head and neck irradiation, repetitive neurotoxic chemotherapy, or hematopoietic stem cell transplantation (HSCT) (10,11,13,14,40–45). Cognitive dysfunction complicating childhood cancer appears to be more frequent and severe than “chemo brain” of adults (46–48). Compared to controls, childhood brain tumor survivors are less likely to marry (49,50), complete high school (51), maintain employment (52–54), or receive appropriate health care (55,56). Brain tumor survivors face additional problems related to motor, sensory, and behavioral disturbances, often culminating in social isolation and failure to attain independence (4,10,52,57,58). Cognitive dysfunction presents at or soon after diagnosis of the cancer, but deficits often emerge insidiously years later. Over the past 40 years, clinical trials modifying cancer treatment have improved overall survival in ALL and central nervous system (CNS) tumors and reduced severity of cognitive dysfunction (4,5,9–11,13,27,44,59–62). This review examines the impact of modern cancer treatment on the epidemiology and pathophysiology of cognitive dysfunction, considers ongoing attempts to monitor outcomes through targeted and feasible neurocognitive assessments, and describes pharmacologic and nonpharmacologic interventions to remediate established cognitive dysfunction. We propose earlier intervention, use of novel intervention approaches, and ways to address accrual and retention to facilitate more robust trials.
Methods
We used PubMed to identify relevant English-language articles published between 1990 and December 2012. In alignment with best practices in search methodology, we aimed to retrieve a comprehensive set of relevant studies using combinations of search terms such as: cancer therapy, brain tumor, leukemia, cognition, cognitive deficits, radiation therapy, chemotherapy, methotrexate, neoplasms, intervention, remediation, and pediatric. We limit citations to seminal reports, primary studies and reviews of the past two decades, and publications of remediation trials and those concerning new strategies. A total of 257 studies were selected for inclusion from 622 searched.
Epidemiology of Cancer-Related Cognitive Dysfunction
Prevalence
Prevalence estimates are derived from a composite of a few prospective longitudinal studies and many cross-sectional observational studies. The latter are characterized by small sample size, heterogeneous tumors, varied control populations, absence of power calculations, disparate assessment tools, and assessment intervals ranging from months to decades after treatment (4,9). Despite methodological limitations, a remarkably consistent picture emerges: prevalence and severity of cognitive dysfunction has declined over the last 50 years. To date, the impact of treatment modification is more noticeable in ALL than in brain tumor survivors.
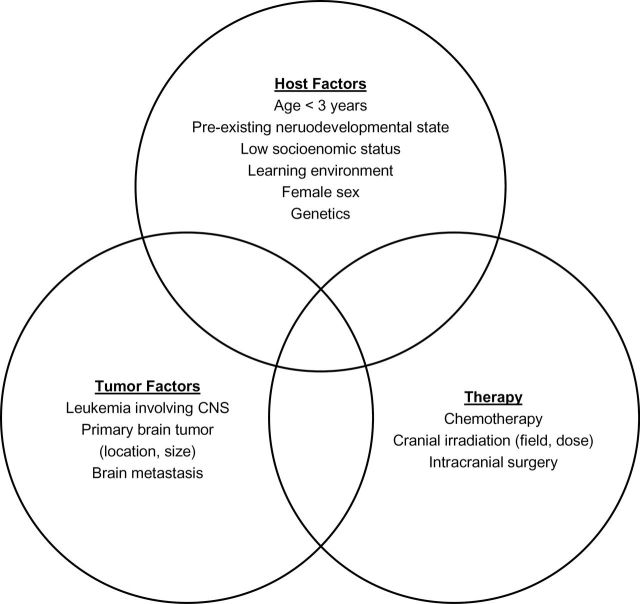
The prevalence of cognitive dysfunction in ALL survivors, measured solely by decline in FSIQ, fell from an estimated 10% to 40% for patients treated in the 1970s and early 1980s to 5% to 10% in the 21st century (62–64). However, deficits in core functions continue to compromise HRQOL and performance for many (5). In contrast, the prevalence of cognitive dysfunction in survivors of childhood brain tumors ranges from 40% to 100% (3–5,65). Risk factors involve interaction among standard variables of host, tumor, and treatment (Figure 1). Risk must be interpreted in the context of the timing and type of assessment used to define cognitive dysfunction: longer follow-up and more detailed and sensitive assessments are likely to find deficits of varying functional importance.
Figure 1.
Factors contributing to risk of cancer-related cognitive dysfunction in childhood cancer survivors. Risk of impairment varies by timing of assessment relative to diagnosis and treatment, and by the assessment battery used. CNS = central nervous system.
Pathogenesis of Pediatric Cancer-Related Cognitive Dysfunction
Host-Related Risk Factors
In children, cognitive dysfunction primarily affects new learning (65). Younger age at treatment is the most important host-related risk factor (Figure 1), explained by the concurrence of cancer injury with critical periods of brain development (66). Basic motor, language, problem-solving and social skills are acquired early in life. By three months of age, a full complement of neurons populates the brain; thereafter, most neuronal proliferation arrests and differentiation of neurons begins within specific germinal centers (67). Synapses reach their maximum number by two years of age, gray matter development peaks at age four, and white matter development continues into the third decade. As the child ages, axons are myelinated, and synaptic contacts are established and refined by waves of overproduction and pruning in varying parts of the growing brain, with the tempo related to gains in cognition (66,68).
Female sex is a risk factor for cognitive dysfunction in some studies of ALL (69–71). There is no clear pathophysiological explanation for observed gender differences, and they are not reported in survivors of other tumors.
Conditions antecedent to the cancer diagnosis also contribute to cognitive dysfunction. Forty to 50% of children with CNS tumors have cognitive impairment or developmental delay pretreatment, and 6% have cognitive abilities in the borderline to extremely low range with IQ < 70 (72). Physical disability and impaired fine motor skills associate with worse performance on neuropsychological assessment (32,73,74). Comorbidities that associate with cognitive dysfunction include seizures, hydrocephalus, meningitis, ventriculitis, cerebrovascular accidents, impaired hearing and/or vision, motor problems (eg, paresis, paralysis, ataxia, and imbalance), behavioral abnormalities, and antecedent learning difficulties or developmental delay (4,57,75).
Genetic risk factors include cancer predisposition syndromes such as neurofibromatosis type 1, tuberous sclerosis, and basal cell nevus syndrome, where underlying cognitive delays or autism confound attribution of effects to tumor and treatment (76). Polymorphisms in the MTHFR gene correlate with risk of inattention in survivors of childhood ALL treated with systemic or intrathecal folate antagonists (77,78). The association between cognitive dysfunction and gene polymorphisms involved in the metabolism of homocysteine (79), methionine synthase glutathione S-transferase, monoamine oxidase, and apo-lipoprotein E4 await confirmation (80).
A limited number of reports of cognitive dysfunction address socioeconomic factors; however, low maternal educational attainment is a consistent association (12,81,82).
Treatment-Related Risk Factors
Radiation Therapy.
Dose and field of cranial irradiation are highly associated with subsequent development of cognitive dysfunction (4,9,12,25,33,44,83). Somnolence syndrome increases the risk of cognitive dysfunction (84). Radiation to temporal regions is associated with problems in memory, social function and general health—radiation to frontal areas with limitations in general health and physical performance (85). In animal models of cranial irradiation therapy (CrRT), spatial learning and memory deficits associate with alterations in glutamate receptors (86,87).
Leukoencephalopathy, a subacute degeneration of white matter, may contribute to radiation-associated cognitive dysfunction (88). Oligodendrocytes and their progenitor cells, the most radiosensitive glial cells, produce myelin. Death of oligodendrocytes, endothelial cell loss, and neural progenitor depletion initiate the release of oxygen species by astrocytes. This elicits an inflammatory response, thereby impairing neurogenesis (88,89). Irradiated rodents show increased hippocampal apoptosis, decreased stem cell numbers and reduced adult neurogenesis (90–92). Even low total doses of radiation can damage the pool of proliferative progenitor cells (93). Vascular injury manifesting five or more years after therapy can also contribute to cognitive dysfunction (88,94). Chronic ischemia or infarcts within the radiation field implicate both large and small caliber arteries (95). Pediatric brain tumor survivors have a 40-fold higher risk of stroke compared to siblings (96,97). Cerebral telangiectasia, cavernomas and aneurysms are recognized long-term complications of CrRT in children (98,99).
Total white matter volume accounts for some of the variance in the relationship between age at radiation therapy and cognitive dysfunction in survivors of ALL (100,101). In brain tumor survivors, disappearance of normal white matter and histologic evidence of demyelination and white matter necrosis on MRI correlate with decreased IQ, executive function deficits, problems with working memory, and overall academic achievement (28,102). Magnetic resonance spectroscopy (MRS) or diffusion tensor imaging (DTI) or tractography detect abnormalities in regions that do not demonstrate changes on conventional MRI (103), as shifts in metabolite ratios may reflect neuronal loss and glial proliferation after injury. Reductions in white matter fractional anisotropy in the corpus collosum or inferior frontal-occipital fasciculus on DTI correlate respectively with reduced processing and motor speed in survivors (104). Hippocampal atrophy and altered signaling in the hippocampus and elsewhere during unsuccessful encoding exercises correlate with impaired memory in survivors of ALL (105).
Chemotherapy.
Neurocognitive sequelae of chemotherapy are less well documented than radiation effects (106). As childhood cancer treatment relies on combinations of drugs, attributing causality to specific agents other than methotrexate is difficult (107). Table 1 lists chemotherapeutic agents commonly associated with complications that may contribute to cognitive dysfunction, including seizures, transient ischemic attacks, encephalopathy, myelopathy, ataxia, posterior reversible leukoencephalopathy, and metabolic encephalopathy. Indirect complications of chemotherapy include infection, neuropathy, coagulopathy, vascular disorders, and poly pharmacy (including antiemetics, analgesics, anxiolytics, and other agents that can compromise mental status). Notably, the risk of neurotoxicity and cognitive dysfunction from new molecularly targeted biological therapies is unfolding, and likely related to penetration of the blood-brain barrier as well as the specificity of the target (107).
Table 1.
Chemotherapy in pediatric cancer associated with cognitive dysfunction*
| Chemotherapy | Acute / subacute CNS effects | Potential long-term CNS effects |
|---|---|---|
| Methotrexate (systemic or intrathecal) |
Seizures Leukoencephalopathy Myelopathy Aseptic meningitis |
Attention deficits Processing speed deficits Seizures |
| Cytosine arabinoside | Cerebellar ataxia Aseptic meningitis Myelopathy Meningismus |
Cognitive deficits Hemiparesis |
| AraG (Compound 506) | Somnolence Seizures |
Unknown |
| Steroids | Sleep disturbance Mood/behavioral disorder PRES Transient psychosis |
Cognitive deficits |
| 5FU | Cerebellar ataxia | Unknown |
| Ifosfamide | Acute encephalopathy Hallucinations/delirium Aseptic meningitis Cranial nerve palsy Movement disorder Seizure |
Cognitive deficits |
| Asparaginase | Thrombotic stroke Encephalopathy |
Unknown |
| Cyclosporine | Leukoencephalopathy | Unknown |
| Cisplatin | Ototoxicity Peripheral neuropathy |
Sensorineural hearing loss |
| Vincristine | Peripheral neuropathy Autonomic/cranial Neuropathy |
Peripheral neuropathy |
| Angiogenesis inhibitors | Hypertension Stroke/bleeding |
Unknown |
* This table is not intended to be comprehensive. Novel targeted agents more recently introduced into practice have not been included. CNS = central nervous system; PRES = posterior reversible encephalopathy syndrome.
The folic acid inhibitor methotrexate is associated with cognitive dysfunction. Folic acid is essential for normal brain development and for DNA synthesis and repair throughout life. Methotrexate penetrates the blood-brain barrier in doses achievable with parenteral, intrathecal, or intraventricular administration (108). Dose, schedule, and route of methotrexate administration correlate with incidence and severity of cognitive dysfunction (6,109–112).
Although multiple systematic reviews and meta-analyses in ALL survivors associate dexamethasone with more cognitive declines than prednisone (113–115), recent primary reports have not confirmed this (82,116,117). In comparing outcomes between CNS prophylaxis with triple intrathecal therapy (ie, methotrexate, cytosine arabinoside, hydrocortisone) vs intrathecal methotrexate alone, the latter has a higher prevalence of processing speed dysfunction (118).
Neurosurgery.
Surgical resection of CNS tumors provides histopathologic specimens and reduces tumor burden. Tumor location determines extent of resection and risk for complications, including altered mental status, stroke, infection, paresis or paralysis, seizures, sensory disturbance, incoordination, and cranial nerve deficits (18,107,119,120). Upon recovery from these events, long-term deficits can persist. While total resection increases the chance of long-term survival, the benefits of aggressive resection must be weighed against risks of disability.
The cerebellum, the most common site of pediatric CNS tumors, is important for cortical development and high-level cognition (121,122). The tumor and its resection disrupt neural circuits connecting prefrontal, superior temporal, posterior parietal, and limbic regions (123). Comparison of preoperative and postoperative neuropsychological function demonstrates that deficits correlate with brainstem infiltration and involvement of the dentate nucleus and are often present preoperatively (48,124). Children with cerebellar low-grade gliomas treated with surgery alone are at increased risk for cognitive, affective, and adaptive deficits (65,125–131). Twenty percent are at risk of postoperative cerebellar mutism syndrome characterized by early onset of mutism, ataxia, hypotonia, and emotional lability. The symptoms of this syndrome diminish with time, but are associated with lasting effects on cognitive dysfunction (126–131). Childhood survivors of infratentorial tumors experience more difficulty than those with supratentorial tumors, attributable to ongoing motor dysfunction and impairment of executive function (128,132,133).
Pre- and postoperative tumor assessment with MRI, use of dissecting microscope, robotic technology, third ventriculostomy, and greater referral to pediatric neurosurgical oncology specialist are improving the therapeutic margin of surgery for pediatric brain tumors (134–136).
Prevention of Cancer-Related Cognitive Dysfunction by Cancer Treatment Modification
With evidence that use of CrRT in ALL was causally related to decline in FSIQ, the dose of prophylactic CrRT was systematically reduced from 2400 cGy in all children with leukemia in the 1980s to abandonment of CrRT for the majority of children in the current era (62,63). While FSIQ for ALL survivors is now within the average range, mean FSIQ is often lower than predicted or lower than those of controls (74,113,115). Review of long-term outcomes demonstrate that some combination of attention, executive function, verbal working memory, processing speed, and fine motor skills are below those of control groups. While replacement of CrRT (with systemic and intrathecal methotrexate) in contemporary therapy has reduced the severity of cognitive dysfunction, the prevalence of attention deficits remains high as 67% (117); 3% to 28% of survivors experience deficits in other domains. Prevalence is consistently higher in those receiving therapy for high risk ALL (82,117,137,138).
In childhood brain tumors, the majority of reports derive from survivors of low-grade gliomas (LGG) or medulloblastoma, the most frequently observed tumors with high survival rates. Among children with CNS tumors, recent meta-analysis reveals severe cognitive dysfunction compared to norms with substantial effect size of -0.83 for FSIQ, -0.93 to -1.22 for core functional domains and -0.45 to 0.63 for academic domains (139). Strategies to reduce cognitive dysfunction in LGGs have included: 1) use of chemotherapy to prevent or postpone the need for cranial irradiation in young children; 2) reducing the field of CrRT with conformal radiation or proton beam radiation therapy; or 3) expectant observation of clinically asymptomatic tumors, especially in children with neurofibromatosis type 1. Sequential assessments of LGG show baseline FSIQ at the low end of average and unchanged six years later (140,141). Age at diagnosis remains the principal predictor of FSIQ (79 in those under the age of five; 96 in older patients). Even when mean FSIQ is normal, twice the number of survivors score one standard deviation below the mean than expected (120). Prospective assessment in children with pilocytic astrocytoma treated with modern therapy reveals that the majority experience progressive deficits in sustained attention and processing speed over time; the most striking deficits occur in children under the age of three and those treated with CrRT (65). While conformal radiation therapy for LGG promises less neurocognitive and neuroendocrine injury (83), its role in younger patients remains to be determined.
Cerebellar medulloblastoma comprises 20% of pediatric CNS tumors. Following curative craniospinal radiation therapy, FSIQ is less than 90 in 90% of survivors (4,5,142–148). The impact of dose reduction of whole brain CrRT from 3600 to 2340 cGy to 1800 cGy remains to be determined. A single-arm study of 2340 cGy shows declines in FSIQ (4.2 points per year) similar to historical controls, with steep declines in children under the age of seven (70). A small study shows preservation of FSIQ one to three years after 1800 cGy (149). Risk stratification, assigning a lower dose to younger children, has not necessarily decreased cognitive dysfunction as the effects of young age at the time of treatment confound assessment of lower-dose CrRT (150). Neither intensity modulation CrRT to the tumor bed (151) nor hyperfractionation of dose has reduced the risk of future cognitive decline (142).
Eliminating CrRT or limiting the field to the tumor bed in infantile tumors of all histologic subtypes is the most encouraging alteration (152,153). Objective assessment at four years shows average cognition similar to those in the HeadStart initiative (154). However, survivors of neonatal tumors continue to fare poorly, with over half having IQ under 70 (155).
Remediation of Established Cognitive Dysfunction
Broad strategies to remediate cognitive dysfunction include both education interventions and pharmacologic treatment. Education interventions encompass school remediation programs, cognitive behavior therapy, training in social skills or specific subjects, and use of computerized cognitive training (Table 2). Historically, pharmacological approaches consisted mostly of classical stimulants, such as methylphenidate, but are evolving to include other classes of drugs (Table 3).
Table 2.
Non-pharmacologic interventions following cancer therapy in childhood survivors
| Intervention | Patient population* | Study design and duration of intervention | Measures | Response | Reference |
|---|---|---|---|---|---|
| Cognitive remediation program | Cancer survivors Age range: 6–22 y (N = 31) |
Pilot: single centerIntervention vs control |
Primary: CPT, Digit Span Secondary: WRAT sentence memory and arithmetic |
Improved CPT scores | (26) |
| Cognitive remediation program | Brain tumors, ALL, bone marrow transplant/ total body irradiation, or non-Hodgkin’s lymphoma ≥ 1 y off therapy Age range: 6–17 y (N = 20) |
Multicenter randomized clinical trialIntervention vs controlTwo-hour cognitive remediation sessions—over 4–5 mo 4–5 mo therapy |
Primary: academic achievement; focused attention; working memory; memory recall; vigilance Secondary: parent/ teacher report on attention; participant self-report |
Improved academics: 0.53; Improved attention by parent Conners’ ratio, but not by teachers; no change in attention, memory, recall or vigilance |
(178) |
| Cognitive remediation program | Brain tumors, ALL ≥ 6 mo off therapy Age range: 7–19 y (N = 12) |
Pilot-Single Center Open 15-session, clinic-based training program over 3–6 mo |
Primary: Digit span; CPT; CVLT-C; WRAT3/WJR; CBCL; SSRS | WJR writing sample and SSRS improved; low participation rate |
(179) |
| School-based math intervention | Children on maintenance phase of treatment for ALL Mean age: 6.7 y (intervention); 6.5 y (standard care) (N = 57 enrolled; 32 completed) |
Two Institutions Intervention vs control 40–50 h direct instruction on math concepts |
Primary: FSIQ, processing speed working memory; visual-motor integration; fine motor speed and dexterity; academic achievement | Improved targeted math skills and visual working memory in intervention group vs decline in the comparison group | (181,251) |
| Compensatory memory device | Brain tumors Age range: adolescent (N = 1) |
Memory notebook with log and calendar, orientation + training | Primary: WRAT, memory | Improved academic achievement; Persistent memory deficits |
(252) |
| Computerized training / Captain’s Log † | Brain tumors; ALL > 1 y off therapy Age range: 10–17 y (N = 9) |
Pilot- Single Institution: Open 50 min a week for 3 mo |
Primary: WISC-WMI; CPRS | Improved working memory on Digit-Span forward; improved inattention | (132) |
| Cogmed RM | Brain tumors; ALL > 1 y off therapyAge: 8–16 y (N = 20) |
Pilot: single institutionIntervention (CogmedRM) vs control group (non-adaptive program)25 training sessions on home-based computerized cognitive training program: 3 months | Primary: CPRS, WRAML2 | Improved visual working memory; decreased learning problems by CPRS | (182) |
| Group skills therapy | Brain tumors Age range: 8–18 y (N = 32) |
Single institution: Open Eight, 2-h weekly group sessionstargeting specific skills |
Primary: SSRS, PedsQLCancer; CBCL; CDI | Improved self-control, social skills, quality of life at 6 months | (37) |
| Group skills therapy | Male brain tumor survivors not on active treatmentAge range: 10–14 y (N = 8) | Sixteen, 1 h group sessions focused on development of social skillsSingle institution: Open Child and parent intervention |
Primary: Child and parent survey on social skills | Improved social skills | (167) |
| Social skills intervention | Brain tumors ≥ 6 mo off therapy Age range: 8–14 y (N = 13) |
Single institution: OpenThree sessions with group social skill training Child and parent intervention |
Primary: SSRS, CBCL, Miami Peds quality of life, IQ Secondary: Program evaluation |
Small/medium effect sizes on social competence, total competence and problem behaviors | (166) |
| Social skills | Pediatric cancer - newly diagnosed Age range: 5–13 y (N = 64) |
Two institutions Intervention vs controlSeveral specific 60-min social skills training vs Standard school reintegration services | Primary: CDI, CBCL, SSSC | Higher perceived classmate and teacher social support, decreased internalizing/externalizing behaviors | (164) |
| Psychological intervention |
Newly diagnosed leukemia/lymphoma, solid tumors, or brain tumors Median age: 5 y (N = 19) |
Pilot Intervention vs control Pre- and 2-mo postintervention |
Primary: anxiety, post-traumatic stress disorder | Reduced anxiety and parental post- traumatic stress disorder symptoms | (253) |
* In many studies, more patients than reported here were screened for eligibility; however, for consistency, we report on only those patients included in the final analysis. ADD = attention deficit disorder; ALL = acute lymphoblastic leukemia; CDI = child development inventory; CPRS = Conners Parent Rating Scale; CPT = Conners Continuous Performance Test; CTRS = Conners Teacher Rating Scale; CVLT = California Verbal Learning Test; CVLT-C = California Verbal Learning Test, Children’s Version; DKEF = Dellis-Kaplan Executive Function Test; FSIQ = full-scale intelligence quotient; IQ = intelligence quotient; SSRS = Social Skills Rating System; WRAT = Wide Range Achievement Test.
† Captain’s Log (http://www.braintrain.com); SSSC =Social Support Scale for Children; WRAML2= Wide Range Assessment of memory and learning 2nd edition; WISC-WMI= Working memory Index.
Table 3.
Pharmacologic interventions following cancer therapy in childhood survivors
| Pharmacologic intervention | Patient population* | Study design and duration of intervention |
Measures | Response | Reference |
|---|---|---|---|---|---|
| Methylphenidate | Children with brain tumors or ALL (N = 12) Age not reported |
Open label 6 mo to 6 y (median 23 mo) |
Primary: attention; concentration; memory; handwriting; organizational ability; behavior; hyperactivity | 75% “good response” 10% “fair response” 10% “poor response” |
(254)† |
| Methylphenidate 0.6mg/kg, 20mg max |
Brain tumors (N = 25) or ALL (N = 7)≥ 24 mo off therapy Age range: 6 mo to 17.5 y |
RDBPC 90 min |
Primary: attention - CPT Secondary: CVLT, VAL |
Improved score on CPT (errors of omission and overall index) | (186)† |
| Methylphenidate 0.3 or 0.6mg/kg (both doses divided daily) |
Brain tumors (N = 51) or ALL (N = 55) ≥ 12 months off therapy Age range: 7–8 years |
RDBPC crossover 3 wK |
Primary: attention – CPRS, CTRS Secondary: SSRS |
Improved attention and concentration; No advantage to higher dose; Response better in subset with preexisting issues |
(255)† (256)† |
| Methylphenidate 0.6mg/kg, 20mg max |
Brain tumors (N = 61) or ALL (N = 61) ≥ 12 mo off therapy Age range: 6–18 y |
RDBPC crossover 2 d |
Primary: CPT, Stroop Secondary: CVLT, VAL, WRAT |
Improved Stroop performance | (1)† |
| Methylphenidate 0.3mg/kg divided daily dose |
Brain tumors (N = 6) ≥3 y off therapy Age range: 8–20 y |
RDBPC crossover 2 wk with a 2 wk washout in between arms |
Primary: RAVL, Trail A, Trail B, CPT | No effect on attention and/or memory | (257)† |
| Donepezil | Brain tumors (N = 11) ≥ 12 mo off therapy Age range: 9–17 y |
Single center, open label 24 wk |
Primary: DKEFs, quality of life | Improved executive function | (211) |
| Growth hormone | Young adult survivors of childhood leukemia with reduced bone mineral density and/ or insulin-like growth factor-1 (N = 20) Age range: 20–29 y |
Single center Pre- and 1- and 2- y postintervention |
Primary: FSIQ, attention, Trail A, Trail B, Digit Span | Improved sustained attention and visual- spatial memory; Worsened verbal memory |
(214) |
* In many studies, more patients than reported here were screened for eligibility; however, for consistency, we report only those included in the final analysis. ADD = attention deficit disorder; ALL = acute lymphoblastic leukemia; CPRS = Conners Parent Rating Scale; CPT = Conners Continuous Performance Test; CTRS = Conners Teacher Rating Scale; CVLT = California Verbal Learning Test; DKEF = Dellis-Kaplan Executive Function Test; FSIQ = full-scale intelligence quotient; IQ = intelligence quotient; RAVL = Ray Auditory Verbal Learning Test; RDBPC = randomized, double-blind, placebo-controlled study; SSRS = Social Skills Rating System; VAL = Visual Auditory Learning Test (subtest from the Woodcock-Johnson battery); WRAT = Wide Range Achievement Test.
† References 1, 186–190 all describe the results of the primary trial of methylphenidate in childhood cancer at differing time points and for different trial outcomes.
Educational Interventions
Provider and parent advocacy is essential to access educational resources such as individualized educational plans (IEP), or classroom and testing accommodations (504 plan) as part of school reintegration. The monograph “Learning and Living with Cancer” (156) educates parents about their options and informs parents and educators about anticipated deficits and need for structured reintegration during and after therapy. The Children’s Oncology Group (COG) and the Task Force on Neurocognitive/Behavioral Complications maintain a comprehensive list of resources for parents, teachers, and school counselors (10,157–159).
School reintegration programs are ubiquitous in North America because legislation mandates educational and special services to minimize gaps in instruction and promote positive academic progress during school absenteeism (53,128,133,134,158,160). However, reintegration programs in the US vary widely in scope and resources as they are administered by the states. Hospital-based programs have largely replaced workshops for peers and educators (160). A pilot study using structured interviews of parents and teachers to compare home-bound, hospital-based, and community-based reintegration concluded that adolescents minimally engaged in school before their cancer diagnosis did best in hospital-based programs and least well in home-based programs. In contrast, high-achieving adolescents engaged in school activities prior to diagnosis did well in all three settings (161).
The proposed standard is staged programs organized by a counselor-liaison to advocate for and interpret neuropsychological evaluations, and to coordinate resources in the community, home and hospital (14,162). Advocacy for vocational rehabilitation and school tracking in courses of study appropriate to a survivor’s cognitive capacity can also facilitate success for adolescents and young adults left with varying levels of cognitive impairment. While studies of school reintegration are needed, they are limited by challenges engaging patients and families even when optimal resources are provided (133).
In addition to attention to academics, neuropsychologists frequently emphasize the importance of training in social skills, especially among children with CNS tumors (37,50,163–166). Four trials on social skill training (Table 2) demonstrate benefit in this survivor population (37,164,166,167).
In a randomized comparison of standard school reintegration vs reintegration plus social skills training in children with non-CNS malignancies, patients in the intervention group perceived higher classmate and teacher social support. Parents reported reduced behavioral problems and improved competence (164). Subsequent follow-up interventions demonstrated improvement of social skills and function with small to medium effect sizes (166,167). In a study where brain tumor survivors received instruction on building self-confidence, making friends, cooperation, managing teasing and bullying, conflict resolution, empathy, and assertion, parents reported benefits (37). Preintervention, parents rated social skills and quality of life lower, while survivors rated themselves as normal. Postintervention, parents reported improvements in self-control and quality of life, although performance had not changed on objective tests. Explanations for the discordance include instrument limitations and that parents’ hopes for improvement are reflected in their evaluation of outcomes (37,166,168).
Cognitive remediation therapy is a structured intervention that uses metacognitive training in problem solving and managing complex tasks through individualized self-monitoring of effectiveness followed by self-correction. Evidence supports the beneficial effects in children after traumatic brain injury or stroke (169–171). Because effects of the overlap in age and the spectrum of progressive disabilities in traumatic brain injury with those observed in cancer-associated cognitive dysfunction, cognitive remediation therapy was investigated in childhood survivors (172–177). A feasibility trial in survivors and caregivers showed statistically significant improvement in focused attention but not in arithmetic computation (26). A follow-up, multicenter randomized trial demonstrated a statistically significant improvement in academic achievement in the cognitive remediation therapy group following a five-month intervention, compared with controls randomized to a wait list (178). Results are tempered by an equivalent improvement in neurocognitive functioning in the control arm, attributed to practice effect. Objective performance scores showed gains preintervention to postintervention in all areas, with improvement in social skills and writing. Parents and patients perceived benefits of the intervention. Cognitive remediation therapy demanded substantial time commitment for therapists, parents, and patients, a limiting factor in studies where only 30% of eligible patients participated and only 60% of participants in the intervention arm completed the entire regimen (179). Importantly, cognitive remediation is variably covered by insurance; hence out of pocket cost limits access.
A feasibility study enrolled three survivors of medulloblastoma to participate in a condensed version of the Swedish Memory and Attention Re-Training (SMART) program, which teaches cognitive skills for the survivors and coaches both parents on stress management (180). Aspects of attention and memory performance improved from pre- to post-training assessment; survivors reported enhancement of social relations and self-image. After training, the stress level of mothers was lower and that of fathers remained low.
To test the hypothesis that mathematics training improves executive function, children with ALL were randomized to intensive individualized training in solving math problems or standard care while on treatment. While the standard care group had higher scores in applied mathematics at baseline, the intervention group improved such that it performed statistically significantly better in applied mathematics and visual memory at the end of intervention and at six-month follow-up. The standard care group did not improve in any area, and declined in seven of 11 domains, illustrating the natural history of cognitive decline. This study shows that early intervention is feasible, beneficial, and durable (181).
A trial of computerized brain training enrolled nine survivors with cognitive dysfunction in a home-based program designed to improve memory, attention, concentration, listening skills, and self-control in people aged six years and older (http://www braintrain.com) (132). Following the intervention, participants exhibited statistically significant increases in working memory, and parents reported reduced problems in attention. Improvements persisted three months later (132,182). A randomized trial of a home-based computerized training program is currently in evaluation as a feasibility study in childhood brain tumor patients following CrRT (NCT01503086).
Pharmacologic Interventions
Current evidence focuses on deficits in attention as a modifiable domain in cognitive dysfunction (183). While some aspects of cognitive dysfunction resemble those of children with attention-deficit disorder (ADD), many survivors lack the typical profile for inattention and/or hyperactivity. The piperidine derivative methylphenidate, a mixed dopaminergic-noradrenergic agonist, enhances function of the fronto-striatal attentional network and is the most studied medication in pediatric ADD.
Because it demonstrates strong dose-response relationships on measures of vigilance, sustained attention, and reaction time in ADD (184,185), methylphenidate and other stimulant medications have been investigated in several studies of childhood cancer survivors with cognitive dysfunction (Table 3). While a single dose of methylphenidate improved attention 90 minutes later (186), a three-week cross-over trial demonstrated improved teacher and parent report of attention with no differences in a 0.3 and 0.6mg/kg dose (187). Male gender, older age at treatment, and higher intellectual functioning at baseline predicted better outcomes. Despite statistically significant improvement in sustained attention, social skills and internalizing and externalizing psychopathology, methylphenidate had no impact on academic productivity (187). An open label trial for patients who demonstrated initial response showed sustained responses among participants after 12 months of continuation therapy, compared with those of nonparticipants (60). Parental, teacher, and patient assessments were aligned in the treatment group, but not in the control group, where parents reported improvement, and teachers and patients did not (60). Based on these studies, the authors conclude that methylphenidate should be the standard of care for children with cognitive dysfunction who show measurable improvement after short-term methylphenidate. Limitations of methylphenidate studies include cohorts that mix brain tumor and ALL survivors and short half-life of the drug. Importantly, a 5% rate of dose-limiting side effects was noted with poorer tolerance in survivors of brain tumors compared to those with leukemia (3,186–189). A COG randomized trial comparing the long-acting methylphenidate with extended-release amoxetine (ACCL0422A) was closed prematurely because of poor accrual, attributable in part to the appearance of black box warnings around the use of methylphenidate.
Modafinil, a dopaminergic central nervous system stimulant, is an alternative to methylphenidate in ADD. Although not Food and Drug Administration (FDA)-approved in children under the age of 16, it is used off-label to treat narcolepsy, excessive daytime sleepiness, and ADD (190–198). Modafinil improves digit span, visual memory and spatial planning capacity among adult volunteers with cancer, with enhanced benefit among those with lower cognitive capacity at baseline (199–201). A randomized clinical trial using modafinil in breast cancer survivors resulted in improved attention and speed of memory; modafinil improved cognition, mood and fatigue in adults with CNS tumors (201). A randomized trial comparing methylphenidate and modafinil in 24 adult survivors of CNS tumors showed benefits of both drugs, with a trend for improved attention with methylphenidate and improved processing speed with modafinil. However, this trial closed after five years for failure to accrue the necessary 75 patients (202). COG is currently evaluating modafinil in a randomized trial among survivors of pediatric CNS tumors (NCT01381718).
Donepezil is an acetylcholinesterase inhibitor with beneficial effects on cognitive, behavioral, and functional symptoms in Alzheimer’s and vascular dementias (203). While donepezil is FDA-approved for Alzheimer’s disease, there are off-label indications for its use in cognitive impairment associated with multiple sclerosis, Parkinson’s disease, multi-infarct dementia, and traumatic brain injury. Donepezil has also been beneficial in improving global function and expressive language in adults with Down syndrome (204,205) and demonstrated promising initial results in children with Down syndrome (206) or autism spectrum disorders (207,208). In a phase II, 24-week, open-label trial of 34 adults with primary brain tumors, donepezil (10mg/day) resulted in improved attention, concentration, language function, verbal and figure memory, and mood (209,210). These results formed the basis of an ongoing phase III trial in survivors of adult brain tumors (NCT00369785) and a feasibility trial in childhood brain tumor survivors (NCT00452868). Pilot data in the latter trial indicate good tolerance of the drug, with efficacy in improving executive function and memory over a six-month, open-label trial (211).
Recombinant human growth hormone (GH) may also have benefits on cognitive function. GH deficiency is sometimes associated with abnormal white matter anisotropy on functional MRI (212) and impaired neural stem cell and myelin generation in rodent models (213). Children with ALL treated with or without CrRT are at risk of GH deficiency (214). Although a meta-analysis found insufficient evidence for beneficial cognitive effects of GH (215), recent observational (216,217) and randomized (218,219) trials report improved cognition in pediatric and adult patients with GH deficiency, adults following traumatic brain injury, and adults with mild cognitive dysfunction with or without GH deficiency. Thirteen young adult survivors of ALL who had either GH deficiency or diminished bone mineral density were treated with two years of GH. Assessment pretreatment and at 12 and 24 months post-treatment showed normal cognition; sustained attention and cognitive-perceptual performance improved progressively at months 12 and 24, while verbal memory declined at 12 months and returned to baseline range by 24 months (214). GH warrants further investigation in randomized clinical trials in children and adults with cancer-associated cognitive dysfunction.
Future Directions
As the childhood cancer survivor population increases, future studies will require: 1) continued refinement of CNS-directed therapies; 2) early intervention with both psychoeducational tools and novel pharmacotherapies; 3) novel pharmacologic and nonpharmacologic neuroprotective strategies during cancer therapy; 4) and resources to assess and individualize neuropsychologic intervention in the highest-risk patients. A common theme across all these is the need for consensus on less burdensome screening assessments for uniformity in practice and in clinical trials.
Assessment of Cognitive Dysfunction
Variable assessment batteries and the high burden of neurocognitive assessment (in terms of time and cost) have been barriers to evaluation of cognitive impact during and following cancer therapeutic trials in children. Although a small number of treatment centers have carried out prospective, longitudinal assessment on the children enrolled on their clinical trials (83,220). COG and other cooperative groups faced challenges in obtaining sequential assessments in multi-institutional randomized phase III trials, even when reduction in cognitive dysfunction is a secondary study aim. Practice effects of sequential testing confound studies and can be addressed by limiting intervals between tests to every six to 12 months or longer (221). Impediments to assessment in trials include cost of testing instruments, limited availability of trained neuropsychologists/psychometricians at each cooperative group participating center, and limited resources for reimbursement of neuropsychologist time. High participant attrition in trials is also a barrier. Traditional neuropsychological batteries take four to seven hours and parents, patients, and treating physicians have competing priorities. Recognizing that brevity and convenience are critical to feasibility, the COG assessment battery, ALTE07C1 (NCT00772200), applied a limited number of age-standardized measurements and is administered by psychologists in less than two hours. Use of the ALTE07C1 battery has resulted in improved compliance with neurocognitive assessments in trials for medulloblastoma, compared with the full neurocognitive battery previously recommended by COG (222). The DIGIVERT panel is also validated for screening survivors for cognitive dysfunction (223). Some screens can be administered by trained research associates rather than psychologists. The Peabody Picture Vocabulary Test can serve as a screen for FSIQ (224). The Trackwell 20-minute screen is a cognitive screening tool suitable for administration within weeks to a few months from diagnosis (225). Computerized assessments with web-based reporting of results offer inexpensive, rapid, high-throughput sequential testing of core domains (226–229). The National Institutes of Health (NIH) toolbox, still in the validation stage, contains standardized measures of neurological and behavioral function with normative values across the lifespan and has been developed to facilitate research (230).
Patient self-report, parent-, and teacher-reported outcomes may have higher acceptance and greater clinical relevance than objective testing, especially for behavioral and social skills. Instruments developed by the NIH Patient Reported Outcomes Measurement Information System (PROMIS) initiative have sound psychometric properties for functional assessment to comparing children with cancer to population norms (231–233). Nonetheless, the sensitivity and specificity of parent- and patient-reported behavioral and neuropsychological function in the pediatric cancer population remain unclear. Overall, investigators report the most deficits, patients report the fewest, and parents tend to report improvement regardless of intervention (37,165,166,234).
While abbreviated assessments facilitate research and can trigger the need for further investigation, none suffices to trigger an individual educational plan in the school systems or replaces a comprehensive assessment. In the practice setting, compliance with neuropsychological recommendations for rehabilitation following assessment is less than 50% (235). COG recommends survivors at risk of cognitive dysfunction undergo full neuropsychological assessment one year after treatment and again at three to five years, or at a point of clinical decline (10,157). Assessment batteries can typically measure FSIQ, academic ability, core functional neurocognitive domains, psychomotor skills, language, behavior and mood. No standard assessment battery is endorsed by the childhood cancer community to date. Insurance coverage of neuropsychological testing is not uniform and educational systems vary in their ability to provide timely testing. Abbreviated computerized testing is attractive, yet its translation into academic, behavioral and functional outcome is unknown. Hence, comparison of computerized assessment with traditional neurocognitive measures remains important. Standardizing the assessments and increasing feasibility of testing and adherence to recommendations is a high priority moving forward in practice and in trials (236).
Early Intervention
Understanding of neural networks and brain plasticity in traumatic brain injury suggest that early intervention (before deficits are crystalized) achieves improved results (237). Figure 2 suggests a conceptual framework for future interventions with nonpharmacologic and pharmacologic neuroprotective approaches to minimize cognitive dysfunction. The trial of mathematical intervention in ALL patients demonstrates that declines may be partially aborted by preemptive intensive educational approaches (181). Results of trials using home-based computerized brain training programs (NCT01503086) may clarify and support the role of such approaches early in therapy.
Figure 2.
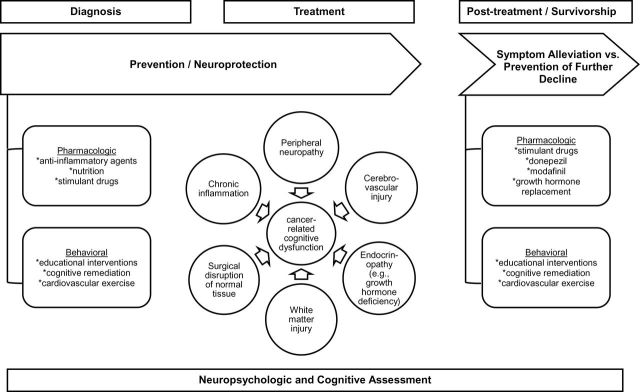
Conceptual approach to design of future interventions for protection and remediation against cancer-related cognitive dysfunction.
Development of reliable blood or imaging biomarkers (238–240) of radiation-associated changes in neurotransmitters and receptors that drive synapse formation or function could facilitate both early identification of neurotoxicity and subsequent monitoring. Potential biomarkers of impending cognitive dysfunction include sequential shifts in inflammatory markers or neurotransmitters, such as phospholipid oxidation profile (241,242), or increased levels of reactive oxidant stress indicators in samples of cerebrospinal fluid (243,244). If confirmed, these biomarkers may also help to identify patients for early pharmacologic trials of agents that approach the inflammation pathway (245).
New Approaches to Intervention
Completing and confirming studies using modafinil, donepezil, or stimulants other than methylphenidate in children should be high priority. Combining strategies may be more successful, such as the use of computerized training and methylphenidate or computerized training and cognitive remediation therapy in adult survivors of brain tumors (246,247). Cardiovascular exercise or yoga as interventions may also be important in children as in adults (248). Recent evidence of white matter changes in cognitive impairment in Hodgkin Lymphoma survivors treated with cardiotoxic therapy raises the role of cardiovascular health in maintenance of cognitive function (249).
Future interventions need to consider the inflammatory response to CrRT and evaluate anti-inflammatory agents to attenuate loss of neuronal precursor cells, mpact on neurogenesis and microenvironmental changes. Strategies to attenuate reactive oxygen species and quench radiation-induced microglial inflammatory injury may provide neuroprotection prior to or during cancer treatment (245). Ultimately, elucidation of the underlying pathophysiology and refinement and streamlining of assessment tools are still required to best utilize imaging or biomarkers of subclinical injury before manifestation of cognitive dysfunction by report or neurocognitive assessment three to five years after the insult.
The design of future interventional trials needs to be robust with standardized goals and clear statistical definition of endpoints, effect size, and accounting for practice effects of repeated testing and washout periods in cross-over studies (1). Strategies to increase compliance and participation are integral to the success of interventions beyond the cure of the tumor and are a challenge common to many areas of cancer survivorship because of study fatigue and time burden. Cooperative groups need to standardize assessment procedures for measurement of impact on cognition across disease groups.
While early intervention is a goal, even simple interventions may not be embraced by children, parents, or physicians. This is evidenced by the suboptimal feasibility of reintegration programs (133), failure of parent follow-up on recommendations for neuropsychological rehabilitation (235), and suboptimal accrual on many pharmacological-based intervention trials. This can only be addressed by education of patients and families and development of compelling clinical trials. Discussing risks of cognitive dysfunction and potential importance of early detection and early intervention should be an integral element of discussion at cancer diagnosis with parents, patients, and providers. Studies that queried parents who do not choose to participate in or drop out of studies find that most parents were too overwhelmed to take on any new responsibilities (37,133,166,250); mothers seem to be especially burdened and especially important in the decision making. Interventions that require additional time in or out of the home and/or in/out of school is a priori an increased burden to families. The whole process needs to be rendered more feasible for everyone involved.
Considerable effort has been expended to make trials in cognitive dysfunction more homogeneous, in order to validate efficacy of a single intervention in a defined population. Exclusion criteria at times limit generalizability and negatively impact accrual. Another strategy for consideration is partnership with other pediatric subspecialties. It could increase accrual and generate more robust studies for medically complex children, in whom progressive cognitive dysfunction complicates survivorship. Radiation injury and folate inhibition are unique to cancer treatment; however, the processes underlying neuronal injury and repair in the developing brain may share features with other brain injury populations, including traumatic brain injury and sickle cell disease.
Conclusions
Ongoing cancer therapy trials aim to reduce neurotoxicity of treatment by modifying CrRT or chemotherapy or by using molecular risk stratification to target less toxic therapy for select patients (82). While large strides have been made, cognitive disabilities will persist in children with primary brain tumors, as CrRT remains a cornerstone of therapy. Therefore, the future lies in pharmacologic or non-pharmacologic interventional trials at earlier time points, in order to prevent or remediate cognitive declines associated with treatment. The overall goal remains improved HRQOL for cancer survivors with cognitive dysfunction and for their families.
Funding
Dr. Lange is supported by the Yetta Dietch Novotny Chair in Pediatric Oncology at the Children’s Hospital of Philadelphia. Dr. Castellino was supported in part by R25CA122061-07 (PI Avis).
We are grateful for the leadership of the Cancer Control (CCL) Committee (L. Sung, MD, PhD) of the Children’s Oncology Group (CCOP Grant U10 CA95861-09; Chair’s Grant U10 CA98543-08; Statistics and Data Center Grant U10 CA98413-08) for facilitating a 2010 review meeting of expert investigators in this area, to foster a multidisciplinary approach to neurologic and neurocognitive risk with childhood cancer therapy.
References
- 1. Conklin HM, Khan RB, Reddick WE, et al. Acute neurocognitive response to methylphenidate among survivors of childhood cancer: a randomized, double-blind, cross-over trial. J Pediatr Psychol. 2007;32(9):1127–1139. [DOI] [PubMed] [Google Scholar]
- 2. Robinson KE, Livesay KL, Campbell LK, et al. Working memory in survivors of childhood acute lymphocytic leukemia: functional neuroimaging analyses. Pediatr Blood Cancer. 2010;54(4):585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mulhern RK, Butler RW. Neurocognitive sequelae of childhood cancers and their treatment. Pediatr Rehabil. 2004;7(1):1–14; discussion 15–16. [DOI] [PubMed] [Google Scholar]
- 4. Duffner PK. Risk factors for cognitive decline in children treated for brain tumors. Eur J Paediatr Neurol. 2010;14(2):106–115. [DOI] [PubMed] [Google Scholar]
- 5. Glauser TA, Packer RJ. Cognitive deficits in long-term survivors of childhood brain tumors. Childs Nerv Syst. 1991;7(1):2–12. [DOI] [PubMed] [Google Scholar]
- 6. Mahoney DH, Jr, Shuster JJ, Nitschke R, et al. Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: an association with intermediate-dose intravenous methotrexate and intrathecal triple therapy--a Pediatric Oncology Group study. J Clin Oncol. 1998;16(5):1712–1722. [DOI] [PubMed] [Google Scholar]
- 7. Mariotto AB, Rowland JH, Yabroff KR, et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1033–1040. [DOI] [PubMed] [Google Scholar]
- 8. Shuper A, Stark B, Kornreich L, et al. Methotrexate treatment protocols and the central nervous system: significant cure with significant neurotoxicity. J Child Neurol. 2000;15(9):573–580. [DOI] [PubMed] [Google Scholar]
- 9. Janzen LA, Spiegler BJ. Neurodevelopmental sequelae of pediatric acute lymphoblastic leukemia and its treatment. Dev Disabil Res Rev. 2008;14(3):185–195. [DOI] [PubMed] [Google Scholar]
- 10. Nathan PC, Patel SK, Dilley K, et al. Guidelines for identification of, advocacy for, and intervention in neurocognitive problems in survivors of childhood cancer: a report from the Children’s Oncology Group. Arch Pediatr Adolesc Med. 2007;161(8):798–806. [DOI] [PubMed] [Google Scholar]
- 11. Butler RW, Mulhern RK. Neurocognitive interventions for children and adolescents surviving cancer. J Pediatr Psychol. 2005;30(1):65–78. [DOI] [PubMed] [Google Scholar]
- 12. Meadows AT, Gordon J, Massari DJ, et al. Declines in IQ scores and cognitive dysfunctions in children with acute lymphocytic leukaemia treated with cranial irradiation. Lancet. 1981;2(8254):1015–1018. [DOI] [PubMed] [Google Scholar]
- 13. Shah AJ, Epport K, Azen C, et al. Progressive declines in neurocognitive function among survivors of hematopoietic stem cell transplantation for pediatric hematologic malignancies. J Pediatr Hematol Oncol. 2008;30(6):411–418. [DOI] [PubMed] [Google Scholar]
- 14. Nazemi KJ, Butler RW. Neuropsychological rehabilitation for survivors of childhood and adolescent brain tumors: a view of the past and a vision for a promising future. J Pediatr Rehabil Med. 2011;4(1):37–46. [DOI] [PubMed] [Google Scholar]
- 15. Kazak AE. Implications of survival: pediatric oncology patients and their families. In: Bearison DJ, Mulhern RK, eds. Pediatric Psychooncology: Psychological Perspectives on Children with Cancer. New York: Oxford University Press; 1994, 171–192. [Google Scholar]
- 16. Carlson-Green B, Morris RD, Krawiecki N. Family and illness predictors of outcome in pediatric brain tumors. J Pediatr Psychol. 1995;20(6):769–784. [DOI] [PubMed] [Google Scholar]
- 17. Barr RD, Simpson T, Whitton A, et al. Health-related quality of life in survivors of tumours of the central nervous system in childhood--a preference-based approach to measurement in a cross-sectional study. Eur J Cancer. 1999;35(2):248–255. [DOI] [PubMed] [Google Scholar]
- 18. Bhat SR, Goodwin TL, Burwinkle TM, et al. Profile of daily life in children with brain tumors: an assessment of health-related quality of life. J Clin Oncol. 2005;23(24):5493–5500. [DOI] [PubMed] [Google Scholar]
- 19. Johnson DL, McCabe MA, Nicholson HS, et al. Quality of long-term survival in young children with medulloblastoma. J Neurosurg. 1994;80(6):1004–1010. [DOI] [PubMed] [Google Scholar]
- 20. Zebrack BJ, Gurney JG, Oeffinger K, et al. Psychological outcomes in long-term survivors of childhood brain cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2004;22(6):999–1006. [DOI] [PubMed] [Google Scholar]
- 21. Reimers TS, Mortensen EL, Nysom K, et al. Health-related quality of life in long-term survivors of childhood brain tumors. Pediatr Blood Cancer. 2009;53(6):1086–1091. [DOI] [PubMed] [Google Scholar]
- 22. Nathan PC, Ford JS, Henderson TO, et al. Health behaviors, medical care, and interventions to promote healthy living in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27(14):2363–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Conklin HM, Li C, Xiong X, et al. Predicting change in academic abilities after conformal radiation therapy for localized ependymoma. J Clin Oncol. 2008;26(24):3965–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harila-Saari AH, Lahteenmaki PM, Pukkala E, et al. Scholastic achievements of childhood leukemia patients: a nationwide, register-based study. J Clin Oncol. 2007;25(23):3518–3524. [DOI] [PubMed] [Google Scholar]
- 25. Mabbott DJ, Spiegler BJ, Greenberg ML, et al. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol. 2005;23(10):2256–2263. [DOI] [PubMed] [Google Scholar]
- 26. Butler RW, Copeland DR. Attentional processes and their remediation in children treated for cancer: a literature review and the development of a therapeutic approach. J Int Neuropsychol Soc. 2002;8(1):115–124. [PubMed] [Google Scholar]
- 27. Mulhern RK, Merchant TE, Gajjar A, et al. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5(7):399–408. [DOI] [PubMed] [Google Scholar]
- 28. Reddick WE, White HA, Glass JO, et al. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer. 2003;97(10):2512–2519. [DOI] [PubMed] [Google Scholar]
- 29. Kaemingk KL, Carey ME, Moore IM, et al. Math weaknesses in survivors of acute lymphoblastic leukemia compared to healthy children. Child Neuropsychol. 2004;10(1):14–23. [DOI] [PubMed] [Google Scholar]
- 30. Butler RW, Haser JK. Neurocognitive effects of treatment for childhood cancer. Ment Retard Dev Disabil Res Rev. 2006;12(3):184–191. [DOI] [PubMed] [Google Scholar]
- 31. Campbell LK, Scaduto M, Sharp W, et al. A meta-analysis of the neurocognitive sequelae of treatment for childhood acute lymphocytic leukemia. Pediatr Blood Cancer. 2007;49(1):65–73. [DOI] [PubMed] [Google Scholar]
- 32. Hockenberry M, Krull K, Moore K, et al. Longitudinal evaluation of fine motor skills in children with leukemia. J Pediatr Hematol Oncol. 2007;29(8):535–539. [DOI] [PubMed] [Google Scholar]
- 33. Duffner PK. Long-term effects of radiation therapy on cognitive and endocrine function in children with leukemia and brain tumors. Neurologist. 2004;10(6):293–310. [DOI] [PubMed] [Google Scholar]
- 34. Merchant TE. Craniopharyngioma radiotherapy: endocrine and cognitive effects. J Pediatr Endocrinol Metab. 2006;19(Suppl 1):439–446. [PubMed] [Google Scholar]
- 35. Schatz J, Kramer JH, Ablin A, et al. Processing speed, working memory, and IQ: a developmental model of cognitive deficits following cranial radiation therapy. Neuropsychology. 2000;14(2):189–200. [DOI] [PubMed] [Google Scholar]
- 36. Maddrey AM, Bergeron JA, Lombardo ER, et al. Neuropsychological performance and quality of life of 10 year survivors of childhood medulloblastoma. J Neurooncol. 2005;72(3):245–253. [DOI] [PubMed] [Google Scholar]
- 37. Barrera M, Schulte F. A group social skills intervention program for survivors of childhood brain tumors. J Pediatr Psychol. 2009;34(10):1108–1118. [DOI] [PubMed] [Google Scholar]
- 38. Kazak AE, Barakat LP, Meeske K, et al. Posttraumatic stress, family functioning, and social support in survivors of childhood leukemia and their mothers and fathers. J Consult Clin Psychol. 1997;65(1):120–129. [DOI] [PubMed] [Google Scholar]
- 39. Stuber ML, Meeske KA, Krull KR, et al. Prevalence and predictors of posttraumatic stress disorder in adult survivors of childhood cancer. Pediatrics. 2010;125(5):e1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krull KR, Okcu MF, Potter B, et al. Screening for neurocognitive impairment in pediatric cancer long-term survivors. J Clin Oncol. 2008;26(25):4138–4143. [DOI] [PubMed] [Google Scholar]
- 41. Parsons SK, Phipps S, Sung L, et al. NCI, NHLBI/PBMTC First International Conference on Late Effects after Pediatric Hematopoietic Cell Transplantation: health-related quality of life, functional, and neurocognitive outcomes. Biol Blood Marrow Transplant. 2012;18(2):162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wasilewski-Masker K, Mertens AC, Patterson B, et al. Severity of health conditions identified in a pediatric cancer survivor program. Pediatr Blood Cancer. 2010;54(7):976–982. [DOI] [PubMed] [Google Scholar]
- 43. Butler RW, Hill JM, Steinherz PG, et al. Neuropsychologic effects of cranial irradiation, intrathecal methotrexate, and systemic methotrexate in childhood cancer. J Clin Oncol. 1994;12(12):2621–2629. [DOI] [PubMed] [Google Scholar]
- 44. Packer RJ. Radiation-induced neurocognitive decline: the risks and benefits of reducing the amount of whole-brain irradiation. Curr Neurol Neurosci Rep. 2002;2(2):131–133. [DOI] [PubMed] [Google Scholar]
- 45. Barrera M, Atenafu E, Andrews GS, et al. Factors related to changes in cognitive, educational and visual motor integration in children who undergo hematopoietic stem cell transplant. J Pediatr Psychol. 2008;33(5):536–546. [DOI] [PubMed] [Google Scholar]
- 46. Tallia H. What’s new concerning the chemobrain? Rev Neurol (Paris). 2013;169(3):216–222. [DOI] [PubMed] [Google Scholar]
- 47. Jim HS, Phillips KM, Chait S, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol. 2012;30(29):3578–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: a review of published studies and recommendations for future research. J Clin Oncol. 2007;25(17):2455–2463. [DOI] [PubMed] [Google Scholar]
- 49. Frobisher C, Lancashire ER, Winter DL, et al. Long-term population-based marriage rates among adult survivors of childhood cancer in Britain. Int J Cancer. 2007;121(4):846–855. [DOI] [PubMed] [Google Scholar]
- 50. Gurney JG, Krull KR, Kadan-Lottick N, et al. Social outcomes in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27(14):2390–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mitby PA, Robison LL, Whitton JA, et al. Utilization of special education services and educational attainment among long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2003;97(4):1115–1126. [DOI] [PubMed] [Google Scholar]
- 52. Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101(13):946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gerhardt CA, Dixon M, Miller K, et al. Educational and occupational outcomes among survivors of childhood cancer during the transition to emerging adulthood. J Dev Behav Pediatr. 2007;28(6):448–455. [DOI] [PubMed] [Google Scholar]
- 54. Kirchhoff AC, Leisenring W, Krull KR, et al. Unemployment among adult survivors of childhood cancer: a report from the childhood cancer survivor study. Med Care. 2010;48(11):1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Henderson TO, Friedman DL, Meadows AT. Childhood cancer survivors: transition to adult-focused risk-based care. Pediatrics. 2010;126(1):129–136. [DOI] [PubMed] [Google Scholar]
- 56. Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290(12):1583–1592. [DOI] [PubMed] [Google Scholar]
- 57. Roncadin C, Dennis M, Greenberg ML, et al. Adverse medical events associated with childhood cerebellar astrocytomas and medulloblastomas: natural history and relation to very long-term neurobehavioral outcome. Childs Nerv Syst. 2008;24(9):995–1002; discussion 1003. [DOI] [PubMed] [Google Scholar]
- 58. Packer RJ, Gurney JG, Punyko JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 2003;21(17):3255–3261. [DOI] [PubMed] [Google Scholar]
- 59. Moleski M. Neuropsychological, neuroanatomical, and neurophysiological consequences of CNS chemotherapy for acute lymphoblastic leukemia. Arch Clin Neuropsychol. 2000;15(7):603–630. [PubMed] [Google Scholar]
- 60. Conklin HM, Reddick WE, Ashford J, et al. Long-term efficacy of methylphenidate in enhancing attention regulation, social skills, and academic abilities of childhood cancer survivors. J Clin Oncol. 2010;28(29):4465–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ullrich NJ, Embry L. Neurocognitive dysfunction in survivors of childhood brain tumors. Semin Pediatr Neurol. 2012;19(1):35–42. [DOI] [PubMed] [Google Scholar]
- 62. Richards S, Pui CH, Gayon P. Systematic review and meta-analysis of randomized trials of central nervous system directed therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60(2):185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9(3):257–268. [DOI] [PubMed] [Google Scholar]
- 64. Pui CH, Thiel E. Central nervous system disease in hematologic malignancies: historical perspective and practical applications. Semin Oncol. 2009;36(4):S2–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Aarsen FK, Paquier PF, Arts WF, et al. Cognitive deficits and predictors 3 years after diagnosis of a pilocytic astrocytoma in childhood. J Clin Oncol. 2009;27(21):3526–3532. [DOI] [PubMed] [Google Scholar]
- 66. Dehorter N, Vinay L, Hammond C, et al. Timing of developmental sequences in different brain structures: physiological and pathological implications. Eur J Neurosci. 2012;35(12):1846–1856. [DOI] [PubMed] [Google Scholar]
- 67. Luciana M, Conklin HM, Hooper CJ, et al. The development of nonverbal working memory and executive control processes in adolescents. Child Dev. 2005;76(3):697–712. [DOI] [PubMed] [Google Scholar]
- 68. Johnston MV. Plasticity in the developing brain: implications for rehabilitation. Dev Disabil Res Rev. 2009;15(2):94–101. [DOI] [PubMed] [Google Scholar]
- 69. Armstrong GT, Sklar CA, Hudson MM, et al. Long-term health status among survivors of childhood cancer: does sex matter? J Clin Oncol. 2007;25(28):4477–4489. [DOI] [PubMed] [Google Scholar]
- 70. Ris MD, Packer R, Goldwein J, et al. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children’s Cancer Group study. J Clin Oncol. 2001;19(15):3470–3476. [DOI] [PubMed] [Google Scholar]
- 71. Robison LL, Nesbit ME, Jr, Sather HN, et al. Factors associated with IQ scores in long-term survivors of childhood acute lymphoblastic leukemia. Am J Pediatr Hematol Oncol. 1984;6(2):115–121. [DOI] [PubMed] [Google Scholar]
- 72. Iuvone L, Peruzzi L, Colosimo C, et al. Pretreatment neuropsychological deficits in children with brain tumors. Neuro Oncol. 2011;13(5):517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rueckriegel SM, Blankenburg F, Henze G, et al. Loss of fine motor function correlates with ataxia and decline of cognition in cerebellar tumor survivors. Pediatr Blood Cancer. 2009;53(3):424–431. [DOI] [PubMed] [Google Scholar]
- 74. Jansen J, Butow PN, van Weert JC, et al. Does age really matter? Recall of information presented to newly referred patients with cancer. J Clin Oncol. 2008;26(33):5450–5457. [DOI] [PubMed] [Google Scholar]
- 75. Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23(6):705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ullrich NJ. Inherited disorders as a factor and predictor of neurodevelopmental outcome in pediatric cancer. Dev Disabil Res Rev. 2008;14:229–237. [DOI] [PubMed] [Google Scholar]
- 77. Krull KR, Brouwers P, Jain N, et al. Folate pathway genetic polymorphisms are related to attention disorders in childhood leukemia survivors. J Pediatr. 2008;152(1):101–105. [DOI] [PubMed] [Google Scholar]
- 78. Kamdar KY, Krull KR, El-Zein RA, et al. Folate pathway polymorphisms predict deficits in attention and processing speed after childhood leukemia therapy. Pediatr Blood Cancer. 2011;57(3):454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cole PD, Beckwith KA, Vijayanathan V, et al. Folate homeostasis in cerebrospinal fluid during therapy for acute lymphoblastic leukemia. Pediatr Neurol. 2009;40(1):34–41. [DOI] [PubMed] [Google Scholar]
- 80. Krull KR, Bhojwani D, Conklin HM, et al. Genetic mediators of neurocognitive outcomes in survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2013;31(17):2182–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Espy KA, Moore IM, Kaufmann PM, et al. Chemotherapeutic CNS prophylaxis and neuropsychologic change in children with acute lymphoblastic leukemia: a prospective study. J Pediatr Psychol. 2001;26(1):1–9. [DOI] [PubMed] [Google Scholar]
- 82. Waber DP, Queally JT, Catania L, et al. Neuropsychological outcomes of standard risk and high risk patients treated for acute lymphoblastic leukemia on Dana-Farber ALL consortium protocol 95-01 at 5 years post-diagnosis. Pediatr Blood Cancer. 2012;58(5):758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Merchant TE, Conklin H, Wu S, et al. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27(22):3691–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ballesteros-Zebadua P, Chavarria A, Celis MA, et al. Radiation-induced neuroinflammation and radiation somnolence syndrome. CNS Neurol Disord Drug Targets. 2012;11(7):937–949. [DOI] [PubMed] [Google Scholar]
- 85. Armstrong GT, Jain N, Liu W, et al. Region-specific radiotherapy and neuropsychological outcomes in adult survivors of childhood CNS malignancies. Neuro Oncol. 2010;12(11):1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shi L, Adams MM, Long A, et al. Spatial learning and memory deficits after whole-brain irradiation are associated with changes in NMDA receptor subunits in the hippocampus. Radiat Res. 2006;166(6):892–899. [DOI] [PubMed] [Google Scholar]
- 87. Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59(17):4383–4391. [PubMed] [Google Scholar]
- 88. Soussain C, Ricard D, Fike JR, et al. CNS complications of radiotherapy and chemotherapy. Lancet. 2009;374(9701):1639–1651. [DOI] [PubMed] [Google Scholar]
- 89. Kim JH, Brown SL, Jenrow KA, et al. Mechanisms of radiation-induced brain toxicity and implications for future clinical trials. J Neurooncol. 2008;87(3):279–286. [DOI] [PubMed] [Google Scholar]
- 90. Parent JM, Tada E, Fike JR, et al. Inhibition of dentate granule cell neurogenesis with brain irradiation does not prevent seizure-induced mossy fiber synaptic reorganization in the rat. J Neurosci. 1999;19(11):4508–4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Peissner W, Kocher M, Treuer H, et al. Ionizing radiation-induced apoptosis of proliferating stem cells in the dentate gyrus of the adult rat hippocampus. Brain Res Mol Brain Res. 1999;71(1):61–68. [DOI] [PubMed] [Google Scholar]
- 92. Tada E, Parent JM, Lowenstein DH, et al. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience. 2000;99(1):33–41. [DOI] [PubMed] [Google Scholar]
- 93. Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16(2):129–134. [DOI] [PubMed] [Google Scholar]
- 94. Poussaint TY, Siffert J, Barnes PD, et al. Hemorrhagic vasculopathy after treatment of central nervous system neoplasia in childhood: diagnosis and follow-up. AJNR Am J Neuroradiol. 1995;16(4):693–699. [PMC free article] [PubMed] [Google Scholar]
- 95. Brown WR, Thore CR, Moody DM, et al. Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res. 2005;164(5):662–668. [DOI] [PubMed] [Google Scholar]
- 96. Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24(33):5277–5282. [DOI] [PubMed] [Google Scholar]
- 97. Gurney JG, Kadan-Lottick NS, Packer RJ, et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer. 2003;97(3):663–673. [DOI] [PubMed] [Google Scholar]
- 98. Baumgartner JE, Ater JL, Ha CS, et al. Pathologically proven cavernous angiomas of the brain following radiation therapy for pediatric brain tumors. Pediatr Neurosurg. 2003;39(4):201–207. [DOI] [PubMed] [Google Scholar]
- 99. Ullrich NJ, Robertson R, Kinnamon DD, et al. Moyamoya following cranial irradiation for primary brain tumors in children. Neurology. 2007;68(12):932–938. [DOI] [PubMed] [Google Scholar]
- 100. Reddick WE, Russell JM, Glass JO, et al. Subtle white matter volume differences in children treated for medulloblastoma with conventional or reduced dose craniospinal irradiation. Magn Reson Imaging. 2000;18(7):787–793. [DOI] [PubMed] [Google Scholar]
- 101. Reddick WE, Shan ZY, Glass JO, et al. Smaller white-matter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia. Cancer. 2006;106(4):941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Brinkman TM, Reddick WE, Luxton J, et al. Cerebral white matter integrity and executive function in adult survivors of childhood medulloblastoma. Neuro Oncol. 2012;14(Suppl 4):iv25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kirov I, Fleysher L, Babb JS, et al. Characterizing ‘mild’ in traumatic brain injury with proton MR spectroscopy in the thalamus: Initial findings. Brain Inj. 2007;21(11):1147–1154. [DOI] [PubMed] [Google Scholar]
- 104. Aukema EJ, Caan MW, Oudhuis N, et al. White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. Int J Radiat Oncol Biol Phys. 2009;74(3):837–843. [DOI] [PubMed] [Google Scholar]
- 105. Monje M, Thomason ME, Rigolo L, et al. Functional and structural differences in the hippocampus associated with memory deficits in adult survivors of acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60(2):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Anderson FS, Kunin-Batson AS, Perkins JL, et al. White versus gray matter function as seen on neuropsychological testing following bone marrow transplant for acute leukemia in childhood. Neuropsychiatr Dis Treat. 2008;4(1):283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wells EM, Nageswara Rao AA, Scafidi J, et al. Neurotoxicity of biologically targeted agents in pediatric cancer trials. Pediatr Neurol. 2012;46(4):212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Blaney SM, Balis FM, Poplack DG. Pharmacologic approaches to the treatment of meningeal malignancy. Oncology (Williston Park). 1991;5(5):107–116; discussion 123, 127. [PubMed] [Google Scholar]
- 109. Carey ME, Hockenberry MJ, Moore IM, et al. Brief report: effect of intravenous methotrexate dose and infusion rate on neuropsychological function one year after diagnosis of acute lymphoblastic leukemia. J Pediatr Psychol. 2007;32(2):189–193. [DOI] [PubMed] [Google Scholar]
- 110. Kaleita TA, Reaman GH, MacLean WE, et al. Neurodevelopmental outcome of infants with acute lymphoblastic leukemia: a Children’s Cancer Group report. Cancer. 1999;85(8):1859–1865. [DOI] [PubMed] [Google Scholar]
- 111. Montour-Proulx I, Kuehn SM, Keene DL, et al. Cognitive changes in children treated for acute lymphoblastic leukemia with chemotherapy only according to the Pediatric Oncology Group 9605 protocol. J Child Neurol. 2005;20(2):129–133. [DOI] [PubMed] [Google Scholar]
- 112. Spiegler BJ, Kennedy K, Maze R, et al. Comparison of long-term neurocognitive outcomes in young children with acute lymphoblastic leukemia treated with cranial radiation or high-dose or very high-dose intravenous methotrexate. J Clin Oncol. 2006;24(24):3858–3864. [DOI] [PubMed] [Google Scholar]
- 113. Peterson CC, Johnson CE, Ramirez LY, et al. A meta-analysis of the neuropsychological sequelae of chemotherapy-only treatment for pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;51(1):99–104. [DOI] [PubMed] [Google Scholar]
- 114. Campbell LK, Scaduto M, Van Slyke D, et al. Executive function, coping, and behavior in survivors of childhood acute lymphocytic leukemia. J Pediatr Psychol. 2009;34(3):317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lofstad GE, Reinfjell T, Hestad K, et al. Cognitive outcome in children and adolescents treated for acute lymphoblastic leukaemia with chemotherapy only. Acta Paediatr. 2009;98(1):180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kadan-Lottick NS, Zeltzer LK, Liu Q, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J Natl Cancer Inst. 2010;102(12):881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Conklin HM, Krull KR, Reddick WE, et al. Cognitive outcomes following contemporary treatment without cranial irradiation for childhood acute lymphoblastic leukemia. J Natl Cancer Inst. 2012;104(18):1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kadan-Lottick NS, Brouwers P, Breiger D, et al. Comparison of neurocognitive functioning in children previously randomly assigned to intrathecal methotrexate compared with triple intrathecal therapy for the treatment of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27(35):5986–5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Chilcote RR, Coccia P, Sather HN, et al. Mediastinal mass in acute lymphoblastic leukemia. Med Pediatr Oncol. 1984;12(1):9–16. [DOI] [PubMed] [Google Scholar]
- 120. Turner CD, Chordas CA, Liptak CC, et al. Medical, psychological, cognitive and educational late-effects in pediatric low-grade glioma survivors treated with surgery only. Pediatr Blood Cancer. 2009;53(3):417–423. [DOI] [PubMed] [Google Scholar]
- 121. Bellebaum C, Daum I. Mechanisms of cerebellar involvement in associative learning. Cortex. 2011;47(1):128–136. [DOI] [PubMed] [Google Scholar]
- 122. Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Ann Rev Neurosci. 2009;32:413–434. [DOI] [PubMed] [Google Scholar]
- 123. De Smet HJ, Baillieux H, Wackenier P, et al. Long-term cognitive deficits following posterior fossa tumor resection: a neuropsychological and functional neuroimaging follow-up study. Neuropsychology. 2009;23(6):694–704. [DOI] [PubMed] [Google Scholar]
- 124. Chen SH, Yang WJ, Fang YP, et al. A genome-wide approach identifies variations in the aspartate metabolism pathway that are associated with asparaginase sensitivity. Leukemia. 2011;25(1):66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Beebe DW, Ris MD, Armstrong FD, et al. Cognitive and adaptive outcome in low-grade pediatric cerebellar astrocytomas: evidence of diminished cognitive and adaptive functioning in National Collaborative Research Studies (CCG 9891/POG 9130). J Clin Oncol. 2005;23(22):5198–5204. [DOI] [PubMed] [Google Scholar]
- 126. Sorich MJ, Pottier N, Pei DQ, et al. In vivo response to methotrexate forecasts outcome of acute lymphoblastic leukemia and has a distinct gene expression profile. PLoS Medicine. 2008;5(4):646–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Inaba H, Khan RB, Laningham FH, et al. Clinical and radiological characteristics of methotrexate-induced acute encephalopathy in pediatric patients with cancer. Ann Oncol. 2008;19(1):178–184. [DOI] [PubMed] [Google Scholar]
- 128. Howard SC, Metzger ML, Wilimas JA, et al. Childhood cancer epidemiology in low-income countries. Cancer. 2008;112(3):461–472. [DOI] [PubMed] [Google Scholar]
- 129. French D, Yang W, Cheng C, et al. Acquired variation outweighs inherited variation in whole genome analysis of methotrexate polyglutamate accumulation in leukemia. Blood. 2009;113(19):4512–4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sandlund JT, Santana VM, Hudson MM, et al. Combination of dexamethasone, high-dose cytarabine, and carboplatin is effective for advanced large-cell non-Hodgkin lymphoma of childhood. Cancer. 2008;113(4):782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Roberson JR, Spraker HL, Shelso J, et al. Clinical consequences of hyperglycemia during remission induction therapy for pediatric acute lymphoblastic leukemia. Leukemia. 2009;23(2):245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hardy KK, Willard VW, Bonner MJ. Computerized cognitive training in survivors of childhood cancer: a pilot study. J Pediatr Oncol Nurs. 2011;28(1):27–33. [DOI] [PubMed] [Google Scholar]
- 133. Annett RD, Erickson SJ. Feasibility of a school reintegration programme for children with acute lymphoblastic leukaemia. Eur J Cancer Care (Engl). 2009;18(4):421–428. [DOI] [PubMed] [Google Scholar]
- 134. Charlton A, Pearson D, Morris-Jones PH. Children’s return to school after treatment for solid tumours. Social science & medicine. 1986;22(12):1337–1346. [DOI] [PubMed] [Google Scholar]
- 135. Ray P, Jallo GI, Kim RY, et al. Endoscopic third ventriculostomy for tumor-related hydrocephalus in a pediatric population. Neurosurg Focus. 2005;19(6):E8. [DOI] [PubMed] [Google Scholar]
- 136. Wisoff JH, Sanford RA, Heier LA, et al. Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children’s Oncology Group. Neurosurgery. 2011;68(6):1548–1554; discussion 1554-1545. [DOI] [PubMed] [Google Scholar]
- 137. Buizer AI, de Sonneville LM, Veerman AJ. Effects of chemotherapy on neurocognitive function in children with acute lymphoblastic leukemia: a critical review of the literature. Pediatr Blood Cancer. 2009;52(4):447–454. [DOI] [PubMed] [Google Scholar]
- 138. Buizer AI, de Sonneville LM, van den Heuvel-Eibrink MM, et al. Chemotherapy and attentional dysfunction in survivors of childhood acute lymphoblastic leukemia: effect of treatment intensity. Pediatr Blood Cancer. 2005;45(3):281–290. [DOI] [PubMed] [Google Scholar]
- 139. Robinson KE, Kuttesch JF, Champion JE, et al. A quantitative meta-analysis of neurocognitive sequelae in survivors of pediatric brain tumors. Pediatr Blood Cancer. 2010;55(3):525–531. [DOI] [PubMed] [Google Scholar]
- 140. Fouladi M, Wallace D, Langston JW, et al. Survival and functional outcome of children with hypothalamic/chiasmatic tumors. Cancer. 2003;97(4):1084–1092. [DOI] [PubMed] [Google Scholar]
- 141. Armstrong GT, Conklin HM, Huang S, et al. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro Oncol. 2011;13(2):223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Carrie C, Grill J, Figarella-Branger D, et al. Online quality control, hyperfractionated radiotherapy alone and reduced boost volume for standard risk medulloblastoma: long-term results of MSFOP 98. J Clin Oncol. 2009;27(11):1879–1883. [DOI] [PubMed] [Google Scholar]
- 143. Landier W, Kinahan KE, Shaw S, et al. Screening for late effects in brain tumor survivors. Cancer Treat Res. 2009;150:389–409. [DOI] [PubMed] [Google Scholar]
- 144. Goldwein JW, Radcliffe J, Packer RJ, et al. Results of a pilot study of low-dose craniospinal radiation therapy plus chemotherapy for children younger than 5 years with primitive neuroectodermal tumors. Cancer. 1993;71(8):2647–2652. [DOI] [PubMed] [Google Scholar]
- 145. Duffner PK, Horowitz ME, Krischer JP, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328(24):1725–1731. [DOI] [PubMed] [Google Scholar]
- 146. Geyer JR, Sposto R, Jennings M, et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children’s Cancer Group. J Clin Oncol. 2005;23(30):7621–7631. [DOI] [PubMed] [Google Scholar]
- 147. Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352(10):978–986. [DOI] [PubMed] [Google Scholar]
- 148. Dhall G, Grodman H, Ji L, et al. Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the “Head Start” I and II protocols. Pediatr Blood Cancer. 2008;50(6):1169–1175. [DOI] [PubMed] [Google Scholar]
- 149. Goldwein JW, Radcliffe J, Johnson J, et al. Updated results of a pilot study of low dose craniospinal irradiation plus chemotherapy for children under five with cerebellar primitive neuroectodermal tumors (medulloblastoma). Int J Radiat Oncol Biol Phys. 1996;34(4):899–904. [DOI] [PubMed] [Google Scholar]
- 150. Palmer SL, Reddick WE, Gajjar A. Understanding the cognitive impact on children who are treated for medulloblastoma. J Pediatr Psychol. 2007;32(9):1040–1049. [DOI] [PubMed] [Google Scholar]
- 151. Jain N, Brouwers P, Okcu MF, et al. Sex-specific attention problems in long-term survivors of pediatric acute lymphoblastic leukemia. Cancer. 2009;115(18):4238–4245. [DOI] [PubMed] [Google Scholar]
- 152. Ashley DM, Merchant TE, Strother D, et al. Induction chemotherapy and conformal radiation therapy for very young children with nonmetastatic medulloblastoma: Children’s Oncology Group study P9934. J Clin Oncol. 2012;30(26):3181–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ashley DM, Riffkin CD, Lovric MM, et al. In vitro sensitivity testing of minimally passaged and uncultured gliomas with TRAIL and/or chemotherapy drugs. Br J Cancer. 2008;99(2):294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Sands SA, Oberg JA, Gardner SL, et al. Neuropsychological functioning of children treated with intensive chemotherapy followed by myeloablative consolidation chemotherapy and autologous hematopoietic cell rescue for newly diagnosed CNS tumors: an analysis of the Head Start II survivors. Pediatr Blood Cancer. 2010;54(3):429–436. [DOI] [PubMed] [Google Scholar]
- 155. Qaddoumi I, Carey SS, Conklin H, et al. Characterization, treatment, and outcome of intracranial neoplasms in the first 120 days of life. J Child Neurol. 2011;26(8):988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Leukemia & Lymphoma Society. Learning and Living with Cancer. 2013. Available at: http://www.lls.org/content/nationalcontent/resourcecenter/freeeducationmaterials/childhoodbloodcancer/pdf/learninglivingwithcancer.pdf Accessed January 10, 2014.
- 157. Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. 2008. Available at: www.survivorshipguidelines.org Accessed February 22, 2013.
- 158. af Sandeberg M, Johansson E, Bjork O, et al. Health-related quality of life relates to school attendance in children on treatment for cancer. J Pediatr Oncol Nurs. 2008;25(5):265–274. [DOI] [PubMed] [Google Scholar]
- 159. Children’s Oncology Group. School Support. Available at: http://www.childrensoncologygroup.org/index.php/school-support Accessed January 10, 2014.
- 160. Charlton A, Larcombe IJ, Meller ST, et al. Absence from school related to cancer and other chronic conditions. Arch Dis Child. 1991;66(10):1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Searle NS, Askins M, Bleyer WA. Homebound schooling is the least favorable option for continued education of adolescent cancer patients: a preliminary report. Med Pediatr Oncol. 2003;40(6):380–384. [DOI] [PubMed] [Google Scholar]
- 162. Bruce BS, Newcombe J, Chapman A. School liaison program for children with brain tumors. J Pediatr Oncol Nurs. 2012;29(1):45–54. [DOI] [PubMed] [Google Scholar]
- 163. Carpentieri SC, Mulhern RK. Patterns of memory dysfunction among children surviving temporal lobe tumors. Arch Clin Neuropsychol. 1993;8(4):345–357. [PubMed] [Google Scholar]
- 164. Varni JW, Katz ER, Colegrove R, Jr, et al. The impact of social skills training on the adjustment of children with newly diagnosed cancer. J Pediatr Psychol. 1993;18(6):751–767. [DOI] [PubMed] [Google Scholar]
- 165. Radcliffe J, Bennett D, Kazak AE, et al. Adjustment in childhood brain tumor survival: child, mother, and teacher report. J Pediatr Psychol. 1996;21(4):529–539. [DOI] [PubMed] [Google Scholar]
- 166. Barakat LP, Hetzke JD, Foley B, et al. Evaluation of a social-skills training group intervention with children treated for brain tumors: a pilot study. J Pediatr Psychol. 2003;28(5):299–307. [DOI] [PubMed] [Google Scholar]
- 167. Die-Trill M, Bromberg J, LaVally B, et al. Development of social skills in boys with brain tumors: a group approach. J Psychosoc Oncol. 1996;14(2):20. [Google Scholar]
- 168. Pai AL, Tackett A, Ittenbach RF, et al. Psychosocial Assessment Tool 2.0_General: validity of a psychosocial risk screener in a pediatric kidney transplant sample. Pediatr Transplant. 2012;16(1):92–98. [DOI] [PubMed] [Google Scholar]
- 169. Cicerone KD, Dahlberg C, Malec JF, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 1998 through 2002. Arch Phys Med Rehabil. 2005;86(8):1681–1692. [DOI] [PubMed] [Google Scholar]
- 170. Galbiati S, Recla M, Pastore V, et al. Attention remediation following traumatic brain injury in childhood and adolescence. Neuropsychology. 2009;23(1):40–49. [DOI] [PubMed] [Google Scholar]
- 171. Catroppa C, Anderson VA, Muscara F, et al. Educational skills: long-term outcome and predictors following paediatric traumatic brain injury. Neuropsychol Rehabil. 2009;19(5):716–732. [DOI] [PubMed] [Google Scholar]
- 172. Fenwick T, Anderson V. Impairments of attention following childhood traumatic brain injury. Child Neuropsychol. 1999;5(4):213–223. [DOI] [PubMed] [Google Scholar]
- 173. Yeates KO, Swift E, Taylor HG, et al. Short- and long-term social outcomes following pediatric traumatic brain injury. J Int Neuropsychol Soc. 2004;10(3):412–426. [DOI] [PubMed] [Google Scholar]
- 174. Gioia GA, Isquith PK. Ecological assessment of executive function in traumatic brain injury. Dev Neuropsychol. 2004;25(1–2):135–158. [DOI] [PubMed] [Google Scholar]
- 175. Anderson V, Catroppa C. Recovery of executive skills following paediatric traumatic brain injury (TBI): a 2 year follow-up. Brain Inj. 2005;19(6):459–470. [DOI] [PubMed] [Google Scholar]
- 176. Anderson V, Catroppa C, Morse S, et al. Functional plasticity or vulnerability after early brain injury? Pediatrics. 2005;116(6):1374–1382. [DOI] [PubMed] [Google Scholar]
- 177. Catroppa C, Anderson VA, Morse SA, et al. Children’s attentional skills 5 years post-TBI. J Pediatr Psychol. 2007;32(3):354–369. [DOI] [PubMed] [Google Scholar]
- 178. Butler RW, Copeland DR, Fairclough DL, et al. A multicenter, randomized clinical trial of a cognitive remediation program for childhood survivors of a pediatric malignancy. J Consult Clin Psychol. 2008;76(3):367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Patel SK, Katz ER, Richardson R, et al. Cognitive and problem solving training in children with cancer: a pilot project. J Pediatr Hematol Oncol. 2009;31(9):670–677. [DOI] [PubMed] [Google Scholar]
- 180. van’t Hooft I, Norberg AL. SMART cognitive training combined with a parental coaching programme for three children treated for medulloblastoma. NeuroRehabilitation. 2010;26(2):105–113. [DOI] [PubMed] [Google Scholar]
- 181. Moore IM, Hockenberry MJ, Anhalt C, et al. Mathematics intervention for prevention of neurocognitive deficits in childhood leukemia. Pediatr Blood Cancer. 2012;59(2):278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Hardy KK, Willard VW, Allen TM, et al. Working memory training in survivors of pediatric cancer: a randomized pilot study. Psycho-oncology. 2013;22(8):1856–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Reddick WE, Conklin HM. Impact of acute lymphoblastic leukemia therapy on attention and working memory in children. Expert Rev Hematol. 2010;3(6):655–659. [DOI] [PubMed] [Google Scholar]
- 184. Chavez B, Sopko MA, Jr, Ehret MJ, et al. An update on central nervous system stimulant formulations in children and adolescents with attention-deficit/hyperactivity disorder. Ann Pharmacother. 2009;43(6):1084–1095. [DOI] [PubMed] [Google Scholar]
- 185. Hanwella R, Senanayake M, de Silva V. Comparative efficacy and acceptability of methylphenidate and atomoxetine in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis. BMC Psychiatry. 2011;11:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Thompson SJ, Leigh L, Christensen R, et al. Immediate neurocognitive effects of methylphenidate on learning-impaired survivors of childhood cancer. J Clin Oncol. 2001;19(6):1802–1808. [DOI] [PubMed] [Google Scholar]
- 187. Conklin HM, Lawford J, Jasper BW, et al. Side effects of methylphenidate in childhood cancer survivors: a randomized placebo-controlled trial. Pediatrics. 2009;124(1):226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Jasper BW, Conklin HM, Lawford J, et al. Growth effects of methylphenidate among childhood cancer survivors: a 12-month case-matched open-label study. Pediatr Blood Cancer. 2009;52(1):39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Butler RW, Sahler OJ, Askins MA, et al. Interventions to improve neuropsychological functioning in childhood cancer survivors. Dev Disabil Res Rev. 2008;14(3):251–258. [DOI] [PubMed] [Google Scholar]
- 190. Biederman J, Pliszka SR. Modafinil improves symptoms of attention-deficit/hyperactivity disorder across subtypes in children and adolescents. J Pediatr. 2008;152(3):394–399. [DOI] [PubMed] [Google Scholar]
- 191. Biederman J, Swanson JM, Wigal SB, et al. Efficacy and safety of modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder: results of a randomized, double-blind, placebo-controlled, flexible-dose study. Pediatrics. 2005;116(6):e777–784. [DOI] [PubMed] [Google Scholar]
- 192. Boellner SW, Earl CQ, Arora S. Modafinil in children and adolescents with attention-deficit/hyperactivity disorder: a preliminary 8-week, open-label study. Curr Med Res Opin. 2006;22(12):2457–2465. [DOI] [PubMed] [Google Scholar]
- 193. Ivanenko A, Tauman R, Gozal D. Modafinil in the treatment of excessive daytime sleepiness in children. Sleep medicine. 2003;4(6):579–582. [DOI] [PubMed] [Google Scholar]
- 194. Morgenthaler TI, Kapur VK, Brown T, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Rugino TA, Samsock TC. Modafinil in children with attention-deficit hyperactivity disorder. Pediatr Neurol. 2003;29(2):136–142. [DOI] [PubMed] [Google Scholar]
- 196. Swanson JM, Greenhill LL, Lopez FA, et al. Modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder: results of a randomized, double-blind, placebo-controlled, fixed-dose study followed by abrupt discontinuation. J Clin Psychiatry. 2006;67(1):137–147. [DOI] [PubMed] [Google Scholar]
- 197. Pataki CS, Feinberg DT, McGough JJ. New drugs for the treatment of attention-deficit/hyperactivity disorder. Expert Opin Emerg Drugs. 2004;9(2):293–302. [DOI] [PubMed] [Google Scholar]
- 198. Shaw EG, Rosdhal R, D’Agostino RB, Jr, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006;24(9):1415–1420. [DOI] [PubMed] [Google Scholar]
- 199. Randall DC, Shneerson JM, File SE. Cognitive effects of modafinil in student volunteers may depend on IQ. Pharmacol Biochem Behav. 2005;82(1):133–139. [DOI] [PubMed] [Google Scholar]
- 200. Turner DC, Robbins TW, Clark L, et al. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology (Berl). 2003;165(3):260–269. [DOI] [PubMed] [Google Scholar]
- 201. Kaleita TA, Wellisch DK, Graham CA, et al. Pilot study of modafinil for treatment of neurobehavioral dysfunction and fatigue in adult patients with brain tumors. J Clin Oncol. 2006;24(18S):1503. [Google Scholar]
- 202. Gehring K, Patwardhan SY, Collins R, et al. A randomized trial on the efficacy of methylphenidate and modafinil for improving cognitive functioning and symptoms in patients with a primary brain tumor. J Neurooncol. 2012;107(1):165–174. [DOI] [PubMed] [Google Scholar]
- 203. Passmore AP, Bayer AJ, Steinhagen-Thiessen E. Cognitive, global, and functional benefits of donepezil in Alzheimer’s disease and vascular dementia: results from large-scale clinical trials. J Neurol Sci. 2005;229–230:141–146. [DOI] [PubMed] [Google Scholar]
- 204. Kishnani PS, Sullivan JA, Walter BK, et al. Cholinergic therapy for Down’s syndrome. Lancet. 1999;353(9158):1064–1065. [DOI] [PubMed] [Google Scholar]
- 205. Kondoh T, Nakashima M, Sasaki H, et al. Pharmacokinetics of donepezil in Down syndrome. Ann Pharmacother. 2005;39(3):572–573. [DOI] [PubMed] [Google Scholar]
- 206. Heller JH, Spiridigliozzi GA, Doraiswamy PM, et al. Donepezil effects on language in children with Down syndrome: results of the first 22-week pilot clinical trial. Am J Med Genet A. 2004;130(3):325–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Hardan AY, Handen BL. A retrospective open trial of adjunctive donepezil in children and adolescents with autistic disorder. J Child Adolesc Psychopharmacol. 2002;12(3):237–241. [DOI] [PubMed] [Google Scholar]
- 208. Chez MG, Tremb RJ, Nowinski CV, et al. Double-blinded placebo-controlled Aricept study in children with autistic-spectrum disorder. Ann Neurol. 2001;49:S95–S96. [Google Scholar]
- 209. Rapp SR, Rosdhal R, D’Agostino RB, et al. Improving cognitive functioning in brain irradiated patients: A Phase II trial of an acetylcholinesterase inhibitor (donepezil). Neuro-oncol. 2004;6:357. [Google Scholar]
- 210. Shaw EG, Rosdhal R, D’Agostino RB, et al. A phase II study of donepezil in irradiated brain tumor patients: effect on health-related quality of life and mood. Neuro-oncol. 2004;6:358. [Google Scholar]
- 211. Castellino SM, Tooze JA, Flowers L, et al. Toxicity and efficacy of the acetylcholinesterase (AChe) inhibitor donepezil in childhood brain tumor survivors: a pilot study. Pediatr Blood Cancer. 2012;59(3):540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Webb EA, O’Reilly MA, Clayden JD, et al. Effect of growth hormone deficiency on brain structure, motor function and cognition. Brain. 2012;135(Pt 1):216–227. [DOI] [PubMed] [Google Scholar]
- 213. Waters MJ, Blackmore DG. Growth hormone (GH), brain development and neural stem cells. Pediatr Endocrinol Rev. 2011;9(2):549–553. [PubMed] [Google Scholar]
- 214. Huisman J, Aukema EJ, Deijen JB, et al. The usefulness of growth hormone treatment for psychological status in young adult survivors of childhood leukaemia: an open-label study. BMC Pediatr. 2008;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Arwert LI, Deijen JB, Witlox J, et al. The influence of growth hormone (GH) substitution on patient-reported outcomes and cognitive functions in GH-deficient patients: a meta-analysis. Growth Horm IGF Res. 2005;15(1):47–54. [DOI] [PubMed] [Google Scholar]
- 216. Reimunde P, Quintana A, Castanon B, et al. Effects of growth hormone (GH) replacement and cognitive rehabilitation in patients with cognitive disorders after traumatic brain injury. Brain Inj. 2011;25(1):65–73. [DOI] [PubMed] [Google Scholar]
- 217. Stephen MD, Varni JW, Limbers CA, et al. Health-related quality of life and cognitive functioning in pediatric short stature: comparison of growth-hormone-naive, growth-hormone-treated, and healthy samples. Eur J Pediatr. 2011;170(3):351–358. [DOI] [PubMed] [Google Scholar]
- 218. Siemensma EP, Tummers-de Lind van Wijngaarden RF, Festen DA, et al. Beneficial effects of growth hormone treatment on cognition in children with Prader-Willi syndrome: a randomized controlled trial and longitudinal study. J Clin Endocrinol Metab. 2012;97(7):2307–2314. [DOI] [PubMed] [Google Scholar]
- 219. Baker LD, Barsness SM, Borson S, et al. Effects of growth hormone-releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults: results of a controlled trial. Arch Neurol. 2012;69(11):1420–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Jansen NC, Kingma A, Schuitema A, et al. Neuropsychological outcome in chemotherapy-only-treated children with acute lymphoblastic leukemia. J Clin Oncol. 2008;26(18):3025–3030. [DOI] [PubMed] [Google Scholar]
- 221. Heilbronner RL, Sweet JJ, Attix DK, et al. Official position of the American Academy of Clinical Neuropsychology on serial neuropsychological assessments: the utility and challenges of repeat test administrations in clinical and forensic contexts. Clin Neuropsychol. 2010;24(8):1267–1278. [DOI] [PubMed] [Google Scholar]
- 222. Embry L, Annett RD, Kunin-Batson A, et al. Implementation of multi-site neurocognitive assessments within a pediatric cooperative group: can it be done? Pediatr Blood Cancer. 2012;59(3):536–539. [DOI] [PubMed] [Google Scholar]
- 223. Krull K, Jain N, Pan Z, et al. Executive functions in aging adult survivors of childhood leukemia. J Clin Oncol. 2010;28(15s):9011. [Google Scholar]
- 224. Castellino SM, Tooze JA, Flowers L, et al. The peabody picture vocabulary test as a pre-screening tool for global cognitive functioning in childhood brain tumor survivors. J Neurooncol. 2011;104(2):559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Pejnovic LP, De Luca CR, Gentle E, et al. Feasibility of neurobehavioral screening following diagnosis of pediatric cancer. Pediatr Blood Cancer. 2012;59(2):295–300. [DOI] [PubMed] [Google Scholar]
- 226. Maruff P, Thomas E, Cysique L, et al. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. 2009;24(2):165–178. [DOI] [PubMed] [Google Scholar]
- 227. Fliessbach K, Rogowski S, Hoppe C, et al. Computer-based assessment of cognitive functions in brain tumor patients. J Neurooncol. 2010;100(3):427–437. [DOI] [PubMed] [Google Scholar]
- 228. Edelstein K, D’Agostino N, Bernstein LJ, et al. Long-term neurocognitive outcomes in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011;33(6):450–458. [DOI] [PubMed] [Google Scholar]
- 229. Iverson GL. Sensitivity of computerized neuropsychological screening in depressed university students. Clin Neuropsychol. 2006;20(4):695–701. [DOI] [PubMed] [Google Scholar]
- 230. National Institutes of Health; Northwestern University. NIH Toolbox: Assessment of neurological and behavioral function. Available at: www.nihtoolbox.org Accessed January 10, 2014.
- 231. Hinds PS, Nuss SL, Ruccione KS, et al. PROMIS pediatric measures in pediatric oncology: Valid and clinically feasible indicators of patient-reported outcomes. Pediatr Blood Cancer. 2013;60(3):402–408. [DOI] [PubMed] [Google Scholar]
- 232. Lai JS, Cella D, Choi S, et al. How item banks and their application can influence measurement practice in rehabilitation medicine: a PROMIS fatigue item bank example. Arch Phys Med Rehabil. 2011;92(10 Suppl):S20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Irwin DE, Gross HE, Stucky BD, et al. Development of six PROMIS pediatrics proxy-report item banks. Health Qual Life Outcomes. 2012;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Quinn GP, Murphy D, Knapp C, et al. Who decides? Decision making and fertility preservation in teens with cancer: a review of the literature. J Adolesc Health. 2011;49(4):337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Quillen J, Crawford E, Plummer B, et al. Parental follow-through of neuropsychological recommendations for childhood-cancer survivors. J Pediatr Oncol Nurs. 2011;28(5):306–310. [DOI] [PubMed] [Google Scholar]
- 236. Noll RB, Patel SK, Embry L, et al. Children’s Oncology Group’s 2013 blueprint for research: Behavioral science. Pediatr Blood Cancer. 2012;60(6):1048–1054. [DOI] [PubMed] [Google Scholar]
- 237. Caroni P, Donato F, Muller D. Structural plasticity upon learning: regulation and functions. Nat Rev Neurosci. 2012;13(7):478–490. [DOI] [PubMed] [Google Scholar]
- 238. Wang JY, Bakhadirov K, Devous MD, Sr, et al. Diffusion tensor tractography of traumatic diffuse axonal injury. Arch Neurol. 2008;65(5):619–626. [DOI] [PubMed] [Google Scholar]
- 239. Huisman TA, Schwamm LH, Schaefer PW, et al. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol. 2004;25(3):370–376. [PMC free article] [PubMed] [Google Scholar]
- 240. Levin HS, Wilde EA, Chu Z, et al. Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J Head Trauma Rehabil. 2008;23(4):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Miketova P, Kaemingk K, Hockenberry M, et al. Oxidative changes in cerebral spinal fluid phosphatidylcholine during treatment for acute lymphoblastic leukemia. Biol Res Nurs. 2005;6(3):187–195. [DOI] [PubMed] [Google Scholar]
- 242. Moore IM, Miketova P, Hockenberry M, et al. Methotrexate-induced alterations in beta-oxidation correlate with cognitive abilities in children with acute lymphoblastic leukemia. Biol Res Nurs. 2008;9(4):311–319. [DOI] [PubMed] [Google Scholar]
- 243. Caron JE, Krull KR, Hockenberry M, et al. Oxidative stress and executive function in children receiving chemotherapy for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2009;53(4):551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Stenzel SL, Krull KR, Hockenberry M, et al. Oxidative stress and neurobehavioral problems in pediatric acute lymphoblastic leukemia patients undergoing chemotherapy. J Pediatr Hematol Oncol. 2009;32(2):113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Ramanan S, Kooshki M, Zhao W, et al. PPARalpha ligands inhibit radiation-induced microglial inflammatory responses by negatively regulating NF-kappaB and AP-1 pathways. Free Radic Biol Med. 2008;45(12):1695–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Gehring K, Sitskoorn MM, Gundy CM, et al. Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol. 2009;27(22):3712–3722. [DOI] [PubMed] [Google Scholar]
- 247. Extermann M, Chen H, Booth-Jones M, et al. Pilot testing of the computerized cognitive test Microcog in chemotherapy-treated older cancer patients. Crit Rev Oncol Hematol. 2005;54(2):137–143. [DOI] [PubMed] [Google Scholar]
- 248. Gehring K, Roukema JA, Sitskoorn MM. Review of recent studies on interventions for cognitive deficits in patients with cancer. Expert Rev Anticancer Ther. 2012;12(2):255–269. [DOI] [PubMed] [Google Scholar]
- 249. Krull KR, Sabin ND, Reddick WE, et al. Neurocognitive function and CNS integrity in adult survivors of childhood hodgkin lymphoma. J Clin Oncol. 2012;30(29):3618–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Kazak AE, Brier M, Alderfer MA, et al. Screening for psychosocial risk in pediatric cancer. Pediatr Blood Cancer. 2012;59(5):822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Moore IM, Espy KA, Kaufmann P, et al. Cognitive consequences and central nervous system injury following treatment for childhood leukemia. Semin Oncol Nurs. 2000;16(4):279–290; discussion 291-279. [DOI] [PubMed] [Google Scholar]
- 252. Kerns KA, Thomson J. Implementation of a compensatory memory system in a school age child with severe memory impairment. Pediatr Rehabil. 1998;2(2):77–87. [DOI] [PubMed] [Google Scholar]
- 253. Kazak AE, Simms S, Alderfer MA, et al. Feasibility and preliminary outcomes from a pilot study of a brief psychological intervention for families of children newly diagnosed with cancer. J Pediatr Psychol. 2005;30(8):644–655. [DOI] [PubMed] [Google Scholar]
- 254. DeLong R, Friedman H, Friedman N, et al. Methylphenidate in neuropsychological sequelae of radiotherapy and chemotherapy of childhood brain tumors and leukemia. J Child Neurol. 1992;7(4):462–463. [DOI] [PubMed] [Google Scholar]
- 255. Mulhern RK, White HA, Glass JO, et al. Attentional functioning and white matter integrity among survivors of malignant brain tumors of childhood. J Int Neuropsychol Soc. 2004;10(2):180–189. [DOI] [PubMed] [Google Scholar]
- 256. Conklin HM, Helton S, Ashford J, et al. Predicting methylphenidate response in long-term survivors of childhood cancer: a randomized, double-blind, placebo-controlled, crossover trial. J Pediatr Psychol. 2010;35(2):144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Torres CF, Korones DN, Palumbo DR, et al. Effect of methylphenidate in the postradiation attention and memory deficits in childrent. Ann Neurol. 1996;40(2):331–332. [Google Scholar]