Abstract
OBJECTIVES
A retrospective database was developed by the European Society of Thoracic Surgeons, collecting patients submitted to surgery for thymic tumours to analyse clinico-pathological prognostic predictors.
METHODS
A total of 2151 incident cases from 35 institutions were collected from 1990 to 2010. Clinical-pathological characteristics were analysed, including age, gender, associated myasthenia gravis stage (Masaoka), World Health Organization histology, type of thymic tumour [thymoma, thymic carcinoma (TC), neuroendocrine thymic tumour (NETT)], type of resection (complete/incomplete), tumour size, adjuvant therapy and recurrence. Primary outcome was overall survival (OS); secondary outcomes were the proportion of incomplete resections, disease-free survival and the cumulative incidence of recurrence (CIR).
RESULTS
A total of 2030 patients were analysed for OS (1798 thymomas, 191 TCs and 41 NETTs). Ten-year OS was 0.73 (95% confidence interval 0.69–0.75). Complete resection (R0) was achieved in 88% of the patients. Ten-year CIR was 0.12 (0.10–0.15). Predictors of shorter OS were increased age (P < 0–001), stage [III vs I HR 2.66, 1.80–3.92; IV vs I hazard ratio (HR) 4.41, 2.67–7.26], TC (HR 2.39, 1.68–3.40) and NETT (HR 2.59, 1.35–4.99) vs thymomas and incomplete resection (HR 1.74, 1.18–2.57). Risk of recurrence increased with tumour size (P = 0.003), stage (III vs I HR 5.67, 2.80–11.45; IV vs I HR 13.08, 5.70–30.03) and NETT (HR 7.18, 3.48–14.82). Analysis using a propensity score indicates that the administration of adjuvant therapy was beneficial in increasing OS (HR 0.69, 0.49–0.97) in R0 resections.
CONCLUSIONS
Masaoka stages III–IV, incomplete resection and non-thymoma histology showed a significant impact in increasing recurrence and in worsening survival. The administration of adjuvant therapy after complete resection is associated with improved survival.
Keywords: Thymoma, Thymic carcinoma, Myasthenia gravis, Neuroendocrine thymic tumours, Staging, Surgery
INTRODUCTION
Thymic malignancies are uncommon tumours, with an estimated incidence of 2.5–3.2/106 people. Thymomas, thymic carcinomas (TC) and neuroendocrine thymic tumours (NETT) are the three most important histological categories.
The rarity of thymic tumours has limited so far the possibility to set homogeneous management protocols as evidenced by a recent survey from the European Society of Thoracic Surgeons (ESTS) [1]. For this reason, the identification of prognostic predictors is of utmost importance to guide the clinician to the most appropriate therapeutic treatment in these rare tumours. A recent review [2] of the available literature evaluating studies reporting multivariable analyses of prognostic factors confirmed the limitations in our current knowledge about prognostic factors in thymic tumours.
Collaborative retrospective databases offer the opportunity to collect a large series of patients in a relatively short time period, and for this reason, they are helpful in rare diseases such as thymic malignancies.
The ESTS retrospective database project was launched in 2011 among ESTS members to collect data of patients submitted to surgical resection of thymic tumours from 1990 to 2010. Follow-up data collection was closed in December 2011. The aim of the present study was to investigate, using the largest database of thymic malignancies ever collected, several clinical-pathological prognostic predictors of incomplete resection, survival and recurrence that have been previously tested with conflicting results in smaller observational studies.
MATERIALS AND METHODS
ESTS is a thoracic surgical organization open to European and non-European members around the world. An enquiry was sent to all ESTS members to ask for participation to the thymic database project and to send their data. Thirty-five institutions responded and joined the project: 27 from Europe, 3 from Asia and 5 from USA/Canada. Institutional Review Board approval was obtained at each institution.
The data fields included demographics, presence of myasthenia gravis (MG), histology [2004 World Health Organization (WHO) classification [3]], tumour size (continuous), stage according to Masaoka [4], completeness of resection, administration of induction or adjuvant treatment, type of surgical procedure, data for survival analysis, cause of death, recurrence, year of surgery and mean number of patients provided by the centres/year (≤4, 5–9, ≥10). Patients operated on or before 2004 were reclassified at each centre using the latest WHO histological classification [3]. Information about the site of recurrence was not sufficient to include this covariate in the predictor analysis.
Overall, data on 2244 patients were collected. Of these, 58 patients with not–otherwise-specified lesions, 19 with undetermined tumours and 16 with insufficient data were excluded, and the remaining 2151 patients form the basis of our report.
Study outcomes
Primary outcome was overall survival (OS) calculated from the date of surgery to the date of death from any cause. Secondary outcomes were the proportion of incomplete resections (microscopically or macroscopically), the disease-free survival (DFS) calculated from the date of surgery to the date of recurrence or death from any cause, and the cumulative incidence of recurrence (CIR) calculated from the date of surgery to the date of recurrence. In order to rule out a potential bias between patients with and without information about recurrence (missing recurrence date or recurrence status) out of the total of R0 resections, we compared OS curves of the two groups. The difference between the curves was not significant [HR 1.19 95% confidence interval (CI) 0.88–1.60; P = 0.25]. We therefore assumed that no significant bias exists between the two populations.
Statistical analysis
OS and DFS were estimated by the Kaplan–Meier product-limit method. CIR was estimated considering death from any cause as a competing event. To account for the heterogeneity across centres, factors associated with OS and DFS were investigated using Cox proportional hazard models with shared frailty. CIR was analysed with proportional hazard frailty models for the subdistribution [5] including the same variables considered for the OS analysis. Factors associated to incomplete resection were investigated using mixed-effect logistic regression models, considering the centre as random effect.
The effect of adjuvant therapy on OS was investigated in patients after R0 resection and by subgroups. In order to adjust this non-randomized comparison and to reduce the loss of power in the subgroup analyses, a propensity score for the likelihood of receiving adjuvant therapy was calculated from eight covariates: age, gender, stage, tumour size, histology, MG, year of intervention and mean annual number of resections. Multivariable Cox proportional hazard models with shared frailty (for centre heterogeneity) were estimated including as predictor the adjuvant therapy along with the propensity score. Effect modifications by subgroups were evaluated by including in the models an interaction term between the covariate indicating the adjuvant therapy and the subgroup covariate of interest, adjusting for propensity score.
In all models fitted in this study, missing data were multiple imputed using the method of chained equations [6]. Combined estimates were obtained from five imputed datasets.
The statistical analysis was performed using STATA (version 11.1) (ice command for multiple imputation) and R (version 2.15.1) (cmprsk and crrSC packages for the competing event analyses).
RESULTS
The median number of patients submitted by each institution was 45 [interquartile range (IQR) 24–73, range 8–257]; 20 institutions (57%) reported <60 cases, 8 (23%) reported 60–99 cases and 7 (20%) reported at least 100 cases. The majority of institutions performed a follow-up schedule based on a 3- to 6-month computed tomography (CT) scan for the first 3 years, followed by annual CT scan lifelong. In most centres, more aggressive thymic tumours (TC and NETT) received a more strict imaging (CT) surveillance. The median follow-up time of surviving patients was 48 months (IQR 21–90). Fixed at 31 December 2011, the end of the follow-up data collection, the completeness of follow-up for the study was 73%.
Study flow of the patient population and patient characteristics
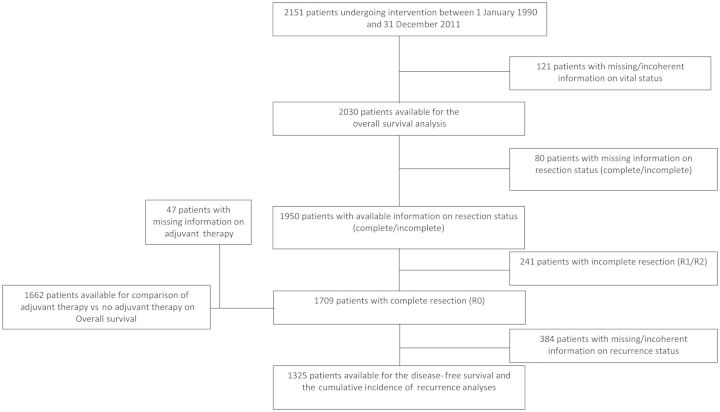
Figure 1 shows the study flow of the 2151 patients in the database; 2030 had sufficient information for OS analysis and 1325 for DFS and CIR analysis.
Figure 1:
Study flow diagram of the patient population for analysis of predictors.
Table 1 shows the patient characteristics and the number and percentages of missing information for the 2030 patients with sufficient information for OS analysis. Of these, there were 1798 thymomas, 191 TCs and 41 NETTs.
Table 1:
Patient characteristics (n = 2030)
| Missing information | ||
|---|---|---|
| Age, median (IQR) | 56 (45;67) | 3 (<1%) |
| Males | 1042 (51%) | 1 (<1%) |
| Myasthenia gravis | 629 (35%) | 243 (12%) |
| T size, median (IQR) | 6 (4;7) | 527 (26%) |
| Masaoka stage | ||
| 1 | 672 (34%) | 34 (2%) |
| 2 | 699 (35%) | |
| 3 | 410 (21%) | |
| 4 | 215 (11%) | |
| Diagnosis | ||
| Thymoma WHO A-AB-B1 | 1018 (50%) | 0 (0%) |
| Thymoma WHO B2-B3 | 780 (38%) | |
| Thymic carcinoma | 191 (9%) | |
| NETT | 41 (2%) | |
| Complete resections (R0) | 1709 (88%) | 80 (4%) |
| Intervention period | ||
| 1990–1995 | 176 (9%) | 0 (0%) |
| 1996–2001 | 395 (19%) | |
| 2002–2007 | 858 (42%) | |
| 2008–2011 | 601 (30%) | |
| Mean number of patients treated yearly by centre | ||
| ≤4 [19 (54%) centres] | 532 (26%) | 0 (0%) |
| 5–9 [11 (31%) centres] | 680 (33%) | |
| ≥10 [ 5 (14%) centres] | 818 (40%) | |
| Induction therapy | 239 (13%) | 154 (8%) |
| CT alone | 170 (9%) | |
| RT alone | 12 (1%) | |
| CT and RT | 57 (3%) | |
| Adjuvant therapy | 853 (44%) | 76 (4%) |
| CT alone | 44 (2%) | |
| RT alone | 566 (29%) | |
| CT and RT | 243 (12%) | |
Data are n (%) unless otherwise indicated. Percentages are calculated on the basis of available data for each characteristics.
CT: chemotherapy; NETT: neuroendocrine thymic tumour; RT: radiotherapy.
A complete resection was achieved in 1709 patients (88%).
The resectability rates were 99, 92, 78 and 53% for stages I, II, III and IV, respectively. The rates were 97, 97, 91, 85, 77, 72 and 69% for types A, AB, B1, B2, B3, TC and NETT
Induction therapy (mostly chemotherapy, 71%) was administered in 239 patients, of whom 186 (80%) were at stage III/IV; adjuvant therapy was administered in 853 patients and consisted of radiotherapy (n = 566), chemotherapy (n = 44) and combined chemo/radiotherapy (n = 243).
Frequency of study end points
Three hundred and twenty-four patients died during the follow-up. Ninety patients had recurrence out of 1325 R0 patients, with complete information about the recurrence status. Two hundred and fourteen patients had either recurrence or died out of the 1325 patients evaluated for DFS and CIR analysis.
Predictors of incomplete resection
In 1709 patients with complete resection, recurrence occurred in 141 cases (8%); it increased with stage (stages I 3%, II 4%, III 22%, IV 40%) and histology (A 3%, AB 5%, B1 8%, B2 11%, B3 14%, TC 30%, NETT 37%).
Among the different examined clinico-pathological variables, the probability of an incomplete resection is higher in male patients (adjOR 1.61, 95% CI 1.18–2.20, P = 0.003); it increases with tumour size (adjOR 1.10 [per 1 cm increase], 95% CI 1.01–1.21, P = 0.031), while it decreases in the presence of MG (adjOR 0.55, 95% CI 0.37–0.81, P = 0.002). With respect to A-AB-B1 thymoma, the probability of an incomplete resection is higher in B2-B3 thymoma (adjOR 5.91, 95% CI 4.02–8.70, P < 0.001), TC (adjOR 10.46, 95% CI 6.22–17.59, P < 0.001) and NETT (adjOR 7.77, 95% CI 3.45–17.51, P < 0.001).
Overall survival and disease-free survival analyses
Figure 2 reports the overall time-to-event curves for the different end points (OS, DFS and CIR). Five- and 10-year OS rates were 0.85 (95% CI 0.83–0.86) and 0.73 (95% CI 0.69–0.75), and DFS rates were 0.84 (95% CI 0.82–0.87) and 0.70 (95% CI 0.65–0.74). Thirty-day mortality was 1% (21 cases).
Figure 2:
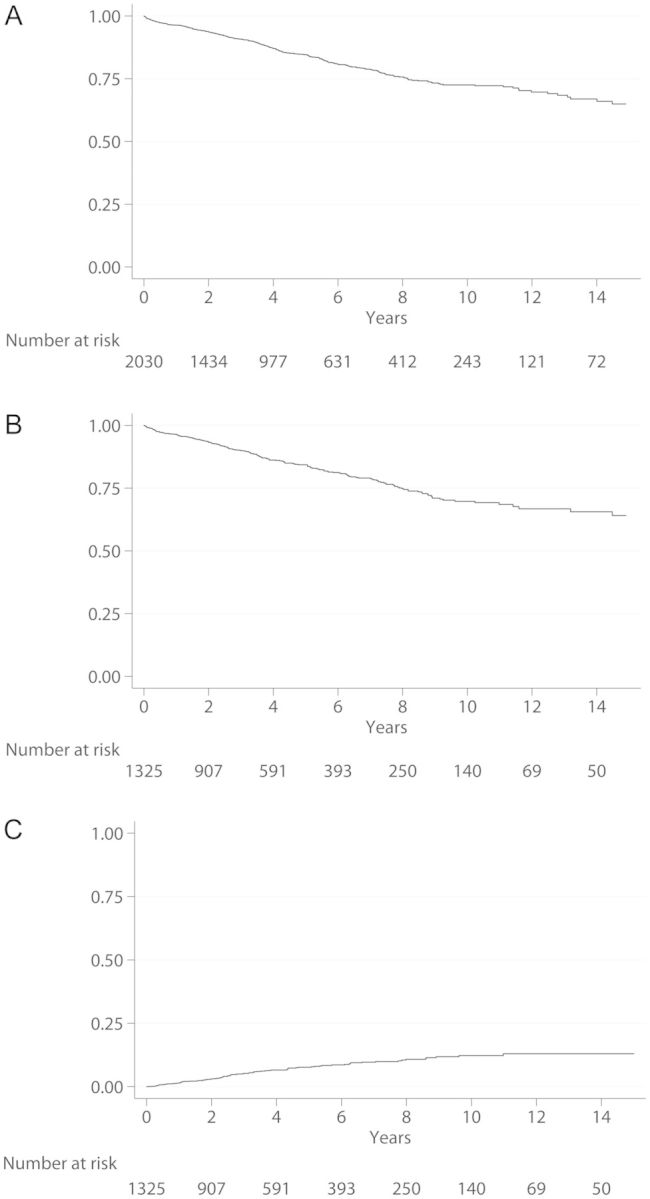
Curves of time-to-event end points.
Table 2 shows the analysis of OS predictors. The results of the DFS analysis paralleled those of OS analysis and were not reported. The risk of mortality increases with age [adjHR (per 5 year increase) 1.19, 95% CI 1.14–1.24, P < 0.001] and with stage (III vs I adjHR 2.66, 95% CI 1.80–3.92, P < 0.001; IV vs I adjHR 4.41, 95% CI 2.67–7.26, P < 0.001). The mortality risk is also higher for TC (adjHR 2.39, 95% CI 1.68–3.40, P < 0.001), NETT (adjHR 2.59, 95% CI 1.35–4.99, P = 0.004) and after incomplete resection (adjHR 1.74, 95% CI 1.18–2.57, P = 0.007); a weak trend towards an increased risk was found in males (adjHR 1.25, 95% CI 0.99–1.58, P = 0.06). No evidence of a major effect modification by histology categories (thymoma, TC and NETT) was found for the evaluated prognostic factors (Table 3).
Table 2:
Analysis of overall survival predictors. Cox proportional hazard model with shared frailty. (n = 2030)
| Univariable analysis |
Multivariable analysisa |
|||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (continuous, per 5 years increase) | 1.16 (1.11–1.21) | <0.001 | 1.19 (1.14–1.24) | <0.001 |
| Male | 1.29 (1.03–1.62) | 0.025 | 1.25 (0.99–1.58) | 0.060 |
| Myasthenia gravis | 0.68 (0.51–0.89) | 0.005 | 1.11 (0.84–1.48) | 0.451 |
| T size (continuous, per 1 cm increase) | 1.07 (1.03–1.11) | 0.001 | 1.04 (1–1.09) | 0.073 |
| Masaoka stage | ||||
| 1 (Ref) | 1 | – | 1 | – |
| 2 | 1.38 (0.96–2.00) | 0.084 | 1.09 (0.75–1.60) | 0.655 |
| 3 | 3.58 (2.51–5.13) | <0.001 | 2.66 (1.80–3.92) | <0.001 |
| 4 | 7.43 (5.16–10.70) | <0.001 | 4.41 (2.67–7.26) | <0.001 |
| Diagnosis | ||||
| Thymoma WHO A-AB-B1 (Ref) | 1 | – | 1 | – |
| Thymoma WHO B2-B3 | 1.40 (1.07–1.82) | 0.013 | 1.09 (0.82–1.46) | 0.553 |
| NETT | 4.76 (2.55–8.87) | <0.001 | 2.59 (1.35–4.99) | 0.004 |
| Thymic carcinoma | 4.49 (3.27–6.15) | <0.001 | 2.9 (1.68–3.40) | <0.001 |
| R status (R+ vs R0) | 3.87 (2.82–5.30) | <0.001 | 1.74 (1.18–2.57) | 0.007 |
| Year of intervention | ||||
| 1990–1995 (Ref) | 1 | – | 1 | – |
| 1996–2001 | 1.25 (0.83–1.86) | 0.282 | 1.09 (0.73–1.62) | 0.690 |
| 2002–2007 | 1.44 (0.93–2.23) | 0.099 | 1.05 (0.68–1.64) | 0.816 |
| 2008–2011 | 1.27 (0.71–2.27) | 0.427 | 1.07 (0.60–1.94) | 0.812 |
| Mean number patients (yearly) | ||||
| ≤4 (Ref) | 1 | – | 1 | – |
| 5–9 | 1.09 (0.55–2.17) | 0.797 | 1.75 (0.89–3.44) | 0.102 |
| ≥10 | 0.64 (0.28–1.50) | 0.309 | 0.92 (0.41–2.06) | 0.838 |
NETT: neuroendocrine thymic tumour.
aAll effects were unadjusted for T size, whereas T size effect was unadjusted for Masaoka stage.
Table 3:
Analysis of overall survival predictors by histology categories of thymic malignancies (thymoma, thymic carcinoma and NETT). Cox proportional hazard model with shared frailty
| Thymoma WHO A-AB-B1 |
Thymoma WHO B2-B3 |
NETT |
Thymic carcinoma |
Interaction, P | |||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||
| Age (continuous, per 5 years increase) | 1.22 (1.13–1.32) | <0.001 | 1.17 (1.09–1.26) | <0.001 | 1.15 (0.92–1.45) | 0.220 | 1.18 (1.09–1.29) | <0.001 | 0.852 |
| Male | 1.62 (1.11–2.38) | 0.013 | 1.00 (0.69–1.46) | 0.987 | 1.54 (0.39–6.07) | 0.540 | 1.18 (0.73–1.89) | 0.502 | 0.345 |
| Myasthenia gravis | 1.19 (0.76–1.85) | 0.446 | 1.07 (0.72–1.60) | 0.734 | 1.99 (0.26–15.41) | 0.507 | 0.99 (0.49–1.98) | 0.975 | 0.903 |
| Tumour size (per 1 cm increase) | 1.06 (0.98–1.14) | 0.135 | 1.05 (0.97–1.14) | 0.199 | 1.01 (0.84–1.21) | 0.957 | 1.02 (0.92–1.13) | 0.699 | 0.899 |
| Masaoka stage | |||||||||
| 1 (Ref) | 1 | – | 1 | – | 1 | – | 1 | – | 0.690 |
| 2 | 1.32 (0.83–2.11) | 0.243 | 0.78 (0.39–1.56) | 0.480 | 1.12 (0.24–5.29) | 0.883 | 1.07 (0.28–4.11) | 0.921 | |
| 3 | 2.13 (1.23–3.72) | 0.007 | 2.87 (1.59–5.19) | <0.001 | 2.30 (0.31–17.14) | 0.412 | 2.88 (0.82–10.15) | 0.100 | |
| 4 | 4.37 (2.16–8.85) | <0.001 | 5.17 (2.48–10.77) | <0.001 | 4.37 (2.16–8.85) | <0.001 | 3.69 (1.05–13.04) | 0.042 | |
| R status (R1-R2 vs R0) | 1.39 (0.73–2.63) | 0.317 | 1.85 (1.12–3.04) | 0.017 | 1.68 (0.49–5.74) | 0.407 | 1.91 (0.97–3.76) | 0.061 | 0.874 |
NETT: neuroendocrine thymic tumour.
Recurrence analysis
Cumulative incidence of recurrence was 0.05 (95% CI 0.04–0.07), 0.08 (95% CI 0.06–0.09) and 0.12 (95% CI 0.10–0.15) at 3, 5 and 10 years, respectively (Fig. 2).
Significant predictors of higher risk of recurrence included (Table 4) young age, non-MG status, increased tumour size, stages III and IV, NETT tumours and the most recent years of intervention (using as reference the earliest period, 1990–1995).
Table 4:
Cumulative incidence of recurrence. Proportional hazard frailty models for the subdistribution on R0 patients (N = 1325)
| HR (95% CI)a | P | |
|---|---|---|
| Age (continuous, per 5 years increase) | 0.91 (0.84–1.00) | 0.039 |
| Male | 0.81 (0.55–1.19) | 0.286 |
| Myasthenia gravis | 0.57 (0.33–0.98) | 0.042 |
| T size (continuous, per 1 cm increase) | 1.16 (1.09–1.24) | 0.003 |
| Masaoka stage | ||
| 1 (Ref) | 1 | – |
| 2 | 1.46 (0.72–2.95) | 0.288 |
| 3 | 5.67 (2.80–11.45) | <0.001 |
| 4 | 13.08 (5.70–30.03) | <0.001 |
| Diagnosis | ||
| Thymoma WHO A-AB-B1 (Ref) | 1 | – |
| Thymoma WHO B2-B3 | 1.22 (0.67–2.20) | 0.515 |
| NETT | 7.18 (3.48–14.82) | <0.001 |
| Thymic carcinoma | 1.50 (0.64–3.49) | 0.349 |
| Year of intervention | ||
| 1990–1995 (Ref) | 1 | – |
| 1996–2001 | 9.43 (2.82–31.51) | <0.001 |
| 2002–2007 | 10.92 (3.32–35.92) | <0.001 |
| 2008–2011 | 8.18 (1.49–44.77) | 0.015 |
| Mean number patients (yearly) treated yearly by centre | ||
| ≤4 (Ref) | 1 | – |
| 5–9 | 1.01 (0.41–2.46) | 0.984 |
| ≥10 | 1.08 (0.56–2.09) | 0.820 |
NETT: neuroendocrine thymic tumour.
aAll effects were unadjusted for T size, whereas T size effect was unadjusted for Masaoka stage.
Adjuvant therapy
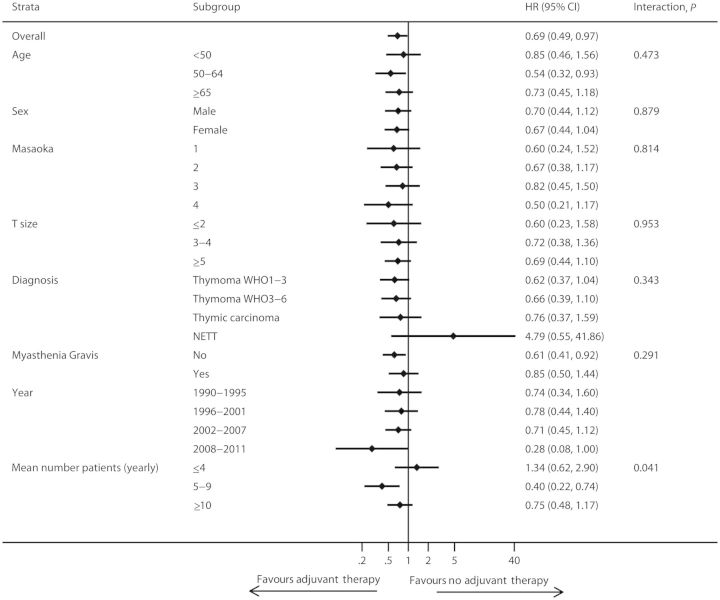
Radiation doses and chemotherapeutic regimens for adjuvant treatments varied among the centres. In general, a total dose of 40–60 Gy was employed for radiotherapy, while cisplatin-based regimens (mostly cisplatin/doxorubicin/cyclophosphamide-CAP, or cisplatin/doxorubicin/cyclophosphamide/vincristine-ADOC) were uniformly used. The analysis was undertaken on the subgroup of patients receiving adjuvant therapy after R0 resection. Complete information was available in 1662 patients out of 1709 R0 resections (Fig. 1). Adjuvant therapy was more frequently administered in younger patients (median age 54 vs 57) and in advanced stages (stage I 75/646, 12%; II 316/612, 52%; III 217/295, 74%; IV 56/91, 62%); the administration was also different according to histology (A-AB-B1 256/920, 28%; B2-B3 326/599, 54%; TC 78/116, 67%; NETT 16/27, 59%) (Table 5). The subgroup analysis was undertaken using a Cox model with shared frailty adjusted for propensity score. Results are reported in Fig. 3 as forest plot. The overall effect of adjuvant therapy on OS was significantly beneficial (HR 0.69, 95% CI 0.49–0.97). No strong evidence of an effect modification by specific subgroup was found. The overall effect of radiotherapy (alone or with chemotherapy) was nearly identical as in the whole adjuvant group (HR 0.66, 95% CI 0.46–0.94).
Table 5:
Patients characteristics according to the administration of adjuvant therapy (R0 patients; n = 1662)
|
na |
Adjuvant therapy administration |
P* | ||
|---|---|---|---|---|
| No (n = 986) | Yes (n = 676) | |||
| Age, median (IQR) | 1659 | 57 (46;68) | 54 (44;64) | <0.001 |
| Males | 1661 | 464 (47%) | 368 (54%) | 0.003 |
| Myasthenia gravis | 1491 | 322 (36%) | 234 (39%) | 0.229 |
| T size, median (IQR) | 1270 | 6 (4;7) | 5 (4;6) | 0.015 |
| Masaoka stage | ||||
| 1 | 1644 | 571 (58%) | 75 (11%) | <0.001 |
| 2 | 296 (30%) | 316 (48%) | ||
| 3 | 78 (8%) | 217 (33%) | ||
| 4 | 35 (4%) | 56 (8%) | ||
| Diagnosis | ||||
| Thymoma WHO A-AB-B1 | 1662 | 664 (67%) | 256 (38%) | <0.001 |
| Thymoma WHO B2–B3 | 273 (28%) | 326 (48%) | ||
| Thymic carcinoma | 38 (4%) | 78 (12%) | ||
| NETT | 11 (1%) | 16 (2%) | ||
NETT: neuroendocrine thymic tumour.
aAnalysis performed on the R0 patients with appropriate information on the administration of adjuvant therapy and on the characteristics of interest.
*P-values based on Mann–Whitney U-test and χ2 test for continuous variables and categorical variables, respectively.
Figure 3:
Forest plot of subgroup analysis for adjuvant vs no adjuvant therapy comparison, adjusted for propensity score (OS). Cox model proportional hazard models with shared frailty. Only R0 patients with available information on adjuvant therapy (n = 1662).
DISCUSSION
The results of our cohort study on patients submitted to surgical resection for thymic malignancies indicate that (i) independent negative predictors of OS were increased age, high Masaoka stages (III/IV), TC, NETT and incomplete resection; (ii) similar predictors for DFS were observed; (iii) adjusted predictors of incomplete resection were male gender, increased tumour size, non-MG status, high-risk thymomas (WHO B2/B3), TC and NETT; (iv) the incidence of recurrence increases with the length of the follow-up. Independent predictors of recurrence were young age, non-MG status, increased tumour size, high Masaoka stages (III/IV) and NETT and (v) exploratory analysis indicates that the administration of adjuvant therapy after complete resection was effective in increasing OS without strong differences among subgroups.
Our analysis showed that several variables are independent predictors of OS, incomplete resection and recurrence, confirming previous smaller observational studies.
Masaoka stage
The clinico-pathological staging system proposed by Masaoka has been repeatedly reported to impact survival and recurrence [7–9]; this is confirmed by our study. Previous reports included stages I and II in the same survival category, as the differences between them were not crucial. In the present study, stages I and II presented similar adjusted hazard ratios, supporting evidence for a redefinition of their identities. In the absence of an officially recognized staging system, therefore, Masaoka stage remains the most reliable way to stage thymic tumours.
Complete resection
In our analysis, macro- and microscopic complete resection (R0) resulted an independent prognostic factor confirming previous reports [9, 10]. This variable was clearly associated with stage and it was strongly prognostic of OS and DFS. An aggressive surgical approach with en bloc removal of the neighbouring organs was recommended to achieve complete resection [11], and this strategy is supported by our findings.
Recurrence
Recurrence has a distinct significance in thymic tumours. It may occur late in the course of the disease, particularly in early-stage tumours. Patients with a recurrence may live for many years owing to the indolent behaviour of most thymomas, and the possibility of reresection and multimodality treatments may significantly prolong survival in these patients [12, 13]. Our study indicates that the risk of recurrence increases with time. This finding corroborates some evidence in smaller studies that lifelong surveillance is warranted after thymic malignancy resection. Recurrence was correlated with advanced stage, increased tumour size and NETT histology. Recurrence was also a robust negative prognostic factor for OS.
Histology
The current WHO classification of thymic malignancies [3] includes two categories: thymoma and TC; each one is further divided into subtypes: 5 for thymoma and 11 for TC, including NETT. TC and NETT have been reported to portend a worse prognosis than thymomas [14–16]. In our study, NETT and TC were strong predictors of reduced OS, DFS and of increased recurrence. The prognostic significance of the different thymoma subtypes has not been confirmed, and a few studies showed that it is primarily related to the worse outcome of B3 tumours. Further, the histological differentiation between B3 and TC is difficult [17], with a reported wide interobserver variability. In the present analysis, high-risk thymomas (B2/B3) did not significantly differ from low-risk thymomas (A/AB/B1) for OS, DFS and recurrence, although B2-B3 thymomas showed an increased risk of being associated with incomplete resection. However, we are aware that the lack of a central review of the pathology specimens in the present series should be taken into account in the interpretation of the obtained results.
Tumour size
The prognostic significance of tumour size has been investigated in thymic neoplasms, and smaller tumours were generally found to be associated with improved survival and decreased risk of recurrence [18]. However, the size thresholds that have been used in these studies were somehow arbitrary. In the present study, we considered tumour size measured as the largest diameter on the surgical specimen as continuous variable. Our analysis indicates that increased tumour size was not a significant predictor of either OS or DFS, although it was found to increase the risk of recurrence and of incomplete resection. This finding may be taken into consideration in the indication of the follow-up schedule of these patients.
Myasthenia gravis
The role of MG has been addressed in several studies [19]; some authors speculate that MG patients are different from non-MG patients [20]. Our results somehow differ from these series, indicating that the presence of MG has no impact on OS and DFS. Non-MG patients, however, are at significantly higher risk of recurrence and of receiving incomplete resections. Although adjusted for other covariates, this finding might be explained by the more strict surveillance of MG patients leading to earlier thymoma detection.
Induction therapy
Administration of induction therapy has been suggested to improve resectability and survival and to decrease recurrence. Retrospective studies [21, 22] showed an encouraging pooled 5-year OS of 78% with a complete resection rate of 72% in locally advanced stages III–IVa thymic tumours, considerably higher than historical series using upfront surgery. Based on these studies, therefore, induction therapy is customarily performed in patients with tumours deemed unresectable at initial surgical evaluation. In the present study, an exploratory analysis of our data indicated a strong biased selection of patients receiving induction treatment that we could not control with the available information, thus preventing the possibility to obtain an unbiased estimate of its efficacy.
Adjuvant therapy
Adjuvant treatment, mostly in the form of radiotherapy or combined chemoradiotherapy, is currently administered in up to 60% of the patients with invasive thymic tumours [1]. This attitude, however, is based on several historical series, and its real impact on survival and recurrence is still debated with no consistent evidence having emerged so far. Kondo et al. [23] found that prophylactic postoperative radiotherapy (PORT) neither prevented recurrence nor increased survival after complete resection of stages II–IV thymic tumours. In a large study based on the surveillance epidemiology and end results database, Forquer et al. [24] found on all-stage thymic tumours (thymoma and thymic carcinomas) that PORT had no or even detrimental effect on local disease (Masaoka I), and a beneficial overall effect on OS in regional disease (stages II–III). Importantly, however, no survival differences were observed after ‘extirpative’ (i.e. R0) surgery. Finally, in a meta-analysis of retrospective studies from 1981 to 2008, Korst et al. [25] found no survival advantage for the use of PORT after complete resection of stages II–III thymomas. The bulk of evidence so far indicates that there is no convincing evidence of a consistent survival advantage of the use of PORT after complete resection of all-stage thymomas. In our study, the analysis of the prognostic effect of adjuvant therapy after complete resection was performed using a propensity score approach, minimizing the bias resulting from unbalanced confounding factors and adjusting the comparison for a large number of covariates in the subgroup analyses. Our results indicate that adjuvant therapy provides an overall beneficial effect on OS, without a strong evidence of an effect modification by specific subgroup. Our finding may therefore support the need for further prospective trials about the role of adjuvant treatment in thymic malignancies
The present study has several strengths and limitations. The main strength is that it is based on the largest database of patients with thymic tumours ever collected. The participating institutions used a homogeneous staging system and histological classification. Several limitations result from the collection of data from centres of different volume activity, expertise and geographic areas and from the lack of a central review of pathology reports. Also, the follow-up was not complete for all patients, and the person-time at risk available for the OS analysis accounted for the 73% of the expected follow-up. Finally, in 15% of R0 recurrent patients, we had no information about the recurrence date, resulting in a loss of statistical power of the analysis, and a consequent theoretical underestimate of the CIR.
In conclusion, despite the previously reported limits, this study on thymic tumours is of interest for its confirmatory results about the prognostic role of several factors, for the evidence of the efficacy of the current standard of care and because it provides suggestions for further investigational studies.
ACKNOWLEDGEMENTS
We thank Gianni Ciccone, from the Department of Epidemiology and Statistics, University of Torino, Italy, for his invaluable help in the statistical analysis of the data. We acknowledge the International Thymic Malignancy Interest Group (ITMIG) for the joint effort in the development of a common platform for the collection of the retrospective data.
Conflict of interest: none declared.
The European Society of Thoracic Surgeons (ESTS) Thymic Working Group: Khaled AlKattan1, Alex Arame2, Majed Refai3, Caterina Casadio4, Paolo Carbognani5, Robert Cerfolio6, Giovanni Donati7, Christophoros N Foroulis8, Cengiz Gebitekin9, David Gomez de Antonio10, Kemp H Kernstine11, Shaf Keshavjee12, Bernhard Moser13, Cosimo Lequaglie14, Moishe Liberman15, Eric Lim16, Andrew G Nicholson16, Loic Lang-Lazdunski17, Maurizio Mancuso18, Nasser Altorki19, Mario Nosotti20, Nuria M Novoa21, Geoffrey Brioude22, Alberto Oliaro23, Pier Luigi Filosso23 , Salvatore Saita24, Marco Scarci25, Jan Schützner26, Alberto Terzi27, Alper Toker28, Hans Van Veer29, Marco Anile30, Erino Rendina31, Luca Voltolini32, Wojciech Zurek33
1King Faisal Specialist Hospital, Alfaisal University, Riyadh, Saudi Arabia
2Hopital Europeen Georges-Pompidou and Hopital Laennec, Paris, France
3Ospedali Riuniti, Ancona, Italy
4University of Eastern Piedmont, Novara, Italy
5University of Parma, Parma, Italy
6University of Alabama at Birmingham, Birmingham, AL, USA
7General Regional Hospital, Aosta, Italy
8Aristotle University of Thessaloniki, A.H.E.P.A. University Hospital, Thessaloniki, Greece
9Uludag University School of Medicine, Bursa, Turkey
10Hospital Universitario Puerta de Hierro Majadahonda, Madrid, Spain
11University of Texas, Southwestern Medical Center and School of Medicine, TX, USA
12Toronto General Hospital, University of Toronto, Toronto, Canada
13Medical University of Vienna, Vienna, Austria
14IRCCS-CROB Centro Riferimento Oncologico della Basilicata, Rionero in Vulture, Italy
15Centre Hospitalier de l'Université de Montréal, University of Montreal, Montreal, Canada
16Royal Brompton and Harefield NHS Foundation Trust and National Heart and Lung Division, Imperial College, London, UK
17Guy's Hospital, London, UK
18SS Antonio e Biagio e Cesare Arrigo Hospital, Alessandria, Italy
19New York Presbyterian Hospital-Weill Cornell Medical Center, NY, USA
20IRCCS Fondazione Cà Granda Ospedale Maggiore Policlinico, Milano, Italy
21University Hospital of Salamanca, IBSAL, Salamanca, Spain
22Hôpital Nord-Aix-Marseille University, Marseille, France
23Department of Surgery, University of Torino, Torino, Italy
24Vittorio Emanuele Hospital, Catania, Italy
25Papworth Hospital NHS Foundation Trust, Papworth Everard, Cambridge, UK
263rd Department of Surgery, First Faculty of Medicine, Charles University in Prague and University Hospital Motol, Prague, Czech Republic
27Thoracic Surgery, Sacred Heart Hospital Negrar, Verona, Italy
28Istanbul University, Istanbul Medical School, Istanbul, Turkey
29University Hospitals Leuven, Leuven, Belgium
30University of Rome SAPIENZA; Policlinico Umberto I; Fondazione Eleonora Lorilard Spencer Cenci; Rome, Italy
31University of Rome SAPIENZA, Ospedale S. Andrea, Fondazione Eleonora Lorilard Spencer Cenci; Rome, Italy
32University Hospital of Siena, Siena, Italy
33Medical University of Gdansk, Gdansk, Poland
REFERENCES
- 1.Ruffini E, Van Raemdonck D, Detterbeck F, Rocco G, Thomas P, Venuta F European Society of Thoracic Surgeons Thymic Questionnaire Working Group. Management of thymic tumors: a survey of current practice among members of the European Society of Thoracic Surgeons. J Thorac Oncol. 2011;6:614–23. doi: 10.1097/JTO.0b013e318207cd74. doi:10.1097/JTO.0b013e318207cd74. [DOI] [PubMed] [Google Scholar]
- 2.Detterbeck F, Youssef S, Ruffini E, Okumura M. A review of prognostic factors in thymic malignancies. J Thorac Oncol. 2011;6(Suppl. 3):S1698–1704. doi: 10.1097/JTO.0b013e31821e7b12. doi:10.1097/JTO.0b013e31821e7b12. [DOI] [PubMed] [Google Scholar]
- 3.Muller-Hermelink HK, Engel P, Harris N. Tumours of the thymus. In: Travis W, Brambilla E, Muller-Hermelink H, editors. Tumours of the Lung, Thymus, Heart. Pathology and Genetics. Lyon: IARC Press; 2004. pp. 145–98. [Google Scholar]
- 4.Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2485–92. doi: 10.1002/1097-0142(19811201)48:11<2485::aid-cncr2820481123>3.0.co;2-r. doi:10.1002/1097-0142(19811201)48:11<2485::AID-CNCR2820481123>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 5.Katsahian S, Resche-Rigon M, Chevret S, Porcher R. Analysing multicentre competing risk data with mixed proportional hazards model for the subdistribution. Stat Med. 2006;225:4267–78. doi: 10.1002/sim.2684. doi:10.1002/sim.2684. [DOI] [PubMed] [Google Scholar]
- 6.Royston P. Multiple imputation of missing values: update of ice. Stata J. 2005;5:527–36. [Google Scholar]
- 7.Rea F, Marulli G, Girardi R, Bortolotti L, Favaretto A, Galligioni A, et al. Long-term survival and prognostic factors in thymic epithelial tumours. Eur J Cardiothorac Surg. 2004;26:412–8. doi: 10.1016/j.ejcts.2004.04.041. doi:10.1016/j.ejcts.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 8.Ruffini E, Filosso PL, Mossetti C, Bruna MC, Novero D, Lista P, et al. Thymoma: inter-relationships among World Health Organization histology, Masaoka staging and myasthenia gravis and their independent prognostic significance: a single-centre experience. Eur J Cardiothorac Surg. 2011;40:146–53. doi: 10.1016/j.ejcts.2010.09.042. doi:10.1016/j.ejcts.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 9.Margaritora S, Cesario A, Cusumano G, Meacci E, D'Angelillo R, Bonassi S, et al. Thirty-five-year follow-up analysis of clinical and pathologic outcomes of thymoma surgery. Ann Thorac Surg. 2010;89:245–52. doi: 10.1016/j.athoracsur.2009.08.074. doi:10.1016/j.athoracsur.2009.08.074. [DOI] [PubMed] [Google Scholar]
- 10.Regnard JF, Magdeleinat P, Dromer C, Dulmet E, de Montpreville V, Levi JF, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg. 1996;112:376–84. doi: 10.1016/S0022-5223(96)70265-9. doi:10.1016/S0022-5223(96)70265-9. [DOI] [PubMed] [Google Scholar]
- 11.Venuta F, Rendina EA, Klepetko W, Rocco G. Surgical management of stage III thymic tumors. Thorac Surg Clin. 2011;21:85–91. doi: 10.1016/j.thorsurg.2010.08.006. doi:10.1016/j.thorsurg.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Ruffini E, Mancuso M, Oliaro A, Casadio C, Cavallo A, Cianci R, et al. Recurrence of thymoma: analysis of clinicopathologic features, treatment, and outcome. J Thorac Cardiovasc Surg. 1997;113:55–63. doi: 10.1016/S0022-5223(97)70399-4. doi:10.1016/S0022-5223(97)70399-4. [DOI] [PubMed] [Google Scholar]
- 13.Ruffini E, Filosso PL, Oliaro A. The role of surgery in recurrent thymic tumors. Thorac Surg Clin. 2009;19:121–31. doi: 10.1016/j.thorsurg.2008.09.005. doi:10.1016/j.thorsurg.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Cardillo G, Carleo F, Giunti R, Lopergolo MG, Salvadori L, De Massimi AR, et al. Predictors of survival in patients with locally advanced thymoma and thymic carcinoma (Masaoka stages III and IVa) Eur J Cardiothorac Surg. 2010;37:819–23. doi: 10.1016/j.ejcts.2009.11.001. doi:10.1016/j.ejcts.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Ruffini E, Oliaro A, Novero D, Campisi P, Filosso PL. Neuroendocrine tumors of the thymus. Thorac Surg Clin. 2011;21:13–23. doi: 10.1016/j.thorsurg.2010.08.013. doi:10.1016/j.thorsurg.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Suster S, Rosai J. Thymic carcinoma. A clinicopathologic study of 60 cases. Cancer. 1991;67:1025–32. doi: 10.1002/1097-0142(19910215)67:4<1025::aid-cncr2820670427>3.0.co;2-f. doi:10.1002/1097-0142(19910215)67:4<1025::AID-CNCR2820670427>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 17.Venuta F, Anile M, Diso D, Vitolo D, Rendina EA, De Giacomo T, et al. Thymoma and thymic carcinoma. Eur J Cardiothorac Surg. 2010;37:13–25. doi: 10.1016/j.ejcts.2009.05.038. doi:10.1016/j.ejcts.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 18.Wright CD, Wain JC, Wong DR, Donahue DM, Gaissert HA, Grillo HC, et al. Predictors of recurrence in thymic tumors: importance of invasion, World Health Organization histology, and size. J Thorac Cardiovasc Surg. 2005;130:1413–21. doi: 10.1016/j.jtcvs.2005.07.026. doi:10.1016/j.jtcvs.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 19.Kondo K, Monden Y. Thymoma and Myasthenia Gravis: a clinical study of 1,089 patients from Japan. Ann Thorac Surg. 2005;79:219–24. doi: 10.1016/j.athoracsur.2004.06.090. doi:10.1016/j.athoracsur.2004.06.090. [DOI] [PubMed] [Google Scholar]
- 20.Lucchi M, Ricciardi R, Melfi F, Duranti L, Basolo F, Palmiero G, et al. Association of thymoma and Myasthenia Gravis: oncological and neurological results of the surgical treatment. Eur J Cardiothorac Surg. 2009;35:812–6. doi: 10.1016/j.ejcts.2009.01.014. doi:10.1016/j.ejcts.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Venuta F, Rendina EA, Coloni GF. Multimodality treatment of thymic tumors. Thorac Surg Clin. 2009;19:71–8. doi: 10.1016/j.thorsurg.2008.09.008. doi:10.1016/j.thorsurg.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Lucchi M, Melfi F, Dini P, Basolo F, Viti A, Givigliano F, et al. Neoadjuvant chemotherapy for stage III and IVA thymomas: a single-institution experience with a long follow-up. J Thorac Oncol. 2006;1:308–13. doi:10.1097/01243894-200605000-00007. [PubMed] [Google Scholar]
- 23.Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1320 patients from Japan. Ann Thorac Surg. 2003;76:878–85. doi: 10.1016/s0003-4975(03)00555-1. doi:10.1016/S0003-4975(03)00555-1. [DOI] [PubMed] [Google Scholar]
- 24.Forquer JA, Rong N, Fakiris AJ, Loehrer PJ, Johnstone PAS. Postoperative radiotherapy after surgical resection of thymoma: differing roles in localized and regional disease. Int J Radiat Oncol Biol Phys. 2010;76:440–5. doi: 10.1016/j.ijrobp.2009.02.016. doi:10.1016/j.ijrobp.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Korst RJ, Kansler AL, Christos PJ, Mandal S. Adjuvant radiotherapy for thymic epithelial tumors: a systematic review and meta-analysis. Ann Thorac Surg. 2009;87:1641–7. doi: 10.1016/j.athoracsur.2008.11.022. doi:10.1016/j.athoracsur.2008.11.022. [DOI] [PubMed] [Google Scholar]