We report causes of 149 deaths that occurred among 661 HIV-infected adults with tuberculosis in a trial assessing the optimal timing for antiretroviral therapy (ART) initiation. Timing of ART, early interruption of cotrimoxazole, and tuberculosis-related factors were determinants of mortality.
Keywords: tuberculosis, HIV, mortality, cause of death, adult
Abstract
Background. Shortening the interval between antituberculosis treatment onset and initiation of antiretroviral therapy (ART) reduces mortality in severely immunocompromised human immunodeficiency virus (HIV)–infected patients with tuberculosis. A better understanding of causes and determinants of death may lead to new strategies to further enhance survival.
Methods. We assessed mortality rates, causes of death, and factors of mortality in Cambodian HIV-infected adults with CD4 count ≤200 cells/µL and tuberculosis, randomized to initiate ART either 2 weeks (early ART) or 8 weeks (late ART) after tuberculosis treatment onset in the CAMELIA clinical trial.
Results. Six hundred sixty-one patients enrolled contributed to 1366.1 person-years of follow-up; 149 (22.5%) died. There were 8.3 deaths per 100 person-years (95% confidence interval [CI], 6.4–10.7) in the early-ART group and 13.8 deaths per 100 person-years (95% CI, 11.2–16.9) in the late-ART group (P = .002). Tuberculosis was the primary cause of death (28%), followed by other HIV-associated conditions (19%). Factors independently associated with mortality in the first 26 weeks were the age, body mass index, hemoglobin, interrupted or ineffective tuberculosis treatment before identification of drug resistance, disseminated tuberculosis, and nontuberculous mycobacterial disease. After 50 weeks in the trial, the most frequent causes of death were non-HIV related or tuberculosis related, including drug toxicity; factors associated with mortality were late ART, loss to follow-up, and absence of cotrimoxazole prophylaxis.
Conclusions. Despite ART introduction, mortality remained high, with tuberculosis as the leading cause of death. Reducing tuberculosis-related mortality remains a challenge in resource-limited settings and requires innovative strategies.
Clinical Trials Registration. NCT00226434.
Despite advances in tuberculosis diagnosis and access to treatment for human immunodeficiency virus (HIV) infection, tuberculosis remains a major cause of death in HIV-infected patients [1, 2]. In 2012, it was estimated that 1.0–1.2 million new tuberculosis cases and 0.32 million tuberculosis-related deaths occurred in HIV-infected persons, accounting for 13% of the global tuberculosis burden and 25% of tuberculosis mortality [1]. Several randomized clinical trials showed the positive impact upon mortality of shortening the delay between tuberculosis treatment onset and initiation of antiretroviral therapy (ART) [3–7]. The level of immunosuppression [3, 4] and the location or severity of tuberculosis disease [6] have been reported to impact the survival benefit gained from earlier ART initiation [3–7].
Notably, in general, data are scarce on tuberculosis-related factors associated with mortality in HIV-infected adults treated for tuberculosis. Observational and autopsy studies showed that tuberculosis itself constitutes the principal cause of death [8–10], but other HIV-related opportunistic infections are frequently reported as the etiology of death. Thus, a deeper understanding of the causes and factors associated with death is needed to develop strategies aiming to further reduce mortality in HIV-infected patients treated for tuberculosis.
Here, we assessed mortality rates, causes, and risk factors of death in HIV-infected patients enrolled in the CAMELIA (Cambodian Early vs Late Introduction of Antiretroviral Therapy) randomized clinical trial. We specifically analyzed (1) early deaths before 26 weeks of follow-up (W26), where mortality is thought to be mainly linked to the severity of initial presentation and expected to be related to HIV and tuberculosis; and (2) late deaths after 50 weeks of follow-up (W50), at a time when most patients would be expected to have been cured from their tuberculosis and to have recovered a level of protective immunity in general [11, 12].
METHODS
Study Design and Patients
CAMELIA was a randomized, open-label clinical trial designed to determine whether early initiation of ART 2 weeks after the onset of tuberculosis treatment, as compared with 8 weeks, would reduce mortality in HIV-infected adults with tuberculosis and advanced immunodeficiency. The inclusion procedures and study design have been described elsewhere [5]. In brief, after signed informed consent, treatment-naive adults with CD4 count ≤200 cells/µL and smear-positive tuberculosis were recruited in 5 Cambodian hospitals from January 2006 to May 2009 and randomized to initiate ART with stavudine, lamivudine, and efavirenz either 2 weeks (early ART) or 8 weeks (late ART) after the initiation of tuberculosis treatment. Tuberculosis treatment consisted of a standard daily regimen of isoniazid, rifampin, ethambutol, and pyrazinamide during the first 2 months followed by isoniazid and rifampin during the following 4 months. Mycobacterial cultures were systematically performed and drugs were available at study sites to adapt treatment in the case of drug resistance or nontuberculous mycobacteria. Patients received cotrimoxazole prophylaxis. Fluconazole was also given when the CD4 count was <100 cells/µL.
Patients had follow-up visits at 2, 4, 8, 10, 14, 18, 22, 26, 34, 42, 50, 58, and 78 weeks after tuberculosis treatment onset, and every 6 months thereafter until the end of the study, which was 50 weeks after the last patient was enrolled. ART could be modified in case of toxicities, and systematic switch from stavudine to zidovudine was encouraged after W50 to minimize the risk of mitochondrial toxicities. In accordance with Cambodian national guidelines [13], cotrimoxazole and fluconazole prophylaxes were discontinued once CD4 cell count was maintained at >200 cells/µL and >100 cells/µL, respectively, for >6 months. All treatments were self-administered, and patients received adherence counseling. The trial was approved by the Cambodian National Ethics Committee for Health Research, and the ethical review boards of the Immune Disease Institute at Harvard Medical School and Médecins Sans Frontières.
Definitions and Outcomes
Paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS) was defined as worsening or emergence of signs or symptoms of tuberculosis (eg, fever, cough, shortness of breath, lymph node, or exacerbation of disease at other extrapulmonary sites) occurring after the initiation of ART, excluding differential diagnoses (poor adherence, drug toxicity, and other diseases). Validation procedures have been described elsewhere [14]. For this analysis, we defined inadequate tuberculosis therapy as either interruption of effective antituberculosis drugs for >3 consecutive days, whatever the reason, or ineffective tuberculosis drugs received before the drug susceptibility testing results for >3 consecutive days. Tuberculosis outcome at W50 was defined as (1) cured or treatment completed: patients who completed tuberculosis treatment and had resolution of symptoms with or without negative sputum smear or culture in the last month of treatment, with no subsequent recurrence; (2) ongoing tuberculosis treatment: patients who were still on tuberculosis treatment for any reason (initial treatment interruption or failure, drug resistance, tuberculosis recurrence); or (3) other: patients known to be alive and either not cured or having completed treatment or not receiving ongoing tuberculosis treatment. Loss to follow-up was defined as absence of known vital status at the end of the study. At W50, patients were classified as having interrupted follow-up if they had not attended protocol visits for 12 weeks or more but were known to be alive beyond this point.
Assessment of Causes of Death
Site investigators primarily assessed the cause of death. When death occurred at home, they were requested to perform an interview of family members to attempt to assess the cause of death. Medical records of all patients who died were subsequently reviewed by 2 investigators not involved in day-to-day clinical management to validate the assigned cause of death. Causes of death were classified into one of the following categories, as suggested previously [8]: tuberculosis, HIV-associated conditions (excluding tuberculosis), tuberculosis or HIV-associated conditions equally likely when it could equally be related to both infections, and nontuberculosis/HIV-associated conditions. The latter was divided into drug toxicity and other nontuberculosis/HIV-associated causes of death. TB-IRIS was classified as an HIV-associated condition.
Statistical Methods
Early mortality was defined as deaths occurring before W26; follow-up was thus censored at W26, or at the date of death, date of withdrawal, or date of last visit for the patients lost to follow-up, whichever occurred first. To identify factors associated with early mortality, the Cox proportional hazard model was used. Those factors investigated included known risk factors of HIV and tuberculosis mortality such as CD4 cell count, body mass index (BMI), hemoglobin count, chest radiographic features, and mycobacterial disease pattern at inclusion, and time-dependent variables such as occurrence of TB-IRIS and inadequate tuberculosis therapy.
Late mortality was defined as death occurring after W50; only patients with a follow-up >50 weeks were considered in this analysis. The Cox model was used to identify factors associated with late mortality. Due to informative missing values in several factors investigated, multiple imputations were performed [15]. A sensitivity analysis was performed, considering only patients with all data available at W50.
For all factors included in the Cox models, the proportional hazard assumption was validated using a test on Schoenfeld residuals. All factors associated with the outcome with a P value <.20 in univariate analysis were entered in the multivariate model. A backward selection procedure was applied to identify factors independently and significantly associated with the outcome. A P value <.05 was considered significant. All analyses were performed using the Stata 12 software (StataCorp, College Station, Texas).
RESULTS
Characteristics of Patients
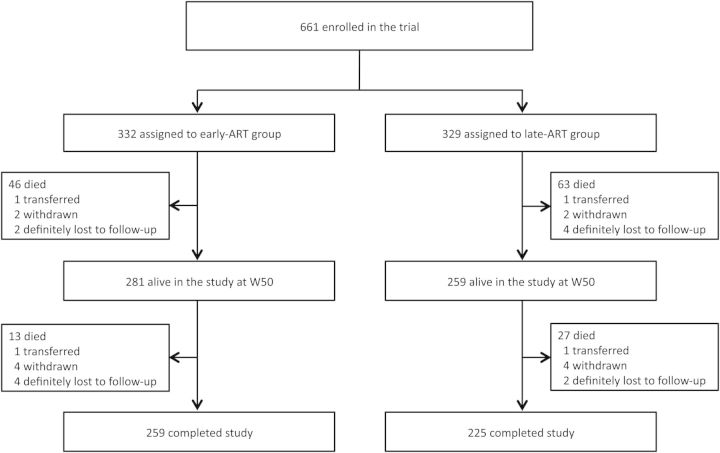
Six hundred sixty-one patients were enrolled in the trial (Figure 1). Their baseline characteristics have been described elsewhere and did not differ between the early-ART and late-ART groups [5]. In summary, patients had advanced immunodeficiency (median CD4 count, 25 cells/µL), low BMI (median, 16.7 kg/m2) (Table 1), and high levels of viremia with median plasma HIV RNA level of 5.64 (interquartile range [IQR], 5.24–6.01) log10 copies/mL. Tuberculosis was culture-confirmed in 577 (87.3%) patients, and 16 (2.4%) patients had nontuberculous mycobacteria disease identified with culture. Patients were followed for a median time of 25 (IQR, 14–36) months with a total of 1366.1 person-years of follow-up accrued during the study.
Figure 1.
Enrollment and follow-up. Abbreviations: ART, antiretroviral therapy; W50, week 50.
Table 1.
Characteristics of Enrolled Patients at 2 Time Points of Specific Interest: Inclusion and After 50 Weeks of Follow-up
| Characteristic | No. (%) of Patients at Inclusion (n = 661) | No. (%) of Patients at Week 50 (n = 540) |
|---|---|---|
| Sex, male | 425 (64.3) | 355 (65.7) |
| Age, y, median (IQR) | 35 (30–41) | 35 (30–41) |
| BMI, kg/m2, median (IQR) | 16.7 (15.2–18.4) | 20.4 (18.8–22.2)a |
| CD4 count, cells/µL | ||
| Median (IQR) | 25 (10–56) | 199 (142–274)b |
| ≤50 | 475 (71.9) | 5 (0.9) |
| 51–200 | 186 (28.1) | 264 (48.9) |
| 201–350 | 0 (0) | 179 (33.1) |
| >350 | 0 (0) | 71 (13.2) |
| Missing | 0 (0) | 21 (3.9) |
| HIV RNA <2.4 log10 copies/mL | 0 (0) | 501 (96.2)c |
| Hemoglobin, g/dL, median (IQR) | 8.7 (7.1–10.3) | 13.4 (12.2–14.5)c |
| Treatment group | ||
| Early ART | 332 (50.2) | 281 (52.0) |
| Late ART | 329 (49.8) | 259 (48.0) |
| Temporarily lost to follow-up at week 50 | NA | 13 (2.4) |
| ART regimen | ||
| Stavudine-lamivudine-efavirenz | 0 (0) | 441 (81.7) |
| Other ART regimens | 0 (0) | 79 (14.6) |
| No ART | 661 (100.0) | 20 (3.7) |
| Cotrimoxazole prophylaxis | 644 (97.4) | 436 (80.7) |
| Fluconazole prophylaxis | 560 (84.7) | 245 (45.4) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable.
a Available for 523 patients.
b Available for 519 patients.
c Available for 518 patients.
At W50, 540 patients were still alive and in the study (Figure 1). Among them, 520 (96.3%) were on ART, 436 (80.7%) and 245 (45.4%) were still receiving cotrimoxazole and fluconazole prophylaxis, respectively, 23 (4.3%) were still on tuberculosis treatment, and 13 (2.4%) had interrupted follow-up (Table 1). Compared to baseline, median weight gain was 9 kg (IQR, 5–12 kg) and median CD4 cell count gain was 164 (IQR, 107–224) cells/µL. Overall, 496 of 513 (96.7%) patients on ART with available measurement had undetectable HIV RNA (<2.4 log10 copies/mL).
Mortality Rates: Sites of Death
One hundred forty-nine patients died during the study: 87 (58.4%) before W26, 22 (14.8%) between W26 and W50, and 40 (26.8%) after W50. Overall, the mortality rate was 10.9 deaths per 100 person-years (95% confidence interval [CI], 9.3–12.8). There were 8.3 deaths per 100 person-years (95% CI, 6.4–10.7) in the early-ART group and 13.8 deaths per 100 person-years (95% CI, 11.2–16.9) in the late-ART group (P = .002). Mortality decreased from 55.9 deaths per 100 person-years (95% CI, 33.1–94.4) in the first 2 weeks to 4.9 deaths per 100 persons-years (95% CI, 3.6–6.7) after W50 (P < .001; Table 2). After W50, mortality remained higher in the late-ART group compared with the early-ART group with rates of 7.0 (95% CI, 4.8–10.3) and 3.0 (95% CI, 1.8–5.2) deaths per 100 person-years, respectively (P = .01). Death occurred during hospitalization in 86 patients (57.7%) and at home in 63 patients (42.3%).
Table 2.
Distribution of Deaths by Period of Time and Treatment Group
| Period | Early ART |
Late ART |
Total |
|||
|---|---|---|---|---|---|---|
| No. | Mortality Rate (95% CI) per 100 PY | No. | Mortality Rate (95% CI) per 100 PY | No. | Mortality Rate (95% CI) per 100 PY | |
| Overall | 59 | 8.3 (6.4–10.7) | 90 | 13.8 (11.2–16.9) | 149 | 10.9 (9.3–12.8) |
| Inclusion–W2 | 7 | 55.6 (26.5–116.7) | 7 | 56.1 (26.8–117.7) | 14 | 55.9 (33.1–94.4) |
| W2–W8 | 10 | 27.3 (14.7–50.8) | 15 | 41.7 (25.1–69.2) | 25 | 34.5 (23.3–51.0) |
| W8–W26 | 20 | 19.4 (12.5–30.1) | 28 | 28.1 (19.4–40.7) | 48 | 23.7 (17.8–13.2) |
| W26–W50 | 9 | 6.8 (3.6–13.1) | 13 | 10.6 (6.2–18.3) | 22 | 8.7 (5.7–3.2) |
| >W50 | 13 | 3.0 (1.8–5.2) | 27 | 7.0 (4.8–10.3) | 40 | 4.9 (3.6–6.7) |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; PY, person-year; W, week.
Causes of Death
Tuberculosis was the most common cause of death (28.2%), followed by HIV-associated conditions (18.8%) (Table 3). There were no significant differences in the distribution of categories of causes of death between the early-ART and late-ART groups (P = .32). Tuberculosis was the most frequent cause of early deaths (37.9%) and also accounted for 17.5% of late deaths. Before W26, the tuberculosis-specific mortality rate was 6.6 (95% CI, 3.5–12.2) deaths per 100 person-years in the early-ART group and 16.2 (95% CI, 10.9–24.2) deaths per 100 person-years in the late-ART group (P = .013). HIV-associated conditions accounted for 18.4% of deaths before W26 and for 7.5% after W50. Six deaths directly attributed to TB-IRIS occurred in the early-ART group. Other non-tuberculosis or HIV-related identified causes of death (gastrointestinal and nontoxic hepatic disorders, neoplasia, suicide, road traffic accident, and stroke) were predominant after W50 (27.5%), followed by drug toxicity (22.5%), including 8 of 9 cases related to lactic acidosis. Individual data for all patients who died after W50 are presented in Supplementary Data.
Table 3.
Causes of Death by Period of Time and Treatment Group
| Cause of Death | Inclusion–W26 |
W26–W50 |
After W50 |
Total (n = 149), No. (%) [Occurring in Hospital] [n = 86], No. | |||
|---|---|---|---|---|---|---|---|
| Early ART (n = 37), | Late ART (n = 50), | Early ART (n = 9), | Late ART (n = 13), | Early ART (n = 13), | Late ART (n = 27), | ||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | ||
| Tuberculosis | 10 (27.0) | 23 (46.0) | 0 (0) | 2 (15.4) | 3 (23.1) | 4 (14.8) | 42 (28.2) [24] |
| Tuberculosis | 10 | 15 | 0 | 1 | 1 | 0 | 27 [14] |
| MDR tuberculosis | 0 | 8 | 0 | 0 | 0 | 1 | 9 [7] |
| Tuberculosis recurrence | 0 | 0 | 0 | 1 | 2 | 3 | 6 [3] |
| HIV-associated conditions | 10 (27.0) | 10 (20.0) | 3 (33.3) | 2 (15.4) | 0 (0) | 3 (11.1) | 28 (18.8) [19] |
| Diarrhea | 1 | 4 | 0 | 1 | 0 | 1 | 7 [4] |
| Nontuberculous mycobacterial disease | 3 | 1 | 1 | 1 | 0 | 0 | 6 [5] |
| TB-IRIS | 4 | 0 | 2 | 0 | 0 | 0 | 6 [4] |
| Progressive multifocal leukoencephalopathy | 1 | 2 | 0 | 0 | 0 | 0 | 3 [2] |
| Cryptococcosis | 1 | 1 | 0 | 0 | 0 | 0 | 2 [2] |
| Encephalitis | 0 | 1 | 0 | 0 | 0 | 1 | 2 [0] |
| Bacterial pneumonia | 0 | 1 | 0 | 0 | 0 | 0 | 1 [1] |
| Pneumocystis jirovecii pneumonia | 0 | 0 | 0 | 0 | 0 | 1 | 1 [1] |
| Tuberculosis or HIV-associated condition equally likely | 7 (18.9) | 7 (14.0) | 3 (33.3) | 2 (15.4) | 3 (23.1) | 1 (3.7) | 23 (15.4) [12] |
| Respiratory distress | 3 | 2 | 1 | 1 | 2 | 1 | 10 [4] |
| Cachexia | 4 | 3 | 0 | 0 | 1 | 0 | 8 [5] |
| Coma | 0 | 2 | 1 | 1 | 0 | 0 | 4 [3] |
| Meningitis | 0 | 0 | 1 | 0 | 0 | 0 | 1 [0] |
| Drug toxicity | 1 (2.7) | 3 (6.0) | 1 (11.1) | 3 (23.1) | 3 (23.1) | 6 (22.2) | 17 (11.4) [15] |
| Lactic acidosis | 0 | 0 | 1 | 1 | 3 | 5 | 10 [8] |
| Hepatotoxicity | 0 | 2 | 0 | 0 | 0 | 0 | 2 [2] |
| Toxic epidermal necrolysis | 0 | 1 | 0 | 1 | 0 | 0 | 2 [2] |
| Drug hypersensitivity | 0 | 0 | 0 | 0 | 0 | 1 | 1 [1] |
| Electrolyte disordersa | 0 | 0 | 0 | 1 | 0 | 0 | 1 [1] |
| Pancytopenia | 1 | 0 | 0 | 0 | 0 | 0 | 1 [1] |
| Other nontuberculosis/HIV-associated conditions | 7 (18.9) | 5 (10.0) | 1 (11.1) | 2 (15.4) | 3 (23.1) | 8 (29.6) | 26 (17.4) [15] |
| Gastrointestinal disorders | 5 | 1 | 0 | 1 | 0 | 2 | 9 [6] |
| Nontoxic hepatic disorder | 1 | 4 | 0 | 0 | 2 | 1 | 8 [7] |
| Neoplasia | 0 | 0 | 0 | 0 | 0 | 3 | 3 [3] |
| Suicide | 1 | 0 | 0 | 1 | 1 | 0 | 3 [1] |
| Road traffic accident | 0 | 0 | 0 | 0 | 0 | 2 | 2 [0] |
| Stroke | 0 | 0 | 1 | 0 | 0 | 0 | 1 [1] |
| Unknown | 2 (5.4) | 2 (4.0) | 1 (1.1) | 2 (15.4) | 1 (7.7) | 5 (18.5) | 13 (8.7) [2] |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; MDR, multidrug resistant; TB-IRIS, paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome; W, week.
a Profound hyponatremia and hypokalemia in the context of unremitting vomiting due to drug intolerance.
Risk Factors of Mortality
Factors independently associated with a higher risk of early death included age ≥40 years, BMI ≤16 kg/m2, hemoglobin <70 g/L, disseminated tuberculosis or nontuberculous mycobacterial disease at enrollment, and inadequate tuberculosis therapy (Table 4).
Table 4.
Risk Factors of Early Deaths (Cox Model)
| Risk Factor | No. of Patients | Deaths, No. (%) | Crude HR on Univariate Analysis (95% CI) | P Value | Adjusted HR on Multivariate Analysis (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Treatment group | .13 | |||||
| Early ART | 332 | 37 (11.1) | 1 | |||
| Late ART | 329 | 50 (15.2) | 1.38 (.91–2.11) | |||
| Sex | .38 | |||||
| Male | 425 | 52 (12.2) | 1 | |||
| Female | 236 | 35 (14.8) | 1.21 (.79–1.86) | |||
| Age at inclusion, y | .04 | .003 | ||||
| ≤29 | 151 | 13 (8.6) | 1 | 1 | ||
| 30–39 | 292 | 36 (12.3) | 1.49 (.79–2.80) | 1.71 (.90–3.24) | ||
| ≥40 | 218 | 38 (17.4) | 2.13 (1.13–3.99) | 2.83 (1.49–5.36) | ||
| BMI at inclusion, kg/m2 | .003 | .02 | ||||
| ≤16.0 | 243 | 47 (19.3) | 2.72 (1.44–5.12) | 2.32 (1.21–4.44) | ||
| 16.1–17.0 | 128 | 14 (10.9) | 1.45 (.67–3.13) | 1.34 (.62–2.92) | ||
| 17.1–18.5 | 135 | 14 (10.4) | 1.36 (.63–2.94) | 1.25 (.57–2.71) | ||
| >18.5 | 155 | 12 (7.7) | 1 | 1 | ||
| CD4 at inclusion, cells/µL | .005 | |||||
| ≤25 | 335 | 58 (17.3) | 3.67 (1.33–10.12) | |||
| 26–50 | 140 | 15 (10.7) | 2.16 (.72–6.51) | |||
| 51–100 | 107 | 10 (9.3) | 1.88 (.59–6.01) | |||
| 101–200 | 79 | 4 (5.1) | 1 | |||
| Hemoglobin at inclusion, g/dL | .001 | .01 | ||||
| ≤7.0 | 165 | 37 (22.4) | 3.45 (1.86–6.38) | 2.48 (1.31–4.72) | ||
| 7.1–10.0 | 301 | 36 (12.0) | 1.72 (.93–3.18) | 1.47 (.79–2.75) | ||
| >10.0 | 195 | 14 (7.2) | 1 | 1 | ||
| Mycobacterial disease pattern | <.001 | <.001 | ||||
| Pulmonary | 442 | 42 (9.5) | 1 | 1 | ||
| Extrapulmonary | 82 | 10 (12.2) | 1.31 (.66–2.62) | 1.21 (.60–2.41) | ||
| Disseminateda | 121 | 30 (24.8) | 2.86 (1.79–4.57) | 2.47 (1.53–4.00) | ||
| Nontuberculous mycobacteria | 16 | 5 (31.2) | 3.93 (1.55–9.93) | 3.46 (1.36–8.86) | ||
| Drug-resistant tuberculosis at inclusion | .23 | |||||
| No | 541 | 69 (12.7) | 1 | |||
| Yes | 107 | 14 (13.1) | 1.01 (.57–1.79) | |||
| Multidrug resistanceb | 13 | 4 (30.8) | 2.77 (1.01–7.59) | |||
| Inadequate tuberculosis therapyc,d | .02 | .03 | ||||
| No | 1 | 1 | ||||
| Yes | 2.25 (1.22–4.17) | 1.96 (1.06–3.63) | ||||
| Chest radiograph at inclusion | .15 | |||||
| Normal | 127 | 18 (14.2) | 1 | |||
| Cavitary pattern | 436 | 58 (13.3) | 0.95 (.56–1.62) | |||
| Abnormal without cavity | 97 | 10 (13.3) | 0.72 (.30–1.57) | |||
| Missing | 1 | 1 (100.0) | 23.10 (3.01–177.54) | |||
| Occurrence of TB-IRISd | .61 | |||||
| No | 1 | |||||
| Yes | 0.84 (.41–1.71) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; HR, hazard ratio; TB-IRIS, paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome.
a Disseminated tuberculosis was defined as pulmonary tuberculosis associated with any type of extrapulmonary tuberculosis.
b Multidrug resistance was defined as resistance to both isoniazid and rifampicin.
c Interruption of effective antituberculosis drugs for >3 consecutive days, whatever the reason, or ineffective tuberculosis drugs received before the drug-susceptibility testing results for >3 consecutive days.
d Time-dependent factor.
Factors assessed at W50 that were independently associated with late death included the late-ART group, interruption in follow-up, and absence of cotrimoxazole prophylaxis (Table 5). When those patients who had interrupted follow-up before W50 were excluded from the analysis, factors independently associated with mortality were the late-ART group (hazard ratio [HR], 2.34; 95% CI, 1.13–4.86), BMI ≤17 kg/m2 (HR, 4.29; 95% CI, 1.53–12.01) and absence of cotrimoxazole prophylaxis (HR, 2.22; 95% CI, 1.01–4.76).
Table 5.
Risk Factors of Late Deaths (Cox Model With Multiple Imputations of Missing Values)
| Risk Factor | Patients, No. | Deaths No. (%) | Crude HR on Univariate Analysis (95% CI) | P Value | Adjusted HR on Multivariate Analysisa (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Treatment group | .01 | .023 | ||||
| Early ART | 281 | 13 (4.6) | 1 | 1 | ||
| Late ART | 259 | 27 (10.4) | 2.31 (1.19–4.48) | 2.28 (1.15–4.50) | ||
| Sex | .28 | |||||
| Male | 355 | 23 (6.5) | 1 | |||
| Female | 185 | 17 (9.2) | 1.41 (.76–2.65) | |||
| Age at inclusion, y | .77 | |||||
| ≤29 | 129 | 11 (8.5) | 1 | |||
| 30–39 | 240 | 16 (6.7) | 0.76 (.35–1.63) | |||
| ≥40 | 171 | 13 (7.6) | 0.88 (.39–1.96) | |||
| BMI at W50b, kg/m2 | .12 | |||||
| ≤17.0 | 30 | 5 (16.7) | 3.46 (1.27–9.40) | |||
| 17.0–18.4 | 76 | 4 (5.3) | 1.00 (.33–3.07) | |||
| 18.5–20.0 | 122 | 9 (7.4) | 1.33 (.58–3.05) | |||
| >20.0 | 295 | 15 (5.1) | 1 | |||
| CD4 at W50b, cells/µL | .31 | |||||
| ≤100 | 49 | 4 (8.2) | 2.16 (.43–10.94) | |||
| 101–200 | 220 | 10 (4.5) | 1.19 (.27–5.29) | |||
| 201–350 | 179 | 17 (9.5) | 2.29 (.58–9.06) | |||
| >350 | 71 | 2 (2.8) | 1 | |||
| Plasma HIV RNA at W50b | .67 | |||||
| Undetectable | 498 | 31 (6.2) | 1 | |||
| ≥2.4 log10 copies/mL | 20 | 2 (10.0) | 1.37 (.33–5.67) | |||
| Hemoglobin at W50b, g/dL | .33 | |||||
| >10.0 | 495 | 30 (6.1) | 1.80 (.55–5.85) | |||
| ≤10.0 | 23 | 3 (13.0) | 1 | |||
| Tuberculosis outcome at W50 | .005 | |||||
| Cured or treatment completed | 512 | 33 (6.4) | 1 | |||
| Ongoing treatment | 19 | 3 (15.8) | 3.03 (.93–9.92) | |||
| Not cured and no treatment | 9 | 4 (44.4) | 7.04 (2.49–19.91) | |||
| Chest radiograph at W50b | .72 | |||||
| Normal | 410 | 23 (5.6) | 1 | |||
| Abnormal | 102 | 8 (7.8) | 1.18 (.46–3.02) | |||
| History of TB-IRIS | .13 | |||||
| No | 405 | 34 (8.4) | 1 | |||
| Yes | 135 | 6 (4.4) | 0.53 (.22–1.27) | |||
| ART at W50 | <.001 | |||||
| Stavudine-lamivudine-efavirenz | 441 | 28 (6.3) | 1 | |||
| Other combination | 79 | 5 (6.3) | 1.00 (.39–2.59) | |||
| None | 20 | 7 (35.0) | 7.22 (3.14–16.58) | |||
| Temporarily lost to follow-up at W50 | <.001 | .01 | ||||
| No | 527 | 33 (6.3) | 1 | 1 | ||
| Yes | 13 | 7 (53.8) | 11.53 (5.09–26.13) | 4.34 (1.53–12.33) | ||
| Fluconazole prophylaxis at W50 | .24 | |||||
| No | 295 | 27 (9.1) | 1.78 (.88–3.59) | |||
| Yes (never interrupted) | 213 | 11 (5.2) | 1 | |||
| Yes (previously interrupted) | 32 | 2 (6.2) | 1.25 (.28–5.62) | |||
| Cotrimoxazole at W50 | .001 | .02 | ||||
| No | 104 | 16 (15.4) | 3.03 (1.61–5.70) | 2.62 (1.19–5.77) | ||
| Yes | 436 | 24 (5.5) | 1 | 1 |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; TB-IRIS, paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome; W50, week 50.
a Multivariate model is stratified on viral load at enrollment for which proportional hazard ratio assumption was not verified.
b Factors with imputed values.
DISCUSSION
This study provides in-depth analysis of mortality in naive HIV-infected adults treated for tuberculosis in the CAMELIA trial. Introduction of ART led to a significant and quick reduction of mortality in adults with tuberculosis and advanced HIV-associated immunodeficiency with early ART initiation, leading to a 34% global reduction in mortality compared with late ART initiation [5]. However, despite prompt tuberculosis treatment initiation and ART initiation, mortality remained high during the first 6 months following tuberculosis diagnosis, and tuberculosis was the leading cause of these deaths. Notably, late mortality among the patients who were still alive at W50 was more than double in those who received late ART or who discontinued cotrimoxazole prophylaxis.
CAMELIA patients presented at a late stage in their HIV disease, with profound immunosuppression, which contributed to the high global mortality initially observed in the study [16, 17]. Mortality before ART initiation was >50 deaths per 100 person-years, consistent with findings from other studies conducted in patients with advanced immunodeficiency [18]. Despite the >10-fold decrease of mortality between enrollment and after W50, and the faster reduction of mortality in patients initiating ART 2 weeks after tuberculosis treatment, mortality remained roughly >20 deaths per 100 patient-years during the first 6 months on ART. Surprisingly, CD4 cell count was not associated with mortality in multivariate analysis. In a study with 72% of patients presenting with a CD4 count <50 cells/µL, mortality may have been mainly driven by poor general condition, reflected by low BMI and anemia, both contributing to increased risk of early death. Notably, global mortality remained relatively high after W50, reaching 4.9 deaths per 100 person-years as compared with 1.0 per 100 person-years in a cohort of Thai patients started on ART at higher CD4 cell counts [19].
Tuberculosis was the main cause of death here, consistent with data from another Southeast Asian cohort study of severely immunocompromised patients that showed that 27% of deaths occurring over 6 months were tuberculosis-related, with an additional 23% potentially related to tuberculosis-associated conditions [8]. Another randomized clinical trial attributed 32% of deaths as being related to tuberculosis over 1 year of follow-up [4]. In our study, although most tuberculosis-associated deaths occurred before week 26, tuberculosis also accounted for 17.5% of the late deaths, mostly due to tuberculosis recurrence. This confirmed the high mortality rates observed in HIV-infected patients with recurrent tuberculosis [20].
Tuberculosis-specific factors that contributed to increased mortality were related to both interruption or inadequacy of treatment and the pattern of disease presentation. Inadequate therapy due to use of a standard tuberculosis treatment in patients who were later found to have drug-resistant tuberculosis or treatment interruption was associated with a 2-fold increased risk of death. Study arm–independent drug-induced hepatitis and rashes were frequent in the study and contributed to treatment interruptions. Furthermore, disseminated tuberculosis, known to be associated with increased mortality [21–23], was associated with a 2-fold increased risk of death. However, extrapulmonary tuberculosis alone or noncavitary or normal chest radiographs were not associated with higher mortality [22].
Residual mortality due to tuberculosis could be reduced by faster access to appropriate treatment through faster diagnosis. Early detection of multidrug resistant tuberculosis is now possible through automated nucleic acid amplification tests (NAATs) [24] or other molecular assays [25] that were not available during the CAMELIA trial. Rapid diagnosis of disseminated tuberculosis by detection of bacteremia on whole-blood NAATs [26], however, may have limited impact, as mortality remains high with the existing standard tuberculosis regimens [23]. Therefore, intensification of tuberculosis treatment with higher doses of rifampicin [27] or innovative regimens [28] should be considered, especially for patients who exhibit severe immunosuppression.
In our study, TB-IRIS accounted for 6 deaths, exclusively in the early-ART group. As expected, early ART initiation led to a higher incidence of TB-IRIS in these severely immunocompromised patients, especially those presenting with disseminated or extrapulmonary tuberculosis [14]. However, the global mortality was lower in this study arm, and the occurrence of TB-IRIS events was not associated with overall mortality.
The survival advantage associated with early ART continued to increase after W50, long after the study intervention. Such a finding was unexpected in a population of patients with a high tuberculosis cure rate, and similarly favorable immunovirological outcomes in both study groups, after roughly 1 year on ART. This difference in mortality raises the possibility that the study intervention led to differences in functional immune recovery with equal absolute lymphocyte counts (Haridas V, Pean P, Jasenosky LD, et al, manuscript in preparation).
Lactic acidosis was an important cause of death after W50. Surprisingly, the occurrence of symptomatic hyperlactatemia on stavudine-containing ART, which was standard of care in Cambodia and used in the trial, was high in CAMELIA [5], as compared with rates observed previously [29, 30]. This high incidence was unexpected in patients with low BMIs [31], which in turn may have contributed to the poor outcomes of lactic acidosis in the trial [32]. One explanation could be the rapid weight gain that was observed during the first year on ART.
Of importance, the interruption of cotrimoxazole prophylaxis before W50 was associated with an approximate 3-fold increase in the risk of death. Most patients with data available at W50 who died later had CD4 counts >200 cells/µL. The use of cotrimoxazole prophylaxis impacts overall mortality, probably due to its role in the prevention of invasive bacterial disease [33, 34], an effect observed up to 72 weeks after ART initiation, even in patients with a CD4 count >200 cells/µL [35]. Despite the small number of obvious infectious causes of death after W50 in the trial, this suggests that the optimal time for discontinuation of cotrimoxazole prophylaxis should be reconsidered in HIV-infected patients with tuberculosis [36, 37].
A major limitation of our study is the lack of autopsies since discrepancies between clinical assessment of cause of death and pathological findings have been reported to be frequent [9]. However, this may have led us to underestimate tuberculosis or other opportunistic infections as causes of death. In CAMELIA, both a limited access to sophisticated diagnostic procedures and a high proportion of deaths occurring at home complicated the assessment of cause of death. Another potential limitation of our study is the severely immunosuppressed nature of the population included in CAMELIA, which may restrict the generalization of our results to all HIV-infected patients with tuberculosis in different settings.
Three trials have gathered strong evidence in favor of early introduction of ART after tuberculosis treatment onset in severely immunocompromised patients [3–5]. Of these, CAMELIA has the longest duration of follow-up. This enabled us to show that the benefit of early ART initiation was prolonged. To improve further long-term outcomes, we propose that the question of the appropriate time for cotrimoxazole prophylaxis interruption should be reconsidered. Tuberculosis played a major role in mortality in this patient series, especially during the first 6 months following diagnosis. Thus, optimized case management should include early ART initiation and opportunistic infection prophylaxis, appropriate management of TB-IRIS and toxicities, and support of adherence to avoid treatment interruption, but also possibly intensification of tuberculosis initial treatment. Together with intensified clinical and microbiological case finding strategies, such innovative approaches may help reduce mortality linked to tuberculosis and HIV.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We are indebted to all patients who participated in the CAMELIA trial. We thank the Cambodian National HIV/AIDS Program, the Cambodian National Tuberculosis Program, Médecins Sans Frontières Belgium, the Institut Pasteur du Cambodge, the Cambodian Health Committee, and the health workers of the 5 hospitals where the CAMELIA trial was conducted (Khmer-Soviet Friendship Hospital, Svay Rieng Provincial Hospital, Calmette Hospital, Takeo Provincial Hospital, and Siem Reap Provincial Hospital), and the Organisation Franco-Cambodgienne de Pneumologie for helpful advice and discussions.
Financial support. This work was supported by the French National Agency for Research on AIDS and Viral Hepatitis (ANRS 1295) and the US National Institutes of Health (NIH), Division of AIDS (CIPRA KH001/DAID-ES ID 10425).
Potential conflicts of interest. O. M. has received grants and support for travel to meetings for the study from NIH and ANRS and received grants from the ANRS for other studies outside of the submitted work. L. B. has received grants from the NIH and ANRS for the submitted work. A. E. G. has received support for travel to meetings for the study from the NIH. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Global tuberculosis report 2013. Available at: http://www.who.int/tb/publications/global_report/en/ Accessed 1 April 2014.
- 2.Straetemans M, Bierrenbach AL, Nagelkerke N, Glaziou P, van der Werf MJ. The effect of tuberculosis on mortality in HIV positive people: a meta-analysis. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365:1492–501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365:1482–91. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanc FX, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365:1471–81. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Török ME, Yen NTB, Chau TTH, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)-associated tuberculous meningitis. Clin Infect Dis. 2011;52:1374–83. doi: 10.1093/cid/cir230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manosuthi W, Mankatitham W, Lueangniyomkul A, et al. Time to initiate antiretroviral therapy between 4 weeks and 12 weeks of tuberculosis treatment in HIV-infected patients: results from the TIME study. J Acquir Immune Defic Syndr. 2012;60:377–83. doi: 10.1097/QAI.0b013e31825b5e06. [DOI] [PubMed] [Google Scholar]
- 8.Cain KP, Anekthananon T, Burapat C, et al. Causes of death in HIV-infected persons who have tuberculosis, Thailand. Emerging Infect Dis. 2009;15:258–64. doi: 10.3201/eid1502.080942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox JA, Lukande RL, Nelson AM, et al. An autopsy study describing causes of death and comparing clinico-pathological findings among hospitalized patients in Kampala, Uganda. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg AE, Lucas S, Tossou O, et al. Autopsy-proven causes of death in HIV-infected patients treated for tuberculosis in Abidjan, Côte d'Ivoire. AIDS. 1995;9:1251–4. doi: 10.1097/00002030-199511000-00006. [DOI] [PubMed] [Google Scholar]
- 11.van Griensven J, Thai S. Predictors of immune recovery and the association with late mortality while on antiretroviral treatment in Cambodia. Trans R Soc Trop Med Hyg. 2011;105:694–703. doi: 10.1016/j.trstmh.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Swaminathan S, Padmapriyadarsini C, Venkatesan P, et al. Efficacy and safety of once-daily nevirapine- or efavirenz-based antiretroviral therapy in HIV-associated tuberculosis: a randomized clinical trial. Clin Infect Dis. 2011;53:716–24. doi: 10.1093/cid/cir447. [DOI] [PubMed] [Google Scholar]
- 13.National Center for HIV/AIDS Dermatology and STD Cambodia. National guidelines for the prophylaxis of opportunistic infections in people living with HIV/AIDS. Phnom Penh, Cambodia: Ministry of Health. 2003. Available at: http://www.nchads.org/Guideline/OI%20Guideline%20Booklet%20Kh.pdf. Accessed 1 April 2014.
- 14.Laureillard D, Marcy O, Madec Y, et al. Paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome after early initiation of antiretroviral therapy in a randomized clinical trial. AIDS. 2013;27:2577–86. doi: 10.1097/01.aids.0000432456.14099.c7. [DOI] [PubMed] [Google Scholar]
- 15.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–42. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 16.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stringer JSA, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 18.Anglaret X, Minga A, Gabillard D, et al. AIDS and non-AIDS morbidity and mortality across the spectrum of CD4 cell counts in HIV-infected adults before starting antiretroviral therapy in Cote d'Ivoire. Clin Infect Dis. 2012;54:714–23. doi: 10.1093/cid/cir898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fregonese F, Collins IJ, Jourdain G, et al. Predictors of 5-year mortality in HIV-infected adults starting highly active antiretroviral therapy in Thailand. J Acquir Immune Defic Syndr. 2012;60:91–8. doi: 10.1097/QAI.0b013e31824bd33f. [DOI] [PubMed] [Google Scholar]
- 20.McGreevy J, Jean Juste MA, Severe P, et al. Outcomes of HIV-infected patients treated for recurrent tuberculosis with the standard retreatment regimen. Int J Tuberc Lung Dis. 2012;16:841–5. doi: 10.5588/ijtld.11.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nahid P, Jarlsberg LG, Rudoy I, et al. Factors associated with mortality in patients with drug-susceptible pulmonary tuberculosis. BMC Infect Dis. 2011;11:1. doi: 10.1186/1471-2334-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Català L, Orcau A, García de Olalla P, Millet JP, Rodríguez-Mondragón A, Caylà JA. Survival of a large cohort of HIV-infected tuberculosis patients in the era of highly active antiretroviral treatment. Int J Tuberc Lung Dis. 2011;15:263–9. i. [PubMed] [Google Scholar]
- 23.Crump JA, Ramadhani HO, Morrissey AB, et al. Bacteremic disseminated tuberculosis in sub-Saharan Africa: a prospective cohort study. Clin Infect Dis. 2012;55:242–50. doi: 10.1093/cid/cis409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tukvadze N, Kempker RR, Kalandadze I, et al. Use of a molecular diagnostic test in AFB smear positive tuberculosis suspects greatly reduces time to detection of multidrug resistant tuberculosis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feasey NA, Banada PP, Howson W, et al. Evaluation of Xpert MTB/RIF for detection of tuberculosis from blood samples of HIV-infected adults confirms Mycobacterium tuberculosis bacteremia as an indicator of poor prognosis. J Clin Microbiol. 2013;51:2311–6. doi: 10.1128/JCM.00330-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steingart KR, Jotblad S, Robsky K, et al. Higher-dose rifampin for the treatment of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis. 2011;15:305–16. [PubMed] [Google Scholar]
- 28.Ruslami R, Ganiem AR, Dian S, et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis. 2013;13:27–35. doi: 10.1016/S1473-3099(12)70264-5. [DOI] [PubMed] [Google Scholar]
- 29.Westreich DJ, Sanne I, Maskew M, et al. Tuberculosis treatment and risk of stavudine substitution in first-line antiretroviral therapy. Clin Infect Dis. 2009;48:1617–23. doi: 10.1086/598977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phan V, Thai S, Choun K, Lynen L, van Griensven J. Incidence of treatment-limiting toxicity with stavudine-based antiretroviral therapy in Cambodia: a retrospective cohort study. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osler M, Stead D, Rebe K, Meintjes G, Boulle A. Risk factors for and clinical characteristics of severe hyperlactataemia in patients receiving antiretroviral therapy: a case-control study. HIV Med. 2010;11:121–9. doi: 10.1111/j.1468-1293.2009.00754.x. [DOI] [PubMed] [Google Scholar]
- 32.Manosuthi W, Prasithsirikul W, Chumpathat N, et al. Risk factors for mortality in symptomatic hyperlactatemia among HIV-infected patients receiving antiretroviral therapy in a resource-limited setting. Int J Infect Dis. 2008;12:582–6. doi: 10.1016/j.ijid.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Anglaret X, Chêne G, Attia A, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Côte d'Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet. 1999;353:1463–8. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 34.Lim P-L, Zhou J, Ditangco RA, et al. Failure to prescribe pneumocystis prophylaxis is associated with increased mortality, even in the cART era: results from the Treat Asia HIV observational database. J Int AIDS Soc. 2012;15:1. doi: 10.1186/1758-2652-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker AS, Ford D, Gilks CF, et al. Daily co-trimoxazole prophylaxis in severely immunosuppressed HIV-infected adults in Africa started on combination antiretroviral therapy: an observational analysis of the DART cohort. Lancet. 2010;375:1278–86. doi: 10.1016/S0140-6736(10)60057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anglaret X, Eholie S. Co-trimoxazole, cART, and non-AIDS infectious diseases. Lancet. 2010;375:1231–3. doi: 10.1016/S0140-6736(10)60200-0. [DOI] [PubMed] [Google Scholar]
- 37.Kumarasamy N, Vallabhaneni S, Cecelia AJ, et al. Safe discontinuation of primary pneumocystis prophylaxis in Southern Indian HIV-infected patients on highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2005;40:377–8. doi: 10.1097/01.qai.0000176591.06549.de. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.