Treatment of uncomplicated malaria with artemether-lumefantrine (AL) or dihydroartemisinin-piperaquine in HIV-infected children on antiretroviral therapy (ART) was safe and efficacious. However there was a high risk of recurrent parasitemia within 28 days following treatment with AL, varying with ART regimen.
Keywords: ACTs, malaria, HIV, children, ART
Abstract
Background. Artemisinin-based combination therapies (ACTs) are highly efficacious and safe, but data from human immunodeficiency virus (HIV)–infected children concurrently receiving antiretroviral therapy (ART) and ACTs are limited.
Methods. We evaluated 28-day outcomes following malaria treatment with artemether-lumefantrine (AL) or dihydroartemisinin-piperaquine (DP) in 2 cohorts of HIV-infected Ugandan children taking various ART regimens. In one cohort, children <6 years of age were randomized to lopinavir/ritonavir (LPV/r) or nonnucleoside reverse transcriptase inhibitor–based ART and treated with AL for uncomplicated malaria. In another cohort, children <12 months of age were started on nevirapine-based ART if they were eligible, and randomized to AL or DP for the treatment of their first and all subsequent uncomplicated malaria episodes.
Results. There were 773 and 165 treatments for malaria with AL and DP, respectively. Initial response to therapy was excellent, with 99% clearance of parasites and <1% risk of repeat therapy within 3 days. Recurrent parasitemia within 28 days was common following AL treatment. The risk of recurrent parasitemia was significantly lower among children taking LPV/r-based ART compared with children taking nevirapine-based ART following AL treatment (15.3% vs 35.5%, P = .009), and those treated with DP compared with AL (8.6% vs 36.2%, P < .001). Both ACT regimens were safe and well tolerated.
Conclusions. Treatment of uncomplicated malaria with AL or DP was efficacious and safe in HIV-infected children taking ART. However, there was a high risk of recurrent parasitemia following AL treatment, which was significantly lower in children taking LPV/r-based ART compared with nevirapine-based ART.
The majority of malaria-endemic countries now recommend artemisinin-based combination therapies (ACTs) for the treatment for uncomplicated falciparum malaria [1]. In sub-Saharan Africa, ACTs selected as first-line regimens include artemether-lumefantrine (AL) and artesunate-amodiaquine (AS-AQ). Dihydroartemisinin-piperaquine (DP), another ACT, was recently added to the World Health Organization (WHO) list of recommended drugs for the treatment of uncomplicated falciparum malaria and has been adopted as a second-line regimen in some African countries [2]. Numerous studies continue to demonstrate excellent efficacy and safety of AL, AS-AQ, and DP in Africa [3–11].
Although there is compelling evidence to support the use of ACTs for malaria treatment in the general population, data evaluating their efficacy and safety in human immunodeficiency virus (HIV)–infected populations are limited. Some studies have shown equivalent antimalarial efficacy in HIV-infected and -uninfected persons [12–14], but others have reported lower efficacy in HIV-infected populations [15–17]. These studies were generally limited to adult populations that received non-ACT antimalarial treatment regimens that are no longer recommended, and did not account for the increasing use of daily trimethoprim-sulfamethoxazole prophylaxis or antiretroviral therapy (ART) among HIV-infected persons in Africa. Moreover, data evaluating the relative safety of ACTs in HIV-infected individuals are even more limited. There is emerging evidence of potential adverse and beneficial pharmacokinetic interactions between various ART regimens and ACTs [18–21].
Currently, WHO guidelines state that there is insufficient information to modify the general malaria treatment recommendations for HIV-infected patients, with the exception that those on zidovudine or efavirenz (EFV) should avoid AQ-containing regimens if possible [22]. The new WHO consolidated HIV treatment guidelines now recommend ART for all children <5 years of age [23]. Thus, it is critical to understand efficacy and safety of ACTs among HIV-infected children.
We evaluated efficacy and safety data of ACTs from 2 longitudinal clinic trials in cohorts of children living in a highly malaria-endemic area of eastern Uganda. In one trial (PROMOTE), HIV-infected children were randomized to either nonnucleoside reverse transcriptase inhibitor (NNRTI)–based or protease inhibitor–based ART, and all episodes of uncomplicated malaria were treated with AL. In the other trial (Tororo Child Cohort [TCC]), HIV-infected children were started on nevirapine (NVP)–based ART if they were eligible and randomized to either AL or DP for the treatment of their first and all subsequent episodes of uncomplicated malaria.
METHODS
Study Setting and Participants
Both studies were conducted in Tororo district, Uganda, a largely rural area with high malaria transmission intensity and an estimated entomological inoculation rate of 591 infective bites per person-year [24]. Details of both studies have been published previously [21, 25–30]. In brief, the PROMOTE study (ClinicalTrials.gov, NCT00978068) included 184 HIV-infected children aged 2 months to <6 years enrolled between October 2009 and October 2011 who were either ART naive and eligible for ART or already receiving ART with viral suppression.
Part of the PROMOTE cohort results have been published elsewhere [21]. In this article, however, we had additional follow-up data of 287 uncomplicated malaria episodes. In addition, we made comparisons of risk of recurrent parasitemia using NVP-based ART group as a baseline, in contrast to the earlier published data where the combined NNRTI-based ART group was used as a baseline. Furthermore, adverse events included in this current data were only assessed for during malaria follow-up as opposed to the entire study duration in the earlier published data.
The TCC study included 48 HIV-infected children aged 6 weeks to 12 months enrolled between August 2007 and April 2008 who were ART naive and an additional 9 children who were HIV exposed (HIV-uninfected children born to HIV-infected mothers) and seroconverted during follow-up.
Study Participant Follow-up
Participants in both studies were given a long-lasting insecticide-treated bed net and trimethoprim-sulfamethoxazole prophylaxis at enrollment and were followed up for all their medical care at a dedicated study clinic open 7 days a week. Parents/guardians were encouraged to bring their children to the clinic whenever they were sick, and after-hours care was provided at Tororo District Hospital. Children who presented with new medical problems underwent standardized medical evaluation. Medications with antimalarial activity were avoided for the treatment of nonmalarial illnesses when possible.
In the PROMOTE study, participants were randomized at enrollment to receive either a protease inhibitor (lopinavir/ritonavir [LPV/r]) plus 2 nucleoside reverse transcriptase inhibitors (NRTIs) or an NNRTI (NVP for children <3 years of age; EFV for children ≥3 years) plus 2 NRTIs. For NRTIs, children received lamivudine plus zidovudine, with stavudine or abacavir replacing zidovudine for children with anemia. In the TCC study, all participants were ART naive at enrollment and those meeting standard WHO criteria during follow-up received NVP plus 2 NRTIs using the same approach as the PROMOTE study.
Malaria Diagnosis, Treatment, and Follow-up
Children who presented with a documented fever (tympanic temperature ≥38.0°C) or history of fever in the previous 24 hours had blood obtained by finger-prick for a thick blood smear. If the smear was positive, the patient was diagnosed with malaria. In the PROMOTE study, patients with uncomplicated malaria were treated with AL. In the TCC study, patients were randomized to receive AL or DP at the time of their first episode of uncomplicated malaria, and they then received the same treatment for all subsequent episodes. In both studies, antimalarials were administered by a study nurse according to weight-based guidelines. Artemether-lumefantrine (tablets of 20 mg of artemether and 120 mg of lumefantrine; Coartem, Novartis), was administered as 1 (5–14 kg) or 2 (15–24 kg) tablets given twice daily for 3 days. Dihydroartemisinin-piperaquine (tablets of 40 mg of dihydroartemisinin and 320 mg of piperaquine; Duocotecxin, Holley Pharm) was given as a total dose of 6.4 mg/kg and 51.2 mg/kg of dihydroartemisinin and piperaquine, respectively, given in 3 equally divided daily doses to the nearest quarter-tablet. Patients were given a glass of milk or asked to breastfeed after each dose of antimalarial medication and observed for 30 minutes with the dose repeated if vomiting occurred. For patients treated with AL, the second daily dose was given at home by the parent or guardian. Patients with complicated malaria were treated with quinine. Patients were instructed to return for follow-up evaluation on days 1, 2, 3, 7, 14, 21, and 28. In the PROMOTE study, complete blood count and alanine aminotransferase (ALT) level were assessed on days 0 and 28. In the TCC study, hemoglobin measurements were assessed on days 0 and 28. Standardized treatment outcomes after 28 days were classified according to WHO guidelines [31].
Laboratory Methods
Thick and thin Giemsa-stained blood smears were used for estimating parasite density and to determine the parasite species, respectively. Parasite densities were calculated as the number of asexual parasites per 200 leukocytes (or per 500 leukocytes, if the count was <10 asexual parasites/200 leukocytes), assuming a leukocyte count of 8000/μL. A blood smear was considered negative when the examination of 100 high-power fields did not reveal asexual parasites. For quality control, all slides were read by a second reader, and a third reader settled any discrepancies.
Statistical Methods
Data were double entered into Access databases and analyzed using Stata version 12 (Stata Corp, College Station, Texas). Risk of recurrent parasitemia within 28 days of initiation of therapy was estimated using the Kaplan-Meier product limit formula, and comparisons were made using the Cox proportional hazards model, controlling for age and with adjustment for repeated measures in the same patient. Associations between ART regimen and the risk of adverse events within 28 days of initiation of therapy with AL from the PROMOTE study were estimated using generalized estimating equations controlling for age and with adjustment for repeated measures in the same patient using exchangeable correlation and robust standard errors. P < .05 was considered statistically significant.
Ethical Approval
In both studies, informed consent was obtained from at least 1 parent or legal guardian of each participant at enrollment. Both studies were approved by the Uganda National Council of Science and Technology, the Makerere University School of Medicine Research Ethics Committee, and the University of California, San Francisco Committee for Human Research. The TCC study was also approved by the institutional review boards of the University of Washington and the Centers for Disease Control and Prevention.
RESULTS
Study Profiles and Characteristics of Study Participants
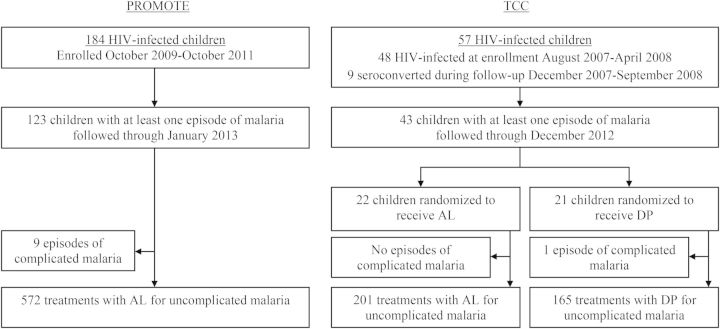
A total of 184 HIV-infected children were enrolled in the PROMOTE study, of whom 123 (66.8%) were diagnosed with at least 1 episode of malaria. A total of 57 HIV-infected children were included in the TCC study, of whom 43 (75.4%) were diagnosed with at least 1 episode of malaria (Figure 1). Characteristics of study participants at enrollment and during follow-up for both studies are presented in Table 1. Compared to those in the TCC study, participants in the PROMOTE study were older at enrollment (mean age, 3.4 vs 0.6 years, P < .001). In the PROMOTE study, a total of 581 treatments were given for malaria, of which 572 were uncomplicated episodes treated with AL and 9 were complicated episodes treated with quinine (6 episodes with danger signs and 3 episodes with severe anemia). In the TCC study, a total of 367 treatments were given for malaria, of which 366 were uncomplicated episodes (201 treated with AL, 165 treated with DP) and 1 was a complicated episode treated with quinine (danger signs without criteria for severe malaria). There were no deaths due to malaria in either study.
Figure 1.
Study profile. Abbreviations: AL, artemether-lumefantrine; DP, dihydroartemisinin-piperaquine; HIV, human immunodeficiency virus; TCC, Tororo Child Cohort.
Table 1.
Characteristics of Study Participants
| Characteristic | PROMOTE (n = 184) | TCC (n = 57) |
|---|---|---|
| At the beginning of the observation period | ||
| Age, y, mean (SD) | 3.4 (1.3) | 0.6 (0.3) |
| Female sex, No. (%) | 92 (49.5) | 28 (49.1) |
| ART naive, No. (%) | 129 (70.1) | 57 (100) |
| WHO stage, No. (%) | ||
| I | 135 (73.4) | 43 (75.4) |
| II | 35 (19.0) | 7 (12.3) |
| III | 3 (1.6) | 7 (12.3) |
| IV | 11 (6.0) | 0 |
| CD4 percentage, median (range) | ||
| ART naive | 16 (2–44) | 21 (2–61) |
| ART experienced | 30 (8–51) | NA |
| Viral load, log10 copies/mL, median (range) | ||
| ART naive | 5.4 (2.6–6.4) | 5.9 (2.8–7.0) |
| ART experienced | All undetectable | NA |
| During follow-up | ||
| Duration of follow-up, y, median (IQR) | 2.1 (1.9–2.7) | 2.2 (1.8–4.3) |
| On ARVs, No. (%) | 184 (100) | 52 (91) |
| Total No. of treatments for malaria | 581 | 367 |
| Malaria treatments per child, median (range) | 1 (0–26) | 2 (0–45) |
| Incidence of malaria per person-year | 1.45 | 2.31 |
Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral; IQR, interquartile range; NA, not applicable; SD, standard deviation; TCC, Tororo Child Cohort; WHO, World Health Organization.
Characteristics of Malaria Episodes Treated With ACTs
In both studies, >93% of episodes of uncomplicated malaria were due to Plasmodium falciparum. Compared with the TCC study, episodes of malaria in the PROMOTE study occurred in older children (mean age, 4.4 vs 2.6 years; P < .001) and with lower geometric mean parasite densities (7792 vs 12 144 asexual parasites/µL; P = .005). All of the episodes in the PROMOTE study occurred in children taking ART, with 43.5%, 38.6%, and 17.8% on LPV/r, NVP, or EFV-based regimen, respectively. In the TCC study, 94.5% of episodes occurred in children taking ART, with all receiving NVP-based regimens (Table 2).
Table 2.
Baseline Characteristics of Individual Malaria Episodes Treated With Artemisinin-Based Combination Therapies
| Characteristic | PROMOTE |
TCC |
|
|---|---|---|---|
| AL (n = 572) | AL (n = 201) | DP (n = 165) | |
| Age, y, mean (SD) | 4.4 (1.6) | 2.5 (1.2) | 2.6 (1.2) |
| Plasmodium species, No. (%) | |||
| P. falciparum | 545 (95.3) | 187 (93.0) | 155 (93.9) |
| P. malariae | 13 (2.3) | 9 (4.5) | 4 (2.4) |
| P. ovale | 13 (2.3) | 5 (2.5) | 6 (3.6) |
| P. vivax | 1 (0.2) | 0 | 0 |
| Temperature °C, mean (SD) | 37.8 (1.0) | 38.1 (1.1) | 38.0 (1.1) |
| Geometric mean parasite density/µL | 7792 | 12 629 | 11 580 |
| Gametocytes present, No. (%) | 71 (12.4) | 9 (4.5) | 15 (9.1) |
| Hemoglobin g/dL, mean (SD) | 10.5 (1.3) | 10.1 (1.4) | 9.6 (1.5) |
| Concomitant ART use, No. (%) | |||
| None | 0 | 12 (6.0) | 8 (4.9) |
| NVP + ZDV + 3TC | 169 (29.6) | 138 (68.7) | 142 (86.1) |
| NVP + d4T + 3TC | 25 (4.4) | 31 (15.4) | 6 (3.6) |
| NVP + ABC + 3TC | 27 (4.7) | 20 (10.0) | 9 (5.5) |
| EFV + ZDV + 3TC | 93 (16.3) | 0 | 0 |
| EFV + ABC + 3TC | 9 (1.6) | 0 | 0 |
| LPV/r + ZDV + 3TC | 242 (42.3) | 0 | 0 |
| LPV/r + ABC + 3TC | 2 (0.4) | 0 | 0 |
| LPV/r + ABC + DDI | 2 (0.4) | 0 | 0 |
| LPV/r + d4T + 3TC | 3 (0.5) | 0 | 0 |
Abbreviations: 3TC, lamivudine; ABC, abacavir; AL, artemether-lumefantrine; ART, antiretroviral therapy; d4T, stavudine; DDI, didanosine; DP,dihydroartemisinin-piperaquine; EFV, efavirenz; LPV/r, lopinavir/ritonavir; NVP, nevirapine; SD, standard deviation; TCC, Tororo Child Cohort; ZDV, zidovudine.
Efficacy Outcomes Following Treatment With ACTs
Combining results for the PROMOTE and TCC studies, initial response to therapy was excellent, with >97% of patients with fever clearance and 99% with parasite clearance by day 3 after initiation of therapy (Table 3). Only 4 early treatment failures were seen during the first 3 days of follow-up (all in the PROMOTE study): 2 patients developed danger signs, 1 patient had a low parasite density of 320 asexual parasites/µL on day 0 that increased to 640 on day 2, and 1 patient had a very high parasite density of 419 360 asexual parasites/µL on day 0 with persistent fever and parasitemia on day 3.
Table 3.
Efficacy Outcomes Following Treatment With Artemisinin-Based Combination Therapies
| Efficacy Outcomes | PROMOTE |
TCC |
|
|---|---|---|---|
| AL (n = 572) | AL (n = 201) | DP (n = 165) | |
| Fever clearancea, No. (%) | |||
| Fever on day 1 | 200 (35.2) | 106 (53.0) | 46 (40.6) |
| Fever on day 2 | 39 (7.0) | 16 (8.0) | 7 (4.2) |
| Fever on day 3 | 17 (3.1) | 3 (1.5) | 5 (3.0) |
| Parasite clearance, No. (%) | |||
| Parasitemia on day 2 | 31 (5.6) | 32 (16.1) | 5 (3.0) |
| Parasitemia on day 3 | 5 (0.9) | 3 (1.5) | 1 (0.6) |
| WHO 28-day outcome, No. (%) | |||
| Lost to follow-up | 4 (0.7) | 2 (1.0) | 2 (1.2) |
| Early treatment failure | 4 (0.7) | 0 | 0 |
| Late clinical failure | 42 (7.3) | 19 (9.5) | 3 (1.8) |
| Late parasitological failure | 123 (21.5) | 53 (26.4) | 11 (6.7) |
| Adequate clinical and parasitological response | 399 (69.8) | 127 (63.2) | 149 (90.3) |
| Gametocytes detected during 28-day follow-up, No. (%) | 97 (17.0) | 15 (7.5) | 30 (18.2) |
| Hemoglobin recoveryb, g/dL, mean (SD) | 0.6 (1.2) | 0.6 (1.5) | 1.0 (1.4) |
Abbreviations: AL, artemether-lumefantrine; DP, dihydroartemisinin-piperaquine; SD, standard deviation; TCC, Tororo Child Cohort; WHO, World Health Organization.
a Subjective fever over previous 24 hours or temperature ≥38.0°C.
b Change in hemoglobin from day 0 to day 28 or day of clinical failure.
In the PROMOTE study, the cumulative risk of recurrent parasitemia after 28 days of follow-up was 29.7% among children treated with AL. In the TCC study, the cumulative risk of recurrent parasitemia was significantly lower among children randomized to DP compared with AL (8.6% vs 36.2%; P < .001). In the PROMOTE study, the choice of ART regimen was associated with the risk of recurrent parasitemia after 28 days of follow-up among children treated with AL (Table 4). Compared with children taking an NVP-based regimen, those taking a LPV/r-based regimen had a significantly lower risk of recurrent parasitemia (15.3% vs 35.5%; P = .009). Children taking an EFV-based regimen had a trend toward a high risk of recurrent parasitemia compared with those taking NVP-based regimen (52.5% vs 35.5%; P = .06).
Table 4.
Variables Associated With Time to Recurrent Parasitemia Within 28 Days Following Therapy
| Category | Group | PROMOTE |
TCC |
||||
|---|---|---|---|---|---|---|---|
| Risk of Failure | HR (95% CI)a | P Value | Risk of Failure | HR (95% CI)a | P Value | ||
| Antimalarial therapy | AL | NA | 36.2% | 1.0 (reference) | … | ||
| DP | NA | 8.6% | 0.20 (.10–.42) | <.001 | |||
| ART regimen | NVP-based | 35.5% | 1.0 (reference) | … | 24.9% | 1.0 (reference) | … |
| EFV-based | 52.5% | 1.76 (.99–3.13) | .06 | NA | |||
| LPV/r-based | 15.3% | 0.39 (.19–.79) | .009 | NA | |||
| None | NA | 5.0% | 0.16 (.02–1.21) | .08 | |||
Abbreviations: AL, artemether-lumefantrine; ART, antiretroviral therapy; CI, confidence interval; DP, dihydroartemisinin-piperaquine; EFV, efavirenz; HR, hazard ratio; LPV/r, lopinavir/ritonavir; NA, not applicable; NVP, nevirapine; TCC, Tororo Child Cohort.
a Controlling for age and repeated measures in the same study participant.
Safety and Tolerability Following Treatment With ACTs
Overall, ACTs were safe and well tolerated in both studies (Table 5). Most adverse events were mild to moderate and either unrelated to study drugs (cough) or commonly associated with malaria (diarrhea, anorexia, vomiting, and malaise). In the TCC study, there were no grade 3–4 adverse events and there was no statistically significant difference in the risk of clinical adverse events between patients treated with AL and DP (Table 5). In the PROMOTE study, among 572 treatments with AL there were 59 grade 3–4 adverse events; all were laboratory abnormalities (52 neutropenia, 3 anemia, 2 thrombocytopenia, and 2 elevated ALT level).
Table 5.
Safety Outcomes Over 28 Days Following Treatment With Artemisinin-Based Combination Therapies
| Selected Adverse Events of Any Severity | PROMOTE |
TCC |
|
|---|---|---|---|
| AL (n = 572) | AL (n = 201) | DP (n = 165) | |
| Cough | 247 (43.2%) | 74 (36.8%) | 64 (38.8%) |
| Diarrhea | 50 (8.7%) | 23 (11.4%) | 27 (16.3%) |
| Anorexia | 45 (7.9%) | 4 (2.0%) | 6 (3.6%) |
| Vomiting | 39 (6.8%) | 18 (9.0%) | 8 (4.9%) |
| Malaise | 35 (6.1%) | 2 (1.0%) | 2 (1.2%) |
| Neutropenia | 156/515 (30.3%) | NA | NA |
| Anemia | 29/522 (5.6%) | NA | NA |
| Thrombocytopenia | 22/522 (4.2%) | NA | NA |
| Elevated alanine aminotransferase | 32/488 (6.6%) | NA | NA |
Data are presented as No. (%).
Abbreviations: AL, artemether-lumefantrine; DP, dihydroartemisinin-piperaquine; NA, not applicable; TCC, Tororo Child Cohort.
In the PROMOTE study, the risk of most adverse events did not differ significantly between children receiving different ART regimens (Table 6). However, EFV-based ART was associated with a significantly reduced risk of neutropenia compared with NVP-based ART (risk ratio [RR], 0.55; 95% confidence interval [CI], .32–.94; P = .03). In addition, those receiving LPV/r-based ART had an 84% reduction in the risk of elevated ALT after malaria treatment compared with those receiving NVP-based ART (RR, 0.16; 95% CI, .06–.43; P < .001).
Table 6.
Associations Between Antiretroviral Therapy Regimen and Risk of Adverse Events Following Treatment With Artemether-Lumefantrine in the PROMOTE Study
| Selected Adverse Events of Any Severity | NVP-Based ART |
EFV-Based ART |
LPV/r-Based ART |
|||
|---|---|---|---|---|---|---|
| RR (95% CI)a | P Value | RR (95% CI)a | P Value | RR (95% CI)a | P Value | |
| Cough | 1.0 (reference) | … | 1.13 (.81–1.59) | .48 | 1.03 (.78–1.35) | .85 |
| Diarrhea | 1.0 (reference) | … | 1.03 (.42–2.53) | .95 | 1.03 (.53–1.99) | .94 |
| Anorexia | 1.0 (reference) | … | 1.20 (.47–3.07) | .70 | 1.50 (.87–2.59) | .14 |
| Vomiting | 1.0 (reference) | … | 0.92 (.38–2.18) | .84 | 0.42 (.15–1.14) | .09 |
| Malaise | 1.0 (reference) | … | 1.69 (.77–3.71) | .19 | 1.00 (.43–2.32) | .99 |
| Neutropenia | 1.0 (reference) | … | 0.55 (.32–.94) | .03 | 0.83 (.60–1.16) | .29 |
| Anemia | 1.0 (reference) | … | 1.88 (.63–5.63) | .26 | 1.24 (.54–2.87) | .61 |
| Thrombocytopenia | 1.0 (reference) | … | 1.98 (.52–7.50) | .32 | 0.40 (.13–1.18) | .10 |
| Elevated ALT | 1.0 (reference) | … | 0.34 (.09–1.36) | .13 | 0.16 (.06–.43) | <.001 |
Abbreviations: ALT, alanine aminotransferase; ART, antiretroviral therapy; CI, confidence interval; EFV, efavirenz; LPV/r, lopinavir/ritonavir; NVP, nevirapine; RR, risk ratio.
a Controlling for age and repeated measures in the same study participant.
DISCUSSION
Treatment of uncomplicated malaria with AL and DP was efficacious and safe in HIV-infected children taking LPV/r or NVP or EFV-based ART. Overall, there was >99% parasite clearance by day 3 in children treated for malaria. In a new era of HIV treatment where nearly all children diagnosed with HIV receive ART, our results add assurance that ACTs can be used to treat uncomplicated falciparum malaria.
In this study that we conducted in a high malaria transmission setting, recurrent parasitemia during 28 days of follow-up was common. Treatment with AL was associated with a higher risk of recurrent parasitemia within 28 days compared to treatment with DP in children taking NVP-based ART. A higher risk of recurrent parasitemia following treatment with AL compared to DP has been reported in several studies of HIV-uninfected children, and has been attributed to the shorter posttreatment prophylactic effect of lumefantrine compared with piperaquine [5, 6, 25, 32, 33]. The lower rates of recurrent parasitemia could favor the use of DP to treat malaria among HIV-infected children in high transmission settings. Studies of drug interactions between LPV/r and DP have not yet been reported. Piperaquine is thought to be metabolized by the CYP3A4 enzyme [34]. Ritonavir would be expected to prolong the exposure to piperaquine, causing a favorable effect in reducing recurrence from malaria reinfection. However, prolonged exposure of malaria parasites to piperaquine monotherapy may also lead to an increase in the selection of resistant parasites. Drug interaction studies between LPV/r and DP would help inform the use of this combination, as access to DP becomes more widespread.
Artemether-lumefantrine remains a much more commonly available ACT for malaria treatment than DP. We earlier reported a reduced risk of recurrent malaria following treatment with AL in children taking LPV/r-based ART compared with NNRTI-based ART [21]. This was explained by interactions between AL and LPV/r that resulted in significantly increased day 7 lumefantrine levels in the LPV/r-based ART group. Ritonavir, a component of LPV/r, is an inhibitor of cytochrome P450 3A4 [35, 36], and has been shown to increase lumefantrine in healthy adult volunteers [20]. NNRTIs are inducers of this enzyme [37–39], and we saw a trend toward increased risk of recurrent parasitema in the EFV-based ART arm compared with the NVP-based ART arm, consistent with higher day 7 lumefantrine levels for children who were on NVP-based compared to EFV-based ART [21]. Of the NNRTIs, EFV remains preferred over NVP for treatment of HIV in children 3 years or older [23]; however, its negative interaction with AL could limit its use in malaria-endemic settings where AL is the first-line treatment for uncomplicated malaria.
Both AL and DP were earlier shown to be safe in HIV-infected and -uninfected children [28], and we saw a few grade 3 or 4 adverse events. Considering laboratory adverse events among children randomized to different ART regimens, LPV/r-based ART was associated with a lower risk of elevated ALT and EFV-based ART was associated with a lower risk of neutropenia, compared with NVP-based ART. Nevirapine is known to cause liver toxicity [40]; we saw 32 adverse events due to elevated ALT levels in NVP-treated children, but only 2 were of grade 3–4 severity.
Our study had some limitations. Genotyping was not done for all episodes of recurrent parasitemia, limiting our ability to differentiate new infections from treatment failure due to recrudescence. However, genotyping of the first 107 episodes of recurrent parasitemia in the PROMOTE cohort demonstrated that all recurrent infections were new, suggesting that recrudescence after therapy was very uncommon [21]. In the PROMOTE cohort, children who were randomized to NNRTI-based ART received EFV-based ART if they were 3 years or older and NVP-based ART if they were <3 years of age. This could have confounded our findings, although age was controlled for in the analysis. Finally, laboratory adverse events were not assessed in the TCC cohort.
In summary, both AL and DP were safe and efficacious among HIV-infected children on LPV/r-based or NVP- or EFV-based ART. In high transmission settings where AL is used as first-line treatment, LPV/r-based ART has a significant advantage over NVP or EFV, being associated with a lower risk of recurrent parasitemia. DP used to treat children receiving an NVP-based ART regimen was associated with the lowest rates of recurrent parasitemia, due to the longer posttreatment prophylactic effect of DP compared with AL. Drug interaction studies between DP and ART will help inform its optimal use in HIV-infected patients.
Notes
Acknowledgments. We are grateful to all the parents and guardians for kindly giving their consent and to the study participants for their cooperation. We also thank all members of the TCC and PROMOTE study teams for their tireless effort and excellent work.
Financial support. The PROMOTE study was supported by the National Institutes of Health (P01 HD059454). The study drug, lopinavir/ritonavir, was donated by Abbott Laboratories. The TCC study was supported by the US President's Emergency Plan for AIDS Relief; Centers for Disease Control and Prevention (cooperative agreement U62P024421); National Center for HIV, Viral Hepatitis, STD, and TB Prevention; Global AIDS Program; and Doris Duke Charitable Foundation (G. D. is a recipient of the Clinical Scientist Development Award).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Country antimalarial drug policies: by region. Available at: http://www.who.int/malaria/am_drug_policies_by_region_afro/en/index.html. Accessed 8 April 2013.
- 2.World Health Organization. World malaria report 2009. Available at: http://www.who.int/malaria/publications/atoz/9789241563901/en/ Accessed 9 April 2013.
- 3.Sagara I, Fofana B, Gaudart J, et al. Repeated artemisinin-based combination therapies in a malaria hyperendemic area of Mali: efficacy, safety, and public health impact. Am J Trop Med Hyg. 2012;87:50–6. doi: 10.4269/ajtmh.2012.11-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abuaku B, Duah N, Quaye L, Quashie N, Koram K. Therapeutic efficacy of artemether-lumefantrine combination in the treatment of uncomplicated malaria among children under five years of age in three ecological zones in Ghana. Malar J. 2012;11:388. doi: 10.1186/1475-2875-11-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nambozi M, Van Geertruyden J-P, Hachizovu S, et al. Safety and efficacy of dihydroartemisinin-piperaquine versus artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Zambian children. Malar J. 2011;10:50. doi: 10.1186/1475-2875-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yavo W, Faye B, Kuete T, et al. Multicentric assessment of the efficacy and tolerability of dihydroartemisinin-piperaquine compared to artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in sub-Saharan Africa. Malar J. 2011;10:198. doi: 10.1186/1475-2875-10-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ndiaye J-LA, Faye B, Gueye A, et al. Repeated treatment of recurrent uncomplicated Plasmodium falciparum malaria in Senegal with fixed-dose artesunate plus amodiaquine versus fixed-dose artemether plus lumefantrine: a randomized, open-label trial. Malar J. 2011;10:237. doi: 10.1186/1475-2875-10-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ngasala BE, Malmberg M, Carlsson AM, et al. Efficacy and effectiveness of artemether-lumefantrine after initial and repeated treatment in children <5 years of age with acute uncomplicated Plasmodium falciparum malaria in rural Tanzania: a randomized trial. Clin Infect Dis. 2011;52:873–82. doi: 10.1093/cid/cir066. [DOI] [PubMed] [Google Scholar]
- 9.Gargano N, Ubben D, Tommasini S, et al. Therapeutic efficacy and safety of dihydroartemisinin-piperaquine versus artesunate-mefloquine in uncomplicated Plasmodium falciparum malaria in India. Malar J. 2012;11:233. doi: 10.1186/1475-2875-11-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charle P, Berzosa P, Lucio A de, Raso J, Nchama GN, Benito A. Artesunate/amodiaquine malaria treatment for Equatorial Guinea (Central Africa) Am J Trop Med Hyg. 2013;88:1087–92. doi: 10.4269/ajtmh.12-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anvikar AR, Sharma B, Shahi BH, et al. Artesunate-amodiaquine fixed dose combination for the treatment of Plasmodium falciparum malaria in India. Malar J. 2012;11:97. doi: 10.1186/1475-2875-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasasira AF, Kamya MR, Achan J, et al. High risk of neutropenia in HIV-infected children following treatment with artesunate plus amodiaquine for uncomplicated malaria in Uganda. Clin Infect Dis. 2008;46:985–91. doi: 10.1086/529192. [DOI] [PubMed] [Google Scholar]
- 13.Byakika-Kibwika P, Ddumba E, Kamya M. Effect of HIV-1 infection on malaria treatment outcome in Ugandan patients. Afr Health Sci. 2007;7:86–92. doi: 10.5555/afhs.2007.7.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamya MR, Gasasira AF, Yeka A, et al. Effect of HIV-1 infection on antimalarial treatment outcomes in Uganda: a population-based study. J Infect Dis. 2006;193:9–15. doi: 10.1086/498577. [DOI] [PubMed] [Google Scholar]
- 15.Geertruyden J-PV, Mulenga M, Mwananyanda L, et al. HIV-1 immune suppression and antimalarial treatment outcome in Zambian adults with uncomplicated malaria. J Infect Dis. 2006;194:917–25. doi: 10.1086/507310. [DOI] [PubMed] [Google Scholar]
- 16.Kamya MR, Kigonya CN, McFarland W. HIV infection may adversely affect clinical response to chloroquine therapy for uncomplicated malaria in children. AIDS. 2001;15:1187–8. doi: 10.1097/00002030-200106150-00019. [DOI] [PubMed] [Google Scholar]
- 17.Birku Y, Mekonnen E, Björkman A, Wolday D. Delayed clearance of Plasmodium falciparum in patients with human immunodeficiency virus co-infection treated with artemisinin. Ethiop Med J. 2002;40(suppl 1):17–26. [PubMed] [Google Scholar]
- 18.Huang L, Parikh S, Rosenthal PJ, et al. Concomitant efavirenz reduces pharmacokinetic exposure to the antimalarial drug artemether-lumefantrine in healthy volunteers. J Acquir Immune Defic Syndr. 2012;61:310–6. doi: 10.1097/QAI.0b013e31826ebb5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byakika-Kibwika P, Lamorde M, Mayito J, et al. Significant pharmacokinetic interactions between artemether/lumefantrine and efavirenz or nevirapine in HIV-infected Ugandan adults. J Antimicrob Chemother. 2012;67:2213–21. doi: 10.1093/jac/dks207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.German P, Parikh S, Lawrence J, et al. Lopinavir/ritonavir affects pharmacokinetic exposure of artemether/lumefantrine in HIV-uninfected healthy volunteers. J Acquir Immune Defic Syndr. 2009;51:424–9. doi: 10.1097/QAI.0b013e3181acb4ff. [DOI] [PubMed] [Google Scholar]
- 21.Achan J, Kakuru A, Ikilezi G, et al. Antiretroviral agents and prevention of malaria in HIV-infected Ugandan children. N Engl J Med. 2012;367:2110–8. doi: 10.1056/NEJMoa1200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Guidelines for the treatment of malaria. 2nd ed. Available at: http://www.who.int/malaria/publications/atoz/9789241547925/en/index.html. Accessed 10 May 2013. [Google Scholar]
- 23.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Available at: http://www.who.int/hiv/pub/guidelines/arv2013/en/ Accessed 19 December 2013.
- 24.Okello PE, Bortel WV, Byaruhanga AM, et al. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–25. [PubMed] [Google Scholar]
- 25.Arinaitwe E, Sandison TG, Wanzira H, et al. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for falciparum malaria: a longitudinal, randomized trial in young Ugandan children. Clin Infect Dis. 2009;49:1629–37. doi: 10.1086/647946. [DOI] [PubMed] [Google Scholar]
- 26.Ikilezi G, Achan J, Kakuru A, et al. Prevalence of asymptomatic parasitemia and gametocytemia among HIV-infected Ugandan children randomized to receive different antiretroviral therapies. Am J Trop Med Hyg. 2013;88:744–6. doi: 10.4269/ajtmh.12-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandison TG, Homsy J, Arinaitwe E, et al. Protective efficacy of co-trimoxazole prophylaxis against malaria in HIV exposed children in rural Uganda: a randomised clinical trial. BMJ. 2011;342 doi: 10.1136/bmj.d1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katrak S, Gasasira A, Arinaitwe E, et al. Safety and tolerability of artemether-lumefantrine versus dihydroartemisinin-piperaquine for malaria in young HIV-infected and uninfected children. Malar J. 2009;8:272. doi: 10.1186/1475-2875-8-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arinaitwe E, Gasasira A, Verret W, et al. The association between malnutrition and the incidence of malaria among young HIV-infected and -uninfected Ugandan children: a prospective study. Malar J. 2012;11:90. doi: 10.1186/1475-2875-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kakuru A, Jagannathan P, Arinaitwe E, et al. The effects of ACT treatment and TS prophylaxis on Plasmodium falciparum gametocytemia in a cohort of young Ugandan children. Am J Trop Med Hyg. 2013;88:736–43. doi: 10.4269/ajtmh.12-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. Available at: http://www.who.int/malaria/publications/atoz/whohtmrbm200350/en/ Accessed 21 May 2013.
- 32.Bassat Q, Mulenga M, Tinto H, et al. Dihydroartemisinin-piperaquine and artemether-lumefantrine for treating uncomplicated malaria in African children: a randomised, non-inferiority trial. PLoS One. 2009;4:e7871. doi: 10.1371/journal.pone.0007871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zwang J, Ashley EA, Karema C, et al. Safety and efficacy of dihydroartemisinin-piperaquine in falciparum malaria: a prospective multi-centre individual patient data analysis. PLoS One. 2009;4:e6358. doi: 10.1371/journal.pone.0006358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee TM-N, Huang L, Johnson MK, et al. In vitro metabolism of piperaquine is primarily mediated by CYP3A4. Xenobiotica. 2012;42:1088–95. doi: 10.3109/00498254.2012.693972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou S-F, Xue CC, Yu X-Q, Li C, Wang G. Clinically important drug interactions potentially involving mechanism-based inhibition of cytochrome P450 3A4 and the role of therapeutic drug monitoring. Ther Drug Monit. 2007;29:687–710. doi: 10.1097/FTD.0b013e31815c16f5. [DOI] [PubMed] [Google Scholar]
- 36.Zhou S, Yung Chan S, Cher Goh B, et al. Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs. Clin Pharmacokinet. 2005;44:279–304. doi: 10.2165/00003088-200544030-00005. [DOI] [PubMed] [Google Scholar]
- 37.Josephson F, Bertilsson L, Böttiger Y, et al. CYP3A induction and inhibition by different antiretroviral regimens reflected by changes in plasma 4beta-hydroxycholesterol levels. Eur J Clin Pharmacol. 2008;64:775–81. doi: 10.1007/s00228-008-0492-8. [DOI] [PubMed] [Google Scholar]
- 38.Fellay J, Marzolini C, Decosterd L, et al. Variations of CYP3A activity induced by antiretroviral treatment in HIV-1 infected patients. Eur J Clin Pharmacol. 2005;60:865–73. doi: 10.1007/s00228-004-0855-8. [DOI] [PubMed] [Google Scholar]
- 39.Ma Q, Okusanya OO, Smith PF, et al. Pharmacokinetic drug interactions with non-nucleoside reverse transcriptase inhibitors. Expert Opin Drug Metab Toxicol. 2005;1:473–85. doi: 10.1517/17425255.1.3.473. [DOI] [PubMed] [Google Scholar]
- 40.Shubber Z, Calmy A, Andrieux-Meyer I, et al. Adverse events associated with nevirapine and efavirenz-based first-line antiretroviral therapy: a systematic review and meta-analysis. AIDS. 2013;27:1403–12. doi: 10.1097/QAD.0b013e32835f1db0. [DOI] [PubMed] [Google Scholar]