One year after illness, children with severe malarial anemia demonstrated significantly lower scores in cognitive ability than community children. Severe malarial anemia may contribute to long-term cognitive impairment in children in sub-Saharan Africa.
Keywords: severe malarial anemia, cerebral malaria, cognitive, impairment
Abstract
Background. Cerebral malaria (CM) is associated with long-term neurocognitive impairment in children ≥5 years of age. No prospective studies to date have assessed neurocognitive impairment in children with CM <5 years of age, or in children with severe malarial anemia (SMA), a form of severe malaria estimated to affect as many as 5 million children annually.
Methods. Children <5 years of age presenting to Mulago Hospital, Kampala, Uganda, with CM (n = 80) or SMA (n = 86) were assessed for overall cognitive ability, attention, and associative memory 1 week after discharge and 6 and 12 months later. The z scores for each domain were computed based on scores of 61 healthy community children (CC), who were also tested at enrollment and 6 and 12 months later. Groups were compared using mixed linear models, adjusted for age, weight for age, and child's education.
Results. At 12 months, children with CM had lower adjusted scores than CC in cognitive ability (P < .001), attention (P = .02), and associative memory, (P = .002). Children with SMA had lower scores than CC in cognitive ability (P = .01) but not attention or associative memory. Cognitive ability scores in children with CM and SMA did not differ significantly.
Conclusions. In children <5 years of age, SMA is associated with long-term impairment in cognitive ability, whereas CM is associated with additional impairment in the areas of attention and associative memory. SMA may be a major contributor to long-term neurocognitive impairment in children in sub-Saharan Africa.
Severe malarial anemia (SMA) and cerebral malaria (CM) are common complications of Plasmodium falciparum malaria. Prospective studies in Ugandan children 5–12 years of age with CM found significant cognitive impairment 2 years after the episode [1, 2]. Retrospective studies suggest that this impairment may last 8 years or longer [3, 4]. The primary areas affected are attention, memory, speech and language, visual spatial ability and executive function [4]. Seizures, hypoglycemia, prolonged coma, and elevated cerebrospinal fluid tumor necrosis factor levels at the time of the CM episode all seem to contribute to the long-term impairment [5]. Emerging evidence suggests that the spectrum of malaria infection, from asymptomatic to severe disease, affects cognitive functions [6–10]. Given the effect on cognition of nonsevere forms of malaria and the association of anemia with impaired cognition [7, 9, 11], SMA, which affects as many as 5 million children annually [12], may also affect cognition. SMA is more common than CM and could significantly hinder children from achieving their full cognitive potential.
SMA and CM both occur most frequently in sub-Saharan African children <5 years of age, but no study to date has prospectively examined neurocognitive outcomes in children with CM <5 years of age or in children of any age with SMA. Critical events in brain growth, including synaptogenesis and myelination, occur between birth and age 5 years [13, 14]. Depending on the location and severity, injuries in this critical period could result in either more severe neurocognitive impairment than injuries sustained at older ages or better recovery due to neural plasticity in the younger age group [15]. In the present study, we prospectively evaluated the risk of neurocognitive impairment in children <5 years old with SMA or CM in the year after their discharge from the hospital.
METHODS
Study Participants
The study was performed at Mulago Hospital, Kampala, Uganda. Children with CM or SMA or community children (CC) were enrolled if they were between 18 months and 5 years of age. CM was defined as (1) coma (Blantyre Coma Scale [BCS] score ≤2), (2) P. falciparum on blood smear, and (3) no other known cause of coma (eg, meningitis, a prolonged postictal state, or hypoglycemia-associated coma reversed by glucose infusion). SMA was defined as the presence of P. falciparum on blood smear in children with a hemoglobin level ≤5 g/dL. Children with CM or SMA were managed according to the Ugandan Ministry of Health treatment guidelines current at the time of the study. These included intravenous quinine treatment followed by oral quinine for severe malaria during hospital admission and artemisinin combination therapy for outpatient follow-up therapy. All children with a hemoglobin levels <5 g/dL (all with SMA, 31 with CM) received a blood transfusion.
The CC were recruited from the nuclear family, extended family, or household compound area of children with CM or SMA. Eligible CC were aged 18 months to 5 years and currently healthy. Parents of children with CM or SMA were given information about the study, asked whether any eligible children were present in their extended family, and requested to bring the eligible children to the center for evaluation. Parents of children in the household compound of a child with CM or SMA were also notified about the study during a home visit. Children were enrolled if they met inclusion criteria and had no exclusion criteria. Exclusion criteria for all children included (1) known chronic illness requiring medical care; (2) known developmental delay; or (3) history of coma, head trauma, hospitalization for malnutrition, or cerebral palsy. Additional exclusion criteria for children with SMA included (1) impaired consciousness at physical examination, (2) other clinical evidence of central nervous system disease, or (3) >1 seizure before admission. Additional exclusion criteria for CC included (1) illness requiring medical care within the previous 4 weeks or (2) major medical or neurologic abnormalities at screening physical examination.
Written informed consent was obtained from parents or guardians of study participants. Ethical approval was granted by the institutional review boards for human studies at Makerere University School of Medicine, University of Minnesota, and Michigan State University.
Clinical and Demographic Assessment
All children underwent a medical history and physical examination. Children with CM were assessed for malaria retinopathy [16] by means of indirect ophthalmoscopy. Nutrition was assessed by height- and weight-for-age z scores (Epi Info version 3.5.3; Centers for Disease Control and Prevention). Emotional stimulation in the home was measured by age-appropriate versions of the Home Observation for the Measurement of the Environment [17]. Socioeconomic status was measured using a scoring system described elsewhere, in which lower scores have been associated with cognitive functioning in healthy Ugandan children ≥5 years old [18].
Cognitive Assessment
Children were tested either a week after discharge (CM or SMA group) or at enrollment (CC) and then 6 and 12 months after enrollment. The Mullen Scales of Early Learning [19] were used to measure cognitive ability. Scores from fine motor, visual reception, receptive language, and expressive language scales were summed to give the early learning composite score, a measure of overall cognitive ability. Associative memory was assessed using the Color Object Association Test [20], in which children are required to associate toys with specific color-coded boxes and scored on the total number of toys placed in the correct boxes. Attention was assessed using the Early Childhood Vigilance Test [21], in which a child was required to focus his or her gaze on cartoons screened on a computer for about 7 minutes; the measure of attention is the percentage of time the child spends gazing at the screen. Neuropsychology testers were blinded to the study group (CM, SMA, or CC) of the child being tested. Pilot testing in healthy Ugandan children showed a broad range of test scores and high reliability for these tests in the present study context. The few other neurocognitive tests previously used in young African children had limitations that made them less suitable for use in the present study (eg, the Bayley Scales of Development is for children <42 months old [22] and the Malawi Developmental Assessment Test [23] does not assess attention and memory, which are affected in CM [1]). The Mullen scales and Color Object Association Test have been successfully used to evaluate interventions to improve cognition in Ugandan children exposed to human immunodeficiency virus (HIV) [24] and to evaluate the effect of maternal health and caregiving on children's cognition [25].
Statistical Analyses
Demographic characteristics were compared using t tests for continuous measures, Pearson χ2 test for categorical variables, or Fisher exact test for binary variables. Age-adjusted z scores for cognitive outcomes were created using the scores of the CC [1, 2]. For each outcome, the z score was computed as (actual score − mean score for child's age)/standard deviation (SD), where the mean score for age and SD were computed by fitting a mixed linear model to data from all available visits for CC (allowing correlated errors for a child's multiple visits); z scores have a mean of 0 and SD 1 in the reference population (CC) over all time points. Groups were then compared according to z scores using all 3 testing times and analyzed using a mixed linear model, which generalizes repeated-measures analysis (again allowing correlated errors for a child's multiple visits). Because CC differed from children with CM or SMA in age, weight-for-age and height-for-age z scores, and child educational level, neurocognitive z scores between groups were compared with adjustment for these characteristics. Sample sizes of 60 CC and 80 children in each of the CM and SMA groups were estimated a priori to give >80% power (α = .05) to detect a 0.5 difference in z scores between each disease group and CC.
For the CM and SMA groups separately, z scores for each cognitive domain were tested for association with clinical features using Pearson correlation for continuous variables and 2-sample t tests for binary variables. To account for multiple testing, differences were considered statistically significant for these tests at P < .01 . Mixed linear models were estimated using the SAS MIXED procedure (version 9.3; SAS Institute) with default settings. Adjusted averages are SAS least-squares means. Other analyses were performed with JMP software (JMP Pro 10.0; SAS Institute).
RESULTS
Clinical and Demographic Characteristics of Study Participants
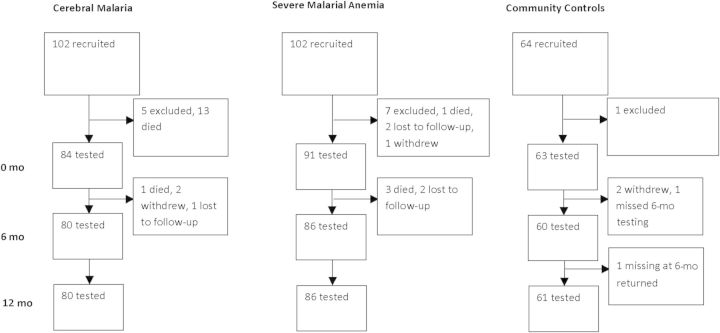
From November 2008 to January 2012, a total of 102 children with CM, 102 with SMA, and 64 CC were recruited. As shown in Figure 1, 13 children met exclusion criteria, 14 died during the initial admission, 4 died during follow-up, 5 were withdrawn from the study, and 5 were lost to follow-up. Eighty children with CM, 86 with SMA, and 61 CC underwent neurocognitive testing at baseline and at 6 and 12 months. Of 681 possible child visits, data were missing for 33 for attention, 14 for associative memory, and 5 for overall cognitive ability. Forty-three children (54%) with CM had retinopathy, and 37 (46%) did not.
Figure 1.
Study design and follow-up.
Demographic, socioeconomic, clinical, and laboratory findings in the study children are summarized in Table 1. Children with SMA were younger than CC and had lower weight-for-age z scores than CC. Compared with the CC, fewer children with CM or SMA had attended preschool. The 3 groups were otherwise similar (Table 1). Hemoglobin SS (1 in CM and 4 in SMA group), HIV infection (2 in CM and 1 in SMA group), and stool helminth infection (Table 1) were uncommon. Mean hemoglobin levels ( ± SD) were lower in children with SMA (3.78 ± 0.85 g/dL) than in those with CM (5.99 ± 2.06 g/dL; P < .001).
Table 1.
Demographic and Clinical Characteristics of Study Children
| Characteristic | CM (n = 80) | SMA (n = 86) | CC (n = 61) | P Valuea |
|---|---|---|---|---|
| Age, mean ± SD, y | 2.8 ± 0.6 | 2.5 ± 0.6 | 2.9 ± 0.7 | <.001b |
| Female sex, No. (%) | 29 (36) | 36 (42) | 27 (44) | .60 |
| Weight for age z score, mean ± SD | −1.2 ± 1.2 | −1.7 ± 1.7 | −0.7 ± 1.3 | <.001c |
| Height for age z score, mean ± SD | −0.5 ± 1.3 | −1.2 ± 1.4 | −1.0 ± 1.4 | .011d |
| Socioeconomic status score, mean ± SD | 8.4 ± 2.5 | 9.3 ± 3.4 | 9.5 ± 3.2 | .06 |
| Home environment z score, mean ± SD | −0.1 ± 1.0 | −0.1 ± 0.8 | 0.0 ± 1.0 | .73 |
| Maternal educational level, No. (%) | ||||
| Primary 6 or lower | 37 (46) | 31 (36) | 24 (39) | .86 |
| Primary 7 | 16 (20) | 17 (20) | 12 (20) | |
| Secondary or higher | 25 (31) | 34 (40) | 22 (36) | |
| Not known | 2 (2) | 4 (5) | 3 (5) | |
| Paternal educational level, No. (%) | ||||
| Primary 6 or lower | 15 (19) | 25 (29) | 9 (15) | .37 |
| Primary 7 | 15 (19) | 7 (8) | 12 (20) | |
| Secondary or higher | 33 (41) | 37 (43) | 26 (43) | |
| Not known | 17 (21) | 17 (20) | 14 (23) | |
| Child with preschool education, No. (%) | 6 (8) | 2 (2) | 14 (23) | .001e |
| Hemoglobin SS, No./total tested, (%) | 1/77 (1) | 4/83 (5) | 0/61 (0) | .23 |
| HIV positive, No./total tested (%) | 2/79 (3) | 1/86 (1) | 0/61 (0) | .63 |
| Stool helminth, No. (%)b | 6/65 (9) | 3/60 (5) | 3/21 (14) | .36 |
Abbreviations: CC, community children; CM, cerebral malaria; HIV, human immunodeficiency virus, SD, standard deviation; SMA, severe malarial anemia.
a P values based on 1-way analysis of variance for continuous variables and Pearson χ2 test for categorical variables other than hemoglobin SS, HIV, and stool helminth infection, which were compared using Fisher exact test.
b SMA group differs from CC and CM groups.
c SMA group differs from CC group.
d SMA group differs from CM group.
e CM and SMA groups differ from CC group. Tukey test was used to compare continuous measures; for child's education, Pearson χ2 test was used to compare pairs of groups, with Bonferroni adjustment.
Long-term Neurocognitive Outcomes in Children With CM and SMA
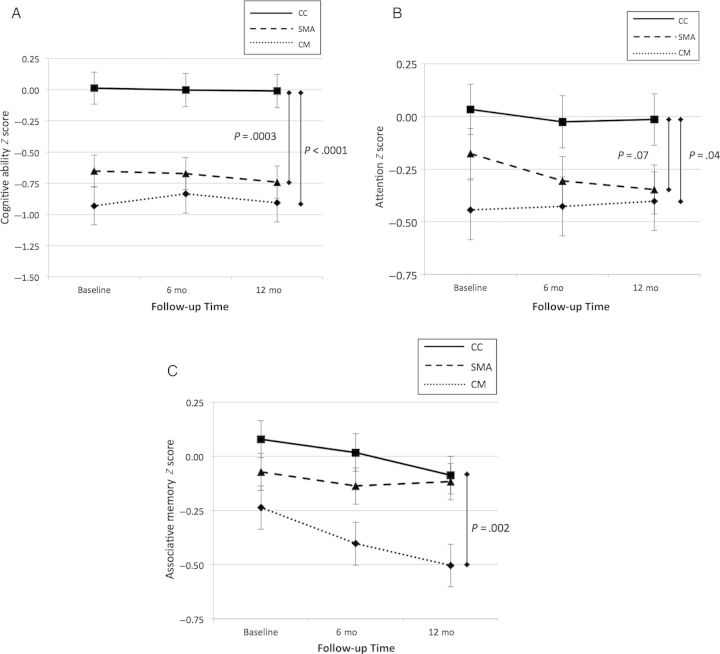
Gross neurologic deficits were present in 43 children (51%) with CM at discharge but had largely resolved at 6-month (4 children, 5%) and 12-month (4 children, 5%) follow-up. However, at 12-month follow-up, children with CM had significantly lower age-adjusted z scores than CC for cognitive ability, attention, and associative memory (Figure 2). Children with SMA had lower adjusted z scores than CC for overall cognitive ability and showed a trend toward lower scores for attention but did not differ for associative memory (Figure 2). The magnitude of difference in cognitive ability or attention scores between children with CM or SMA compared with CC did not change significantly over the study period (Figure 2; P > .70 for tests of the interaction of study group and visit for all 3 outcomes). The differences in age-adjusted neurocognitive z scores between children with CM or SMA and CC remained after additional adjustment for weight-for-age and height-for-age z scores, and child preschool education, the only factors that differed significantly between study groups (Table 2). Adjusted scores for cognitive ability and attention did not differ significantly between children with CM and SMA (P > .05 for both), but children with CM had significantly lower associative memory scores than those with SMA (P = .001). Comparing adjusted scores between groups using all 3 testing time points, children with CM had lower scores than CC in overall cognitive ability, attention, and associative memory, whereas children with SMA had lower scores than CC in overall cognitive ability and attention (Table 2). Analyses were also conducted excluding children with any preschool education, children with hemoglobin SS or children with HIV infection. Differences between groups remained significant after these groups were excluded (Supplementary Tables 1–3).
Figure 2.
Age-adjusted neurocognitive test scores in children with cerebral malaria (CM) or severe malarial anemia (SMA) and in community children (CC) at baseline and at 6 and 12 months for cognitive ability (A), attention (B), and associative memory (C). Scores are given as means with standard errors of the mean.
Table 2.
Neurocognitive Outcome z Scores in Children With CM or SMA and in CC at 12-Month Follow-up and for All Study Visitsa
| Score and Outcome | CM | SMA | CC | CM − CC |
SMA − CC |
||
|---|---|---|---|---|---|---|---|
| Differenceb | P Value | Differenceb | P Value | ||||
| z Score at 12 mo, mean ± SE | |||||||
| Cognitive ability | −0.96 ± 0.13 | −0.63 ± 0.13 | −0.11 ± 0.16 | −0.85 ± 0.21 | <.001 | −0.52 ± 0.21 | .01 |
| Attention | −0.45 ± 0.12 | −0.32 ± 0.12 | 0.01 ± 0.14 | −0.46 ± 0.19 | .02 | −0.33 ± 0.19 | .08 |
| Associative memory | −0.52 ± 0.09 | −0.09 ± 0.08 | −0.10 ± 0.10 | −0.41 ± 0.14 | .002 | 0.02 ± 0.13 | .89 |
| z Score for all visits, mean ± SEc | |||||||
| Cognitive ability | −0.94 ± 0.12 | −0.57 ± 0.12 | −0.10 ± 0.14 | −0.85 ± 0.19 | <.001 | −0.48 ± 0.19 | .01 |
| Attention | −0.47 ± 0.09 | −0.25 ± 0.09 | 0.02 ± 0.11 | −0.49 ± 0.14 | <.001 | −0.27 ± 0.14 | .05 |
| Associative memory | −0.39 ± 0.06 | −0.08 ± 0.06 | −0.02 ± 0.07 | −0.38 ± 0.09 | <.001 | −0.07 ± 0.09 | .49 |
Abbreviations: CC, community children; CM, cerebral malaria; SE, standard error; SMA, severe malarial anemia.
a Adjusted for age, nutrition, and child educational level. Age-adjusted neurocognitive z scores were calculated using CC as the comparator group. Nutrition was adjusted for using for weight-for-age and height for age z scores.
b Estimated mean difference ± SE.
c Mixed linear model estimates of mean z scores at enrollment and 6- and 12-month visits. The mixed linear model analysis is a generalization of repeated-measures analysis.
Clinical Features Associated With Neurocognitive Outcomes
Clinical features assessed as risk factors for adverse neurocognitive outcomes for children with CM or SMA included hypoglycemia (glucose < 2.2 mmol/L), days of fever, use of antimalarial medications before admission, prior seizure history, prior hospitalization, malnutrition, admission temperature, systolic and diastolic blood pressure, hypoxia (pulse oxygen saturation <92%), deep respirations, lactic acidosis (lactic acid level ≥5 mmol/L), hyperparasitemia (>250 000 parasites/µL), hemoglobin level, white blood cell count, platelet count, and presence of bacteremia. For children with CM, additional clinical features included presence and number of seizures before admission, BCS score, coma duration, abnormal posturing, presence and number of seizures after admission, and abnormal neurologic examination findings at discharge or 6- or 12-month follow-up.
In children with SMA, no clinical features were associated with worsened neurocognitive outcomes. In children with CM, decreased cognitive ability was seen in children with longer coma duration, lower BCS score, more seizures, or neurologic deficits at 6- or 12-month follow-up. Decreased attention was seen in children with longer coma duration or neurologic deficits at 6-month follow-up (Tables 3 and 4). The presence of retinopathy did not modify the effect of CM for any neurocognitive outcome (all P > .05), and children with or without retinopathy had similar z scores for all cognitive outcomes (all P > .23).
Table 3.
Categorical Variables Significantly Associated With Cognitive Ability or Attention in Children With Cerebral Malaria
| Variable | Cognitive Ability |
Attention |
||||
|---|---|---|---|---|---|---|
| Group | Mean (SE) | P Valuea | Group | Mean (SE) | P Valuea | |
| BCS scoreb | 1 (n = 17) | −2.04 (0.37) | <.001 | … | … | … |
| 2 (n = 61) | −0.52 (0.20) | |||||
| Neurologic deficit at 6 mo | Yes (n = 4) | −6.16 (0.55) | <.001 | Yes (n = 4) | −1.86 (0.47) | .002 |
| No (n = 75) | −0.59 (0.1) | No (n = 72) | −0.33 (0.11) | |||
| Neurologic deficit at 12 mo | Yes (n = 3) | −6.78 (0.67) | <.001 | … | … | … |
| No (n = 76) | −0.64 (0.13) | … | … | |||
Abbreviations: BCS, Blantyre Coma Scale; SE, standard error.
a Groups were compared using t tests or Pearson correlation to zero.
b In 2 children the BCS score was recorded as ≤2, but the exact score was not recorded. The BCS score was 1 or 2 in all children with a recorded score (none had a score of 0).
Table 4.
Continuous Variables Significantly Associated With Cognitive Ability or Attention in Children With Cerebral Malaria
| Variable | Cognitive Ability |
Attention |
||
|---|---|---|---|---|
| Pearson Correlation (r) | P Valuea | Pearson Correlation (r) | P Valuea | |
| Coma duration after admission (hours) | −0.59 | <.001 | −0.45 | <.001 |
| No. of seizures during admission | −0.68 | <.001 | … | … |
a P value for comparison of Pearson correlation coefficient with 0.
DISCUSSION
To our knowledge, the present study demonstrates for the first time that SMA, a form of severe malaria that affects between 1.5 and 5 million children in sub-Saharan Africa annually [12], is associated with long-term impairment in overall cognitive ability. The z scores for cognitive ability at 12-month follow-up, adjusted for potential confounding factors, were almost a full SD (−0.85) lower in children with CM compared with CC, and half a SD lower (−0.52) in children with SMA, analogous to a difference of 13 and 8 IQ points as a result of CM and SMA, respectively. Given the large number of children who have SMA each year and the size of the effect on overall cognitive ability in children with SMA in the present study, SMA may be a major contributor to long-term neurocognitive impairment in African children.
Previous prospective studies have documented that although gross neurologic deficits in children with CM largely resolve over time [1, 26], CM is associated with long-term neurocognitive impairment [1, 2], neurologic disability [27], and developmental deficits [28] in children ≥3 years of age. The present study confirms these findings in children with CM as young as 18 months and adds to these findings a new finding of long-term cognitive impairment in children with SMA. In the present study, children with CM had significantly reduced memory scores, which were not seen in children with SMA, and of the clinical factors measured, those associated with neurocognitive deficits occurred only in children with CM (eg, coma and seizures). These findings suggest that the pathways by which SMA and CM lead to neurocognitive impairment may differ and demonstrate the importance of testing multiple neurocognitive domains when assessing neurodevelopmental sequelae of severe illness.
In previous studies, cerebrospinal fluid tumor necrosis factor levels were associated with neurocognitive impairment in attention and working memory in children with CM [29], suggesting that central nervous system inflammation may play a role in neurocognitive impairment in children with CM. A prior study demonstrated no difference in neurologic outcomes in children with CM with vs without retinopathy [30]. We found, similarly, no differences in neurocognitive outcomes in children with CM with or without retinopathy, although our study lacked the power to detect small differences in neurocognitive outcomes. Cognitive impairment in children with SMA could be due to iron deficiency or metabolic acidosis, both of which are common in SMA [31, 32] and are associated with cognitive impairment in other disease processes [5, 33]. Cognitive impairment in children with SMA could also be due to the systemic inflammation seen in SMA [34] or to a direct effect of low hemoglobin on cognition [11]. The present study did not find an association between cognitive outcomes and either hemoglobin level or lactic acidosis in children with SMA or CM, but base deficit, which we did not measure, may be a better measure of metabolic acidosis in severe malaria [35], and a single hemoglobin value may not adequately reflect hemoglobin levels over the length of follow-up. Iron deficiency was not assessed in this study, but memory deficits, which are common in children with iron deficiency [36], were not present in children with SMA in the present study, suggesting that additional pathways may be involved in neurocognitive impairment in SMA.
Differences between study groups in neurocognitive scores did not change significantly over the 1-year period of evaluation, indicating that cognitive damage associated with CM or SMA occurs early and does not change greatly in the first year after the episode. These findings suggest that interventions given early in the treatment course may be most effective in reducing long-term neurodevelopmental sequelae. Further studies will be required to assess whether impairment worsens over subsequent years. Coma duration and number of seizures were strongly associated with cognitive outcomes in children with CM, suggesting that more effective seizure prevention could be an effective intervention to decrease cognitive impairment. Prior studies attempting to reduce seizures in children with CM with phenobarbital led to increased mortality [37], but new antiepileptic medications such as levetiracetam, which do not lead to respiratory depression [38], may provide a better option to prevent seizures and possibly reduce long-term neurocognitive impairment in CM.
Many factors can affect cognition in children, and in studies of severe malaria it is impossible to conduct in-depth neurocognitive testing before the disease episode. We attempted to address this limitation by excluding children with known prior neurologic disability; by choosing CC from the extended household or neighborhood of children with CM or SMA, to minimize differences between groups in education, socioeconomic status, and malaria exposure; and by conducting the most comprehensive assessment to date of other factors that may affect cognitive outcomes, including parental and child educational levels, child nutrition, child home environment, family socioeconomic status, and presence of HIV or hemoglobin SS. Differences between groups in neurocognitive outcome scores were not altered by adjusting potential confounding factors that differed between children with CM or SMA and CC or after exclusion of children with factors that might strongly affect cognition (eg, HIV, hemoglobin SS, and preschool education).
In summary, the present study demonstrates that SMA and CM are associated with neurocognitive impairment in children <5 years of age. SMA affects overall cognitive ability, whereas CM affects multiple areas of neurocognitive function, including cognitive ability, attention, and associative memory. Further study is required to determine the mechanisms by which CM and SMA lead to neurocognitive impairment, because this knowledge could lead to adjunctive neuroprotective therapy for children with severe malaria and decrease the long-term neurocognitive sequelae. These findings provide strong further rationale for malaria elimination programs, because they indicate that eliminating malaria may significantly decrease the burden of neurocognitive impairment in sub-Saharan African children.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the children and their parents who participated in this study, the study team for their dedicated effort in treating the children and collecting the data, and Brianna Yund for scoring the Early Childhood Vigilance Test videos.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institute of Neurological Disorders and Stroke and the Fogarty International Center (grants R01NS055349 and D43 NS078280).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Boivin MJ, Bangirana P, Byarugaba J, et al. Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics. 2007;119:e360–6. doi: 10.1542/peds.2006-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.John CC, Bangirana P, Byarugaba J, et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122:e92–9. doi: 10.1542/peds.2007-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter JA, Ross AJ, Neville BG, et al. Developmental impairments following severe falciparum malaria in children. Trop Med Int Health. 2005;10:3–10. doi: 10.1111/j.1365-3156.2004.01345.x. [DOI] [PubMed] [Google Scholar]
- 4.Kihara M, Carter JA, Newton CR. The effect of Plasmodium falciparum on cognition: a systematic review. Trop Med Int Health. 2006;11:386–97. doi: 10.1111/j.1365-3156.2006.01579.x. [DOI] [PubMed] [Google Scholar]
- 5.Idro R, Marsh K, John CC, Newton CR. Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr Res. 2010;68:267–74. doi: 10.1203/PDR.0b013e3181eee738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Serouri AW, Grantham-McGregor SM, Greenwood B, Costello A. Impact of asymptomatic malaria parasitaemia on cognitive function and school achievement of schoolchildren in the Yemen Republic. Parasitology. 2000;121:337–45. doi: 10.1017/s0031182099006502. [DOI] [PubMed] [Google Scholar]
- 7.Fernando D, de Silva D, Wickremasinghe R. Short-term impact of an acute attack of malaria on the cognitive performance of schoolchildren living in a malaria-endemic area of Sri Lanka. Trans R Soc Trop Med Hyg. 2003;97:633–9. doi: 10.1016/s0035-9203(03)80093-7. [DOI] [PubMed] [Google Scholar]
- 8.Holding PA, Stevenson J, Peshu N, Marsh K. Cognitive sequelae of severe malaria with impaired consciousness. Trans R Soc Trop Med Hyg. 1999;93:529–34. doi: 10.1016/s0035-9203(99)90368-1. [DOI] [PubMed] [Google Scholar]
- 9.Nankabirwa J, Wandera B, Kiwanuka N, Staedke SG, Kamya MR, Brooker SJ. Asymptomatic Plasmodium infection and cognition among primary schoolchildren in a high malaria transmission setting in Uganda. Am J Trop Med Hyg. 2013;88:1102–8. doi: 10.4269/ajtmh.12-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thuilliez J, Sissoko MS, Toure OB, Kamate P, Berthélemy J-C, Doumbo OK. Malaria and primary education in Mali: a longitudinal study in the village of Donéguébougou. Soc Sci Med. 2010;71:324–34. doi: 10.1016/j.socscimed.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olney DK, Pollitt E, Kariger PK, et al. Young Zanzibari children with iron deficiency, iron deficiency anemia, stunting, or malaria have lower motor activity scores and spend less time in locomotion. J Nutr. 2007;137:2756–62. doi: 10.1093/jn/137.12.2756. [DOI] [PubMed] [Google Scholar]
- 12.Murphy SC, Breman JG. Gaps in the childhood malaria burden in Africa: cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycemia, and complications of pregnancy. Am J Trop Med Hyg. 2001;64(1-2 Suppl):57–67. doi: 10.4269/ajtmh.2001.64.57. [DOI] [PubMed] [Google Scholar]
- 13.Kolb B, Whishaw IQ. Fundamentals of human neuropsychology. 6th ed. New York, NY: Worth Publishers; 2009. [Google Scholar]
- 14.Kostovic I, Judas M, Petanjek Z. Structural development of the human prefrontal cortex. In: Nelson CA, Luciana M, editors. 2nd ed. Massachusetts Institute of Technology; 2008. pp. 213–36. Handbook of Developmental Cognitive Neuroscience. [Google Scholar]
- 15.Anderson V, Spencer-Smith M, Wood A. Do children really recover better? neurobehavioural plasticity after early brain insult. Brain. 2011;134:2197–221. doi: 10.1093/brain/awr103. [DOI] [PubMed] [Google Scholar]
- 16.Birbeck GL, Beare N, Lewallen S, et al. Identification of malaria retinopathy improves the specificity of the clinical diagnosis of cerebral malaria: findings from a prospective cohort study. Am J Trop Med Hyg. 2010;82:231–4. doi: 10.4269/ajtmh.2010.09-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caldwell BM, Bradley RH. Home inventory administration manual. 3rd ed. Little Rock, AR: University of Arkansas; 2001. [Google Scholar]
- 18.Bangirana P, John CC, Idro R, et al. Socioeconomic predictors of cognition in Ugandan children: implications for community interventions. PLoS One. 2009;4:e7898. doi: 10.1371/journal.pone.0007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullen EM. Mullen Scales of Early Learning: AGS edition. 1995. American Guidance Services, Inc, Circle Pines, MN. [Google Scholar]
- 20.Jordan CM, Johnson AL, Hughes SJ, Shapiro EG. The Color Object Association Test (COAT): the development of a new measure of declarative memory for 18- to 36-month-old toddlers. Child Neuropsychol. 2008;14:21–41. doi: 10.1080/09297040601100430. [DOI] [PubMed] [Google Scholar]
- 21.Goldman DZ, Shapiro EG, Nelson CA. Measurement of vigilance in 2-year-old children. Dev Neuropsychol. 2004;25:227–50. doi: 10.1207/s15326942dn2503_1. [DOI] [PubMed] [Google Scholar]
- 22.Carlo WA, Goudar SS, Pasha O, et al. Randomized trial of early developmental intervention on outcomes in children after birth asphyxia in developing countries. J Pediatr. 2013;162:705–12 e3. doi: 10.1016/j.jpeds.2012.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gladstone M, Lancaster GA, Umar E, et al. The Malawi Developmental Assessment Tool (MDAT): the creation, validation, and reliability of a tool to assess child development in rural African settings. PLoS Med. 2010;7:e1000273. doi: 10.1371/journal.pmed.1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boivin MJ, Bangirana P, Nakasujja N, et al. A year-long caregiver training program to improve neurocognition in preschool Ugandan HIV-exposed children. J Dev Behav Pediatr. 2013;34:269–78. doi: 10.1097/DBP.0b013e318285fba9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bodeau-Livinec F, Cot M, Koura G, Boivin M. Assessing the effects of maternal anemia on child development in Benin. In: Boivin MJ, Giordani B, editors. New York, NY: Springer; 2013. pp. 203–14. Neuropsychology of Children in Africa: Perspectives on Risk and Resilience. [Google Scholar]
- 26.van Hensbroek MB, Palmer A, Jaffar S, Schneider G, Kwiatkowski D. Residual neurologic sequelae after childhood cerebral malaria. J Pediatr. 1997;131(1 Pt 1):125–9. doi: 10.1016/s0022-3476(97)70135-5. [DOI] [PubMed] [Google Scholar]
- 27.Birbeck GL, Molyneux ME, Kaplan PW, et al. Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol. 2010;9:1173–81. doi: 10.1016/S1474-4422(10)70270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boivin MJ, Gladstone MJ, Vokhiwa M, et al. Developmental outcomes in Malawian children with retinopathy positive cerebral malaria. Trop Med Int Health. 2011;16:263–71. doi: 10.1111/j.1365-3156.2010.02704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.John CC, Panoskaltsis-Mortari A, Opoka RO, et al. Cerebrospinal fluid cytokine levels and cognitive impairment in cerebral malaria. Am J Trop Med Hyg. 2008;78:198–205. [PMC free article] [PubMed] [Google Scholar]
- 30.Postels DG, Taylor TE, Molyneux M, et al. Neurologic outcomes in retinopathy-negative cerebral malaria survivors. Neurology. 2012;79:1268–72. doi: 10.1212/WNL.0b013e31826aacd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cserti-Gazdewich CM, Dhabangi A, Musoke C, et al. Inter-relationships of cardinal features and outcomes of symptomatic pediatric Plasmodium falciparum malaria in 1,933 children in Kampala, Uganda. Am J Trop Med Hyg. 2013;88:747–56. doi: 10.4269/ajtmh.12-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Mast Q, Van Dongen-Lases EC, Swinkels DW, et al. Mild increases in serum hepcidin and interleukin-6 concentrations impair iron incorporation in haemoglobin during an experimental human malaria infection. Br J Haematol. 2009;145:657–64. doi: 10.1111/j.1365-2141.2009.07664.x. [DOI] [PubMed] [Google Scholar]
- 33.Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131(2S-2):649S–66S. doi: 10.1093/jn/131.2.649S. discussion 66S-68S. [DOI] [PubMed] [Google Scholar]
- 34.Perkins DJ, Were T, Davenport GC, Kempaiah P, Hittner JB, Ong'echa JM. Severe malarial anemia: innate immunity and pathogenesis. Int J Biol Sci. 2011;7:1427–42. doi: 10.7150/ijbs.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Seidlein L, Olaosebikan R, Hendriksen ICE, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54:1080–90. doi: 10.1093/cid/cis034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siddappa AM, Georgieff MK, Wewerka S, Worwa C, Nelson CA, Deregnier RA. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr Res. 2004;55:1034–41. doi: 10.1203/01.pdr.0000127021.38207.62. [DOI] [PubMed] [Google Scholar]
- 37.Crawley J, Waruiru C, Mithwani S, et al. Effect of phenobarbital on seizure frequency and mortality in childhood cerebral malaria: a randomised, controlled intervention study. Lancet. 2000;355:701–6. doi: 10.1016/S0140-6736(99)07148-2. [DOI] [PubMed] [Google Scholar]
- 38.Cormier J, Chu CJ. Safety and efficacy of levetiracetam for the treatment of partial onset seizures in children from one month of age. Neuropsychiatr Dis Treat. 2013;9:295. doi: 10.2147/NDT.S30224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.