Following virologic failure on a first-line protease inhibitor–based regimen, if no or limited drug resistance is detected, remaining on the same regimen coupled with strategies to improve adherence is a reasonable and effective approach.
Keywords: first-line, protease inhibitor, virologic failure, antiretroviral therapy
Abstract
Background. Virologic failure (VF) on a first-line ritonavir-boosted protease inhibitor (PI/r) regimen is associated with low rates of resistance, but optimal management after failure is unknown.
Methods. The analysis included participants in randomized trials who experienced VF on a first-line regimen of PI/r plus 2 nucleoside reverse transcriptase inhibitors (NRTIs) and had at least 24 weeks of follow-up after VF. Antiretroviral management and virologic suppression (human immunodeficiency virus type 1 [HIV-1] RNA <400 copies/mL) after VF were assessed.
Results. Of 209 participants, only 1 participant had major PI-associated treatment-emergent mutations at first-line VF. The most common treatment approach after VF (66%) was to continue the same regimen. The virologic suppression rate 24 weeks after VF was 64% for these participants, compared with 72% for those who changed regimens (P = .19). Participants remaining on the same regimen had lower NRTI resistance rates (11% vs 30%; P = .003) and higher CD4+ cell counts (median, 275 vs 213 cells/µL; P = .005) at VF than those who changed. Among participants remaining on their first-line regimen, factors at or before VF significantly associated with subsequent virologic suppression were achieving HIV-1 RNA <400 copies/mL before VF (odds ratio [OR], 3.39 [95% confidence interval {CI}, 1.32–8.73]) and lower HIV-1 RNA at VF (OR for <10 000 vs ≥10 000 copies/mL, 3.35 [95% CI, 1.40–8.01]). Better adherence after VF was also associated with subsequent suppression (OR for <100% vs 100%, 0.38 [95% CI, .15–.97]). For participants who changed regimens, achieving HIV-1 RNA <400 copies/mL before VF also predicted subsequent suppression.
Conclusions. For participants failing first-line PI/r with no or limited drug resistance, remaining on the same regimen is a reasonable approach. Improving adherence is important to subsequent treatment success.
Antiretroviral therapy (ART) that includes a ritonavir-boosted protease inhibitor (PI/r) plus 2 nucleoside reverse transcriptase inhibitors (NRTIs) is among the first-line therapies recommended for human immunodeficiency virus (HIV) infection in current treatment guidelines [1, 2]. Although the effectiveness of PI/r-based regimens as initial therapy is well established, about 10%–20% of patients experience virologic failure (VF) within 2 years [3–6].
A distinctive characteristic of PI/r regimens is that those who experience VF rarely have detectable PI resistance [7–10]. As a result, clinicians theoretically have the option of continuing the same regimen or modifying the treatment by changing to a different PI/r or introducing a new drug class, such as a nonnucleoside reverse transcriptase inhibitor (NNRTI). It is important to understand the outcome of different management strategies after failure of PI/r-based first-line therapy, but few data exist, as many first-line studies terminate follow-up soon after a participant reaches the primary VF endpoint. Furthermore, the number of participants experiencing failure in any one study is small.
This analysis evaluated therapeutic approaches and outcomes among participants in 3 large randomized clinical trials undertaken by the AIDS Clinical Trials Group (ACTG) who experienced VF on first-line PI/r-based ART.
METHODS
Study Population
The study included all 3 randomized trials of initial ART conducted by the ACTG that included a PI/r regimen and was completed by June 2013. The study population included participants who experienced VF on first-line PI/r plus 2 NRTIs while participating in ACTG A5142 [3], A5202 [4], or A5208 trial 2 [5]; A5208 trial 1 [11] was not included, as participants in that study had to have prior single-dose nevirapine exposure. The design and main results of these studies have been previously published [3–5]. The ACTG A5142 and A5202 trials enrolled men and women in the United States. A5208 trial 2 enrolled women in eastern and southern Africa without prior single-dose nevirapine exposure. These studies included randomization to lopinavir/ritonavir (LPV/r) (A5142 and A5208) or atazanavir/ritonavir (ATV/r) (A5202) as first-line PI/r, with 1 of the following combinations of 2 NRTIs: lamivudine plus either tenofovir, zidovudine, or stavudine in A5142; tenofovir plus emtricitabine or lamivudine plus abacavir in A5202; and tenofovir plus emtricitabine in A5208.
Participants who changed to a regimen other than PI/r plus 2 NRTIs prior to first-line VF and those who had <24 weeks of follow-up after failure were excluded from the analysis.
First-line Virologic Failure and ART Management
HIV type 1 (HIV-1) RNA was measured in plasma using the Ultrasensitive Roche Amplicor Monitor V1.5 in A5142 and A5202, and the standard Roche Amplicor Monitor V1.5 in A5208. First-line VF was defined similarly with little variation among the 3 studies (Table 1).
Table 1.
Definition of First-line Virologic Failure, Protocol-Suggested Postvirologic Failure Management, and Available Antiretroviral Therapy and Major Resistance Mutation by ACTG Study
| Definition | A5142 | A5202 | A5208 |
|---|---|---|---|
| Definition of first-line virologic failure | HIV-1 RNA <1 log10 copies/mL below baseline at/after 8 wk of ART or ≥200 copies/mL at/after 32 wk confirmation of VF was required in a subsequent plasma sample |
HIV-1 RNA ≥1000 copies/mL at/after 16 wk or ≥200 copies/mL at/after 24 wk; confirmation of VF was required in a subsequent plasma sample | HIV-1 RNA <1 log10 copies/mL below baseline at/after 12 wk or ≥400 copies/mL at/after 24 wk; confirmation of VF was required in a subsequent plasma sample |
| Protocol suggested management on first-line virologic failure | Suggested second-line regimen is EFV + additional agents selected by genotypic resistance result Alternatively, any regimen may be chosen based on the genotypic resistance test results | Subjects may remain on their study regimen in consultation with their primary care provider. If the CD4 count or the HIV-1 RNA returns to the baseline level, subjects will be strongly advised to change therapy according to resistance test result | Suggested second-line regimen is NVP-containing regimen, but switching to a second-line regimen is not mandatory. Participants may remain on the step 1 regimen at the discretion of the participant and site investigator |
| Study-provided ART | EFV, LPV/r, d4T, TDF | ABC/3TC, FTC/TDF, 3TC/ZDV, LPV/r, ABC, ATV, ddI, EFV, FTC, FPV, 3TC, d4T, RTV, TDF, ZDV | NVP, LPV/r, FTC, TDF, FTC/TDF, ddI, and ZDV |
| Definition of major resistance mutation | IAS-USA (2006 version) [12] | IAS-USA (2008 version) [13], as well as T69D, L74I, and G190C/E/Q/T/V for reverse transcriptase, and L24I, F53L, I54V/A/T/S, G73C/S/T/A, and N88D for protease | IAS-USA (2008 version) [13] |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ACTG, AIDS Clinical Trials Group; ART, antiretroviral therapy; ATV, atazanavir; d4T, stavudine; ddI, didanosine; EFV, efavirenz; FPV, fosamprenavir; FTC, emtricitabine; HIV-1, human immunodeficiency virus type 1; IAS, International AIDS Society; LPV/r, lopinavir/ritonavir; NVP, nevirapine; RTV, ritonavir; TDF, tenofovir; ZDV, zidovudine.
Decisions regarding ART management after first-line VF were made by site clinicians and participants. Real-time drug resistance testing at VF was available in A5142 and A5202, but was done retrospectively using stored samples at the end of A5208; pretreatment drug resistance testing using stored samples was performed retrospectively for participants experiencing VF in all 3 studies. Major resistance mutations were defined mainly based on International Antiviral Society (IAS)–USA [12, 13], and the details are shown in Table 1 along with the protocol-suggested management on first-line virologic failure and study-provided ART.
Statistical Methods
The primary endpoint of the analysis was virologic suppression, defined as HIV-1 RNA <400 copies/mL, at 24 weeks after confirmation of first-line VF. Missing values were considered as lack of suppression unless the last HIV-1 RNA before 24 weeks was <400 copies/mL. For participants who changed ART within 24 weeks after first-line VF confirmation, virologic suppression at 24 weeks after regimen change was also examined.
The following variables were evaluated for their association with ART management following VF and with subsequent virologic suppression: first-line ART regimen, HIV-1 RNA, and CD4 cell count at ART initiation and at VF, HIV-1 RNA <400 copies/mL at any time prior to VF, weeks from ART initiation to VF, change in CD4 count from ART initiation to VF, age and drug resistance at VF, and last available self-reported adherence (in the prior 4 days) within 24 weeks prior to VF. Sex and race were only examined among A5142 and A5202 participants because A5208 only included African women, and all were black. The association between resistance and ART management was also only examined among A5142 and A5202 participants, as real-time resistance results were not available in A5208 to guide ART management. Participants who switched to a nonstandard second-line regimen after VF (including a nonboosted PI or both an NNRTI and a PI/r) were included in analyses of ART management after VF but were excluded from analyses examining subsequent suppression.
All analyses were stratified by ACTG study. To compare characteristics and virologic suppression rates between participants remaining on the same regimen vs changing regimen, and among different regimens for those who changed regimens, van Elteran and Cochran-Mantel-Haenszel tests were used for continuous and categorical variables, respectively. To evaluate factors associated with subsequent virologic suppression, multivariable logistic regression was constructed by stepwise variable selection with P < .05 required for entry and subsequent retention. The following sensitivity analyses were conducted: excluding participants whose HIV-1 RNA at VF was <400 copies/mL for both initial and confirmatory measurements; excluding participants from resource-limited settings (A5208); and defining virologic suppression as HIV-1 RNA <200 copies/mL instead of <400 copies/mL (limited to A5142 and A5202 because A5208 used the assay with lower limit of quantification of 400 copies/mL). All analyses were performed using SAS software, version 9.2.
RESULTS
Characteristics at Pretreatment and at First-line Virologic Failure
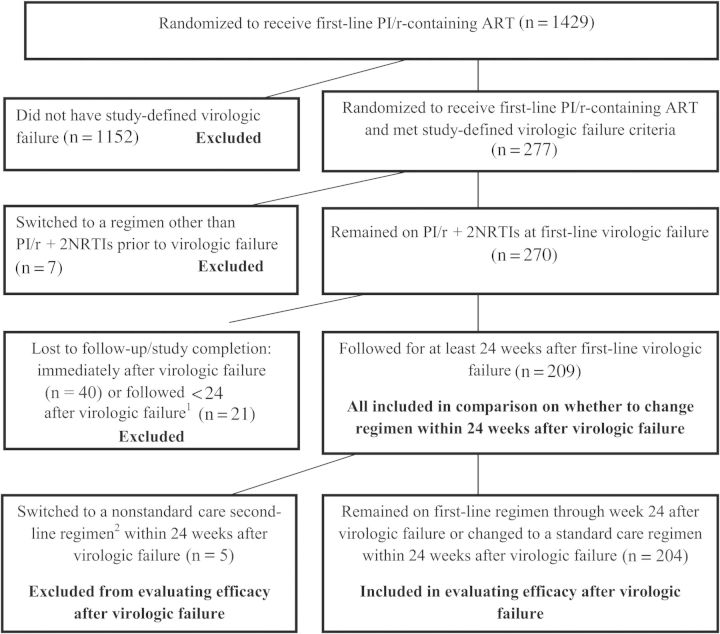
Among the 1429 participants randomized to receive a first-line PI/r-based regimen, 277 (19%) experienced study-defined VF. Seven were not eligible for this analysis because they switched to a regimen other than PI/r + 2 NRTIs prior to VF. Sixty-one were excluded from the analysis because they were not followed (n = 40) or had <24 weeks of follow-up (n = 21) after VF, either because of study closure or because of loss to follow-up (Figure 1).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram for inclusion and exclusion criteria. 1Among the 21 patients with some follow-up, 16 stayed on their first-line ritonavir-boosted protease inhibitor (PI/r)–based regimen; 13 of the 21 (62%) had HIV-1 RNA <400 copies/mL at their last available measurement after initial virologic failure. 2The 5 participants who changed to a nonstandard regimen included 3 who changed to an unboosted protease inhibitor–containing regimen and 2 who changed to a PI/r + nonnucleoside reverse transcriptase regimen. Abbreviations: ART, antiretroviral therapy; HIV-1, human immunodeficiency virus type 1; NRTI, nucleoside reverse transcriptase inhibitor; PI/r, ritonavir-boosted protease inhibitor.
Among the 209 participants included, 67 (32%) were from A5142 and 107 (51%) were from A5202 (thus 83% enrolled in the United States), and 35 (17%) were from A5208 (enrolled in Africa). Overall, 43% of participants were female, the median pretreatment HIV-1 RNA was 4.8 log10 copies/mL, and median CD4 count was 118 cells/µL; 49% received LPV/r and 51% received ATV/r as the PI/r component of first-line ART (Table 2).
Table 2.
Characteristics of Study Participants at Initiation of Antiretroviral Therapy and at First-line Virologic Failure by Study
| Characteristic | Statistics/Levels | A5142 (N = 67) | A5202 (N = 107) | A5208 (N = 35) | Overall (N = 209) |
|---|---|---|---|---|---|
| Characteristics prior to starting ART | |||||
| Sex | Female | 24 (36%) | 31 (29%) | 35 (100%) | 90 (43%) |
| Age, y | Median (quartiles) | 38 (32–42) | 37 (31–44) | 31 (26–38) | 37 (30–42) |
| CD4 count, cells/µL | Median (quartiles) | 141 (46–248) | 107 (32–302) | 137 (80–178) | 118 (42–253) |
| HIV-1 RNA, log10 copies/mL | Median (quartiles) | 4.8 (4.4–5.2) | 4.8 (4.5–5.4) | 5.4 (4.8–5.8) | 4.8 (4.5–5.5) |
| First-line ART | LPV/r + 3TC + TDF | 20 (30%) | 0 (0%) | 0 (0%) | 20 (10%) |
| LPV/r + 3TC + ZDV | 28 (42%) | 0 (0%) | 0 (0%) | 28 (13%) | |
| LPV/r + 3TC + D4T | 19 (28%) | 0 (0%) | 0 (0%) | 19 (9%) | |
| LPV/r + TDF + FTC | 0 (0%) | 0 (0%) | 35 (100%) | 35 (17%) | |
| ATV/r + 3TC + ABC | 0 (0%) | 64 (60%) | 0 (0%) | 64 (31%) | |
| ATV/r + TDF + FTC | 0 (0%) | 43 (40%) | 0 (0%) | 43 (21%) | |
| Characteristics at first-line virologic failure | |||||
| Age, y | Median (quartiles) | 39 (34–43) | 39 (33–46) | 33 (28–39) | 38 (32–44) |
| Achieved HIV-1 RNA <400 copies/mL at any time prior to initial failure | Yes | 53 (79%) | 85 (79%) | 26 (74%) | 164 (78%) |
| Weeks from ART initiation | Median (quartiles) | 39 (14–76) | 37 (24–84) | 48 (13–72) | 39 (18–76) |
| HIV-1 RNA, log10 copies/mL | Median (quartiles) | 3.8 (3.2–4.7) | 3.8 (3.0–4.6) | 4.2 (3.3–5.0) | 3.9 (3.2–4.7) |
| CD4 count, cells/µL | Median (quartiles) | 251 (187–370) | 269 (156–425) | 180 (129–316) | 246 (160–404) |
| CD4 count change from ART initiation, cells/µL | Median (quartiles) | 95 (50–243) | 100 (39–182) | 74 (6–201) | 96 (35–190) |
| Self-reported adherence within 4 d Prior to clinic visit | Not on ART | 6 (9%) | 12 (11%) | 1 (3%) | 19 (9%) |
| <100% | 23 (34%) | 18 (17%) | 7 (20%) | 48 (23%) | |
| 100% | 33 (49%) | 70 (65%) | 27 (77%) | 130 (62%) | |
| Unknown | 5 (13%) | 7 (2%) | 0 | 12 (6%) | |
| Drug resistancea | Major PI-associatedb | 0 (0%) | 5 (5%) | 0 (0%) | 5 (3%) |
| Minor PI-associated | 43 (88%) | 96 (90%) | 32 (100%) | 171 (91%) | |
| NNRTI-associatedc | 1 (2%) | 3 (3%) | 3 (9%) | 7 (4%) | |
| NRTI- associatedd | 12 (24%) | 20 (19%) | 6 (19%) | 38 (20%) | |
| Not available | 18 | 0 | 3 | 21 | |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; ATV/r, ritonavir-boosted atazanavir; d4T, stavudine; FTC, emtricitabine; HIV-1, human immunodeficiency virus type 1; LPV/r, ritonavir-boosted lopinavir; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; TDF, tenofovir; ZDV, zidovudine.
a Resistance mutations were defined as those listed by the International Antiviral Society (IAS)–USA for A5142 (2006 version) [12] and A5208 (2008 version) [13]; for A5202, they were defined as those listed by the IAS-USA 2008 version [13], as well as T69D, L74I, and G190C/E/Q/T/V for major reverse transcriptase–associated mutations, and L24I, F53L, I54V/A/T/S, G73C/S/T/A, and N88D for major protease-associated mutations.
b Only 1 of the 5 participants selected a major protease-associated resistance mutation (N88S) after treatment initiation; the other 4 participants had mutations (I54V, L90M, L33F, and G73A/L90M, respectively) prior to starting treatment.
c All 7 participants had NNRTI-associated resistance mutations before treatment initiation.
d Fifteen participants selected NRTI-associated resistance mutations after treatment initiation; the other 23 participants had mutations present prior to starting treatment. Among the 38 participants with NRTI-associated resistance mutations, 2 (5%) had K65R mutation, 20 (53%) had M184V mutation, and 3 (8%) had thymidine-associated mutations.
Median time from ART initiation to confirmation of VF was 39 weeks, and 78% of participants achieved HIV-1 RNA <400 copies/mL at some time prior to VF. At VF, the median HIV-1 RNA was 3.9 log10 copies/mL, the median CD4 count was 246 cells/µL, and the median CD4 count increase from pretreatment was 96 cells/µL. Self-reported ART adherence within the 4 days prior to VF was 100% for 62% of participants and <100% for 23% (9% reported not being on ART, and 6% had no report). Of the 209 participants, 188 had drug resistance results available at VF (Table 2). Only 1 participant selected a new major PI-associated mutation since ART initiation (0.5%), although another 4 participants had such mutations at baseline; 15 (9%) selected new NRTI-associated mutations, with 23 having these at baseline; and 7 (4%) had NNRTI-associated mutations, all present at baseline. Among the 61 participants who were excluded, resistance was also infrequent at VF: none had major PI-associated resistance and 7% and 4% had NRTI and NNRTI resistance, respectively. Compared with the 209 participants included, the participants excluded did, however, have significantly higher median pretreatment CD4 counts (277 vs 165 cells/µL; P < .001) and lower median pretreatment HIV-1 RNA (4.66 vs 4.84 log10 copies/mL; P = .006).
ART Regimen After First-line Virologic Failure
Participants who did not change ART regimen through 24 weeks after first-line VF confirmation were defined as remaining on their first-line regimen, whereas participants who changed regimens within 24 weeks were classified by the type of their first regimen change. Among the 209 participants, 137 (66%) remained on their first-line regimen, 28 (13%) changed to an NNRTI-based regimen (with or without NRTI change), 14 (7%) changed to a different PI/r (with or without NRTI change), 25 (12%) only changed 1 or both of their NRTIs, and 5 (2%) changed to a nonstandard second-line regimen (2 changed to a PI/r + NNRTI + NRTI regimen, and 3 changed to an unboosted PI + NRTI regimen) (Table 3). The proportion of participants remaining on their first-line regimen differed significantly among the 3 studies: 51% for A5142, 69% for A5202, and 83% for A5208 (P = .003).
Table 3.
Antiretroviral Therapy Regimen Within 24 Weeks After First-line Virologic Failure Confirmation
| Regimen | A5142 (N = 67) | A5202 (N = 107) | A5208 (N = 35) | Overall (N = 209) |
|---|---|---|---|---|
| No change in ART | 34 (51%) | 74 (69%) | 29 (83%) | 137 (66%) |
| Changed to an NNRTI-containing regimen | 15 (22%) | 9 (8%) | 4 (11%) | 28 (13%) |
| Changed to ART including a different PI/r | 6 (9%) | 8 (7%) | 0 (0%) | 14 (7%) |
| Changed NRTI(s) only | 8 (12%) | 15 (14%) | 2 (6%) | 25 (12%) |
| Changed to nonstandard second-line regimena | 4 (6%) | 1 (1%) | 0 (0%) | 5 (2%) |
Abbreviations: ART, antiretroviral therapy; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
a The 5 participants who changed to a nonstandard second-line regimen included 2 who changed to a ritonavir-boosted PI + NNRTI-based regimen and 3 who changed to a PI regimen without ritonavir boosting.
Participants remaining on their first-line regimen had a higher CD4 count at VF than those who changed (median, 275 vs 213 cells/µL; P = .005). Among A5142 and A5202 participants with real-time resistance test results available at VF, those remaining on their first-line regimen were less likely to have NRTI resistance mutations (11% vs 30%; P = .003). There were no significant differences for other factors evaluated.
Virologic Suppression After First-line Virologic Failure
At 24 weeks after confirmation of first-line VF, 136 of 204 participants (67%) had HIV-1 RNA <400 copies/mL (excluding the 5 on nonstandard regimens; Table 4): 88 (64%) of those who remained on their first-line regimen and 48 (72%) of those who changed (odds ratio [OR], 0.74 [95% confidence interval {CI}, .48–1.16]; P = .19; if adjusted for CD4 count and presence of NRTI resistance mutations at VF: OR, 0.75 [95% CI, .36–1.58]; P = .45). Among the 146 participants without NRTI-, NNRTI-, or major PI-associated resistance mutations detected, the proportion with virologic suppression was 62% for those who remained on their first-line ART and 61% for those who changed (OR, 0.97 [95% CI, .61–1.54]; P = .89).
Table 4.
Virologic and Immunologic Outcome at 24 Weeks After First-line Virologic Failure Confirmation
| Change ART Within 24 wk Post–Virologic Failure | A5142 | A5202 | A5208 | Overall | P Valuea |
|---|---|---|---|---|---|
| HIV-1 RNA <400 copies/mL | |||||
| No change in ART | 20/34 (59%) | 45/74 (61%) | 23/29 (79%) | 88/137 (64%) | .19 |
| ART changedb | 21/29 (72%) | 22/32 (69%) | 5/6 (83%) | 48/67 (72%) | |
| Total | 41/63 (65%) | 67/106 (63%) | 28/35 (80%) | 136/204 (67%) | |
| HIV-1 RNA <200 copies/mL | |||||
| No change in ART | 20/34 (59%) | 42/74 (57%) | Not availablec | 62/108 (57%) | .34 |
| ART changedb | 20/29 (69%) | 20/32 (63%) | Not available | 40/61 (66%) | |
| Total | 40/63 (63%) | 62/106 (58%) | Not available | 102/169 (60%) | |
| CD4+ cell count, cells/µL | |||||
| No change in ART | 304 (221–461) | 313 (205–520) | 268 (205–437) | 304 (205–469) | .02 |
| ART changedb | 308 (218–412) | 253 (160–341) | 207 (177–200) | 273 (194–351) | |
| Total | 304 (221–445) | 286 (194–463) | 256 (197–432) | 287 (198–439) | |
| CD4+ cell count change, cells/µL, since first-line virologic failure confirmation | |||||
| No change in ART | 24 (−33–102) | 19 (−93–96) | 73 (6–149) | 34 (−40–100) | .34 |
| ART changedb | 30 (−6–119) | 65 (−18–88) | −3 (26–51) | 44 (−19–87) | |
| Total | 30 (−25–113) | 43 (−33–93) | 51 (−23–97) | 38 (−27–96) | |
Data are presented as No. (%) or median (first quartile - third quartile).
Abbreviations: ART, antiretroviral therapy; HIV-1, human immunodeficiency virus type 1.
a The P value was from the test comparing outcomes in participants who remained on the first-line regimen and those who changed regimen after first-line failure.
b Five participants who changed to a nonstandard care second-line regimen (3 changed to a unboosted protease inhibitor [PI] regimen and 2 changed to a ritonavir-boosted PI plus nonnucleoside reverse transcriptase inhibitor regimen) after first-line virologic failure were excluded from outcome evaluation.
c Not available for A5208 participants because the lower limit of quantification of the assay used in A5208 was 400 copies/mL.
Among participants from A5142 and A5202 (which used a more sensitive HIV-1 RNA assay), 62 participants (57%) who remained on their first-line ART and 40 participants (69%) who changed had HIV-1 RNA <200 copies/mL at 24 weeks after first-line VF confirmation (P = .34) (Table 4).
Among the 137 participants remaining on their first-line regimen, the following factors were significantly associated with higher odds of virologic suppression at week 24 after first-line VF in univariate analysis: greater increase in CD4 count and longer time from ART initiation to VF, HIV-1 RNA <400 copies/mL at any time prior to VF, and HIV-1 RNA <10 000 copies/mL at VF confirmation. Two variables remained significantly associated in multivariate analysis: HIV-1 RNA <400 copies/mL at any time prior to VF (OR, 3.39 vs ≥400 copies/mL; P = .011), and HIV-1 RNA <10 000 copies/mL at VF (OR, 3.35 vs ≥10 000 copies/mL; P = .007) (Table 5). No significant interaction was detected between these 2 variables.
Table 5.
Variables Associated With HIV-1 RNA <400 Copies/mL at Week 24 After Confirmation of First-line Virologic Failure for Participants Remaining on First-line Antiretroviral Therapy
| Univariate Modela |
Multivariable Modelb |
||||
|---|---|---|---|---|---|
| Variable | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Sex | Female vs Male | 0.89 (.38–2.05) | .78 | ||
| NRTI in initial regimen | Lamivudine + otherc vs tenofovir + emtricitabine | 1.02 (.40–2.58) | .97 | ||
| HIV-1 RNA at ART initiation | Per 1 log10 copies/mL higher | 1.27 (.78–2.09) | .34 | ||
| CD4 count at ART initiation | Per 100 cells/µL higher | 1.02 (.82–1.27) | .89 | ||
| Age at virologic failure | Per 10 ys increase | 1.00 (.97–1.04) | .83 | ||
| CD4 change from baseline to virologic failure confirmation | Per 100 cells/µL increase | 1.41 (1.06–1.87) | .02 | ||
| Self-reported adherence at virologic failure | <100% vs 100% | 1.53 (.59–3.96) | .39 | ||
| Not on ARV vs 100% | 0.95 (.36–2.47) | .91 | |||
| CD4 count at virologic failure confirmation | Per 100 cells/µL higher | 0.98 (.79–1.21) | .84 | ||
| Achieved HIV-1 RNA <400 copies/mL at any time prior to initial failure | Yes vs No | 4.57 (1.83–11.38) | .001 | 3.39 (1.32–8.73) | .011 |
| HIV-1 RNA at virologic failure confirmation | <10 000 vs ≥10 000 copies/mL | 4.21 (1.83–9.67) | <.001 | 3.35 (1.40–8.01) | .007 |
| Any NRTI resistanced at virologic failure | Yes vs No | 4.05 (.88–18.73) | .07 | ||
| Any minor PI resistanced at virologic failure | Yes vs No | 0.49 (.09–2.57) | .40 | ||
| Time from ART initiation to virologic failure | Per 10 wks increase | 1.13 (1.02–1.26) | .02 | ||
Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral; CI, confidence interval; HIV-1, human immunodeficiency virus type 1; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
a Both univariate model and multivariable model was stratified by study.
b Multivariable model was selected by stepwise procedure with entry and inclusion, P < .05.
c “Other” refers to one of abacavir, tenofovir, zidovudine, or stavudine.
d Resistance mutations were defined as those listed by the International Antiviral Society (IAS)–USA for A5142 (2006 version) [12] and A5208 (2008 version) [13]; for A5202, they were defined as those listed by the IAS-USA 2008 version [13], as well as T69D, L74I, and G190C/E/Q/T/V for major reverse transcriptase–associated mutations, and L24I, F53L, I54V/A/T/S, G73C/S/T/A, and N88D for major protease-associated mutations.
Among the 137 participants remaining on their first-line regimen, 126 (92%) had self-reported adherence data available after VF: 84 (67%) reported 100% adherence during the 4 days prior to evaluation, 35 (28%) reported <100% adherence, and 7 (6%) were not on ART at the evaluation. The virologic suppression rate was 75% for participants who reported 100% adherence, compared with 54% for participants reporting <100% adherence (OR, 0.38; P = .044), and 15% for participants not on ART (OR, 0.06; P = .016) (Table 6). Similar results were found when adjusted for whether or not a participant achieved HIV-1 RNA <400 copies/mL prior to VF and for HIV-1 RNA at VF.
Table 6.
Self-Reported Adherence of Study Treatment After First-line Virologic Failure and Association With Virologic Suppression (HIV-1 RNA <400 Copies/mL) at Week 24 After First-line Virologic Failure for Participants Remaining on the First-line Regimen
| Adherence Within the Past 4 d | No. | Suppression Rate | Univariate Analysisa |
Adjusted Analysisb |
||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |||
| 100% | 84 | 63 (75%) | Reference level | Reference level | ||
| <100% | 35 | 19 (54%) | 0.43 (.19–.99) | .046 | 0.38 (.15–.97) | .044 |
| Not on ART | 7 | 1 (14%) | 0.07 (.01–.58) | .014 | 0.06 (.01–.59) | .016 |
Abbreviations: ACTG, AIDS Clinical Trials Group; ART, antiretroviral therapy; CI, confidence interval; HIV-1, human immunodeficiency virus type 1; OR, odds ratio.
a Both univariate and adjusted analysis was stratified by ACTG study.
b Analysis was adjusted by whether achieving HIV-1 RNA <400 copies/mL at any time prior to initial failure and whether HIV-1 RNA at first-line virologic failure <10 000 copies/mL. In this model, achieving HIV-1 RNA <400 copies/mL at any time prior to initial failure remained significant (odds ratio [OR], 5.01 [95% CI, 1.77–14.24]; P = .002), but not HIV-1 RNA <10 000 copies/mL at the time of virologic failure (OR, 2.41 [95% CI, .81–5.98]; P = .12).
For the 67 participants who changed ART within 24 weeks after first-line VF, the median time from VF confirmation to regimen change was 10 weeks. Forty-three (64%) had HIV-1 RNA <400 copies/mL at 24 weeks after regimen change, including 20 (71%) of 28 participants who switched to a NNRTI-containing regimen, 6 (44%) of 14 who changed to a different PI/r, and 18 (72%) of 25 who only changed 1 or more NRTIs (P = .39). Achieving HIV-1 RNA <400 copies/mL prior to VF was the only significant factor associated with higher suppression rate at 24 weeks after regimen change (OR, 6.50 [95% CI, 1.91–22.11]; P = .003).
Results consistent with those reported above were observed in sensitivity analyses, with the exception that, in the analysis restricted to A5142 and A5202 participants in which virologic suppression was defined as HIV-1 RNA <200 copies/mL, virologic suppression rates at 24 weeks after regimen change differed significantly by type of change (67% of the 15 participants who changed to a NNRTI-based regimen, 33% of the 6 participants who changed to another PI/r, and 57% of the 7 participants who only changed NRTIs; P = .028).
DISCUSSION
This study provides important information that adds to the current knowledge regarding management of ART after VF on first-line PI/r plus 2 NRTIs. Of note, our study suggests that a large proportion of patients failing these regimens can subsequently achieve virologic suppression without changing their ART regimen, particularly if no resistance is detected, if virologic suppression was ever achieved prior to VF, and if self-reported treatment adherence is good.
Our study included participants in randomized trials who experienced VF on PI/r-based regimens. The proportion of participants with treatment-emergent mutations at VF was low—0.5% for major PI-associated and 9% for NRTI-associated mutations. The most common therapeutic strategy within 24 weeks after VF (66%) was to continue the same first-line regimen; 64% of participants doing this achieved HIV-1 RNA <400 copies/mL after 24 weeks. This did not differ significantly from the rate (72%) among those who changed regimen. Because the proportion of participants with resistance-associated mutations was higher among those who changed regimen vs those who did not, we also evaluated virologic suppression rates among the subgroup of participants with no such mutations detected at first-line VF; subsequent suppression rates were almost identical in those who remained on their initial ART regimen vs those who changed (62% vs 61%).
The rarity of major PI-associated resistance mutations following VF on PI/r regimens in this study is consistent with findings in other studies [7–10], resulting from several possible mechanisms: one is a high genetic barrier to resistance and the higher drug concentration achieved with ritonavir boosting [7]; another is that PIs have inhibitory effects in multiple steps in the viral life cycle and act like multiple drugs in one [14]; and a third one is the limited time period during which resistance can be selected due to the short pharmacokinetic half-lives of PI/r regimens allowing rebound of susceptible virus [15]. This raises the possibility, as seen in our study, for subsequent virologic suppression with continued use of the same regimen. Other studies have also shown that delay in treatment switch after failing first-line PI/r does not have substantial impact on subsequent outcome [16, 17]. In the absence of measurable drug resistance, continuing a first-line PI/r regimen might therefore be a reasonable approach, especially for those with lower HIV-1 RNA at VF and successful suppression of HIV-1 RNA prior to VF, as suggested by our multivariate analysis (Table 5). However, these findings should not undermine the importance of resistance testing at the time of VF on a first-line PI/r-based regimen; although the rate of PI resistance was low in our study, there was a higher rate of NRTI resistance. Our study was not able to examine the impact that NRTI resistance had on outcome because these participants were more likely to have regimen changes following VF, and the number with NRTI resistance who remained on the same PI/r-based regimen was small.
For participants who changed ART regimens after first-line VF, no difference in rates of virologic suppression to <400 copies/mL after regimen change was detected among different regimens. However, our study had limited sample size for participants who changed regimens after failing first-line PI/r, restricting the power to detect possible differences among regimens. A sensitivity analysis did, however, suggest that changing to a different PI/r-containing regimen gave a lower rate of suppression to <200 copies/mL than other regimen changes. The latter is similar to the findings in a German cohort, which suggested that switching to a NNRTI-based regimen had improved durability compared with switching to a different PI after VF [18]. However, the newer PI darunavir was not used in these studies, and its use might give better outcomes. As for participants who did not change regimen, achieving an HIV-1 RNA <400 copies/mL prior to initial VF was a significant predictor of subsequent suppression among those who changed regimens. Such prior suppression may be a marker of adequate adherence that is realizable again following VF whether or not a regimen is changed. This is consistent with other studies that have shown that improved adherence after first-line VF is important for the subsequent virologic suppression with or without regimen change [17, 19, 20], and emphasizes the need for ongoing efforts to promote good adherence following VF.
Our study has some limitations, and the findings need to be interpreted with caution. First, our study was observational and involved follow-up of participants in clinical trials. Hence, ART management after first-line VF was not randomized, and treatment options as well as the definition of VF and assays used varied among the 3 studies and between study sites. Identification of VF and subsequent treatment management may have been quicker in these trials than would occur in practice. Also, data were not collected to allow an evaluation of the extent to which a physician's assessment of a patient's adherence to treatment and low-grade PI/r-related toxicity might have determined the approach to treatment management following VF. Second, despite the fact that we combined data from >200 participants from 3 large clinical trials who experienced first-line VF, this sample size likely provides inadequate power to identify some factors that might be associated with clinically relevant differences in outcome. Third, 23% of participants who experienced VF on a PI/r-based regimen were excluded because of limited or no follow-up after VF. The excluded participants had higher CD4 count and lower HIV-1 RNA prior to starting ART than those included but, like those included, had limited resistance at first-line VF. Although we cannot fully assess the impact of these exclusions on our results, among the 21 excluded who had some follow-up, a similar percentage (62%) achieved HIV-1 RNA <400 copies/mL at their last available measurement after initial VF as among included participants at 24 weeks after VF (67%).
CONCLUSIONS
Our findings suggest that if no or limited drug resistance is detected at VF on a first-line PI/r-containing regimen, remaining on the same regimen after VF coupled with strategies to improve adherence could be a reasonable and effective approach to achieving virologic suppression. Further evaluation of approaches to treatment management following VF on a first-line PI/r-containing regimen is warranted.
Notes
Acknowledgments. We thank the study volunteers who participated in AIDS Clinical Trials Group (ACTG) A5142, A5202, and A5208; the ACTG clinical units that enrolled patients in these trials; Frontier Science Foundation for data management; other members of the A5142, A5202, and A5208 protocol teams; and pharmaceutical companies that provided drugs for these studies: Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Boehringer Ingelheim Pharmaceuticals, and Merck & Co.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Financial support. This work was supported by the Statistical and Data Management Center of the AIDS Clinical Trials Group, under the National Institute of Allergy and Infectious Diseases (grant number 1 UM1 AI068634 and the Leadership grant number 1 UM1 AI068636). R. M. G. received support from the National Center for Advancing Translational Sciences (grant number UL1RR024996) and the National Institute of Allergy and Infectious Diseases (grant number UM1AI069419). R. H. received support from National Institute of Allergy and Infectious Diseases (grant number AI064086, AI069432, and AI36214). P. E. S. received support from National Institute of Allergy and Infectious Diseases UM1AI069472 and UM1AI069412). E. S. D. received support from National Institute of Allergy and Infectious Diseases (grant number A1069424) and UCLA CTSI (grant number TR000124). S. A. R. received support from National Institute of Allergy and Infectious Diseases (grant number UM1 AI069494).
Potential conflicts of interest. M. D. H. reports previously being a paid member of data monitoring committees for Boehringer Ingelheim, Medicines Development, Pfizer, and Tibotec. R. H. reports having received honoraria or consultant fees from BMS, Gilead Sciences, and Jannsen, and research support (to UCSD) from Abbott, GlaxoSmithKline, Merck, and Pfizer. R. M. G. reports serving as a coinvestigator on clinical trials sponsored by GlaxoSmithKline and ViiV (with research grants to Weill Cornell Medical College). E. S. D. reports consultant or scientific advisory board fees from Abbvie, Bristol-Myers Squibb, Gilead, Merck, Teva, and ViiV, as well as research support from Bristol-Myers Squibb, Gilead, and ViiV. P. E. S. has served as a consultant or scientific advisory board member for Abbott, BMS, Gilead, GSK, Merck, and Janssen, and has received grant support for research from BMS, Gilead, and GSK. J. S. C. has served as a consultant to ViiV and has received grant support to UCLA from Merck. T. B. C. reports consultant fees from Gilead Sciences. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Department of Health and Human Services; 2013. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf . Accessed 28 January 2014. [Google Scholar]
- 2.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 3.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daar ES, Tierney C, Fischl MA, et al. Atazanavir plus ritonavir or efavirenz as part of 3-drug regimen for initial treatment of HIV-1. Ann Intern Med. 2011;154:445–56. doi: 10.1059/0003-4819-154-7-201104050-00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lockman S, Hughes M, Sawe F, et al. Nevirapine- versus lopinavir/ritonavir-based initial therapy for HIV-1 infection among women in Africa: a randomized trial. PLoS Med. 2012;9:e1001236. doi: 10.1371/journal.pmed.1001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malan DR, Krantz E, David N, et al. Efficacy and safety of atazanavir, with or without ritonavir, as part of once-daily highly active antiretroviral therapy regimens in antiretroviral-naïve patients. J Acquir Immune Defic Syndr. 2008;47:161–7. doi: 10.1097/QAI.0b013e31815ace6a. [DOI] [PubMed] [Google Scholar]
- 7.Lathouwers E, De Meyer S, Dierynck I, et al. Virological characterization of patients failing darunavir/ritonavir or lopinavir/ritonavir treatment in the ARTEMIS study: 96-week analysis. Antivir Ther. 2011;16:99–108. doi: 10.3851/IMP1719. [DOI] [PubMed] [Google Scholar]
- 8.Wallis CL, Mellors JW, Venter WD, et al. Protease inhibitor resistance is uncommon in HIV-1 subtype C infected patients on failing second-line lopinavir/r-containing antiretroviral therapy in South Africa. AIDS Res Treat. 2011;2011:769627. doi: 10.1155/2011/769627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lataillade M, Chiarella J, Yang R, et al. Virologic failures on initial boosted-PI regimen infrequently possess low-level variants with major PI resistance mutations by ultra-deep sequencing. PLoS One. 2011;7:e30118. doi: 10.1371/journal.pone.0030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lillemark MR, Gerstoft J, Obel N, et al. Characterization of HIV-1 from patients with virological failure to a boosted protease inhibitor regimen. J Med Virol. 2011;83:377–83. doi: 10.1002/jmv.21997. [DOI] [PubMed] [Google Scholar]
- 11.Lockman S, Hughes M, McIntyre J, et al. Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med. 2010;363:1499–509. doi: 10.1056/NEJMoa0906626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: fall 2006. Top HIV Med. 2006;14:125–30. [PubMed] [Google Scholar]
- 13.Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: spring 2008. Top HIV Med. 2008;16:62–8. doi: 10.1007/s11750-007-0034-z. [DOI] [PubMed] [Google Scholar]
- 14.Rabi SA, Laird GM, Gurand CM, et al. Multi-step inhibition explains HIV-1 protease inhibitor pharmacodynamics and resistance. J Clin Invest. 2013;123:3848–60. doi: 10.1172/JCI67399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbloom DI, Hill AL, Rabi SA, Siliciano RF, Nowak MA. Antiretroviral dynamics determines HIV evolution and predicts therapy outcome. Nat Med. 2012;18:1378–85. doi: 10.1038/nm.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen ML, van der Laan MJ, Napravnik S, Eron JJ, Moore RD, Deeks SG. Long-term consequences of the delay between virologic failure of highly active antiretroviral therapy and regimen modification. AIDS. 2008;22:2097–106. doi: 10.1097/QAD.0b013e32830f97e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pujades-Rodríguez M, Balkan S, Arnould L, Brinkhof MA, Calmy A AIDS Working Group of MSF. Treatment failure and mortality factors in patients receiving second-line HIV therapy in resource-limited countries. JAMA. 2010;304:303–12. doi: 10.1001/jama.2010.980. [DOI] [PubMed] [Google Scholar]
- 18.Brunner J, Seybold U, Gunsenheimer-Bartmeyer B, Hamouda O, Bogner JR ClinSurv-Studiengemeinschaft (gleichwertiger Beitrag) Long-term efficacy of second-line treatment of HIV infection after class change following virological failure on protease inhibitor-based therapy [in German] Dtsch Med Wochenschr. 2010;135:1166–70. doi: 10.1055/s-0030-1255124. [DOI] [PubMed] [Google Scholar]
- 19.Murphy RA, Sunpath H, Castilla C, et al. Second-line antiretroviral therapy: long-term outcomes in South Africa. J Acquir Immune Defic Syndr. 2012;61:158–63. doi: 10.1097/QAI.0b013e3182615ad1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta RK, Goodall RL, Ranopa M, Kityo C, et al. High rate of HIV resuppression after viral failure on first-line antiretroviral therapy in the absence of switch to second-line therapy. Clin Infect Dis. 2014;58:1023–6. doi: 10.1093/cid/cit933. [DOI] [PMC free article] [PubMed] [Google Scholar]