We collected paired sera from live poultry workers in southern China and identified a high risk of asymptomatic influenza A(H7N9) virus infection, particularly in females and those with longer exposure to poultry.
Keywords: influenza A(H7N9), poultry workers, seroprevalence
Abstract
Background. Confirmed cases of avian influenza A(H7N9) virus infection in humans continue to occur in mainland China. Few confirmed cases have occurred in poultry workers despite potentially higher rates of exposure.
Methods. A serological survey was conducted in May and December 2013 in poultry market workers, and in March and September 2013 in the general population. Blood samples were collected and tested for antibodies to H7N9 and H5N1 viruses by hemagglutination inhibition (HI) assays. Multivariable analysis was employed to identify risk factors related to H7N9 infection indicated by serology among poultry workers.
Results. In the poultry workers, 36 of 501 (7.2%) in May and 56 of 375 (14.9%) in December had HI antibody titers ≥1:160 to H7N9. Of 96 individuals who participated in both surveys, 52 (54.2%) workers had a ≥4-fold rise in H7N9 antibody titers from May to December. In a multivariable analysis, female sex (odds ratio [OR], 2.713; 95% confidence interval [CI], 1.098–6.705) and ≥10 years of occupational exposure (OR, 3.592; 95% CI, 1.246–10.354) were identified as risk factors for infection. Seroprevalence against H5N1 at ≥1:160 was low in May (4/501 [0.8%]) and December (3/375 [0.8%]). In the general population, 0 of 417 individuals in March and 0 of 408 individuals in September had antibody titers ≥1:160 to H7N9 or to H5N1.
Conclusions. Although none of the participants in our study had virologically confirmed H7N9 infection, the high proportion of poultry workers with serologic evidence of H7N9 infection between May and December 2013 suggests a substantial risk of mild H7N9 infections in this group, supporting stricter control measures in live poultry markets.
A novel avian influenza A(H7N9) virus emerged in February 2013 and caused more than 100 laboratory-confirmed human infections in the Yangtze River Delta region in spring 2013 [1, 2]. Although few cases were confirmed in the summer of 2013, human cases resurged in the winter of 2013–2014 with >270 human cases confirmed since 1 January 2014. Since December 2013, most confirmed cases in southern China have been reported in Guangdong province. As more and more confirmed H7N9 cases have been reported, almost all with serious illness requiring hospitalization, the extent of mild infections remains uncertain. Of 139 confirmed cases reported by 1 December 2013, only 6% occurred in persons with occupational exposure to live poultry [1], despite potentially substantial rates of exposure to the virus among this group. On the other hand, mild cases have been detected through sentinel surveillance of influenza-like illnesses [3, 4], indicative of a much larger number of similar cases requiring medical attention in the general population [5], and there could potentially be an even greater number of subclinical infections [6]. Serologic studies are required to identify asymptomatic infections in the population, and we established such a study in 2013.
A few studies have reported low levels of antibody to H7N9 virus among poultry workers before [7] and after the spring 2013 epidemic [8], but more recent data have not been reported in the literature. Two confirmed human cases of H7N9 reported in Hong Kong on 2 and 6 December 2013 reported potential exposure in Shenzhen, at a time when confirmed cases had not yet been reported in humans in Shenzhen although there had been sporadic detections of the virus in live poultry markets [9]. However, on 19 December 2013, the first human case in the city was reported. Having collected sera from poultry workers during the spring outbreak, we immediately collected more sera in late December 2013. The objectives of our study were to examine the seroprevalence of antibody to H7N9 among the general population and among poultry market workers before and during a local H7N9 epidemic, and to identify risk factors for H7N9 infection based on serology.
METHODS
Study Design
The serological surveys of poultry market workers were conducted in May and December 2013. During the first survey conducted in May, poultry market workers were recruited from retail and wholesale poultry markets in all 10 districts of Shenzhen. After obtaining informed consent, a blood sample was drawn from each participant, and a detailed questionnaire (see Supplementary Appendix) was used to collect information about demographic characteristics, history of exposure to poultry, and history of seasonal influenza vaccination. For the second survey in December, poultry market workers were recruited in 7 districts where environmental swabs of poultry market had just tested positive for H7N9 virus, and a shorter questionnaire was used to collect information about demographic characteristics and types of exposure to poultry. For the second survey, we attempted to find and recruit as many participants of the first survey as possible in those poultry markets sampled in both surveys. Nasal swabs were collected from the participating poultry workers in the first survey but not in the second survey.
The cross-sectional serological surveys in the general population were conducted in March and September 2013. In each survey, we aimed to recruit 70 subjects in each of 4 age groups (0–4, 5–14, 15–24, and ≥60 years), plus 140 subjects aged 25–59 years, for a total sample size of 420 subjects. The sex ratio of subjects was controlled at 1:1. These subjects were recruited from the people receiving routine medical examinations in hospital, and from the children in primary school and middle school while a vaccination campaign was conducted simultaneously. In addition to the blood sample, age and sex of participants was also recorded.
Ethical Approval
Ethical approval was obtained from ethics committee of the Shenzhen Center for Disease Control and Prevention.
Laboratory Analysis
A modified horse red blood cell (RBC) hemagglutination inhibition (HI) assay was used to detect immune response to novel influenza A(H7N9) virus and avian influenza A(H5N1) virus infection. Laboratory procedure was developed by the Chinese National Influenza Center [10]. The antigens of A/Anhui/1/2013(H7N9) and A/Shenzhen/01/2011(H5N1) were used for HI test. Horse RBCs displayed a high proportion of sialic acid α2,3-Gal binding, which is preferential for avian influenza virus. It is observed that use of horse RBCs significantly increased the sensitivity of detection of HI antibodies in the sera of confirmed H7N9 cases compared with the use of turkey RBCs, thus the World Health Organization recommended that horse RBCs should be used to detect HI antibodies for H7N9 virus infection [10]. Before the HI assay was conducted, serum specimens were adsorbed with horse RBCs and treated with receptor-destroying enzyme to remove nonspecific agglutinins and virus inhibitions. Sera were tested in doubling dilutions starting at 1:20, and only titers of ≥1:160 were considered seropositive for H7N9. Nasal swabs collected in the first survey were tested for H7N9 virus using real-time reverse transcription polymerase chain reaction (RT-PCR).
Statistical Analysis
Data were analyzed using SPSS software, version 16.0 (SPSS, Chicago, Illinois). 95% confidence interval of proportions was estimated using binomial distributions, and proportions were compared using χ2 tests and Fisher exact tests. Univariable and multivariable logistic regression analyses were performed to identify risk factors for serologic evidence of H7N9 infection. In univariate analysis, variables with a P value <.10 were selected for inclusion in multivariable analysis. Serologic evidence of H7N9 infection, termed “seroconversion” for simplicity, was indicated by a ≥4-fold rise in antibody against H7N9 measured by HI in paired sera plus a titer ≥1: 40 in the second specimen.
RESULTS
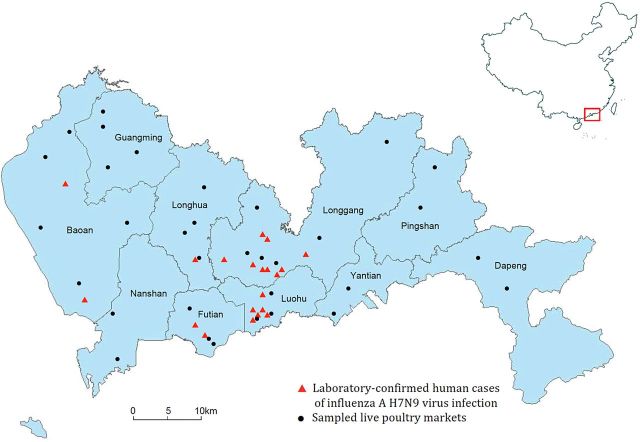
The timeline of our study is shown in Figure 1. A total of 501 serum specimens were obtained from workers in 17 poultry markets in May and 375 specimens were obtained from workers in 24 poultry markets in December. The location of sampled live poultry markets is shown in Figure 2. The subjects in the first and second surveys had the same median age of 41 years. The proportion of women in the second survey was higher than that in the first survey (47.7% vs 40.7%, respectively; P < .05). In the first survey, 345 (68.9%) of the poultry workers were enrolled from the 7 districts where environmental swabs of poultry market subsequently tested positive for H7N9 virus in December 2013. In addition, 41.3% of subjects in the first survey were collected from 5 districts in Shenzhen City where no laboratory-confirmed human cases had been reported by the end of February 2014. In the first survey, 197 (40.6%) subjects reported receipt of seasonal influenza vaccination in the past year (Table 1). All 501 nasal swabs collected from participants in the first survey tested negative for H7N9 using real time RT-PCR.
Figure 1.
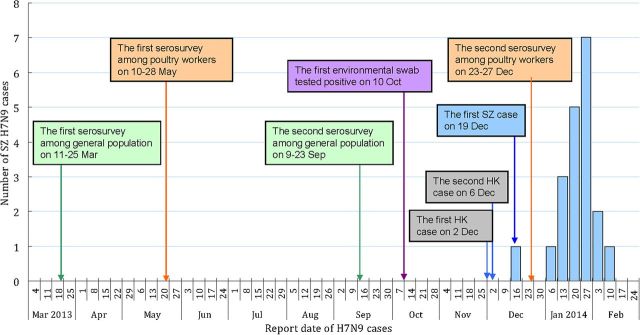
Timeline of sera collection in poultry workers and the general population in 2013, reports of detections of influenza A(H7N9) virus in environmental samples, reports of human cases in Hong Kong (suspected to have been exposed to H7N9 virus in or near live poultry markets in Shenzhen), and reports of human cases in Shenzhen. Abbreviations: HK, Hong Kong; SZ, Shenzhen.
Figure 2.
Location of laboratory-confirmed cases of influenza A(H7N9) virus infection and sampled live poultry markets in 10 districts of Shenzhen.
Table 1.
Characteristics of Poultry Workers in Serological Surveys Conducted in May and December 2013
| Characteristics | First Serosurvey, No. (n = 501) | Second Serosurvey, No. (n = 375) | P Value |
|---|---|---|---|
| Age, y, median (range) | 41 (16–67) | 41 (6–60) | .827 |
| Sex | |||
| Male | 297 (59.3%) | 196 (52.3%) | <.05 |
| Female | 204 (40.7%) | 179 (47.7%) | |
| District | |||
| With confirmed cases | 294 (58.7%) | 316 (84.3%) | <.001 |
| Without confirmed cases | 207 (41.3%) | 59 (15.7%) | |
| With environmental swabs tested positive for H7N9 | 345 (68.9%) | 375 (100%) | <.001 |
| Without environmental swabs tested positive for H7N9 | 156 (31.1%) | 0 (0%) | |
| Type of market | |||
| Retail | 258 (51.5%) | 375 (100%) | <.001 |
| Wholesale | 243 (48.5%) | 0 (0%) | |
| Duration of having job related to selling poultry, median (IQR) | 6.0 (8.00) | NA | |
| Seasonal vaccination in the previous year | NA | ||
| Yes | 197 (40.6%) | ||
| No | 288 (59.4%) | ||
Abbreviations: IQR, interquartile range; NA, not available.
In the first survey, 109 (21.8%) individuals and 36 (7.2%) had H7N9 antibody titers ≥1:80 and ≥1:160, respectively, by HI. In the second survey, 211 (56.3%) and 56 (14.9%) individuals had H7N9 antibody titers ≥1:80 and ≥1:160, respectively, by HI (Table 2). The seroprevalence of H7N9 antibody ≥1:80 or ≥1:160 by age and sex were not statistically significantly different in the first and second surveys. In the first survey, the seroprevalence of H7N9 antibody ≥1:80 or ≥1:160 among poultry workers in wholesale poultry markets was significantly lower than those working in retail poultry markets (P < .05). The poultry workers who reported receipt of seasonal influenza vaccination in the past year had significantly lower probability of having H7N9 antibody titer ≥1:80 or ≥1:160, compared with poultry workers not reporting receipt of vaccination.
Table 2.
Seroprevalence of Influenza A(H7N9) Virus Antibody Titers ≥1:80 or ≥1:160, by Characteristic, Among Poultry Workers in Serological Surveys Conducted in May and December 2013
| Characteristic | First Serosurvey Positive, No. (%, 95% CI) (n = 501) |
Second Serosurvey Positive, No. (%, 95% CI) (n = 375) |
||
|---|---|---|---|---|
| ≥1:80 | ≥1:160 | ≥1:80 | ≥1:160 | |
| Age, y | ||||
| <40 | 43 (19.5, 14.5–25.4) | 11 (5.0, 2.5–8.8) | 84 (49.7, 41.9–57.5) | 23 (13.6, 8.8–19.7) |
| ≥40 | 66 (23.5, 18.7–28.9) | 25 (8.9, 5.8–12.9) | 127 (61.7, 54.6–68.3) | 33 (16.0, 11.3–21.8) |
| Sex | ||||
| Male | 61 (20.5, 16.1–25.6) | 18 (6.1, 3.6–9.4) | 110 (56.1, 48.9–63.2) | 26 (13.2, 8.9–18.9) |
| Female | 48 (23.5, 17.9–30.0) | 18 (8.8, 5.3–13.6) | 101 (56.4, 48.9–63.8) | 30 (16.8, 11.3–22.2) |
| District | ||||
| With confirmed cases | 31 (10.5, 7.3–14.6) | 6 (2.0, .8–4.4) | 161 (50.9, 45.3–56.6) | 31 (9.8, 6.5–12.1) |
| Without confirmed cases | 78 (37.7, 31.1–44.7) | 30 (14.5, 10.0–20.0) | 50 (84.7, 73.0–92.8) | 25 (42.4, 29.6–55.9) |
| With environmental swabs tested positive for H7N9 | 62 (18.0, 14.1–22.4) | 19 (5.5, 3.4–8.5) | 211 (56.3, 51.1–61.4) | 56 (14.9, 11.5–19.0) |
| Without environmental swabs tested positive for H7N9 | 47 (30.1, 23.1–38.0) | 17 (10.9, 6.0–16.9) | 0 | 0 |
| Type of market | ||||
| Retail | 81 (39.1, 32.4–46.1) | 31 (15.0, 10.4–20.6) | 211 (56.3, 51.1–61.4) | 56 (14.9, 11.5–19.0) |
| Wholesale | 28 (9.5, 6.4–13.5) | 5 (1.7, .6–3.9) | 0 | 0 |
| Duration of having job related to selling poultry, y | NA | |||
| <10 | 76 (20.3, 16.4–24.8) | 20 (5.3, 3.1–8.1) | ||
| ≥10 | 26 (26.6, 18.3–36.8) | 13 (13.4, 6.6–20.1) | ||
| Seasonal vaccination in the previous year | NA | |||
| Yes | 20 (10.1, 6.3–15.1) | 4 (2.0, .6–5.1) | ||
| No | 88 (30.2, 25.0–35.9) | 32 (11.1, 7.7–15.3) | ||
| Total | 109 (21.8, 18.2–25.6) | 36 (7.2, 5.1–9.8) | 211 (56.3, 51.1–61.4) | 56 (14.9, 11.5–19.0) |
Abbreviations: CI, confidence interval; NA, not available.
Of 96 poultry market workers who participated in both surveys, 7 (7.3%) participants in the first survey and 15 (15.6%) individuals in the second survey had HI antibody titers ≥1:160, including 3 individuals who had titers ≥1:160 in both surveys. Among the 12 who developed HI antibody titers ≥1:160 between May and December, in May, 5 had titers of <1:20, 3 had titers of 1:40, and 4 had titers of 1:80. Among the 96 workers, 52 (54.2%) had seroconversion—that is, a titer of ≥1:40 in December and a ≥4-fold rise in H7N9 antibody titer between May and December. The characteristics and exposure histories of these 96 workers in relation to the risk of seroconversion are shown in Table 3. The risk of seroconversion among female poultry workers and poultry workers with occupational exposure history of ≥10 years was significantly higher than that among male poultry workers and poultry workers with occupational exposure history of <10 years, respectively. In multivariable analysis, female poultry workers (odds ratio [OR], 2.713; 95% confidence interval [CI], 1.098–6.705) and poultry workers with occupational exposure history of ≥10 years (OR, 3.592; 95% CI, 1.246–10.354) both remained significantly associated with seroconversion (Table 4). Regarding antibody against H5N1, 4 of 501 (0.8%) individuals in the first survey and 3 of 375 (0.8%) individuals in the second survey had antibody titers ≥1:160 by HI.
Table 3.
Characteristics of 96 Poultry Workers With Paired Serum in Serological Surveys Conducted in May and December 2013
| Characteristics | Seroconversion, No.(%, 95% CI) (n = 52) | OR (95% CI) | P Value |
|---|---|---|---|
| Age, y | |||
| <40 | 22 (48.9, 33.7–64.2) | Reference | |
| ≥40 | 30 (58.8, 44.1–72.4) | 1.364 (.608–3.057) | .452 |
| Sex | |||
| Male | 17 (38.6, 24.3–54.5) | Reference | |
| Female | 35 (67.3, 52.9–79.7) | 3.270 (1.413–7.567) | <.01 |
| District | |||
| Without confirmed cases | 6 (11.5, 2.8–20.2) | Reference | |
| With confirmed cases | 46 (88.5, 79.8–97.2) | 2.255 (.747–6.808) | .149 |
| Seasonal vaccination in the previous year | |||
| Yes | 27 (52.9, 39.3–66.5) | Reference | |
| No | 24 (47.1, 33.5–60.7) | 0.667 (.291–1.525) | .337 |
| Exposure to poultry | |||
| Feeding | |||
| No | 48 (53.3, 42.5–63.9) | Reference | |
| Yes | 4 (66.7, 22.3–95.7) | 1.750 (.305–10.042) | .530 |
| Cleaning henhouse | |||
| No | 39 (50.0, 38.5–61.5) | Reference | |
| Yes | 13 (72.2, 46.5–90.3) | 2.600 (.846–7.991) | .095 |
| Eating | |||
| No | 50 (59.5, 48.3–70.1) | Reference | |
| Yes | 2 (16.7, 2.1–48.4) | 0.136 (.028–.660) | <.05 |
| Processing (killing, plucking, washing, cutting, curing, cooking) | |||
| No | 41 (54.7, 42.8–66.2) | Reference | |
| Yes | 11 (52.4, 29.8–74.3) | 0.912 (.346–2.405) | .853 |
| Transportation | |||
| No | 47 (52.8, 42.0–63.5) | Reference | |
| Yes | 5 (71.4, 29.0–96.3) | 2.234 (.411–12.129) | .352 |
| Capturing | |||
| No | 42 (51.9, 40.5–63.1) | Reference | |
| Yes | 10 (66.7, 38.4–88.2) | 1.857 (.583–5.916) | .295 |
| Selling | |||
| No | 12 (60, 36.1–80.9) | Reference | |
| Yes | 40 (52.6, 40.8–64.2) | 0.741 (.282–2.017) | .557 |
| Years of exposure | |||
| <10 y | 29 (47.5, 34.6–60.7) | Reference | |
| ≥10 y | 22 (78.6, 59.1–91.7) | 4.046 (1.440–11.369) | <.01 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Table 4.
Multivariable Analyses of Risk Factors Associated With Influenza A(H7N9) Virus Infection Among 96 Poultry Workers in Serological Surveys Conducted in May and December 2013
| Characteristics | Adjusted OR (95% CI) | P Value |
|---|---|---|
| Sex | ||
| Male | Reference | |
| Female | 2.713 (1.098–6.705) | .031 |
| Years of exposure | ||
| <10 y | Reference | |
| ≥10 y | 3.592 (1.246–10.354) | .018 |
Abbreviations: CI, confidence interval; OR, odds ratio.
A total of 825 serum specimens were obtained from the 2 cross-sectional studies in the general population, with 417 in March and 408 in September 2013. All specimens had H7N9 antibody titers of <1:160 by HI (Table 5). Only 2 individuals from the September survey had antibody titers of 1:80, and 13 individuals from September survey had antibody titers of 1:40 to H7N9. All specimens had H5N1 antibody titers <1:160 by HI (Table 5). Only 1 individual from the September survey had antibody titers of 1:80, and 16 individuals from the September survey had antibody titers of 1:40 to H5N1.
Table 5.
Characteristics and Antibodies Against Influenza A(H7N9) and A(H5N1) Viruses Among General Population in Serological Surveys Conducted in March and September 2013
| Characteristics | March Serosurvey (n = 417), No. (%) |
September Serosurvey (n = 408), No. (%) |
||||
|---|---|---|---|---|---|---|
| Overall | H7N9 Titer ≥1:80 | H5N1 Titer ≥1:80 | Overall | H7N9 Titer ≥1:80 | H5N1 Titer ≥1:80 | |
| Age, y | ||||||
| 0–4 | 61 (14.6) | 0 | 0 | 59 (14.5) | 0 | 0 |
| 5–14 | 87 (20.9) | 0 | 0 | 73 (17.9) | 0 | 0 |
| 15–24 | 65 (15.6) | 0 | 0 | 56 (13.7) | 0 | 0 |
| 25–59 | 152 (36.5) | 0 | 0 | 154 (37.7) | 2 | 1 |
| ≥60 | 52 (12.5) | 0 | 0 | 66 (16.2) | 0 | 0 |
| Sex | ||||||
| Male | 203 (48.7) | 0 | 0 | 194 (47.5) | 0 | 0 |
| Female | 214 (51.3) | 0 | 0 | 214 (52.5) | 0 | 0 |
| District | ||||||
| With environmental swabs tested positive for H7N9 | 357 (85.6) | 0 | 0 | 348 (85.3) | 0 | 0 |
| Without environmental swabs tested positive for H7N9 | 60 (14.4) | 0 | 0 | 60 (14.7) | 0 | 0 |
| Total | 417 | 0 | 0 | 408 | 2 | 1 |
DISCUSSION
Our study identified a substantial increase in seroprevalence of antibody against H7N9 among poultry workers in Shenzhen, from 7.2% to 14.9% between May and December 2013. Furthermore, 52 of 96 (54.2%) poultry workers who provided samples in both studies had reliable serologic evidence of H7N9 infection during the period between May and December based on ≥4-fold rises in antibody titer. At the time we collected the second sets of sera in December, H7N9 viruses were detected in environmental samples in some poultry markets two months earlier, and a human case had just been reported, consistent with gradually increasing prevalence of H7N9 in poultry as the winter progressed. It can therefore be hypothesized that risk of infection in poultry workers could have further increased in early 2014. We are not aware of other reports of such high risk of H7N9 infection among poultry workers as demonstrated here. One study in Zhejiang province reported that 1.3% of poultry workers had H7N9 antibody titers ≥1:160 by HI in April–May 2013 [8]. An earlier study reported that 0 of 1554 poultry workers in eastern China had H7N9 antibody titers ≥1:160 by HI between January and November 2012 [7]. A study of H7N7 in the Netherlands did identify similarly high levels of seropositivity, albeit at very low antibody titers, among exposed poultry workers [11].
Prior studies on serological responses in confirmed human cases of H7N9 reported generally low antibody titers in convalescence, with <50% of cases reaching convalescent titers ≥1:160 [8, 12, 13], although this might be partly attributed to the older age of many of those cases and immunosenescence. On the other hand, poultry workers may have had prior exposures to other avian viruses including H7 subtypes, leading to increased boosting of anti-H7 antibody after an H7N9 infection. We identified 21.8% of poultry workers with H7N9 antibody titers of 1:80 in May 2013, and a very substantial increase to 56.3% in December 2013, whereas very few adults in the general population had antibody titers at this level.
We identified 2 risk factors for serologic evidence of H7N9 infection based on a ≥4-fold rise in antibody titers in paired sera—namely, female sex and a longer history of occupational exposure (Table 4). The increased risk in females could be related to the types of duties, with women more often responsible for selling poultry, defeathering and cleaning, and men more likely to be responsible for transporting and slaughtering poultry. However we did not identify any specific types of exposure associated with a higher risk of infection in the univariate analyses (Table 3), and females generally report greater use of preventive measures [14]. While there have been a greater number of confirmed H7N9 cases in men vs women in the general population, relatively few confirmed H7N9 cases have occurred in live poultry workers, and patterns of exposure by sex could be quite different. Longer history of occupational exposure could be associated with reduced precautionary behaviors, or could partially reflect confounding by age if older persons were more susceptible to infection.
In addition, we identified that working in a retail rather than a wholesale market was associated with increased risk of antibody titer ≥1:160 in May 2013 (Table 2). The wholesale poultry market functions as a distribution center of live poultry. Batches of live poultry are transported to market from other places and sold to retailers approximately 1–3 days after arrival. The short-term stay at wholesale market could limit the workers' contact with live poultry. Furthermore, wholesale market staff usually clean and disinfect common area of the market twice a week, providing the owners of poultry houses with free detergents and requiring them to disinfect poultry house when every batch of live poultry is sold. Therefore shorter contact with poultry and better disinfection measures could have reduced the risk for wholesale market workers compared with retail market workers. One previous study found that poultry retailers had much higher anti-H9 antibody seroprevalence compared with wholesalers (15.5% vs 6.6%) [15].
In contrast to poultry workers, we did not find evidence of H7N9 infections among the general population (Table 5), and the estimated seroprevalence of antibody titers ≥1:160 in our September 2013 survey was 0% (95% CI, 0%–.87%), indicating that a large epidemic of asymptomatic infections had not occurred by that date. However, we cannot rule out a smaller number of asymptomatic infections, and larger serological studies would be needed to reduce our upper bound of 0.87%. Although some family clusters of cases have been reported [1], the critical sites on the H7N9 genome have remained unchanged, and H7N9 has not yet developed the capability to transmit easily from human to human. Our finding is consistent with a study from Zhejiang province in April–May 2013, which reported that none of 1129 general individuals were seropositive to H7N9 at titers ≥1:160, and only 9 (0.8%) had HI titers of 1:40 [8].
Our study has a number of limitations. We did not conduct intensive follow-up of the poultry workers or the general population to identify influenza-like illnesses or to virologically confirm H7N9 infections during our study period, and our inferences on the incidence of H7N9 infections are based only on serological evidence. Antibody to H7N9 measured by HI may indicate antibody generated after H7N9 infection, or cross-reactive antibody after a different influenza virus infection. We also investigated seroprevalence of antibody to H5N1, and found low seroprevalence, similar to other studies [15, 16]. Finally, we did not collect detailed exposure information to allow us to assess the association between seroprevalence and the frequency or duration of exposure to different types of poultry.
Whereas the identification of mild H7N9 infections through sentinel surveillance [3, 4] indicates that the number of H7N9 infections substantially exceeds the number of laboratory-confirmed cases [5], our results are consistent with a substantial number of H7N9 infections among poultry workers despite relatively few confirmed cases having occurred in these persons [1, 2]. Interventions in retail markets may be particularly important in controlling risk. Increases in the incidence of human cases in Shenzhen in January and February 2014 suggest that prevalence in poultry may have also increased during this period, compared with the preceding months, and further serological studies in poultry workers and the general population would be valuable to improve our understanding of the number of infections that have occurred in the present winter outbreak.
Finally, seasonal influenza vaccination is recommended for persons with occupational exposure to poultry, to reduce the risk of joint infections with human and avian viruses and consequent risk of virus reassortment or recombination, and our study demonstrates the importance of promoting seasonal influenza vaccination in poultry workers in Shenzhen, who faced a high risk of H7N9 infection in 2013.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the Chinese National Influenza Center (World Health Organization Influenza Reference Laboratory) for providing H7N9 antigen, and staff members of 10 district Centers for Disease Control and Prevention of Shenzhen for collecting blood samples and related information.
Author contributions. J. C., H. M., and R. Z. designed and supervised the study. S. F., X. L., X. W., B. P., W. W., and Y. T. conducted HI tests. X. W., S. F., X. L., X. T., J. H., X. X., S. M., and D. K. gathered data. X. T., X. W., C. X., and B. J. C. analyzed data. X. W., C. X., B. J. C., and X. T. drafted the article, and all authors contributed to review and revision and have seen and approved the final version.
Financial support. C. X. and B. J. C. received financial support from the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant number U54 GM088558) and the Area of Excellence Scheme of the Hong Kong University Grants Committee (grant number AoE/M-12/06).
Potential conflicts of interest. B. J. C. reports receipt of research funding from MedImmune Inc and Sanofi Pasteur, and consults for Crucell NV. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Li Q, Zhou L, Zhou M, et al. Epidemiology of human infections with avian influenza A(H7N9) virus in China. N Engl J Med. 2014;370:520–32. doi: 10.1056/NEJMoa1304617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowling BJ, Jin L, Lau EH, et al. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population-based study of laboratory-confirmed cases. Lancet. 2013;382:129–37. doi: 10.1016/S0140-6736(13)61171-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu C, Havers F, Wang L, et al. Monitoring avian influenza A(H7N9) virus through national influenza-like illness surveillance, China. Emerg Infect Dis. 2013;19:1289–92. doi: 10.3201/eid1908.130662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ip DK, Liao Q, Wu P, et al. Detection of mild to moderate influenza A/H7N9 infection by China's national sentinel surveillance system for influenza-like illness: case series. BMJ. 2013;346:f3693. doi: 10.1136/bmj.f3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu H, Cowling BJ, Feng L, et al. Human infection with avian influenza A H7N9 virus: an assessment of clinical severity. Lancet. 2013;382:138–45. doi: 10.1016/S0140-6736(13)61207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uyeki TM, Cox NJ. Global concerns regarding novel influenza A(H7N9) virus infections. N Engl J Med. 2013;368:1862–4. doi: 10.1056/NEJMp1304661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai T, Zhou J, Shu Y. Serologic study for influenza A (H7N9) among high-risk groups in China. N Engl J Med. 2013;368:2339–40. doi: 10.1056/NEJMc1305865. [DOI] [PubMed] [Google Scholar]
- 8.Yang S, Chen Y, Cui D, et al. Avian-origin influenza A(H7N9) infection in influenza A(H7N9)-affected areas of China: a serological study. J Infect Dis. 2014;209:265–9. doi: 10.1093/infdis/jit430. [DOI] [PubMed] [Google Scholar]
- 9.Avian influenza report, vol 9, no. 49, reporting period 1–7 December 2013 (week 49) 2013. Available at: http://www.chp.gov.hk/files/pdf/2013_avian_influenza_report_vol9_wk49.pdf. Accessed 24 February 2013.
- 10.Serological detection of avian influenza A(H7N9) virus infections by modified horse red blood cells haemagglutination-inhibition assay. Available at: http://www.who.int/influenza/gisrs_laboratory/cnic_serological_diagnosis_hai_a_h7n9_20131220.pdf . Accessed 27 May 2013.
- 11.Meijer A, Bosman A, van de Kamp EE, Wilbrink B, Du Ry van Beest Holle M, Koopmans M. Measurement of antibodies to avian influenza virus A(H7N7) in humans by hemagglutination inhibition test. J Virol Methods. 2006;132:113–20. doi: 10.1016/j.jviromet.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Zhang A, Huang Y, Tian D, et al. Kinetics of serological responses in influenza A(H7N9)-infected patients correlate with clinical outcome in China, 2013. Euro Surveill. 2013;18:20657. doi: 10.2807/1560-7917.es2013.18.50.20657. [DOI] [PubMed] [Google Scholar]
- 13.Lin PH, Chao TL, Kuo SW, et al. Virological, serological, and antiviral studies in an imported human case of avian influenza A(H7N9) virus in Taiwan. Clin Infect Dis. 2014;58:242–6. doi: 10.1093/cid/cit638. [DOI] [PubMed] [Google Scholar]
- 14.Li T, Feng J, Qing P, et al. Attitudes, practices and information needs regarding novel influenza A (H7N9) among employees of food production and operation in Guangzhou, southern China: a cross-sectional study. BMC Infect Dis. 2014;14:4. doi: 10.1186/1471-2334-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M, Fu CX, Zheng BJ. Antibodies against H5 and H9 avian influenza among poultry workers in China. N Engl J Med. 2009;360:2583–4. doi: 10.1056/NEJMc0900358. [DOI] [PubMed] [Google Scholar]
- 16.Huo X, Zu R, Qi X, et al. Seroprevalence of avian influenza A (H5N1) virus among poultry workers in Jiangsu Province, China: an observational study. BMC Infect Dis. 2012;12:93. doi: 10.1186/1471-2334-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.