Abstract
Atherothrombosis is no longer considered solely a disorder of lipoprotein accumulation in the arterial wall. Rather, the initiation and progression of atherosclerotic lesions is currently understood to have major inflammatory influences that encompass components of both the innate and acquired immune systems. Promising clinical data for ‘upstream’ biomarkers of inflammation such as interleukin-6 (IL-6) as well as ‘downstream’ biomarkers such as C-reactive protein, observations regarding cholesterol crystals as an activator of the IL-1β generating inflammasome, and recent Mendelian randomization data for the IL-6 receptor support the hypothesis that inflammatory mediators of atherosclerosis may converge on the central IL-1, tumour necrosis factor (TNF-α), IL-6 signalling pathway. On this basis, emerging anti-inflammatory approaches to vascular protection can be categorized into two broad groups, those that target the central IL-6 inflammatory signalling pathway and those that do not. Large-scale Phase III trials are now underway with agents that lead to marked reductions in IL-6 and C-reactive protein (such as canakinumab and methotrexate) as well as with agents that impact on diverse non-IL-6-dependent pathways (such as varespladib and darapladib). Both approaches have the potential to benefit patients and reduce vascular events. However, care should be taken when interpreting these trials as outcomes for agents that target IL-6 signalling are unlikely to be informative for therapies that target alternative pathways, and vice versa. As the inflammatory system is redundant, compensatory, and crucial for survival, evaluation of risks as well as benefits must drive the development of agents in this class.
Keywords: Inflammation, Atherosclerosis, C reactive protein, Interleukin-6, Inflammasome, Methotrexate, Canakinumab, Salsalate, Darapladib, Colchicine
Introduction
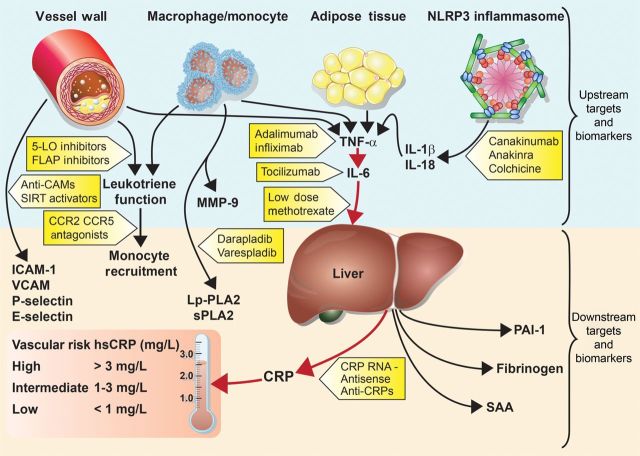
In addition to being a disorder of lipid accumulation, atherothrombosis is understood to have major inflammatory influences that interact with atherogenic lipoproteins to accelerate disease progression, ultimately leading to plaque rupture and clinical events.1 Multiple cell types deriving from monocyte/macrophage lines have been implicated in atherogenesis, as have several chemokines, cytokines, and adhesion molecules that derive from the vessel wall or from tissue sources that associate with vascular risk such as central adipose.2,3 Partly on this basis, it has been hypothesized that targeting different inflammatory pathways might have efficacy in the treatment and prevention of cardiovascular disease.4 In particular, as outlined in Figure 1, several emerging lines of evidence support the hypothesis that inhibition of the central immune pathway linking interleukin-1 (IL-1), tumour necrosis factor (TNF-α), and IL-6 might serve as a potent target for atherothrombotic protection.
Figure 1.
Inflammatory pathways as potential targets for atherosclerotic therapies. IL-1b, interleukin-1-beta; IL-18, interleukin-18; IL-6, interleukin-6; TNF-α, tumour necrosis factor-alpha; MMP-9, matrix metalloproteinase-9; Lp-PLA2, lipoprotein-associated phospholipase A2; sPLA2, secretory phospholipase A2; ICAM-1, intercellular adhesion molecule type 1; VCAM, vascular cellular adhesion molecule; PAI-1, plasminogen activator inhibitor type-1; SAA, serum amyloid A; CRP, C-reactive protein; hsCRP, high-sensitivity C-reactive protein.; 5-LO, 5-lipoxygenase; FLAP, 5-lipoxygenase-activating protein; SIRT1, sirtuin-1; CCR2/CCR5, chemokine receptor types 2 and 5.4
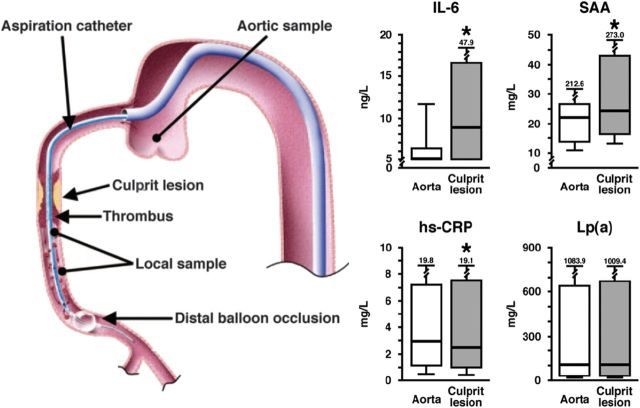
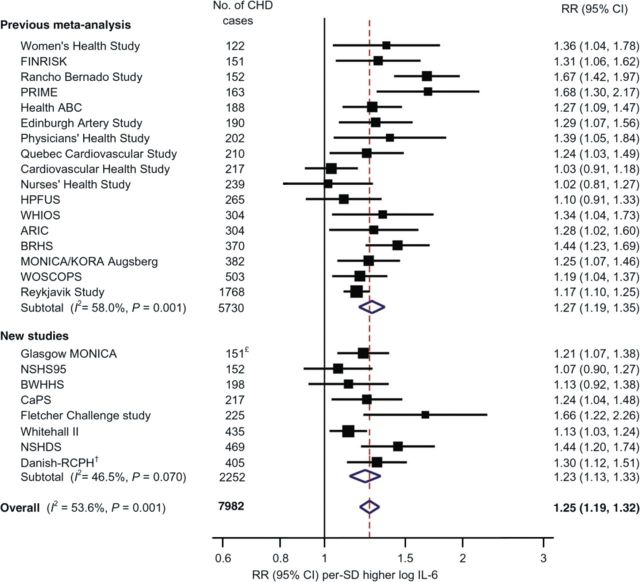
First, of data linking inflammatory biomarkers to future vascular events, evidence is most robust for the ‘upstream’ biomarkers TNF-α and IL-65 which lead to hepatic production of the clinically useful ‘downstream’ biomarker high-sensitivity (hs) C-reactive protein.4 Indeed, IL-6 is highly up-regulated at the site of coronary occlusion in patients with ST-segment elevation infarction, while C-reactive protein is not6 (Figure 2). In comprehensive meta-analyses, C-reactive protein has been shown to add as much to vascular risk prediction as either total or HDL-cholesterol. While hs C-reactive protein is more stable than IL-6 and thus easier to use in clinical practice, epidemiological data consistently show IL-6 levels to correlate with future vascular risk (Figure 3).7 In recent overviews, of several inter-correlated measures of inflammation, only two biomarkers—downstream C-reactive protein (hazard ratio 1.69 per 1 SD higher baseline level) and upstream TNF-α (hazard ratio 1.32 per 1 SD higher baseline level)-remained independently significant predictors of future vascular risk.7
Figure 2.
Interleukin-6 and serum amyloid A levels at the site of coronary occlusion and in the peripheral blood.6
Figure 3.
Plasma level of interleukin-6 and the risk of future vascular events.7
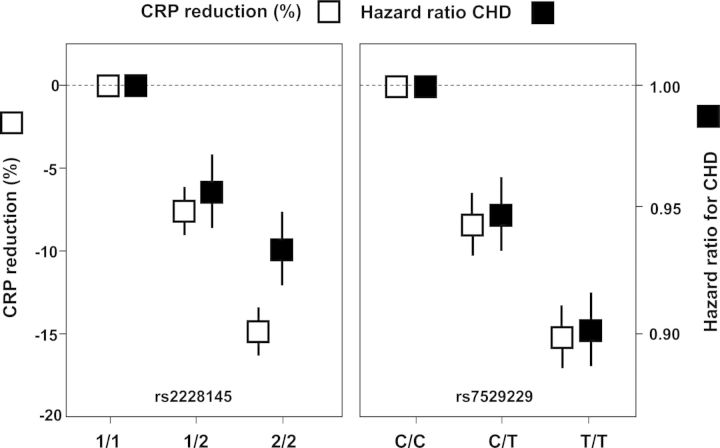
Secondly, two Mendelian randomization studies demonstrate that genetic polymorphism in the IL-6 receptor signalling pathway concordantly leads to reduced plasma levels of C-reactive protein and to reduced vascular risk8,9 (Figure 4). While such data do not prove a causal relationship between inflammation and vascular events, they do provide elegant support for the concept that targeting IL-6 signalling may be a novel method for atheroprotection.
Figure 4.
Genetic polymorphism in the IL-6 regulatory pathway associate with lifelong lower levels of C-reactive protein and with concordantly lower vascular event rates.8,9
Thirdly, as will be discussed in greater detail, recent evidence has directly linked deposition of cholesterol crystals to activation of the IL-1β producing nucleotide-binding leucine-rich repeat-containing pyrin receptor (NLRP3) inflammasome.10,11 Since IL-1β production leads to increased levels of IL-6 and C-reactive protein, these data provide a mechanistic link between early deposition of cholesterol crystals within the vessel wall to the macrophage–monocyte interactions that initiate fatty streaks and promote local atherosclerotic progression.
Fourthly, while statins are powerful LDL-lowering drugs proven to reduce vascular risk in many patient groups, these agents also reduce C-reactive protein.12,13 Statins reduce C-reactive protein in relation to potency and across dose ranges, but the magnitude of C-reactive protein reduction for individuals cannot be predicted on the basis of LDL reduction. Polymorphisms that influence statin-induced changes in LDL-cholesterol are separate and distinct from those that influence statin-induced changes in C-reactive protein.14,15 The JUPITER primary prevention trial showed that providing statin therapy to individuals with high levels of inflammation prevents cardiovascular events and reduces all-cause mortality, even if those individuals already have low levels of LDL-cholesterol.16 JUPITER also demonstrated that statins reduce venous thrombosis, an intriguing finding as there are no atherosclerotic plaques to rupture in the venous wall and LDL-cholesterol has little relation to stasis-induced thrombosis.17 Further, the greatest absolute risk reductions associated with statin therapy in JUPITER were observed among those with the highest levels of inflammation at a trial entry.18 As previously shown in the PROVE-IT TIMI 22 trial, the greatest relative risk reductions were also seen among those who not only reduced LDL-cholesterol <70 mg/dL, but who also reduced hs C-reactive protein below 1 mg/L.19
These and other observations surrounding C-reactive protein and statin therapy provide evidence that lipid lowering is in part an anti-inflammatory therapy.20 Intensification of statin therapy results in rapid reduction of atherosclerotic inflammation as imaged by FDG-PET/CT.21 Mechanisms for the anti-inflammatory effects of statin therapy include impaired prenylation of small G proteins and the expression of transcription factors such as Kruppel-like factor 2 which promotes transcription of genes sets with anti-thrombotic, anti-inflammatory, and anti-proliferative functions.22,23
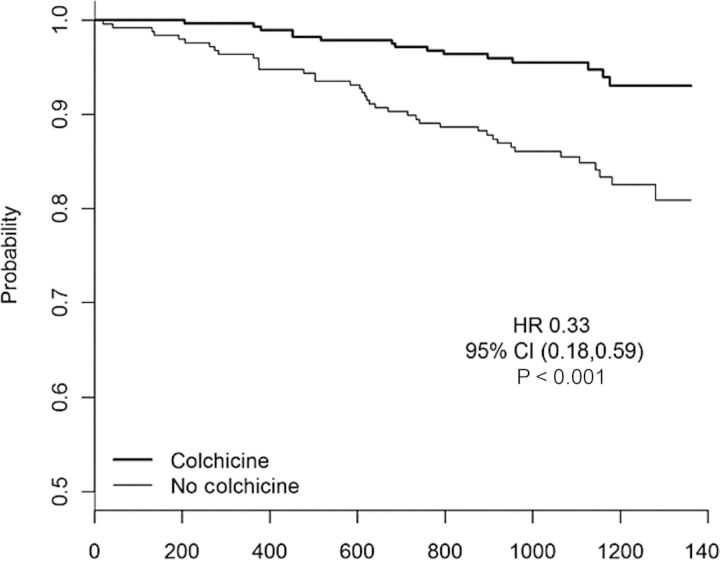
On the basis of these accumulating data, it is convenient to categorize anti-inflammatory agents undergoing evaluation as potential atheroprotective drugs into two groups, those that do and do not target the central IL-1, TNF-α, and IL-6 pathway. As shown in Figure 5, Phase III trials are now underway with anti-inflammatory agents that lead to marked reductions in IL-6 and C-reactive protein (such as canakinumab and methotrexate) as well as with agents that do not (such as varespladib and darapladib). Care must be taken when interpreting these studies as outcomes for drugs that target IL-6 signalling may be uninformative for agents that do not, and vice versa. Examples of these two groups of agents and their potential to impact on vascular risk are reviewed below.
Figure 5.
Phase III trials of anti-inflammatory agents under evaluation in cardiovascular disease. Trials are grouped by agents that do and do not impact primarily on the central IL-1, TNF-α, IL-6 signalling system.
Agents that inhibit the central interleukin-1, tumour necrosis factor-α, interleukin-6 regulatory pathway
Interleukin-1 antagonists and the nucleotide-binding leucine-rich repeat-containing pyrin receptor 3 inflammasome
Interleukins are critical mediators of the systemic anti-inflammatory response. Of inflammatory molecules implicated in atherothrombosis, IL-1 plays a prominent role as it sits proximal to the classical IL-6 signalling cascade. Thus, IL-1 has long been considered a target for novel vasculoprotective agents.24
The IL-1 type 1 receptor is impacted upon by two activators (IL-1α and IL-1β) and by the naturally occurring inhibitor, IL-1 receptor antagonist (IL-1Ra) that competitively blocks binding of IL-1α and β to the type 1 receptor. In a clinically important additional level of regulation, innate immune cells including monocytes initially produce IL-1β as an inactive precursor (pro-IL-1β) that requires proteolytic cleavage to attain biological activity. This is typically mediated by a complex of intracellular proteins known as the NLRP3 inflammasome which, in response to the presence of crystalline structures, leads to activation of caspase-1 (also known as IL-1β-converting enzyme).25 While this mechanism of IL-1β activation was originally described as a danger signal triggered by exogenous crystals such as alum, silica, or asbestos, the same NLRP3 inflammasome is also activated by endogenous agents such as uric acid and cholesterol when they move from a soluble to crystalline form.10,11 The recognition that cholesterol crystals can activate IL-1β production has provided a common hypothesis linking hyperlipidaemia to vascular inflammation.
Activated macrophages within atheromatous plaques express IL-1 resulting in smooth muscle proliferation, recruitment of additional inflammatory cell lines into the plaque and endothelial wall, a triggering of an auto-inductive pro-inflammatory process, and critical regulation of upstream induction of IL-6 and downstream production of C-reactive protein.7,26 Atheroprone oscillatory flow has recently been shown to additionally increase NLRP3 expression, activation of caspase-1, and IL-1β production.27
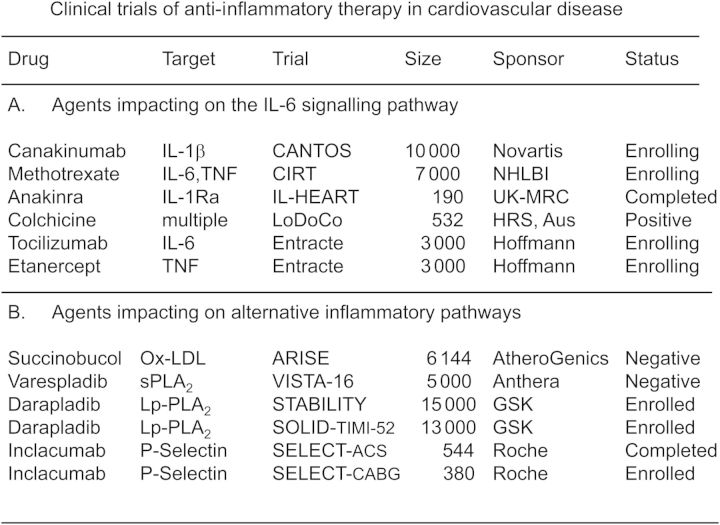
On the basis of these data, a pivotal trial in secondary prevention has been initiated known as the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS).28 Canakinumab is a human monoclonal anti-human IL-1β antibody indicated for the treatment of several rare IL-1β over-expression disorders. In high vascular risk patients, canakinumab produces dose-dependent reductions of >50% for IL-6 and C-reactive protein, as well as a 20% reduction in fibrinogen, a third mediator of thrombosis and inflammation29 (Figure 6). In clinical data among those with rheumatoid arthritis (RA) and diabetes, canakinumab has shown little effect on lipid levels or platelet function. Thus, canakinumab provides a novel method to directly test the inflammatory hypothesis of atherothrombosis by inhibiting the central IL-1, TNF-α, and IL-6 pathway without confounding effect on lipids or coagulation.
Figure 6.
Phase II data demonstrating dose-dependent effects of canakinumab, a monoclonal antibody targeting IL-1β, on plasma levels of fibrinogen, IL-6, and C-reactive protein.29
Now fully enrolled, CANTOS includes 10 000 participants with stable coronary artery disease who remain at high inflammatory risk due to a persistent elevation of C-reactive protein (>2 mg/L) despite usual therapy, including statins. CANTOS is an event-driven trial in which the primary endpoint is a reduction in rates of recurrent major cardiovascular events. Critical secondary endpoints include evaluations of all-cause mortality and a slowing of diabetic progression. This latter endpoint reflects the recognition that diabetes is also an inflammatory disorder driven in part by IL-1 activation.30
At least two other IL-1 antibodies are in development as potential agents for atherothrombosis. In addition, the exogenous IL-1Ra anakinra (an approved agent for RA which has been shown to improve vascular function in that setting).31,32 Anakinra also has efficacy in diabetes by reducing HbA1c, the pro-insulin:insulin, IL-6, and C-reactive protein.33
Methotrexate and the tumour necrosis factor and interleukin-6 inhibitors
Patients with systemic inflammatory disorders such as RA have TNF-α-induced endothelial dysfunction which can be reversed by the specific antibody infliximab34 as well as increased vascular event rates. Observational data suggest that these rates decline when patients are treated with agents that inhibit the IL-6 signalling system. A recent meta-analysis reported that patients with RA or psoriatic arthritis (PsA) taking low-dose methotrexate (LDM) have a 21% lower risk of future cardiovascular events.35 Similar data have been presented for RA patients taking TNF-inhibitors such as etanercept and infliximab or the IL-6 inhibitor tocilizumab. Interleukin -6 inhibition with tocilizumab has been reported to improve endothelial function and reduces arterial stiffness.36 However, as tocilizumab increases LDL-cholesterol levels, studies of this agent to date have largely focused on cardiovascular safety rather than efficacy. Among RA patients treated with TNF-inhibitors, modest improvements in cardiovascular risk profile have been observed in some studies.37 In the setting of heart failure, however, etanercept failed to improve clinical outcomes and was associated with infection and adverse lipoprotein changes.38
To address some of these issues, the ENTRACTE investigators are undertaking a randomized, open-label clinical trial comparing the effects of IL-6 receptor blockade with tocilizumab to TNF-inhibition with etanercept on the rate of vascular events among 3000 patients with moderate-to-severe RA (ClinicalTrials.gov Identifier: NCT01331837). Rheumatoid arthritis patients 50 years of age or greater with an inadequate clinical response to at least one non-biological disease-modifying anti-rheumatic drug and a history of coronary heart disease or multiple cardiovascular risk factors are eligible for ENTRACTE. This trial has no placebo group as all enrolled patients have a systemic inflammatory disorder requiring treatment; whether results from ENTRACTE will generalize to populations without clinically manifest inflammatory disorders is thus uncertain.
Similarly, everolimus used in transplantation medicine has the potential to reduce inflammation via the mTOR pathwys. The CLEVER-ACS trial (ClinicalTrials.gov Identifier: NCT01529554) is about to enroll patients with ST-segment elevation myocardial infarction to determine whether such an anti-inflammatory therapy will reduce infarct size, remodelling and clinical evetns in this patient population.
In 2013, the National Heart Lung and Blood Institute initiated the Cardiovascular Inflammation Reduction Trial (CIRT) in which 7000 patients with chronic atherosclerosis and either diabetes or metabolic syndrome will be randomized to usual care plus placebo or to usual care plus LDM.39 Low-dose methotrexate (15–20 mg per week) is the treatment of choice for RA and PsA, and is taken safely on a long-term basis by several hundred thousand patients worldwide. Among those with RA and PsA, LDM reduces IL-6 and C-reactive protein and thus impacts directly on the central IL-6 signalling pathway. Emerging evidence suggests that at least part of the anti-inflammatory and atheroprotective effects of LDM result from increased adenosine release and subsequent antagonism of the adenosine A2A receptor.40 Stimulation of this receptor induces the expression of several proteins associated with reverse cholesterol transport including 27-hydroxylase and the ATP-binding cassette transporter, preventing formation of foam cells from human macrophages.41 A2A stimulation also attenuates inflammation directly and reduces the expression of adhesion molecules such as intercellular adhesion molecule type 1 (ICAM) and vascular cellular adhesion molecule (VCAM) commonly associated with atheroma progression.42 Methorexate also may have direct anti-atherosclerotic effects; in the cholesterol-fed rabbit model, weekly i.v. methotrexate reduced new atheroma formation by 75% and reduced multiple biomarkers of macrophage function within the vessel wall.43 The primary endpoint of CIRT, like that of CANTOS and ENTRACTE, is recurrent non-fatal cardiovascular events and cardiovascular mortality. An important pre-specified secondary endpoint of CIRT is a reduction in the progression to diabetes (among those with metabolic syndrome) and reduced need for hypolipidaemic agents (among those who are already diabetic). Cardiovascular Inflammation Reduction Trial employs a central titration algorithm to ensure identical allocation of LDM (or placebo) at all study sites, and has ongoing monitoring for side effects such as infection or bone-marrow suppression.
Agents that inhibit alternative inflammatory pathways
Secretory phospolipase inhibitors
Members of the phospholipase A2 superfamily including lipoprotein-associated phopsholipase A2 (Lp-PLA2) and secretory PLA2 (sPLA2) are characterized by their ability to hydrolyse phospholipid molecules at the sn-2 position, a process that results in the production of potentially atherogenic lipid fractions and enhances oxidant stress.44 Both Lp-PLA2 and sPLA2 derive largely from macrophages and circulate in plasma complexed with LDL- and HDL-cholesterol. sPLA2-modified lipoprotein and Lp-PLA2-modified lipoproteins have been shown to associate with oxidized LDL particles capable of activating multiple inflammatory pathways with potential relevance for atherogenesis and plaque rupture.45 Clinically, plasma measurement of sPLA2 activation as well as Lp-PLA2 mass and Lp-PLA2 activity associate with elevated cardiovascular risk.46,47 However, the utility of commercial measures for these biomarkers remains controversial.48 Once patients are treated with high-dose statin therapy, there is little evidence that levels of Lp-PLA2 associate with residual vascular risk.49 Whether secretory phospholipases play a causal role in atherothrombosis has also recently been challenged; as with C-reactive protein, Mendelian randomization studies for sPLA2 have not found evidence, suggesting a direct causal relationship.50 In contrast to canakinumab, anakinra, and methotrexate, it is unlikely that secretory phospholipase inhibitors impact directly on the central IL-6 regulatory pathway as neither varespladib (a non-specific sPLA2 inhibitor) nor darapladib (a targeted LpPLA2 inhibitor) has robust effects on IL-6 or C-reactive protein. In the FRANCIS trial,51 on-treatment IL-6 levels were 3.2 pg/mL in the varespladib group and 3.0 pg/mL in the placebo group, a non-significant difference. Similarly, no significant differences in on-treatment C-reactive protein levels were observed in the PLASMA I52 or PLASMA II studies.53
With regard to darapladib, no significant effects on C-reactive protein when compared with placebo were found in phase II dose finding studies54 or in the larger 300 patient Integrated Biomarker and Imaging Study (IBIS-2) which evaluated the effects of darapladib on IVUS-based plaque characteristics.55 In IBIS-2, no effect of darapladib was observed on the co-primary endpoints of plasma C-reactive protein concentration or coronary atheroma deformability as evaluated by palpography. However, a secondary endpoint of lipid necrotic core progression as assessed by virtual histology was improved in the active treatment group (P = 0.01). Thus, varespladib and darapladib are effective agents for reducing sPLA2 and Lp-PLA2, respectively, but do not appear to impact greatly on the central IL-6 to C-reactive protein signalling pathway. As such, trials of these agents are of biological importance as they target a specific non-IL-6-dependent pathway hypothesized to be relevant to vascular inflammation.
To date, three major Phase III outcome trials have been initiated testing the impact of either varespladib or darapladib on recurrent vascular events. One of these trials, the 5000 participant VISTA-16 was recently stopped early by its Data and Safety Monitoring Board for futility.56 The 15 000 participant STABILITY trial found a small but non-significant reduction in vascular events among those with stable atherosclerosis, while the SOLID-TIMI-52 trial of 13 000 patients with acute ischaemia is ongoing.57,58
Vascular-targeted anti-oxidants
Part of the atherosclerotic hazard associated with secretory phospholipases is the result of production of lipid oxidation products and oxidative stress. An additional agent with anti-oxidant and potential anti-inflammatory properties to reach Phase II trial evaluation has been succinobucol, a monosuccinic acid ester of probucol, itself a lipid-lowering agent that failed to gain wide clinical use in part due to adverse effects on the QT segment.
Investigators in the Aggressive Reduction in Inflammation Stops Events (ARISE) trial randomly allocated 6144 patients with recent ischaemia to either succinobucol or to placebo and followed participants for the primary endpoint of cardiovascular death, resuscitated cardiac arrest, myocardial infarction, stroke, unstable angina, or revascularization.59 No benefit on this primary endpoint was observed (HR: 1.00, 95% CI: 0.89–1.13, P = 0.96), but adverse events in terms of haemorrhage, lipid levels, hypertension, and atrial fibrillation were increased. A modest benefit was present for succinobucol for a secondary endpoint limited to cardiovascular death, myocardial infarction, stroke, and cardiovascular arrest (HR: 0·81, 95% CI: 0.68–0.98, P = 0.029) and a tertiary endpoint of new-onset diabetes developed in fewer patients without diabetes at baseline in the succinobucol group than in the placebo group (HR: 0.37, 95% CI: 0.24–0.56, P < 0.001). Succinobucol modestly reduced haemoglobin A1c. In a second trial conducted among 232 patients undergoing elective percutaneous coronary interventions, succinubocol 280 mg daily had no effect on plaque volume or atherosclerotic regression as evaluated by intravascular ultrasound.60 In this study, as in ARISE, succinobucol was not shown to reduce either IL-6 or C-reactive protein. Thus, trial data for succinubocol are not informative as a test of the central IL-6 regulatory pathway. Due largely to the adverse effect profile observed in ARISE, no further development of this agent is underway.
Adhesion molecule inhibitors
Adhesion molecules such as ICAM-1 and VCAM-1 serve critical roles in the adhesion and transmigration of leucocytes across the endothelial wall, an early step in the formation of the atherosclerotic plaque. Epidemiological data have long shown strong positive associations between soluble levels of these adhesion molecules and future vascular events and thus multiple adhesion molecules serve as potential vasculoprotective targets for atherothrombosis.61
Leucocyte tethering and rolling along the vascular endothelium is also mediated by a related class of cell surface glycoproteins known as selectins that are more typically expressed by platelets.62 In particular, P-selectin has been shown to mediate multiple cell–cell interactions relevant to the initiation and progression of atherosclerotic plaques, an effect postulated to be of greatest importance at the time of plaque rupture.63 In man, the expression of P-selectin is increased in atherosclerotic plaque, is found in higher levels in the plasma of those with unstable angina, and at least in the setting of dialysis, is associated with increased vascular mortality.64 P-selectin antagonism has also been found to reduce thrombus formation in humans.65
Following this lead, 544 patients with non-ST-segment elevation myocardial infarction scheduled for angiography were randomly allocated in the SELECT-ACS trial to placebo or to inclacumab (a recombinant monoclonal antibody against P-selectin) at a dose of either 5 or 20 mg/kg given as a single 1-hour infusion. While there was no effect compared with placebo at the 5 mg inclacumab dose, those allocated to 20 mg inclacumab had moderately reduced myocardial damage as measured by indices of troponin and CK-MB release.66 These Phase II data provide optimism that novel anti-thrombotic approaches that additionally impact on cell–cell interactions might prove efficacious in some cardiovascular settings. A similar Phase II trial of inclacumab is ongoing among patients scheduled for coronary artery bypass surgery (SELECT-CABG).
Leukotriene inhibitors
Derived from arachidonic acid, the leukotrienes comprise a group of eicosanoids capable of activating multiple inflammatory pathways. Among potential leukotriene modifiers being considered in vascular disease, inhibitors of the 5-lipoxygenase (5-LO) pathway have been most aggressively pursued.67,68 This reflects, in part, observations of increased 5-LO expression in those with unstable atheroma and among those with chronic ischaemia. Interest in inhibiting 5-LO also increased after reports that polymorphism in the 5-LO activating protein gene results in an increased risk of myocardial infarction.69 To date, one clinical 5-LO inhibitor (Atreleuton,VIA-2291) has been evaluated in the setting of carotid disease and in an imaging trial conducted in the setting of acute coronary ischaemia.70 In the carotid study, 50 patients undergoing elective carotid endarterectomy were treated for 12 weeks with either 100 mg of VIA-2291 or placebo; in this study, the primary endpoint of per cent reduction in macrophage inflammatory cells in excised plaque tissue did not differ between the two treatment groups. In the acute coronary ischaemia study, 191 patients were randomly assigned to receive 25, 50, or 100 mg VIA-2291 or placebo daily for 12 weeks. The primary study endpoint, whole blood stimulated leukotriene LTB4 at a trough drug level, was reduced in all active treatment groups (P < 0.001) and a significant reduction of urine leukotriene LTE4 was observed compared with placebo. In a subset limited to 60 compliant patients who had coronary CT exams before and after treatment, new coronary plaques were less frequent in the active treatment group when compared with placebo.
Serpins and sirtuins
Serine protease inhibitors (serpins) are a family of common glycoproteins that exhibit complex effects on thrombosis in man, but in virus vectors exhibit anti-thrombotic and anti-inflammatory properties.71 In particular, infusion of the viral serpin Serp-1 results in inhibition of tissue-type and urokinase type plasminogen activation and in animal models has resulted in reduced neointimal proliferation and inhibition of monocytes/macrophage recruitment to the site of tissue injury following experimental balloon angioplasty.72 Recently, Tardif et al. performed a Phase II study of the myxoma virus-derived serpin Serp-1 among 48 patients with ACS undergoing percutaneous coronary intervention.73 Treatment was given by i.v. bolus of 5 or 15 μg/kg Serp-1 (or placebo) before intervention and 24–48 h after. In this study, the primary endpoint of in-stent neointimal hyperplasia evaluated by intravascular ultrasound did not differ after 6 months. However, Serp-1 did reduce markers of cardiac damage such as troponin I and CKMB levels and showed a trend towards clinical benefit although the number of events recorded was small. Of interest Serp-1 did not have impact on circulating inflammatory biomarkers in this initial evaluation.
Sirtuins comprise a family of NAD+-dependent deacetylases that regulate multiple cellular processes related to ageing, apoptosis, atherosclerosis, and inflammation.74,75 Of these enzymes, SIRT1, a histone deacetylase, has gained interest in vascular disease as it exerts anti-inflammatory effects via NFkB, inhibits atherothrombosis, and promotes angiogenesis.76,77 In apo-E-deficient mice, over-expression of SIRT1 decreases atherosclerotic progression.78 On the other hand, pharmacological SIRT1 activation in ApoE−/− mice provides atheroprotection by reducing hepatic PCSK9 secretion and enhancing LDL-R expression.79 Consistent with the common soil hypothesis linking atherothrombosis to insulin resistance, genetic variation in sirtuins has been associated with type 2 diabetes and obesity.80 On this basis, sirtuin activators have been hypothesized as a potential novel strategy to prevent atherosclerosis and treat diabetes,81 an intriguing issue as small molecule activators of SIRT1 are available. In addition, as the metabolite NAD+ has been shown to result in sirtuin activation, there is renewed interest in the administration of NAD+ precursors such as nicotinic acid and nicotinamide as therapeutic agents in the prevention of recurrent vascular disease.
The re-purposing of generic anti-inflammatory agents
As described above, the generic agent LDM is currently being evaluated by the National Heart Lung and Blood Institute as a potential anti-atherosclerotic agent. Several other generic anti-inflammatory drugs including aspirin, salsalate, colchicine, and hydroxychloroquine have also been considered for evaluation in the setting of atherosclerosis.
Aspirin and salsalate
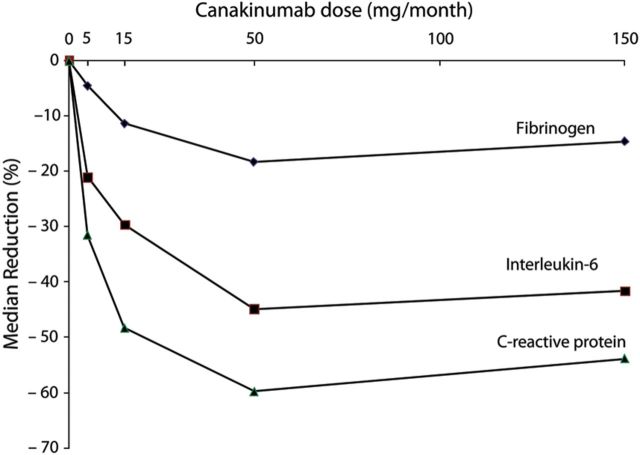
Aspirin, an acetylated form of salicylate, has both anti-thrombotic and anti-inflammatory effects, the latter due to the fact that the acetyl group covalently modifies serine at the active site of cyclo-oxygenase enzymes. In 1997, an analysis of inflammation performed within the Physicians Health Study demonstrated that the magnitude of cardiovascular risk reduction associated with random allocation to aspirin when compared with placebo was greatest among those with the highest baseline levels of C-reactive protein and became sequentially smaller as the levels of C-reactive protein dropped (P-trend across quartiles < 0.001)82 (Figure 7). This differential benefit of aspirin on first-ever vascular events was not related to anti-platelet effects as these did not vary across levels of C-reactive protein. Thus, this study of one of the oldest drugs used in medicine effectively became the first clinical demonstration that anti-inflammatory therapies might be effective for the prevention of cardiovascular disease.
Figure 7.
Effects of aspirin when compared with placebo in the primary prevention of future vascular events, stratified by the baseline level of C-reactive protein.82
Unlike aspirin, salicylate (salsalate) is not acetylated. Thus, salsalate is not a cyclo-oxygenase inhibitor and does not impact upon bleeding times or platelet aggregation. Salsalate, however, is an effective anti-inflammatory widely used to treat joint pain and that modestly reduces leucocyte counts likely as a result of nuclear factor-κβ effects. Whether or not salsalate might reduce cardiovascular event rates is unknown. However, as shown in stages 1 and 2 of the TINSAL-T2D trials conducted among diabetics, salsalate safely reduces haemoglobin A1c as well as C-reactive protein, and has systemic anti-inflammatory effects as reflected in reduced circulating leucocyte, neutrophil, and lymphocyte.83,84 Modest but not dose-limiting tinnitus was observed in these studies. As an inexpensive and otherwise well-tolerated generic agent, salsalate remains a candidate anti-inflammatory agent worthy of testing in future vascular inflammation reduction trials.
Colchicine and hydroxychloroquine
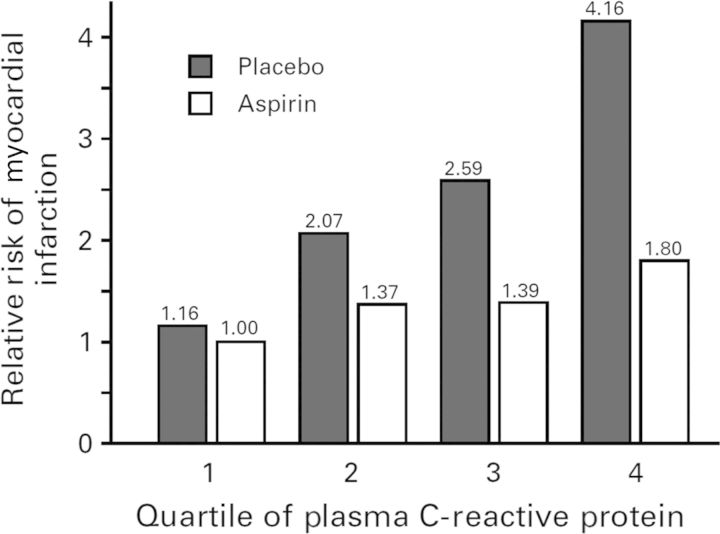
Other than methotrexate, perhaps the most provocative data supporting the concept of ‘re-purposing’ old anti-inflammatory medications for a new role in vasculoprotection derives from recent data regarding colchicine, an agent long used to treat gout. Colchicine has several anti-inflammatory properties including anti-tubulin effects that inhibit neutrophil function as well as modest effects on the NLRP3 inflammasome, a mechanism consistent with its effects on gout, a classical disorder of crystal deposition. After demonstrating that colchicine reduces C-reactive protein independent of aspirin and statins,85 the LoDoCo Investigators assigned 532 patients with stable coronary artery disease to receive either colchicine 0.5 mg per day for at least 30 days or to non-placebo control treatment using a PROBE open-label study design.86 Although >20% of those allocated to colchicine were intolerant of treatment due to anticipated adverse gastrointestinal effects and stopped treatment either early (n = 32) or late (n = 30) in the trial, active treatment nonetheless markedly reduced the primary trial endpoint of recurrent acute coronary syndrome, cardiac arrest or non-embolic stroke (HR: 0.33, 95% CI: 0.18–0.59, P = 0.001), with most of the effect due to reduction in hospitalization for ACS (Figure 8). Benefits appeared early after trial initiation, were sustained throughout the median 3-year follow-up period, and were consistent in all major subgroups considered. Large-scale, fully blinded trials of colchicine in secondary prevention are thus warranted.
Figure 8.
Primary result of the LoDoCo trial of colchicine in the secondary prevention of vascular events.86
Finally, the anti-malarial drug hydroxychloroquine has long been used to reduce inflammation in patients with RA and lupus. Part of this anti-inflammatory effect is related to acidic alteration of the pH within lysosomes, an effect that diminishes proteolysis and may result in reduced immune cell function. Hydroxychloroquine also inhibits stimulation of toll-like receptor-9 and can produce an inhibition of the innate immune response.87 Like salsalate, hydroxychloroquine has been shown to reduce haemoglobin A1c in refractory diabetic patients.88 However, rare reports in lupus patients of hydroxychloroquine-induced cardiomyopathy have limited further evaluation of this agent in vascular patients.
High-density lipoprotein
HDL-cholesterol has a variety of anti-inflammatory effects such as reduction of the interaction of white blood cells with the endothelium, reduction in VCAM and ICAM expression in the presence of cytokines such as TNF-α, reduction in the production of reactive oxygen species by endothelial cells and inhibition of apoptosis.89,90 However, these anti-inflammatory effects are lost in HDL-C obtained from patients with coronary artery disease, acute coronary syndromes, and renal insufficiency,91 at least in part due to reduced PON-1 activity. To date, trials aimed at increasing HDL-C in order to reduce clinical events have been negative92 or neutral.93
Adaptive immunity and vaccination against atherosclerosis
In addition to innate immunity, adaptive immunity plays a substantive role in atherothrombosis and antigen-presenting dendritic cells, T lymphocytes, B lymphocytes, and immunoglobulins are all found within vascular plaque.94 Several laboratories have thus aggressively pursued vaccination strategies as a novel method to reduce atherosclerosis. While early targets for these vaccines used LDL as an immunogen,95 more recent work has focused on epitopes to oxidized LDL or to specific peptide sequences of apolipoprotein B 100, the principle apoprotein carried within LDL-cholesterol. In mouse models of hypercholesterolaemia, it has also recently become apparent that TH1 cells appear to increase atherosclerotic progression, whereas T regulatory cells may have a role in disease regression.96,97 All of these observations, as well as initial success in mouse models, have provided impetus to take potential atherosclerosis vaccines based on apo B peptides and other antigens into human clinical testing.98
Limitations and future directions
As several commentators have noted, the inflammatory system is simultaneously redundant, compensatory, and crucial for survival.99 As such, anti-inflammatory therapies in the setting of chronic disorders pose substantive challenges. Further, evaluation of risks as well as benefits must drive the development of agents in this class. As shown by adverse data for TNF-inhibitors in heart failure and the finding of increased vascular risk among those taking COX-2 inhibitors, there may be unintended consequences of inflammation suppression. However, as proven among these with RA and inflammatory bowel disease, long-term treatment with systemic anti-inflammatory agents can be accomplished safely. In addition to the therapies described here, multiple alternative approaches to inflammation inhibition are being developed. These include targeted steroid delivery systems such as Nanocort; the infusion of reconstituted HDL-cholesterol; and emerging imaging-based approaches to inflammation detection and targeted intervention.100–102 Genetic studies are also pointing to new directions for vascular inflammation inhibition. In one recent genome-wide association study, major histocompatibility complex genes that regulate inflammation and T-cell responses were found to significantly associate with vascular risk.103 The next 5 years will see publication of several massive trials directly testing the inflammatory hypothesis of atherosclerosis.104 The core results of these trials—including those that do and do not inhibit the central IL-1, TNF-α, and IL-6 pathway—will tell us a great deal about whether anti-inflammatory therapies will eventually become a cornerstone of vascular risk reduction. If successful, these trials will usher in a new era in which the treatment of chronic vascular disease moves beyond the reduction of LDL-cholesterol alone.
Funding
P.M.R. is supported by investigator-initiated research grants from AstraZeneca, Novartis, AMGEN, and the National Heart Lung and Blood Institutes (HL 101422, HL101389).
Conflict of interest: P.M.R. has served as a consultant to ISIS, Vascular Biogenics, Amgen, Pfizer, and Boston Heart, and is listed as a co-inventor on patents held by the Brigham and Women's hospital that relate to the use of inflammatory biomarkers in cardiovascular disease and diabetes that have been licensed to AstraZeneca and Seimens. Neither P.M.R. nor the Brigham and Women's Hospital receive royalty payments related to the use of these biomarkers in either the CIRT or CANTOS trials.
References
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghattas A, Griffiths HR, Devitt A, Lip GY, Shantsila E. Monocytes in coronary artery disease and atherosclerosis: where are we now? JACC. 2013;62:1541–1551. doi: 10.1016/j.jacc.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM. Targeting inflammatory pathways for the treatment of cardiovascular disease. Eur Heart J. 2014;35:540–543. doi: 10.1093/eurheartj/eht398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 6.Maier W, Altwegg LA, Corti R, Gay S, Hersberger M, Maly FE, Sutsch G, Roffi M, Neidhart M, Eberli FR, Tanner FC, Gobbi S, von Eckardstein A, Luscher TF. Inflammatory markers at the site of ruptured plaque in acute myocardial infarction: locally increased interleukin-6 and serum amyloid A but decreased C-reactive protein. Circulation. 2005;111:1355–1361. doi: 10.1161/01.CIR.0000158479.58589.0A. [DOI] [PubMed] [Google Scholar]
- 7.Kaptoge S, Seshasai SR, Gao P, Freitag DF, Butterworth AS, Borglykke A, Di Angelantonio E, Gudnason V, Rumley A, Lowe GD, Jorgensen T, Danesh J. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35:578–589. doi: 10.1093/eurheartj/eht367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hingorani AD, Casas JP. The interleukin-6 receptor as a target for prevention of coronary heart disease: a Mendelian randomisation analysis. Lancet. 2012;379:1214–1224. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, Gao P, Saleheen D, Rendon A, Nelson CP, Braund PS, Hall AS, Chasman DI, Tybjaerg-Hansen A, Chambers JC, Benjamin EJ, Franks PW, Clarke R, Wilde AA, Trip MD, Steri M, Witteman JC, Qi L, van der Schoot CE, de Faire U, Erdmann J, Stringham HM, Koenig W, Rader DJ, Melzer D, Reich D, Psaty BM, Kleber ME, Panagiotakos DB, Willeit J, Wennberg P, Woodward M, Adamovic S, Rimm EB, Meade TW, Gillum RF, Shaffer JA, Hofman A, Onat A, Sundstrom J, Wassertheil-Smoller S, Mellstrom D, Gallacher J, Cushman M, Tracy RP, Kauhanen J, Karlsson M, Salonen JT, Wilhelmsen L, Amouyel P, Cantin B, Best LG, Ben-Shlomo Y, Manson JE, Davey-Smith G, de Bakker PI, O'Donnell CJ, Wilson JF, Wilson AG, Assimes TL, Jansson JO, Ohlsson C, Tivesten A, Ljunggren O, Reilly MP, Hamsten A, Ingelsson E, Cambien F, Hung J, Thomas GN, Boehnke M, Schunkert H, Asselbergs FW, Kastelein JJ, Gudnason V, Salomaa V, Harris TB, Kooner JS, Allin KH, Nordestgaard BG, Hopewell JC, Goodall AH, Ridker PM, Holm H, Watkins H, Ouwehand WH, Samani NJ, Kaptoge S, Di Angelantonio E, Harari O, Danesh J. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–1213. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the Pravastatin Inflammation/CRP Evaluation (PRINCE), a randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100:230–235. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 14.Chasman DI, Giulianini F, MacFadyen J, Barratt BJ, Nyberg F, Ridker PM. Genetic determinants of statin-induced low-density lipoprotein cholesterol reduction: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Circ Cardiovasc Genet. 2012;5:257–264. doi: 10.1161/CIRCGENETICS.111.961144. [DOI] [PubMed] [Google Scholar]
- 15.Chu AY, Guilianini F, Barratt BJ, Nyberg F, Chasman DI, Ridker PM. Pharmacogenetic determinants of statin-induced reductions in C-reactive protein. Circ Cardiovasc Genet. 2012;5:58–65. doi: 10.1161/CIRCGENETICS.111.961847. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 17.Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Ridker PM. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–1861. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ridker PM, MacFadyen J, Libby P, Glynn RJ. Relation of baseline high-sensitivity C-reactive protein level to cardiovascular outcomes with rosuvastatin in the Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) Am J Cardiol. 2010;106:204–209. doi: 10.1016/j.amjcard.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, Macfadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 20.Braunwald E. Creating controversy where none exists: the important role of C-reactive protein in the CARE, AFCAPS/TexCAPS, PROVE IT, REVERSAL, A to Z, JUPITER, HEART PROTECTION, and ASCOT trials. Eur Heart J. 2012;33:430–432. doi: 10.1093/eurheartj/ehr310. [DOI] [PubMed] [Google Scholar]
- 21.Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, Subramanian SS, Abdelbaky A, Rudd JH, Farkouh ME, Nunes IO, Beals CR, Shankar SS. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62:909–917. doi: 10.1016/j.jacc.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 22.Bu DX, Griffin G, Lichtman AH. Mechanisms for the anti-inflammatory effects of statins. Curr Opin Lipidol. 2011;22:165–170. doi: 10.1097/MOL.0b013e3283453e41. [DOI] [PubMed] [Google Scholar]
- 23.Sen-Banerjee S, Mir S, Lin Z, Hamik A, Atkins GB, Das H, Banerjee P, Kumar A, Jain MK. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112:720–726. doi: 10.1161/CIRCULATIONAHA.104.525774. [DOI] [PubMed] [Google Scholar]
- 24.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Ann Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 25.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 26.Fearon WF, Fearon DT. Inflammation and cardiovascular disease: role of the interleukin-1 receptor antagonist. Circulation. 2008;117:2577–2579. doi: 10.1161/CIRCULATIONAHA.108.772491. [DOI] [PubMed] [Google Scholar]
- 27.Xiao H, Lu M, Lin TY, Chen Z, Chen G, Wang WC, Marin T, Shentu TP, Wen L, Gongol B, Sun W, Liang X, Chen J, Huang HD, Pedra JH, Johnson DA, Shyy JY. Sterol regulatory element binding protein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility. Circulation. 2013;128:632–642. doi: 10.1161/CIRCULATIONAHA.113.002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, Thuren T. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–2748. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 30.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 31.Ikonomidis I, Lekakis JP, Nikolaou M, Paraskevaidis I, Andreadou I, Kaplanoglou T, Katsimbri P, Skarantavos G, Soucacos PN, Kremastinos DT. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation. 2008;117:2662–2669. doi: 10.1161/CIRCULATIONAHA.107.731877. [DOI] [PubMed] [Google Scholar]
- 32.Crossman DC, Morton AC, Gunn JP, Greenwood JP, Hall AS, Fox KA, Lucking AJ, Flather MD, Lees B, Foley CE. Investigation of the effect of Interleukin-1 receptor antagonist (IL-1ra) on markers of inflammation in non-ST elevation acute coronary syndromes (The MRC-ILA-HEART Study) Trials. 2008;9:8. doi: 10.1186/1745-6215-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 34.Hurlimann D, Forster A, Noll G, Enseleit F, Chenevard R, Distler O, Bechir M, Spieker LE, Neidhart M, Michel BA, Gay RE, Luscher TF, Gay S, Ruschitzka F. Anti-tumor necrosis factor-alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation. 2002;106:2184–2187. doi: 10.1161/01.cir.0000037521.71373.44. [DOI] [PubMed] [Google Scholar]
- 35.Micha R, Imamura F, Wyler von Ballmoos M, Solomon DH, Hernan MA, Ridker PM, Mozaffarian D. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362–1370. doi: 10.1016/j.amjcard.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Protogerou AD, Zampeli E, Fragiadaki K, Stamatelopoulos K, Papamichael C, Sfikakis PP. A pilot study of endothelial dysfunction and aortic stiffness after interleukin-6 receptor inhibition in rheumatoid arthritis. Atherosclerosis. 2011;219:734–736. doi: 10.1016/j.atherosclerosis.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Popa C, Netea MG, Radstake T, Van der Meer JW, Stalenhoef AF, van Riel PL, Barerra P. Influence of anti-tumour necrosis factor therapy on cardiovascular risk factors in patients with active rheumatoid arthritis. Ann Rheum Dis. 2005;64:303–305. doi: 10.1136/ard.2004.023119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 39.Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AA, Glynn RJ, Ridker PM. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199–207. doi: 10.1016/j.ahj.2013.03.018. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiss AB, Rahman MM, Chan ES, Montesinos MC, Awadallah NW, Cronstein BN. Adenosine A2A receptor occupancy stimulates expression of proteins involved in reverse cholesterol transport and inhibits foam cell formation in macrophages. J Leuk Biol. 2004;76:727–734. doi: 10.1189/jlb.0204107. [DOI] [PubMed] [Google Scholar]
- 41.Reiss AB, Carsons SE, Anwar K, Rao S, Edelman SD, Zhang H, Fernandez P, Cronstein BN, Chan ES. Atheroprotective effects of methotrexate on reverse cholesterol transport proteins and foam cell transformation in human THP-1 monocyte/macrophages. Arthritis Rheum. 2008;58:3675–3683. doi: 10.1002/art.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McPherson JA, Barringhaus KG, Bishop GG, Sanders JM, Rieger JM, Hesselbacher SE, Gimple LW, Powers ER, Macdonald T, Sullivan G, Linden J, Sarembock IJ. Adenosine A(2A) receptor stimulation reduces inflammation and neointimal growth in a murine carotid ligation model. Arterioscler Thromb Vasc Biol. 2001;21:791–796. doi: 10.1161/01.atv.21.5.791. [DOI] [PubMed] [Google Scholar]
- 43.Bulgarelli A, Martins Dias AA, Caramelli B, Maranhao RC. Treatment with methotrexate inhibits atherogenesis in cholesterol-fed rabbits. J Cardiovasc Pharmacol. 2012;59:308–314. doi: 10.1097/FJC.0b013e318241c385. [DOI] [PubMed] [Google Scholar]
- 44.Rosenson RS, Stafforini DM. Modulation of oxidative stress, inflammation, and atherosclerosis by lipoprotein-associated phospholipase A2. J Lipid Res. 2012;53:1767–1782. doi: 10.1194/jlr.R024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenson RS. Phospholipase A2 inhibition and atherosclerotic vascular disease: prospects for targeting secretory and lipoprotein-associated phospholipase A2 enzymes. Curr Opin Lipidol. 2010;21:473–480. doi: 10.1097/MOL.0b013e32833eb581. [DOI] [PubMed] [Google Scholar]
- 46.Thompson A, Gao P, Orfei L, Watson S, Di Angelantonio E, Kaptoge S, Ballantyne C, Cannon CP, Criqui M, Cushman M, Hofman A, Packard C, Thompson SG, Collins R, Danesh J. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–1544. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenson RS, Hurt-Camejo E. Phospholipase A2 enzymes and the risk of atherosclerosis. Eur Heart J. 2012;33:2899–2909. doi: 10.1093/eurheartj/ehs148. [DOI] [PubMed] [Google Scholar]
- 48.Stein EA. Lipoprotein-associated phospholipase A(2) measurements: mass, activity, but little productivity. Clin Chem. 2012;58:814–817. doi: 10.1373/clinchem.2012.183475. [DOI] [PubMed] [Google Scholar]
- 49.Ridker PM, MacFadyen JG, Wolfert RL, Koenig W. Relationship of lipoprotein-associated phospholipase A(2) mass and activity with incident vascular events among primary prevention patients allocated to placebo or to statin therapy: an analysis from the JUPITER trial. Clin Chem. 2012;58:877–886. doi: 10.1373/clinchem.2011.180281. [DOI] [PubMed] [Google Scholar]
- 50.Holmes MV, Simon T, Exeter HJ, Hingorani AD, Sabatine MS, Mallat Z, Casas JP, Talmud PJ. Reply: limits of Mendelian randomization analyses in selection of secretory phospholipase A2-IIA as a valid therapeutic target for prevention of cardiovascular disease. J Am Coll Cardiol. 2014;63:943. doi: 10.1016/j.jacc.2013.10.070. [DOI] [PubMed] [Google Scholar]
- 51.Rosenson RS, Hislop C, Elliott M, Stasiv Y, Goulder M, Waters D. Effects of varespladib methyl on biomarkers and major cardiovascular events in acute coronary syndrome patients. J Am Coll Cardiol. 2010;56:1079–1088. doi: 10.1016/j.jacc.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 52.Rosenson RS, Hislop C, McConnell D, Elliott M, Stasiv Y, Wang N, Waters DD. Effects of 1-H-indole-3-glyoxamide (A-002) on concentration of secretory phospholipase A2 (PLASMA study): a phase II double-blind, randomised, placebo-controlled trial. Lancet. 2009;373:649–658. doi: 10.1016/S0140-6736(09)60403-7. [DOI] [PubMed] [Google Scholar]
- 53.Rosenson RS, Elliott M, Stasiv Y, Hislop C, Investigators PI. Randomized trial of an inhibitor of secretory phospholipase A2 on atherogenic lipoprotein subclasses in statin-treated patients with coronary heart disease. Eur Heart J. 2011;32:999–1005. doi: 10.1093/eurheartj/ehq374. [DOI] [PubMed] [Google Scholar]
- 54.Mohler ER, III, Ballantyne CM, Davidson MH, Hanefeld M, Ruilope LM, Johnson JL, Zalewski A. The effect of darapladib on plasma lipoprotein-associated phospholipase A2 activity and cardiovascular biomarkers in patients with stable coronary heart disease or coronary heart disease risk equivalent: the results of a multicenter, randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 2008;51:1632–1641. doi: 10.1016/j.jacc.2007.11.079. [DOI] [PubMed] [Google Scholar]
- 55.Serruys PW, Garcia-Garcia HM, Buszman P, Erne P, Verheye S, Aschermann M, Duckers H, Bleie O, Dudek D, Botker HE, von Birgelen C, D'Amico D, Hutchinson T, Zambanini A, Mastik F, van Es GA, van der Steen AF, Vince DG, Ganz P, Hamm CW, Wijns W, Zalewski A. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation. 2008;118:1172–1182. doi: 10.1161/CIRCULATIONAHA.108.771899. [DOI] [PubMed] [Google Scholar]
- 56.Nicholls SJ, Kastelein JJ, Schwartz GG, Bash D, Rosenson RS, Cavender MA, Brennan DM, Koenig W, Jukema JW, Nambi V, Wright RS, Menon V, Lincoff AM, Nissen SE. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA. 2014;311:252–262. doi: 10.1001/jama.2013.282836. [DOI] [PubMed] [Google Scholar]
- 57.O'Donoghue ML, Braunwald E, White HD, Serruys P, Steg PG, Hochman J, Maggioni AP, Bode C, Weaver D, Johnson JL, Cicconetti G, Lukas MA, Tarka E, Cannon CP. Study design and rationale for the Stabilization of pLaques usIng Darapladib-Thrombolysis in Myocardial Infarction (SOLID-TIMI 52) trial in patients after an acute coronary syndrome. Am Heart J. 2011;162:613–619. doi: 10.1016/j.ahj.2011.07.018. e1. [DOI] [PubMed] [Google Scholar]
- 58.White H, Held C, Stewart R, Watson D, Harrington R, Budaj A, Steg PG, Cannon CP, Krug-Gourley S, Wittes J, Trivedi T, Tarka E, Wallentin L. Study design and rationale for the clinical outcomes of the STABILITY Trial (STabilization of Atherosclerotic plaque By Initiation of darapLadIb TherapY) comparing darapladib versus placebo in patients with coronary heart disease. Am Heart J. 2010;160:655–661. doi: 10.1016/j.ahj.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 59.Tardif JC, McMurray JJ, Klug E, Small R, Schumi J, Choi J, Cooper J, Scott R, Lewis EF, L'Allier PL, Pfeffer MA. Effects of succinobucol (AGI-1067) after an acute coronary syndrome: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:1761–1768. doi: 10.1016/S0140-6736(08)60763-1. [DOI] [PubMed] [Google Scholar]
- 60.Tardif JC, Gregoire J, L'Allier PL, Ibrahim R, Anderson TJ, Reeves F, Title LM, Schampaert E, LeMay M, Lesperance J, Scott R, Guertin MC, Brennan ML, Hazen SL, Bertrand OF. Effects of the antioxidant succinobucol (AGI-1067) on human atherosclerosis in a randomized clinical trial. Atherosclerosis. 2008;197:480–486. doi: 10.1016/j.atherosclerosis.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 61.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 62.Frenette PS, Denis CV, Weiss L, Jurk K, Subbarao S, Kehrel B, Hartwig JH, Vestweber D, Wagner DD. P-Selectin glycoprotein ligand 1 (PSGL-1) is expressed on platelets and can mediate platelet-endothelial interactions in vivo. J Exp Med. 2000;191:1413–1422. doi: 10.1084/jem.191.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. The combined role of P- and E-selectins in atherosclerosis. J Clin Invest. 1998;102:145–152. doi: 10.1172/JCI3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scialla JJ, Plantinga LC, Kao WH, Jaar B, Powe NR, Parekh RS. Soluble P-selectin levels are associated with cardiovascular mortality and sudden cardiac death in male dialysis patients. Am J Nephrol. 2011;33:224–230. doi: 10.1159/000324517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chelliah R, Lucking AJ, Tattersall L, Daga S, Beresford-Cleary NJ, Cortas K, Fox KA, Feuerstein GZ, Connolly TM, Newby DE. P-selectin antagonism reduces thrombus formation in humans. J Thromb Haemost. 2009;7:1915–1919. doi: 10.1111/j.1538-7836.2009.03587.x. [DOI] [PubMed] [Google Scholar]
- 66.Tardif JC, Tanguay JF, Wright SS, Duchatelle V, Petroni T, Gregoire JC, Ibrahim R, Heinonen TM, Robb S, Bertrand OF, Cournoyer D, Johnson D, Mann J, Guertin MC, L'Allier PL. Effects of the P-selectin antagonist inclacumab on myocardial damage after percutaneous coronary intervention for non-ST-segment elevation myocardial infarction: results of the SELECT-ACS trial. J Am Coll Cardiol. 2013;61:2048–2055. doi: 10.1016/j.jacc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 67.De Caterina R, Zampolli A. From asthma to atherosclerosis--5-lipoxygenase, leukotrienes, and inflammation. N Engl J Med. 2004;350:4–7. doi: 10.1056/NEJMp038190. [DOI] [PubMed] [Google Scholar]
- 68.Funk CD. Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nat Rev Drug Discov. 2005;4:664–672. doi: 10.1038/nrd1796. [DOI] [PubMed] [Google Scholar]
- 69.Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, Samani NJ, Gudmundsson G, Grant SF, Thorgeirsson G, Sveinbjornsdottir S, Valdimarsson EM, Matthiasson SE, Johannsson H, Gudmundsdottir O, Gurney ME, Sainz J, Thorhallsdottir M, Andresdottir M, Frigge ML, Topol EJ, Kong A, Gudnason V, Hakonarson H, Gulcher JR, Stefansson K. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004;36:233–239. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- 70.Tardif JC, L'Allier PL, Ibrahim R, Gregoire JC, Nozza A, Cossette M, Kouz S, Lavoie MA, Paquin J, Brotz TM, Taub R, Pressacco J. Treatment with 5-lipoxygenase inhibitor VIA-2291 (Atreleuton) in patients with recent acute coronary syndrome. Circ Cardiovasc Imaging. 2010;3:298–307. doi: 10.1161/CIRCIMAGING.110.937169. [DOI] [PubMed] [Google Scholar]
- 71.Richardson J, Viswanathan K, Lucas A. Serpins, the vasculature, and viral therapeutics. Front Biosci. 2006;11:1042–1056. doi: 10.2741/1862. [DOI] [PubMed] [Google Scholar]
- 72.Lucas A, Liu L, Macen J, Nash P, Dai E, Stewart M, Graham K, Etches W, Boshkov L, Nation PN, Humen D, Hobman ML, McFadden G. Virus-encoded serine proteinase inhibitor SERP-1 inhibits atherosclerotic plaque development after balloon angioplasty. Circulation. 1996;94:2890–2900. doi: 10.1161/01.cir.94.11.2890. [DOI] [PubMed] [Google Scholar]
- 73.Tardif JC, L'Allier PL, Gregoire J, Ibrahim R, McFadden G, Kostuk W, Knudtson M, Labinaz M, Waksman R, Pepine CJ, Macaulay C, Guertin MC, Lucas A. A randomized controlled, phase 2 trial of the viral serpin Serp-1 in patients with acute coronary syndromes undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2010;3:543–548. doi: 10.1161/CIRCINTERVENTIONS.110.953885. [DOI] [PubMed] [Google Scholar]
- 74.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Ann Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stein S, Lohmann C, Schafer N, Hofmann J, Rohrer L, Besler C, Rothgiesser KM, Becher B, Hottiger MO, Boren J, McBurney MW, Landmesser U, Luscher TF, Matter CM. SIRT1 decreases Lox-1-mediated foam cell formation in atherogenesis. Eur Heart J. 2010;31:2301–2309. doi: 10.1093/eurheartj/ehq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Breitenstein A, Stein S, Holy EW, Camici GG, Lohmann C, Akhmedov A, Spescha R, Elliott PJ, Westphal CH, Matter CM, Luscher TF, Tanner FC. Sirt1 inhibition promotes in vivo arterial thrombosis and tissue factor expression in stimulated cells. Cardiovasc Res. 2011;89:464–472. doi: 10.1093/cvr/cvq339. [DOI] [PubMed] [Google Scholar]
- 78.Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, Wei YS, Cai H, Liu DP, Liang CC. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80:191–199. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miranda MX, van Tits LJ, Lohmann C, Arsiwala T, Winnik S, Tailleux A, Stein S, Gomes AP, Suri V, Ellis JL, Lutz TA, Hottiger MO, Sinclair DA, Auwerx J, Schoonjans K, Staels B, Luscher TF, Matter CM. The Sirt1 activator SRT3025 provides atheroprotection in Apoe−/− mice by reducing hepatic Pcsk9 secretion and enhancing Ldlr expression. Eur Heart J. 2014 doi: 10.1093/eurheartj/ehu095. doi:10.1093/eurheartj/ehj095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Biason-Lauber A, Boni-Schnetzler M, Hubbard BP, Bouzakri K, Brunner A, Cavelti-Weder C, Keller C, Meyer-Boni M, Meier DT, Brorsson C, Timper K, Leibowitz G, Patrignani A, Bruggmann R, Boily G, Zulewski H, Geier A, Cermak JM, Elliott P, Ellis JL, Westphal C, Knobel U, Eloranta JJ, Kerr-Conte J, Pattou F, Konrad D, Matter CM, Fontana A, Rogler G, Schlapbach R, Regairaz C, Carballido JM, Glaser B, McBurney MW, Pociot F, Sinclair DA, Donath MY. Identification of a SIRT1 mutation in a family with type 1 diabetes. Cell Metab. 2013;17:448–455. doi: 10.1016/j.cmet.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brandes RP. Activating SIRT1: a new strategy to prevent atherosclerosis? Cardiovasc Res. 2008;80:163–164. doi: 10.1093/cvr/cvn245. [DOI] [PubMed] [Google Scholar]
- 82.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 83.Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010;152:346–357. doi: 10.1059/0003-4819-152-6-201003160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goldfine AB, Fonseca V, Jablonski KA, Chen YD, Tipton L, Staten MA, Shoelson SE. Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2013;159:1–12. doi: 10.7326/0003-4819-159-1-201307020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nidorf M, Thompson PL. Effect of colchicine (0.5 mg twice daily) on high-sensitivity C-reactive protein independent of aspirin and atorvastatin in patients with stable coronary artery disease. Am J Cardiol. 2007;99:805–807. doi: 10.1016/j.amjcard.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 86.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. JACC. 2013;61:404–410. doi: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 87.Takeda K, Kaisho T, Akira S. Toll-like receptors. Ann Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 88.Gerstein HC, Thorpe KE, Taylor DW, Haynes RB. The effectiveness of hydroxychloroquine in patients with type 2 diabetes mellitus who are refractory to sulfonylureas—a randomized trial. Diabetes Res Clin Pract. 2002;55:209–219. doi: 10.1016/s0168-8227(01)00325-4. [DOI] [PubMed] [Google Scholar]
- 89.Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, Chroni A, Yonekawa K, Stein S, Schaefer N, Mueller M, Akhmedov A, Daniil G, Manes C, Templin C, Wyss C, Maier W, Tanner FC, Matter CM, Corti R, Furlong C, Lusis AJ, von Eckardstein A, Fogelman AM, Luscher TF, Landmesser U. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest. 2011;121:2693–2708. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luscher TF, Landmesser U, von Eckardstein A, Fogelman AM. High-density lipoprotein: vascular protective effects, dysfunction, and potential as therapeutic target. Circ Res. 2014;114:171–182. doi: 10.1161/CIRCRESAHA.114.300935. [DOI] [PubMed] [Google Scholar]
- 91.Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Krankel N, Kania G, Zewinger S, Akhmedov A, Shi Y, Martin T, Perisa D, Winnik S, Muller MF, Sester U, Wernicke G, Jung A, Gutteck U, Eriksson U, Geisel J, Deanfield J, von Eckardstein A, Luscher TF, Fliser D, Bahlmann FH, Landmesser U. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity. 2013;38:754–768. doi: 10.1016/j.immuni.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 92.Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, Kastelein JJ, Bittner V, Fruchart JC. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–1310. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 93.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 94.Niessner A, Weyand CM. Dendritic cells in atherosclerotic disease. Clin Immunol. 2009;134:25–32. doi: 10.1016/j.clim.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Palinski W, Miller E, Witzum JL. Immunization of low density lipoprotein (LDL) receptor deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci USA. 1995;92:821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hermansson A, Ketelhuth DFJ, Strodthoff D, Wurm M, Hansson EM, Nicoletti A, Paulsson-Berne G, Hansson GK. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J Exp Med. 2010;207:1081–1093. doi: 10.1084/jem.20092243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nilsson J, Bjorkbacka H, Fredrikson GN. Apolipoprotein B100 autoimmunity and atherosclerosis: disease mechanisms and therapeutic potential. Curr Opin Lipidol. 2012;23:422–428. doi: 10.1097/MOL.0b013e328356ec7c. [DOI] [PubMed] [Google Scholar]
- 98.Chyu KY, Nilsson J, Sha PK. Immunization for atherosclerosis. Cur Atheroscler Rep. 2007;9:104–109. doi: 10.1007/s11883-007-0005-8. [DOI] [PubMed] [Google Scholar]
- 99.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nilsson J, Wigren M, Shah PK. Vaccines against atherosclerosis. Expert Rev Vaccines. 2013;12:311–321. doi: 10.1586/erv.13.4. [DOI] [PubMed] [Google Scholar]
- 101.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 102.Quillard T, Libby P. Molecular imaging of atherosclerosis for improving diagnostic and therapeutic development. Circ Res. 2012;111:231–244. doi: 10.1161/CIRCRESAHA.112.268144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Davies RW, Wells GA, Stewart AF, Erdmann J, Shah SH, Ferguson JF, Hall AS, Anand SS, Burnett MS, Epstein SE, Dandona S, Chen L, Nahrstaedt J, Loley C, Konig IR, Kraus WE, Granger CB, Engert JC, Hengstenberg C, Wichmann HE, Schreiber S, Tang WH, Ellis SG, Rader DJ, Hazen SL, Reilly MP, Samani NJ, Schunkert H, Roberts R, McPherson R. A genome-wide association study for coronary artery disease identifies a novel susceptibility locus in the major histocompatibility complex. Circ Cardiovasc Genet. 2012;5:217–225. doi: 10.1161/CIRCGENETICS.111.961243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Couzin-Frankel J. Cardiovascular disease. Massive trials to test inflammation hypothesis. Science. 2012;337:1158. doi: 10.1126/science.337.6099.1158. [DOI] [PubMed] [Google Scholar]