Abstract
Human adipose tissue-derived multilineage progenitor cells (hADMPCs) are attractive for cell therapy and tissue engineering because of their multipotency and ease of isolation without serial ethical issues. However, their limited in vitro lifespan in culture systems hinders their therapeutic application. Some somatic stem cells, including hADMPCs, are known to be localized in hypoxic regions; thus, hypoxia may be beneficial for ex vivo culture of these stem cells. These cells exhibit a high level of glycolytic metabolism in the presence of high oxygen levels and further increase their glycolysis rate under hypoxia. However, the physiological role of glycolytic activation and its regulatory mechanisms are still incompletely understood. Here, we show that Notch signaling is required for glycolysis regulation under hypoxic conditions. Our results demonstrate that 5% O2 dramatically increased the glycolysis rate, improved the proliferation efficiency, prevented senescence, and maintained the multipotency of hADMPCs. Intriguingly, these effects were not mediated by hypoxia-inducible factor (HIF), but rather by the Notch signaling pathway. Five percent O2 significantly increased the level of activated Notch1 and expression of its downstream gene, HES1. Furthermore, 5% O2 markedly increased glucose consumption and lactate production of hADMPCs, which decreased back to normoxic levels on treatment with a γ-secretase inhibitor. We also found that HES1 was involved in induction of GLUT3, TPI, and PGK1 in addition to reduction of TIGAR and SCO2 expression. These results clearly suggest that Notch signaling regulates glycolysis under hypoxic conditions and, thus, likely affects the cell lifespan via glycolysis.
Introduction
Human adipose tissue-derived mesenchymal stem cells (MSCs), also referred to as human adipose tissue-derived multilineage progenitor cells (hADMPCs), are multipotent stem cells that can differentiate into various types of cells, including hepatocytes [1], cardiomyoblasts [2], pancreatic cells [3], and neuronal cells [4–6]. They can be easily and safely obtained from lipoaspirate without posing serious ethical issues and can also be expanded ex vivo under appropriate culture conditions. Moreover, MSCs, including hADMPCs, have the ability to migrate to injured areas and secrete a wide variety of cytokines and growth factors that are necessary for tissue regeneration [7–11]. In addition, due to their hypoimmunogenicity and immunomodulatory effects, hADMPCs are good candidates as gene delivery vehicles for therapeutic purposes [12]. Thus, hADMPCs are attractive seeding cells for cell therapy and tissue engineering. However, similar to other somatic stem cells or primary cells, hADMPCs have limited growth potential and ultimately stop proliferation as a result of cellular senescence [13], which hinders their therapeutic application.
Conversely, embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) are immortal under standard culture conditions. Recently, several groups have reported that these cells greatly rely on glycolysis for energy production even under high-oxygen conditions [14–16]. This phenomenon is known as the Warburg effect and was originally described for cancer cells by Otto Warburg in the 1920s [17]. Although mitochondrial respiration is more efficient than glycolysis in generating ATP (net yield of 30 ATPs vs. 2 ATPs), glycolysis is able to produce ATP considerably faster than mitochondrial respiration as long as glucose supplies are adequate. Thus, a metabolic shift from mitochondrial respiration to glycolysis would provide a growth advantage for actively proliferating cells. Moreover, Kondoh et al. demonstrated that enhanced glycolysis is also involved in cellular immortalization through reduction of intrinsic reactive oxygen species (ROS) production [14,18,19]. Since accumulation of intrinsic ROS levels could be a major reason for replicative senescence [20], enhancing glycolysis in cultured cells might improve the quality of the cells by suppressing premature senescence. One candidate method for induction of glycolysis is application of low-oxygen conditions to activate the transcription factor, hypoxia-inducible factor (HIF). HIF-1 is known to increase the expression of most glycolytic enzymes and the glucose transporters GLUT1 and GLUT3 [20]. Thus, several studies have reported that hypoxia is beneficial for the maintenance of hESCs in a pluripotent state [21,22]. Moreover, low oxygen tension has been reported to enhance the generation of iPSCs both from mouse and human primary fibroblasts [23].
Recently, hypoxic culture conditions have also been reported to confer a growth advantage, prevent premature senescence, and maintain undifferentiated states in somatic stem cells; for example, hematopoietic stem cells (HSCs) [24], neural stem cells [25], and bone marrow-derived MSCs [26]. These stem cells reside in their local microenvironments called the “stem cell niche,” where the oxygen tension is relatively low (in the range of 1%–9%). Thus, hypoxic culture may be beneficial to these stem cells with regard to in vitro proliferation, cell survival, and differentiation. Takubo et al. reported that HSCs activated Pdk through HIF1α in hypoxic culture conditions, resulting in maintenance of glycolytic flow and suppression of the influx of glycolytic metabolites into mitochondria, and this glycolytic metabolic state was shown to be indispensable for the maintenance of HSCs [27]. Several studies have reported that MSCs exhibit a high level of glycolytic metabolism in the presence of high oxygen levels and further increase their rate of glycolysis on culture under hypoxia [28,29]. However, a relationship between beneficial effects of hypoxic conditions and metabolic status in addition to involvement of HIFs in the metabolic changes has not been investigated in these reports.
In this study, we aimed at investigating the effect of 5% oxygen on hADMPCs. Our results demonstrate that culture under 5% oxygen increased the glycolysis rate, improved the proliferation efficiency, prevented the cellular senescence, and maintained the undifferentiated status of hADMPCs. Intriguingly, these effects were not mediated by HIF, but rather by Notch signaling, an important signaling pathway required for the development of many cell types and maintenance of stem cells [30,31]. Five percent oxygen activated Notch signaling, resulting in the upregulation of SLC2A3, TPI, and PGK1 in addition to the downregulation of TIGAR and SCO2, which may contribute to the increase in the glycolysis rate. These observations, thus, provide new regulatory mechanisms for stemness maintenance obtained under 5% oxygen conditions.
Materials and Methods
Adipose tissue samples
Subcutaneous adipose tissue samples (10–50 g each) were resected during plastic surgery from five female and two male patients (age 20–60 years) as discarded tissue. The study protocol was approved by the Review Board for Human Research of Kobe University Graduate School of Medicine Foundation for Biomedical Research and Innovation, Osaka City University Graduate School of Medicine, and Kinki University Pharmaceutical Research and Technology Institute (reference number: 12-043). Each subject provided signed informed consent.
Cell culture
hADMPCs were isolated as previously reported [11,32–34] and maintained in a medium containing 60% DMEM low glucose, 40% MCDB-201 medium (Sigma Aldrich), 1×insulin-transferrin-selenium (Life Technologies), 1 nM dexamethasone (Sigma Aldrich), 100 mM ascorbic acid 2-phosphate (Wako), 10 ng/mL epidermal growth factor (PeproTech), and 5% fetal bovine serum. The cells were plated to a density of 5×103 cells/cm2 on fibronectin-coated dishes, and the medium was replaced every 2 days. For hypoxic culture, cells were cultured in a gas mixture composed of 90% N2, 5% CO2, and 5% O2. For maintenance of the hypoxic gas mixture, a ProOx C21 carbon dioxide and oxygen controller and a C-Chamber (Biospherix) were used.
Senescence-associated β-galactosidase staining
Cells were fixed with 2% paraformaldehyde/0.2% glutaradehyde for 5 min at room temperature and then washed twice with phosphate-buffered saline (PBS). The cells were then incubated overnight at 37°C with fresh senescence-associated β-galactosidase (SA-β-Gal) chromogenic substrate solution (1 mg/mL Bluo-gal (Life Technologies), 40 mM citric acid (pH 6.0), 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM NaCl, and 2 mM MgCl2).
Measurement of ROS production
Cells were harvested and incubated with 10 μM 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA). The amount of intracellular ROS production was proportional to the green fluorescence, as analyzed using a Guava EasyCyte 8HT flow cytometer (Millipore) using an argon laser at 488 nm and a 525/30 nm band pass filter, and dead cells were excluded using the Live/Dead Fixable Far Red Dead Cell Stain Kit (Life Technologies).
EdU proliferation assay
For assessment of cell proliferation, hADMPCs were seeded on a fibronectin-coated six-well plate at a density of 5×103 cells/cm2 and cultured for 3 days. Cell proliferation was detected by incorporating of 5-ethynyl-2′-deoxyuridine (EdU) and using the Click-iT EdU Alexa Fluor 488 Flow Cytometry Assay Kit (Life Technologies). Briefly, according to the manufacturer's protocol, cells were incubated with 10 μM EdU for 2 h before fixation, permeabilized, and stained with EdU. EdU-positive cells were then analyzed using the 488 nm laser of a Guava EasyCyte 8HT flow cytometer (Millipore).
Flow cytometry analysis
Flow cytometry analysis was performed as previously described [34]. Briefly, hADMPCs were harvested and re-suspended in staining buffer (PBS containing 1% BSA, 2 mM EDTA, and 0.01% sodium azide) at a density of 1×106 cells/mL, incubated for 20 min with a fluorescein isothiocyanate (FITC)-conjugated antibody against CD49b or CD98 (BioLegend) or a phycoerythrin (PE)-conjugated antibody against CD10, CD13, CD29, CD44, CD49a, CD49c, CD49d, CD49e, CD51/61, CD73, CD90, CD105, CD117, SSEA4, HLA-A,B,C (BioLegend), CD133/1 (Miltenyi Biotec), or CD166 (Beckman Coulter). Nonspecific staining was assessed using relevant isotype controls. Dead cells were excluded using the Live/Dead Fixable Far Red Dead Cell Stain Kit (Life Technologies). FlowJo software was used for quantitative analysis.
RNA extraction, cDNA generation, and quantitative polymerase chain reaction
Total RNA was extracted using the RNeasy Mini Kit (Qiagen) accroding to the manufacturer's instructions. cDNA was generated from 1 μg of total RNA using the Verso cDNA Synthesis Kit (Thermo Scientific) and purified using the MinElute PCR Purification Kit (Qiagen). Quantitative polymerase chain reaction (Q-PCR) analysis was conducted using the SsoFast EvaGreen supermix (Bio-Rad) according to the manufacturer's protocols. The relative expression value for each gene was calculated using the ΔΔCt method, and the most reliable internal control gene was determined using geNorm Software (http://medgen.ugent.be/∼jvdesomp/genorm/). Details of the primers used in these experiments are available on request.
Western blot analysis
Whole cell extracts were prepared by washing cells with ice-cold PBS and lysing them with M-PER Mammalian Protein Extraction Reagent (Thermo Scientific Pierce) according to the manufacturer's instructions. Nuclear and cytosolic extracts were prepared as follows. Cells were washed with ice-cold PBS and lysed with lysis buffer (50 mM Tris-HCl (pH 7.5), 0.5% Triton X-100, 137.5 mM NaCl, 10% glycerol, 5 mM EDTA, 1 mM sodium vanadate, 50 mM sodium fluoride, 10 mM sodium pyrophosphate, and protease inhibitor cocktail). Then, insoluble nuclei were isolated by centrifugation and lysed with lysis buffer containing 0.5% SDS. Equal amounts of proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene fluoride membranes (Immobilon-P; Millipore), and probed with antibodies against cleaved Notch1 (#2421; Cell Signaling Technology), HIF-1α (#610959; BD Bioscience), hypoxia-inducible factor 2α (MAB3472; Millipore), Akt (#9272; Cell Signaling Technology), and phospho Akt (Ser473) (#4060; Cell Signaling Technology). Horseradish peroxidase (HRP)-conjugated anti-mouse or -rabbit IgG antibody (Cell Signaling Technology) was used as a secondary antibody, and immunoreactive bands were visualized using Immobilon Western Chemiluminescent HRP substrate (Millipore). The band intensity was measured using the ImageJ software.
Fluorescence microscopy
Phase-contrast and fluorescence images were obtained using a fluorescence microscope (BZ-9000; Keyence) using BZ Analyzer Software (Keyence).
Adipogenic, osteogenic, and chondrogenic differentiation procedures
For adipogenic differentiation, cells were cultured in differentiation medium (Zen-Bio). After 7 days, half of the medium was exchanged for adipocyte medium (Zen-Bio) and this was repeated every 3 days. Three weeks after differentiation, adipogenic differentiation was confirmed by a microscopic observation of intracellular lipid droplets with the aid of Oil Red O staining. Osteogenic differentiation was induced by culturing the cells in osteocyte differentiation medium (Zen-Bio). Differentiation was examined by Alizarin Red staining. For chondrogenic differentiation, 2×105 hADMSCs were centrifuged at 400 g for 10 min. The resulting pellets were cultured in chondrogenic medium (Lonza) for 21 days. The pellets were fixed with 4% paraformaldehyde in PBS, embedded in OCT, frozen, and sectioned at 8 μm. The sections were incubated with PBSMT (PBS containing 0.1% Triton X-100, and 2% skim milk) for 1 h at room temperature, and then incubated with a mouse monoclonal antibody against type II collagen (Abcam) for 1 h. After washing with PBS, cells were incubated with Alexa 546-conjugated anti-mouse IgG to identify chondrocytes (Life Technologies). The cells were counterstained with 4′-6-diamidino-2-phenylindole (DAPI) (Life Technologies) to identify cellular nuclei. The sections were also stained with 1% alcian blue (Sigma Aldrich) in 3% acetic acid, pH 2.5 for 30 min.
Determination of HK, PFK, LDH, PDH, and Cox IV activities
Cells (2×106) were lysed, and HK, PFK, LDH, or PDH activity was measured using the Hexokinase Colorimetric Activity Kit, Phosphofructokinase (PFK) Activity Colorimetric Assay Kit, Lactate Dehydrogenase (LDH) Activity Assay Kit, or Pyruvate Dehydrogenase Activity Colorimetric Assay Kit (all from BioVision), respectively, according to the manufacturer's instructions. To measure Cox IV activity, mitochondria were isolated from 2×107 cells using a Mitochondria Isolation Kit (Thermo Scientific) and lysed with buffer containing n-Dodecyl β-D-maltoside, followed by measurement with the Mitochondria Activity Assay (Cytochrome C Oxidase Activity Assay) Kit (BioChain Institute), according to the manufacturer's instructions.
Results
5% oxygen hypoxic culture condition increases proliferation capacity and decreases senescence
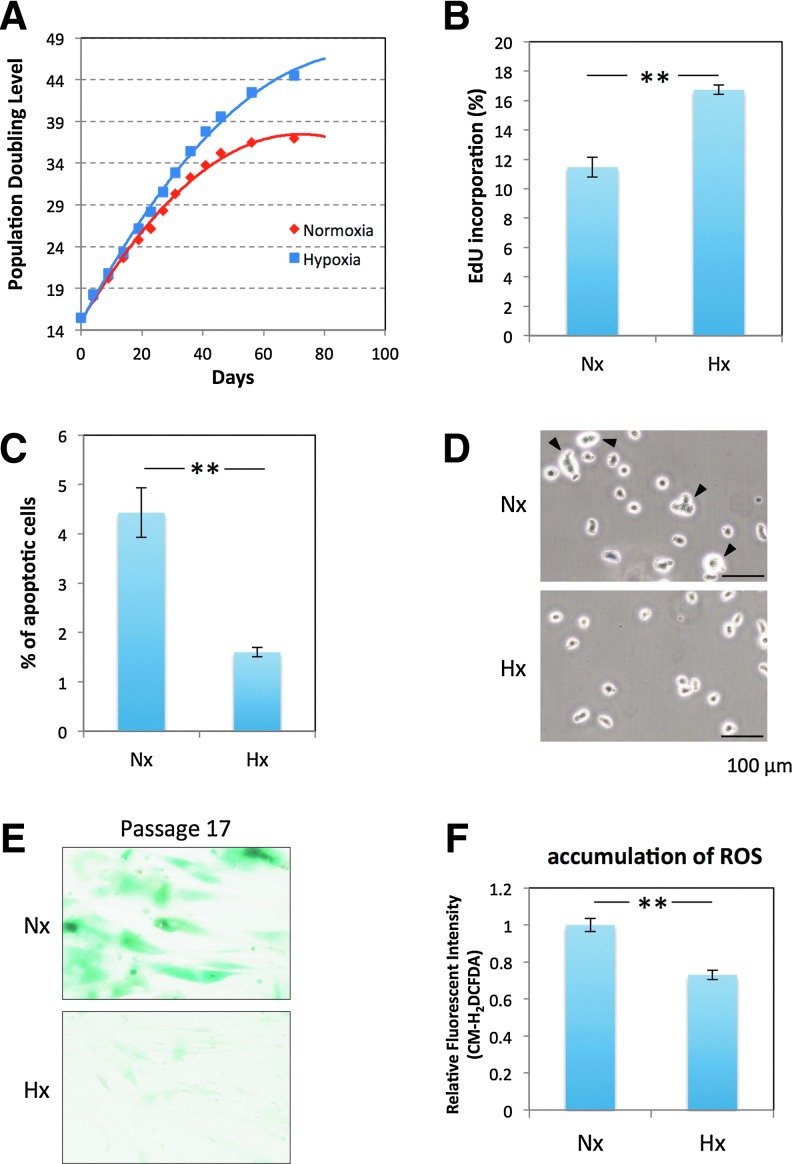
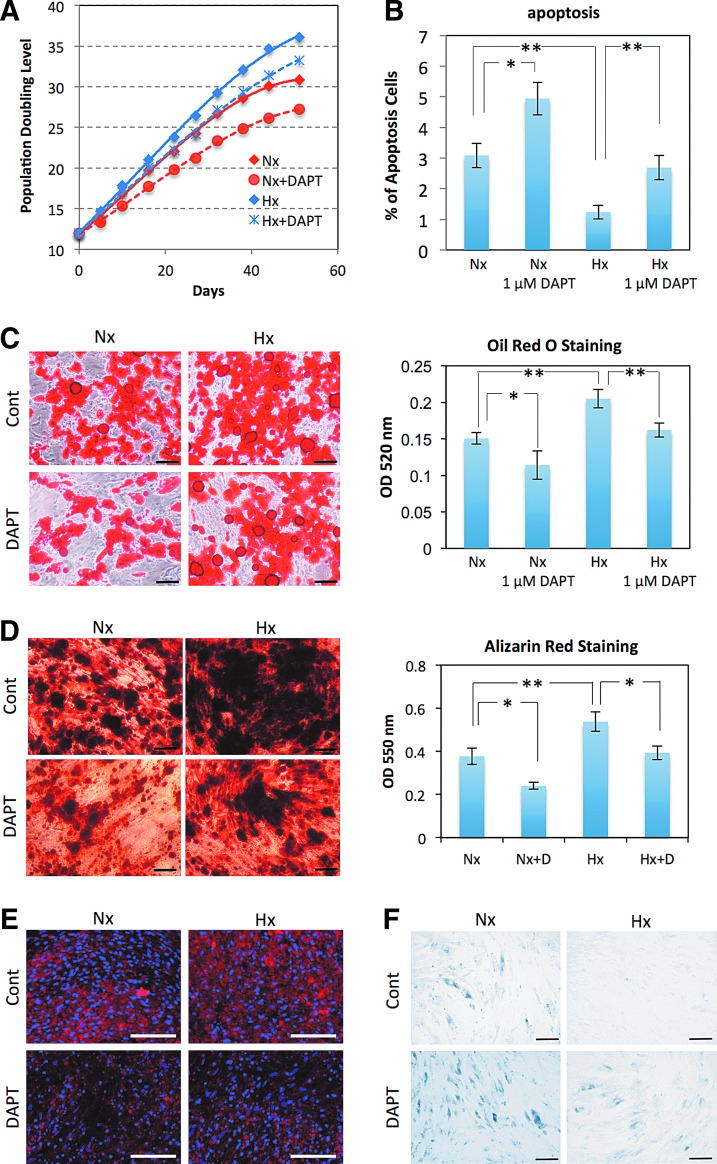
hADMPCs were cultured under 20% oxygen (normoxia; Nx) or 5% oxygen (hypoxia; Hx), and their proliferation capacities were examined based on the relationship between the number of cultivation days and the population doubling level (PDL). Nx-cultured hADMPCs ceased proliferation at a PDL of 35–40 (between 46–70 days), whereas continuous cell proliferation beyond 45 PDL was observed when hADMPCs were cultured in the Hx condition (Fig. 1A). To investigate whether this increase of PDL in the Hx culture condition resulted from an increase in cell cycle progression and increase in survival rates, EdU, an alternative to 5-bromo-2′-deoxyuridine (BrdU), was incorporated into the genomic DNA of the hADMPCs, and the amount of incorporated EdU was quantified by flow cytometry. As shown in Fig.1B, the EdU incorporation rate was significantly higher in Hx-cultured hADMPCs than in Nx-cultured hADMPCs, suggesting that cell growth was increased in the Hx culture condition. In addition, measurement of DNA content in hADMPCs revealed a slight but significant decrease of sub-G1 peaks, which indicates the existence of apoptotic cells with degraded DNA, when the cells were cultured in the Hx condition (Fig. 1C). These data suggest that the Hx culture condition increases the proliferation capacity of hADMPCs by promoting their cell growth and survival rates. We also found that Nx-cultured hADMPCs were larger with a more irregular shape (Fig. 1D), which suggests that the Hx culture condition prevented hADMPCs from entering senescence [35]. To further investigate this phenomenon, cellular senescence was measured by staining for SA-β-Gal, which revealed that SA-β-Gal activity was increased in Nx-cultured hADMPCs at passage 17 (Fig. 1E). Since it has been hypothesized that senescence results from oxidative stress [20], accumulation of ROS in hADMPCs was detected using the nonfluorescent probe, CM-H2DCFDA. Flow cytometry analysis revealed that ROS were generated at higher levels in hADMPCs when cultured in the Nx condition (Fig. 1F), suggesting that reduced production of ROS in the Hx condition may prevent the cells from entering replicative senescence.
FIG. 1.
Hypoxia increases proliferation capacity and decreases senescence in tissue-derived multilineage progenitor cells (hADMPCs). (A) Growth profiles of hADMPCs under normoxic (red square) and hypoxic (blue square) conditions. The population doubling level (PDL) was determined to be 0 when cells were isolated from human adipose tissue. Cells were maintained until they reached PDL13–15 (passage 3) and then split into four aliquots of equal cell densities. PDL was calculated based on the total cell number at each passage. (B) Detection of normoxic (Nx) and hypoxic (Hx) cells by flow cytometry after incorporation of EdU. (C) Percentages of apoptotic cells with sub-G1 DNA under Nx and Hx conditions. The results are presented as the mean of three independent experiments. (D) hADMPCs cultured under Nx and Hx conditions were harvested by trypsin-EDTA and then imaged using a phase-contrast microscope. Arrowheads indicate cells with a larger and more irregular shape. (E) Cells expanded under Nx and Hx conditions were stained with SA-β-gal. (F) Cellular reactive oxygen species detection by the oxidative stress indicator CM-H2DCFDA in hADMPCs under Nx or Hx. Data are presented as the mean fluorescence intensity of three independent experiments. Error bars indicate SD. **P<0.01 indicates significant difference (independent t-test) between Nx and Hx. Scale bars; 100 μm. Color images available online at www.liebertpub.com/scd
Hypoxic culture maintains some MSC properties and increases differentiation
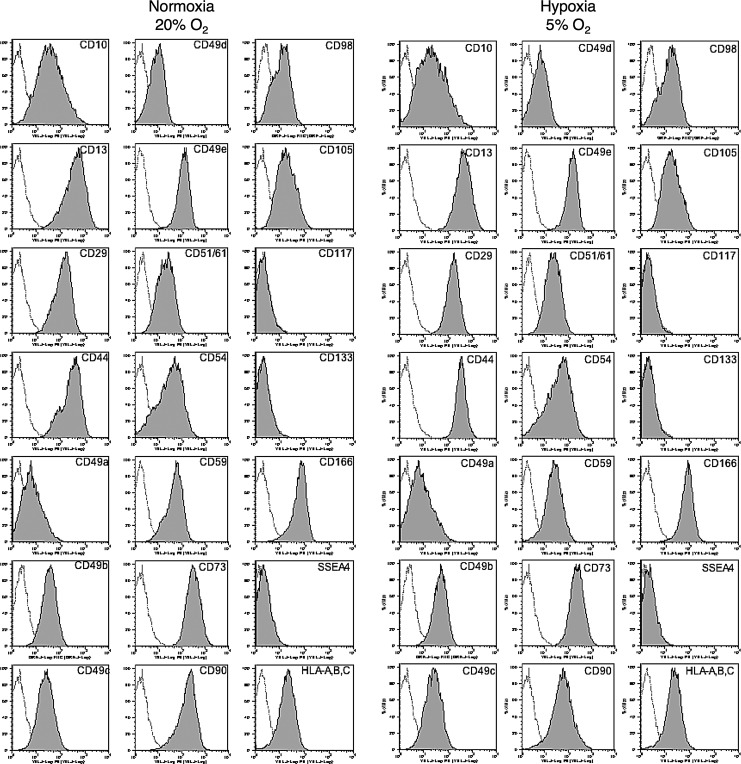
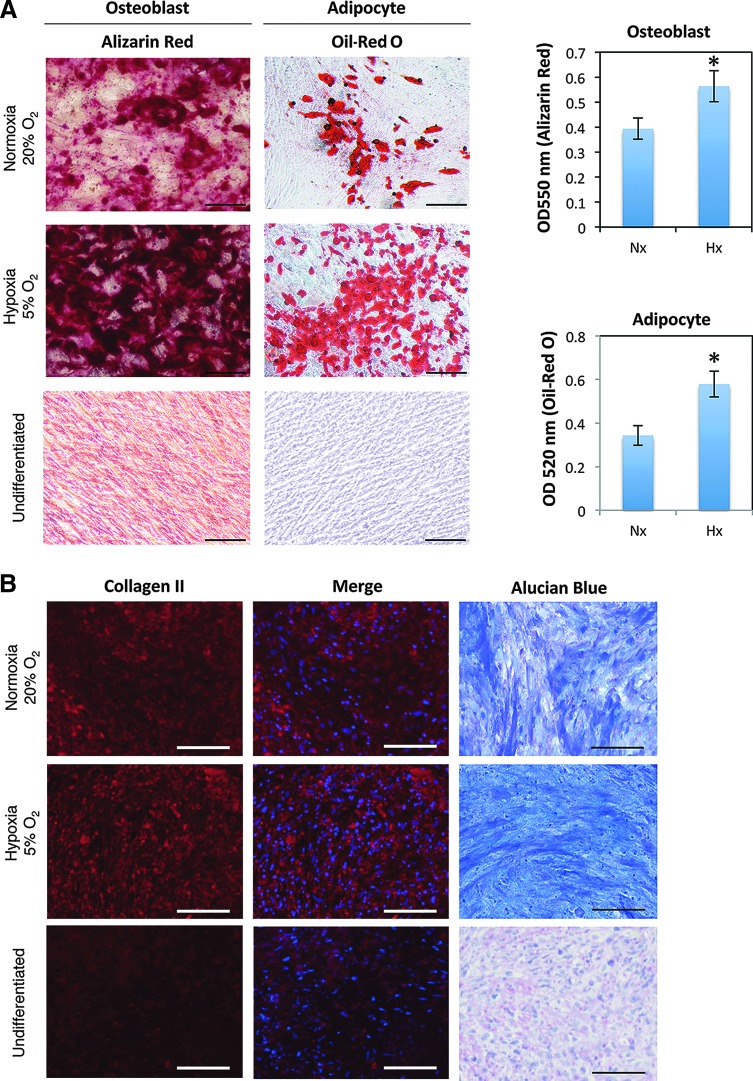
We then examined the cell properties of hADMPCs under Nx and Hx conditions. Initially, cell surface antigens expressed on hADMPCs were analyzed by flow cytometry. No significant difference in expression profile between hADMPCs cultured in Nx and Hx was observed; the cells were consistently positive for CD10, CD13, CD29, CD44, CD49a, CD49b, CD49c, CD49d, CD49e, CD51/61, CD54, CD59, CD73, CD90, CD98, CD105, CD166, and HLA-A, B, C, but negative for CD34, CD45, CD117, and CD133 (Fig. 2 and data not shown). These data were consistent with previous reports describing the expression profiles of cell surface markers of hMSCs [36,37]. To further examine the stem cell properties of hADMPCs, their potential for differentiation into adipocyte, osteocyte, and chondrocyte lineages was analyzed at passage 8. Hx-cultured hADMPCs presented enhanced differentiation into various lineages (Fig. 3A, B), indicating that the Hx culture condition improved the stem cell properties of hADMPCs.
FIG. 2.
Hypoxic culture maintains mesenchymal stem cell properties. hADMPCs cultured under normoxia (20% O2) or hypoxia (5% O2) were labeled with antibodies against the indicated antigens and analyzed by flow cytometry. Representative histograms are shown. The respective isotype control is shown as a gray line.
FIG. 3.
Hypoxic culture enhances stem cell properties. hADMPCs were expanded under normoxic and hypoxic conditions. (A) Normoxic (20% O2) and hypoxic (5% O2) cells at passage 8 were induced for 3 weeks to differentiate into osteoblasts and adipocytes and stained with Alizarin Red and Oil-Red O, respectively. The stained dye was extracted, and OD values were measured and plotted as the means of three independent experiments±SD. *P<0.05. Scale bars, 200 μm. (B) Normoxic (20% O2) and hypoxic (5% O2) cells at passage 8 were induced for 3 weeks to differentiate to chondrocytes, and immunofluorescent analysis of collagen II (red) and Alucian Blue staining were performed. The blue signals indicate nuclear staining. Scale bars, 100 μm. Noninduced control cultures in growth medium without adipogenic, osteogenic or chondrogenic differentiation stimuli are shown (Undifferentiated). Color images available online at www.liebertpub.com/scd
Hypoxic culture condition activates Notch signaling
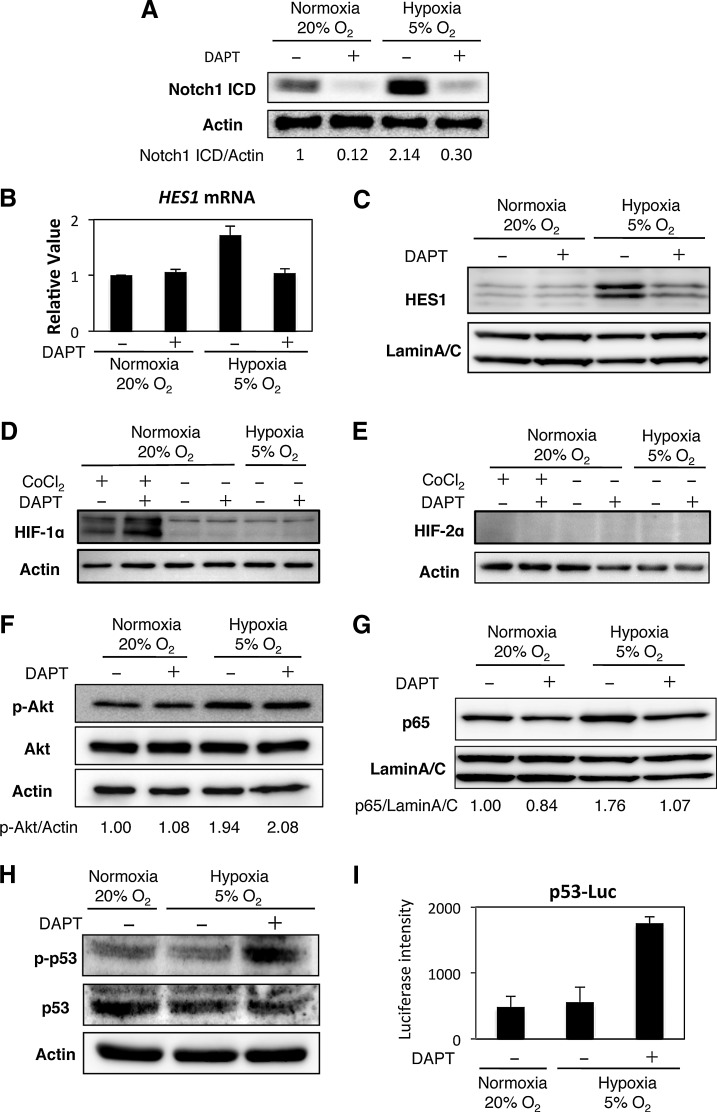
To reveal the molecular mechanism by which the Hx culture condition increased the proliferative capacity and maintained the stem cell properties of hADMPCs, we next examined Notch signaling, which is required for maintaining stem-cell features of various types of stem cells [30,31]. As expected, levels of cleaved NOTCH1, an activated form of NOTCH1, were significantly increased (greater than twofold) in the Hx culture condition (Fig. 4A). Q-PCR analysis revealed that HES1, a downstream target of Notch signaling, was upregulated in Hx-cultured hADMPCs, which also indicated that Notch signaling was activated in the Hx culture condition (Fig. 4B). Administration of the γ-secretase inhibitor DAPT at 1 μM, which was sufficient to inhibit the proteolytic cleavage of NOTCH1 (Fig. 4A), decreased the Hx-induced expression of HES1 at both mRNA and protein levels (Fig. 4B, C). These data indicate that Hx increased the expression of HES1 through activation of Notch signaling. It has been reported that Notch signaling and hypoxia-inducible factor (HIF) undergo crosstalk in hypoxic cells [38–41]. Therefore, HIF-1α and HIF-2α protein levels in hADMPCs were analyzed by western blotting. HIF-1α was stabilized when a chemical hypoxia-mimicking agent, cobalt chloride, was applied in the culture; whereas no obvious increase of HIF-1α was observed in the Hx culture condition (Fig. 4D). However, we did not detect any HIF-2α expression even in the presence of cobalt chloride (Fig. 4E). Q-PCR analysis revealed that HIF2A mRNA was not expressed in these cells (data not shown). From these results, we concluded that neither HIF-1α nor HIF-2α was involved in the Hx-induced increase in the proliferative capacity and stem cell properties of hADMPCs.
FIG. 4.
Hypoxic culture condition activates Notch signaling but not HIF proteins. hADMPCs were expanded under normoxic (20% O2) and hypoxic (5% O2) conditions. DAPT (1 μM) was added to inhibit Notch signaling. (A) Western blot analysis of intracellular domain of Notch1 (Notch1 ICD) expression. Actin served as the loading control. Numbers below blots indicate relative band intensities as determined by ImageJ software. (B) Q-PCR analysis of HES1. Each expression value was calculated with the ΔΔCt method using UBE2D2 as an internal control. (C) Western blot analysis of HES1 in nuclear fractions of hADMPCs. Lamin A/C served as the loading control. (D, E) Western blot analysis of HIF-1α (D) and HIF-2α (E). Cobalt chloride (CoCl2) was added at a concentration of 100 μM to stabilize HIF proteins (positive control). (F) Western blot analysis of phosphorylated Akt (p-Akt) and Akt. Actin served as the loading control. Numbers below blots indicate relative band intensities as determined by ImageJ software. (G) Western blot analysis of nuclear localization of p65. Lamin A/C served as the loading control. Numbers below blots indicate relative band intensities as determined by ImageJ software. (H) Western blot analysis of phosphorylated p53 (p-p53) and p53. Actin served as the loading control. (I) Activity of p53 was measured by the p53-luciferase reporter assay. Relative luciferase activity was determined from three independent experiments and normalized to pGL4.74 activity.
To identify the signaling responsible for the observed effect, we next examined the Akt, NF-κB, and p53 signaling pathways. It has been reported that hypoxic conditions induce the activation of Akt and NF-κB signaling [42,43]. In addition, hypoxic conditions have been shown to inhibit p53 activity [44], and crosstalk between these pathways and Notch signaling has also been demonstrated [41,45–47]. As shown in Fig. 4F, the Hx condition increased Akt phosphorylation, which was not decreased by DAPT treatment. These data demonstrate that 5% oxygen activated Akt signaling but not via Notch signaling. Similarly, the hypoxic condition induced nuclear accumulation of p65, which was inhibited by DAPT treatment (Fig. 4G). These data suggest that NF-κB signaling is regulated by Notch signaling in hADMPCs. Furthermore, p53 was not activated under the 5% oxygen condition as assessed by detection of phospho-p53 and a p53 reporter assay. However, DAPT treatment significantly increased p53 activity (Fig. 4H, I).
Notch signaling is indispensable for acquisition of the advantageous properties of hADMPCs
We next examined the roles of Notch signaling in the proliferative capacity and stem cell properties of hADMPCs in the Hx culture condition. To inhibit Notch signaling, DAPT was added to the medium at a final concentration of 1 μM. DAPT treatment significantly decreased the PDL when hADMPCs were cultured under either 20% or 5% oxygen (Fig. 5A). Intriguingly, measurement of the DNA content in hADMPCs revealed that inhibition of Notch signaling by 1 μM DAPT significantly attenuated the decrease in apoptotic cells in the Hx condition (Fig. 5B). These data suggest that 5% oxygen increases the proliferation capacity of hADMPCs through Notch signaling by promoting their survival. To examine whether Notch signaling affects the stem cell properties of hADMPCs, differentiation into adipocyte, osteocyte, and chondrocyte lineages was analyzed at passage 8. Hx-cultured hADMPCs underwent greater differentiation into all lineages as described in Fig. 3, whereas application of a Notch inhibitor significantly decreased the differentiation capacity to all lineages (Fig. 5C–E). In addition, SA-β-Gal staining revealed that inhibition of Notch signaling by DAPT remarkably promoted senescence in both the Nx and Hx culture conditions, suggesting that the suppression of replicative senescence observed in the Hx condition is mediated by Notch signaling (Fig. 5F).
FIG. 5.
Notch signaling is indispensable for acquisition of the advantageous properties of hADMPCs. hADMPCs were expanded under normoxic (20% O2; Nx) and hypoxic (5% O2; Hx) conditions. DAPT (1 μM) was added to inhibit Notch signaling. (A) Growth profiles of hADMPCs under Nx (red) and Hx (blue) conditions. Solid lines represent control cells, and dotted lines represent DAPT-treated cells. The number of population doublings was calculated based on the total cell number at each passage. (B) Percentages of apoptotic cells with sub-G1 DNA. Results are presented as the mean of three independent experiments±SD. (C, D) hADMPCs at passage 8 were induced for 3 weeks to differentiate into adipocytes (C) and osteoblasts (D) and stained with Oil Red O and Alizarin Red, respectively. The stained dye was extracted, and OD values were measured and plotted as the means of three independent experiments±SD. (E) hADMPCs at passage 8 were induced for 3 weeks to differentiate into chondrocytes, and an immunofluorescent analysis of collagen II (red) was performed. The blue signals indicate nuclear staining. (F) hADMPCs were stained with SA-β-gal. *P<0.05 and **P<0.01 indicate significant differences (independent t-test) between Nx and Hx. Scale bars; 100 μm. Color images available online at www.liebertpub.com/scd
Glycolysis is enhanced in the 5% oxygen condition through Notch signaling
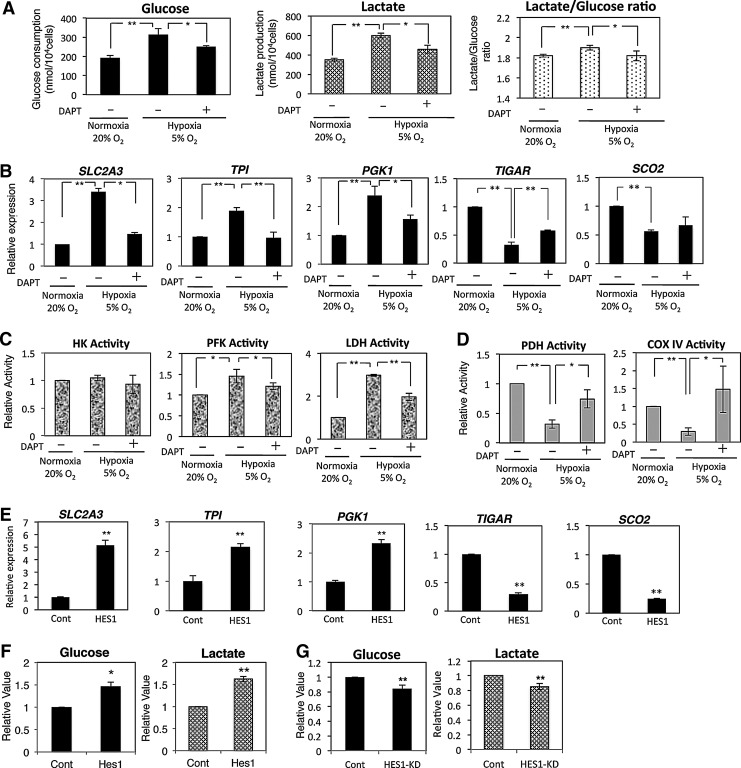
Recent studies suggest that the metabolic shift from aerobic mitochondrial respiration to glycolysis extends the life span possibly via reduction of intrinsic ROS production [18,19]. Our results demonstrate that the 5% oxygen condition reduced ROS accumulation in hADMPCs (Fig. 1F). In addition, the relationship between Notch signaling and glycolysis has been recently established [48,49]. We, therefore, considered glycolytic flux by measuring the glucose consumption and lactate production of hADMPCs in the Nx or Hx culture conditions. As shown in Fig. 6A, glucose consumption and lactate production were elevated in the Hx culture condition, indicating that a metabolic shift to glycolysis occurred when hADMPCs were cultured in 5% oxygen. In contrast, the Notch inhibitor DAPT markedly reduced glycolytic flux as assessed by glucose consumption and lactate production (Fig. 6A). To identify the genes responsible for the glycolytic change, we performed a Q-PCR analysis. As shown in Fig. 6B, SLC2A3, TPI, and PGK1, encoding glycolytic enzymes, were upregulated in the 5% oxygen condition; whereas these genes were suppressed by DAPT treatment. Interestingly, Hes1 transduction by an adenoviral vector markedly induced the mRNA expression of the same genes (Fig. 6E). In addition, SCO2, a positive modulator of aerobic respiration, and TIGAR, a negative regulator of glycolysis, were transcriptionally downregulated in the 5% oxygen condition; whereas DAPT treatment partially restored the expression level (Fig. 6B). Adenoviral expression of Hes1 dramatically reduced SCO2 and TIGAR expression (Fig. 6E), which suggests that the Notch-Hes1 signaling modulates the metabolic pathway. We also measured the activities of key enzymes in glycolysis. Hexokinase activity was not changed under hypoxic conditions; however, PFK and LDH were activated in 5% oxygen condition, which was attenuated by Notch inhibition (Fig. 6C). In addition, pyruvate dehydrogenase (PDH) and cytochrome c oxidase (Complex IV) activity assays showed that mitochondrial respiration decreased under the hypoxic condition and that DAPT treatment restored it (Fig. 6D). Moreover, glycolytic flux in Hes1-expressing hADMPCs was positively correlated with the expression of these glycolytic genes as assessed by glucose consumption and lactate production (Fig. 6F). In contrast, HES1 knockdown by adenoviral transduction of HES1 RNAi resulted in a significant reduction of glycolytic flux (Fig. 6G), demonstrating that HES1 is involved in the regulation of glycolysis.
FIG. 6.
Glycolysis is enhanced under 5% oxygen through Notch signaling. (A–D) hADMPCs were expanded under normoxic (20% O2) and hypoxic (5% O2) conditions. DAPT (1 μM) was added to inhibit Notch signaling. (A) Glucose consumption and lactate production of hADMPCs were measured and plotted as the means of three independent experiments±SD. (B) Relative mRNA expression of SLC2A3, TPI, PGK1, TIGAR, and SCO2 in hADMPCs. Each expression value was calculated with the ΔΔCt method using UBE2D2 as an internal control. (C, D) Hexokinase (HK), phosphofructokinase (PFK), lactate dehydrogenase (LDH) (C), pyruvate dehydrogenase (PDH), and Complex IV (Cox IV) (D) activities were measured and the value of relative activity was plotted as the means of three independent experiments±SD. (E, F) hADMPCs were transduced with either mock (Cont) or HES1 and then cultured for 3 days. (E) Relative mRNA expression of SLC2A3, TPI, PGK1, TIGAR, and SCO2 in hADMPCs. Each expression value was calculated with the ΔΔCt method using UBE2D2 as an internal control. (F) Glucose consumption and lactate production of hADMPCs were measured and plotted as the means of three independent experiments±SD. (G) hADMPCs were transduced with either scrambled control RNAi (Cont) or RNAi against HES1 (HES1-KD), and then cultured for 3 days. Glucose consumption and lactate production of hADMPCs were measured and plotted as the means of three independent experiments±SD. **P<0.01. *0.01<P<0.05.
Glycolysis supports the proliferation of hADMPCs
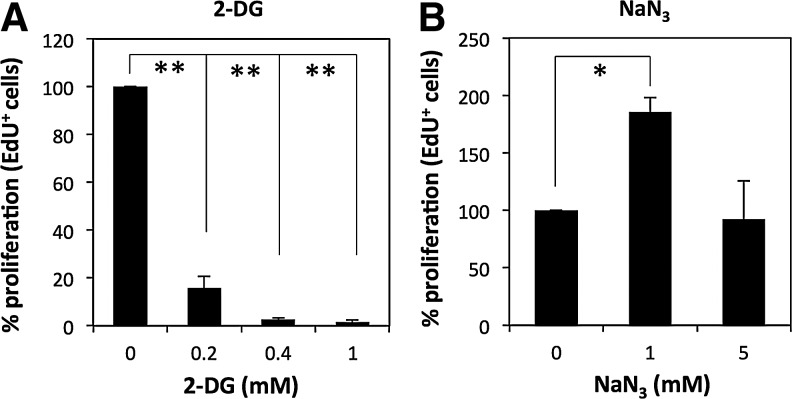
To determine whether aerobic glycolysis is important for the proliferation of hADMPCs, hADMPCs were treated with the glycolytic inhibitor 2-deoxy-D-glucose (2-DG) or the respiration inhibitor sodium azide (NaN3). We found that hADMPCs were sensitive to treatment with 2-DG even at a low concentration of 0.2 mM (Fig. 7A). In contrast, treatment of hADMPCs with NaN3 rather increased their proliferation at the concentration of 1 mM and supported their proliferation even at the concentration of 5 mM (Fig. 7B). These data suggest that the proliferation of hADMPCs is compromised when aerobic glycolysis is blocked.
FIG. 7.
Glycolysis supports proliferation of hADMPCs. hADMPCs were treated with 0, 0.2, 0.4, and 1 mM 2-deoxy-D-glucose (2-DG) (A) or 0, 1, and 5 mM sodium azide (NaN3) (B) for 24 h. Cells were then allowed to incorporate EdU for 2 h, and the EdU-positive cells were analyzed by flow cytometry. The percentages for the 0 mM control were plotted as the means of three independent experiments±SD. *P<0.05; **P<0.01.
Discussion
Recent evidence suggests that hypoxic culture conditions confer a growth advantage, prevent premature senescence, and maintain undifferentiated states in ESCs, iPSCs, and somatic stem cells. However, the molecular mechanism underlying the beneficial effects of culturing these cells at low oxygen conditions remains unclear. Our findings prompted us to hypothesize that Notch signaling in physiological hypoxic conditions (5% O2) contributes to these effects on hADMPCs by modulating glycolytic flux.
We found that 5% O2 significantly increased the proliferation capacity, decreased apoptosis, and inhibited senescence of hADMPCs (Fig. 1). Moreover, 5% O2 improved the differentiation of hADMPCs without affecting the expression of their cell surface markers (Figs. 2 and 3). Welford et al. reported that HIF-1α delays premature senescence of mouse embryonic fibroblasts under hypoxic conditions (2% O2) [50]. Tsai et al. reported that hypoxia (1% O2) inhibits senescence and maintains MSC properties through accumulation of HIF-1α [26]. Hypoxia was recently reported to enhance the undifferentiated status and stem cell properties in various stem and precursor cell populations via the interaction of HIF with the Notch intracellular domain to activate Notch-responsive promoters [38]. In the current study, the effects observed in 5% O2 condition were independent of HIF proteins, because accumulation of HIF-1α and HIF-2α was not observed (Fig. 4). Instead, our findings suggest that 5% O2 activated Notch signaling, which contributed advantageous effects of hypoxic culture on hADMPCs. A pharmacological inhibitor of Notch signaling, DAPT, abrogated the hypoxic-induced Notch activation, increased proliferation capacity and lifespan, maintenance of stem cell properties, and prevention of senescence (Figs. 4 and 5). Moreover, we also found that 5% O2 enhanced glucose consumption and lactate production, and these effects were also attenuated by Notch inhibition (Fig. 6A) and knockdown of HES1 (Fig. 6G). Previously, it has been reported that Notch signaling promotes glycolysis by activating the PI(3)K-Akt pathway [48,49]. However, our results indicate that Akt signaling was not activated by Notch signaling, because DAPT did not attenuate hypoxia-induced Akt phosphorylation (Fig. 4F). Although Akt is unlikely to be regulated by Notch signaling in hADMPCs, it is obvious in our data that Akt signaling was activated by 5% O2. Therefore, we could not rule out the possibility that the promotion of glycolysis in the 5% O2 condition was caused by Akt signaling.
Recent evidence suggests that Notch signaling acts as a metabolic switch [48,51]. Zhou et al. demonstrated that hairy, a basic helix-loop-helix transcriptional repressor regulated by Notch signaling, was upregulated and genes encoding metabolic enzymes, including TCA cycle enzymes and respiratory chain complexes, were downregulated in hypoxia-tolerant flies. Intriguingly, they also found that hairy-binding elements were present in the regulatory region of the downregulated metabolic genes. Their work, thus, provides new evidence that hairy acts as a metabolic switch [51]. Landor et al. demonstrated that both hyper- and hypoactive Notch signaling induced glycolysis, albeit by different mechanisms. They showed that Notch activation increased glycolysis through activation of PI3K-AKT signaling, whereas decreased Notch activity inhibited mitochondrial function in a p53-dependent manner in MCF7 breast cancer cell lines [48]. Consistent with their reports, our findings that Notch signaling promoted activity of some glycolysis enzymes and inhibited mitochondrial activity (Fig. 6) also suggest that Notch signaling functioned as a metabolic switch. While our data showed that Notch inhibition by DAPT resulted in reduced glycolysis (Fig. 6A–C), induction of mitochondrial function (Fig. 6D) and activation of p53 (Fig. 4H, I) are not consistent with the report of Landor el al. This contradiction might be explained by the expression level of endogenous Notch. Landor et al. showed that in breast cancer MDA-M-231 cells, which showed higher endogenous Notch activity, high glucose uptake, and lactate production than MCF7 breast cancer cell lines, Notch inhibition by DAPT significantly reduced glucose consumption and lactate production [48]. As shown in Fig. 4A, we observed that hADMPCs in 5% O2 displayed high Notch activity. Moreover, the lactate-to-glucose ratio was 1.8–1.9 in hADMPCs, suggesting that hADMPCs largely rely on glycolysis for energy production (Fig. 6A). In addition, it was reported that hMSCs showed a higher glycolytic rate than primary human fibroblast [52]. It appears that hADMPCs cultured under hypoxic conditions might possess cell properties similar to MDA-M-231 cells or MCF7 cells, in which stable expression of constructs NICD1-GFP produces high Notch activity.
Nuclear translocation of p65 was observed in hypoxic conditions, demonstrating that NF-κB is a direct target of Notch signaling (Fig. 4G). Intriguingly, the hypoxic culture conditions in this study upregulated several genes encoding glycolytic enzymes (SLC2A3, TPI, and PGK1); whereas the expression of these genes was suppressed by Notch inhibition. In addition, Hes1 transduction induced mRNA expression of the same genes (Fig. 6). It was previously reported that SLC2A3 expression was regulated by p65/NF-κB signaling, and that Notch/Hes1 is able to induce the activation of the NF-κB pathway in human T-ALL lines and animal disease models [53]. Espinosa et al. demonstrated that Hes1 directly targeted the deubiquitinase CYLD, resulting in deubiquitination and inactivation of TAK1 and IKK, degradation of IκBa, and activation of NF-κB signaling [53]. In our systems, however, we did not observe repression of CYLD mRNA in Hes1-overexpressing hADMPCs (data not shown). While PGK1 mRNA has been reported to be upregulated by NF-κB, it has not clearly been shown to be controlled by NF-κB despite the presence of an NF-κB site in the promoter [54]. Although modulation of TPI expression by NF-κB has not been reported, we found several NF-κB binding sites on the human TPI promoter (data not shown). Since NF-κB is likely to be one of the responsible signals for hypoxic-induced glycolysis [53], further analysis will be required to determine the mechanism by which NF-κB signaling is induced by Notch signaling. In addition, it will be important to investigate whether NF-κB is really responsible for the observed glycolysis and whether it regulates the expression of SLC2A3, TPI, and PGK1 in hADMPCs under 5% oxygen.
In addition, SCO2, a positive modulator of aerobic respiration, and TIGAR, a negative regulator of glycolysis, were transcriptionally downregulated in the 5% oxygen condition; whereas DAPT treatment partially restored expression (Fig. 6B). We observed some glycolysis and mitochondrial enzyme activity and found that the activities of COX IV and PFK were consistent with gene expression data (Fig. 6C, D). Adenoviral expression of Hes1 dramatically reduced SCO2 and TIGAR expression (Fig. 6E), which suggests that Notch-Hes1 signaling modulates the metabolic pathway. Intriguingly, our results also indicate that Hes1 could suppress the expression of TIGAR and SCO2, a p53 target gene. It has been reported that Notch signaling suppresses p53 in lymphomagenesis [46]. Moreover, Kim et al. reported that NICD1 inhibits p53 phosphorylation and represses p53 transactivation by interacting with p53 [47]. In addition, DAPT treatment resulted in the enhancement of p53 activity in the hypoxic conditions (Fig. 4H, I). Therefore, it is possible that p53 activation was regulated by Notch signaling in hADMPCs, although we did not observe a decrease in p53 activity in hypoxic conditions in this study (Fig. 4). Further analysis will be required to determine whether p53 activity is suppressed in hypoxic conditions over a longer period of culture.
Cells undergoing active proliferation utilize large amounts of glucose through glycolysis, producing pyruvate for use in substrates (amino acids and lipids) and the pentose shunt for use in nucleic acid substrates, and also producing NADPH as a reducing agent to counter oxidative stress [18,55]. In the current study, 5% O2 actually increased proliferation and decreased the accumulation of ROS, which may be involved in the reduction of senescence (Fig. 1). Since accumulation of endogenous ROS might be a major reason for replicative senescence [20], enhancing glycolysis in cultured cells may improve the quality of the cells by suppressing premature senescence. Kondoh et al. demonstrated that enhanced glycolysis is involved in cellular immortalization through reduction of intrinsic ROS production [14,18,19]. Therefore, it is possible that the extension of lifespan observed in our experimental conditions was caused by the reduction of intracellular ROS levels through enhanced glycolysis by Notch signaling. Our data indicate that aerobic glycolysis is utilized for proliferation of hADMPCs, because the glycolytic inhibitor 2-DG attenuates the proliferation rate of hADMPCs (Fig. 7A). Intriguingly, the aerobic respiration block by NaN3 did not decrease the proliferation; rather, it increased proliferation at a low concentration (Fig. 7B), which may support our data indicating that the metabolic switch from mitochondrial respiration to glycolysis provides a growth advantage to hADMPCs. However, the question of whether the enhanced glycolysis really contributes to the prolonged lifespan in hADMPCs remains to be determined in this study.
In the current study, the molecular mechanism for how Notch signaling is activated in 5% O2 conditions was explored. It has been reported that Notch1 activity is influenced by oxygen concentration [40,41,56]. In melanoma cells, hypoxia (2% O2) resulted in increased expression of Notch1 by HIF-1α and also by Akt through NF-κB activity [41]. Similarly, in hypoxic breast cancer cells, Notch ligand JAG2 was shown to be transcriptionally activated by hypoxia (1% O2) in an HIF-1α-dependent manner, resulting in an elevation of Notch signaling [40]. In contrast, in hESCs continuously cultured in 5% O2, alteration of the Notch pathway seems to be independent of HIF-1α [56]. In our system, Notch1 activation was not likely dependent on HIF-1α and HIF-2α, because these proteins did not accumulate in the Hx condition. In contrast, our results indicate that the 5% O2 condition activated Akt and NF-κB signaling (Fig. 4), which suggests that these molecules may activate Notch signaling in hADMPCs. NF-κB was previously shown to increase Notch1 activity indirectly by increasing the expression of Notch ligand Jagged1 in HeLa, lymphoma, and myeloma cells [57]. In addition, Akt regulated Notch1 by increasing Notch1 transcription through the activity of NF-κB in melanoma cells [41]. Further analysis is required to clarify the mechanism underlying this phenomenon.
In conclusion, the 5% oxygen condition conferred a growth advantage through a metabolic shift to glycolysis, improved the proliferation efficiency, prevented the cellular senescence, and maintained the undifferentiated status of hADMPCs. These observations, thus, provide new regulatory mechanisms for the maintenance of stemness observed in 5% oxygen conditions. In addition, our study sheds new light on the regulation of replicative senescence, which might have an impact for quality control of hADMPC preparations used for therapeutic applications.
Acknowledgments
The authors would like to thank Koichi Sakaguchi, Mio Oishi, Mika Uemura, and Kei Sawaragi for technical support. This work was supported by MEXT KAKENHI grant number 24791927 to H.M. This work was also supported in part by grants from the Ministry of Health, Labor, and Welfare of Japan and a grant from the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO).
Author Disclosure Statement
The authors declare no conflict of interest. No competing financial interests exist.
References
- 1.Okura H, Komoda H, Saga A, Kakuta-Yamamoto A, Hamada Y, Fumimoto Y, Lee CM, Ichinose A, Sawa Y. and Matsuyama A. (2010). Properties of hepatocyte-like cell clusters from human adipose tissue-derived mesenchymal stem cells. Tissue Eng Part C Methods 16:761–770 [DOI] [PubMed] [Google Scholar]
- 2.Okura H, Matsuyama A, Lee CM, Saga A, Kakuta-Yamamoto A, Nagao A, Sougawa N, Sekiya N, Takekita K, et al. (2010). Cardiomyoblast-like cells differentiated from human adipose tissue-derived mesenchymal stem cells improve left ventricular dysfunction and survival in a rat myocardial infarction model. Tissue Eng Part C Methods 16:417–425 [DOI] [PubMed] [Google Scholar]
- 3.Okura H, Komoda H, Fumimoto Y, Lee CM, Nishida T, Sawa Y. and Matsuyama A. (2009). Transdifferentiation of human adipose tissue-derived stromal cells into insulin-producing clusters. J Artif Organs 12:123–130 [DOI] [PubMed] [Google Scholar]
- 4.Safford KM, Safford SD, Gimble JM, Shetty AK. and Rice HE. (2004). Characterization of neuronal/glial differentiation of murine adipose-derived adult stromal cells. Exp Neurol 187:319–328 [DOI] [PubMed] [Google Scholar]
- 5.Leu S, Lin YC, Yuen CM, Yen CH, Kao YH, Sun CK. and Yip HK. (2010). Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J Transl Med 8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikegame Y, Yamashita K, Hayashi S, Mizuno H, Tawada M, You F, Yamada K, Tanaka Y, Egashira Y, et al. (2011). Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 13:675–685 [DOI] [PubMed] [Google Scholar]
- 7.Tan B, Luan Z, Wei X, He Y, Wei G, Johnstone BH, Farlow M. and Du Y. (2011). AMP-activated kinase mediates adipose stem cell-stimulated neuritogenesis of PC12 cells. Neuroscience 181:40–47 [DOI] [PubMed] [Google Scholar]
- 8.Reid AJ, Sun M, Wiberg M, Downes S, Terenghi G. and Kingham PJ. (2011). Nerve repair with adipose-derived stem cells protects dorsal root ganglia neurons from apoptosis. Neuroscience 199:515–522 [DOI] [PubMed] [Google Scholar]
- 9.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV. and March KL. (2004). Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109:1292–1298 [DOI] [PubMed] [Google Scholar]
- 10.Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, Park BS. and Sung JH. (2009). Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen 17:540–547 [DOI] [PubMed] [Google Scholar]
- 11.Moriyama M, Moriyama H, Ueda A, Nishibata Y, Okura H, Ichinose A, Matsuyama A. and Hayakawa T. (2012). Human adipose tissue-derived multilineage progenitor cells exposed to oxidative stress induce neurite outgrowth in PC12 cells through p38 MAPK signaling. BMC Cell Biol 13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H, Ye Z. and Mahato RI. (2011). Genetically modified mesenchymal stem cells for improved islet transplantation. Mol Pharm 8:1458–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V. and Ho AD. (2008). Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One 3:e2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondoh H, Lleonart ME, Nakashima Y, Yokode M, Tanaka M, Bernard D, Gil J. and Beach D. (2007). A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid Redox Signal 9:293–299 [DOI] [PubMed] [Google Scholar]
- 15.Prigione A, Fauler B, Lurz R, Lehrach H. and Adjaye J. (2010). The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells 28:721–733 [DOI] [PubMed] [Google Scholar]
- 16.Varum S, Rodrigues AS, Moura MB, Momcilovic O, At Easley C, Ramalho-Santos J, Van Houten B. and Schatten G. (2011). Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One 6:e20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warburg O, Wind F. and Negelein E. (1927). The Metabolism of tumors in the body. J Gen Physiol 8:519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kondoh H. (2008). Cellular life span and the Warburg effect. Exp Cell Res 314:1923–1928 [DOI] [PubMed] [Google Scholar]
- 19.Kondoh H, Lleonart ME, Gil J, Wang J, Degan P, Peters G, Martinez D, Carnero A. and Beach D. (2005). Glycolytic enzymes can modulate cellular life span. Cancer Res 65:177–185 [PubMed] [Google Scholar]
- 20.Beckman KB. and Ames BN. (1998). The free radical theory of aging matures. Physiol Rev 78:547–581 [DOI] [PubMed] [Google Scholar]
- 21.Ezashi T, Das P. and Roberts RM. (2005). Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A 102:4783–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forristal CE, Wright KL, Hanley NA, Oreffo RO. and Houghton FD. (2010). Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction 139:85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida Y, Takahashi K, Okita K, Ichisaka T. and Yamanaka S. (2009). Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell 5:237–241 [DOI] [PubMed] [Google Scholar]
- 24.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M. and Suda T. (2010). Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell 7:391–402 [DOI] [PubMed] [Google Scholar]
- 25.Santilli G, Lamorte G, Carlessi L, Ferrari D, Rota Nodari L, Binda E, Delia D, Vescovi AL. and De Filippis L. (2010). Mild hypoxia enhances proliferation and multipotency of human neural stem cells. PLoS One 5:e8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai CC, Chen YJ, Yew TL, Chen LL, Wang JY, Chiu CH. and Hung SC. (2011). Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood 117:459–469 [DOI] [PubMed] [Google Scholar]
- 27.Takubo K, Nagamatsu G, Kobayashi CI, Nakamura-Ishizu A, Kobayashi H, Ikeda E, Goda N, Rahimi Y, Johnson RS, et al. (2013). Regulation of glycolysis by pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell 12:49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grayson WL, Zhao F, Izadpanah R, Bunnell B. and Ma T. (2006). Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol 207:331–339 [DOI] [PubMed] [Google Scholar]
- 29.Wang DW, Fermor B, Gimble JM, Awad HA. and Guilak F. (2005). Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J Cell Physiol 204:184–191 [DOI] [PubMed] [Google Scholar]
- 30.Moriyama M, Osawa M, Mak SS, Ohtsuka T, Yamamoto N, Han H, Delmas V, Kageyama R, Beermann F, Larue L. and Nishikawa S. (2006). Notch signaling via Hes1 transcription factor maintains survival of melanoblasts and melanocyte stem cells. J Cell Biol 173:333–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiba S. (2006). Notch signaling in stem cell systems. Stem Cells 24:2437–2447 [DOI] [PubMed] [Google Scholar]
- 32.Okura H, Saga A, Fumimoto Y, Soeda M, Moriyama M, Moriyama H, Nagai K, Lee CM, Yamashita S, et al. (2011). Transplantation of human adipose tissue-derived multilineage progenitor cells reduces serum cholesterol in hyperlipidemic Watanabe rabbits. Tissue Eng Part C Methods 17:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saga A, Okura H, Soeda M, Tani J, Fumimoto Y, Komoda H, Moriyama M, Moriyama H, Yamashita S, et al. (2011). HMG-CoA reductase inhibitor augments the serum total cholesterol-lowering effect of human adipose tissue-derived multilineage progenitor cells in hyperlipidemic homozygous Watanabe rabbits. Biochem Biophys Res Commun 412:50–54 [DOI] [PubMed] [Google Scholar]
- 34.Moriyama H, Moriyama M, Sawaragi K, Okura H, Ichinose A, Matsuyama A. and Hayakawa T. (2013). Tightly regulated and homogeneous transgene expression in human adipose-derived mesenchymal stem cells by lentivirus with tet-off system. PLoS One 8:e66274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG. and Prockop DJ. (2002). Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells 20:530–541 [DOI] [PubMed] [Google Scholar]
- 36.Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W. and Ho AD. (2005). Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 33:1402–1416 [DOI] [PubMed] [Google Scholar]
- 37.Hass R, Kasper C, Bohm S. and Jacobs R. (2011). Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U. and Bondesson M. (2005). Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell 9:617–628 [DOI] [PubMed] [Google Scholar]
- 39.Zheng X, Linke S, Dias JM, Zheng X, Gradin K, Wallis TP, Hamilton BR, Gustafsson M, Ruas JL, et al. (2008). Interaction with factor inhibiting HIF-1 defines an additional mode of cross-coupling between the Notch and hypoxia signaling pathways. Proc Natl Acad Sci U S A 105:3368–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pietras A, von Stedingk K, Lindgren D, Pahlman S. and Axelson H. (2011). JAG2 induction in hypoxic tumor cells alters Notch signaling and enhances endothelial cell tube formation. Mol Cancer Res 9:626–636 [DOI] [PubMed] [Google Scholar]
- 41.Bedogni B, Warneke JA, Nickoloff BJ, Giaccia AJ. and Powell MB. (2008). Notch1 is an effector of Akt and hypoxia in melanoma development. J Clin Invest 118:3660–3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beitner-Johnson D, Rust RT, Hsieh TC. and Millhorn DE. (2001). Hypoxia activates Akt and induces phosphorylation of GSK-3 in PC12 cells. Cell Signal 13:23–27 [DOI] [PubMed] [Google Scholar]
- 43.Culver C, Sundqvist A, Mudie S, Melvin A, Xirodimas D. and Rocha S. (2010). Mechanism of hypoxia-induced NF-kappaB. Mol Cell Biol 30:4901–4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rohwer N, Dame C, Haugstetter A, Wiedenmann B, Detjen K, Schmitt CA. and Cramer T. (2010). Hypoxia-inducible factor 1alpha determines gastric cancer chemosensitivity via modulation of p53 and NF-kappaB. PLoS One 5:e12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Espinosa L, Cathelin S, D'Altri T, Trimarchi T, Statnikov A, Guiu J, Rodilla V, Ingles-Esteve J, Nomdedeu J, et al. (2010). The Notch/Hes1 pathway sustains NF-kappaB activation through CYLD repression in T cell leukemia. Cancer Cell 18:268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beverly LJ, Felsher DW. and Capobianco AJ. (2005). Suppression of p53 by Notch in lymphomagenesis: implications for initiation and regression. Cancer Res 65:7159–7168 [DOI] [PubMed] [Google Scholar]
- 47.Kim SB, Chae GW, Lee J, Park J, Tak H, Chung JH, Park TG, Ahn JK. and Joe CO. (2007). Activated Notch1 interacts with p53 to inhibit its phosphorylation and transactivation. Cell Death Differ 14:982–991 [DOI] [PubMed] [Google Scholar]
- 48.Landor SK, Mutvei AP, Mamaeva V, Jin S, Busk M, Borra R, Gronroos TJ, Kronqvist P, Lendahl U. and Sahlgren CM. (2011). Hypo- and hyperactivated Notch signaling induce a glycolytic switch through distinct mechanisms. Proc Natl Acad Sci U S A 108:18814–18819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ciofani M. and Zuniga-Pflucker JC. (2005). Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat Immunol 6:881–888 [DOI] [PubMed] [Google Scholar]
- 50.Welford SM, Bedogni B, Gradin K, Poellinger L, Broome Powell M. and Giaccia AJ. (2006). HIF1alpha delays premature senescence through the activation of MIF. Genes Dev 20:3366–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou D, Xue J, Lai JC, Schork NJ, White KP. and Haddad GG. (2008). Mechanisms underlying hypoxia tolerance in Drosophila melanogaster: hairy as a metabolic switch. PLoS Genet 4:e1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Funes JM, Quintero M, Henderson S, Martinez D, Qureshi U, Westwood C, Clements MO, Bourboulia D, Pedley RB, Moncada S. and Boshoff C. (2007). Transformation of human mesenchymal stem cells increases their dependency on oxidative phosphorylation for energy production. Proc Natl Acad Sci U S A 104:6223–6228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawauchi K, Araki K, Tobiume K. and Tanaka N. (2008). p53 regulates glucose metabolism through an IKK-NF-kappaB pathway and inhibits cell transformation. Nat Cell Biol 10:611–618 [DOI] [PubMed] [Google Scholar]
- 54.Carter KL, Cahir-McFarland E. and Kieff E. (2002). Epstein-barr virus-induced changes in B-lymphocyte gene expression. J Virol 76:10427–10436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ak P. and Levine AJ. (2010). p53 and NF-kappaB: different strategies for responding to stress lead to a functional antagonism. FASEB J 24:3643–3652 [DOI] [PubMed] [Google Scholar]
- 56.Prasad SM, Czepiel M, Cetinkaya C, Smigielska K, Weli SC, Lysdahl H, Gabrielsen A, Petersen K, Ehlers N, et al. (2009). Continuous hypoxic culturing maintains activation of Notch and allows long-term propagation of human embryonic stem cells without spontaneous differentiation. Cell Prolif 42:63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bash J, Zong WX, Banga S, Rivera A, Ballard DW, Ron Y. and Gelinas C. (1999). Rel/NF-kappaB can trigger the Notch signaling pathway by inducing the expression of Jagged1, a ligand for Notch receptors. EMBO J 18:2803–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]