Abstract
Background
Since the 1970s, CHOP chemotherapy has been the standard treatment for patients with diffuse large B-cell lymphoma (DLBCL). In 2002, randomized trials changed this standard by demonstrating that adding rituximab immunotherapy to CHOP improved survival. However, how these results influenced chemo-immunotherapy adoption in clinical practice remains unclear.
Methods
Using the National Cancer Database to compare chemo-immunotherapy use with chemotherapy alone, we collected data on demographics, stage, health insurance, area-level socio-economic status (SES), facility characteristics, and type of treatment for DLBCL patients diagnosed in the United States 2001-2004. Multivariable log binomial models examined associations between race, insurance, and treatment allocation, adjusting for covariates.
Results
Among 38,002 patients with DLBCL, 27% received chemo-immunotherapy and 50% chemotherapy alone. Patients who had localized disease, were diagnosed in 2001, black, uninsured/Medicaid insured, or lower SES were less likely to receive any form of chemotherapy (all p<0.0001). Patients who were diagnosed 2001, black [relative risk (RR) 0.83, 95% Confidence Interval (CI) 0.78-0.89], >60 years (RR 0.94, 95% CI 0.90-0.98), or had localized disease (RR 0.89, 95% CI 0.86-0.92) were less likely to receive chemo-immunotherapy. Receiving treatment at high DLBCL volume teaching/research facilities was associated with the greatest likelihood of chemo-immunotherapy (RR 1.69, 95% CI 1.52-1.89).
Conclusions
Black DLBCL patients were less likely to receive chemotherapy or chemo-immunotherapy during this period.
Impact
This large national cohort study demonstrates disparities in the diffusion of chemo-immunotherapy for DLBCL. Improving DLBCL outcomes will require efforts to extend access to proven advances in therapy to all segments of the population.
Keywords: Diffuse large B-cell lymphoma, Non-Hodgkin Lymphoma, Lymphoma, Healthcare Disparities, Immunotherapy, Rituximab, Chemo-immunotherapy
Introduction
With an estimated 66,360 new cases diagnosed in the United States (US) in 2011, non-Hodgkin lymphoma (NHL) is the sixth most common cancer.(1) Diffuse large B-cell lymphoma (DLBCL) is the most commonly occurring subtype of NHL in the US, comprising approximately one third of all adult lymphomas. The natural history of DLBCL is aggressive, with a median survival of less than one year in untreated patients.(2) The combination chemotherapy regimen of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) remained the standard therapy for DLBCL for decades following its development in the 1970s.(3, 4) Even when compared with more intensive chemotherapy regimens, the standard CHOP regimen produced similar survival outcomes and was better tolerated.(3)
In 1997, rituximab, the first monoclonal antibody used as an anti-cancer therapy, was approved for use by the Food and Drug Administration for follicular lymphoma, and this immunotherapy was soon applied to DLBCL and other B-cell NHLs.(5, 6) In 2002, a randomized clinical trial in patients >60 years of age with advanced-stage DLBCL demonstrated that when rituximab was added to standard CHOP chemotherapy the complete response rate improved from 63% (CHOP alone) to 76% (p=0.005), and 2-year overall survival (OS) improved from 57% to 70% (p=0.007).(7) Follow-up data from this trial and other randomized, controlled clinical trials confirmed the benefits of combined chemo-immunotherapy for DLBCL.(8-10) For patients with limited-stage DLBCL chemotherapy given with rituximab for three cycles followed by radiation has produced 7-year OS rates of up to 95%,(11, 12) and has become the acknowledged standard of care for this group as well.(13)
However, it remains unclear what the timing of adoption of chemo-immunotherapy as the standard for DLBCL has been in the US and what factors have influenced who received this combination when it was first adopted. To address these issues we sought to: 1) describe the clinical and demographic features of patients with DLBCL who received combination chemo-immunotherapy during the time period of its initial adoption, 2) assess the differences between patients with DLBCL who received chemo-immunotherapy and those who did not, and 3) examine time trends in the use of chemo-immunotherapy for patients with DLBCL.
Patients and Methods
Study Population and Patient Selection
Data from the National Cancer Data Base (NCDB), a nationwide, hospital-based cancer registry jointly sponsored by the American Cancer Society and the American College of Surgeons, were utilized to examine chemo-immunotherapy use. The NCDB contains approximately 20 million records from more than 1,400 Commission-on-Cancer (CoC) approved hospital-based cancer programs in the US and Puerto Rico. While the NCDB accrues cancer cases arising in the hospital-based setting, this database collects patient-level data from encounters that occur in both the inpatient and outpatient setting. Because cancer diagnoses are determined by pathologists and pathology departments that are largely hospital-based, diagnoses of DLBCL and treatment of DLBCL that occurs in the outpatient setting would be captured in the NCDB as long as the pathologist rendering the diagnoses performs services in a CoC hospital. Each record contains standardized data elements on patient demographics, tumor characteristics (including stage and histopathology), and first course of therapy. Approximately 75% of all newly diagnosed cases of cancer in the US are captured at the institutional level and includes inpatient and outpatient data reported to the NCDB. The NCDB also contains information on patient insurance status, county of residence, facility type in which patients were treated, and an encrypted facility identifier. Because no patient, provider, or hospital identifiers were examined and no protected health information was reviewed, institutional review board approval was not required for this study.
Patients were eligible for study inclusion if they were aged 18 years or older, diagnosed with International Classification of Diseases for Oncology (ICD-O) (14) codes 9679 or 9680 between January 1, 2001, and December 31, 2004, and received all or part of their first course of treatment at the reporting facility in the inpatient or outpatient setting.. All data refer to neoplasms with malignant behavior. Data were abstracted using coding guidelines documented in the Facility Oncology Registry Data Standards (FORDS) manual.
Among the 51,207 patients identified with DLBCL, 38,002 met all eligibility criteria (Supplementary Fig. S1). Patients were excluded if their age was <18 or >101 years, or if there were missing data on age, gender, race/ethnicity, insurance, region of residence, chemotherapy, or immunotherapy. Missing data on race was the most common reason for exclusion (n=4527, 8.84%). Non-Medicare/Medicaid government-funded plans comprised 0.3% of the population and were excluded from analyses because they provided insufficient data to be separately analyzed.
Study Variables
Administration of chemotherapy, immunotherapy, and radiation therapy were captured using the FORDS. To examine recent treatment practice patterns that were in accordance with state-of-the-art available data in 2002 we examined factors associated with chemotherapy plus immunotherapy versus chemotherapy alone. The independent variables included in this analysis were drawn from four sources: patient-level demographics, clinical characteristics, facility-level variables, and area-level information. Patient-level demographic variables included: age at diagnosis, gender, race/ethnicity, and primary payer or insurance type at diagnosis. Clinical characteristics captured at the patient level included: lymphoma stage, primary site, and year of diagnosis. Treatment-level or facility-level characteristics included: the volume of lymphoma patients at the treatment facility by facility type. Area-level characteristics were based on the patient's residence and included: census region and education level in patient's zip code. Since all area-level measures of socioeconomic status (SES) were highly correlated, educational level was selected as a single marker of SES.
Three types of treatment facilities were described according to the classification system employed by the College of Surgeons Commission on Cancer's approvals program; these are community cancer programs, comprehensive community cancer programs, and teaching/research centers. Community hospitals treat at least 300 cancer cases a year and have a full range of services for cancer care but patients need referral for portions of their treatment. Comprehensive community cancer centers are facilities that offer the same range of services as the community hospitals but have at least 750 annual cancer cases and conduct weekly cancer conferences. Teaching/research facilities differ from community cancer facilities in that the teaching/research facilities have residency programs and ongoing cancer research. Twenty-nine of the 39 National Cancer Institute designated Comprehensive Cancer Programs participate in the CoC approvals program and are included among teaching/research facilities in this study.
Treatment volume for each facility was calculated by counting all DLBCL cases diagnosed during 2001-2004. The facilities were then divided into low or high volume based on the median by facility type, which was 13 cases over the study period for community hospitals, 37 for comprehensive community cancer centers and 55 for teaching/research facilities. Patient residence was based on the reported state of residence at diagnosis and categorized as West, Midwest, Northeast, or South. Area-based indicator of patient education, were derived at the zip code level from 2000 US Census data and included as quartiles of the observed distribution in the general US population. The proportion of the population in a patient's zip code of residence who did not have a high school diploma was stratified as ≥29%, 20%–28.9%, 14%–19.9%, <14%, or missing. To evaluate how representative our cohort of patients was to a population based sample, we compared our study cohort to Surveillance, Epidemiology, and End Results 17 (SEER) cases diagnosed with DLBCL (ICD-0 codes 9679 and 9680) between 2001 and 2004. To assess the capture of immunotherapy and chemotherapy in the NCDB, we compared rates of chemotherapy and immunotherapy among NCDB patients ≥65 years to patients in a linked SEER-Medicare dataset. SEER-Medicare was projected to have more complete data on both chemotherapy and immunotherapy since these are billed separately and rituximab has a specific Healthcare Common Procedure Coding System code.
Statistical Analyses
Analyses were performed with SAS software (version 9.2; SAS Statistical Institute, Cary, NC). Chi-square tests were employed to analyze the relationship between race, other covariates and the outcome variables of interest. Because all other variables examined were statistically significant in univariate analyses, multivariable log binomial models were used to generate risk ratio estimates (α=0.05). Multivariable log binomial models were relied upon considering the common outcome in our study.(15) Interactions between race and all other covariates were tested by comparing -2 Log Likelihood Chi-Square values from Chunk Tests. Time trends in the utilization of chemo-immunotherapy for patients with DLBCL were tracked from 1998 to 2004 and compared by race. The Cochran Armitage test for trend was used to examine time trends in the utilization of chemo-immunotherapy for DLBCL.
Results
Patients with DLBCL in NCDB and SEER were similar in terms of their age, gender, year of diagnosis, and primary site of involvement (Table 1). Fewer DLBCL patients in the NCDB cohort had unknown stage. A greater percentage of DLBCL cases in the NCDB cohort were from the South and Northeast regions and relatively fewer cases were from the West. No data are available in SEER regarding the primary payer, facility characteristics, use of chemo-immunotherapy, or area-level measures of SES during this time period. Male patients that were uninsured or with Medicaid insurance presented at a younger age compared to patients with private insurance [median age 48, 47 years vs. 55 (p=<0.0001)] and more commonly presented with advanced stage disease [stage III/IV 46.1%, 51.9% vs. 39.5% (p=<0.0001)]. Similarly, females that were uninsured or with Medicaid insurance were younger [median age 54, 52 years vs. 56 (p=<0.0001)] and more commonly presented with advanced stage disease [38.6%, 42.9% vs. 36.8% (p=<0.0001)]. In accordance with our prior findings using SEER data(16) black patients presented with DLBCL a decade younger, and more commonly had advanced stage disease. In NCDB, black and Hispanic patients more commonly were uninsured or had Medicaid insurance, and were more commonly treated at a low volume comprehensive cancer facility (Table 2).
Table 1. Comparisons of NCDB and SEER DLBCL patients.
| Patient Characteristics | NCDB 2001-2004 (n=38,002) |
SEER
17 2001-2004 (n=19,172) |
|---|---|---|
| Median age years (IQR) | 68 (54-78) | 68 (53-78) |
| Age >60 years | 25133 (66.14) | 12431 (64.84) |
| Sex, female | 18173 (47.82) | 8999 (46.94) |
| Race/Ethnicity* | ||
| White | 31671 (83.34) | 14298 (74.58) |
| Hispanic | 2056 (5.41) | 2079 (10.84) |
| Black | 3001 (7.9) | 1302 (6.79) |
| Other | 1274 (3.35) | 1390 (7.25) |
| Unknown | 0 | 103 (0.54) |
| Stage | ||
| I/II | 17327 (45.59) | 6924 (36.12) |
| III/IV | 15670 (41.23) | 6324 (32.99) |
| Unknown | 5005 (13.17) | 5924 (30.90) |
| Diagnosis Year | ||
| 2001 | 8014 (21.09) | 4622 (24.11) |
| 2002 | 8889 (23.39) | 4669 (24.35) |
| 2003 | 10361 (27.26) | 4873 (25.42) |
| 2004 | 10738 (28.26) | 5008 (26.12) |
| Primary Site | ||
| Lymph node | 23174 (60.98) | 11855 (61.83) |
| Extra-nodal | 14828 (39.02) | 7317 (38.17) |
| Region | ||
| Northeast | 8703 (22.9) | 1092 (5.70) |
| Midwest | 9782 (25.74) | 4519 (22.93) |
| South | 13054 (34.35) | 2799 (14.20) |
| West | 6463 (17.01) | 10762 (54.60) |
Abbreviations: IQR, inter-quartile range
Table 2. Patient, area level and facility characteristics by race/ethnicity, n=38,002.
| Patient Characteristics | Non-Hispanic White (%) | Hispanic (%) | Black (%) | Other (%) | p-value across groups | p-value Black vs. White |
|---|---|---|---|---|---|---|
| Median age years (IQR) | 70 (57-79) | 59 (44-73) | 53 (42-68) | 44 (50-75) | ||
| Age >60 years | 22248 (70.25) | 1011(49.17) | 1129 (37.62) | 745(58.48) | <.0001 | <.0001 |
| Sex, female | 15225(48.07) | 935(45.48) | 1382(46.05) | 631(49.53) | 0.0142 | 0.0341 |
| Stage | <.0001 | <.0001 | ||||
| I/II | 14605(46.11) | 871(42.36) | 1220(40.65) | 631(49.53) | ||
| III/IV | 12957(40.91) | 870(42.32) | 1335 (44.49) | 508 (39.87) | ||
| Unknown | 4109 (12.97) | 315 (15.32) | 446 (14.86) | 135 (10.60) | ||
| Diagnosis Year | <.0001 | 0.0585 | ||||
| 2001 | 6769 (21.37) | 404 (19.65) | 600 (19.99) | 241 (18.92) | ||
| 2002 | 7535 (23.79) | 401 (19.50) | 692(23.06) | 261(20.49) | ||
| 2003 | 8591 (27.13) | 556 (27.04) | 814 (27.12) | 400 (31.40) | ||
| 2004 | 8776 (27.71) | 695 (33.80) | 895 (29.82) | 372(29.20) | ||
| Primary Site | <.0001 | 0.0288 | ||||
| Lymph node | 19376 (61.18) | 1194 (58.07) | 1897 (63.21) | 707 (55.49) | ||
| Extra-nodal | 12295 (38.82) | 862 (41.93) | 1104 (36.79) | 567 (44.51) | ||
| Primary payer | <.0001 | <.0001 | ||||
| Uninsured | 793 (2.50) | 260 (12.65) | 286 (9.53) | 75 (5.89) | ||
| Medicaid | 1080 (3.41) | 373 (18.14) | 518 (17.26) | 157 (12.32) | ||
| Medicare 18-64 | 930 (2.94) | 76 (3.70) | 217 (7.23) | 37 (2.90) | ||
| Medicare ≥65 | 16797 (53.04) | 603 (29.33) | 729 (24.29) | 439 (34.46) | ||
| Private | 12071 (38.11) | 744 (36.19) | 1251 (41.69) | 566 (44.43) | ||
| Region | <.0001 | <.0001 | ||||
| Northeast | 7432 (23.47) | 354 (17.22) | 658 (21.93) | 259 (20.33) | ||
| Midwest | 8778 (27.72) | 226 (10.99) | 595 (19.83) | 183 (14.36) | ||
| South | 10485 (33.11) | 755 (36.72) | 1566 (52.18) | 248 (19.47) | ||
| West | 4976 (15.71) | 721 (35.07) | 182 (6.06) | 584 (45.84) | ||
| Median No High School | <.0001 | <.0001 | ||||
| ≥29% | 3688 (11.64) | 876 (42.61) | 1142 (38.05) | 229 (17.97) | ||
| 20-28.9% | 6607 (20.86) | 432 (21.01) | 862 (28.72) | 270 (21.19) | ||
| 14-19.9% | 7737 (24.43) | 311 (15.13) | 440 (14.66) | 222 (17.43) | ||
| <14% | 11718 (37.00) | 333 (16.20) | 384 (12.80) | 475 (37.28) | ||
| Missing | 1921 (6.07) | 104 (5.06) | 173 (5.76) | 78 (6.12) | ||
| Treatment Type | ||||||
| Any Treatment | 26662 (84.18) | 1649 (80.20) | 2510 (83.64) | 1046 (82.10) | <.0001 | 0.4344 |
| Any Chemotherapy | 24469 (77.26) | 1534 (74.61) | 2355 (78.47) | 954 (74.88) | <.0001 | <.0001 |
| Chemotherapy+Immunotherapya | 8759 (35.80) | 499 (32.53) | 644 (27.35) | 332 (34.80) | <.0001 | <.0001 |
| Facility Characteristics | <.0001 | <.0001 | ||||
| High Volume Community | 1260 (3.98) | 85 (4.13) | 104 (3.47) | 42 (3.30) | ||
| High Volume Comprehensive | 3881 (12.25) | 231 (11.24) | 236 (7.86) | 119 (9.34) | ||
| High Volume Tch/Research | 4405 (13.91) | 213 (10.36) | 290 (9.66) | 202 (15.86) | ||
| Low Volume Community | 10500 (33.15) | 547 (26.61) | 644 (21.46) | 304 (23.86) | ||
| Low Volume Comprehensive | 2234 (7.05) | 273 (13.28) | 641 (21.36) | 138 (10.83) | ||
| Low Volume Tch/Research | 8231 (25.99) | 629 (30.59) | 987 (32.89) | 439 (34.46) | ||
| Missing facility type | 1160 (3.66) | 78 (3.79) | 99 (3.30) | 30 (2.35) |
Frequency and percentage of chemotherapy+immunotherapy only includes the 29,312 patients that received chemotherapy
Abbreviations: Tch, teaching
Within the NCDB, 10,234 patients were identified who received combination chemo-immunotherapy and 19,078 who received chemotherapy alone. There were statistically significant differences between these groups in terms of stage at diagnosis, diagnosis year, race, primary payer, region of the US, facility type, and measures of SES (Table 3). The probability of receiving chemotherapy among DLBCL patients age 65 and older was similar in NCDB (71.1%) and SEER-Medicare (68.1%). Although, the proportion of patients who received chemotherapy plus immunotherapy was lower among NCDB patients (24.8%) compared to SEER-Medicare (46.3%), the under ascertainment of immunotherapy in NCDB appeared to be non-differential with respect to race. The ratio of chemo-immunotherapy use among black relative to white patients was similar in NCDB (0.80) and SEER-Medicare (0.77). Additionally, the proportion of patients receiving no treatment was similar between NCDB patients ≥65 years (19.9%) and SEER-Medicare patients (21%).
Table 3. Receipt of chemotherapy by patient, area level and facility characteristics.
| Patient Characteristics | Chemotherapy Alone
(%) (n=19,078) |
Chemo-immunotherapy
(%) (n=10,234) |
|---|---|---|
| Median age years (IQR) | 66 (52-76) | 66 (52-76) |
| Age >60 years | 11924 (62.5) | 6409 (62.62) |
| Sex, female | 8950 (46.91) | 4780 (46.71) |
| Race/Ethnicity | ||
| White | 15710 (82.35) | 8759 (85.59) |
| Hispanic | 1035 (5.43) | 499 (4.88) |
| Black | 1711 (8.97) | 644 (6.29) |
| Other | 622 (3.26) | 332 (3.24) |
| Unknown | 0 | 0 |
| Stage | ||
| I/II | 8793 (46.09) | 4524 (44.21) |
| III/IV | 7659 (40.15) | 5083 (49.67) |
| Unknown | 2626 (13.76) | 627 (6.13) |
| Diagnosis Year | ||
| 2001 | 5585 (29.27) | 1414 (13.82) |
| 2002 | 4222 (22.13) | 2227 (21.76) |
| 2003 | 4615 (24.19) | 3175 (31.02) |
| 2004 | 4656 (24.41) | 3418 (33.4) |
| Primary Site | ||
| Lymph node | 11742 (61.55) | 6949 (67.9) |
| Extra-nodal | 7336 (38.45) | 3285 (32.1) |
| Primary payer | ||
| Uninsured | 804 (4.21) | 332 (3.24) |
| Medicaid | 1146 (6.01) | 532 (5.2) |
| Medicare 18-64 | 657 (3.44) | 300 (2.93) |
| Medicare ≥65 | 8535 (44.74) | 4660 (45.53) |
| Private | 7936 (41.6) | 4410 (43.09) |
| Region | ||
| Northeast | 4180 (21.91) | 2507 (24.5) |
| Midwest | 4980 (26.1) | 2859 (27.94) |
| South | 7068 (37.05) | 2951 (28.84) |
| West | 2850 (14.94) | 1917 (18.73) |
| Median No High School | ||
| ≥29% | 3207 (16.81) | 1269 (12.4) |
| 20-28.9% | 4188 (21.95) | 2128 (20.79) |
| 14-19.9% | 4393 (23.03) | 2389 (23.34) |
| <14% | 6156 (32.27) | 3836 (37.48) |
| Missing | 1134 (5.94) | 612 (5.98) |
| Facility Characteristics | ||
| High Volume Community | 2370 (12.42) | 964 (9.42) |
| High Volume Comprehensive | 5697 (29.86) | 3493 (34.13) |
| High Volume Tch/Research | 4814 (25.23) | 3359 (32.82) |
| Low Volume Community | 850 (4.46) | 257 (2.51) |
| Low Volume Comprehensive | 2790 (14.62) | 1071 (10.47) |
| Low Volume Tch/Research | 1922 (10.07) | 637 (6.22) |
| Missing facility type | 635 (3.33) | 453 (4.43) |
In the multivariable log binomial models, year of diagnosis, stage, race, age >60 years, region, area-level educational status, and facility characteristics were significant predictors of receiving combination chemo-immunotherapy versus chemotherapy alone and were significant predictors of receiving any chemotherapy (alone or with immunotherapy) versus no chemotherapy (Table 4). There was no association between insurance status and receipt of chemo-immunotherapy versus chemotherapy alone; however, insurance was associated with receipt of chemotherapy versus no chemotherapy. Patients who were black (relative risk [RR] 0.83, 95% Confidence Interval [CI] 0.78-0.89), >60 years (RR 0.94, 95% CI 0.90-0.98), had limited stage disease (RR 0.89, 95% CI 0.86-0.92) or missing staging information (RR 0.54, 95% CI 0.50-0.58), were diagnosed in 2001-2002, or were treated at a facility other than a high volume teaching/research facility were less likely to receive chemo-immunotherapy. Patients who were black (RR 1.14, 95% CI 1.05-1.25), Hispanic (RR 1.23, 95% CI 1.13-1.35), or >60 years (RR 1.39, 95% CI 1.30-1.50) were more likely to receive no form of treatment following their diagnosis. Additionally, patients without insurance or insurance other than private were more likely to receive no treatment, as were patients treated at low volume facilities.
Table 4. Factors associated with likelihood of any chemotherapy versus none and chemo-immunotherapy versus chemotherapy alone.
| Any Chemotherapy vs. no chemotherapyn=38,002 | Chemo-immunotherapy vs. Chemotherapy alonen=29,312 | |||
|---|---|---|---|---|
|
|
|
|||
| Parameter | Risk Ratio | 95% CI | Risk Ratio | 95% CI |
| Insurance Status | ||||
| Private | 1.00 | 1.00 | ||
| Uninsured | 0.96 | 0.93-0.99 | 0.92 | 0.84-1.01 |
| Medicaid | 0.95 | 0.93-0.99 | 0.93 | 0.87-1.00 |
| Medicare 18-64 | 0.92 | 0.88-0.95 | 0.93 | 0.85-1.02 |
| Medicare ≥65 | 0.94 | 0.93-0.96 | 1.04 | 1.00-1.09 |
| Race | ||||
| White | 1.00 | 1.00 | ||
| Hispanic | 0.97 | 0.95-1.00 | 0.94 | 0.87-1.01 |
| Black | 0.98 | 0.95-1.00 | 0.83 | 0.78-0.89 |
| Other | 0.99 | 0.95-1.02 | 0.93 | 0.85-1.01 |
| Age | ||||
| 18-59 | 1.00 | 1.00 | ||
| >60 | 0.93 | 0.91-0.94 | 0.94 | 0.90-0.98 |
| Gender | ||||
| Male | 1.00 | 1.00 | ||
| Female | 0.99 | 0.98-1.00 | 1.00 | 0.97-1.03 |
| Region | ||||
| South | 1.00 | 1.00 | ||
| Northeast | 1.00 | 0.99-1.02 | 1.20 | 1.15-1.25 |
| Midwest | 1.03 | 1.01-1.05 | 1.18 | 1.13-1.23 |
| West | 0.98 | 0.96-1.00 | 1.25 | 1.20-1.31 |
| Stage | ||||
| III/IV | 1.00 | 1.00 | ||
| I/II | 0.97 | 0.96-0.98 | 0.89 | 0.86-0.92 |
| Missing | 0.93 | 0.91-0.94 | 0.54 | 0.50-0.58 |
| Primary Site | ||||
| Extra-nodal | 1.00 | 1.00 | ||
| Lymph node | 0.95 | 0.94-0.96 | 0.87 | 0.84-0.90 |
| Year of Diagnosis | ||||
| 2004 | 1.00 | 1.00 | ||
| 2003 | 0.89 | 0.87-0.90 | 1.03 | 1.00-1.07 |
| 2002 | 0.9 | 0.88-0.91 | 0.87 | 0.83-0.90 |
| 2001 | 0.88 | 0.87-0.90 | 0.50 | 0.48-0.53 |
| Percentage of census region with No High School Degree | ||||
| <14% | 1.00 | 1.00 | ||
| 14-19% | 1.01 | 0.99-1.02 | 0.96 | 0.93-1.00 |
| 20-28.9% | 1.00 | 0.99-1.02 | 0.98 | 0.94-1.02 |
| ≥29% | 1.00 | 0.99-1.02 | 0.88 | 0.83-0.92 |
| Missing | 1.00 | 1.01 | 0.94-1.08 | |
| Facility Characteristics | ||||
| High Volume Tch/Research | 1.00 | 1.00 | ||
| High Volume Community | 0.97 | 0.96-0.99 | 0.72 | 0.68-0.77 |
| High Volume Comprehensive | 0.99 | 0.97-1.00 | 0.94 | 0.90-0.97 |
| Low Volume Community | 0.97 | 0.94-1.00 | 0.59 | 0.53-0.66 |
| Low Volume Comprehensive | 0.98 | 0.96-1.00 | 0.70 | 0.66-0.74 |
| Low Volume Tch/Research | 0.99 | 0.96-1.00 | 0.66 | 0.61-0.71 |
| Missing | 1.00 | 0.97-1.03 | 1.03 | 0.96-1.10 |
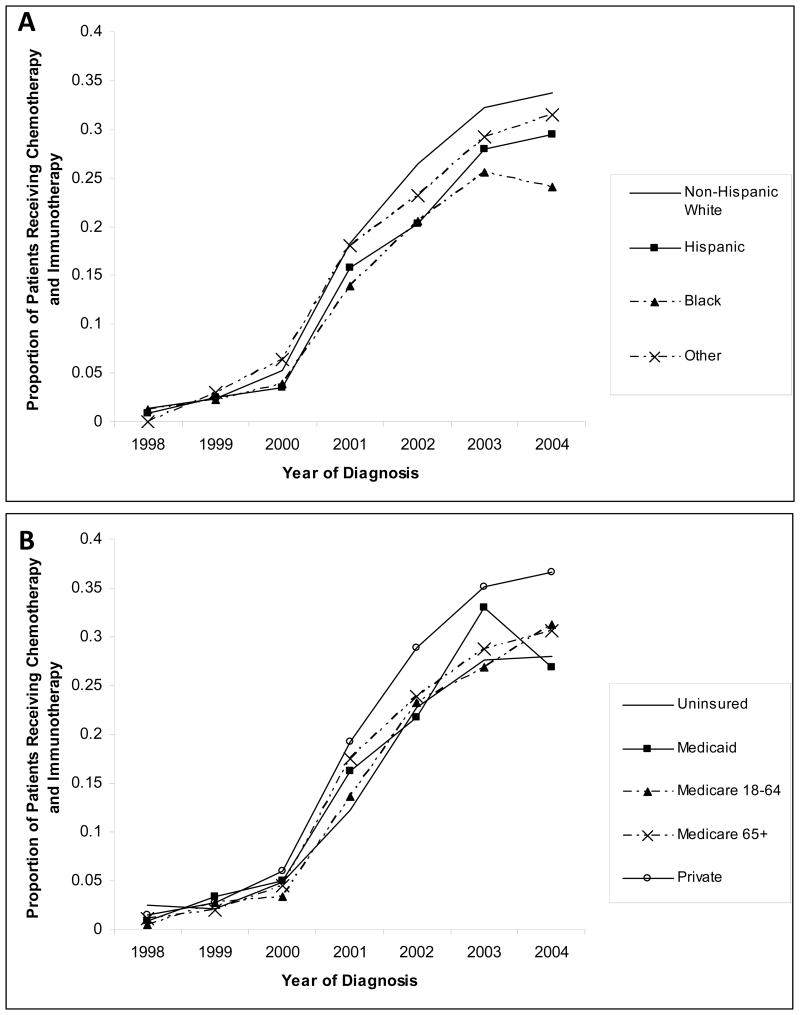
From 1998 to 2004 the proportion of patients who received chemo-immunotherapy in NCDB increased dramatically from 0.01% to 32.6% (Cochran Armitage Z=-78.48, p<0.0001). The increase in the utilization of chemo-immunotherapy over this time period occurred for patients across all racial categories (Figure 1A), but disparities existed throughout this period. In 2004, 23.6% of black patients and 33.2% of white patients received chemo-immunotherapy as the initial treatment for DLBCL. Similarly, in 2004 26.6% of Medicaid patients and 27.3% of uninsured patients received chemo-immunotherapy, compared with 35.9% of privately insured patients (Figure 1B).
Figure 1. Receipt of Chemotherapy and Immunotherapy.
This figure provides the distribution of therapy received by patients in the NCDB between 1998-2004 (A) by Race/Ethnicity; (B) by Insurance Status.
Discussion
Although lymphoma represents 5% of all cancers in the US, it is estimated that $4.6 billion per year is spent in the US on treatment for lymphoma.(17) This high cost of lymphoma care fortunately has been associated with improvements in outcomes. The CHOP regimen has been the foundation of therapy for DLBCL for several decades, despite attempts to improve outcomes with more intensive treatments.(3) When the Groupe d'Etude de Lymphome d'Adultes (GELA) reported the first randomized controlled trial demonstrating the benefit of adding rituximab to CHOP chemotherapy for the treatment of patients ≥60 years of age with newly diagnosed DLBCL, the standard of care began to change. Additional data from the MabThera International Trial demonstrated that patients ≤60 years chemo-immunotherapy experienced improved 3-year event-free survival from 59% (chemotherapy alone) to 79% (log-rank p<0.0001), and increased 3-year OS from 83% to 93% (log-rank p=0.0001).(18) The results from the GELA trial in older patients were confirmed by a US Intergroup trial,(10) the German Lymphoma Study Group RICOVER-60 trial,(19, 20) and additional follow-up of the GELA study.(8) Furthermore, a population-based study comparing adult patients in British Columbia with DLBCL treated when the province standard therapy was CHOP or CHOP-like chemotherapy to patients treated under an updated policy that recommended chemotherapy with rituximab as standard of care showed improved progression-free survival and OS among patients treated under the updated policy.(21) While the final publication of some of these results occurred following our study period, substantial data were presented earlier at peer-reviewed international meetings describing the benefits of chemo-immunotherapy.(5, 6, 22)
Following the initial FDA approval of rituximab in 1997, the proportion of patients who received chemo-immunotherapy for DLBCL increased dramatically from 1998 to 2004. In this study, we found that while increase in the use of chemo-immunotherapy occurred for patients across all racial and SES categories, uninsured, Medicaid, black, and lower SES patients were less likely to receive chemo-immunotherapy in the 2001-2004 time period. In multivariable models, age >60 years, stage, race, region, SES and facility characteristics remained significant predictors of receiving chemo-immunotherapy, but insurance status did not. However, insurance status was likely not a significant predictor of chemo-immunotherapy because uninsured patients were less likely to receive any treatment at all, making chemotherapy a poor comparator. The reduced use of chemo-immunotherapy in older DLBCL patients is particularly interesting given that the benefits of chemo-immunotherapy were first demonstrated in patients > 60 years of age.(7) Follow-up national cohort studies are needed to determine whether this was a time-limited phenomenon or may reflect a bias toward limiting therapy in older individuals. Racial and SES disparities in the utilization of novel technologies or therapies for cancer patients have previously been demonstrated.(23) Further studies are necessary to disentangle the interacting effects of race and individual level SES in national cohort studies of lymphoma treatments and outcomes and to assess the influence that patient-level variables like social support, emotional support, and informational support have on lymphoma treatment selection.(24) Because individual level SES is not available in the NCDB, we were not able to examine the complex relationship between race and SES on lymphoma treatments. However, our analysis suggests that even when controlling for other covariates, age, race, and area-level SES remain important predictors of who received chemo-immunotherapy for DLBCL. Unfortunately, additional follow-up of these trends is not possible using the NCDB because coding of rituximab immunotherapy changed following this period.
In addition we found that patients who received treatment at a high lymphoma volume teaching/research center or comprehensive cancer center were more likely to receive chemo-immunotherapy. High-volume research and teaching facilities appeared to be early adopters of this innovation and may be able to more promptly apply treatment advances to individual patients. Understanding factors that influence the adoption and use of innovative treatment strategies(25) at these facilities may provide insight into measures that can be applied to speed the diffusion of innovative treatments to other segments of the US population.
It is important to note some limitations of this study. Because registry data do not contain the specific type of chemotherapy or immunotherapy administered, we relied upon general codes to indicate if chemotherapy and/or immunotherapy were administered. While rituximab was the major form of immunotherapy administered during this time, it is possible that other forms of immunotherapy (e.g. interferon) could have been coded as well. However, the utilization of these therapies in the US during this time period was limited. Moreover, area-level measures of SES were available in this dataset but patient-level measures of SES were not. In other studies where patient-level SES were collected with area-level SES, the latter appeared to underestimate the effect of SES.(26) Because cancer registry data lack information on all relevant clinical data such as International Prognostic Index (IPI) score,(27) our analyses could not examine the impact of these factors on chemo-immunotherapy use. However, where possible we did integrate components of the IPI (age > 60, stage III/IV) as covariates in our regression models. Our study is also subject to the effects of potential misclassification and under reporting previously described in cancer registry studies, including for race/ethnicity and treatment. However, CoC approved facilities are thought to have more complete data on treatment.(28) Ascertainment of patients for this study was based on a diagnosis of DLBCL being rendered at a CoC hospital facility, including patients who received care in both the inpatient and outpatient settings. This would reduce the possibility of selection bias due to more black patients being treated as inpatients due to advanced disease compared to white patients.
When we compared patients in our cohort aged >65 to SEER-Medicare, the rates of chemotherapy use and no treatment were similar. However, the rate of chemo-immunotherapy among NCDB patients was lower than that observed among SEER-Medicare patients, which may reflect improved receipt of chemo-immunotherapy among the Medicare population and/or an under-ascertainment of immunotherapy in NCDB. However, the under-ascertainment of immunotherapy did not vary by race. Moreover, because black patients less commonly present with DLBCL over the age of 65 years, SEER-Medicare linked data may be a less appropriate dataset for examining racial disparities in DLBCL treatment, further accentuating the value of our analysis using the NCDB.
Despite these limitations, this study has several strengths. First, the NCDB provided a large national cohort of patients with validated information on demographic and clinical characteristics at diagnosis. Moreover, it provides data on insurance status and area-level SES, allowing discrimination between the effects of race, insurance status, and SES on treatment administration. Finally, this study used a cohort of patients from over 1,350 institutions across the US over a four-year time period surrounding the demonstration that rituximab with chemotherapy improved outcomes over chemotherapy alone. This allowed us to examine the diffusion of this innovative treatment strategy across segments of the US DLBCL population and to explore factors that predict who received chemo-immunotherapy.
Other studies have also demonstrated that while advances in therapy have produced improvements in survival for patients with NHL, disparities exist in treatment and outcomes in the US.(29-31) In particular, a SEER-Medicare analysis by Wang et al. showed that older African American patients with NHL were less likely to receive chemotherapy than Caucasian patients.(31) While lower SES was predictive of all-cause and NHL-specific mortality, after controlling for differences in treatment, comorbidity, and socioeconomic status, race was not. In this study by Wang and colleagues, 72% of African Americans resided in areas with the poorest quartile of SES as compared with 22% of Caucasians, making it difficult to separate the effects of race and SES on treatment selection and outcome. More importantly, the study did not distinguish among NHL subtypes, rendering their findings regarding treatment selection and survival difficult to interpret since these vary markedly by lymphoma subtype. In our study, black patients were younger (median age 53 vs. 70 years), more likely to present with stage III/IV disease (44.5% vs. 40.9%), more likely to be uninsured (9.5% vs. 2.5%) or Medicaid insured (17.3% vs. 3.4%) and more likely to reside in a zip code where ≥29% of the population had no high school diploma (38.1% vs. 11.6%) when compared with white patients (all p<0.0001). However, there was no effect modification between SES and insurance on race after controlling for demographic (gender, age), clinical (diagnosis year, primary site, and stage), and facility level factors. Moreover, our study focused solely on patients with DLBCL eliminating the effect that known racial differences in NHL prevalence would have on our findings.(16, 32)
These results support our prior findings that black patients with DLBCL in the US present at a younger age and with more advanced stage disease when compared with other racial/ethnic groups.(16) Among 37,009 DLBCL cases diagnosed from 1992 to 2005 in the SEER registry, 65% of black patients compared to 37% of whites presented at age ≤60 years (median age 51 vs 68, p<0.001). Moreover, 54% of black compared with 47% of white patients presented with stage III/IV disease (p=0.05) and 5-year survival rates were 38% vs. 46% (p=0.02). These differences in presentation did not arise through an association with HIV,(33) which was also demonstrated in the NCDB (data not shown).
Do disparities in treatment outcomes result from inequalities in the utilization of standard therapies for lymphoma? While a number variables may influence disparities in treatment outcomes including patient-related, provider-related, healthcare system-related, and societal factors (34-37), prior studies investigating healthcare disparities in cancer patients indicate that equal treatment yields equal outcomes.(38-41) For lymphoma patients limited data exist regarding disparities in treatment utilization that are linked to treatment outcomes. A recent SEER analysis comparing survival trends among patients with DLCBL from 1973 to 2004 demonstrated improved median OS in the era of immunotherapy [2000-2004 median OS 47 months, (p=0.005)], however this benefit was not maintained across race with white patients having significantly better outcomes [47 months vs. 29 months (p=0.001)].(42) Moreover, an Emory University cohort study that examined 361 white and 123 black DLBCL patients indicated that racial differences in DLBCL outcomes occur even when the same treatment is given to black and white patients.(43) Although there were no racial differences in the use of R-CHOP therapy, white race predicted for improved 2-year OS (odds ratio=1.77; 95% CI 1.15-2.73). Because there are known biological subgroups of DLBCL that are associated with differences in treatment response and survival,(44) performing meaningful DLBCL disparities research in the future will require collection of biological specimens to ascertain the interactions between, race, SES, treatment disparities, and outcomes.
Conclusions
Effective lymphoma care involves the provision of appropriate and beneficial services to cancer patients based on scientific knowledge. Quality of care needs to be defined and studied to ensure that patients with DLBCL and other cancers receive the best available standard of care. While our study demonstrated that the use of chemo-immunotherapy rose during the period immediately following proof of its benefit, improving outcomes for patients with lymphoma in the US will require increased attention on efforts to extend the benefits of proven advances in therapy to all segments of the population.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Katherine Virgo and Dr. John Bian for analyzing the rates of chemotherapy and immunotherapy in SEER-Medicare data
Grant Support: This work was supported by Dr. Flowers' Georgia Cancer Coalition Distinguished Scientist Award, American Society of Hematology Amos Medical Faculty Development Award, and funding from the National Cancer Institute R21CA158686.
Footnotes
Conflict of Interests: Dr. Flowers has served as a consultant for Spectrum, Celgene, Optum Rx, Seattle Genetics, Allos, Genentech/Roche (unpaid), and Millennium/Takeda (unpaid). Dr. Flowers leads research studies that are supported by Spectrum, Novartis, Millennium/Takeda, and Gilead.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Flowers CR, Sinha R, Vose JM. Improving outcomes for patients with diffuse large B-cell lymphoma. CA Cancer J Clin. 2010;60:393–408. doi: 10.3322/caac.20087. [DOI] [PubMed] [Google Scholar]
- 3.Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993;328:1002–6. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 4.McKelvey EM, Gottlieb JA, Wilson HE, Haut A, Talley RW, Stephens R, et al. Hydroxyldaunomycin (Adriamycin) combination chemotherapy in malignant lymphoma. Cancer. 1976;38:1484–93. doi: 10.1002/1097-0142(197610)38:4<1484::aid-cncr2820380407>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: A multicenter phase II study. Blood. 1998;92:1927–1932. [PubMed] [Google Scholar]
- 6.Coiffier B, Lepage E, Herbrecht R, Tilly H, Solal-Celigny P, Munck JN, et al. Mabthera (Rituximab) plus CHOP is superior to CHOP alone in elderly patients with diffuse large-B-cell lymphoma (DLCL): Interim results of a randomized GELA trial. Blood. 2000;96:223A. Abstract #950. [Google Scholar]
- 7.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 8.Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116:2040–5. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfreundschuh MG, Trumper L, Ma D, Österborg A, Pettengell R, Trneny M, et al. Randomized intergroup trial of first line treatment for patients <=60 years with diffuse large B-cell non-Hodgkin's lymphoma (DLBCL) with a CHOP-like regimen with or without the anti-CD20 antibody rituximab – early stopping after the first interim analysis. Proc Amer Soc Clin Oncol. 2004 Abstract #6500. [Google Scholar]
- 10.Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–7. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 11.Fisher RI, Miller TP, O'Connor OA. Diffuse aggressive lymphoma. Hematology (Am Soc Hematol Educ Program) 2004:221–36. doi: 10.1182/asheducation-2004.1.221. [DOI] [PubMed] [Google Scholar]
- 12.Miller TP, Dahlberg S, Cassady JR, Adelstein DJ, Spier CM, Grogan TM, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin's lymphoma. N Engl J Med. 1998;339:21–6. doi: 10.1056/NEJM199807023390104. [DOI] [PubMed] [Google Scholar]
- 13.Zelenetz AD, Abramson JS, Advani RH, Andreadis CB, Bartlett N, Bellam N, et al. NCCN Clinical Practice Guidelines in Oncology: Non-Hodgkin's lymphoma v.1.2012. National Comprehensive Cancer Network, Inc.; 2012. [DOI] [PubMed] [Google Scholar]
- 14.ICD-O International Classification of Diseases for Oncology. Geneva: World Health Organization; 1976. [Google Scholar]
- 15.Deddens JA, Petersen MR. Approaches for estimating prevalence ratios. Occup Environ Med. 2008;65:481, 501–6. doi: 10.1136/oem.2007.034777. [DOI] [PubMed] [Google Scholar]
- 16.Shenoy PJ, Malik N, Nooka A, Sinha R, Ward KC, Brawley OW, et al. Racial differences in the presentation and outcomes of diffuse large B-cell lymphoma in the United States. Cancer. 2010 doi: 10.1002/cncr.25765. [DOI] [PubMed] [Google Scholar]
- 17.Brown ML, Riley GF, Schussler N, Etzioni R. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40:IV-104–17. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- 18.Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–91. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 19.Pfreundschuh M, Kloess M, Zeynalova S, Lengfelder E, Franke A, Reiser M, et al. Six vs eight cycles of bi-weekly CHOP-14 with or without rituximab for elderly patients with diffuse large B-cell lymphoma (DLBCL): Results of the completed RICOVER-60 trial of the german high-grade non-Hodgkin lymphoma study group (DSHNHL) Blood. 2006;108:205. [Google Scholar]
- 20.Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60) Lancet Oncology. 2008;9:105–116. doi: 10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 21.Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–33. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 22.Vose JM, Link BK, Grossbard ML, Czuczman M, Grillo-Lopez A, Gilman P, et al. Phase II study of rituximab in combination with chop chemotherapy in patients with previously untreated, aggressive non-Hodgkin's lymphoma. J Clin Oncol. 2001;19:389–97. doi: 10.1200/JCO.2001.19.2.389. [DOI] [PubMed] [Google Scholar]
- 23.Chen AY, Halpern MT, Schrag NM, Stewart A, Leitch M, Ward E. Disparities and trends in sentinel lymph node biopsy among early-stage breast cancer patients (1998-2005) J Natl Cancer Inst. 2008;100:462–74. doi: 10.1093/jnci/djn057. [DOI] [PubMed] [Google Scholar]
- 24.Glover R, Shenoy P, Kharod GA, Schaefer A, Bumpers K, Berry JTM, et al. Patterns of Social Support among Lymphoma Patients Considering Stem Cell Transplantation. Social Work in Health Care. 2011;50:815–827. doi: 10.1080/00981389.2011.595889. [DOI] [PubMed] [Google Scholar]
- 25.Flowers CR, Melmon KL. Clinical investigators as critical determinants in pharmaceutical innovation. Nat Med. 1997;3:136–43. doi: 10.1038/nm0297-136. [DOI] [PubMed] [Google Scholar]
- 26.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–10. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–94. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 28.Pappas DP, Garbus JE, Feuerman M, Reed WP. Improving uniformity of care for colorectal cancers through National Quality Forum quality indicators at a Commission on Cancer-accredited community based teaching hospital. Surg Oncol Clin N Am. 2011;20:587–96. x. doi: 10.1016/j.soc.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 29.The A, Li Y, Reddy V, Davis R, Baird M, Foran J. A Comparative Study of Diffuse Large B-Cell Lymphoma (DLBCL) between African Americans and Caucasians: Single-Center Experience at the University of Alabama at Birmingham (UAB) Blood (ASH Annual Meeting Abstracts) 2007;110:4430. [Google Scholar]
- 30.Vance KT, Kilgore ML, Yun H, Gary LC, Foran JM. Race and Non-Hodgkin's Lymphoma: Adverse Impact of Race and Treatment Delays on Survival. A SEER-Medicare Population Study (1995 2003) Blood (ASH Annual Meeting Abstracts) 2007;110:3578. [Google Scholar]
- 31.Wang M, Burau KD, Fang S, Wang H, Du XL. Ethnic variations in diagnosis, treatment, socioeconomic status, and survival in a large population-based cohort of elderly patients with non-Hodgkin lymphoma. Cancer. 2008;113:3231–41. doi: 10.1002/cncr.23914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flowers CR, Glover R, Lonial S, Brawley OW. Racial differences in the incidence and outcomes for patients with hematological malignancies. Curr Probl Cancer. 2007;31:182–201. doi: 10.1016/j.currproblcancer.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Malik N, Shenoy PJ, Bumpers K, Sinha R, Flowers CR. Racial Differences in the Presentation and Outcomes of Diffuse Large B-Cell Lymphoma in the United States. Blood (ASH Annual Meeting Abstracts) 2009;114 doi: 10.1002/cncr.25765. Abstract#898. [DOI] [PubMed] [Google Scholar]
- 34.Powe NR, Cooper LA. Diversifying the racial and ethnic composition of the physician workforce. Ann Intern Med. 2004;141:223–4. doi: 10.7326/0003-4819-141-3-200408030-00013. [DOI] [PubMed] [Google Scholar]
- 35.Lavizzo-Mourey R, Lumpkin JR. From unequal treatment to quality care. Ann Intern Med. 2004;141:221. doi: 10.7326/0003-4819-141-3-200408030-00011. [DOI] [PubMed] [Google Scholar]
- 36.Groman R, Ginsburg J. Racial and ethnic disparities in health care: a position paper of the American College of Physicians. Ann Intern Med. 2004;141:226–32. doi: 10.7326/0003-4819-141-3-200408030-00015. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan SH, Greenfield S. The Patient's role in reducing disparities. Ann Intern Med. 2004;141:222–3. doi: 10.7326/0003-4819-141-3-200408030-00012. [DOI] [PubMed] [Google Scholar]
- 38.Hayes N, Rollins R, Weinberg A, Brawley O, Baquet C, Kaur JS, et al. Cancer-related disparities: weathering the perfect storm through comprehensive cancer control approaches. Cancer Causes Control. 2005;16(Suppl 1):41–50. doi: 10.1007/s10552-005-0487-z. [DOI] [PubMed] [Google Scholar]
- 39.Reddy S, Shapiro M, Morton R, Jr, Brawley OW. Prostate cancer in black and white Americans. Cancer Metastasis Rev. 2003;22:83–6. doi: 10.1023/a:1022216119066. [DOI] [PubMed] [Google Scholar]
- 40.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. Jama. 2002;287:2106–13. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 41.Brawley OW. Lung cancer and race: equal treatment yields equal outcome among equal patients, but there is no equal treatment. J Clin Oncol. 2006;24:332–3. doi: 10.1200/JCO.2005.03.7077. [DOI] [PubMed] [Google Scholar]
- 42.Komrokji RS, Al Ali NH, Beg MS, Safa MM, Rollison D, Kharfan-Dabaja M, et al. Outcome of diffuse large B-Cell lymphoma in the United States has improved over time but racial disparities remain: review of SEER data. Clin Lymphoma Myeloma Leuk. 2011;11:257–60. doi: 10.1016/j.clml.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Shenoy PJ, Bumpers K, King N, Huang T, Malik N, Sinha R, et al. Black/White Differences in the Treatment and Outcomes of Diffuse Large B Cell Lymphoma: A Matched Cohort Analysis. ASH Annual Meeting Abstracts. 2009;114:1392. [Google Scholar]
- 44.Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–47. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.