Abstract
The zebrafish (Danio rerio) has been used extensively as a model system for developmental studies but, unlike most teleost fish, it grows in a determinate-like manner. A close relative, the giant danio (Devario cf. aequipinnatus), grows indeterminately, displaying both hyperplasia and hypertrophy of skeletal myofibers as an adult. To better understand adult muscle hyperplasia, a postlarval/postnatal process that closely resembles secondary myogenesis during development, we characterized the expression of Pax3/7, c-Met, syndecan-4, Myf5, MyoD1, myogenin, and myostatin during in vitro myogenesis, a technique that allows for the complete progression of myogenic precursor cells to myotubes. Pax7 appears to be expressed only in newly activated MPCs while Pax3 is expressed through most of the myogenic program, as are c-Met and syndecan-4. MyoD1 appears important in all stages of myogenesis, while Myf5 is likely expressed at low to background levels, and myogenin expression is enriched in myotubes. Myostatin, like MyoD1, appears to be ubiquitous at all stages. This is the first comprehensive report of key myogenic factor expression patterns in an indeterminate teleost, one that strongly suggests that Pax3 and/or Myf5 may be involved in the regulation of this paradigm. Further, it validates this species as a model organism for studying adult myogenesis in vitro, especially mechanisms underlying nascent myofiber recruitment.
Keywords: Myogenesis, Zebrafish, Giant danio, Indeterminate growth, Pax3
Introduction
The zebrafish (Danio rerio) is a well-established model organism for investigations in developmental biology. More recently, the zebrafish has been utilized in studies of muscle genomics, somite formation, myotome development, muscle fiber specification, and muscle differentiation, as reviewed in Sparrow et al. (2008) and Buckingham and Vincent (2009). However, the small size of this species has limited in vitro studies of myogenic precursor cell (MPC) physiology due to amount of tissue needed to isolate sufficient numbers of these cells for in vitro work. Additionally, the zebrafish exhibits determinate-like somatic growth, with hypertrophy of existing myofibers being the primary source of skeletal muscle growth during the postlarval period (van Raamsdonk et al. 1982; Biga and Goetz 2006). While the ex utero development of zebrafish may make it a good model organism for studies of embryonic and larval muscle development, these characteristics have limited the usefulness of this organism in investigating mechanisms of postlarval myogenesis. However, the clade Danioninae contains both small and large danio species, leading to the prediction that several, if not many, relatives of the zebrafish are indeterminate growers. In 2006, Biga and Goetz demonstrated this very fact in the giant danio (Devario cf. aequipinnatus), the most closely related cyprinid widely available in the United States (Meyer et al. 1993; Tang et al. 2010). In addition to its larger size (~8–10 cm), this species exhibits true indeterminate growth, augmenting musculature in the postlarval period through both nascent myofiber recruitment (hyperplasia) and hypertrophy (Biga and Goetz 2006).
Past investigations of myogenic precursor cell physiology in teleost fish have proven difficult. No continuous piscine myogenic cell line is available, despite numerous attempts at immortalization (Hightower and Renfro 1988). Primary myoblast culture systems have been developed and described in a variety of species, including salmonids (Greenlee et al. 1995; Matschak and Stickland 1995; Rescan et al. 1995), other cyprinids (Koumans et al. 1990; Sepich et al. 1994), and ictalurids (Mulvaney and Cyrino 1995). However, it is unclear whether the isolated cells are true MPCs or undifferentiated myoblasts, largely due to the general lack of evidence based on molecular markers. It has been well demonstrated that these cells are at least of the myogenic lineage, as myosin heavy chain is expressed by myotubes in vitro (Greenlee et al. 1995; Rescan et al. 1995; Fauconneau and Paboeuf 2000). To better understand teleost MPCs and to validate the primary culture system for investigations of piscine muscle biology, we present evidence demonstrating progression of the myogenic program in cultured giant danio MPCs isolated from adult animals. For the first time, we demonstrate putative evidence of protein expression of MPC-specific factors (Pax3, Pax7, syndecan-4, and c-Met), myogenic regulatory factors (MyoD1, Myf5, and myogenin), and myostatin, a TGF-β family member which acts as a negative regulator of muscle growth, in an indeterminate-growing organism, a species which can be directly juxtaposed against an accepted model organism, the zebrafish.
Materials and Methods
Animals
Adult (>1 yr of age) giant danio (Devario cf. aequipinnatus) were obtained from commercial tropical fish dealers throughout the United States. Upon arrival, fish were maintained in a recirculating aquatic system (dechlorinated city water) at 26°C under a 14 L/10D photoperiod. Fish were fed once daily ad libitum. All animal care and experimental procedures described herein were approved in advance of experimentation by the Institutional Animal Care and Use Committee at North Dakota State University, Fargo.
Primary myoblast culture system
Following a protocol developed by Rescan et al. (1995) and used extensively by fish muscle biologists, primary myoblasts were isolated from adult fish. Fish were euthanized by tricaine methanesulfonate (>300 mg/mL; AVMA, Pittsburg, PA), submerged in 70% ethanol for 30 s, and de-scaled. Epaxial, fast-glycolytic (‘white’) muscle tissue (5 g) was sterilely dissected free of slow-oxidative (‘red’) muscle tissue and immediately placed in chilled (~4°C) isolation media (9 mM NaHCO3, 20 mM HEPES, 1%v/v penicillin/streptomycin/fungizone, 0.15%v/v gentamicin in Dulbecco’s modified Eagle’s medium supplemented with 15%v/v donor equine serum). Following mechanical dissociation to a slurry consistency, muscle tissue was twice washed with washing media (9 mM NaHCO3, 20 mM HEPES, 1%v/v penicillin/streptomycin/fungizone, 0.15%v/v gentamicin DMEM) and centrifuged at 300×g for 5 min. Muscle tissue was then enzymatically digested in 0.2% (w/v) collagenase type IV (Worthington Biochemical, Worcester, MA) for 90 min followed by two washes and centrifugations at 300×g. Two trypsin (MP Biomedical, Portland, OR; 0.1%w/v in 9 mM NaHCO3, 20 mM HEPES, 1%v/v PSF DMEM) digestions were completed next, followed by triturations and filtrations through 100 and 40 μm cell strainers (BD, New York, NY). Isolated cells were then counted using a hemocytometer and the trypan blue exclusion method. Isolated cells were plated on poly-L-lysine-treated (Sigma, Palo Alto, CA), laminin-coated (BD) plates at a density of 2×106 cells per milliliter. Cultures were incubated at 26°C in complete media (9 mM NaHCO3, 20 mM HEPES, 1%v/v PSF DMEM supplemented with 10%v/v characterized fetal bovine serum; HyClone, Ithaca, NY) under normal atmospheric conditions without CO2 supplementation for 9–11 d. Media was changed daily for the first 4 d of culture; thereafter, media was changed every other day to control cell proliferation and differentiation. Prior to media changes, cultures were rinsed with washing media for 30 s to 1 min to remove non-adherent cells and debris. All cell cultures were performed in triplicate.
Immunocytochemistry
At the appropriate stages, cells were fixed and probed with antibodies following a standard immunocytochemistry protocol. Briefly, cultures were washed in DMEM three times, and fixed in 4% formaldehyde (16% formaldehyde diluted in phosphate-buffered saline; Pierce, Englewood, CO) for 10 min. Cells were then permeabilized by incubation in 0.5% (v/v) Triton X-100 dissolved in PBS for 10 min at room temperature with gentle rocking. Cultures were blocked in 2% (w/v) bovine serum albumin in PBS 0.2% (v/v) Tween-20 for 24 h. Cultures were then rinsed twice with PBS-0.1% (v/v) Tween-20 (hereinafter PBST) followed by incubation with primary antibodies in 3% BSA (w/v) PBST for 24 h at 4°C with gentle rocking. Monoclonal mouse anti-Pax3 (ascites, 1:40 dilution) developed by C. P. Ordahl and monoclonal mouse anti-Pax7 (ascites, 1:40 dilution) developed by A. Kawakami were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. Polyclonal rabbit anti-MyoD1 (C-20; 1:20 dilution), polyclonal rabbit anti-myogenin (M-225; 1:20 dilution) and polyclonal rabbit anti-Met (C-28; 1:20 dilution) antibodies were obtained from Santa Cruz Biotechnology, Santa Cruz, CA. Polyclonal rabbit anti-syndecan-4 (AB71439; 1:40 dilution) antibody was obtained from Abcam, Cambridge, MA. A polyclonal rabbit antibody raised against recombinant brook trout (Salvelinus fontanalis) myostatin (Roberts and Goetz 2003; Biga et al. 2004) was used to detect danionin myostatin (1:50 dilution). Polyclonal rabbit anti-Myf5 (Z-FISH™ Anti-Myf-5 IN; 1:20) was used to detect endogenous myogenic factor-5 protein and was obtained from AnaSpec EGT Group, Fremont, CA. Following incubation with primary antibody, secondary antibody incubation was carried out in 1% BSA PBST for 2 h at room temperature. Rabbit antibodies were detected using a fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit antibody (Vector Laboratories) and mouse antibodies were detected using a Texas Red-conjugated rabbit anti-mouse antibody (Abcam). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole in mounting medium (Vectashield with DAPI; Vector Laboratories, Burlingame, CA). Immunolabeled cells were microphotographed under a Leica DM IL inverted microscope at ×40 magnification and Image Q imaging system, generously provided by the CORE Biology Facility at North Dakota State University, Fargo. For quantification purposes, cells in three fields (randomly chosen, ×40 magnification) were evaluated for factor expression (i.e., positive staining for Pax3, Pax7, c-Met, syndecan-4, MyoD1, Myf5, myogenin, and myostatin) per day of culture, normalized to total cells present in that field. ImageJ (http://rsb.info.nih.gov/ij/, available from the National Institutes of Health, Baltimore, MD) was used to determine both numbers of positive cells and total cells per field.
Proliferation studies
To assess the proliferative ability of MPCs and myoblasts, isolated cells were plated with 10 μM 5-bromodeoxyuridine (BrdU; Sigma) in complete media (see above) for 24 h throughout the culture period. Following BrdU exposure, cultures were fixed in 4% (w/v) paraformaldehyde in phosphate-buffered saline for 20 min, rinsed twice with PBS, permeabilized with 0.5% (v/v) Triton X-100 in PBS for 20 min, denatured in 3 N HCl for 7 min, washed with PBS twice, and finally blocked in 3% (w/v) BSA in PBS-0.1% Triton X-100 for 24 h. Following blocking, cultures were rinsed twice with PBS followed by incubation with anti-BrdU clone BU33 (1:200, Sigma-Aldrich, Cambridge, MA) in 1% (w/v) BSA PBS-0.1% Triton X-100 for 1 h at room temperature and 23 h at 4°C. Proliferative cells were detected with a secondary Texas Red-conjugated rabbit anti-mouse antibody (Abcam) in 1% BSA PBS-0.1% Triton X-100 for 2 h at room temperature. Counterstaining and visualization were completed as described above.
Statistical analysis
All cell cultures were performed in triplicate and all analyses were conducted from three randomly chosen fields of exact size from each culture. For each factor examined, percent positive cells per day (e.g.,% Pax3+ cells on day 2) were analyzed by one-way ANOVA for effect of day, with application of Bonferroni’s post hoc test as necessary to detect differences between d (with significance determined at P<0.05). For proliferation studies, one-way ANOVA was utilized to test for differences between d, with overall effect of d considered at P<0.05 and differences between d considered significant at P<0.05 following Bonferonni’s post hoc test. GraphPad Prism 5 (www.graphpad.com) was used to conduct all statistical tests.
Results
Myogenesis follows a progression of cell commitment beginning with myogenic precursor cells that become determined as myoblasts and differentiate into myotubes and then functional myofibers. Many studies have utilized primary myoblast cultures to examine various aspects of myogenesis in teleosts; however, this culture system is poorly defined. To address this, we characterized isolated MPCs from giant danio in vitro as they proliferate and differentiate into myotubes. Because most teleost fish are indeterminate growers, we chose to characterize the in vitro myogenesis of this danionin, as it is likely to be more representative of teleost fish in general than its close relative, the zebrafish.
Paired-box transcription factors are present throughout myogenesis
Both Pax3 and Pax7 are detectable during myogenesis in giant danio (Figs. 1 and 2); however, the expression profiles of these transcription factors are quite different. Upon seeding of adult-derived MPCs collected from giant danio, the expression of Pax7 is rapidly downregulated (>99% at 2.5 h versus 72.4±24.0% at 5 h post-seeding; Fig. 1) and undetectable during myoblast proliferation stages and myotube formation (days 1–9; Fig. 2). Conversely, putative Pax3 expression appears to be highly expressed throughout myoblast proliferation and even into differentiation (50.2±17.7% Pax3+ nuclei on day 1 of culture, with expression gradually increasing through myogenesis and peaking at 94.4±9.7% of nuclei at day 9 of culture; Fig. 2).
Figure 1.
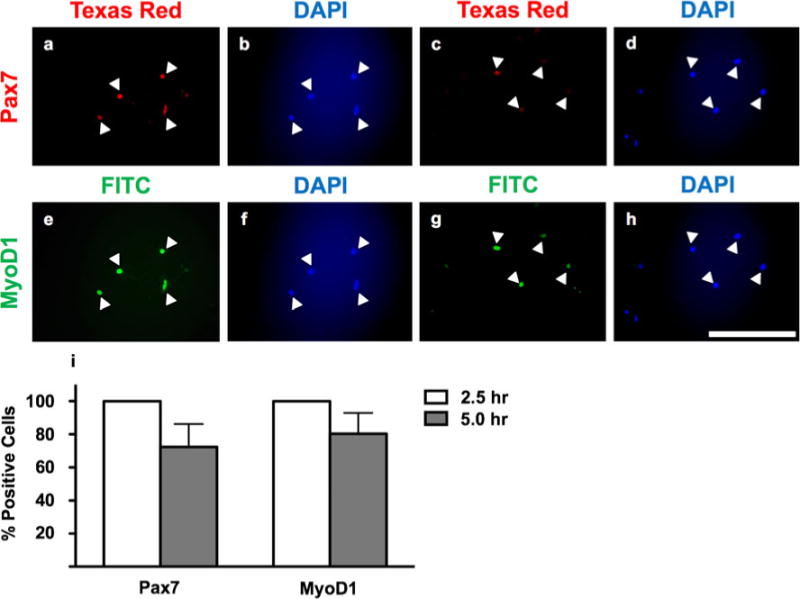
Immunocytochemistry of Pax7 and MyoD1 expression in myogenic precursor cells cultured for 2.5 h (a, b; e, f) and 5 h (c, d; g, h) post-isolation. Anti-Pax7 detected by Texas Red fluorophore and anti-MyoD1 detected by FITC fluorophore. Total nuclei detected by DAPI. a, b; c, d; e, f; and g, h represent identical fields. White arrowheads indicate the same cells between pairs of identical fields. Images are representative of duplicated staining across triplicate experiments. Scale=100 μm. Quantification of Pax7 and MyoD1 expression (i), Pax7: One-way ANOVA, P<0.001 (different from days 1–9); MyoD1: one-way ANOVA, P=0.4643 (not different from days 1–9), n=18.
Figure 2.
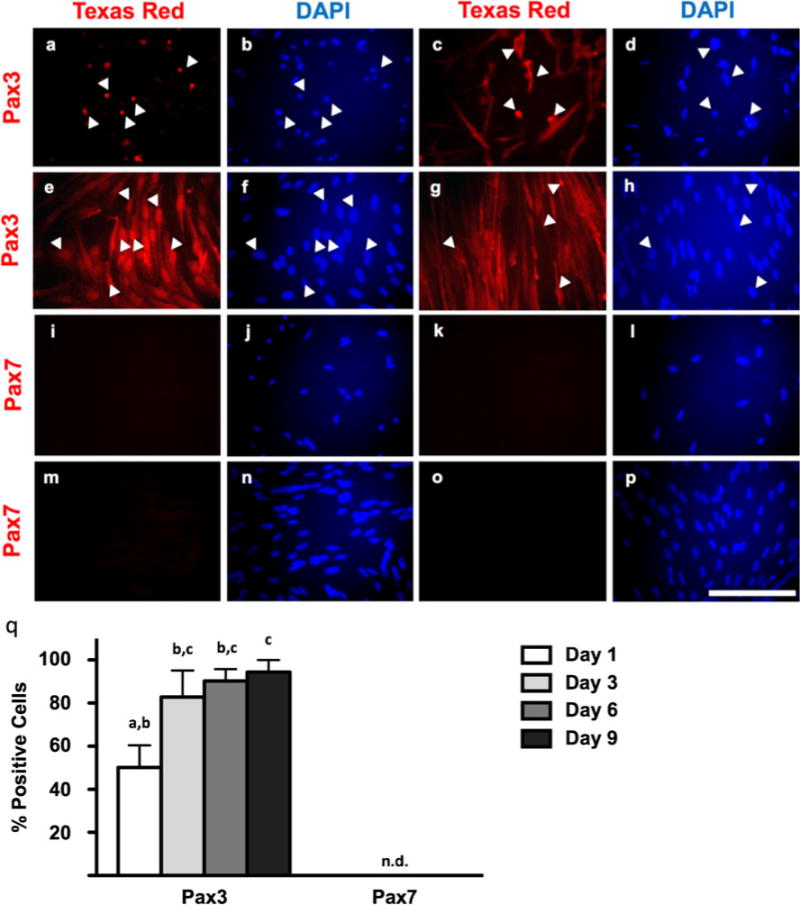
Immunocytochemistry of Pax3 and Pax7 expression in myogenic precursor cells, early-stage myoblasts, late-stage myoblasts, and differentiating myotubes at days 1–2 (a, b; i, j), days 3–4 (c, d; k, l), days 6–7 (e, f; m, n), and days 9–10 (g, h; o, p) of culture, respectively. Anti-Pax3 and anti-Pax7 detected by Texas Red fluorophore. Total nuclei detected by DAPI. a, b; c, d; e, f; (g, h; i, j; k, l; m; n; and o, p represent identical fields. White arrowheads indicate the same cells between pairs of identical fields. Images are representative of duplicated staining across triplicate experiments. Scale=100 μm. Quantification of Pax3 and Pax7 across culture period (q). Pax3: one-way ANOVA, P=0.0024, n=12; Pax7: one-way ANOVA, P<0.0001, n=12. Different letters indicate statistical differences at P<0.05 by Bonferroni’s post hoc test.
Myogenic precursor cell markers are expressed during myogenic progression
In addition to being Pax3+/Pax7+, isolated MPCs from giant danio are also c-Met+ (the receptor for hepatocyte growth factor/scatter factor), where c-Met expression is highest at seeding and, interestingly, in myotubes (95.6±7.7% c-Met+ on day 1 versus 99.0±1.6% on day 9 of culture; Fig. 3). Intense expression of c-Met is limited to MPCs and early proliferative myoblasts (days 1–6), but some diffuse localization as cells progress into differentiation (days 7–9). c-Met expression remains high throughout myogenesis with several small c-Met+ cells being present in myotubes in close proximity to larger c-Met+ cells (Fig. 3a–h).
Figure 3.
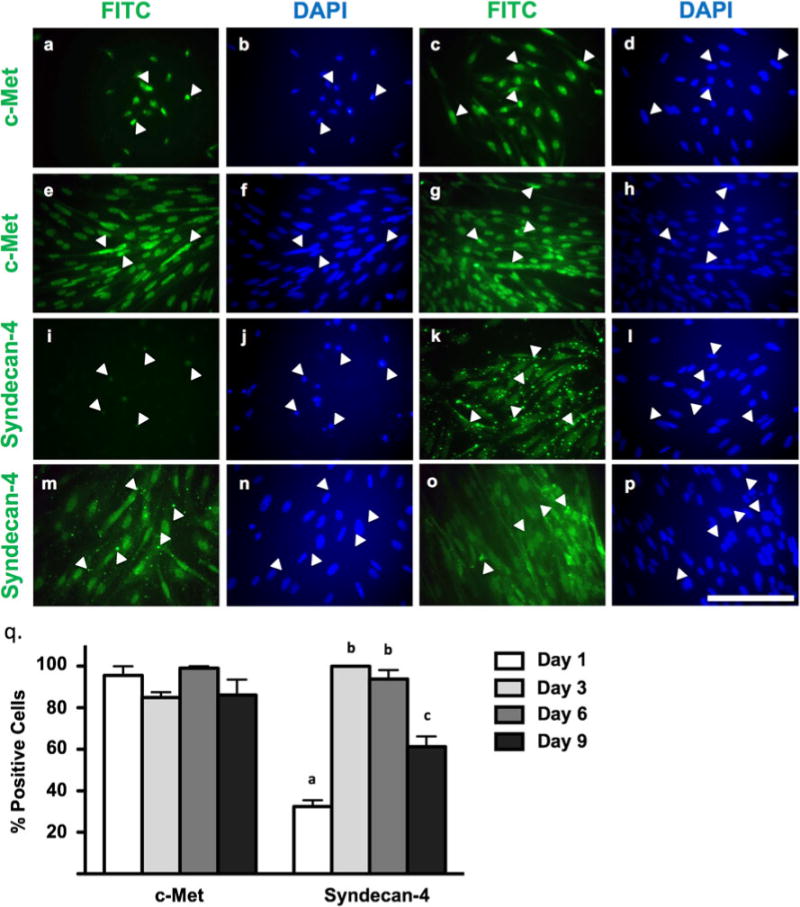
Immunocytochemistry of c-Met and syndecan-4 expression in myogenic precursor cells, early-stage myoblasts, late-stage myoblasts, and differentiating myotubes at days 1–2 (a, b; i, j), days 3–4 (c, d; k, l), days 6–7 (e, f; m, n), and days 9–10 (g, h; o, p) of culture, respectively. Anti-c-Met and anti-syndecan-4 detected by fluorescein isothiocyanate (FITC) fluorophore. Total nuclei detected by DAPI. a, b; c, d; e, f; g, h; i, j; k, l; m; n; and o, p represent identical fields. White arrowheads indicate the same cells between pairs of identical fields. Images are representative of duplicated staining across triplicate experiments. Scale=100 μm. Quantification of c-Met and syndecan-4 across culture period (q). c-Met: one-way ANOVA, P=0.2523, n=12; syndecan-4: one-way ANOVA, P<0.0001, n=12. Different letters indicate statistical differences at P<0.05 by Bonferroni’s post hoc test.
Syndecan-4, a heparan sulfate proteoglycan, is also present throughout in vitro myogenesis in giant danio (Fig. 3i–p). At seeding (day 1), a small fraction of cells (32.47±5.3%) positively stain for syndecan-4, but this expression increases as cells become elongated (days 3–4) where syndecan-4 is detected in all myoblasts with strong aggregated staining (>99%). This highly organized staining decreases as myoblasts progress through myogenesis and staining becomes more diffuse. During differentiation, syndecan-4 staining is reduced compared to late-stage myoblasts, with some aggregates detected (61.3±8.6% of cells syndecan-4+ on day 9 of culture; Fig. 3).
Myogenic regulatory factor expression is present during myoblast proliferation and differentiation
Cells at seeding appear to be Pax3+/Pax7+/MyoD1+, indicating commitment to the myogenic lineage upon seeding (data not shown). At seeding, most cells are MyoD1+ (Fig. 4i–p, y) and this expression level continues throughout myogenesis (89.0± 11.6% of nuclei MyoD1+ on day 1 of culture versus 86.2±14.6% on day 9). In differentiating myotubes, clusters of nuclei exhibit intense staining for MyoD1. Myf5 is also present at seeding; however, these cells are very rare (Fig. 4a–h). This low level of Myf5 expression (as determined by very weak fluorescence, close to background levels) continues throughout myogenesis, indicating that Myf5+ cells are rare in giant danio MPCs and in vitro myogenesis (17±3.5% of nuclei Myf5+ on day 1 of culture). In contrast, myogenin expression is detected in MPCs (81.4±17.2%, days 1–2), with a similar profile in proliferative myoblasts (>72.4±7.8%, days 3–6; Fig. 4q–x, y). Staining is primarily nuclear, with some diffuse fluorescence in the cytoplasm. Myogenin expression becomes more detectable in late-stage myoblasts or myotubes as cells begin to terminally differentiate, where more intense expression is also apparent (80.4±17.4% of nuclei myogenin+).
Figure 4.
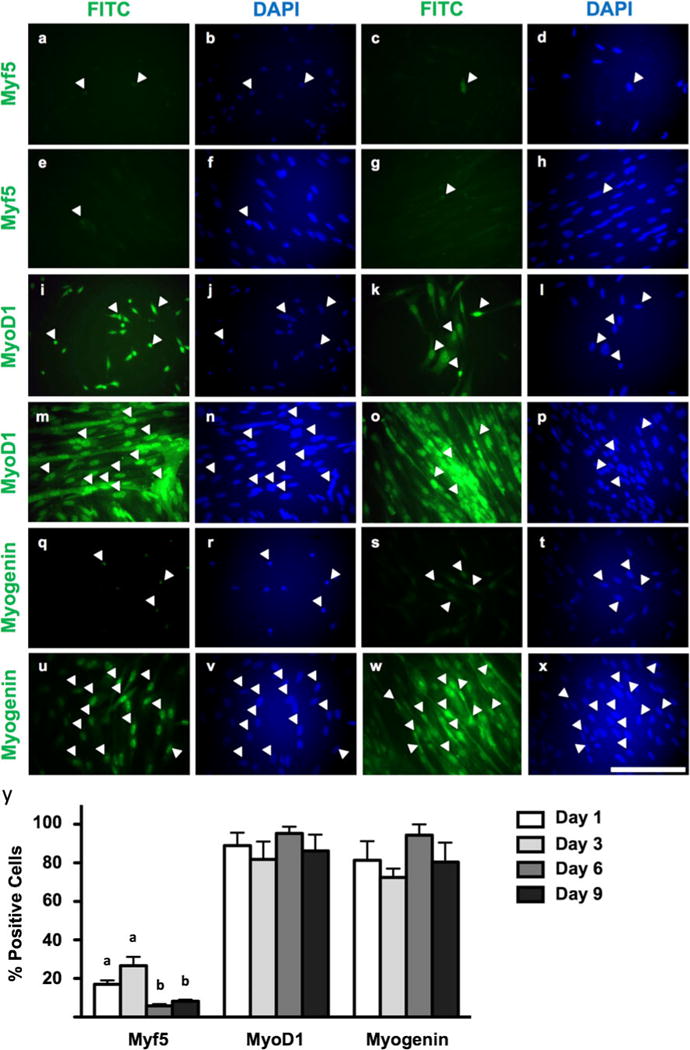
Immunocytochemistry of Myf5, MyoD1, and myogenin expression in myogenic precursor cells, early-stage myoblasts, late-stage myoblasts, and differentiating myotubes at days 1–2 (a, b; i, j; q, r), days 3–4 (c, d; k, l; s, t), days 6–7 (e, f; m, n; u, v), and days 9–10 (g, h; o, p; w, x) of culture, respectively. Anti-Myf5, anti-MyoD1, and anti-myogenin detected by fluorescein isothiocyanate (FITC) fluorophore. Total nuclei detected by DAPI. a, b; c, d; e, f; g, h; i, j; k, l; m; n; o, p; q, r; s, t; u, v; and w, x represent identical fields. White arrowheads indicate the same cells between pairs of identical fields. Images are representative of duplicated staining across triplicate experiments. Scale=100 μm. Quantification of Myf5, MyoD1, and myogenin expression across culture period (y). Myf5: one-way ANOVA, P=0.0205, n=12; MyoD1: one-way ANOVA, P=0.4643, n=12; myogenin: one-way ANOVA, P=0.3392. Different letters indicate statistical differences at P<0.05 by Bonferroni’s post hoc test.
Myostatin, a TGF-β family member, expression is detected throughout myogenesis
Myostatin expression is detected in seeded myocytes as early as day 1, as evidenced by strong staining in newly seeded cells (74.2±23.0%; Fig. 5). As MPCs progress to myoblasts, myostatin expression remains present in >99% of cells, with some intense cytoplasmic staining (Fig. 5). The strong immunofluorescence exhibited in MPCs continues throughout the myogenic program, even in terminally differentiating myotubes.
Figure 5.
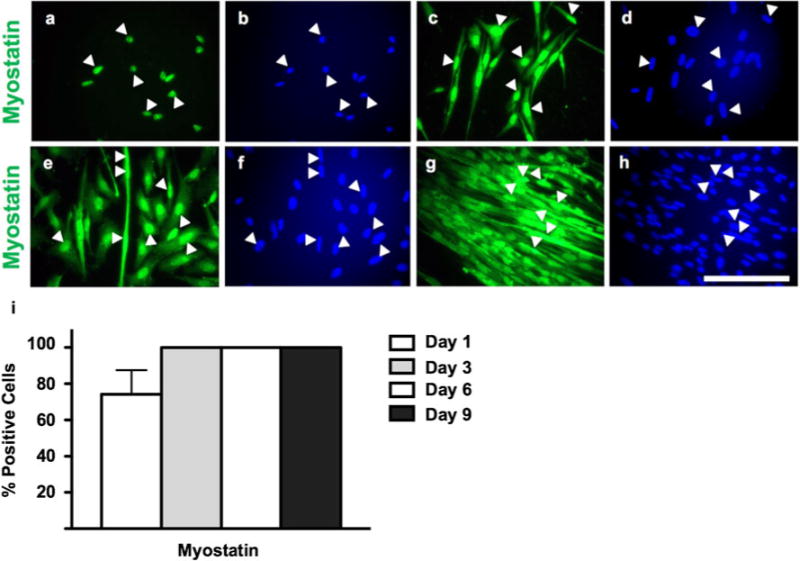
Immunocytochemistry of myostatin expression in myogenic precursor cells (a, b) and early-stage myoblasts at days 3–4 (c, d), respectively; and late-stage myoblasts and differentiating myotubes at day 6 (e, f) and day 9 (g, h) of culture, respectively. Anti-myostatin detected by fluorescein isothiocyanate (FITC) fluorophore. Total nuclei detected by DAPI. a, b; c, d; e, f; and g, h represent identical fields. White arrowheads indicate the same cells between pairs of identical fields. Images are representative of duplicated staining across triplicate experiments. Scale=100 μm. Quantification of myostatin expression (i), myostatin: one-way ANOVA, P=0.0589, n=12.
Proliferation increases as myogenic precursor cells progress to myotubes
Between seeding and day 1 (at 2× 106 cells/mL), there is a marked decrease in total cells present across fields of view. Further, on day 1 of culture, MPCs do not appear to proliferate (Fig. 6a). However, as these cells progress to myotubes, proliferation rates (as measured by the incorporation of 5-bromodeoxyuridine, BrdU, during the S-phase of the cell cycle) increase, with the most rapid increase on day 2 of culture (>3,000% over day 1; Fig. 6b) and a subsequent small decrease observed at day 3. The highest rate of cell proliferation occurs on day 9 of culture (P<0.001), with cells likely undergoing apoptosis or senescence following this point as this marks the end of the culture period.
Figure 6.
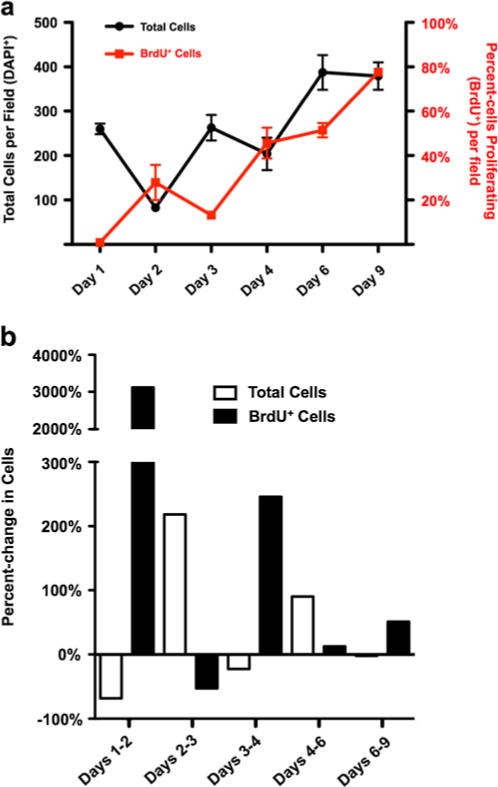
a, Total giant danio MPCs and myoblasts detected in vitro by 4′,6-diamidino-2-phenylindole nuclear staining over the culture period (black) and proliferation of giant danio myogenic precursor cells and myoblasts as determined by the percentage of 5-bromodeoxyuridine (BrdU)-positive nuclei (red), *P<0.0001 by one-way ANOVA; and b, percent-change in total cells present (white) or BrdU+ cells (black) between X and Y days (i.e., days X–Y).
Discussion
Skeletal muscle contains multinucleated myofibers that arise from embryonic muscle cells called myoblasts. Myoblasts primarily develop during embryogenesis from the paraxial mesoderm of somites that later form the dermomyotome. Eventually, myogenic cells delaminate and undergo elongation and terminal differentiation to form the functional myotome (Rescan 2005; Scaal and Wiegreffe 2006; Biressi et al. 2007; Stellabotte and Devoto 2007). Postlarvally, muscle cell proliferation leading to skeletal muscle growth and regeneration is the result of the activation of adult skeletal muscle-specific stem cells referred to as myogenic precursor cells (in fish) or myosatellite cells in most other animals, including mammals (Moss and Leblond 1971; Lemischka 1999; Charge and Rudnicki 2004; Zammit et al. 2006a). Following proper stimuli, such as exercise or injury to underlying myofibers, myogenic precursor cells become active, proliferate, and can fuse with existing myofibers to become new myonuclei (Moss and Leblond 1971; Seale and Rudnicki 2000; Hawke and Garry 2001), a process referred to as hypertrophy.
While hypertrophy of existing myofibers is the primary skeletal muscle growth mechanism exhibited by terrestrial mammals and zebrafish, many teleost fish are able to undergo postlarval muscle fiber hyperplasia as well (Mommsen 2001). To accomplish this, MPCs proliferate to generate fields of myoblasts that, in turn, form nascent myofibers. In adult teleost fish, small diameter fibers can be seen interspersed among larger diameter fibers, a phenotype referred to as mosaic hyperplasia (Alfei et al. 1989; Rescan 2005). Adult giant danio, even at 1–2 yr of age, can resume growth in response to a stimulus (e.g., growth hormone treatment) followed by mosaic hyperplasia, a characteristic not seen to an appreciable degree in zebrafish (Biga and Goetz 2006). Although it is hypothesized that MPCs play a role in this mosaic hyperplasia, the mechanisms underlying this process are unknown. Isolating MPCs from these fish is possible and describing their behavior in vitro is the first step to understanding the fate choice(s) exhibited by these cells. We hypothesize that MPC-specific markers (paired box factors Pax3 and Pax7, the receptor for hepatocyte growth factor, c-Met, and the proteoglycan syndecan-4) and myogenic regulatory factors (MyoD1, Myf5, and myogenin) determine whether a MPC (or the resulting myoblasts, rather) fuses with other myoblasts or with existing myofibers. The differential expression of these factors documented here suggests that they may in fact play a role in determining a hypertrophic and/or hyperplastic phenotype.
Paired-box transcription factors exhibit differential expression and indicate the presence of myogenic precursor cells throughout the primary culture system
Myosatellite cells in mammals are characterized by the expression of Pax7 (Seale et al. 2000) and CD34, but do not express detectable levels of Myf5 or MyoD1 proteins (Beauchamp et al. 2000). In mammals, Pax7 is expressed by quiescent, activated, and proliferating myosatellite cells, but not in myonuclei (Seale et al. 2000; Collins et al. 2005; Relaix et al. 2005; Shefer et al. 2006; Zammit et al. 2006b). Pax7 was thought to be required for the specification of myosatellite cells (Seale et al. 2004), although other work has shed doubt on this hypothesis (Oustanina et al. 2004). In zebrafish, Pax7+ nuclei have been identified in the trunk musculature (Stellabotte et al. 2007) and in pectoral fin buds (Patterson et al. 2008), utilizing the same Pax7 antibody detailed in this report. In adult giant danio (as opposed to the aforementioned larval zebrafish), Pax7 appears to be present in MPCs and quickly downregulated as cells progress to myoblasts. Pax3, a paralog to Pax7, is believed to be important in limb muscle development (Relaix et al. 2004) and is expressed sporadically in mammalian myosatellite cells, particularly in the diaphragm (Relaix et al. 2006). In avian muscle, MPCs located in muscle spindles also express Pax3 and may be involved in maintaining myofibers (Kirkpatrick et al. 2010). Pax3 may also play a role in inducing differentiation (Young and Wagers 2010), a finding supported by positive Pax3 staining in differentiating giant danio myoblasts. Interestingly, in the immortalized C2C12 myogenic cell line commonly used in investigations of myogenesis, Pax3 attenuates differentiation, while ectopic expression induces differentiation in primary murine myoblasts (Young and Wagers 2010).
In fish, investigations of Pax genes and their functions have just begun. In indeterminate-growing Atlantic salmon (Salmo salar) primary myoblast cultures, Pax7 mRNA peaks around day 8 of culture and the authors attributed this to cell population renewal (Bower and Johnston 2010). Similarly, giant danio cell proliferation peaks around day 9; however, we have been unable to detect Pax7 expression in myotubes. It is important to note that the antibody used to detect Pax7 expression in this study was made against chicken (Gallus gallus) Pax7, although it has been used in zebrafish (Nyholm et al. 2007; Seger et al. 2011) and Xenopus studies (Vivien et al. 2012). In zebrafish, several isoforms and splice variants of Pax7 and Pax3 have been identified (Seo et al. 1998). The most obvious reason for the lack of Pax7 expression is that myotubes (at least in mammals) do not express this protein (Seale et al. 2000). However, it is also possible that the Pax7 protein expressed by giant danio MPCs is an isoform unrecognized by this antibody or that Pax7a and Pax7b mRNAs are targeted by miRNAs, as was recently demonstrated with its paralog, Pax3 (Boutet et al. 2012). Further, Pax3 may be more applicable to adult growth and myofiber maintenance (Kirkpatrick et al. 2010), as studies in larval zebrafish have revealed that Pax7+ MPCs appear to respond to injury while Pax3+ MPCs remain largely unchanged (Seger et al. 2011). This postulation appears to be supported by recent work in mice, demonstrating that, while Pax7 is critical during the fetal and neonatal periods, it is dispensable in adult animals (Lepper et al. 2009).
MPC-specific markers are expressed differentially during in vitro myogenesis
The proto-oncogene c-Met, a receptor tyrosine kinase specific for hepatocyte growth factor/scatter factor (HGF/SF), has been identified in MPCs (Allen et al. 1995; Cornelison and Wold 1997), immortal myoblasts of the C2C12 cell line (Anastasi et al. 1997) and in MyoD1−/− primary myoblasts (Sabourin et al. 1999). Other works have demonstrated that HGF/SF (ligand for c-Met) functions in a concentration-dependent manner, with low concentrations promoting activation (Tatsumi et al. 1998) and high concentrations yielding quiescence (Yamada et al. 2010) of myosatellite cells in mammals. c-Met reactivity in MPCs has been identified in another indeterminate growing teleost, the Atlantic salmon (Johnston et al. 2000). Of proliferating cells in these fish (as determined by PCNA expression), 78% were c-Met+ (Johnston et al. 2003). In giant danio, c-Met is expressed in myoblasts and myotubes in a duplex of puncti, suggesting possible dimerization (Fig. 4). In some instances, immunostaining for c-Met appears nuclear; however, staining could indicate peripheral localization and does not rule out cytoplasmic or plasma membrane localization. The two-dimensional nature of our microscopy confounds our ability to describe this pattern of staining further; however, preliminary evidence generated using a Zeiss inverted Axio Observer Z1 imaging microscope and z-stacking (generously provided by the Advanced Imaging and Microscopy core center of North Dakota State University) indicates that this staining is intracellular, but not nuclear (data not shown). Primary myoblasts adhere to the culture substratum and therefore are not suspended in the culture medium. Previous c-Met localization demonstrates a halo-like or punctuated expression patterns surrounding the nuclei in suspended cells (Knudsen et al. 2009), suggesting an important potential feature of relative association of c-Met and the nucleus. The relative lack of cytoplasm in these cells may lead to a potential in vitro artifact in primary myoblasts that appears as nuclear staining, when in fact the staining is more likely membranous with close proximity to the nucleus. In human cells reared in suspension, c-Met expression was shown to be similar using the same antibody employed in our study (C-28; see “Materials and Methods” above), verifying the usefulness of this antibody in piscine systems. While it would be beneficial to compare c-Met immunoreactivity in Atlantic salmon, the Johnston et al. (2003) report only showed cell numbers and not actual immunocytochemical images, although they did use an antibody provided by the same manufacturer.
In giant danio, syndecan-4 is highest in early- and late-stage myoblasts (days 3–8; Fig. 3) during culture and decreases as cells progress to myotubes. This proteoglycan has been used as a marker for myosatellite cells (Cornelison et al. 2001) and may be indirectly involved with HGF/SF signaling through c-Met, as syndencan-4−/− animals possess myosatellite cells with decreased c-Met expression (Cornelison et al. 2004). Murine syndecan-4−/− myosatellite cells continue to express c-Met in vitro; however, they fail to enter the myogenic program (Cornelison et al. 2004), suggesting a specific role for syndecan-4 in myogenesis. Syndecan-4 marks Pax7+ and c-Met+ myosatellite cells (Bosnakovski et al. 2008; Tanaka et al. 2009). In giant danio culture, presence of syndecan-4+ (namely at the myotube stage) might suggest involvement with self-renewal of the MPC niche (Cornelison et al. 2004; Day et al. 2009).
Myogenic regulatory factor expression indicates myogenic lineage progression and myoblast proliferation and differentiation
MyoD1, the most studied myogenic regulatory factor (MRF), appears to be the archetypal myogenic basic-helix-loop-helix transcription factor and is expressed in organisms ranging from Drosophila melanogaster (Wei et al. 2007) to humans (Scrable et al. 1990). In giant danio, at least in vitro, this MRF appears to be constitutively expressed in myoblasts. This appears to be consistent with other primary culture systems isolated from mammalian and avian species (Halevy et al. 2004; Yablonka-Reuveni and Anderson 2006). In addition, this demonstrates known myogenic protein expression in MPCs and myoblasts isolated by our protocol from adult giant danio (Fig. 4), confirming the myogenic nature of this system. In addition, MyoD1 is present in later stage (which appear to be differentiated) myoblasts and co-expressed with Pax3 in giant danio. In mammalian and avian primary myoblast culture systems, Pax7+/MyoD1+ cells are present during the myoblast stage of myogenesis. In day 7 and 11 chicken myoblast cultures, Pax7 is co-expressed with MyoD1 and myogenin (Halevy et al. 2004); however, many studies, including that by Halevy and colleagues, report much longer culture periods than our cells appear to tolerate. The late expression of MyoD1 seen in day 9 differentiating cells may be due to multiple isoforms of this MRF. In salmonid fishes, there are at least three known MyoD1 isoforms that are differentially regulated during myoblast proliferation and differentiation (Macqueen and Johnston 2008). Zebrafish appear to possess only one MyoD isoform, although the unsequenced genome of the giant danio may reveal a similar pattern of isoform diversification as that seen in salmonids and fishes like medaka, Takifugu, and Tetraodon (Macqueen and Johnston 2008). Additionally, the polyclonal antibody used to detect MyoD1 expression in this study most likely does not distinguish between different MyoD1 isoforms; therefore, the constitutive expression observed might be confounded by variation in isoform expression.
Compared to other systems (i.e., mammalian primary myoblasts), Myf5 expression is isolated and limited in giant danio myoblasts (if present at all, as expression is more consistent with background than true staining), with expression peaking early (albeit sparsely) with proliferation of myoblasts followed by little to no expression in differentiating cells (Fig. 6). The antisera used in this study was raised against a Myf5 epitope of zebrafish origin (see “Materials and Methods”), and sequence analysis from our laboratory indicates that the giant danio Myf5 ortholog is highly conserved between these two species (data not shown). Further, Myf5 specificity has been demonstrated by Western blot and in vitro using zebrafish primary myoblasts (data not shown). Myf5 is thought to set the irreversible commitment to myogenesis, as opposed to self-renewal (Kuang et al. 2007). Interestingly, lower levels of Myf5 have been shown to result in a ‘priming’ effect in MPCs and that haploinsufficiency of Myf5 results in enhanced self-renewal (Gayraud-Morel et al. 2012). At all stages of adult giant danio myogenesis, the nearly absent expression of Myf5 may suggest a more ‘potent’ nature of these cells, as compared to those isolated from mammalian systems. Alternatively, studies in embryonic zebrafish have suggested that MyoD1 is important in the specification of fast muscle MPCs (Coutelle et al. 2001), which may explain the apparent dominant role of MyoD1 in our fast-glycolytic (‘white’) muscle-derived culture system. Unlike in mammalian systems, slow- and fast-twitch muscle fibers can be separated at the macroscopic level in giant danio and other teleosts, making the study of fish musculature even more attractive.
Myogenin expression appears to be low (but present) in MPCs and continues to increase in later-stage cells. Studies in mammalian systems have identified myogenin as a basic helix-loop-helix transcription factor upregulated by MyoD1 early in differentiation (Cao et al. 2006). Knockout studies in mice have demonstrated the critical role for myogenin in differentiation, as myogenin−/− embryos do not develop differentiated muscle (Nabeshima et al. 1993). In mammals, over-expression of Pax7 results in the attenuation of myogenin expression in myotubes (Olguin and Olwin 2004), suggesting that myogenin leads to cell cycle withdrawal. In giant danio myoblasts, myogenin expression appears much sooner than expected, suggesting that myogenin may play a more diverse role in teleost fish. Further, proliferation rates of myoblasts increase throughout the culture period, as demonstrated by Fig. 6. This trend is not seen in mammalian species and may implicate myogenin in a novel regulatory mechanism related to indeterminate growth. Data from amphibians (Xenopus laevis) also suggest that myogenin activates structural genes (Charbonnier et al. 2002) and we speculate this is likely the case in giant danio under in vivo conditions. We have not observed spontaneous contraction of myotubes in vitro and this appears to hold true for other teleost species as well, supporting our finding that myotubes may not fully differentiate in vitro. The apparent expression of myogenin in giant danio myoblast cultures may also be fiber-type-specific, as myogenin mRNA localizes to ‘red,’ slow-oxidative myofibers in rainbow trout and is relatively scarce in ‘white,’ fast-glycolytic muscle (Rescan et al. 1995). However, studies in zebrafish have demonstrated that myogenin expression plays an important role in the formation of these fast myofibers (Hinits et al. 2009). Thus, it is important to distinguish our study from previous investigations of myogenin expression. The fish from which MPCs were isolated for this work were adult fish while the zebrafish studies we examined in the literature involved embryonic and larval fish (zebrafish) or very young juvenile fish (salmonids, such as rainbow trout). It has been well-demonstrated that embryonic and larval myogenesis are quite different from postembryonic, postnatal/postlarval myogenesis (Le Grand and Rudnicki 2007).
Myostatin, a TGF-β family member, appears to be expressed despite cell stage
Interestingly, myostatin, a member of the TGF-β superfamily (McPherron et al. 1997), appears to be expressed throughout the giant danio myogenic program, at apparently high levels (Fig. 5). Previous studies indicate that myostatin is important in maintaining MPC quiescence (McCroskery et al. 2003; McFarland et al. 2006; McFarlane et al. 2008) and may explain the apparently high expression levels in day 1 MPCs. Several reports have described the potential for myostatin to being sequestered to the cell membrane via proteogylcans, like decorin, and this mechanism may be responsible for local functional regulation (Miura et al. 2006; Nishimura et al. 2007; Zhu et al. 2007; Kishioka et al. 2008). In fish, myostatin function is less clear. Myostatin mRNA data from our laboratory (data not shown) suggest a decrease in myostatin protein levels would be detected during rapid proliferation; however, the actual pattern of myostatin protein expression does not support this finding, as myostatin protein does not appear to decrease in proliferating cells. Further, the high levels of expression, including what may at first appear to be nuclear, are likely due to a similar phenomenon as that seen with c-Met staining (see above). This antibody, however, is of piscine origin (raised against a brook trout epitope of myostatin-1b; see “Materials and Methods”) and the high degree of conservation between salmonid and danionin myostatin orthologs (>75% identity) validates the use of this antibody. Of course, expression does not necessarily equal activity. It is likely that a portion of the detected myostatin in vitro is sequestered by proteoglycans during proliferating stages, as suggested by mammalian experiments (Kishioka et al. 2008). It is therefore hypothesized that a proteoglycan, like decorin, may be acting as a sequestering molecule at these stages (Nishimura et al. 2007). In other fishes, such as Sparus aurata, myostatin expression was detectable at day 11 of culture (Funkenstein et al. 2006), a finding similar to ours. In salmonids, myostatin protein has been identified in vitro (Ostbye et al. 2007) but most studies have used quantitative PCR analysis to study myostatin function at the tissue level and not at the single cell level, as we report here.
Isolated satellite cells proliferate following seeding and continue to proliferate through differentiation
Data from other teleost fishes indicate that MPCs proliferate in vitro, reaching a plateau near the point where cultured cells begin to apoptose or undergo senescence (Greenlee et al. 1995; Rescan et al. 1995; Codina et al. 2008; Levesque et al. 2008; Bower and Johnston 2010). However, other reports have indicated that MPCs do not proliferate under culture conditions (Koumans et al. 1990; Matschak and Stickland 1995). In giant danio, total cells present in vitro markedly decreases between days 1 and 2 of culture (Fig. 6). We attribute this to our method of cell isolation. Unlike methods employed in better-characterized model organisms such as mice, isolation of fish MPCs relies on physical methods, not flow cytometry based on surface protein or transgenic GFP expression. It is likely that myofibroblasts are also isolated using our protocol; however, 90–100% of cells per field at day 2 of culture are MyoD1+, indicating that they are not fibroblasts. Observations from our laboratory indicate that these fibroblasts are only present during days 1 and 2 of culture (data not shown), as they are readily identified by their ‘star-like’ morphology. Further, our in vitro system relies on laminin, a protein for which MPCs have a much greater affinity than fibroblasts. Thorough washing of newly seeded cells is an effective method for removing myofibroblasts, as well. Alternatively, these cells may represent a subpopulation of MPCs that our culture conditions do not support. In mammals, such MPC populations have been described (Biressi and Rando 2010).
From total cell counts, it appears that two ‘waves’ of proliferation occur during giant danio myogenesis in vitro. Days 3 and 4 of culture are representative of the ‘early-myoblast’ stage, as determined by the size (i.e., small) and spindle-like shape of the myoblasts. Day 6–9 myoblasts are much larger, although they possess a similar spindle-like morphology. Cell count data indicate a proliferative expansion precedes each of these periods (Fig. 6). Before the ‘early’-myoblast period, proliferation (as judged by cells present in culture) increases by 218%, with a smaller yet important increase of 90% prior to the ‘late-stage’ myoblast period.
Giant danio myoblasts proliferate throughout culture, as demonstrated by BrdU incorporation (Fig. 6). In vitro, giant danio myoblasts reach a proliferative plateau around day 9 of culture. A small, yet statistically insignificant, decrease in proliferation appears to be seen between days 2 and 3. Otherwise, proliferation rapidly increases throughout myogenesis. Putative Pax3 expression appears to be present throughout proliferation and may contribute to the high level of proliferation seen in most stages of in vitro myogenesis, especially in late-stage myoblasts, as suggested by studies in mice (Conboy and Rando 2002). Interestingly, myogenin expression in mammalian myoblasts is associated with subsequent cell cycle withdrawal and fusion (Andres and Walsh 1996). While we did not double-label cells to detect myogenin+/BrdU+ myoblasts, the high degree of myogenin+ myoblasts in later stages of culture (>99%, days 6–11) and the high degree of BrdU incorporation (>51%; Fig. 6) strongly suggests that a significant portion of the proliferation fraction of cells are both expressing the MRF myogenin and undergoing DNA synthesis.
Conclusions
While it may be tempting to draw conclusions by juxtaposing determinate-growing terrestrial organisms like mice and fowl and aquatic indeterminate-growing giant danio, a multitude of confounding variables make such a comparison difficult. The surprising expression pattern of Pax3 suggests an ‘embryonic-like’ nature of giant danio MPCs. This expression pattern, coupled with the embryonic morphology of these cells (Cossu and Biressi 2005; Biressi et al. 2007) and their largely Pax7-independent proliferation, may suggest that giant danio MPCs retain the ability to fuse with existing fibers or form nascent fibers, as fetal myoblasts do during secondary myogenesis in the mouse (Stockdale 1992) because they maintain the regulatory pathways present during embryogenesis (see Fig. 7 for working model). Further, Kirkpatrick et al. (2010) identified intrafusal Pax3+ nuclei in chickens and suggested that these nuclei may belong to MPCs responsible for maintaining muscle mass. Concurring with our data, the authors even described these cells and the myofibers associated with them as “immature” (Kirkpatrick et al. 2010). As giant danio and most species of teleost fish are able to grow throughout their lifespans, Pax3 may play an important role in these organisms’ ability to increase muscle mass via nascent myofiber recruitment, despite the aging process. This, coupled with scarce expression of Myf5, if any at all, may suggest a mechanism for self-renewal of MPCs in indeterminate-growing species. Further, the high degree of proliferating myoblasts expressing myogenin may point to a novel function for these proteins in indeterminate-growing species. However, we are unaware of any data from adult zebrafish demonstrating the expression of Pax3 and/or myogenin in vitro or in vivo, making characterization of adult D. rerio myogenesis a priority.
Figure 7.
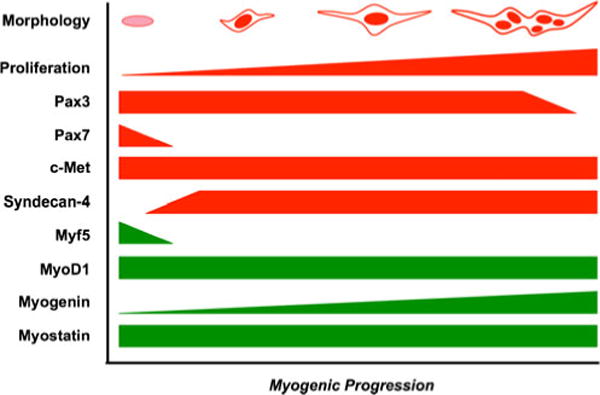
Schematic of relative cell proliferation (red) and expression levels of paired-box factors, myogenic precursor cell-specific markers (red), myogenic regulatory factors, and myostatin (green) in giant danio myogenic precursor cells, myoblasts, and differentiating myotubes.
Acknowledgments
We would like to thank Drs. Josep Planas and Juan Castillo for their assistance and direction with the primary myoblast cultures, as well as Zachary Fowler, Brooke Franzen, Nathan Froehlich, Kira Marshall, Ethan Remily, and Sinibaldo Romero for their technical assistance in isolating MPCs from numerous fish. Thanks are also due to Dr. Jodie Haring, Dr. Joseph Provost, and Naomi Light for their assistance in cell imaging. Funds for this work were provided to PRB by the Center for Protease Research NIH Grant # 2P20 RR015566, NIH NIAMS Grant # R03AR055350, and NDSU Advance FORWARD NSF Grant #HRD-0811239. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Contributor Information
Jacob Michael Froehlich, Department of Biology, University of Alabama at Birmingham, 1300 University Blvd CH464, Birmingham, AL 35294, USA.
Nicholas J. Galt, Department of Biology, University of Alabama at Birmingham, 1300 University Blvd CH464, Birmingham, AL 35294, USA
Matthew J. Charging, Department of Biological Sciences, North Dakota State University, Fargo, ND, USA
Ben M. Meyer, Department of Biological Sciences, North Dakota State University, Fargo, ND, USA
Peggy R. Biga, Email: pegbiga@uab.edu, Department of Biology, University of Alabama at Birmingham, 1300 University Blvd CH464, Birmingham, AL 35294, USA
References
- Alfei L, Maggi F, Parvopassu F, Bertoncello G, De Vita R. Postlarval muscle growth in fish: a DNA flow cytometric and morphometric analysis. Basic Appl Histochem. 1989;33(2):147–158. [PubMed] [Google Scholar]
- Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol. 1995;165(2):307–312. doi: 10.1002/jcp.1041650211. [DOI] [PubMed] [Google Scholar]
- Anastasi S, Giordano S, Sthandier O, Gambarotta G, Maione R, Comoglio P, Amati P. A natural hepatocyte growth factor/scatter factor autocrine loop in myoblast cells and the effect of the constitutive Met kinase activation on myogenic differentiation. J Cell Biol. 1997;137(5):1057–1068. doi: 10.1083/jcb.137.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres V, Walsh K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol. 1996;132(4):657–666. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151(6):1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biga PR, Cain KD, Hardy RW, Schelling GT, Overturf K, Roberts SB, Goetz FW, Ott TL. Growth hormone differentially regulates muscle myostatin1 and −2 and increases circulating cortisol in rainbow trout (Oncorhynchus mykiss) Gen Comp Endocrinol. 2004;138(1):32–41. doi: 10.1016/j.ygcen.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Biga PR, Goetz FW. Zebrafish and giant danio as models for muscle growth: determinate vs. indeterminate growth as determined by morphometric analysis. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1327–1337. doi: 10.1152/ajpregu.00905.2005. [DOI] [PubMed] [Google Scholar]
- Biressi S, Molinaro M, Cossu G. Cellular heterogeneity during vertebrate skeletal muscle development. Dev Biol. 2007;308(2):281–293. doi: 10.1016/j.ydbio.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Biressi S, Rando TA. Heterogeneity in the muscle satellite cell population. Semin Cell Dev Biol. 2010;21(8):845–854. doi: 10.1016/j.semcdb.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnakovski D, Xu Z, Li W, Thet S, Cleaver O, Perlingeiro RC, Kyba M. Prospective isolation of skeletal muscle stem cells with a Pax7 reporter. 3204. 2008;12:3194. doi: 10.1634/stemcells.2007-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutet SC, Cheung TH, Quach NL, Liu L, Prescott SL, Edalati A, Iori K, Rando TA. Alternative polyadenylation mediates microRNA regulation of muscle stem cell function. Cell Stem Cell. 2012;10(3):327–336. doi: 10.1016/j.stem.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower NI, Johnston IA. Paralogs of Atlantic salmon myoblast determination factor genes are distinctly regulated in proliferating and differentiating myogenic cells. Am J Physiol Regul Integr Comp Physiol. 2010;298(6):R1615–1626. doi: 10.1152/ajpregu.00114.2010. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Vincent SD. Distinct and dynamic myogenic populations in the vertebrate embryo. Curr Opin Genet Dev. 2009;19(5):444–453. doi: 10.1016/j.gde.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Cao Y, Kumar RM, Penn BH, Berkes CA, Kooperberg C, Boyer LA, Young RA, Tapscott SJ. Global and gene-specific analyses show distinct roles for Myod and Myog at a common set of promoters. EMBO J. 2006;25(3):502–511. doi: 10.1038/sj.emboj.7600958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonnier F, Gaspera BD, Armand AS, Van der Laarse WJ, Launay T, Becker C, Gallien CL, Chanoine C. Two myogenin-related genes are differentially expressed in Xenopus laevis myogenesis and differ in their ability to transactivate muscle structural genes. J Biol Chem. 2002;277(2):1139–1147. doi: 10.1074/jbc.M107018200. [DOI] [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84(1):209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Codina M, Garcia dela serrana D, Sanchez-Gurmaches J, Montserrat N, Chistyakova O, Navarro I, Gutierrez J. Metabolic and mitogenic effects of IGF-II in rainbow trout (Oncorhynchus mykiss) myocytes in culture and the role of IGF-II in the PI3K/Akt and MAPK signalling pathways. Gen Comp Endocrinol. 2008;157(2):116–124. doi: 10.1016/j.ygcen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122(2):289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3(3):397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol. 2001;239(1):79–94. doi: 10.1006/dbio.2001.0416. [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Wilcox-Adelman SA, Goetinck PF, Rauvala H, Rapraeger AC, Olwin BB. Essential and separable roles for Syndecan-3 and Syndecan-4 in skeletal muscle development and regeneration. Genes Dev. 2004;18(18):2231–2236. doi: 10.1101/gad.1214204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191(2):270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Cossu G, Biressi S. Satellite cells, myoblasts and other occasional myogenic progenitors: possible origin, phenotypic features and role in muscle regeneration. Semin Cell Dev Biol. 2005;16(4–5):623–631. doi: 10.1016/j.semcdb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Coutelle O, Blagden CS, Hampson R, Halai C, Rigby PW, Hughes SM. Hedgehog signalling is required for maintenance of myf5 and myoD expression and timely terminal differentiation in zebrafish adaxial myogenesis. Dev Biol. 2001;236(1):136–150. doi: 10.1006/dbio.2001.0193. [DOI] [PubMed] [Google Scholar]
- Day K, Paterson B, Yablonka-Reuveni Z. A distinct profile of myogenic regulatory factor detection within Pax7+cells at S phase supports a unique role of Myf5 during posthatch chicken myogenesis. Dev Dyn. 2009;238(4):1001–1009. doi: 10.1002/dvdy.21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauconneau B, Paboeuf G. Effect of fasting and refeeding on in vitro muscle cell proliferation in rainbow trout (Oncorhynchus mykiss) Cell Tissue Res. 2000;301(3):459–463. doi: 10.1007/s004419900168. [DOI] [PubMed] [Google Scholar]
- Funkenstein B, Balas V, Skopal T, Radaelli G, Rowlerson A. Long-term culture of muscle explants from. Sparus aurata Tissue Cell. 2006;38(6):399–415. doi: 10.1016/j.tice.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Gayraud-Morel B, Chretien F, Jory A, Sambasivan R, Negroni E, Flamant P, Soubigou G, Coppee JY, Di Santo J, Cumano A, Mouly V, Tajbakhsh S. Myf5 haploinsufficiency reveals distinct cell fate potentials for adult skeletal muscle stem cells. J Cell Sci. 2012;125(Pt 7):1738–1749. doi: 10.1242/jcs.097006. [DOI] [PubMed] [Google Scholar]
- Greenlee A, Dodson M, Yablonka-Reuveni Z, Kersten C, Cloud J. In vitro differentiation of myoblast from skeletal muscle of rainbow trout. J Fish Biol. 1995;46:731–747. [Google Scholar]
- Halevy O, Piestun Y, Allouh MZ, Rosser BW, Rinkevich Y, Reshef R, Rozenboim I, Wleklinski-Lee M, Yablonka-Reuveni Z. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev Dyn. 2004;231(3):489–502. doi: 10.1002/dvdy.20151. [DOI] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91(2):534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Hightower LE, Renfro JL. Recent applications of fish cell culture to biomedical research. J Exp Zool. 1988;248(3):290–302. doi: 10.1002/jez.1402480307. [DOI] [PubMed] [Google Scholar]
- Hinits Y, Osborn DP, Hughes SM. Differential requirements for myogenic regulatory factors distinguish medial and lateral somitic, cranial and fin muscle fibre populations. Development. 2009;136(3):403–414. doi: 10.1242/dev.028019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston IA, Manthri S, Smart A, Campbell P, Nickell D, Alderson R. Plasticity of muscle fibre number in seawater stages of Atlantic salmon in response to photoperiod manipulation. J Exp Biol. 2003;206(Pt 19):3425–3435. doi: 10.1242/jeb.00577. [DOI] [PubMed] [Google Scholar]
- Johnston IA, McLay HA, Abercromby M, Robins D. Phenotypic plasticity of early myogenesis and satellite cell numbers in Atlantic salmon spawning in upland and lowland tributaries of a river system. J Exp Biol. 2000;203(Pt 17):2539–2552. doi: 10.1242/jeb.203.17.2539. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick LJ, Yablonka-Reuveni Z, Rosser BW. Retention of Pax3 expression in satellite cells of muscle spindles. J Histochem Cytochem. 2010;58(4):317–327. doi: 10.1369/jhc.2009.954792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishioka Y, Thomas M, Wakamatsu J, Hattori A, Sharma M, Kambadur R, Nishimura T. Decorin enhances the proliferation and differentiation of myogenic cells through suppressing myostatin activity. J Cell Physiol. 2008;215(3):856–867. doi: 10.1002/jcp.21371. [DOI] [PubMed] [Google Scholar]
- Knudsen BS, Zhao P, Resau J, Cottingham S, Gherardi E, Xu E, Berghuis B, Daugherty J, Grabinski T, Toro J, Giambernardi T, Skinner RS, Gross M, Hudson E, Kort E, Lengyel E, Ventura A, West RA, Xie Q, Hay R, Woude GV, Cao B. A novel multipurpose monoclonal antibody for evaluating human c-Met expression in preclinical and clinical settings. Appl Immunohistochem Mol Morphol. 2009;17(1):57–67. doi: 10.1097/PAI.0b013e3181816ae2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumans JTM, Akster H, Dulos G, Osse JWM. Myosatellite cells of Cyprinid carpio(Teleosti) in vitro: isolation, recognition, and differentiation. Cell Tissue Res. 1990;261:173–181. [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129(5):999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand F, Rudnicki MA. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol. 2007;19(6):628–633. doi: 10.1016/j.ceb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemischka I. The power of stem cells reconsidered? Proc Natl Acad Sci U S A. 1999;96(25):14193–14195. doi: 10.1073/pnas.96.25.14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Conway SJ, Fan CM. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. 2009;460(7255):627–631. doi: 10.1038/nature08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque HM, Shears MA, Fletcher GL, Moon TW. Myogenesis and muscle metabolism in juvenile Atlantic salmon (Salmo salar) made transgenic for growth hormone. J Exp Biol. 2008;211(Pt 1):128–137. doi: 10.1242/jeb.006890. [DOI] [PubMed] [Google Scholar]
- Macqueen DJ, Johnston IA. An update on MyoD evolution in teleosts and a proposed consensus nomenclature to accommodate the tetraploidization of different vertebrate genomes. PLoS One. 2008;3(2):e1567. doi: 10.1371/journal.pone.0001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschak TW, Stickland NC. The growth of Atlantic salmon (Salmo salar L.) myosatellite cells in culture at two different temperatures. Experientia. 1995;51(3):260–266. doi: 10.1007/BF01931109. [DOI] [PubMed] [Google Scholar]
- McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162(6):1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane C, Hennebry A, Thomas M, Plummer E, Ling N, Sharma M, Kambadur R. Myostatin signals through Pax7 to regulate satellite cell self-renewal. Exp Cell Res. 2008;314(2):317–329. doi: 10.1016/j.yexcr.2007.09.012. [DOI] [PubMed] [Google Scholar]
- McFarland DC, Velleman SG, Pesall JE, Liu C. Effect of myostatin on turkey myogenic satellite cells and embryonic myoblasts. Comp Biochem Physiol A Mol Integr Physiol. 2006;144(4):501–508. doi: 10.1016/j.cbpa.2006.04.020. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387(6628):83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- Meyer A, Biermann CH, Orti G. The phylogenetic position of the zebrafish (Danio rerio), a model system in developmental biology: an invitation to the comparative method. Proc Biol Sci. 1993;252(1335):231–236. doi: 10.1098/rspb.1993.0070. [DOI] [PubMed] [Google Scholar]
- Miura T, Kishioka Y, Wakamatsu J, Hattori A, Hennebry A, Berry CJ, Sharma M, Kambadur R, Nishimura T. Decorin binds myostatin and modulates its activity to muscle cells. Biochem Biophys Res Commun. 2006;340(2):675–680. doi: 10.1016/j.bbrc.2005.12.060. [DOI] [PubMed] [Google Scholar]
- Mommsen TP. Paradigms of growth in fish. Comp Biochem Physiol B Biochem Mol Biol. 2001;129:2–3. 207–219. doi: 10.1016/s1096-4959(01)00312-8. [DOI] [PubMed] [Google Scholar]
- Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971;170(4):421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Mulvaney DR, Cyrino JEP. Establishment of channel catfish satellite cell cultures. Basic Appl Myol. 1995;5(1):65–70. [Google Scholar]
- Nabeshima Y, Hanaoka K, Hayasaka M, Esumi E, Li S, Nonaka I. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature. 1993;6437:532–535. doi: 10.1038/364532a0. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Oyama K, Kishioka Y, Wakamatsu J, Hattori A. Spatiotemporal expression of decorin and myostatin during rat skeletal muscle development. Biochem Biophys Res Commun. 2007;361(4):896–902. doi: 10.1016/j.bbrc.2007.07.104. [DOI] [PubMed] [Google Scholar]
- Nyholm MK, Wu SF, Dorsky RI, Grinblat Y. The zebrafish zic2a–zic5 gene pair acts downstream of canonical Wnt signaling to control cell proliferation in the developing tectum. Development. 2007;134(4):735–746. doi: 10.1242/dev.02756. [DOI] [PubMed] [Google Scholar]
- Olguin HC, Olwin BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol. 2004;275(2):375–388. doi: 10.1016/j.ydbio.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostbye TK, Bardal T, Vegusdal A, Frang OT, Kjorsvik E, Andersen O. Molecular cloning of the Atlantic salmon activin receptor IIB cDNA—localization of the receptor and myostatin in vivo and in vitro in muscle cells. Comp Biochem Physiol Part D Genomics Proteomics. 2007;2(2):101–111. doi: 10.1016/j.cbd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J. 2004;23(16):3430–3439. doi: 10.1038/sj.emboj.7600346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SE, Mook LB, Devoto SH. Growth in the larval zebrafish pectoral fin and trunk musculature. Dev Dyn. 2008;237(2):307–315. doi: 10.1002/dvdy.21400. [DOI] [PubMed] [Google Scholar]
- Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172(1):91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M. Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes Dev. 2004;18(9):1088–1105. doi: 10.1101/gad.301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham MA. Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435(7044):948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- Rescan PY. Muscle growth patterns and regulation during fish ontogeny. Gen Comp Endocrinol. 2005;142:1–2. 111–116. doi: 10.1016/j.ygcen.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Rescan PY, Gauvry L, Paboeuf G. A gene with homology to myogenin is expressed in developing myotomal musculature of the rainbow trout and in vitro during the conversion of myosatellite cells to myotubes. FEBS Lett. 1995;362(1):89–92. doi: 10.1016/0014-5793(95)00215-u. [DOI] [PubMed] [Google Scholar]
- Roberts SB, Goetz FW. Myostatin protein and RNA transcript levels in adult and developing brook trout. Mol Cell Endocrinol. 2003;210:1–2. 9–20. doi: 10.1016/j.mce.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Sabourin LA, Girgis-Gabardo A, Seale P, Asakura A, Rudnicki MA. Reduced differentiation potential of primary MyoD−/− myogenic cells derived from adult skeletal muscle. J Cell Biol. 1999;144(4):631–643. doi: 10.1083/jcb.144.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaal M, Wiegreffe C. Somite compartments in anamniotes. Anat Embryol (Berl) 2006;211(Suppl 1):9–19. doi: 10.1007/s00429-006-0127-8. [DOI] [PubMed] [Google Scholar]
- Scrable HJ, Johnson DK, Rinchik EM, Cavenee WK. Rhabdomyosarcoma-associated locus and MYOD1 are syntenic but separate loci on the short arm of human chromosome 11. Proc Natl Acad Sci U S A. 1990;87(6):2182–2186. doi: 10.1073/pnas.87.6.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Ishibashi J, Scime A, Rudnicki MA. Pax7 is necessary and sufficient for the myogenic specification of CD45+:Sca1+stem cells from injured muscle. PLoS Biol. 2004;2(5):E130. doi: 10.1371/journal.pbio.0020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Rudnicki MA. A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev Biol. 2000;218(2):115–124. doi: 10.1006/dbio.1999.9565. [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102(6):777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Seger C, Hargrave M, Wang X, Chai RJ, Elworthy S, Ingham PW. Analysis of Pax7 expressing myogenic cells in zebrafish muscle development, injury, and models of disease. Dev Dyn. 2011;240(11):2440–2451. doi: 10.1002/dvdy.22745. [DOI] [PubMed] [Google Scholar]
- Seo HC, Saetre BO, Havik B, Ellingsen S, Fjose A. The zebrafish Pax3 and Pax7 homologues are highly conserved, encode multiple isoforms and show dynamic segment-like expression in the developing brain. Mech Dev. 1998;70:1–2. 49–63. doi: 10.1016/s0925-4773(97)00175-5. [DOI] [PubMed] [Google Scholar]
- Sepich DS, Ho RK, Westerfield M. Autonomous expression of the nic1 acetylcholine receptor mutation in zebrafish muscle cells. Dev Biol. 1994;161(1):84–90. doi: 10.1006/dbio.1994.1010. [DOI] [PubMed] [Google Scholar]
- Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294(1):50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow J, Hughes SM, Segalat L. Other model organisms for sarcomeric muscle diseases. Adv Exp Med Biol. 2008;642:192–206. doi: 10.1007/978-0-387-84847-1_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellabotte F, Devoto SH. The teleost dermomyotome. Dev Dyn. 2007;236(9):2432–2443. doi: 10.1002/dvdy.21253. [DOI] [PubMed] [Google Scholar]
- Stellabotte F, Dobbs-McAuliffe B, Fernandez DA, Feng X, Devoto SH. Dynamic somite cell rearrangements lead to distinct waves of myotome growth. Development. 2007;134(7):1253–1257. doi: 10.1242/dev.000067. [DOI] [PubMed] [Google Scholar]
- Stockdale FE. Myogenic cell lineages. Dev Biol. 1992;154(2):284–298. doi: 10.1016/0012-1606(92)90068-r. [DOI] [PubMed] [Google Scholar]
- Tanaka KK, Hall JK, Troy AA, Cornelison DD, Majka SM, Olwin BB. Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell. 2009;4(3):217–225. doi: 10.1016/j.stem.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang KL, Agnew MK, Hirt MV, Sado T, Schneider LM, Freyhof J, Sulaiman Z, Swartz E, Vidthayanon C, Miya M, Saitoh K, Simons AM, Wood RM, Mayden RL. Systematics of the subfamily Danioninae (Teleostei: Cypriniformes: Cyprinidae) Mol Phylogenet Evol. 2010;57(1):189–214. doi: 10.1016/j.ympev.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194(1):114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- van Raamsdonk W, van’t Veer L, Veeken K, te Kronnie T, de Jager S. Fiber type differentiation in fish. Mol Physiol. 1982;2:31–47. [Google Scholar]
- Vivien C, Scerbo P, Girardot F, Le Blay K, Demeneix BA, Coen L. Non-viral expression of mouse Oct4, Sox2, and Klf4 transcription factors efficiently reprograms tadpole muscle fibers in vivo. J Biol Chem. 2012;287(10):7427–7435. doi: 10.1074/jbc.M111.324368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Rong Y, Paterson BM. Stereotypic founder cell patterning and embryonic muscle formation in Drosophila require nautilus (MyoD) gene function. Proc Natl Acad Sci U S A. 2007;104(13):5461–5466. doi: 10.1073/pnas.0608739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Anderson JE. Satellite cells from dystrophic (mdx) mice display accelerated differentiation in primary cultures and in isolated myofibers. Dev Dyn. 2006;235(1):203–212. doi: 10.1002/dvdy.20602. [DOI] [PubMed] [Google Scholar]
- Yamada M, Tatsumi R, Yamanouchi K, Hosoyama T, Shiratsuchi S, Sato A, Mizunoya W, Ikeuchi Y, Furuse M, Allen RE. High concentrations of HGF inhibit skeletal muscle satellite cell proliferation in vitro by inducing expression of myostatin: a possible mechanism for reestablishing satellite cell quiescence in vivo. Am J Physiol Cell Physiol. 2010;298(3):C465–476. doi: 10.1152/ajpcell.00449.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AP, Wagers AJ. Pax3 induces differentiation of juvenile skeletal muscle stem cells without transcriptional upregulation of canonical myogenic regulatory factors. J Cell Sci. 2010;123(Pt 15):2632–2639. doi: 10.1242/jcs.061606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 2006a;54(11):1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Relaix F, Nagata Y, Ruiz AP, Collins CA, Partridge TA, Beauchamp JR. Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci. 2006b;119(Pt 9):1824–1832. doi: 10.1242/jcs.02908. [DOI] [PubMed] [Google Scholar]
- Zhu J, Li Y, Shen W, Qiao C, Ambrosio F, Lavasani M, Nozaki M, Branca MF, Huard J. Relationships between transforming growth factor-beta1, myostatin, and decorin: implications for skeletal muscle fibrosis. J Biol Chem. 2007;282(35):25852–25863. doi: 10.1074/jbc.M704146200. [DOI] [PubMed] [Google Scholar]


