Abstract
Clozapine is a particularly effective antipsychotic medication but its use is curtailed by the risk of clozapine-induced agranulocytosis/granulocytopenia (CIAG), a severe adverse drug reaction occurring in up to 1% of treated individuals. Identifying genetic risk factors for CIAG could enable safer and more widespread use of clozapine. Here we perform the largest and most comprehensive genetic study of CIAG to date by interrogating 163 cases using genome-wide genotyping and whole-exome sequencing. We find that two loci in the major histocompatibility complex are independently associated with CIAG: a single amino acid in HLA-DQB1 (126Q) (P=4.7×10−14, odds ratio, OR=0.19, 95% CI 0.12–0.29) and an amino acid change in the extracellular binding pocket of HLA-B (158T) (P=6.4×10−10, OR=3.3, 95% CI 2.3–4.9). These associations dovetail with the roles of these genes in immunogenetic phenotypes and adverse drug responses for other medications, and provide insight into the pathophysiology of CIAG.
Introduction
Clozapine-induced agranulocytosis/granulocytopenia (CIAG) is a rare adverse drug event that occurs in 1% of treated individuals and can be fatal if not detected early1. As a result, clozapine is underused despite its superior efficacy and particular utility in treatment-resistant schizophrenia2. Our goal is to identify genetic risk factors for CIAG that could enable safer and increased use of clozapine.
Because of the specific nature of this adverse drug response and the role of the major histocompatibility complex (MHC) in adverse drug responses, candidate gene studies have focused on human leukocyte antigen (HLA) alleles in CIAG.3, 4, 5, 6, 7, 8 A small exome sequencing study of CIAG was unrevealing9. While nominally significant HLA associations have been reported, no study to our knowledge has reported unequivocal statistical significance or the dissection of the complex linkage disequilibrium (LD) relationships in the MHC.
Using multiple ascertainment schemes, we assemble the largest CIAG cohort to date (163 cases, 54.0% never previously reported, Supplementary Table 1). We conduct a genome survey using whole-exome sequencing and genome-wide genotyping to test for association with CIAG (Figure 1, Supplementary Table 2). We show CIAG is associated with two independent amino acid changes in HLA-B (158T) and HLA-DQB1 (126Q). However, the odds ratios for these risk factors do not suggest immediate clinical application in predictive testing.
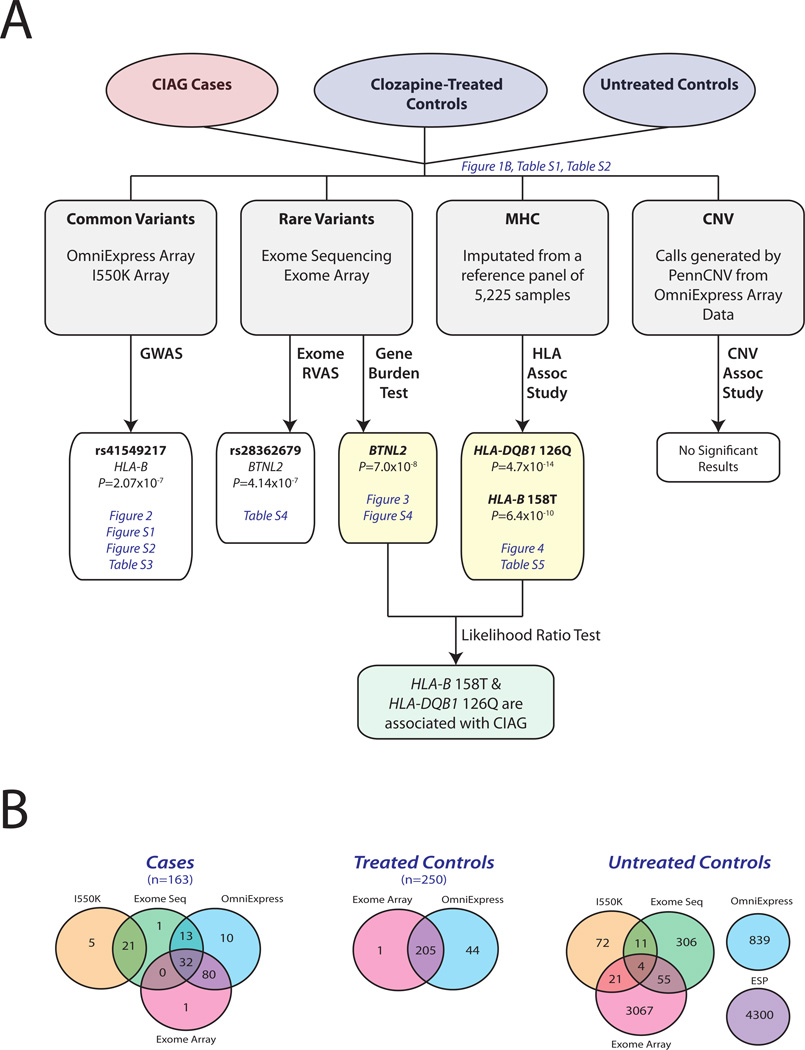
Figure 1. Study Design.
Our study design consisted of looking for genetic associations with CIAG across the minor allele frequency spectrum. (A) First, we recruited CIAG cases (red circle) and both treated and untreated controls (blue circles). Next, we used multiple technologies to genotype variants including genotyping arrays for both common and rare variants, whole-exome sequencing, imputation of classical HLA 4-digit alleles and amino acids in the major histocompatibility complex, and copy number variants. Lastly, we performed association tests for each class of variants. Results that exceeded the multiple testing threshold are colored in yellow. References to figures and tables are listed in blue italics. (B) We used multiple technologies to genotype variants including whole-exome sequencing (green), exome array (pink), OmniExpress (blue) and Illumina 550K genome-wide arrays (orange). In addition, we used allele counts from the Exome Sequencing Project as extra control chromosomes (purple). As demonstrated by the Venn diagrams, the majority of cases (n=163) and treated controls (n=250) were genotyped on more than one platform.
Results
Genome-wide common variant association study
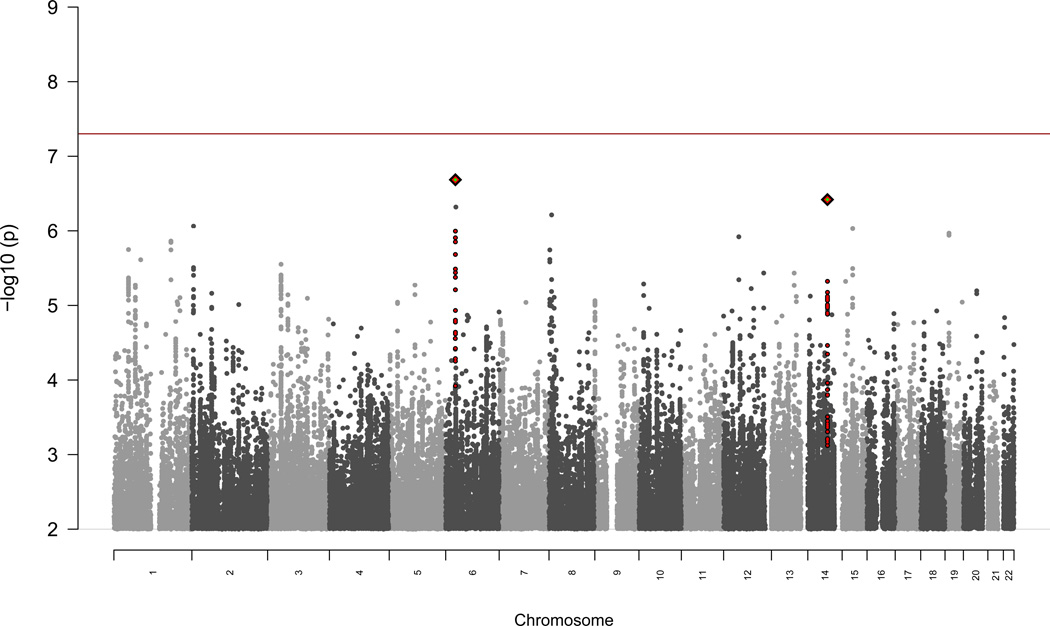
We performed a genome-wide association study of 8,809,853 genotyped and imputed common variants in 161 CIAG cases (2 additional cases had exome sequencing or exome array data) and 1,196 controls of European ancestry. After controlling for ancestry (Supplementary Fig. 1), no loci exceeded genome-wide significance (P < 5×10−8, Figure 2). Our sample size was powered to detect common genetic variants with effect sizes of clinical utility (e.g., power was >99% for an odds ratio > 4 and MAF > 10%) (Supplementary Fig. 2). The SNP with the best evidence for association was in an intron in HLA-B (rs41549217, P=2.07×10−7, OR=4.66, 95% CI 2.6–8.3, Supplementary Data 1). We specifically examined the distribution of p-values of SNPs previously shown to be associated with immune-mediated diseases, but did not detect any enrichment (Supplementary Fig. 3). Evaluation of copy number variation did not reveal any associations with CIAG. No protein encoded by a gene implicated by GWAS is known to bind clozapine10.
Figure 2. GWAS Manhattan Plot.
We performed a genome-wide association study for 161 cases and 1196 controls. We did not find any loci that exceeded the threshold for genome-wide significance of 5×10−8 (red line). The most significant SNP is rs41549217, in an intron of HLA-B (P=2.07×10−7, logistic regression).
Exome-wide rare variant association study
We tested protein-coding variants (MAF < 5%) for association using whole-exome sequencing data from 67 CIAG cases and 376 untreated population controls. For variants that were also genotyped on the exome array, we merged the sequencing data with exome array data from 81 CIAG cases and 3,294 controls of European ancestry. We also used raw count data from ~4,300 samples of European ancestry from the NHLBI Exome Sequencing Project as an additional control reference to increase statistical power11. The top finding is a low frequency missense variant in BTNL2 annotated as benign by PolyPhen-2 (rs28362679, P=4.14×10−7, OR=3.9, 95% CI 1.2–12.9, Supplementary Data 2).
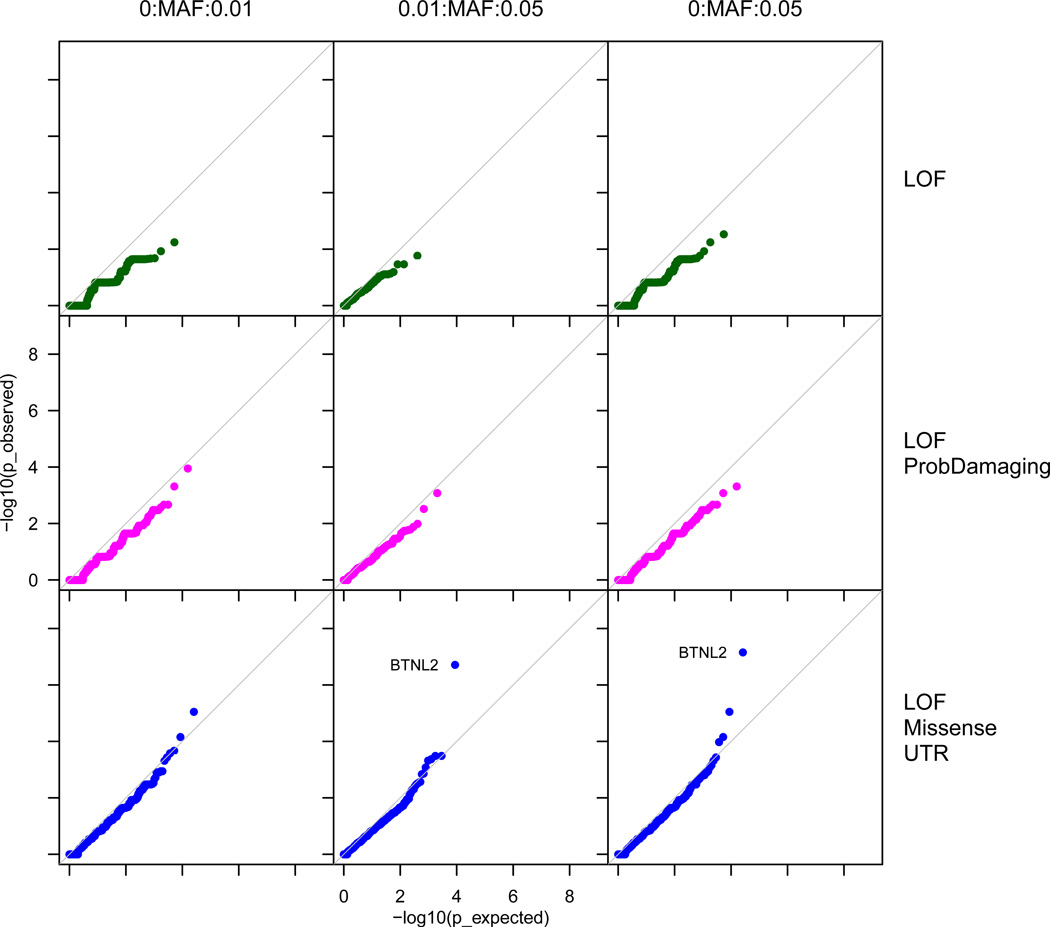
Next, we used a burden test to evaluate whether cases were more likely to be carriers of a functional variant in a gene compared to controls in the exome sequencing data. We divided rare variants into different functional categories and MAF ranges and found that BTNL2 (P=7.0×10−8) was the only gene to exceed a conservative significance threshold of P=2.5×10−6, corresponding to a Bonferroni correction of 20,000 genes tested12 (Figure 3). None of the protein products of these genes is known to bind clozapine10. The BTNL2 signal is driven by two independent variants: the top SNP noted above (rs28362679) and a conditionally independent missense variant annotated as benign by PolyPhen-2 (rs143211074; P=6.0×10−6, OR=4.4, 95% CI 1.10–17.8). These associations replicated using the exome array and NHLBI Exome Sequencing Project data (Supplementary Fig. 4). BTNL2 is in the MHC region and is in strong LD with HLA-DQB1 and HLA-DRB1, which have been associated with autoimmune diseases and adverse drug reactions including CIAG7, 13. BTNL2 codes for the butyrophilin-like protein 2, a member of the immunoglobulin gene superfamily with a role in regulating T-cell activation14. Given its location in a region of very high LD, the BTNL2 association could implicate it directly or reflect indirect associations with classical HLA alleles.
Figure 3. Gene-Burden Test Results.
We evaluated whether cases were more likely to carry rare functional variants in a gene compared to controls using sequencing data from 67 cases and 376 controls. Variants were annotated using the Variant Effect Predictor tool and divided into three sets of severity. The most severe category consists of loss-of-function (LOF) variants annotated as being nonsense and splice site (green). The intermediate category included all LOF variants as well as missense variants annotated as probably damaging by PolyPhen-2 (magenta). The least severe category consisted of all LOF, missense, and untranslated region (UTR) variants (blue). We had varying minor allele frequency (MAF) cutoffs with the most restrictive being 0–1% MAF and the most inclusive being 0–5%. For each MAF and variant set, we assessed whether any genes deviated from expectation as demonstrated by the diagonal line. The only gene that showed enrichment was BTNL2 (P=7.0×10−8, Fisher’s Exact Test).
Classical HLA allele imputation
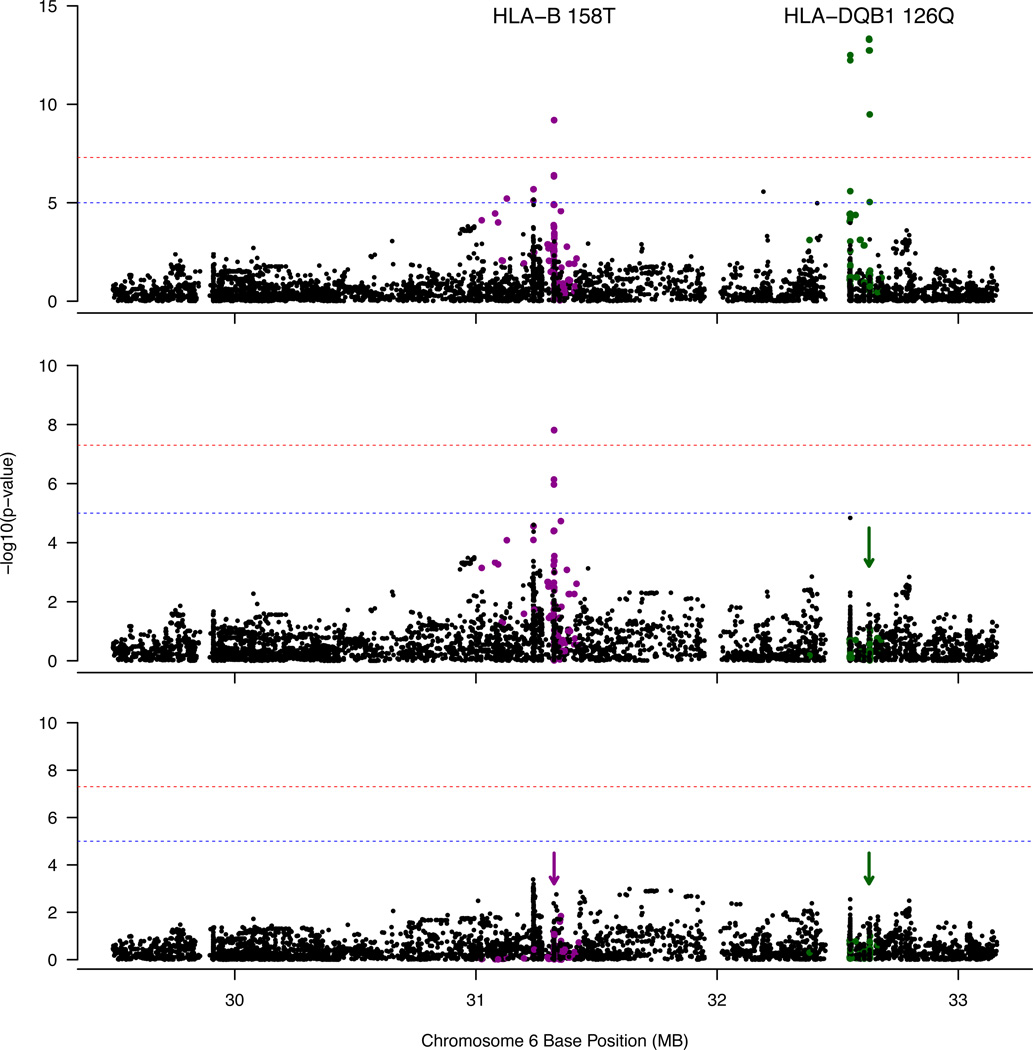
We imputed four-digit classical HLA alleles and amino acid sequences in 162 CIAG cases and 4,319 controls of European ancestry15 from a reference panel of 5,225 individuals using SNP2HLA15. The cases, mainly of northwestern European and Ashkenazi Jewish ancestry, were well-matched to controls (Supplementary Fig. 5). Two independent loci are associated with CIAG after multiple-testing correction (Figure 4). The top signal is an amino acid change in HLA-DQB1 (126Q) that is in strong LD with HLA-DQB1*05:02 (P=4.7×10−14, OR=0.19, 95% CI 0.12–0.29). After conditioning on HLA-DQB1 126Q, a genome-wide significant signal in HLA-B remains (threonine to alanine at residue 158, Pcond =1.54×10−8, OR=3.11, 95% CI 2.1–4.6). No other statistically significant loci remain after conditioning on both HLA-DQB1 126Q and HLA-B 158T (Supplementary Data 3).
Figure 4. Manhattan Plot of the MHC Region from HLA Imputation.
This figure shows the association results of CIAG to imputed HLA classical alleles, amino acids, and SNPs for 161 cases and 4300 controls. (A) The top signal is from a single amino acid in HLA-DQB1 (126Q) (P=4.7×10−14, OR=0.19, logistic regression). All variants with an R2 > 0.1 with HLA-DQB1 126Q are colored green. (B) After conditioning on HLA-DQB1 126Q as demonstrated by the green arrow, the top signal is a single amino acid in HLA-B (158T) (Pcond =1.54×10−8, OR=3.11, logistic regression). All variants with an R2 > 0.1 with HLA-B 158T are colored purple. (C) After conditioning on HLA-DQB1 126 Q and HLA-B 158T (purple and green arrows), no genome-wide significant loci remain.
HLA-DQB1 and HLA-B explains the MHC signal better than BTNL2
To determine whether the BTNL2 signal is independent of the HLA-B 158T and HLA-DQB1 126Q signals, we analyzed samples with imputed HLA alleles and genotyped BTNL2 variants (146 CIAG cases and 3,364 controls). In conditional analyses, we observed that the most associated BTNL2 variant (rs28362679) was in high LD with HLA-DQB1 126Q (R2=0.618) and the other BTNL2 variant (rs143211074) had a low correlation with HLA-B 158T (R2=0.042) (Supplementary Fig. 6). To determine whether the association in the region was better explained by the two variants in BTNL2 or the two HLA-alleles, we compared the likelihoods of models with HLA-DQB1 126Q and HLA-B 158T predicting CIAG compared to a model with the two BTNL2 variants. The combination of HLA-DQB1 126Q and HLA-B 158T is 27,000 times more likely to explain the MHC association with CIAG than the two BTNL2 variants. We also looked at whether a model including one or both BTNL2 variants in addition to HLA-DQB1 126Q and HLA-B 158T explained the association better than HLA-B and HLA-DQB1 alone. We found including rs143211074 in the model was 13 times more likely to explain the association than the two-component model of HLA-B and HLA-DQB1, but there was no improvement when we added rs28362679. These results indicate rs143211074 could be independently associated with CIAG, but the more likely explanation is rs143211074 is tagging an HLA haplotype that is not genome-wide significant on its own (given that rs143211074 and HLA-DRB1*04:02 are highly correlated with R2=0.77).
HLA sequence alignment
We used the IMGT/HLA database16 (http://www.ebi.ac.uk/ipd/imgt/hla) to determine which classical alleles correspond to HLA-DQB1 126Q and HLA-B 158T and to align these results with previous candidate gene studies of CIAG. Since having a glutamine at position 126 in HLA-DQB1 is protective for CIAG, the most common high-risk allele is HLA-DQB1*05:02 which has a histidine at position 126. This is consistent with prior reports of an association of CIAG with HLA-DQB1 6672G>C (a variant in strong LD with HLA-DQB1*05:02)4, 5. HLA-B*38, HLA-B*39, and HLA-B*67 are the most common alleles with a threonine at position 158 in the protein sequence (consistent with previous reports although none met contemporary significance levels)3, 4, 8. Our results cannot differentiate whether the amino acids we identified are causal or whether the overall configuration of these genetically related isoforms confers risk for CIAG.
CIAG severity by HLA-B and HLA-DQB1 carrier status
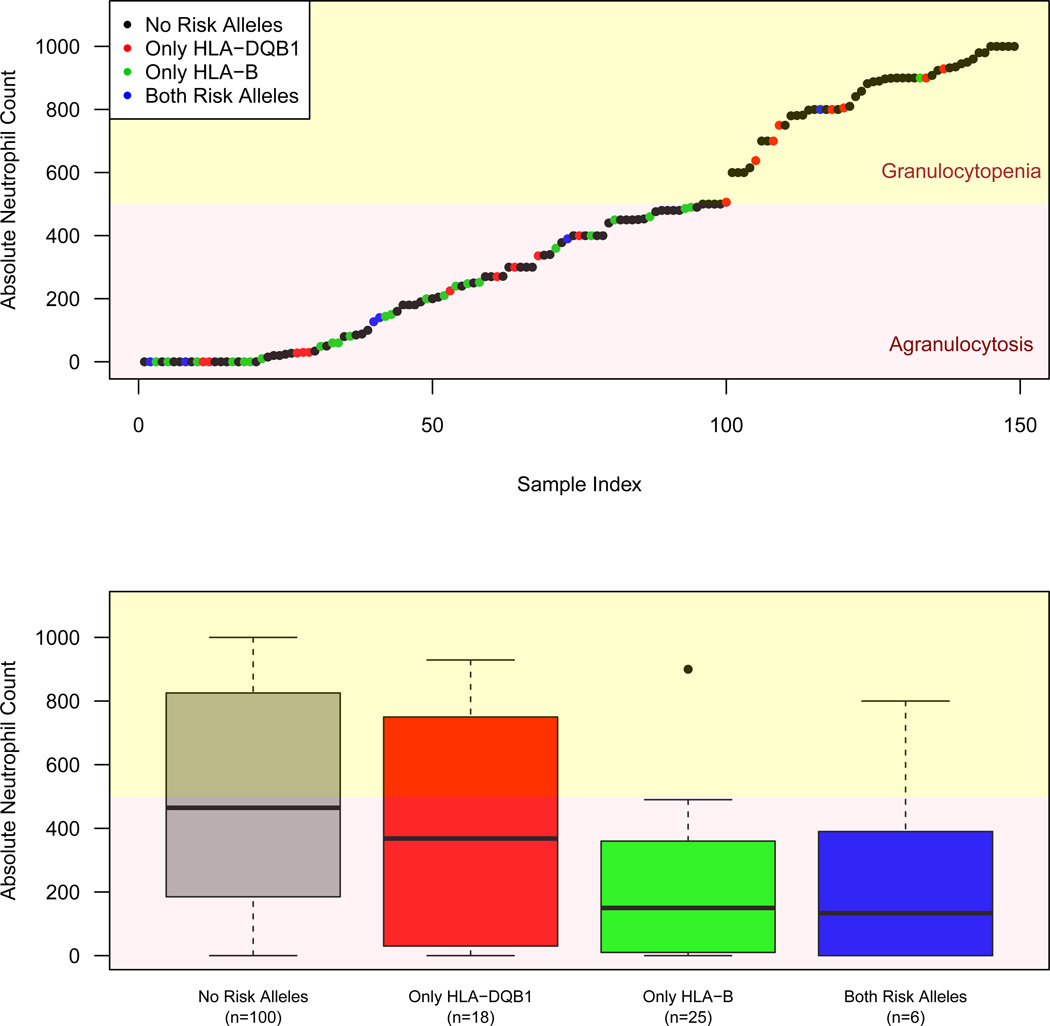
Finally, we tested for differences in CIAG severity (as indexed by minimum absolute neutrophil count, ANC) according to risk allele status of HLA-DQB1 and HLA-B among 149 cases with these data. Cases who were carriers of HLA-B 158T demonstrated significantly lower ANC than non-carriers (P<0.01, Figure 5). However, as nearly all cases with substantial Ashkenazi Jewish ancestry carried the HLA-B 158T allele (Supplementary Fig. 7), it is not possible to determine conclusively whether some of this signal could be driven by an unexplored feature of ascertainment or population difference. Hence we repeated the analysis after excluding cases with Ashkenazi Jewish ancestry (PC1>0.02) and subsequently correcting the phenotype for ancestry and sample cohort and observed a consistent effect but of reduced significance (P=0.049) owing to reduced power because of the number of 158T alleles excluded (Supplementary Fig. 8). Similar analyses of time to onset of CIAG were not significant (data not shown).
Figure 5. Absolute Neutrophil Count Stratified by HLA Risk Allele Carrier Status.
(A) For cases in which the lowest recorded absolute neutrophil count (ANC) data were available, we ordered samples by the ANC and colored the points by their imputed carrier status of HLA-DQB1 126Q and HLA-B 158T (black=no risk alleles, red=only a carrier of HLA-DQB1 risk alleles as tagged by 126Q, green=only a carrier of HLA-B 158T, blue=carrier of both HLA-DQB1 and HLA-B risk alleles). The background colors demonstrate the clinical definitions for agranulocytosis (red: ANC<500) and severe granulocytopenia (yellow: 500≤ANC≤1000). (B) The same data are shown as a box plot to demonstrate that HLA-B 158T carriers demonstrate a trend towards lower ANC than non-carriers of HLA-B 158T.
Modeling of clozapine binding to HLA-B
The associations of HLA-DQB1 and HLA-B with CIAG are biologically plausible. HLA-DQB1 is part of the MHC Class II antigen-presentation pathway and has been associated with autoimmune diseases (e.g., rheumatoid arthritis)17 and a range of immunogenetic phenomena (e.g., allergic sensitization, human papillomavirus seropositivity, and chronic hepatitis B infection)18, 19, 20. HLA-B is an important component of the MHC Class I antigen-presentation pathway and a “hot spot” for serious adverse drug reactions including flucloxacillin-induced liver injury,21 carbamazepine-induced Stevens-Johnson syndrome22, and abacavir hypersensitivity syndrome23. For several of these adverse drug reactions, the mechanism appears to be off-target binding of a drug molecule to the antigen-binding cleft of the HLA-B protein23.
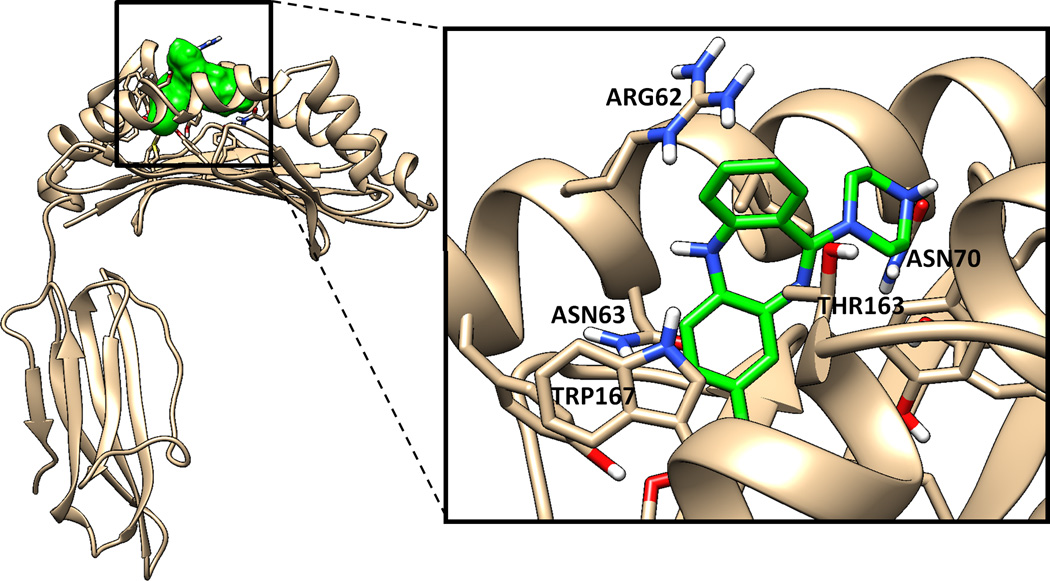
Similar to what has been done for abacavir23, we used a molecular docking approach to determine that clozapine binds with relatively high affinity to the putative HLA-B*39 antigen-presenting peptide groove when there is a threonine at position 158 (Figure 6). Docking simulations also suggested a potential for clozapine to bind in the pockets of HLA-B*57:01 and HLA-B*35:08 with predicted affinities much higher than that for multiple other HLA-B variants (Supplementary Fig. 9). As a negative control, docking studies showed that clozapine had low binding affinities for the antigen-presenting pockets of HLA-A proteins.
Figure 6. Predicted binding pose of clozapine in the antigen-presenting pocket of HLA-B* 39.
Docking of clozapine into the putative antigen binding pocket of the homology model of HLA-B39 identified a binding pose (shown in this figure) with the highest predicted binding affinity. Residues in the protein binding pocket involved in specific interactions with clozapine are annotated.
Discussion
In conclusion, we identified two independent loci in the MHC region that are associated with CIAG: HLA-DQB1 and HLA-B. These findings are consistent with previous results but the evidence is now much stronger in this comprehensive analysis. Moreover, we localized the signal to two amino acids (HLA-DQB1 126Q and HLA-B 158T), but cannot distinguish whether these amino acids are causal or if the overall configuration of the alleles confers risk for CIAG. Although these data greatly clarify the contributions of HLA variation to CIAG, the odds ratios do not immediately suggest clinical application in screening. If we consider a genetic test that was positive if an individual carried either risk factor, the sensitivity and specificity of such a test would be 0.36 and 0.89. Because more than half of cases do not carry these two relatively infrequent risk factors, a predictive test based solely on HLA-DQB1 and HLA-B could not define a truly “safe” group for clozapine use. We note that 7 CIAG cases (4.3%) carried two risk alleles, suggesting that this combination had particularly high risk compared to the population expectation of 0.3–0.4%. These genetic insights could further understanding of the biological process underlying CIAG and, as other non-genetic and genetic risk factors for CIAG are identified, constitute an important component of tests to screen patients for the safer use of clozapine.
Methods
Ethical Statement
The study was approved by Institutional Review Boards at the University of North Carolina (http://research.unc.edu/offices/human-research-ethics) and at Massachusetts General Hospital (http://healthcare.partners.org/phsirb). Research was carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants (or legal guardians, if applicable).
Sample Description
We formed the Clozapine-Induced Agranulocytosis Consortium (CIAC) to further this effort by bringing together nearly all investigators in the area and to ascertain new cases. CIAG (clozapine-induced agranulocytosis/granulocytopenia) cases developed agranulocytosis (absolute neutrophil count, ANC < 500/mm3) or granulocytopenia (500 ≤ ANC ≤ 1,000/mm3) while taking clozapine and without a known medical condition that increases risk for agranulocytosis (e.g., hematological disorder or autoimmune disease). There were 98 cases with agranulocytosis (60.1%), 61 cases with granulocytopenia (37.4%), and 4 cases (2.5%) included on the basis of clinical judgment due to a precipitous drop in ANC to < 1,500/mm3 while being intensively monitored. We allowed any primary psychiatric diagnosis (although most subjects had schizophrenia or schizoaffective disorder) and any reported ancestry was allowed. Heterogeneity due to ANC threshold, diagnosis, and ancestry was addressed analytically.
Subjects were identified in two ways. Opportunistic ascertainment was via a broad survey of colleagues to identify all worldwide samples in existing CIAG collections that allowed rapid and efficient identification of existing CIAG cases and controls exposed to clozapine without CIAG. This included cases from the International Severe Adverse Events Consortium (iSAEC,), http://www.saeconsortium.org). Systematic assessment included identification of additional cases from the Novartis and Teva clozapine registries (https://www.clozarilcare.com/care, https://www.clozapineregistry.com), and the electronic pharmaceutical record from the Pennsylvania state hospital system. Briefly, we contacted the physician of record to confirm an episode of CIAG, the physician then obtained permission from the potential subject to release contact information, and we then contacted the subject to attempt recruitment into this study.
We attempted to obtain and review medical records for all subjects. Using a standardized review form, we abstracted demographic data, clinical diagnosis, and clozapine dosage, exposure duration, and the lowest ANC. Two senior CIAC clinicians evaluated all available data to verify CIAG case status. Most DNA samples were extracted from peripheral venous blood; a subset of DNA samples were from lymphoblastoid cell lines or oral sources. Most samples were subjected to standard quality control procedures (DNA quantification, electrophoresis to assess degradation, and genotyping a “fingerprint” of 25 common SNPs using a Sequenom iPLEX panel). Genotype data confirmed that all subjects were independent and of European ancestry (see below).
A combination of clozapine-treated and untreated controls was used to increase statistical power. Clozapine-treated controls had received clozapine for over one year with no documented ANC < 1,500/mm3 and no medical condition with an increased risk of agranulocytosis. Ascertainment of clozapine-treated controls was performed in the same manner as for the cases. Untreated controls were broadly consented population controls ascertained in unrelated disease and cohort studies. Allele frequency data from the NHLBI Exome Sequencing Project (ESP) were also used.
Generation of Genome-wide Array Data
385 samples from CIAC (135 cases and 249 treated controls) were genotyped on the Illumina OmniExpress array at Duke University. We supplemented the CIAC data with 839 untreated population controls from the United Kingdom Blood Service cohort who were genotyped on the Illumina OmniExpress platform at the Broad Institute. In addition, 26 case samples from the International Serious Adverse Events Consortium (iSAEC, http://www.saeconsortium.org) were genotyped at Duke University. These data were merged with 108 untreated population controls from the NIMH repository genotyped on Illumina arrays at the University of Michigan. Genotype calls were generated using Illumina’s GenomeStudio software. All samples had a call rate > 98% and an inbreeding coefficient between −0.2 and 0.2. SNPs were included if the call rate was > 98%, were in Hardy-Weinberg equilibrium (Pcontrol > 1×10−6, Pcase > 1×10−10), and the call rate difference between cases and controls was < 0.02. Samples were unrelated and of European ancestry via multidimensional scaling24. We used SHAPEIT25, 26 and IMPUTE227 to impute genotypes for all SNPs using 1000 Genomes Project data as a reference panel. The imputation reference set was 2,186 phased haplotypes from the 1000 Genomes Project (August 2012, 30,069,288 variants, release “v3.macGT1”, http://mathgen.stats.ox.ac.uk/impute/data_download_1000G_phase1_integrated.html). Imputation was done for each platform separately (OmniExpress, I550K) to avoid platform-based batch effects in imputation.
Generation of Whole-Exome Sequencing Data
We sequenced 41 CIAC cases and 26 iSAEC cases at Duke University. Exome sequence from CIAC cases was captured using Agilent SureSelect 50MB kits (Santa Clara, CA) and sequenced using Illumina HiSeq 2000 (San Diego, CA) for paired-end 100 bp reads. The iSAEC samples were sequenced several years earlier using Agilent SureSelect kits (Santa Clara, CA) and Illumina GAII for paired-end reads (San Diego, CA). For controls, we used data from 376 phenotypically normal controls who had been exome sequenced at the Broad Institute28. All raw FASTQ data were processed using the same Picard pipeline29, 30 and genotype calls were generated for all data jointly using the GATK Unified Genotyper tool31 in order to avoid batch effects.
Individual genotype calls were filtered by requiring read depth ≥ 10, genotype quality score ≥ 30, and meeting specified allelic balance criteria (based on genotype: for 0/0 0–0.3; for 0/1 0.3–0.7; and for 1/1 0.7–1.0). All variants were required to have a GATK PASS rating, to be biallelic, not to have a significant difference in call rate between cohorts (P > 0.01) (eliminates biases due to a variant being sequenced in one cohort but not another), and not to be an insertion/deletion variant. Samples were required to have a call rate > 95%, < 600 singleton calls, and a Ti:Tv ratio between 3.1 and 3.4. The first ten principal components were computed using PLINK with 14,466 independent variants with a MAF > 5% and not located in the MHC region.
Generation of Exome Array Data
We genotyped deleterious exonic variation and a comprehensive set of MHC tag SNPs using the Illumina HumanExome array (v1.0, http://genome.sph.umich.edu/wiki/Exome_Chip_Design). Initial genotyping was conducted at Duke University and genotype calls were made using GenomeStudio (v2010.3, genotyping module v1.8.4) using the default Illumina cluster file. Samples were removed for missingness > 1% (based on common SNPs with MAF > 5%) or genome-wide heterozygosity outside the range of 0.1–1.2% (based on rarer SNPs with MAF < 5%) using the original GenomeStudio calls. Cases that failed quality control (N=23) were re-genotyped at the Broad Institute on the Illumina HumanExome array (v1.1); genotype calling and quality control were the same as for the Duke data except that a custom cluster file and a missingness threshold > 2% were used. We then used zCall to recover rare genotypes.32 After removing poorly performing samples, individual SNPs from the Duke data were included if they had a call rate > 97% from the original GenomeStudio calls, a HWE p-value > 1×10−5 and a call rate > 99.5% calculated from the zCalls. For the Broad data, more lenient call rate thresholds were used due to the small number of samples (GenomeStudio calls > 75%, zCalls > 95%).
The passing exome array data were merged with population controls of European ancestry broadly consented for biomedical studies. These controls included samples from the NIMH repository (n=1,037)33, POPRES (n=703)21, the Prospective Registry in IBD Study (n=254)34, the 1000 Genomes Project (n=449)35, and controls ascertained for an age-related macular degeneration study (n=704)36. Quality control for the shared controls was similar to the steps for the CIAC data, except that a call rate of 99% was used36. The final dataset included only SNPs passing quality control in all cohorts. We removed one member of putative relative pairs (π̂ > 0.2) and used EIGENSOFT37 to calculate the first 10 principal components based on 16,621 SNPs (MAF > 5%, excluding the extended MHC region (chr6:25–35 mb), and following LD pruning). 213/3366 controls were ascertained for not having CIAG.
HLA Allele Imputation
HLA classical alleles and amino acids were imputed using BEAGLE38 for exome array data or IMPUTE227 for genome array data with a reference set of 5225 samples from the Type 1 Diabetes Genetics Consortium15. The imputation accuracy using this reference panel is extremely high. For example, the SNP2HLA authors found the accuracy of HLA-B*38:01 was 99.8%, HLA-B*39:01 was 99.8%, and HLA-B*39:06 was 99.9% using the same set of MHC tag SNPs present on the Illumina HumanExome array compared to direct genotyping of these variants15. We also confirmed the accuracy of imputation of HLA-DQB1*05:02 by SNP2HLA using existing data for 19 samples that had PCR-based sequencing of HLA-DQB1 6672G>C, which is in strong LD with HLA-DQB1*05:027: 37/38 imputed HLA-DQB1*05:02 calls were concordant with the PCR-based sequencing results.
Genome-wide common variant association study
Principal components (PCs) were calculated using EigenSoft with the merged OmniExpress and I550K/I610K imputed datasets. SNPs used to calculate the principal components were independent (LD pruned) and did not include the MHC (chr6:25–35 Mb). We tested the first 20 principal components for phenotype association (using logistic regression) and evaluated their impact on the genome-wide test statistics using λ, a measure of how much the observed distribution of P-values deviates from the expected distribution of P-values. Five principal components (1–4 and 12) were included in all association analyses. The first four PCs were selected because it is standard practice to include the first four PCs in analyses to account for ancestry and, of PC5–PC20, PC12 was the only PC associated with case-control status (P<0.05). We performed a mega-analysis between the OmniExpress data and the I550K/I610K imputed dosage data using PLINK with principal components (described above) as covariates for logistic regression. SNPs with an INFO score ≥ 0.6 (metric of imputation quality for a SNP) and MAF ≥ 1% in controls were considered passing. We did not observe any batch effects due to multiple platforms or confounding due to population ancestry as indicated by a value of λ = 1.047 and no false positive results (Supplementary Fig. 2).
Immune-mediated SNP Enrichment
To determine whether our GWAS results show any indication of signal in loci known to be associated with autoimmune diseases, we randomly drew P-values from the GWAS results above to create an expected null distribution. In order to make this comparison more representative in terms of power per SNP, random draws were only taken from the subset of variants with MAF > 0.05 and INFO > 0.8 (6,752,294 SNPs). We drew 100 sets of 838 SNPs and compared the distribution of p-values to those observed in a curated list of 838 associations to immune-mediated and autoimmune disease curated from the NHGRI GWAS catalog. The comparison demonstrated that CIAG association to known immune-mediated SNPs was not distinguishable from random draws of SNPs.
Exome-wide rare variant association study
Because we had data from different platforms for rare exonic variants (exome sequencing, array, and allele counts from the Exome Sequencing Project11, ESP, available at http://evs.gs.washington.edu/EVS), we used the following procedure to combine allele counts. If a variant was assayed on multiple platforms, we ensured the MAFs between the controls did not differ (P > 0.001). If the MAFs in controls were not compatible, we removed data in the following order: ESP, exome array, and exome sequencing. For each variant, we added allele counts for each technology in both cases and controls and used Fisher’s exact test on the combined sample to test for significance.
Variants were functionally annotated using the Variant Effect Predictor tool from Ensembl39. We then divided the annotations into three categories. The most restrictive grouping included all nonsense and splice-region variants. The intermediate grouping included all nonsense, splice-region, and missense variants annotated as probably damaging by PolyPhen-240. The least restrictive grouping included all nonsense, splice-region, missense, and untranslated region variants. To test for an excess burden of rare and functional variants, we used Fisher’s exact test where the variable of interest is the number of carriers of a functional variant. We used the number of carriers of a functional variant instead of the total minor allele count as the testing variable to account for LD between variants.
MHC Association Analysis
We used imputed HLA classical alleles from all samples that were genotyped on the exome array. For the remaining samples that were not genotyped on the exome array, we imputed HLA classical alleles from either OmniExpress or I500K. Principal components were calculated from 6000 independent markers that were present on all three platforms to obtain one consistent set of principal components for all samples using PLINK (Supplementary Fig. 5). We merged the imputed dosage data from all three platforms to do a mega-analysis and used logistic regression to calculate association test statistics for each imputed allele using the first 10 principal components as covariates in PLINK to account for any confounding due to population structure. For conditional analyses, we used the dosage of the conditioning marker as an additional covariate in the analysis.
Model Comparison
We used R to compute the log-likelihood of a logistic regression model consisting of either the top two BTNL2 missense variants or HLA-DQB1 126Q and HLA-B 158T dosages with the first ten principal components as covariates in 144 cases and 3,355 controls. We also explored whether adding one or both BTNL2 variants improved the model consisting of HLA-DQB1 126Q and HLA-B 158T.
Linkage Disequilibrium Calculations
We used PLINK to calculate the R2 between each pair of variants in both cases and controls who had BTNL2 and HLA imputation data (n=146 cases and 3,364 controls). Haploview41 was used to plot the LD between the top BTNL2 variants, HLA-B 158T, HLA-DQB1 126Q alleles, and the top HLA-DRB1 alleles (*04:02 and *16:01).
Wilcoxon-Rank Sum Tests
We used a Wilcoxon-Rank Sum test in R to determine whether the ANC distribution between carriers and non-carriers of HLA risk alleles was different in cases who had a lowest recorded ANC ≤ 1000 (n=149). To correct for covariates, we used the residuals of the regression between ANC and the principal components and cohort indicator as the quantitative variable for the Wilcoxon-rank sum test (Supplementary Fig. 8). For samples with dosage, treatment duration, and minimum ANC available (n = 92), we calculated the correlation between these variables. ANC is moderately correlated with treatment duration (R2 = 0.165) and weakly correlated with clozapine dosage (R2 = 0.035). Treatment duration is moderately correlated with dosage (R2 = 0.229). Carrier status was determined using the best guess genotype for the HLA-B 158T and HLA-DQB1 126Q alleles.
Copy Number Variation Analysis
To generate CNV genotypes, we applied PennCNV to the Illumina OmniExpress data. We removed any samples that had a log R ratio standard deviation > 0.3 or that were above the 95th percentile for the number of CNV calls leaving 134 cases and 242 controls. CNVs included for analysis were required to have > 10 SNP probes, > 20 kb in length, < 50% overlap with large genome gaps, and with MAF < 1% in controls. We used PLINK to test for CIAG association using the “--cnv-test-2sided” command with significance determined using 100,000 permutations. The family-wise corrected P-values were examined. No rare CNV was significantly associated with CIAG (data not shown).
Molecular Docking
We used the Maestro 2012 software suite (Schrödinger, http://www.schrodinger.com) to build a homology model of HLA-B39 using the protein structures of HLA-B*08 (Protein Data Bank, PDB, http://www.wwpdb.org, code = 3SPV, resolution 1.30Å) and HLA-B*41 (PDB code = 3LN4, resolution 1.30 Å) as templates. We also used a multiple sequence alignment encompassing HLA-B*08, B41, and B38. Clozapine was docked into the antigen-presenting site of the predicted structure of HLA-B39 using the Glide program in extra-precision docking mode (Glide XP). Ionizable groups were first converted to their most probable charged forms at pH 7.4 using the LIGPREP software within Maestro. The binding region was defined by a 10Å × 10Å × 10Å box centered on the B39 pocket. A scaling factor of 0.8 was applied to the van der Waals radii. Default settings were used for all the remaining parameters. The docking poses were energy-minimized with Macromodel in the OPLS2005 force field with flexible ligand and rigid receptor. The refined poses were re-ranked on the basis of the calculated interaction energy. The lowest-energy pose was selected and rescored in the active site using GlideScore XP. Clozapine was also docked in the antigen-presenting sites of HLA-B*57:01 (PDB code = 3VRI), HLA-B*35:08 (PDB code = 3BWA), and the binding pockets of other HLA-A and HLA-B proteins.
Supplementary Material
Acknowledgments
We thank Drs Thomas Lehner (NIMH) and David Goldstein (Duke University) for their help on this project. We acknowledge the assistance of Dr Michael Karukin (Teva Pharmaceuticals) and Drs Rafael Muniz and Vinod Kumar (Novartis) for help in accessing US national clozapine registries. We thank Drs Stanton Gerson (Department of Hematology, Case Western Reserve), Armond Goldman (Department of Immunology, University of Texas Galveston), and Nancy Berliner (Department of Hematology, Harvard University) for providing input on mechanisms of agranulocytosis. MP is NIHR Senior Investigator. This project was funded by R01 MH080403 (PFS, EVDO) from the US National Institute of Mental Health via the American Recovery and Reinvestment Act of 2009. DF and AT acknowledge support from National Science Foundation grant ABI 10-567.
LFJ has received grant support from Genentech, Amgen, and Sunovion in the past three years, and is a member of a Data Monitoring Committee for Janssen. AH is an employee of iSAEC, a 501(c)(3) non-profit research organization. JAL serves on the Advisory Board of EnVivo and Intracellular Therapies and does not receive direct financial compensation or salary support for his participation. He receives grant support from Biomarin, EnVivo, Genentech, Novartis, Psychogenics and Sunovion; is a member of the Advisory Board of Pear Therapeutics and holds a financial interest; and holds a patent from Repligen.
Footnotes
Author Contributions
The study was designed by LFJ, JLK, JAL, MJD, and PFS with additional input by EJCGVDO. Cases and controls were ascertained and phenotyped by LFJ, IC, MD, EG, DCG, ALH, DLK, AKM, JN, MP, DR, TW, DLL, and RCJ. Most genomic assays were done under the supervision of KS with some done by CH. Data analysis was conducted by JIG, LD, HH, ML, BMN, SR, and JPS. Molecular docking modeling was done by DF and AT. All authors discussed the results and implications and commented on the manuscript at all stages.
Conflicts of Interest
The remaining authors declare no competing financial interests.
References
- 1.Alvir JM, Lieberman JA, Safferman AZ, Schwimmer JL, Schaaf JA. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. The New England journal of medicine. 1993;329:162–167. doi: 10.1056/NEJM199307153290303. [DOI] [PubMed] [Google Scholar]
- 2.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman JA. HLA-B38, DR4, DQw3 and Clozapine-Induced Agranulocytosis in Jewish Patients With Schizophrenia. Archives of General Psychiatry. 1990;47:945. doi: 10.1001/archpsyc.1990.01810220061007. [DOI] [PubMed] [Google Scholar]
- 4.Yunis JJ, Corzo D, Salazar M, Lieberman JA, Howard A, Yunis EJ. HLA associations in clozapine-induced agranulocytosis. Blood. 1995;86:1177–1183. [PubMed] [Google Scholar]
- 5.Dettling M, Cascorbi I, Roots I, Mueller-Oerlinghausen B. Genetic determinants of clozapine-induced agranulocytosis: recent results of HLA subtyping in a non-jewish caucasian sample. Arch Gen Psychiatry. 2001;58:93–94. doi: 10.1001/archpsyc.58.1.93. [DOI] [PubMed] [Google Scholar]
- 6.Dettling M, Cascorbi I, Opgen-Rhein C, Schaub R. Clozapine-induced agranulocytosis in schizophrenic Caucasians: confirming clues for associations with human leukocyte class I and II antigens. The pharmacogenomics journal. 2007;7:325–332. doi: 10.1038/sj.tpj.6500423. [DOI] [PubMed] [Google Scholar]
- 7.Athanasiou MC, et al. Candidate gene analysis identifies a polymorphism in HLA-DQB1 associated with clozapine-induced agranulocytosis. The Journal of clinical psychiatry. 2011;72:458–463. doi: 10.4088/JCP.09m05527yel. [DOI] [PubMed] [Google Scholar]
- 8.Valevski A, et al. HLA-B38 and clozapine-induced agranulocytosis in Israeli Jewish schizophrenic patients. European journal of immunogenetics : official journal of the British Society for Histocompatibility and Immunogenetics. 1998;25:11–13. doi: 10.1046/j.1365-2370.1998.00091.x. [DOI] [PubMed] [Google Scholar]
- 9.Tiwari AK, et al. Exome sequence analysis of Finnish patients with clozapine-induced agranulocytosis. Molecular psychiatry. 2013 doi: 10.1038/mp.2013.74. [DOI] [PubMed] [Google Scholar]
- 10.Yadav PN, et al. The presynaptic component of the serotonergic system is required for clozapine's efficacy. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:638–651. doi: 10.1038/npp.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NHLBI Exome Sequencing Project. Exome Variant Server. 2011 http://evs.gs.washington.edu/EVS/ [Google Scholar]
- 12.MacArthur DG, et al. Guidelines for investigating causality of sequence variants in human disease. Nature. 2014;508:469–476. doi: 10.1038/nature13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei C-Y, Lee M-TM, Chen Y-T. Pharmacogenomics of adverse drug reactions: implementing personalized medicine. Human Molecular Genetics. 2012;21:R58–R65. doi: 10.1093/hmg/dds341. [DOI] [PubMed] [Google Scholar]
- 14.Mitsunaga S, et al. Exome sequencing identifies novel rheumatoid arthritis-susceptible variants in the BTNL2. Journal of human genetics. 2013;58:210–215. doi: 10.1038/jhg.2013.2. [DOI] [PubMed] [Google Scholar]
- 15.Jia X, et al. Imputing amino acid polymorphisms in human leukocyte antigens. PloS one. 2013;8:e64683. doi: 10.1371/journal.pone.0064683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson J, Halliwell JA, McWilliam H, Lopez R, Parham P, Marsh SG. The IMGT/HLA database. Nucleic Acids Res. 2013;41:D1222–D1227. doi: 10.1093/nar/gks949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Negi S, et al. A genome-wide association study reveals ARL15, a novel non-HLA susceptibility gene for rheumatoid arthritis in North Indians. Arthritis and rheumatism. 2013;65:3026–3035. doi: 10.1002/art.38110. [DOI] [PubMed] [Google Scholar]
- 18.Bonnelykke K, et al. Meta-analysis of genome-wide association studies identifies ten loci influencing allergic sensitization. Nat Genet. 2013;45:902–906. doi: 10.1038/ng.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubicz R, et al. A genome-wide integrative genomic study localizes genetic factors influencing antibodies against Epstein-Barr virus nuclear antigen 1 (EBNA-1) PLoS genetics. 2013;9:e1003147. doi: 10.1371/journal.pgen.1003147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mbarek H, et al. A genome-wide association study of chronic hepatitis B identified novel risk locus in a Japanese population. Hum Mol Genet. 2011;20:3884–3892. doi: 10.1093/hmg/ddr301. [DOI] [PubMed] [Google Scholar]
- 21.Daly AK, et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nature Genetics. 2009;41:816–819. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- 22.Chung WH, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428:486. doi: 10.1038/428486a. [DOI] [PubMed] [Google Scholar]
- 23.Illing PT, et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature. 2012;486:554–558. doi: 10.1038/nature11147. [DOI] [PubMed] [Google Scholar]
- 24.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delaneau O, Marchini J, Zagury JF. A linear complexity phasing method for thousands of genomes. Nature methods. 2012;9:179–181. doi: 10.1038/nmeth.1785. [DOI] [PubMed] [Google Scholar]
- 26.Delaneau O, Zagury JF, Marchini J. Improved whole-chromosome phasing for disease and population genetic studies. Nature methods. 2013;10:5–6. doi: 10.1038/nmeth.2307. [DOI] [PubMed] [Google Scholar]
- 27.Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet. 2012;44:955–959. doi: 10.1038/ng.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim ET, et al. Rare complete knockouts in humans: population distribution and significant role in autism spectrum disorders. Neuron. 2013;77:235–242. doi: 10.1016/j.neuron.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DePristo MA, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nature Genetics. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldstein JI, et al. zCall: a rare variant caller for array-based genotyping: Genetics and population analysis. Bioinformatics. 2012;28:2543–2545. doi: 10.1093/bioinformatics/bts479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sklar P, et al. Whole-genome association study of bipolar disorder. Molecular psychiatry. 2008;13:558–569. doi: 10.1038/sj.mp.4002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivas MA, et al. Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat Genet. 2011;43:1066–1073. doi: 10.1038/ng.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abecasis GR, et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seddon JM, et al. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat Genet. 2013;45:1366–1370. doi: 10.1038/ng.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 38.Browning BL, Browning SR. A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. American journal of human genetics. 2009;84:210–223. doi: 10.1016/j.ajhg.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLaren W, Pritchard B, Rios D, Chen Y, Flicek P, Cunningham F. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics. 2010;26:2069–2070. doi: 10.1093/bioinformatics/btq330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adzhubei IA, et al. A method and server for predicting damaging missense mutations. Nature methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.