Summary
In this issue of Cancer Discovery, AI-Ahmadie and colleagues identify a somatic mutation in the Rad50 gene as a likely contributing factor to an unusual curative response to systemic combination therapy employing the DNA-damaging agent, irinotecan, and a checkpoint kinase 1(Chk1) inhibitor in a patient with recurrent, metastatic small-cell cancer. This study highlights the importance of in-depth analysis of exceptional responders to chemo and targeted therapy in early phase clinical trials and opens new avenues for developing cancer genome-based combination therapy to improve the efficacy of traditional chemotherapy through synthetically lethal interactions.
Clinical trials play a critical role in translating the cutting-edge discoveries of cancer research from bench to bedside. The criteria to evaluate the clinical success of candidate drugs are based on the frequency of disease regression or prolonged median progression-free survival. Drugs that fail to induce these desired clinical outcomes in a significant number of patients are often withdrawn from further clinical evaluation. However, the heterogeneous treatment response in patients has long been recognized in clinical trials. In the same clinical trial, some patients can exhibit exquisite sensitivity and/or durable responses to anti-cancer treatment, and are referred to as “exceptional responders,” while others show no clinical benefit and display progression of disease (1). It typically remains unexplored why a subgroup of patients have outlier responses in what are otherwise considered failed clinical trials. However, investigating the molecular markers and mechanism(s) associated with these exceptional responses provides the potential to revitalize drugs that failed in clinical trials for the majority of patients, but would benefit an identifiable subgroup of patients. Further, these studies have the potential to contribute to the identification of rational drug combinations that can extend the utility of chemotherapies and targeted therapeutics. Thus, it is of critical theoretical and translational importance to identify mechanisms underlying exceptional responders.
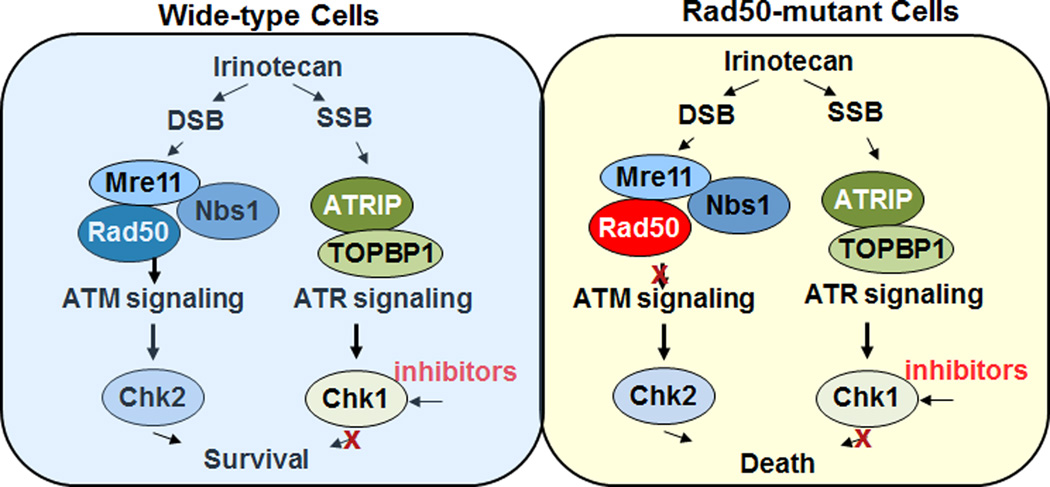
Although it remains a clinical challenge to attain curative therapeutic response in patients with metastatic solid tumors, particularly in early phase clinical trials, a 51-year old women with recurrent, metastatic small-cell cancer achieved a complete and durable response in a phase 1 clinical trial of AZD7762, an ATP-competitive checkpoint kinase inhibitor (Chk1/2) in combination with irinotecan, a topoisomerase I inhibitor (Topo I). To investigate the genetic basis of this outlier example of curative systemic cancer therapy, AI-Ahmadie and colleagues performed whole-genome sequencing (WGS) of tumor samples and identified a Rad50 (L1237F) mutation as a potential contributor to the curative response (2). Rad50 is a component of the multifunctional protein complex MRN (Mre11-Rad50-Nbs1) that detects DNA double strand breaks (DSBs), activates the ATM checkpoint kinase and promotes DSB repair (Figure 1). The Rad50 L1237F mutation is located in the D-loop of the ATPase domain near the C-terminal of Rad50, which based on studies from the team appears to be a hypomorphic mutation with the gene product retaining residual function. The authors found that the impaired function of the MRN complex due to this mutation leads to a synthetic lethality in cancer cells when they were treated with Chk1 inhibition and DNA-damaging chemotherapy irinotecan (Fig. 1).
Figure 1.
The impaired function of the MRN complex due to a somatic mutation of Rad50 leads to a synthetic lethality in cancer cells when they are treated with Chk1 inhibitor and DNA-damaging chemotherapy irinotecan.
As tumors can harbor thousands of mutations, it is technically challenging to sort out the most relevant mutations of potential functional and biological significance in exceptional responders to chemotherapy. Al-Ahmadie and colleagues provide valuable insights into delineating tumor genome sequencing data. Firstly, they sequenced the recurrent tumor specimen obtained after standard chemotherapy, but before trial enrollment. They then confirmed the mutations in the diagnostic tumor samples collected pre-chemotherapy. By comparing chemo-treated tumor and treatment-naïve tumor, the authors selected mutations that arose early in molecular time, which were more likely to exert biological driving effects on tumor development. Secondly, they performed an integrated analysis using the mutation, DNA copy number, and tumor clonality data with pathway analysis relevant to the mechanism of drug action to prioritize genomic aberrations. Thirdly, considering heterogeneity of tumor cells, they confirmed whether the mutation was a prevalent mutation and found that the Rad50 L1237F mutation was detected in the majority of tumor cells. Fourthly, they analyzed protein structure to identify the potential functional impact of mutations in the tumor. Finally, the authors took advantage of the evolutionary conservation of Rad50 function by efficiently modeling its mutations in the S. cerevisiae model system, then recapitulated the findings in mammalian cells.
Mechanistically, irinotecan inhibits Topo I by reversibly abolishing the religation activity of Topo I to generate single strand -DNA breaks (SSBs). When replication forks collide with the SSBs, the stalled and collapsed replication forks result in formation of DSBs. Thus, irinotecan-Mechanistically, irinotecan inhibits Topo I by reversibly abolishing the religation activity of Topo I to generate single strand -DNA breaks (SSBs). When replication forks collide with the SSBs, the stalled and collapsed replication forks result in formation of DSBs. Thus, irinotecan-induced DSBs are largely replication dependent (3). In wild-type cells, the presence of SSBs and DSBs activates both ataxia telangiectasia mutated (ATM) and ATM and Rad3-related (ATR) kinases-mediated signaling pathways, which enable cells to survive DNA damage by coordinating DNA damage response, the cell cycle checkpoint and DNA repair (4). Although extensive cross-talk and overlapping functions have been found between these two pathways, in general, activation of ATM is largely dependent on the proper function of the MRN complex in response to DSBs, which in turn activates Chk2. In response to SSB, ATR activation is facilitated by proteins ATRIP and TOPBP1, which regulate the downstream checkpoint protein Chk1(4). In contrast, in Rad50-mutant tumor cells, the ATM-dependent signaling pathway is compromised due to impaired function of MRN complex. Thus, inhibition of Chk1 further blocks the ATR signaling pathway, which can lead to a deleterious effect on cell survival. This synthetic lethal interaction in Rad50 deficient cells likely explains the exceptional response observed in this patient with a Rad50 mutation. In contrast, wild-type cells can cope with Chk1 inhibition due to functional compensation from the ATM signaling pathway. Functional redundancy between the ATM and ATR signaling transduction pathways also potentially explains why the majority of patients in this clinical trial did not show therapeutic benefits from the Chk1 inhibitor and irinotecan combination. Although the authors provide compelling data demonstrating that irinotecan and Chk1 inhibition generates a synthetic lethal response in cells with Rad50 defects, including the specific aberration identified in this patient’s tumor, the results remain an association without direct proof. The cogent hypothesis developed from this single exceptional responder is sufficient to warrant exploration in clinical trials based on multiplex genomic testing for defects in Rad50 and potentially the MRN complex; however, due to the paucity of Rad50 aberrations in any single cancer lineage, this will require a concerted pancancer community effort combined with comprehensive genomic testing of all patients eligible for clinical trials.
Previous studies of exceptional responders have identified response biomarkers for targeted therapy such as activating mutations of mTOR and TSC1 mutations for the mTOR inhibitor everolimus or EGFR mutations for the EGFR inhibitor gefitinib (5–9). In these studies, genomics-based approaches have been successfully used as a valuable tool to explain the mechanisms underlying exceptional responses to targeted therapeutics and to these resulting hypotheses by studies matching targeted therapy with actionable molecular targets. Compared to these studies, Al-Ahmadie and colleagues demonstrated the impact of extensive genetic analysis on an unusual response to traditional DNA-damaging chemotherapy, although in the current lexicon it is indeed likely that a Top1 inhibitor would be classified as a targeted therapy. Rather than targeting the mutation itself, the study identifies a mechanism-based combination therapy to target the therapeutic vulnerability rendered by the genetic mutation, a compelling example of synthetic lethality. Thus this study provides us with a new conceptual framework to develop systematic combination therapy through genetic and chemical drug-induced synthetically lethal interaction, which is especially important for optimizing responses to standard DNA-damaging treatments.
In patients receiving radiation therapy and conventional chemotherapy, DNA damage causes cell-cycle arrest and cell death either directly or following DNA replication during the S phase of the cell cycle, where cell death can be induced when cells attempt to replicate damaged DNA. Thus DNA-damaging treatments are more toxic to replicating cells than to non-replicating cells. The therapeutic window for DNA-damaging treatments is offered, in part, by more rapid growth of cancer cells than their normal counterparts. However, a lack of robustness, decreased cell survival signals or a loss of alternative DNA repair pathways due to the underlying genomic instability of tumors may also be key components. As exemplified by the study of Al-Ahmadie and colleagues, the efficacy of DNA-damaging treatment can be modulated by genetic alterations in DDR and DNA repair pathways in cancer cells or alternatively by chemical inhibitors of these pathways proving an approach to generalize responsiveness in patients with intact DDR and DNA repair pathways. DDR and DNA repair networks are composed of many parallel pathways to maintain genomic integrity, which have both distinct and overlapping functions such as ATM and ATR signaling pathways. As it constitutes a barrier of tumorigenesis, genome maintenance mechanisms are often breached by cancer cells through alterations of components of DDR and repair genes during tumor development. These genetic changes themselves may not be actionable targets (e.g., the Rad50 mutation in this study). However, they can render cancer cells reliant on a reduced set of DDR and DNA repair pathways for survival in the presence of DNA-damaging treatments, which can be subsequently targeted by chemical inhibitors such as Chk1 inhibitor in this case, or potentially an ATR inhibitor, to enhance treatment efficacy. Through a synthetic lethal interaction, the combination of genetic alteration and chemical inhibition of DDR and DNA repair pathway may transform traditionally DNA-damaging treatments that are not targeted to specific oncogenes in a patient’s tumor into new therapeutic regimens, which are highly effective and selective for a specific subgroup of patients.
References
- 1.Mullard A. Learning from exceptional drug responders. Nat Rev Drug Discov. 2014;13:401–402. doi: 10.1038/nrd4338. [DOI] [PubMed] [Google Scholar]
- 2.Al-Ahmadie H, Iyer G, Hohl M, Asthana S, Inagaki A, Schultz N, et al. Synthetic lethality in ATM-deficient RAD50-mutant tumors underlie outlier response to cancer therapy. Cancer Discov. 2014 doi: 10.1158/2159-8290.CD-14-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 4.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iyer G, Hanrahan AJ, Milowsky MI, Al-Ahmadie H, Scott SN, Janakiraman M, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338:221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 7.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 8.Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–1167. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 9.Wagle N, Grabiner BC, Van Allen EM, Hodis E, Jacobus S, Supko JG, et al. Activating mTOR mutations in a patient with an extraordinary response on a phase I trial of everolimus and pazopanib. Cancer Discov. 2014;4:546–553. doi: 10.1158/2159-8290.CD-13-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]