Abstract
Aim
Our objective was to describe the association between voriconazole concentrations and CYP2C19 diplotypes in pediatric cancer patients, including children homozygous for the CYP2C19*17 gain-of-function allele.
Materials & methods
A linear mixed effect model compared voriconazole dose-corrected trough concentrations (n = 142) among CYP2C19 diplotypes in 33 patients (aged 1–19 years). Voriconazole pharmacokinetics was described by a two-compartment model with Michaelis−Menten elimination.
Results
Age (p = 0.05) and CYP2C19 diplotype (p = 0.002) were associated with voriconazole concentrations. CYP2C19*17 homozygotes never attained therapeutic concentrations, and had lower dose-corrected voriconazole concentrations (median: 0.01 µg/ml/mg/kg; p = 0.02) than CYP2C19*1 homozygotes (median: 0.07 µg/ml/mg/kg). Modeling indicates that higher doses may produce therapeutic concentrations in younger children and in those with a CYP2C19*17/*17 diplotype.
Conclusion
Younger age and the presence of CYP2C19 gain-of-function alleles were associated with subtherapeutic voriconazole concentrations. Starting doses based on age and CYP2C19 status could increase the number of patients achieving therapeutic voriconazole exposure.
Keywords: antifungals, CYP2C19, personalized medicine, pharmacogenetics, voriconazole
Background
Pediatric cancer patients who experience prolonged periods of immunosuppression caused by myeloablative hematopoietic stem cell transplantation or myelosuppressive chemotherapy are highly susceptible to invasive fungal infections [1–3] . Voriconazole is a triazole antifungal agent that has potent activity against a broad spectrum of clinically important pathogens, and is recommended as a primary treatment for invasive aspergillosis in immunocompromised patients [4–8]. Because invasive fungal infections are associated with significant morbidity and mortality, promptly attaining therapeutic voriconazole plasma concentrations is imperative for achieving a favorable response [9–11]. An initial low voriconazole plasma concentration, even when therapeutic drug monitoring is subsequently utilized to target a specific concentration, may be a risk factor for increased mortality [12]. However, elevated voriconazole concentrations can result in adverse effects such as neurotoxicity and hepatotoxicity [13–17]. Pediatric patients have large interindividual variation in voriconazole pharmacokinetic parameters, which may contribute to delays in achieving appropriate voriconazole concentrations [3,7,18,19]. Identifying patient characteristics, such as genetic variants in pharmacogenes, that influence voriconazole plasma concentrations will facilitate the individualization of voriconazole dosing, allowing for faster achievement of therapeutic concentrations.
Voriconazole exhibits nonlinear pharmacokinetics, possibly due to saturable metabolism [3,18,20–22]. Children have lower voriconazole plasma concentrations than adults when administered weight-equivalent doses, which may be partially explained by decreased voriconazole oral bioavailability in children [22–24]. The role of gastrointestinal transporters or metabolism in voriconazole absorption is not clear [21]. Age-dependent differences in voriconazole plasma concentrations are also explained by pediatric patients having a higher elimination capacity of voriconazole due to increased voriconazole metabolism [3,7,19,25]. Voriconazole is metabolized by CYP2C19, CYP3A4, and to a lesser extent by CYP2C9, to compounds that have minimal antifungal activity [22,26]. CYP2C19 and FMO3 have been demonstrated to contribute to voriconazole metabolism in human liver microsomes [3,26,27]. CYP2C19 is a highly polymorphic pharmacogene, and genetic variants in the CYP2C19 gene locus may alter CYP2C19 substrate metabolism resulting in interindividual phenotypic variability [28–30]. Therefore, CYP2C19 genetic variants may have a clinically important impact on voriconazole concentrations in pediatric patients [3,31].
Limited data are available describing the correlation between CYP2C19 genetic variants and voriconazole plasma concentrations in pediatric patients [3,18,19,31]. CYP2C19 diplotypes predictive of intermediate or poor metabolism have been demonstrated to be associated with elevated voriconazole plasma concentrations when compared with pediatric patients with normal (extensive) CYP2C19 metabolism [3,31]. However, other studies have suggested that CYP2C19 polymorphisms may not be predictive of voriconazole plasma concentrations in a clinical setting [18,19]. Previous investigations either did not include patients who carried the CYP2C19*17 allele, which is responsible for CYP2C19 ultrarapid metabolism, or combined extensive and ultrarapid metabolizers into one category. Therefore, there is a lack of data in pediatric patients to illustrate whether CYP2C19 ultrarapid metabolizers have decreased voriconazole plasma concentrations with standard doses. In this retrospective study focusing on immunocompromised pediatric patients, we present data describing the correlation between voriconazole plasma concentrations and CYP2C19 diplotypes that are representative of all four phenotypic groups (i.e., ultrarapid, extensive, intermediate and poor metabolizers), including individuals homozygous for the CYP2C19*17 gain-of-function allele.
Materials & methods
Study design & patient population
This study was designed as a single-center retrospective review focusing on immunocompromised patients with cancer treated at St Jude Children’s Research Hospital (TN USA). Patients were prescribed voriconazole for either antifungal prophylaxis or treatment of an invasive fungal infection. Every patient genotyped for CYP2C19 who was prescribed oral voriconazole prior to 20 March 2013 and had at least one voriconazole plasma trough concentration determined was eligible for study inclusion. Any patient with an ambiguous CYP2C19 diplotype or any patient carrying a CYP2C19 allele of uncharacterized enzymatic function was excluded owing to the inability to clearly assign a phenotype. Individual voriconazole plasma trough concentrations were excluded if the concentration was obtained while a patient was on continuous oral feeds, the voriconazole concentration was not a trough, or the voriconazole concentration was not obtained at steady state. To be considered a trough concentration, the blood sample for voriconazole analysis must have been obtained within 2 h of the scheduled trough. Patients were considered to be at steady state after 5 days of voriconazole treatment without a loading dose or after 2 days of treatment following a loading dose [22]. Five individuals received intravenous voriconazole before being switched to an oral formulation; these patients must have been taking oral voriconazole for at least 2 days for the trough concentrations to be considered for analysis. The initial recommended voriconazole maintenance dose in patients 12 years of age and older was 400 mg/day (200 mg administered twice daily) [32–35], and in those less than 12 years of age the initial recommended voriconazole maintenance dose was 14 mg/kg/day (7 mg/kg administered twice daily) [23,32,34,36,37]. Although patients were counseled not to take oral voriconazole within 2 h of food, confirmation of the timing of voriconazole administration in relation to meals was not available. All patients were enrolled on an institutional review board-approved research protocol, Pharmacogenetic Determinants of Treatment Response in Children (PGEN5).
Every voriconazole plasma trough concentration (µg/ml) and the corresponding daily dose (mg/kg) was recorded along with the covariates age, ancestry, gender, and any prescribed drug documented to alter voriconazole plasma concentrations [4,22,38,39]. Ancestry was determined using DMET Plus (Affymetrix, CA, USA) genotyping results by applying a naive Bayesian classifier to cluster patients into four major groups (i.e., African, Asian, European or Hispanic ancestry) based on population-specific allele frequencies provided by Affymetrix. A particular ancestry group was assigned if the posterior probability was greater than 90%.
Genotyping & phenotype assignment
Genotyping was performed at the Medical College of Wisconsin (WI, USA) in a Clinical Laboratory Improvement Amendments-certified laboratory using the DMET Plus array. Previously, the DMET Plus genotyping results were demonstrated to be concordant with orthogonal genotyping methods [40]. DMET Plus interrogates 18 CYP2C19 genetic variants, which are translated into 16 possible CYP2C19 star (*)-alleles using the DMET Console Software (version 1.2; Affymetrix). Four CYP2C19 star-alleles (CYP2C19*1, *2A, *2B and *17) were observed in the patients meeting inclusion criteria. CYP2C19*1 is assigned by default when no genetic variants are detected, CYP2C19*2A is defined by rs4244285 (c.681G>A), CYP2C19*2B is defined by rs4244285 (c.681G>A) and rs17878459 (c.276G>C) and CYP2C19*17 is defined by rs12248560 (c. −806C>T). Nucleotide coordinates are annotated to GenBank CYP2C19 mRNA sequence M61854.1[41].
CYP2C19 phenotype assignment was based on Clinical Pharmacogenetics Implementation Consortium guidelines [28,30]. Patients with a CYP2C19*17/*17 or *1/*17 diplotype are predicted to be ultrarapid metabolizers, CYP2C19*1/*1 patients are predicted to be extensive metabolizers, patients with either a CYP2C19*1/*2A or *1/*2B diplotype are predicted to be intermediate metabolizers, and patients with a *2A/*2A diplotype are predicted to be poor metabolizers. Extensive metabolizers are considered to have normal, or wild-type, CYP2C19 metabolic activity.
Analysis of voriconazole plasma concentrations
Except for one sample, voriconazole plasma concentrations were measured at St Jude in a Clinical Laboratory Improvement Amendments-certified laboratory by reverse-phase HPLC with UV detection. Samples underwent liquid–liquid extraction in methyl tert-butyl ether and evaporation under nitrogen, followed by reconstitution in a mobile phase consisting of 20 mM sodium acetate (pH 4.88) in acetonitrile at a ratio of 49:51 (v:v). Analytical chromatography was performed by injecting the samples onto a YMC™C8 (YMC America, Inc. PA, USA) reverse phase column (150 mm × 4.6 mm, 3.0 µm particle size) at a flow rate of 1.0 ml/min and a temperature of 35°C using isocratic elution as the mode of separation. Voriconazole concentrations were calculated by comparing the peak height ratio of the drug with the internal standard ketoconazole against a four-point calibration curve. The lower limit of voriconazole detection was 0.025 µg/ml and the upper limit of detection was 30 µg/ml. As part of our validation process, selected patient samples (n = 23) were measured in our laboratory and were also sent to an external reference laboratory (ARUP Laboratories, UT, USA) that used liquid chromatography– tandem mass spectrometry (LC–MS/MS). The median percentage difference between measurements using the two methods was 6% (range: 0.3–21; n = 23). One sample was analyzed outside of St Jude by a reference laboratory that used HPLC for analysis.
Pharmacokinetic analysis
For purposes of estimating doses needed to achieve therapeutic concentrations, a two-compartment nonlinear pharmacokinetic model with Michaelis–Menten elimination was used to describe the pharmacokinetics of voriconazole, as has been used previously [23,36,42,43]. Because only voriconazole plasma trough concentrations were measured in this study, the absorption rate (Ka), bioavailability (f), Michaelis–Menten half-saturation (Km), volume of distribution of the peripheral compartment (Vp), and intercompartmental clearance (Q) values were obtained from a previously reported voriconazole population pharmacokinetic analysis [23]. The volume of the central compartment (V) and Michaelis–Menten maximum activity (Vmax) along with interindividual and interoccasion variability were estimated using nonlinear mixed-effects modeling as implemented in Monolix (V4.2). The covariate effects of age and CYP2C19 diplotype were included in the parameter Vmax. The model was internally tested to determine how well the post hoc estimated pharmacokinetic parameters could predict trough concentrations at higher doses. Specifically, for those patients who had multiple voriconazole trough concentrations reported, we used each patient’s post hoc estimated pharmaco kinetic parameters determined at each of their lower voriconazole doses to predict the trough concentration at the highest prescribed dose. The predicted trough concentrations were then compared with observed trough concentrations corresponding to the highest prescribed voriconazole dose. Simulation studies using each individual’s post hoc estimated pharmacokinetic parameters were used to determine a voriconazole dose for each diplotype/age group that increased the percentage of predicted day 5 voriconazole trough concentrations within the goal therapeutic range of 1–6 µg/ml relative to a fixed dose.
Statistical analysis
The χ2 test was used to compare patient characteristics between age groups. A linear mixed-effects model was used to compare the relationship between CYPC19 diplotypes and voriconazole trough concentrations corrected for daily voriconazole dose. Linear mixed-effects modeling of all the log2 transformed voriconazole trough concentrations corrected for daily dose was also used to identify covariates (age, ancestry, gender, CYP2C19 diplotype and interacting drugs) that may influence voriconazole plasma concentrations, with CYP2C19 diplotypes treated as an ordinal variable. For pharmacokinetic analysis, the McNemar’s χ2 test was used to compare the number of voriconazole troughs predicted to be within the therapeutic range based on extrapolated doses versus the observed number of voriconazole troughs in the therapeutic range. The significance of covariates that may affect Vmax was determined using the χ2 test (to compare the difference in the −2 log-likelihood between the two hierarchical models) and the t-test (to determine if the covariate term was significantly different from zero).
Results
The clinical and demographic characteristics of the 33 patients who qualified for this study are summarized in Table 1. The study population was stratified by age (i.e., <12 years and >12 years) to determine if there was equal representation of younger and older patients among the covariates ancestry, gender and CYP2C19 diplotypes. There were no significant differences between the two age groups in any of the covariates. Overall, 58% of patients were 11 years of age and younger and 42% of patients were 12 years or older. The observed allele frequencies for CYP2C19*1, *2 and *17 were 59.1, 16.7 and 24.2%, respectively. Based on average allele frequencies observed in European, Hispanic and African ancestry groups, the expected allele frequencies of our patient population for CYP2C19*1,*2 and *17 is approximately 64, 15 and 20%, respectively [28].
Table 1.
Patient characteristics (n = 33).
| Characteristic | All patients | <12 years of age | ≥ 12 years of age | p-value |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Female | 14 (42.4) | 9 | 5 | 0.72 |
| Male | 19 (57.6) | 10 | 9 | |
| Ancestry, n (%) | ||||
| African | 6 (18.2) | 4 | 2 | 0.97† |
| European | 23 (69.6) | 12 | 11 | |
| Hispanic | 2 (6.1) | 2 | 0 | |
| Multiple race | 2 (6.1) | 1 | 1 | |
| CYP2C19 diplotype, n (%) | ||||
| *17/*17 | 4 (12.1) | 2 | 2 | 0.15‡ |
| *1/*17 | 8 (24.3) | 6 | 2 | |
| *1/*1 | 11 (33. 3) | 8 | 3 | |
| *1/*2A*1/*2B | 9 (27.3) | 3 | 6 | |
| *2A/*2A | 1 (3.0) | 0 | 1 | |
| Primary diagnosis, n (%) | ||||
| Acute lymphoblastic leukemia | 12 (36.4) | 7 | 5 | 0.48§ |
| Acute myeloid leukemia | 13 (39.4) | 6 | 7 | |
| Non-Hodgkin lymphoma | 3 (9.1) | 2 | 1 | |
| Other¶ | 5 (15.1) | 4 | 1 | |
| Age, n (%) | 19 (57.6) | 14 (42.4) | ||
| Median (years) | 9.0 | |||
| Range (years) | 1–19 | |||
| Weight | ||||
| Median (kg) | 19.4 | 71.6 | ||
| Range (kg) | 8.6–57.8 | 4 0 –110 | ||
Hispanic and multiple race were combined for statistical analysis
CYP2C19*1/*2A,*1/*2B and *2A/*2A diplotype groups were combined for statistical analysis
Acute lymphoblastic and myeloid leukemia groups were combined for statistical analysis.
Ganglioglioma, Ewing’s sarcoma, germ cell tumor, Hodgkin lymphoma and pineoblastoma
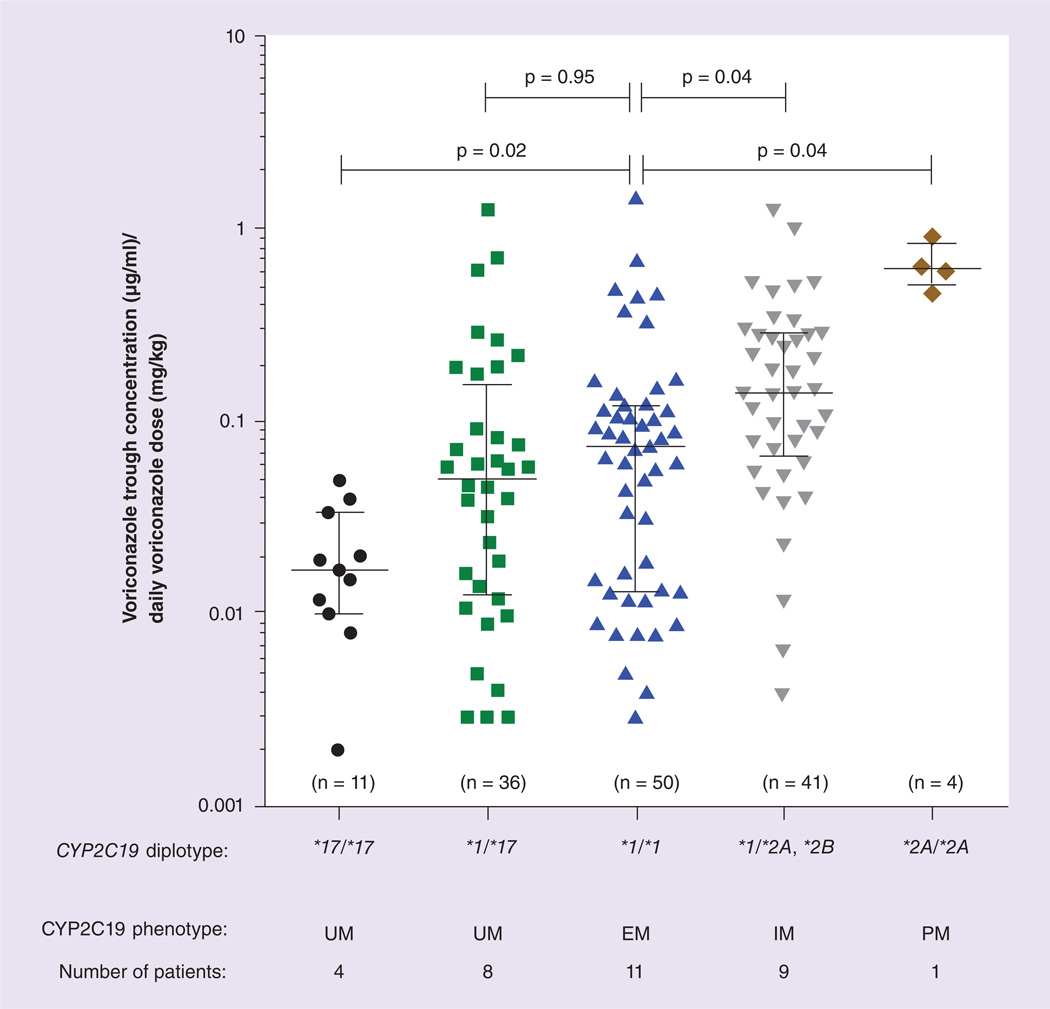
The voriconazole doses administered ranged from 2.6 to 41.2 mg/kg/day among those aged less than 12 years; doses ranged from 3.6 to 16.1 mg/kg/day for those children aged 12 years or older (Table 2). A total of 142 voriconazole plasma trough concentrations were analyzed, with a median of three voriconazole concentrations per patient (range: 1–15). The relationship between individual voriconazole trough concentrations corrected for the daily voriconazole dose and CYP2C19 diplotypes is depicted in Figure 1. Patients homozygous for CYP2C19*17 had lower dose-normalized trough voriconazole concentrations (median: 0.01 µg/ml/mg/kg; range: 0.002–0.05; p = 0.02) and patients with a CYP2C19*1/*2A, *1/*2B diplotype (median: 0.14 µg/ml/mg/kg; range: 0.004–1.24; p = 0.04) or CYP2C19*2A/*2A diplotype (median: 0.62 µg/ml/mg/kg; range:0.47–0.91; p = 0.04) had higher dose-normalized trough voriconazole concentrations than those with a CYP2C19*1/*1 diplotype (median: 0.07 µg/ml/mg/kg; range: 0.003–1.47). Patients heterozygous for CYP2C19*17 did not have significantly lower dose-normalized trough voriconazole concentrations (median: 0.05 µg/ml/mg/kg; range: 0.003–1.26; p = 0.95) than those with a CYP2C19*1/ *1 diplotype.
Table 2.
Observed median voriconazole dose and corresponding median trough concentration stratified by CYP2C19 diplotype and age, and extrapolated voriconazole dose predicted to achieve a steady-state trough concentration of 1–6 µg/ml.
| CYP2C19 diplotype | Observed median voriconazole dose mg/kg/day (range) |
Observed median voriconazole trough concentration, µg/ml (range) |
Extrapolated voriconazole dose to achieve a therapeutic concentration in majority of patients, mg/kg/day |
Predicted median voriconazole trough concentration based on the extrapolated dose, µg/ml (range) |
|---|---|---|---|---|
| Patients <12 years of age | ||||
| CYP2C19*171*17 (n = 2)† | 14.05(9.3–22.2) | 0.17(0.030.38) | 36 | 0.88(0.44–1.22) |
| CYP2C19*1/*17 (n = 6) | 19.2(8.6–33.1) | 0.90(0.06–10.89) | 30 | 1.72 (0.09–49.65) |
| CYP2C19*1/*1 (n = 8) | 14.9(2.6–41.2) | 0.82 (0.03–20.17) | 20 | 1.89(0.11–34.07) |
| CYP2C19*1/*2A,*1/*2B (n = 3) | 23 (15.9–34.6) | 2.71 (0.20–8.46) | 18 | 1.82 (0.66–5.70) |
| CYP2C19*2A/*2A (n = 0) | - | - | - | - |
| Patients ≥12 years of age | ||||
| CYP2C19*17/*17(n = 2) | 7.6(6.2–11.5) | 0.30(0.05–0.49) | 28 | 1.76(1.13–3.03) |
| CYP2C19*1/*17(n = 2) | 9.5 (6.3–13.6) | 1.73(0.03–6.99) | 14 | 3.22(0.06–10.11) |
| CYP2C19*1/*1 (n = 3) | 8.3(3.9–16.1) | 1.39(0.05–3.57) | 14 | 2.21 (0.19–8.10) |
| CYP2C19*1/*2A,*1/*2B (n = 6) | 8.3(3.6–16.0) | 1.64(0.03–11.21) | 10 | 2.66(0.06–12.41) |
| CYP2C19*2A/*2A (n = 1) | 5.9(4.2–6.3) | 3.69 (2.97–3.86) | 4 | 2.30(1.80–3.35) |
(n) represents the number of patients per group.
Figure 1. Linear mixed-effects model analysis of the relationship between CYP2C19 diplotypes and voriconazole trough concentrations corrected for daily voriconazole dose.
A total of 142 voriconazole trough concentrations grouped by CYP2C19 diplotype were measured in 33 children, where (n) is the number of voriconazole concentrations measured in each diplotype group.
EM: Extensive metabolizer; IM: Intermediate metabolizer; PM: Poor metabolizer; UM: Ultrarapid metabolizer.
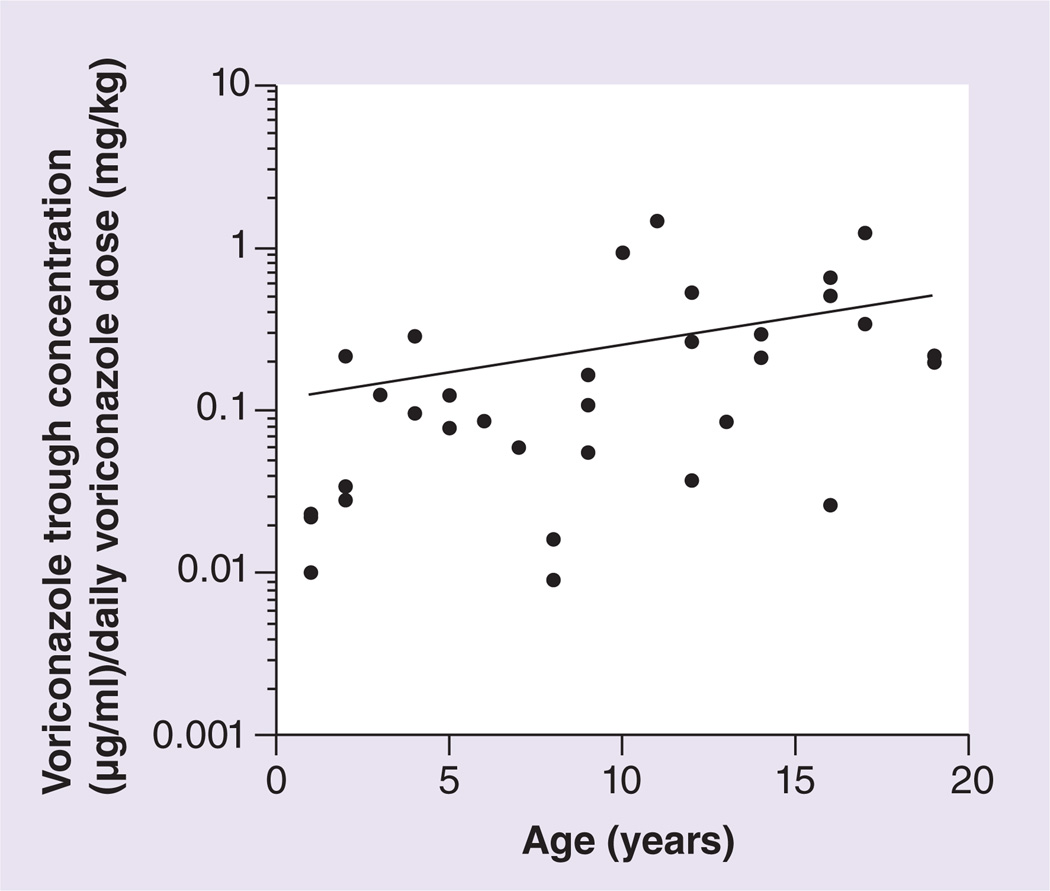
Other antifungals (fluconazole and posaconazole), proton pump inhibitors (omeprazole and pantoprazole) and steroids (dexamethasone, hydrocortisone and methylprednisolone) were prescribed to patients included in this study, all of which may alter voriconazole plasma concentrations. For approximately 7% of trough concentrations obtained from CYP2C19*1/*17 or CYP2C19*1/*2A, *1/*2B patients, fluconazole or posaconazole was coadministered with voriconazole; these agents were not concurrently taken with voriconazole in CYPC19*1/*1 patients. Proton pump inhibitors were coadministered with voriconazole for 58% of the trough concentrations obtained from CYP2C19*1/*17 patients, 24% of trough concentrations obtained from CYP2C19*1/*1 patients, and 39% of trough concentrations obtained from CYP2C19*1/*2A, *1/*2B patients. Steroids were coadministered with voriconazole for 11% of the trough concentrations obtained from CYP2C19*1/*17 patients, 8% of trough concentrations obtained from CYP2C19*1/*1 patients, and 37% of trough concentrations obtained from CYP2C19*1/*2A, *1/*2B patients. Although one might predict steroids would induce metabolism of voriconazole (and thus decrease trough levels), those carrying a low function CYP2C19 allele still tended to have higher trough concentrations despite a slightly higher frequency of steroid use. Those who were either a CYP2C19*17 homozygote or CYP2C19*2A homozygote were not administered any known CYP2C19 inducers or inhibitors. With this small sample size, a multivariate analysis detected no significant effect of these medications, nor ancestry and gender, on voriconazole trough concentrations corrected for daily dose, but CYP2C19 diplotype and age did have a significant impact (Table 3). Consistent with the previously established relationship between older age and voriconazole plasma concentrations, there was a correlation between age and higher voriconazole trough concentrations corrected for daily dose (Figure 2) [3,21,24,36].
Table 3.
Characteristics associated with dose-corrected voriconazole trough concentrations.
| Covariate | Coeffcient | p-value† |
|---|---|---|
| CYP2C19 diplotype‡ | 0.85 | 0.002 |
| Age | 0.08 | 0.05 |
| Ancestry | ||
| Caucasian | – | – |
| African–American | − 0.18 | NS |
| Hispanic | 0.58 | NS |
| Multiple race | 1.06 | NS |
| Gender | ||
| Female | – | – |
| Male | 0.22 | NS |
| Antifungals | ||
| Not concomitantly administered | – | – |
| Concomitantly administered | 0.83 | NS |
| Proton pump inhibitors | ||
| Not concomitantly administered | – | – |
| Concomitantly administered | −0.30 | NS |
| Steroids | ||
| Not concomitantly administered | – | – |
| Concomitantly administered | 0.64 | NS |
The p-value indicates if the covariate significantly affected dose-corrected voriconazole plasma concentrations in a linear mixed-effects model.
CYP2C19 diplotypes were treated as an ordinal variable. Increasing numerical ordinal scores are representative of a predicted decrease in CYP2C19 catalytic activity (i.e., CYP2C19*2A/*2A > CYP2C19*1/*2A,*1/*2B > CYP2C19*1/*1 > CYP2C19*1/*17 > CYP2C19*17/*17).
NS: Not significant.
Figure 2.
Correlation of age with voriconazole trough concentrations corrected for daily voriconazole dose (r2 = 0.16; p < 0.05).
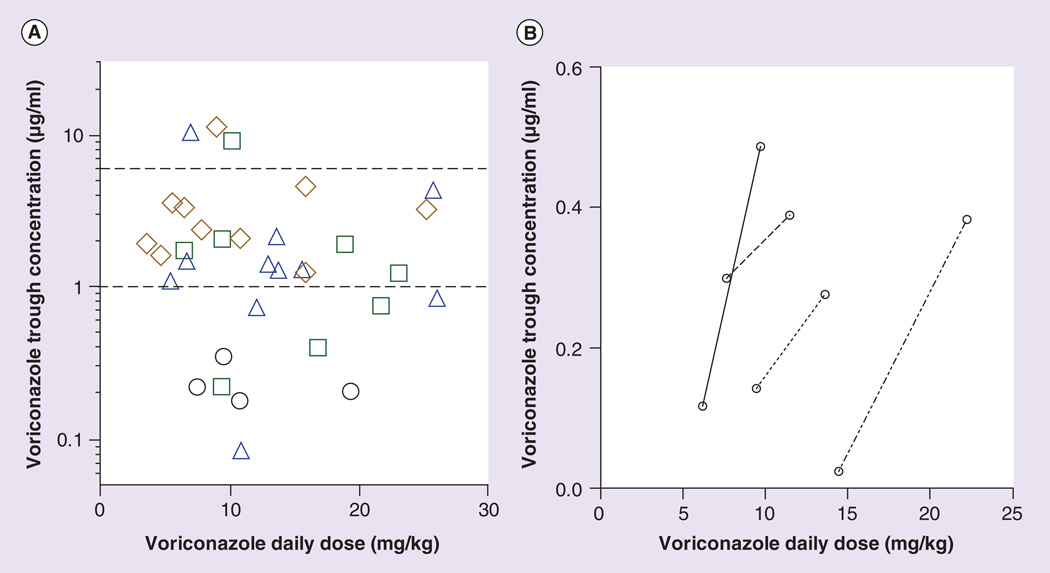
Voriconazole plasma trough concentrations between 1 and 6 µg/ml were considered to be therapeutic. Therapeutic voriconazole concentrations were not observed in any of the four patients homozygous for CYP2C19*17 (Figure 3A); however, increasing the voriconazole dose in these patients yielded higher voriconazole concentrations than did lower doses (Figure 3B), suggesting that higher voriconazole doses may overcome ultrarapid metabolism caused by the CYP2C19*17 allele. Approx- imately 38% of patients heterozygous for CYP2C19*17 had a mean voriconazole concentration that was sub-therapeutic, and 27% of CYP2C19*1 homozygotes had a mean concentration that was subtherapeutic. Because only one patient was homozygous for CYP2C19*2A, the CYP2C19*1/*2A, *1/*2B and *2A/*2A diplotype groups were combined for determining the number of patients outside therapeutic voriconazole trough concentrations. None of the patients with these diplotypes had a mean voriconazole concentration that was sub-therapeutic, with one patient having a supratherapeutic concentration (Figure 3A).
Figure 3. Relationship between CYP2C19 diplotypes and voriconazole trough concentrations.
(A) A scatter plot of the mean voriconazole trough concentration per patient versus the mean voriconazole daily dose. Patients with a CYP2C19*17/*17 diplotype (predicted ultrarapid metabolizers) are represented by black circles, patients with a CYP2C19*1/*17 diplotype (predicted ultrarapid metabolizers) are represented by green squares, patients with a CYP2C19*1/*1 diplotype (predicted extensive metabolizers) are represented by blue triangles, and patients with a CYP2C19*1/*2A*1/*2B or *2A/*2A diplotype (predicted intermediate or poor metabolizers) are represented by brown diamonds. The voriconazole plasma concentrations located between the dotted lines are considered to be therapeutic concentrations. (B) A plot of the first initial voriconazole trough concentration and the last measured voriconazole concentration versus daily dose of the four CYP2C19*17 homozygous patients. Please see color figure at www.futuremedicine.com/doi/pdf/10.2217/pgs.14.53
The population pharmacokinetic model, using the parameters in Table 4, resulted in a relationship between the observed trough concentrations and the predicted trough concentrations with an r2 = 0.97, p < 10−3, and a relative mean absolute error (expressed as a percentage of the predicted concentration) of 19%. Consistent with the observed voriconazole trough concentrations, the population pharmacokinetic parameter Vmax decreased with age (p < 1 × 10−7) and was significantly higher in the CYP2C19*17/*17 patients (p = 0.002; Table 4). Internal testing of our population pharmacokinetic model was performed by using post hoc estimated pharmacokinetic parameters determined at lower voriconazole doses to predict trough concentrations at the highest prescribed doses. Comparing predicted voriconazole concentrations to observed concentrations, our population pharmacokinetic model predicted trough concentrations with a median error of −0.3 µg/ml, a 26% error relative to observed concentrations.
Table 4.
Population pharmacokinetic parameters.
| Parameter | Parameter value | RSE (%) | IIV (%) | IOV (%) | p-value† |
|---|---|---|---|---|---|
| Estimated parameters | |||||
| V (l/kg) | 3.37 | 43 | 169 | 161 | – |
| Vmax (µg/h/kg) | 3.36 | 27 | 42 | 39 | – |
| Effect of covariates on Vmaxठ| |||||
| Age | − 0 .147 | 18 | – | – | <1 × 10−7 |
| CYP2C19*17/*17 | 1.27 | 32 | – | – | 0.002 |
| CYP2C19*1/*17 | − 0 .147 | 212 | – | – | NS |
| CYP2C19*1/*2A*1/*2B | −0.444 | 70 | – | – | NS |
| CYP2C19*2A/*2A | − 0. 419 | 362 | – | – | NS |
| Fixed parameters | |||||
| Ka (l/h) | 0.849 | ||||
| f | 0.446 | ||||
| Km (µg/ml) | 3 | ||||
| Q (l/h/kg) | 0.609 | ||||
| Vp(l/kg) | 2 .17 | ||||
The p-value indicates if the covariate significantly affected V max
CYP2C19*1/*1 reference diplotype.
Covariate model: Vmax = θeb1·age+bi·(CYP2C19i) where CYP2C19i = (*17/*17,*1/*17,*1/*2A,*2A/*2A).
f: Bioavailability; IIV: Interindividual variability; IOV: Interoccasion variability; Ka: Absorption rate; Km: Michaelis–Menten half-saturation; NS: Not significant; RSE: Relative standard error; Q: Intercompartmental clearance; Vp: Volume of the peripheral compartment; Vmax: Michaelis–Menten maximum activity.
Using each individual’s post hoc estimated pharmacokinetic parameters, a voriconazole daily dose was extrapolated for each CYP2C19 diplotype/age group to increase the number of voriconazole troughs predicted to be in the therapeutic range. Table 2 shows the median (range) of the predicted trough concentrations based on these extrapolated doses. The proportion of voriconazole troughs within the therapeutic range using extrapolated doses (60%) was predicted to be higher (p < 0.03) than the proportion observed to be within the therapeutic range with standard dosing (46.5%), while achieving fewer troughs (28%) below the therapeutic range (compared with 44.4% observed) and maintaining a similar percentage above the therapeutic range (12% compared with 9.2% observed). Considering the CYP2C19*17/*17 patients, all 11 observed voriconazole troughs were less than 1 µg/ml (median trough: 0.21 µg/ml). However, simulations using extrapolated doses (based on age and diplotype) of 36 mg/kg/day for those less than 12 years of age and 28 mg/kg/day for those 12 years of age or greater predicted that eight of the 11 trough concentrations (73%) in this group would be in the therapeutic range (median trough = 0.88 µg/ml for age <12 years and median trough = 1.76 µg/ml for age ≥12 years; Table 2), with three troughs predicted to be below the therapeutic range.
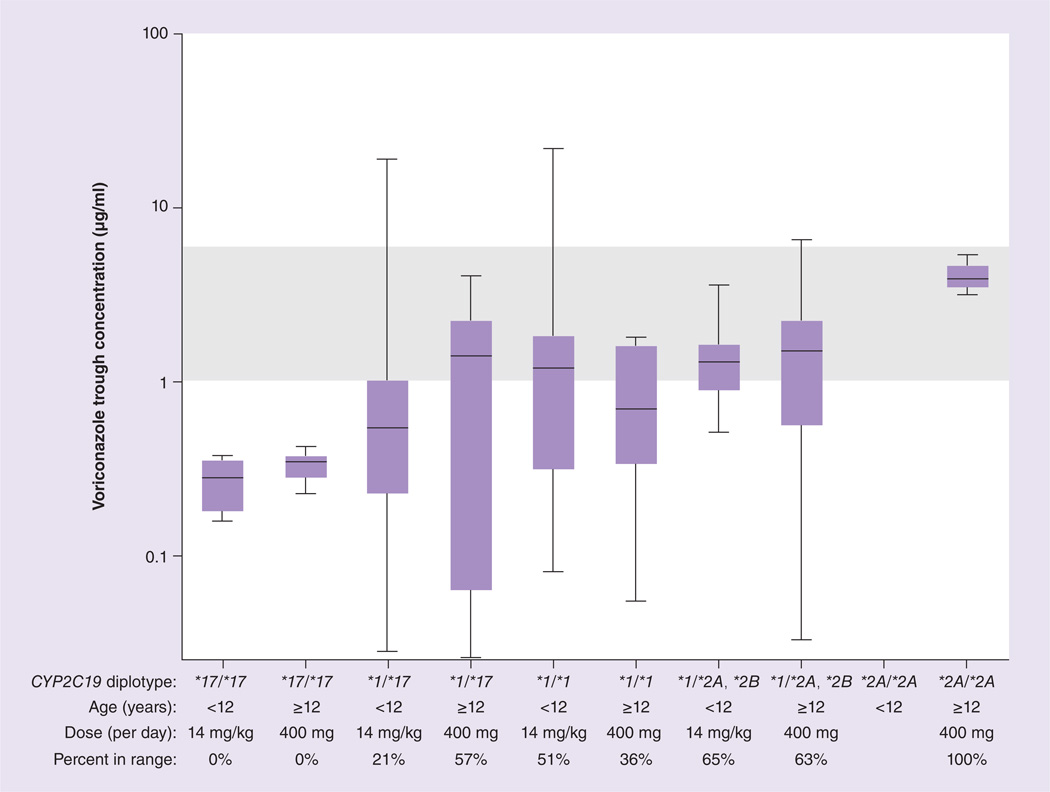
Based on population pharmacokinetic parameters (Table 4), voriconazole trough concentrations for each CYP2C19 diplotype/age group were simulated for the initial maintenance voriconazole doses currently used at St Jude (14 mg/kg/day for patients less than 12 years of age and 400 mg/day for patients 12 years of age or greater; Figure 4) and for the extrapolated voriconazole doses based on CYP2C19 diplotype and age (Figure 5). The diplotype/age-based voriconazole extrapolated doses were predicted to yield a higher percentage of trough concentrations in the therapeutic range than the predictions based on the standard initial St Jude maintenance doses (p = 1 × 10−10).
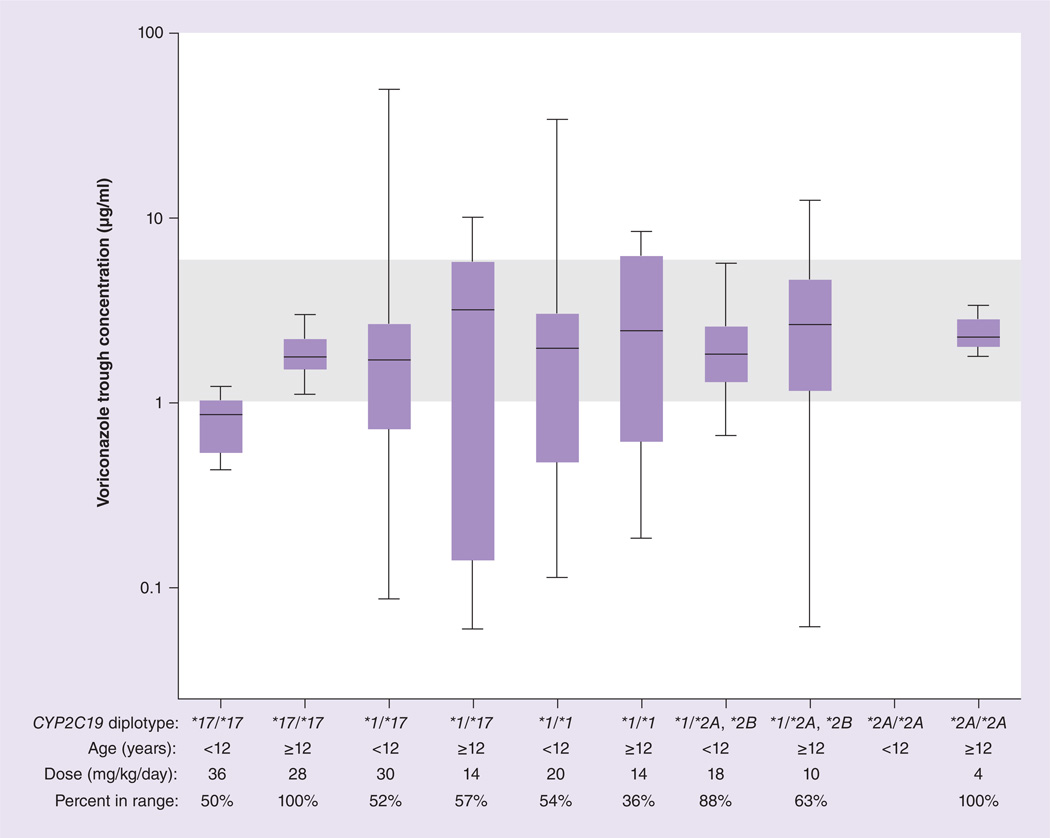
Figure 4. Voriconazole trough concentrations observed for actual voriconazole doses prescribed and percentage of patients in the goal therapeutic range of 1–6 µg/ml (shaded area).
Boxes indicate 25th to 75th percentile, and whiskers indicate the minimum and maximum values.
Figure 5. Simulated voriconazole doses based on age and CYP2C19 diplotype that are predicted to result in trough concentrations in the therapeutic range of 1–6 µg/ml (shaded area).
Variability in each diplotype group is based on actual pharmacokinetic variability observed in patients in each group. Boxes indicate 25th to 75th percentile, and whiskers indicate the minimum and maximum values.
Discussion
In both adult and pediatric populations, CYP2C19 intermediate and poor metabolizers have been demonstrated to have elevated voriconazole plasma concentrations when compared with extensive metabolizers [3,31,44–47]. However, the clinical importance of CYP2C19 phenotypic variability in relation to voriconazole treatment is controversial. It has been proposed that in a clinical setting, covariates such as comorbidities or concomitant drugs may diminish the influence of CYP2C19 polymorphisms on voriconazole concentrations [18,19]. In our clinical setting, we were able to detect that CYP2C19 diplotype was significantly associated with voriconazole plasma concentrations in immunocompromised pediatric patients, even in the presence of covariates such as concomitantly administered drugs. Individuals predicted to be CYP2C19 intermediate or poor metabolizers had higher dose-corrected voriconazole trough concentrations versus extensive metabolizers, which is consistent with a recent study in pediatric patients [31].
There are limited data in adults, and a lack of data in pediatrics, demonstrating that CYP2C19 ultrarapid metabolizers have decreased voriconazole plasma concentrations when compared with extensive metabolizers [46–49]. In a study of healthy Chinese adults, it was shown that even those with only one CYP2C19*17 allele (CYP2C19*1/*17) had approximately 50% lower voriconazole exposure versus CYP2C19*1 homozygotes [50]. Our data in a pediatric population demonstrates that the CYP2C19*17 allele is associated with lower dose-corrected voriconazole trough concentrations when compared with CYP2C19*1 homozygotes, especially in those homozygous for the CYP2C19*17 allele.
Because nontherapeutic voriconazole plasma concentrations are associated with unfavorable treatment outcomes, we investigated whether CYP2C19 polymorphisms were correlated with either supratherapeutic or subtherapeutic voriconazole concentrations [9–11,51]. A therapeutic range for voriconazole has not been well defined, but generally trough plasma concentrations less than 1 µg/ml have been associated with treatment failure and concentrations greater than 6 µg/ml have been associated with adverse effects such as neurotoxicity [9,10,14,38,52]. There is increasing evidence to support a minimum therapeutic voriconazole concentration of 2 µg/ml [9,51,53], but for the purpose of our study a trough concentration of 1 µg/ml was considered therapeutic. Only three patients in our study had mean voriconazole concentrations above the therapeutic range, but the group most likely to have supratherapeutic concentrations, CYP2C19 poor metabolizers, was not well represented in this study. The four patients included in our study who were homozygous for CYP2C19*17 never attained therapeutic voriconazole concentrations at any dosage. Additionally, every patient homozygous for CYP2C19*17 had an initial steady-state voriconazole trough concentration that was less than 0.35 µg/ml, which may be a risk factor for increased mortality [12] . Higher voriconazole concentrations were observed in CYP2C19*17 homozygous patients with increased voriconazole doses (Figure 3B), although doses as high as those predicted to be necessary to yield therapeutic voriconazole concentrations (Table 2) were not used.
All children at least 12 years of age in this study weighed a minimum of 40 kg, therefore the initial recommended voriconazole maintenance dose for our patients 12 years of age and older in this study was 400 mg/day [32–35] with a recommended maximum daily dose of 600 mg/day [34]. In those less than 12 years of age, the initial recommended maintenance dose is less clear with suggested maintenance doses ranging from 8 to 18 mg/kg/day [54,35]. There is evidence to support an initial voriconazole maintenance dose of 14 mg/kg/day in patients less than 12 years of age [23,32,34–36], and this is generally the initial maintenance dose utilized at St Jude. We used pharmacokinetic modeling for the purpose of extrapolating a voriconazole dose predicted to achieve a plasma trough concentration of 1–6 µg/ml in patients stratified by CYP2C19 diplotype and age (Table 2 & Figure 5) [23,36,42,41,36]. Because initial low voriconazole trough concentrations are associated with unfavorable outcomes, a voriconazole starting dose high enough to achieve therapeutic concentrations may be of benefit [12,51,53,55]. Our predictions suggest that, except for CYP2C19 poor metabolizers, most children <12 years of age should receive starting doses of voriconazole above the recommended dose of 14 mg/kg/day, and for all children, doses based on age and CYP2C19 status should theoretically decrease variability in voriconazole trough concentrations. For patients in our study who were CYP2C19*17 homozygotes, an extrapolated voriconazole dose that is approximately three-times higher than the initial recommended maintenance dose (14 mg/kg/day for those aged less than 12 years and 400 mg/day for those aged 12 years and older) was predicted to be necessary to yield a steady-state trough concentration of 1–6 µg/ml. The extrapolated voriconazole dose for CYP2C19*17 homozygotes aged 12 years and older in this study is similar to, though higher than, the dose that was required (800 mg/day) to reach a trough concentration of 1–2 µg/ml in an adolescent cystic fibrosis patient who was homozygous for CYP2C19*17 [46]. For the purpose of pharmacokinetic modeling, the voriconazole dosage (Table 2) was expressed as mg/kg/day in individuals aged 12 years and older. Because we stratified our patient population by CYP2C19 diplotype and age we had relatively few patients in each CYP2C19 diplotype/age group. Other factors that may influence our population pharmacokinetic model include the inability to determine if all patients were in a fasting state. Our patient population ranged from 1 to 19 years of age. Younger individuals may have lower voriconazole bioavailability [22–24], though it is unclear if decreased bioavailability is due to increased CYP2C19 expression, differences in intestinal metabolism or drug transport, or differences in hepatic blood flow [7,23]. Correlation of treatment outcomes or voriconazole-induced adverse events with CYP2C19 diplotypes was not investigated in this retrospective analysis.
Steroids may upregulate CYP2C19 expression through a glucocorticoid-response element found in its promoter region, thereby resulting in decreased voriconazole concentrations [38,49,56], but in our study, steroid use was more common in the diplotypes with higher voriconazole concentrations, suggesting that genotype may have been more important than concurrent drug use in this case. Coadministration of voriconazole with CYP2C19 substrates such as proton pump inhibitors or other antifungals may increase voriconazole plasma concentrations due to competitive inhibition [38]; however, an analysis of over 3300 voriconazole concentrations obtained from 240 patients did not identify proton pump inhibitors as a significant covariate influencing voriconazole metabolism [49]. Although concurrent drugs are likely to interact with voriconazole, we found no evidence of pharmacokinetic interactions in our study, though our study was not powered to detect such differences. Similar to our study, an investigation of 406 voriconazole concentrations obtained from 151 patients found that CYP2C19 genotype greatly influenced voriconazole pharmacokinetics while proton pump inhibitors or steroids did not significantly influence voriconazole concentrations, though study size hampered covariate analysis [57]. Nonetheless, steroids, proton pump inhibitors or other antifungals were not predictive of voriconazole concentrations, whereas, in agreement with prior studies [3,21,24,36,51], age and CYP2C19 status were.
Conclusion
In this relatively small cohort of immunosuppressed patients exposed to multiple concomitant drugs, we found that CYP2C19 diplotype was significantly associated with voriconazole trough concentrations in pediatric patients, even when adjusting for age and other clinical covariates. We suggest that tailoring the starting dosage of voriconazole, based on age and CYP2C19 diplotype, is a reasonable approach to attempt to reach therapeutic voriconazole concentrations, particularly when accompanied by therapeutic drug monitoring of voriconazole plasma concentrations.
Future perspective
There is a growing body of evidence demonstrating that CYP2C19 genetic variants and age influence voriconazole plasma concentrations. There is substantial evidence for interpatient variability, and for pharmacodynamic associations between response/toxicity and voriconazole concentrations. Future evaluations of whether dosing based on age and CYP2C19 status yields a higher proportion of patients being in the goal range for voriconazole are needed.
Executive summary.
Background
Pediatric patients have large interindividual variation in voriconazole pharmacokinetic parameters, which may contribute to delays in achieving therapeutic voriconazole trough concentrations.
CYP2C19 is one of the main enzymes responsible for the metabolism of voriconazole, and genetic variants in the CYP2C19 gene locus may alter voriconazole metabolism thus contributing to the observed inter individual variations in voriconazole trough concentrations.
Results
CYP2C19*17 homozygotes had lower dose-corrected voriconazole trough concentrations than extensive metabolizers (CYP2C19*1/*1) and never attained therapeutic voriconazole trough concentrations
Increasing the voriconazole dose in CYP2C19*17/*17 patients yielded higher voriconazole concentrations, suggesting that higher voriconazole doses in these patients may at least partly overcome their pharmacokinetic disadvantage.
Intermediate and poor metabolizers (CYP2C19*1/*2A,*1/*2B) had higher dose-corrected voriconazole trough concentrations than extensive metabolizers.
Conclusion
CYP2C19 diplotypes were significantly associated with voriconazole trough concentrations in pediatric patients, even when adjusting for age and other clinical covariates.
Our data support the utilization of CYP2C19 genotyping and age to individualize starting doses of voriconazole.
Acknowledgments
Supported by NCI grants CA 36401 and CA 21765, NIH/NIGMS Pharmacogenomics Research Network (U01 GM92666), and by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved
References
Papers of special note have been highlighted as:
• of interest
- 1.Groll AH, Kurz M, Schneider W, et al. Five-year-survey of invasive aspergillosis in a paediatric cancer centre. Epidemiology, management and long-term survival. Mycoses. 1999;42:7–8. doi: 10.1046/j.1439-0507.1999.00496.x. 431–442. [DOI] [PubMed] [Google Scholar]
- 2.Walsh TJ, Gonzalez C, Lyman CA, Chanock SJ, Pizzo PA. Invasive fungal infections in children: recent advances in diagnosis and treatment. Adv. Pediatr. Infect. Dis. 1996;11:187–290. [PubMed] [Google Scholar]
- 3.Walsh TJ, Karlsson MO, Driscoll T, et al. Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration. Antimicrob. Agents Chemother. 2004;48(6):2166–2172. doi: 10.1128/AAC.48.6.2166-2172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandherr M, Maschmeyer G. Pharmacology and metabolism of voriconazole and posaconazole in the treatment of invasive aspergillosis: review of the literature. Eur. J. Med. Res. 2011;16(4):139–144. doi: 10.1186/2047-783X-16-4-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purkins L, Wood N, Greenhalgh K, Allen MJ, Oliver SD. Voriconazole, a novel wide-spectrum triazole: oral pharmacokinetics and safety. Br. J. Clin. Pharmacol. 2003;56(Suppl. 1):10–16. doi: 10.1046/j.1365-2125.2003.01993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 2008;46(3):327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 7.Walsh TJ, Driscoll T, Milligan PA, et al. Pharmacokinetics, safety, and tolerability of voriconazole in immunocompromised children. Antimicrob. Agents Chemother. 2010;54(10):4116–4123. doi: 10.1128/AAC.00896-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wingard JR, Carter SL, Walsh TJ, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116(24):5111–5118. doi: 10.1182/blood-2010-02-268151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith J, Safdar N, Knasinski V, et al. Voriconazole therapeutic drug monitoring. Antimicrob. Agents Chemother. 2006;50(4):1570–1572. doi: 10.1128/AAC.50.4.1570-1572.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 2008;46(2):201–211. doi: 10.1086/524669. [DOI] [PubMed] [Google Scholar]
- 11.Goodwin ML, Drew RH. Antifungal serum concentration monitoring: an update. J. Antimicrob. Chemother. 2008;61(1):17–25. doi: 10.1093/jac/dkm389. [DOI] [PubMed] [Google Scholar]
- 12.Miyakis S, Van Hal SJ, Ray J, Marriott D. Voriconazole concentrations and outcome of invasive fungal infections. Clin. Microbiol. Infect. 2010;16(7):927–933. doi: 10.1111/j.1469-0691.2009.02990.x. • Demonstrates that initial low voriconazole trough concentrations are associated with mortality.
- 13.Philips JA, Marty FM, Stone RM, Koplan BA, Katz JT, Baden LR. Torsades de pointes associated with voriconazole use. Transpl. Infect. Dis. 2007;9(1):33–36. doi: 10.1111/j.1399-3062.2006.00160.x. [DOI] [PubMed] [Google Scholar]
- 14.Fallon RM, Girotto JE. A review of clinical experience with newer antifungals in children. J. Pediatr. Pharmacol. Ther. 2008;13(3):124–140. doi: 10.5863/1551-6776-13.3.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alffenaar JW, De Vos T, Uges DR, Daenen SM. High voriconazole trough levels in relation to hepatic function: how to adjust the dosage? Br. J. Clin. Pharmacol. 2009;67(2):262–263. doi: 10.1111/j.1365-2125.2008.03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyd AE, Modi S, Howard SJ, Moore CB, Keevil BG, Denning DW. Adverse reactions to voriconazole. Clin. Infect. Dis. 2004;39(8):1241–1244. doi: 10.1086/424662. [DOI] [PubMed] [Google Scholar]
- 17.Zonios DI, Gea-Banacloche J, Childs R, Bennett JE. Hallucinations during voriconazole therapy. Clin. Infect. Dis. 2008;47(1):e7–e10. doi: 10.1086/588844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Driscoll TA, Frangoul H, Nemecek ER, et al. Comparison of pharmacokinetics and safety of voriconazole intravenous-to-oral switch in immunocompromised adolescents and healthy adults. Antimicrob. Agents Chemother. 2011;55(12):5780–5789. doi: 10.1128/AAC.05010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Driscoll TA, Yu LC, Frangoul H, et al. Comparison of pharmacokinetics and safety of voriconazole intravenous-to-oral switch in immunocompromised children and healthy adults. Antimicrob. Agents Chemother. 2011;55(12):5770–5779. doi: 10.1128/AAC.00531-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazarus HM, Blumer JL, Yanovich S, Schlamm H, Romero A. Safety and pharmacokinetics of oral voriconazole in patients at risk of fungal infection: a dose escalation study. J. Clin. Pharmacol. 2002;42(4):395–402. [PubMed] [Google Scholar]
- 21.Leveque D, Nivoix Y, Jehl F, Herbrecht R. Clinical pharmacokinetics of voriconazole. Inter. J. Antimicrob. Agents. 2006;27(4):274–284. doi: 10.1016/j.ijantimicag.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Theuretzbacher U, Ihle F, Derendorf H. Pharmacokinetic/ pharmacodynamic profile of voriconazole. Clin. Pharmacokinet. 2006;45(7):649–663. doi: 10.2165/00003088-200645070-00002. [DOI] [PubMed] [Google Scholar]
- 23.Karlsson MO, Lutsar I, Milligan PA. Population pharmacokinetic analysis of voriconazole plasma concentration data from pediatric studies. Antimicrob. Agents Chemother. 2009;53(3):935–944. doi: 10.1128/AAC.00751-08. • Describes voriconazole population pharmacokinetic parameters in pediatric patients.
- 24.Shima H, Miharu M, Osumi T, Takahashi T, Shimada H. Differences in voriconazole trough plasma concentrations per oral dosages between children younger and older than 3 years of age. Pediatr. Blood Cancer. 2010;54(7):1050–1052. doi: 10.1002/pbc.22451. [DOI] [PubMed] [Google Scholar]
- 25.Michael C, Bierbach U, Frenzel K, et al. Voriconazole pharmacokinetics and safety in immunocompromised children compared to adult patients. Antimicrob. Agents Chemother. 2010;54(8):3225–3232. doi: 10.1128/AAC.01731-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyland R, Jones BC, Smith DA. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab. Dispos. 2003;31(5):540–547. doi: 10.1124/dmd.31.5.540. [DOI] [PubMed] [Google Scholar]
- 27.Yanni SB, Annaert PP, Augustijns P, Ibrahim JG, Benjamin DK, Jr, Thakker DR. In vitro hepatic metabolism explains higher clearance of voriconazole in children versus adults: role of CYP2C19 and flavin-containing monooxygenase 3. Drug Metab. Dispos. 2010;38(1):25–31. doi: 10.1124/dmd.109.029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott SA, Sangkuhl K, Gardner EE, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clin. Pharmacol. Ther. 2011;90(2):328–332. doi: 10.1038/clpt.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott SA, Sangkuhl K, Shuldiner AR, et al. PharmGKB summary: very important pharmacogene information for cytochrome P450, family 2, subfamily C, polypeptide 19. Pharmacogenet. Genomics. 2012;22(2):159–165. doi: 10.1097/FPC.0b013e32834d4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hicks JK, Swen JJ, Thorn CF, et al. Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin. Pharmacol. Ther. 2013;93(5):402–408. doi: 10.1038/clpt.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narita A, Muramatsu H, Sakaguchi H, et al. Correlation of CYP2C19 phenotype with voriconazole plasma concentration in children. J. Pediatr. Hematol. Oncol. 2013;35(5):e219–e223. doi: 10.1097/MPH.0b013e3182880eaa. • Demonstrates that intermediate and poor metabolizers have elevated voriconazole trough concentrations in a pediatric population.
- 32.Pieper S, Kolve H, Gumbinger HG, Goletz G, Wurthwein G, Groll AH. Monitoring of voriconazole plasma concentrations in immunocompromised paediatric patients. J. Antimicrob. Chemother. 2012;67(11):2717–2724. doi: 10.1093/jac/dks258. [DOI] [PubMed] [Google Scholar]
- 33.Geist MJ, Egerer G, Burhenne J, Riedel KD, Weiss J, Mikus G. Steady-state pharmacokinetics and metabolism of voriconazole in patients. J. Antimicrob. Chemother. 2013;68(11):2592–2299. doi: 10.1093/jac/dkt229. [DOI] [PubMed] [Google Scholar]
- 34.Voriconazole. In. Lexi-Comp Online. Hudson, OH, USA: Lexi-Comp, Inc; www.lexi.com. [Google Scholar]
- 35.Voriconazole. In. European Medicines Agency Online. Voriconazole Accord: EPAR-Product Information. www.ema.europa.eu/ema.
- 36.Neely M, Rushing T, Kovacs A, Jelliffe R, Hoffman J. Voriconazole pharmacokinetics and pharmacodynamics in children. Clin. Infect. Dis. 2010;50(1):27–36. doi: 10.1086/648679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prasad PA, Coffin SE, Leckerman KH, Walsh TJ, Zaoutis TE. Pediatric antifungal utilization: new drugs, new trends. Pediatr. Infect. Dis. J. 2008;27(12):1083–1088. doi: 10.1097/INF.0b013e31817eeee5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dolton MJ, Ray JE, Chen SC, Ng K, Pont LG, McLachlan AJ. Multicenter study of voriconazole pharmacokinetics and therapeutic drug monitoring. Antimicrob. Agents Chemother. 2012;56(9):4793–4799. doi: 10.1128/AAC.00626-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purkins L, Wood N, Ghahramani P, Love ER, Eve MD, Fielding A. Coadministration of voriconazole and phenytoin: pharmacokinetic interaction, safety, and toleration. Br. J. Clin. Pharmacol. 2003;56(Suppl. 1):37–44. doi: 10.1046/j.1365-2125.2003.01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandez CA, Smith C, Yang W, et al. Concordance of DMET plus genotyping results with those of orthogonal genotyping methods. Clin. Pharmacol. Ther. 2012;92(3):360–365. doi: 10.1038/clpt.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CYP2C19 allele nomenclature. www.cypalleles.ki.se/cyp2c19.htm.
- 42.Hope WW. Population pharmacokinetics of voriconazole in adults. Antimicrob. Agents Chemother. 2012;56(1):526–531. doi: 10.1128/AAC.00702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friberg LE, Ravva P, Karlsson MO, Liu P. Integrated population pharmacokinetic analysis of voriconazole in children, adolescents, and adults. Antimicrob. Agents Chemother. 2012;56(6):3032–3042. doi: 10.1128/AAC.05761-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scholz I, Oberwittler H, Riedel KD, et al. Pharmacokinetics, metabolism and bioavailability of the triazole antifungal agent voriconazole in relation to CYP2C19 genotype. Br. J. Clin. Pharmacol. 2009;68(6):906–915. doi: 10.1111/j.1365-2125.2009.03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ikeda Y, Umemura K, Kondo K, Sekiguchi K, Miyoshi S, Nakashima M. Pharmacokinetics of voriconazole and cytochrome P450 2C19 genetic status. Clin. Pharmacol. Ther. 2004;75(6):587–588. doi: 10.1016/j.clpt.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Berge M, Guillemain R, Tregouet DA, et al. Effect of cytochrome P450 2C19 genotype on voriconazole exposure in cystic fibrosis lung transplant patients. Eur. J. Clin. Pharmacol. 2011;67(3):253–260. doi: 10.1007/s00228-010-0914-2. [DOI] [PubMed] [Google Scholar]
- 47.Weiss J, Ten Hoevel MM, Burhenne J, et al. CYP2C19 genotype is a major factor contributing to the highly variable pharmacokinetics of voriconazole. J. Clin. Pharmacol. 2009;49(2):196–204. doi: 10.1177/0091270008327537. [DOI] [PubMed] [Google Scholar]
- 48.Malingre MM, Godschalk PC, Klein SK. A case report of voriconazole therapy failure in a homozygous ultrarapid CYP2C19*17/*17 patient comedicated with carbamazepine. Br. J. Clin. Pharmacol. 2012;74(1):205–206. doi: 10.1111/j.1365-2125.2011.04156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dolton MJ, Mikus G, Weiss J, Ray JE, McLachlan AJ. Understanding variability with voriconazole using a population pharmacokinetic approach: implications for optimal dosing. J. Antimicrob. Chemother. 2014 doi: 10.1093/jac/dku031. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 50.Wang G, Lei HP, Li Z, et al. The CYP2C19 ultra-rapid metabolizer genotype influences the pharmacokinetics of voriconazole in healthy male volunteers. Eur. J. Clin. Pharmacol. 2009;65(3):281–285. doi: 10.1007/s00228-008-0574-7. [DOI] [PubMed] [Google Scholar]
- 51.Owusu Obeng A, Egelund EF, Alsultan A, Peloquin CA, Johnson JA. CYP2C19 polymorphisms and therapeutic drug monitoring of voriconazole: are we ready for clinical implementation of pharmacogenomics? Pharmacotherapy. 2014 doi: 10.1002/phar.1400. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imhof A, Schaer DJ, Schanz U, Schwarz U. Neurological adverse events to voriconazole: evidence for therapeutic drug monitoring. Swiss Med. Wkly. 2006;136:45–46. doi: 10.4414/smw.2006.11547. 739–742. [DOI] [PubMed] [Google Scholar]
- 53.Andes D, Pascual A, Marchetti O. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob. Agents Chemother. 2009;53(1):24–34. doi: 10.1128/AAC.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steinbach WJ. Antifungal agents in children. Pediatr. Clin. North Am. 2005;52(3):895–915. doi: 10.1016/j.pcl.2005.02.009. viii. [DOI] [PubMed] [Google Scholar]
- 55.Dolton MJ, McLachlan AJ. Clinical importance of the CYP2C19*17 variant allele for voriconazole. Br. J. Clin. Pharmacol. 2011;71(1):137–138. doi: 10.1111/j.1365-2125.2010.03801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Y, Ferguson SS, Negishi M, Goldstein JA. Identification of constitutive androstane receptor and glucocorticoid receptor binding sites in the CYP2C19 promoter. Mol. Pharmacol. 2003;64(2):316–324. doi: 10.1124/mol.64.2.316. [DOI] [PubMed] [Google Scholar]
- 57.Wang T, Chen S, Sun J, et al. Identification of factors influencing the pharmacokinetics of voriconazole and the optimization of dosage regimens based on Monte Carlo simulation in patients with invasive fungal infections. J. Antimicrob. Chemother. 2014;69(2):463–470. doi: 10.1093/jac/dkt369. [DOI] [PubMed] [Google Scholar]