Abstract
Aim:
To explore the signal transducer and activator of transcription 3 (STAT3) signaling pathway, especially STAT3 acetylation, in angiotensin II (Ang II)-induced pro-fibrotic responses in renal tubular epithelial cells.
Methods:
Rat renal tubular epithelial cell line (NRK-52E) was used. STAT3 acetylation and phosphorylation, as well as the expression of fibronectin, collagen IV and transforming growth factor-β1 (TGF-β1) were examined using Western blotting. The level and localization of STAT3 phosphorylation on Tyr705 were detected with fluorescence immunocytochemistry. The cells were transfected with a plasmid vector carrying p300 gene or siRNA targeting p300 to regulate p300 expression.
Results:
Overexpression of p300 significantly increased STAT3 acetylation on Lys685, STAT3 phosphorylation on Tyr705, and the expression of TGF-β1, collagen IV and fibronectin in the cells. Treatment of the cells with Ang II (1 μmol/L) significantly increased STAT3 phosphorylation on Tyr705 through JAK2 activation, and dose-dependently increased the expression of fibronectin, collagen IV and TGF-β1. Pretreatment with curcumin, an inhibitor of JAK2 and p300, blocked Ang II-induced effects. Knockdown of p300 significantly decreased STAT3 acetylation on Lys685, and abolished Ang II-stimulated STAT3 phosphorylation on Tyr705, whereas pretreatment of the cells with C646, a selective inhibitor of p300, inhibited Ang II-induced STAT3 nuclear translocation and the expression of TGF-β1, collagen IV and fibronectin. Pretreatment of the cells with AG490, a JAK2 inhibitor, markedly inhibited Ang II-induced STAT3 phosphorylation on Tyr705 and fibronectin expression.
Conclusion:
p300-dependent STAT3 acetylation is necessary for Ang II-induced STAT3 phosphorylation and the consequent pro-fibrotic responses in renal tubular epithelial cells in vitro.
Keywords: renal fibrosis, angiotensin II, renal tubular epithelial cells, STAT3, JAK2, extracellular matrix, p300, curcumin, C646, AG490
Introduction
Renal fibrosis, a common feature in most cases of chronic kidney disease (CKD), is characterized by glomerulosclerosis and tubulointerstitial fibrosis. The progression of renal fibrosis may lead to the loss of functional renal parenchyma that ultimately contributes to end-stage kidney failure1,2. Various major causes can induce renal fibrosis, including the dysfunction of renal tubular epithelial cells, which plays a crucial role for fibrogenesis and progression. Tubular epithelial cells undergoing pro-fibrotic cellular changes, such as the increased expression of transforming growth factor-β1 (TGF-β1) and extracellular matrix (ECM) deposition, can contribute to the early development and progression of renal interstitial fibrosis3,4,5,6,7,8,9; however, there is an ongoing debate on type II epithelial-to-mesenchymal transition (EMT), which proposes that injured epithelial cells can be the direct precursors of myofibroblasts.
A number of proteins, cytokines and growth factors may be involved in the progression of renal interstitial fibrosis. Increasing evidence suggests that angiotensin II (Ang II), one of the primary intermediates of the rennin-angiotensin system (RAS), is capable of orchestrating further inflammation and fibrosis under pathophysiological circumstances10,11. Ang II is one of the main pathogenetic mediators of renal interstitial fibrosis in various CKD conditions, including obstructive nephropathy12,13,14.
The Janus kinase family (JAK)/signal transducers and activators of transcription (STAT) signaling pathway is thought to contribute to a pleiotropic cascade essential for a wide range of signal transduction cytokines and for the expression of growth factors15. JAK2/STAT3 is one of the best characterized signaling pathways confirmed to be the downstream mediator of Ang II16,17,18,19,20. STAT3 plays a crucial role in promoting cell cycle progression and preventing apoptosis21,22. In recent years, STAT3 has been increasingly implicated in the progression of renal fibrogenesis, especially in the obstructed kidney9,23,24,25. Both Ang II and STAT3 have been confirmed to mediate kidney dysfunction separately. However, the role of the Ang II/STAT3 signaling pathway in renal fibrogenesis is still uncertain.
STAT3 activation requires phosphorylation on tyrosine 705 (Tyr705) and serine 727 (Ser727)26. It can also be reversibly acetylated on lysine 685 (Lys685). The acetylation of this single lysine residue regulated by the histone acetyltransferase cAMP response element binding protein-binding protein (CBP)/p300 and histone deacetylases (HDACs) contributes significantly to STAT3 dimerization and transcription27,28,29. Transcriptional coactivator p300 and its homolog CBP are capable of regulating many cellular processes via their substrates and downstream coactivators, including hypoxia-inducible factor-1 (HIF-1) and STAT329,30,31,32. Furthermore, strong evidence demonstrates that p300 promotes the expression of fibronectin, a hallmark of ECM, which associates with cell adhesion and tissue fibrosis33,34,35,36,37.
Previous studies have revealed that STAT3 acetylation plays an important role in psoriasis38, cardiotoxicity39, lymphoid malignancies40, hepatocellular carcinoma41 and renal fibrosis24,42,43. In this study, we investigated whether STAT3 acetylation was involved in STAT3 mediated pro-fibrotic cellular changes in renal tubular epithelial cells. Understanding the role of the Ang II/STAT3 signaling pathway in pro-fibrotic responses and the relationship of this pathway with p300 may have potential for both the prevention and treatment of renal fibrosis.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM), recombinant human angiotensin II, nicotinamide, curcumin and AG490 were purchased from Sigma-Aldrich (Saint Louis, Missouri, USA). C646 was purchased from ChemBridge (San Diego, CA, USA). Fetal bovine serum (FBS) was obtained from Sijiqing Biological Engineering Materials Co (Hangzhou, China). The BCA Protein Assay Kit was acquired from Shenergy Biocolor BioScience and Technology (Shanghai, China). The anti-fibronectin antibody was obtained from Sigma-Aldrich (Saint Louis, USA). The anti-TGF-β1 antibody was obtained from Bioworld Technology, Inc (Nantong, China). The anti-collagen IV antibody was attained from Abcam (Cambridge, MA, USA). The anti-phospho-STAT3 (Tyr705), anti-phospho-STAT3 (Ser727), anti-acetyl-STAT3 (K685), anti-STAT3 and anti-phospho-JAK2 (Tyr1007/1008) antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). The anti-JAK2 antibody was obtained from Millipore (Billerica, MA, USA). The anti-p300 antibody was obtained from Santa Cruz Biotechnologies, Inc (Santa Cruz, CA, USA). The anti-β-actin antibody, anti-GAPDH antibody, horseradish peroxidase-conjugated secondary antibodies, Cy3-conjugated secondary antibody, DAPI and an enhanced chemiluminescence (ECL) detection kit were acquired from Beyotime Institute of Biotechnology (Nantong, China). Polyvinylidene difluoride (PVDF) membranes were obtained from Millipore (Billerica, MA, USA). Proteinase inhibitor and phosphatase inhibitor were obtained from Roche (Mannheim, Germany). All other chemicals and reagents used were of analytical grade.
Cell culture
The rat renal tubular epithelial cell line (NRK-52E) was purchased from the Institute of Biochemistry and Cell Biology (Shanghai, China) and cultured in DMEM media containing 10% FBS in an atmosphere of 5% CO2 at 37 °C. To obtain quiescent cells, cells were starved in a medium containing 0.5% FBS for 24 h before the start of the experiments. Cells were stimulated with Ang II at different concentrations for a designated time period before harvesting. When necessary, cells were pretreated with the diluent (DMSO) or curcumin (12.5 μmol/L), AG490 (12.5 μmol/L) and C646 (10 μmol/L) 60 min before the application of Ang II. Each experiment was repeated at least three times.
Western blotting
Cell lysate homogenates were prepared as described previously44. In brief, the cells were lysed in 1×sodium dodecyl sulfate (SDS) and supplemented with proteinase inhibitor and phosphatase inhibitor. Protein concentrations were determined by a BCA Protein Assay Kit according to the manufacturer's instructions. An equal amount (40 μg per sample) of total protein was loaded and separated by electrophoresing with a 10% SDS-PAGE gel and then electrophoretically transblotted onto a PVDF membrane. After the transfer, the membrane was blocked with Tris-buffered saline containing 5% skim milk and 0.1% Tween (TBS/Tween) for 1 h at room temperature. The membranes were then incubated with primary antibody at 4 °C overnight [antibody type: anti-fibronectin, 1:20000; anti-collagen IV, 1:1000; anti-TGF-β1, 1:1000; anti-phospho-STAT3 (Tyr705), 1:1000; anti-phospho-STAT3 (Ser727), 1:1000; anti-acetyl-STAT3 (Lys685), 1:500; anti-STAT3, 1:2000; anti-phospho-JAK2 (Tyr1007/1008), 1:1000; anti-JAK2, 1:1000; anti-β-actin, 1:5000; anti-GAPDH, 1:5000]. On the next day, the membranes were washed 3 times with TBS/Tween and incubated with horseradish peroxidase-conjugated secondary antibodies for 2 h. After an additional 3 washes with TBS/Tween, the hybridizing bands were developed using the ECL detection kit according to the manufacturer's instructions. The signals were then imaged with the ImageQuant LAS4000 mini system (GE Healthcare). The specific bands representing the target proteins were analyzed by densitometry and normalized to β-actin, STAT3 or JAK2 signals. The relative protein levels were expressed as fold-induction over controls.
p300 small interference RNA (siRNA) and overexpression
An siRNA targeting p300 was purchased from Ribobio Company (Guangzhou, China) (5′-GCAAGCAGUCAUCUAUUUAdTdT-3′). A nonsilencing siRNA oligonucleotide that does not recognize any known homolog of mammalian genes (Ribobio, Guangzhou, China) was used as a negative control. NRK-52E cells were transfected with p300 siRNA (50 nmol/L) or control siRNA (50 nmol/L) facilitated by Lipofectamine 2000 Reagent (Invitrogen, USA) according to the manufacturer's instructions. Forty-eight hours after transfection, the cells were subjected to Ang II stimulation for 60 min. The plasmids used were kindly provided by Prof Chen YU. NRK-52E cells grown in 6-well plates were transfected with the pcDNA3.0-p300 plasmid (2 μg) or with the pcDNA3.0 plasmid (2 μg) facilitated by Lipofectamine 2000 Reagent (Invitrogen). Cells were harvested for immunoblotting analysis 48 h after transfection.
Fluorescence immunocytochemistry
To observe the distribution of phospho-STAT3-(Tyr705), NRK-52E cells grown in 24-well plates were pretreated with the diluent (DMSO) or C646 (10 μmol/L) for 60 min before Ang II stimulation. The cells were then fixed in 4% paraformaldehyde for 30 min, followed by permeabilizing with 0.1% (v/v) Triton X-100 and incubated in PBS containing 5% BSA for 60 min at room temperature. Cells were then treated overnight with anti-phospho-STAT3 (Tyr705) primary antibody at 4 °C. The next day, cells were washed with PBS 3 times and incubated with Cy3-labeled goat anti-rabbit IgG antibody at room temperature for 2 h. After an additional 3 washes with PBS, cells were stained with DAPI for 15 min and visualized and photographed under fluorescence microscopy.
Statistical analysis
Data are presented as the mean±SEM with statistical analyses performed using one-way analysis of variance and subsequently with Tukey's multiple comparison test. The differences between two groups were compared by a Student's t-test. P<0.05 was considered a statistically significant difference between mean values.
Results
p300 induced pro-fibrotic responses and STAT3 activation in tubular epithelial cells
Western blot analysis showed that the overexpression of p300 by the transfection of a recombinant plasmid carrying the p300 gene increased the p300 protein level significantly in NRK-52E cells (Figure 1A). This result was accompanied by the up-regulation of STAT3 acetylation on Lys685 (Figure 1B) and phosphorylation on Tyr705 (Figure 1B). Furthermore, p300 also increased fibronectin, collagen IV and TGF-β1 protein levels (Figure 2).
Figure 1.
Effect of p300 overexpression on signal transducer and activator of transcription 3 (STAT3) activation in NRK-52E cells. Cells were transfected with 2 μg of pcDNA3.0-p300 plasmid or 2 μg of pcDNA3.0 plasmid for 48 h. p300 (A), acetyl-STAT3 (Lys685) (B) and phospho-STAT3 (Tyr705) (B) levels were detected by Western blotting. Data are presented as the mean±SEM of 3 experiments. bP<0.05, cP<0.01 compared with control (pcDNA3.0 plasmid).
Figure 2.
Effect of p300 overexpression on pro-fibrotic responses in NRK-52E cells. Cells were transfected with 2 μg of pcDNA3.0-p300 plasmid or 2 μg of pcDNA3.0 plasmid for 48 h. Fibronectin, collagen IV and transforming growth factor-β1 (TGF-β1) were detected by Western blotting. Data are presented as the mean±SEM of 3 experiments. bP<0.05, cP<0.01 compared with control (pcDNA3.0 plasmid).
Ang II induced pro-fibrotic responses and STAT3 phosphorylation on Tyr705 in tubular epithelial cells
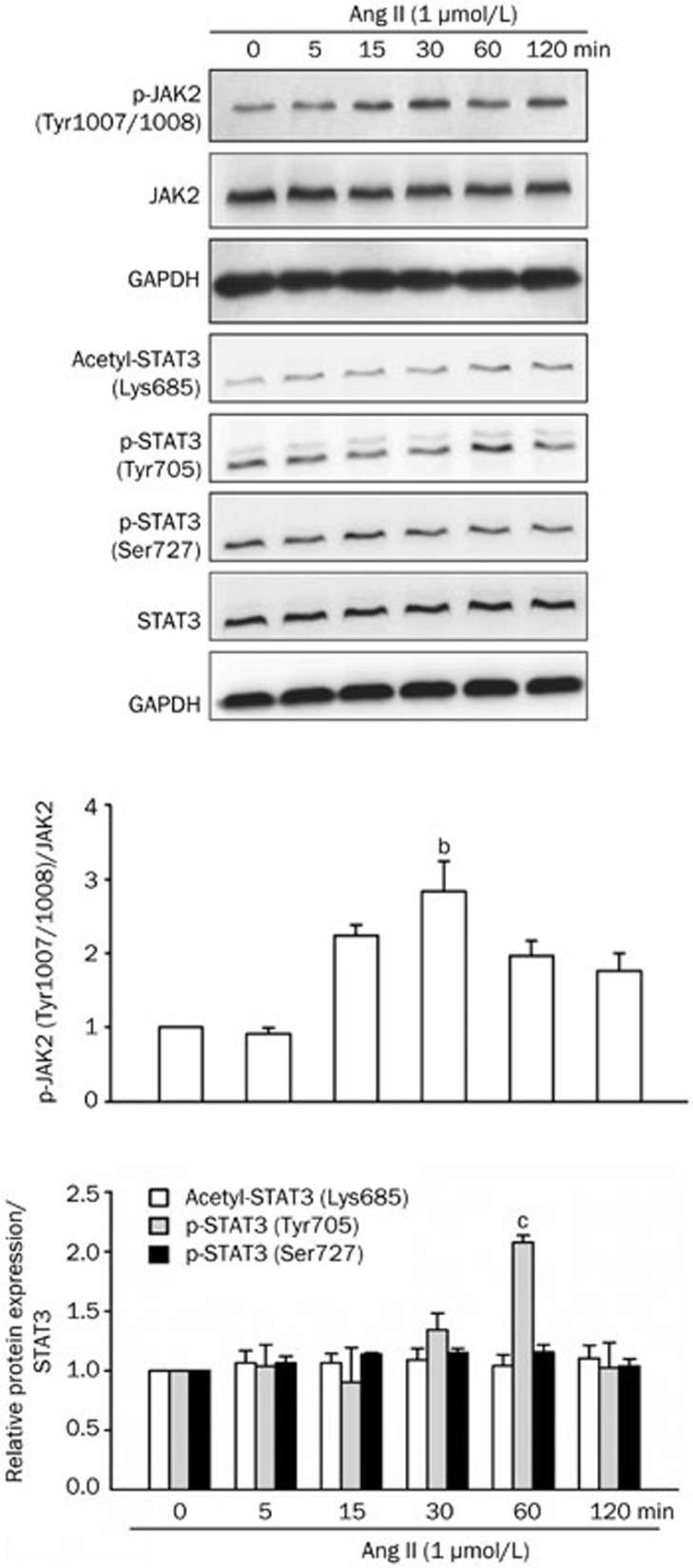
Western blot analysis demonstrated that the treatment of NRK-52E cells with Ang II for 48 h increased fibronectin, collagen IV and TGF-β1 protein levels in a dose-dependent manner. Ang II at 1 μmol/L significantly up-regulated these pro-fibrotic hallmarks (Figure 3). Investigation on the possible intracellular molecular cascades revealed that the phosphorylation of the JAK2/STAT3 signaling pathway was rapidly enhanced in NRK-52E cells after Ang II application. JAK2 phosphorylation was up-regulated 30 min after Ang II treatment (Figure 4). STAT3 phosphorylation on Tyr705 was also enhanced, peaking at 60 min after Ang II treatment (Figure 4). Ang II did not affect STAT3 Ser727 phosphorylation or Lys685 acetylation, which was observed constitutively in NRK-52E cells (Figure 4).
Figure 3.
Effect of angiotensin II (Ang II) on pro-fibrotic gene expression in NRK-52E cells. Cells were treated with Ang II at the indicated concentrations for 48 h. Fibronectin, collagen IV and TGF-β1 were detected by Western blot analysis. Media containing Ang II were changed every 24 h. Data are presented as the mean±SEM of 3 experiments. bP<0.05, cP<0.01 compared with control [Ang II (0 μmol/L)].
Figure 4.
Effect of Ang II on Janus kinase 2 (JAK2)/STAT3 signaling pathway activation in NRK-52E cells. Cells were treated with Ang II (1 μmol/L) for 0–120 min. Phospho-JAK2 (Tyr1007/1008), acetyl-STAT3 (Lys685), phospho-STAT3 (Tyr705) and phospho-STAT3 (Ser727) were analyzed by Western blot analysis. Data are presented as the mean±SEM of 3 or 4 experiments. bP<0.05, cP<0.01 vs control [Ang II (0 min)].
Curcumin opposed pro-fibrotic responses induced by Ang II in tubular epithelial cells via the inhibition of STAT3 acetylation and phosphorylation
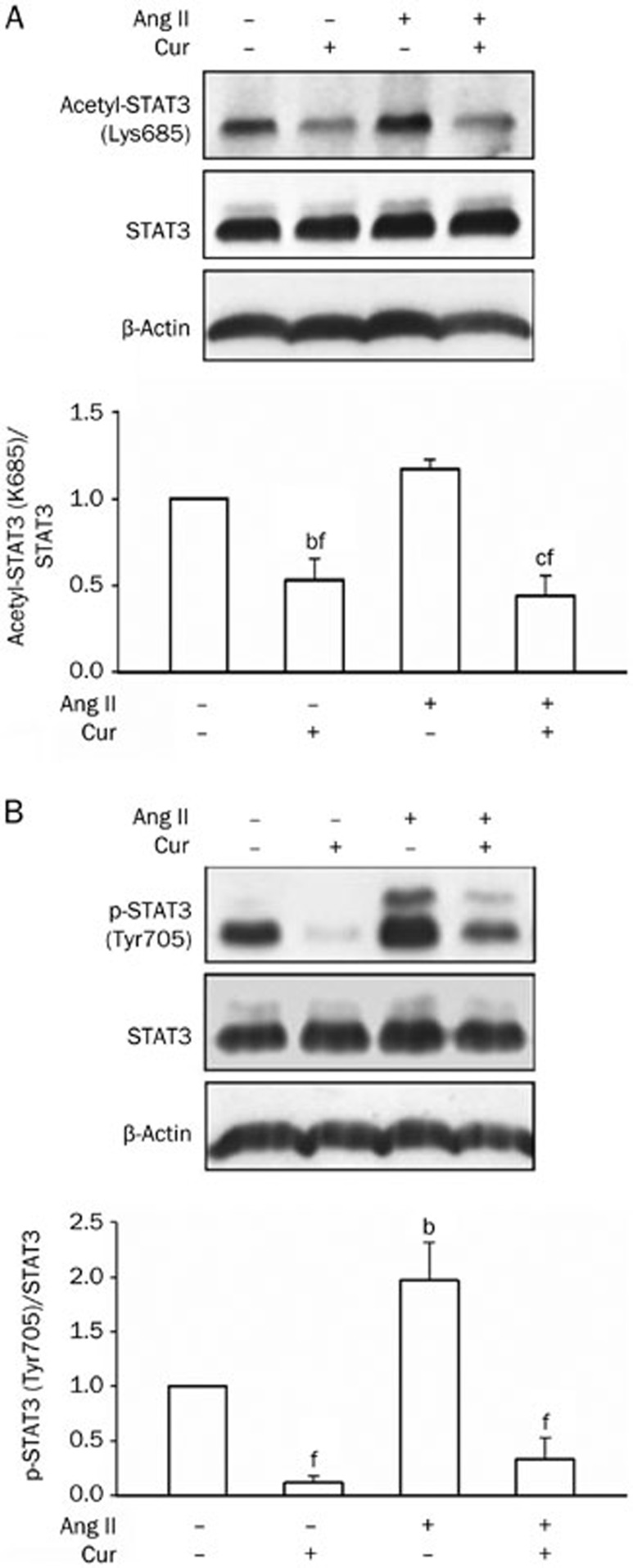
To examine whether the STAT3 signaling pathway is involved in Ang II-induced pro-fibrotic responses, curcumin, an inhibitor of p300 and JAK245,46,47,48,49, was employed in the experiment. The pretreatment of cells with curcumin for 60 min reduced the STAT3 acetylation level (Figure 5A) and prevented Ang II-induced up-regulation of STAT3 Tyr705 phosphorylation (Figure 5B). Curcumin also attenuated the Ang II-driven up-regulation of fibronectin, collagen IV and TGF-β1 (Figure 6), indicating that the STAT3 pathway was involved in Ang II-induced pro-fibrotic responses in tubular epithelial cells.
Figure 5.
Effect of curcumin (Cur) on STAT3 acetylation and Ang II-induced STAT3 phosphorylation in NRK-52E cells. After a 1 h treatment with Ang II (1 μmol/L) with or without a 1 h Cur (12.5 μmol/L) pretreatment, acetyl-STAT3 (Lys685) (A) and phospho-STAT3 (Tyr705) (B) were analyzed by Western blot analysis. Data are presented as the mean±SEM of 3–4 experiments. bP<0.05, cP<0.01 compared with control [Ang II (−) and Cur (−)]. fP<0.01 compared with Ang II only.
Figure 6.
Effect of Cur on Ang II-induced expression of pro-fibrotic genes in NRK-52E cells. After a 48 h treatment with Ang II (1 μmol/L) with or without a 1-h Cur (12.5 μmol/L) pretreatment, fibronectin, collagen IV, and TGF-β1 were analyzed by Western blot analysis. Media containing Ang II or Cur were changed every 24 h. Data are presented as the mean±SEM of 3 experiments. bP<0.05, cP<0.01 compared with control [Ang II (−) and Cur (−)]. fP<0.01 compared with Ang II only.
The inhibition of p300-dependent STAT3 Lys685 acetylation opposed STAT3 Tyr705 phosphorylation and pro-fibrotic responses regulated by Ang II in tubular epithelial cells
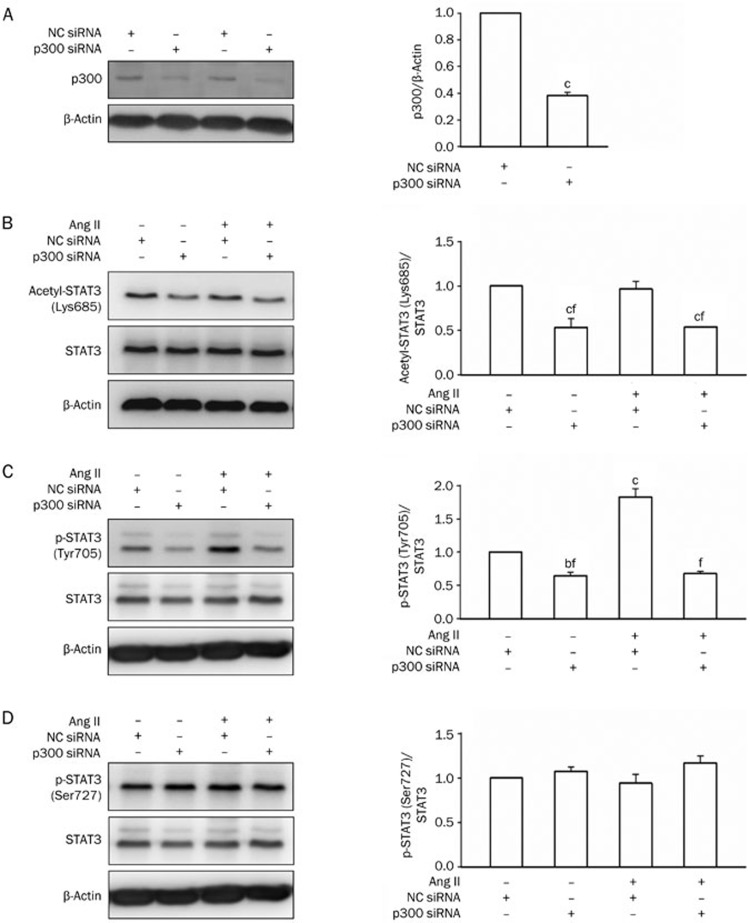
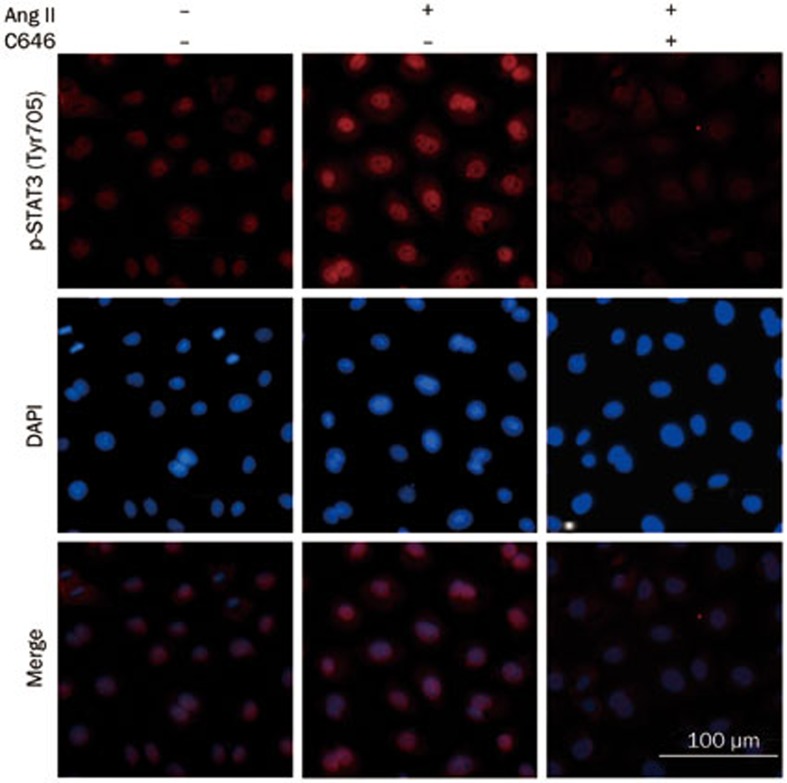
siRNA targeted against p300 efficiently decreased p300 protein levels, but control siRNA did not have the same effect (Figure 7A). The knockdown of p300 decreased STAT3 Lys685 acetylation (Figure 7B) and abolished the Ang II-stimulated up-regulation of STAT3 Tyr705 phosphorylation (Figure 7C), but not Ser727 phosphorylation (Figure 7D). The pretreatment of cells with C646, a selective inhibitor of p30050,51,52, rapidly inhibited Ang II-induced STAT3 nuclear translocation, which was proportional to STAT3 Tyr705 phosphorylation (Figure 8). C646 also attenuated fibronectin, collagen IV and TGF-β1 expression induced by Ang II (Figure 9).
Figure 7.
Effect of p300 knockdown on STAT3 acetylation and Ang II-induced STAT3 phosphorylation in NRK-52E cells. Cells were transfected with p300 siRNA (50 nmol/L) or NC siRNA (50 nmol/L) for 48 h and then treated with Ang II (1 μmol/L) for 1 h. p300 (A), acetyl-STAT3 (Lys685) (B), phospho-STAT3 (Tyr705) (C) and phospho-STAT3 (Ser727) (D) were detected by Western blot analysis. Data are presented as the mean±SEM of 3 experiments. bP<0.05, cP<0.01 compared with control [Ang II (−) and NC siRNA (+)]. fP<0.01 compared with Ang II [Ang II (+) and NC siRNA (+)].
Figure 8.
Effect of C646 on Ang II-induced STAT3 Tyr705 phosphorylation and nuclear translocation in NRK-52E cells. Cells were treated with Ang II (1 μmol/L) for 1 h in the presence or absence of a 1-h C646 (10 μmol/L) pretreatment. Cells were stained with an anti-phospho-STAT3 (Tyr705) antibody and then subjected to microscopic analysis (×800).
Figure 9.
Effect of C646 on Ang II-induced pro-fibrotic responses in NRK-52E cells. Cells were treated with Ang II (1 μmol/L) for 48 h with or without a 1-h pretreatment with C646 (10 μmol/L). Fibronectin, collagen IV and TGF-β1 were analyzed by Western blot analysis. Media containing Ang II or C646 were changed every 24 h. Data are presented as the mean±SEM of 6 experiments. bP<0.05, cP<0.01 compared with control [Ang II (−) and C646 (−)]. fP<0.01 compared with Ang II only.
The inhibition of STAT3 Tyr705 phosphorylation opposed STAT3-dependent pro-fibrotic responses regulated by Ang II in tubular epithelial cells
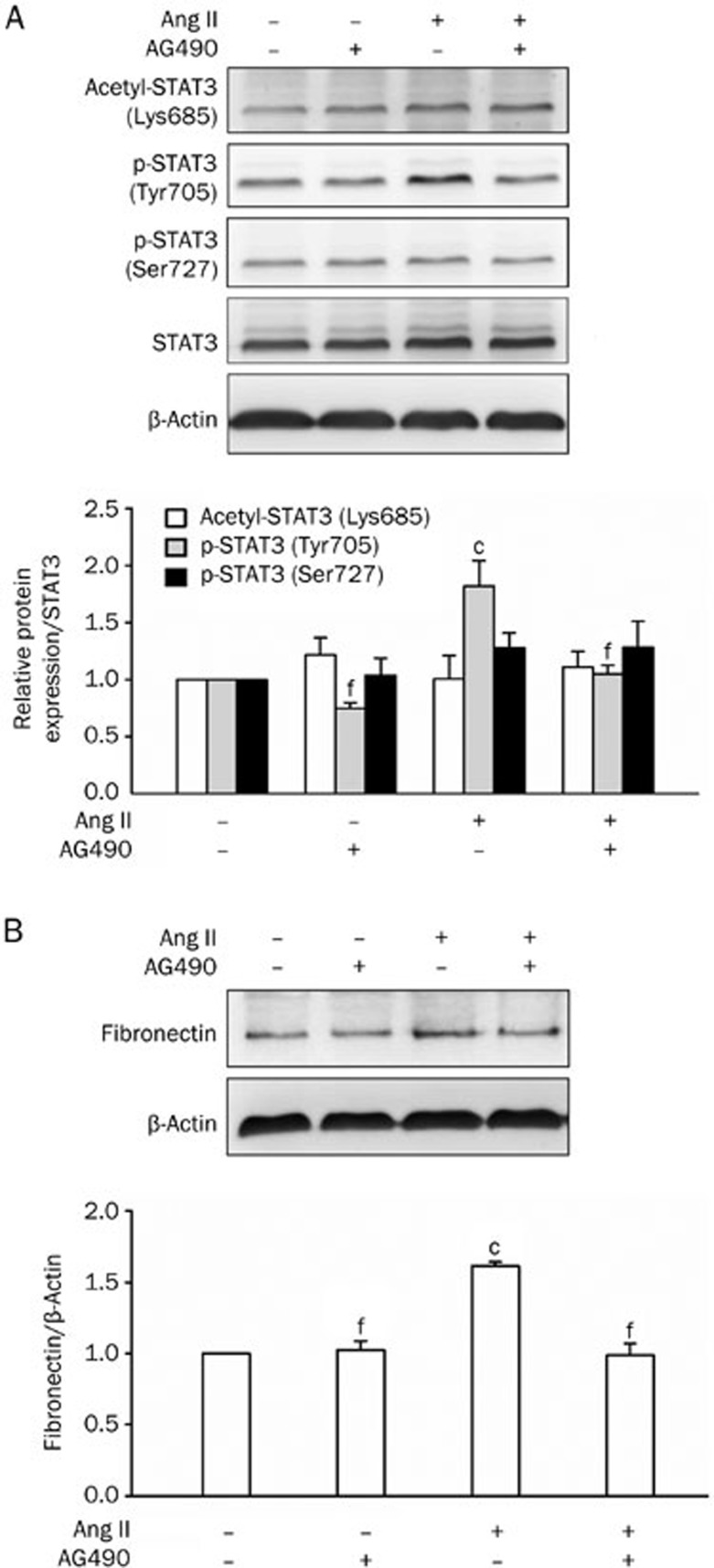
AG490, a tyrosine kinase tyrphostin that inhibits the activity of JAK253,54,55,56, suppressed Ang II-induced STAT3 phosphorylation on Tyr705 (Figure 10A). The pretreatment of cells with AG490 also abolished downstream fibronectin expression (Figure 10B). However, AG490 did not affect STAT3 Ser727 phosphorylation or Lys685 acetylation (Figure 10A).
Figure 10.
Effect of AG490 on Ang II-induced STAT3 activation and expression of pro-fibrotic gene in NRK-52E cells. (A) Cells were treated with Ang II (1 μmol/L) for 1-h with or without a 1 h pretreatment of AG490 (12.5 μmol/L). Acetyl-STAT3 (Lys685), phospho-STAT3 (Tyr705) and phospho-STAT3 (Ser727) were detected by Western blot analysis. Data are presented as the mean±SEM of 3 experiments. cP<0.01, compared with control [Ang II (−) and AG490 (−)]. fP<0.01 compared with Ang II only. (B) Cells were treated with Ang II (1 μmol/L) for 48 h with or without a 1-h pretreatment of AG490 (12.5 μmol/L). Fibronectin was analyzed by Western blot analysis. Media containing Ang II or AG490 were changed every 24 h. Data are presented as the mean±SEM of 3 experiments. cP<0.01, compared with control [Ang II (−) and AG490 (−)]. fP<0.01 compared with Ang II only.
Discussion
In the present study, we investigated the role of the STAT3 signaling pathway in pro-fibrotic cellular changes, which is potentially related to the pathogenesis of renal fibrosis, and its relationship with p300 in tubular epithelial cells. Previous studies have revealed that p300 increases fibronectin protein levels by directly promoting fibronectin gene expression33,34,35,36,37. To identify the role of p300 in fibrogenesis, we first detected fibrotic hallmarks after p300 overexpression in tubular epithelial cells. The overexpression of p300 induced an up-regulation of fibronectin, collagen IV and TGF-β1. Elevated p300 also led to the accumulation of STAT3 Lys685 acetylation and Tyr705 phosphorylation.
Ang II is one of the best characterized upstream mediators of pro-fibrotic response stimulation and STAT3 phosphorylation via JAK2 in various cell types16,17,18,19,20. We observed a correlation between STAT3 signaling and pro-fibrotic changes after Ang II application. Ang II treatment induced JAK2 activation and increased STAT3 phosphorylation on Tyr705, accompanied by the up-regulation of TGF-β1, collagen IV and fibronectin protein levels. Because the dependence of the Ang II-induced pro-fibrotic responses on STAT3 signaling is unclear, cells were treated with Ang II in the presence or absence of curcumin, an inhibitor of JAK2 and p30045,46,47,48,49. Our results revealed that curcumin led to the deacetylation and dephosphorylation of STAT3, which was associated with the repression of pro-fibrotic responses induced by Ang II. These results suggested that STAT3 signaling is necessary for Ang II-induced pro-fibrotic cellular changes.
STAT3 is one of the major transcription factors that interacts with p300 and plays a critical role in the recruitment of p300 and RNA polymerase to the promoters of target genes, such as angiotensinogen29,57 and socs332. Furthermore, STAT3-driven target gene expression requires p300-mediated STAT3 acetylation32,57. Thus, we hypothesized that STAT3 was involved in Ang II-induced fibrogenesis via JAK2-dependent Tyr705 phosphorylation, and STAT3 acetylation also played a role. The major STAT3 acetylation site is located at its C-terminal position, Lys685, which can enhance its DNA binding activity and transactivation activity27. We discovered that both the inhibition of p300 by C646 and the knockdown of p300 by siRNA suppressed Ang II-induced STAT3 Tyr705 phosphorylation, while C646 also exhibited a suppressing effect against Ang II-induced pro-fibrotic responses. These findings revealed that the constitutive STAT3 Lys685 acetylation in tubular epithelial cells was necessary for STAT3 Tyr705 phosphorylation and transactivation of downstream pro-fibrotic genes. Importantly, p300 siRNA did not affect STAT3 phosphorylation on Ser727, arguing that the effect of p300 inhibition was at least partly selective.
In addition, we investigated whether the JAK2-dependent STAT3 Tyr705 phosphorylation was involved in Ang II-induced pro-fibrotic responses. AG490, a selective inhibitor of JAK253,54,55,56, provided a suppressing effect on Ang II-induced fibronectin up-regulation through STAT3 dephosphorylation on Tyr705, but not Ser727. Similarly, AG490 did not affect STAT3 Lys685 acetylation.
Our findings suggest the possibility that STAT3 acetylation facilitates the phosphorylation on its special site. This result is consistent with a previous study that reports that SIRT1, a class III protein deacetylase, opposes STAT3 Tyr705 phosphorylation and STAT3-dependent effects of IL-22 by STAT3 deacetylation38. The stabilization of STAT3 can be sustained by p300-dependent acetylation that can block STAT3 degradation through the ubiquitination-mediated proteasomal pathway39. In addition, a recent study has provided in vitro evidence that SIRT1 activators can induce STAT3 deacetylation on Lys685, associated with STAT3 Tyr705 dephosphorylation, which leads to the repression of NF-κB/STAT3 complex binding to the GADD45G gene promoter in Ly3 cells40. Therefore, our observations suggest that STAT3 acetylation on Lys685 is required for Ang II-induced STAT3 Tyr705 phosphorylation and STAT3-dependent downstream fibrotic responses.
In summary, this study provides the evidence of STAT3-mediated pro-fibrotic cellular changes via p300-dependent acetylation on Lys685 in renal tubular epithelial cells. Thus, the inhibition or prevention of p300-dependent STAT3 acetylation may provide a novel therapeutic strategy for CKD-associated fibrosis.
Author contribution
Yu HUANG, Chen YU, Wei ZHANG, and Li-min LU designed the research project; Jun NI, Yang SHEN, and Zhen WANG performed experiments; De-cui SHAO and Jia LIU analyzed the data; Ya-li KONG and Lan-jun FU contributed reagents and materials; Jun NI, Li ZHOU, and Hong XUE wrote the manuscript.
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China to Li-min LU (No 81070577, 81170636), Hong XUE (No 81000280), Wei ZHANG (No 81100531) and Chen YU (No 81070547).
References
- Eddy AA. The STAT3 NH2-terminal domain stabilizes enhanceosome assembly by interacting with the p300 bromodomain. Adv Chronic Kidney Dis. 2005;12:353–65. doi: 10.1074/jbc.M805941200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–7. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- Matsui F, Rhee A, Hile KL, Zhang H, Meldrum KK. IL-18 induces profibrotic renal tubular cell injury via STAT3 activation. Am J Physiol Renal Physiol. 2013;305:F1014–F1021. doi: 10.1152/ajprenal.00620.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–50. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- Rastaldi MP, Ferrario F, Giardino L, Dell'Antonio G, Grillo C, Grillo P, et al. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int. 2002;62:137–46. doi: 10.1046/j.1523-1755.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–8. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- Ivanova L, Butt MJ, Matsell DG. Mesenchymal transition in kidney collecting duct epithelial cells. Am J Physiol Renal Physiol. 2008;294:F1238–F1248. doi: 10.1152/ajprenal.00326.2007. [DOI] [PubMed] [Google Scholar]
- Ranganathan P, Jayakumar C, Ramesh G. Proximal tubule-specific overexpression of netrin-1 suppresses acute kidney injury-induced interstitial fibrosis and glomerulosclerosis through suppression of IL-6/STAT3 signaling. Am J Physiol Renal Physiol. 2013;304:F1054–F1065. doi: 10.1152/ajprenal.00650.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Guo D, Chen CB, Wang W, Schuster M, Loibner H, et al. Prevention of angiotensin II-mediated renal oxidative stress, inflammation, and fibrosis by angiotensin-converting enzyme 2. Hypertension. 2011;57:314–22. doi: 10.1161/HYPERTENSIONAHA.110.164244. [DOI] [PubMed] [Google Scholar]
- Li YQ, Li XB, Guo SJ, Chu SL, Gao PJ, Zhu DL, et al. Apocynin attenuates oxidative stress and cardiac fibrosis in angiotensin II-induced cardiac diastolic dysfunction in mice. Acta Pharmacol Sin. 2013;34:352–9. doi: 10.1038/aps.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Miyajima A, Kikuchi E, Kozakai N, Kosaka T, Ieda M, et al. Renal damage inhibited in mice lacking angiotensinogen gene subjected to unilateral ureteral obstruction. Urology. 2009;74:938–43. doi: 10.1016/j.urology.2009.02.059. [DOI] [PubMed] [Google Scholar]
- Grande MT, Perez-Barriocanal F, Lopez-Novoa JM. Role of inflammation in tubulo-interstitial damage associated to obstructive nephropathy. J Inflamm (Lond) 2010;7:19. doi: 10.1186/1476-9255-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SC, Lai KN. The pathogenic role of the renal proximal tubular cell in diabetic nephropathy. Nephrol Dial Transplant. 2012;27:3049–56. doi: 10.1093/ndt/gfs260. [DOI] [PubMed] [Google Scholar]
- Schindler CW. Series introduction. JAK-STAT signaling in human disease. J Clin Invest. 2002;109:1133–7. doi: 10.1172/JCI15644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama H, Fukuda K, Pan J, Makino S, Sano M, Takahashi T, et al. Biphasic activation of the JAK/STAT pathway by angiotensin II in rat cardiomyocytes. Circ Res. 1998;82:244–50. doi: 10.1161/01.res.82.2.244. [DOI] [PubMed] [Google Scholar]
- Weng YI, Aroor AR, Shukla SD. Ethanol inhibition of angiotensin II-stimulated Tyr705 and Ser727 STAT3 phosphorylation in cultured rat hepatocytes: relevance to activation of p42/44 mitogen-activated protein kinase. Alcohol. 2008;42:397–406. doi: 10.1016/j.alcohol.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Kandalam U, Clark MA. Angiotensin II activates JAK2/STAT3 pathway and induces interleukin-6 production in cultured rat brainstem astrocytes. Regul Pept. 2010;159:110–6. doi: 10.1016/j.regpep.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Amiri F, Shaw S, Wang X, Tang J, Waller JL, Eaton DC, et al. Angiotensin II activation of the JAK/STAT pathway in mesangial cells is altered by high glucose. Kidney Int. 2002;61:1605–16. doi: 10.1046/j.1523-1755.2002.00311.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang J, Zhou F, Wang X, Feng Z. STAT proteins mediate angiotensin II-induced production of TIMP-1 in human proximal tubular epithelial cells. Kidney Int. 2003;64:459–67. doi: 10.1046/j.1523-1755.2003.00133.x. [DOI] [PubMed] [Google Scholar]
- Bromberg J, Darnell JJ. The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–73. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- Liu DB, Hu GY, Long GX, Qiu H, Mei Q, Hu GQ. Celecoxib induces apoptosis and cell-cycle arrest in nasopharyngeal carcinoma cell lines via inhibition of STAT3 phosphorylation. Acta Pharmacol Sin. 2012;33:682–90. doi: 10.1038/aps.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratsune M, Masaki T, Hirai T, Kiribayashi K, Yokoyama Y, Arakawa T, et al. Signal transducer and activator of transcription 3 involvement in the development of renal interstitial fibrosis after unilateral ureteral obstruction. Nephrology (Carlton) 2007;12:565–71. doi: 10.1111/j.1440-1797.2007.00881.x. [DOI] [PubMed] [Google Scholar]
- Pang M, Kothapally J, Mao H, Tolbert E, Ponnusamy M, Chin YE, et al. Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol. 2009;297:F996–F1005. doi: 10.1152/ajprenal.00282.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Guo JK, Pang M, Tolbert E, Ponnusamy M, Gong R, et al. Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol. 2012;23:854–67. doi: 10.1681/ASN.2011050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Zhong Z, Darnell JJ. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–50. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- Wang R, Cherukuri P, Luo J. Activation of Stat3 sequence-specific DNA binding and transcription by p300/CREB-binding protein-mediated acetylation. J Biol Chem. 2005;280:11528–34. doi: 10.1074/jbc.M413930200. [DOI] [PubMed] [Google Scholar]
- Yuan Z L, Guan Y J, Chatterjee D, Chin YE. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science. 2005;307:269–73. doi: 10.1126/science.1105166. [DOI] [PubMed] [Google Scholar]
- Ray S, Sherman CT, Lu M, Brasier AR. Angiotensinogen gene expression is dependent on signal transducer and activator of transcription 3-mediated p300/cAMP response element binding protein-binding protein coactivator recruitment and histone acetyltransferase activity. Mol Endocrinol. 2002;16:824–36. doi: 10.1210/mend.16.4.0811. [DOI] [PubMed] [Google Scholar]
- Huang C, Han Y, Wang Y, Sun X, Yan S, Yeh ET, et al. SENP3 is responsible for HIF-1 transactivation under mild oxidative stress via p300 de-SUMOylation. EMBO J. 2009;28:2748–62. doi: 10.1038/emboj.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yang J, Yang K, Cang H, Huang XZ, Li H, et al. The biphasic redox sensing of SENP3 accounts for the HIF-1 transcriptional activity shift by oxidative stress. Acta Pharmacol Sin. 2012;33:953–63. doi: 10.1038/aps.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T, Ray S, Lee C, Brasier AR. The STAT3 NH2-terminal domain stabilizes enhanceosome assembly by interacting with the p300 bromodomain. J Biol Chem. 2008;283:30725–34. doi: 10.1074/jbc.M805941200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Chen S, Xin X, Chiu J, Khan ZA, Chakrabarti S. Diabetes-induced extracellular matrix protein expression is mediated by transcription coactivator p300. Diabetes. 2006;55:3104–11. doi: 10.2337/db06-0519. [DOI] [PubMed] [Google Scholar]
- Chiu J, Khan ZA, Farhangkhoee H, Chakrabarti S. Curcumin prevents diabetes-associated abnormalities in the kidneys by inhibiting p300 and nuclear factor-kappaB. Nutrition. 2009;25:964–72. doi: 10.1016/j.nut.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Chen S, Feng B, George B, Chakrabarti R, Chen M, Chakrabarti S. Transcriptional coactivator p300 regulates glucose-induced gene expression in endothelial cells. Am J Physiol Endocrinol Metab. 2010;298:E127–E137. doi: 10.1152/ajpendo.00432.2009. [DOI] [PubMed] [Google Scholar]
- Feng B, Chen S, Mcarthur K, Wu Y, Sen S, Ding Q, et al. miR-146a-Mediated extracellular matrix protein production in chronic diabetes complications. Diabetes. 2011;60:2975–84. doi: 10.2337/db11-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua P, Feng W, Rezonzew G, Chumley P, Jaimes EA. The transcription factor ETS-1 regulates angiotensin II-stimulated fibronectin production in mesangial cells. Am J Physiol Renal Physiol. 2012;302:F1418–F1429. doi: 10.1152/ajprenal.00477.2011. [DOI] [PubMed] [Google Scholar]
- Sestito R, Madonna S, Scarponi C, Cianfarani F, Failla CM, Cavani A, et al. STAT3-dependent effects of IL-22 in human keratinocytes are counterregulated by sirtuin 1 through a direct inhibition of STAT3 acetylation. FASEB J. 2011;25:916–27. doi: 10.1096/fj.10-172288. [DOI] [PubMed] [Google Scholar]
- Jain S, Wei J, Mitrani LR, Bishopric NH. Auto-acetylation stabilizes p300 in cardiac myocytes during acute oxidative stress, promoting STAT3 accumulation and cell survival. Breast Cancer Res Treat. 2012;135:103–14. doi: 10.1007/s10549-012-2069-6. [DOI] [PubMed] [Google Scholar]
- Scuto A, Kirschbaum M, Buettner R, Kujawski M, Cermak JM, Atadja P, et al. SIRT1 activation enhances HDAC inhibition-mediated upregulation of GADD45G by repressing the binding of NF-kappaB/STAT3 complex to its promoter in malignant lymphoid cells. Cell Death Dis. 2013;4:e635. doi: 10.1038/cddis.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi G, Chatterjee S, Rajendran P, Li F, Shanmugam MK, Wong K F, et al. Inhibition of STAT3 dimerization and acetylation by garcinol suppresses the growth of human hepatocellular carcinoma in vitro and in vivo. Mol Cancer. 2014;13:66. doi: 10.1186/1476-4598-13-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang M, Ma L, Liu N, Ponnusamy M, Zhao TC, Yan H, et al. Histone deacetylase 1/2 mediates proliferation of renal interstitial fibroblasts and expression of cell cycle proteins. J Cell Biochem. 2011;112:2138–48. doi: 10.1002/jcb.23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J, Shen Y, Wang Z, Shao DC, Liu J, Fu LJ, et al. STAT3 acetylation is associated with angiotesin II-induced murine renal fibrosis. Acta Pharmacol Sin. 2014;35:1045–54. doi: 10.1038/aps.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Zhang L, Shao Y, Xue H, Zhou L, Wang XF, et al. Angiotensin II type 2 receptor mediated angiotensin II and high glucose induced decrease in renal prorenin/renin receptor expression. Mol Cell Endocrinol. 2010;315:188–94. doi: 10.1016/j.mce.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Marcu MG, Jung YJ, Lee S, Chung EJ, Lee MJ, Trepel J, et al. Curcumin is an inhibitor of p300 histone acetylatransferase. Med Chem. 2006;2:169–74. doi: 10.2174/157340606776056133. [DOI] [PubMed] [Google Scholar]
- Sunagawa Y, Morimoto T, Wada H, Takaya T, Katanasaka Y, Kawamura T, et al. A natural p300-specific histone acetyltransferase inhibitor, curcumin, in addition to angiotensin-converting enzyme inhibitor, exerts beneficial effects on left ventricular systolic function after myocardial infarction in rats. Circ J. 2011;75:2151–9. doi: 10.1253/circj.cj-10-1072. [DOI] [PubMed] [Google Scholar]
- Kim HY, Park EJ, Joe EH, Jou I. Curcumin suppresses Janus kinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J Immunol. 2003;171:6072–9. doi: 10.4049/jimmunol.171.11.6072. [DOI] [PubMed] [Google Scholar]
- Duan W, Yang Y, Yan J, Yu S, Liu J, Zhou J, et al. The effects of curcumin post-treatment against myocardial ischemia and reperfusion by activation of the JAK2/STAT3 signaling pathway. Basic Res Cardiol. 2012;107:263. doi: 10.1007/s00395-012-0263-7. [DOI] [PubMed] [Google Scholar]
- Hendrayani SF, Al-Khalaf HH, Aboussekhra A. Curcumin triggers p16-dependent senescence in active breast cancer-associated fibroblasts and suppresses their paracrine procarcinogenic effects. Neoplasia. 2013;15:631–40. doi: 10.1593/neo.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EM, Yan G, Mukherjee C, Orry A, Wang L, Holbert MA, et al. Virtual ligand screening of the p300/CBP histone acetyltransferase: identification of a selective small molecule inhibitor. Chem Biol. 2010;17:471–82. doi: 10.1016/j.chembiol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XN, Lin J, Ning QY, Gao L, Yao YS, Zhou JH, et al. A histone acetyltransferase p300 inhibitor C646 induces cell cycle arrest and apoptosis selectively in AML1-ETO-positive AML cells. PLoS One. 2013;8:e 55481. doi: 10.1371/journal.pone.0055481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G, Eller MS, Elm C, Larocca CA, Ryu B, Panova IP, et al. Selective inhibition of p300 HAT blocks cell cycle progression, induces cellular senescence, and inhibits the DNA damage response in melanoma cells. J Invest Dermatol. 2013;133:2444–52. doi: 10.1038/jid.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, et al. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996;379:645–8. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- De Vos J, Jourdan M, Tarte K, Jasmin C, Klein B. JAK2 tyrosine kinase inhibitor tyrphostin AG490 downregulates the mitogen-activated protein kinase (MAPK) and signal transducer and activator of transcription (STAT) pathways and induces apoptosis in myeloma cells. Br J Haematol. 2000;109:823–8. doi: 10.1046/j.1365-2141.2000.02127.x. [DOI] [PubMed] [Google Scholar]
- Qian C, Wang J, Yao J, Wang L, Xue M, Liu W, et al. Involvement of nuclear JAK2 signaling in AG490-induced apoptosis of gastric cancer cells. Anat Rec (Hoboken) 2013;296:1865–73. doi: 10.1002/ar.22820. [DOI] [PubMed] [Google Scholar]
- Guo D, Li JR, Wang Y, Lei L S, Yu CL, Chen NN. Cyclovirobuxinum D suppresses lipopolysaccharide-induced inflammatory responses in murine macrophages in vitro by blocking JAK-STAT signaling pathway. Acta Pharmacol Sin. 2014;35:770–8. doi: 10.1038/aps.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Boldogh I, Brasier AR. STAT3 NH2-terminal acetylation is activated by the hepatic acute-phase response and required for IL-6 induction of angiotensinogen. Gastroenterology. 2005;129:1616–32. doi: 10.1053/j.gastro.2005.07.055. [DOI] [PubMed] [Google Scholar]