Abstract
Rickettsial infections represent a major cause of non-malarial febrile illnesses among the residents of Southeast Asia and returned travelers from that region. There are several challenges in recognition, diagnosis, and management of rickettsioses endemic to Southeast Asia. This review focuses on the prevalent rickettsial infections, namely, murine typhus (Rickettsia typhi), scrub typhus (Orientia tsutsugamushi), and members of spotted fever group rickettsiae. Information on epidemiology and regional variance in the prevalence of rickettsial infections is analyzed. Clinical characteristics of main groups of rickettsioses, unusual presentations, and common pitfalls in diagnosis are further discussed. In particular, relevant epidemiologic and clinical aspects on emerging spotted fever group rickettsiae in the region, such as Rickettsia honei, R. felis, R. japonica, and R. helvetica, are presented. Furthermore, challenges in laboratory diagnosis and management aspects of rickettsial infections unique to Southeast Asia are discussed, and data on emerging resistance to antimicrobial drugs and treatment/prevention options are also reviewed.
Introduction
Although mortality rates for malaria have decreased globally, non-malarial infections remain a major cause of morbidity and mortality in many parts of Southeast Asia.1 Overall, rickettsial infections rate as the second most frequently reported infections for non-malarial febrile illnesses among residents of Southeast Asia, just after dengue.1 Rickettsioses also remain a substantial risk among refugee populations,2 particularly those living along Thailand–Myanmar border, where they may contribute up to 33% of febrile illness presentations.3 They are also associated with stillbirth in pregnancy or low birth weight in infants.4
Conversely, Southeast Asia is a region of growing tourism. Although rich in natural, cultural, and historical attractions, increasing business and investment opportunities and the relative ease of air travel mean that millions of tourists from all over the world travel to Southeast Asia yearly for a wide range of urban and ecotourism activities. For these tourists returning home, imported fevers are a common occurrence and rickettsial infections are a relatively frequent contributor (2–3.3% of all imported fevers).5–7 Rickettsial infections ranked fourth among the identifiable causes of systemic febrile illness in returned travelers from Southeast Asia (16 [2.9%] of 547 identified etiologies).8
Detailed epidemiology and understanding on the burden of rickettsioses in Southeast Asia has been limited. Hundreds of fatal cases and thousands of non-fatal cases probably go undiagnosed in the region each year.9 Under-recognition and under-testing of potential cases, lack of facilities for testing and non-standardized reporting systems likely contribute to the underestimation of true epidemiology.5,10 Physicians caring for the local populace and febrile returned travelers face the extremely difficult task of recognizing and differentiating rickettsial infections from other equally prevalent and important diseases.11 The greatest challenge lies in diagnosing rickettsioses in a timely manner early in the course, when antibiotic use is most effective.12
This report aims to provide a comprehensive review on the prevalence of rickettsial infections in Southeast Asia, with particular emphasis on the characteristics and clinical features of Rickettsia typhi, Orientia tsutsugamushi, and spotted fever group rickettsiae (SFGR). This report also reviews rickettsial diagnostics, associated challenges in resource-limited settings of Southeast Asia, and available treatment options. The aim overall is to provide awareness and understanding of rickettsial diseases to the physicians caring for the local populace and returned travelers from Southeast Asia. Other related vector-borne infections, such as leptospirosis, Q fever, erlichiosis and bartonellosis, are not within the scope of this review.
Overview
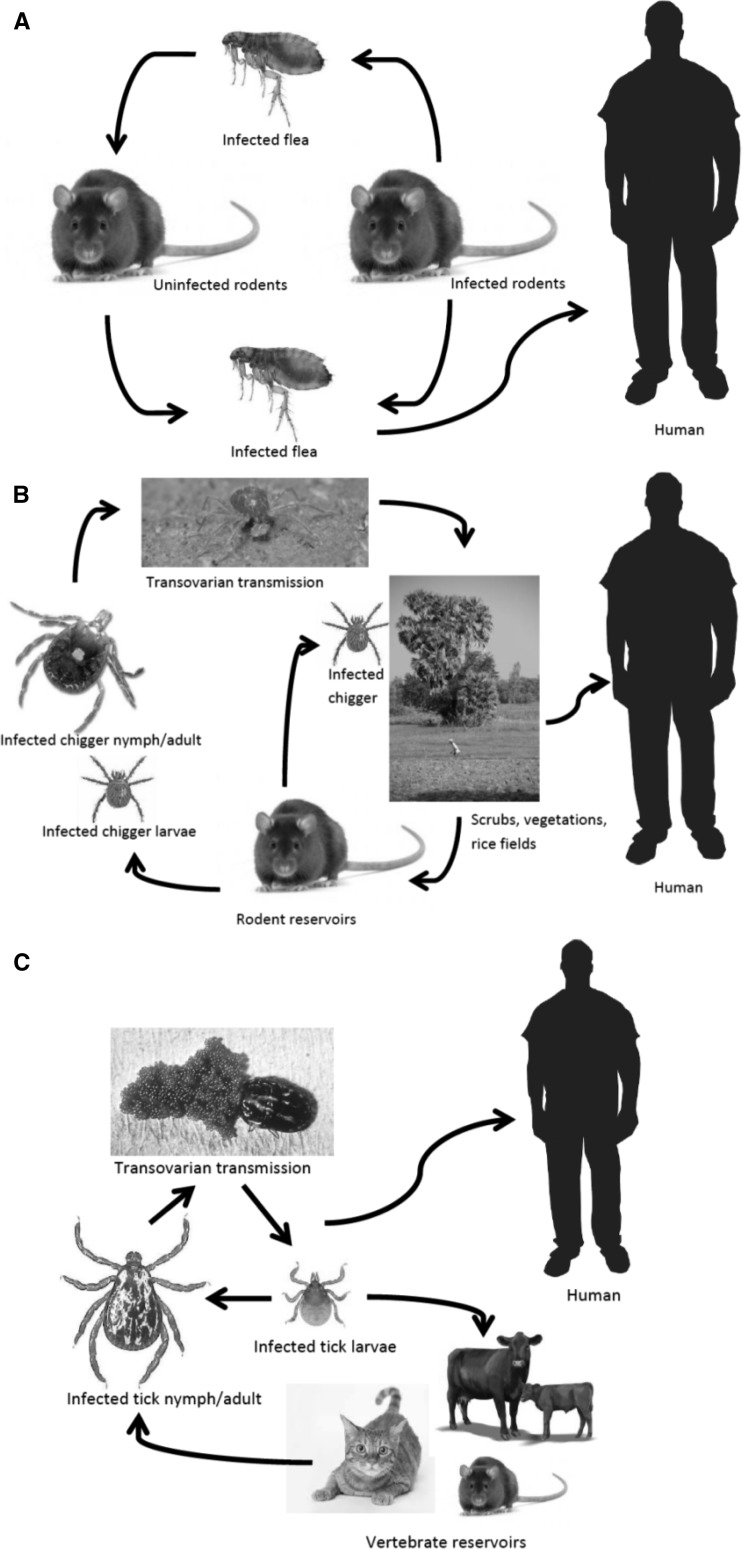
Rickettsiae are small (0.3 × 1–2 μm) obligate intracellular, gram-negative rods that can cause disease in humans as accidental hosts. The pathogenic species of Rickettsia and Orientia are maintained in animal and arthropod reservoirs and are transmitted by arthropod vectors, such as ticks, mites, lice, and fleas (Figure 1). Generally, species within the genus Rickettsia can be subdivided into two groups: the typhus group (containing two species: endemic murine typhus R. typhi and epidemic typhus R. prowazekii) and the spotted fever group (SFG). The list of SFG Rickettsia species recognized as causing human infections is increasing. The genus Orientia contains only two species: O. tsutsugamushi and O. chuto.13
Figure 1.
Simplified diagrams on the life cycles of rickettsiae. A, R. typhi, B, O. tsutsugamushi, and C, spotted fever group rickettsiae. Image source: Google images (www.google.com).
Among residents of Southeast Asia, R. typhi, O. tsutsugamushi, and some members of SFG Rickettsia, including R. honei, are important rickettsial species that cause human diseases. Amongst returned travelers with rickettsial infections, R. typhi and O. tsutsugamushi infections are largely found in persons who visited Southeast Asia.14,15
Epidemiology
The nations of Southeast Asia are Brunei, Cambodia, East Timor (Timor Leste), Indonesia, Laos, Malaysia, Myanmar (Burma), the Philippines, Singapore, Thailand and Vietnam. A summary of clinical studies published within the last 15 years (1999–2014) with regards to prevalence of rickettsial infections in SEA is provided in Supplemental Table. Most studies originated in Thailand; published epidemiologic data are not available for Brunei, East Timor, Myanmar, and Vietnam. Singapore reported 41 serologically confirmed cases of rickettsial diseases in the country during 1999–2000; most were murine typhus.16–18 Further information on the reported prevalence of rickettsial infections specific to the Mekong delta region from all published literature is outlined by Acestor and others.1 Incident rates of rickettsial infections in Southeast Asia are largely unknown and may be increasing in some countries, partially attributable to improved diagnostic methods.18,19
An important aspect to note regarding these epidemiology studies is that although variations in seroprevalence rates may exist between countries and also within each country, such figures need to be interpreted with caution because of several limitations, such as different study populations (e.g., all acute undifferentiated fevers versus exclusion of patients with malaria, which affect the overall reported proportions), variations in serologic methods used, and the diagnostic criteria for seropositivity (single positive titer values versus ≥ 4-fold increase in titers for paired serum samples).20 In addition, in those studies that used a single positive titer as diagnostic criteria, the values ranged from 1:50 to 1:400. Therefore, a direct comparison between the studies is not valid. In addition, there have only been few prevalence studies using the polymerase chain reaction (PCR) method (Supplemental Table) because of its limited availability.
Nevertheless, some useful conclusions can be drawn. First, overall rickettsial infections in Southeast Asia are probably more common than previously perceived, and a significant proportion of patients were found to be seropositive for any rickettsial infections at low titers, many of whom likely had previous infections/exposures.21–24 Second, when relative contributions from the 3 rickettsial groups were compared, it was found that murine typhus was more commonly reported among the urban dwelling patients and scrub typhus and SFGR infections were more prevalent among rural patients.22,25 Geographically, as noted previously, scrub typhus was prevalent in northern and northeastern regions of Thailand, and incidence rates may be as high as 16 cases/100,000 population per year in some areas such as Chiang Mai.26–29 Socioeconomically, agricultural, plantation, or forest workers in Thailand, Malaysia, and Indonesia were frequently affected by scrub typhus and SFGR infections.21,23,27,30,31 Third, despite their predilection for certain geographic areas or human populations, none of the 3 rickettsial groups discriminated between urban and rural dwellers (or tourists). Thus, equal consideration should be given for testing of all rickettsial groups in patients with suspected diseases.
The main reservoirs and vectors for rickettsioses found in Southeast Asia are summarized in Table 1 . A number of rodent and ectoparasite studies may also shed further light on human epidemiology.32–38 Rodents were found to be important reservoirs for SFGR and murine typhus; high seropositive rates (up to 39.1% for SFGR) were observed in some parts of rural Indonesia.32,33 Infection of rat flea Xenopsylla cheopis with R. felis has also been found in Indonesia, which increases the possibility of human infections with R. felis in the region, although no clinical case has been reported to date.35,37 Ectoparasite field surveys of villages in Laos and Malaysian Borneo of domestic animals also showed significantly high overall R. felis DNA detection rates of 76.6% and 74.4%, respectively.36 However, in contrast, another study in peninsula Malaysia showed a lower rate (2.9%) of R. felis DNA detection.38 In addition, previously unrecognized vectors or rickettsial species have also been reported in these studies. Studies in rural mountainous areas of Thailand demonstrated that Bandicota species and Rattus argentiventer may be the main rodent vectors for transmission of SFGR (R. japonica and R. honei)34 and O. tsutsugamushi.29 Another study in Thailand found 3 novel phylogentically distinct SFGR DNAs (ATT, HOT1 and HOT2) in 30% of Amblyomma testudinarium and 16.8% of Hemaphysalis ornithophila tick species collected from animals and vegetation in Khao Yai National Park.39 However, it is unclear if these newly discovered SFGR could cause human disease and whether the ticks species carrying these rickettsiae could feed on humans as a host.39
Table 1.
Summary of prevalent rickettsiae in Southeast Asia, their reservoirs, and vectors for disease transmission
| Rickettsiae | Main reservoirs | Main vectors |
|---|---|---|
| Typhus group | ||
| Murine typhus (R. typhi) | Rats (Rattus rattus, Rattus norvegicus, other Rattus sp.)12,32,33,37 | Xenopsylla cheopis37 |
| Scrub typhus (O. tsutsugamushi) | Rats (Rattus sp.and Bandicota sp.)29,33 | Trombiculid mites (larval stage)29 |
| Spotted fever group | ||
| R. honei | Rats (Rattus sp. and Bandicota indica)32,34,73 | Ixodes granulatus, Ixodes sp., Rhipicephalus sp.73 |
| R. felis | Rats (Rattus sp.) and shrews (Suncus murinus),37 domestic cats, dogs, cows and pigs36,38 | Ctenocephalides orientis, C. felis felis,36 X. cheopis12,35,37 |
| R. conorii subsp. indica | Rats (Rattus sp.)32 | R. sanguineus2 |
| R. helvetica | Unknown | Ixodes sp.3,62,76 |
| R. japonica | Rats (Rattus sp. and B. indica)34 | Various species of animal ticks2 |
Despite extensive data, these epidemiologic studies may not have captured the true prevalence and regional variance of rickettsioses in Southeast Asia. In addition, prevalence studies in popular tourist destinations are lacking. Thus, risks to visitors are unknown. References to case reports of infections in returned travelers from different parts of Southeast Asia are provided below in relevant sections. Further co-ordinated effort is urgently needed among nations in Southeast Asia to ascertain regional prevalence.
Immunopathogenesis
Rickettsiae attach and enter the host cell receptors by means of surface proteins ompB and ompA in SFGR.40 Rickettsial infections are vasculotrophic in nature and current hypothesis regarding immunopathogenic mechanisms concerns that of oxidative stress, leading to endothelial cell injury, increased microvascular permeability, and possibly development of a procoagulant state.40 Rickettsia-infected endothelium produces interleukin-6 (IL-6), IL-8, and monocyte chemoattractant protein-1.40 Host immune responses determine the clinical severity and other clinical predictors include older age, glucose-6-phosphate dehydrogenase deficiency, diabetes, and male sex.41 Notably, an in vitro study showed that scrub typhus infection was associated with more prominent levels of inflammatory cytokines production and more pronounced activation of coagulation pathways compared with murine typhus.42 Further studies have suggested that scrub typhus infection induces type 1 immune response and results in increases in levels of interferon-γ, tumor necrosis factor-α, IL-12p40, IL-18, and IL-15.43 These intrinsic differences in host responses may help explain why scrub typhus can produce more severe disease and deranged biochemical parameters compared with murine typhus or SFGR.
Cytotoxic CD8+ T cells clear acute rickettsial infections and CD4+ and CD8+ T cells are involved in the differentiation of B lymphocytes to plasma cells and the production of antibodies against ompA and ompB, which are protective against reinfection.44 Antibodies against rickettsial lipopolysaccharides are also produced but they do not appear to confer any protection.45
Clinical Features
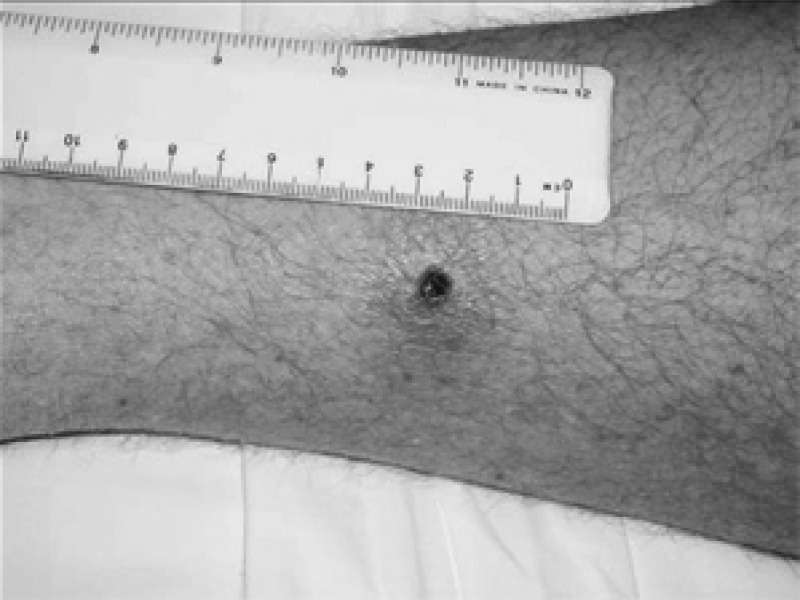
Clinical manifestations of most rickettsioses represent a continuous spectrum.40 Rickettsial infections generally begin as an acute, non-specific, febrile illness often accompanied by headache, myalgia, nausea, and vomiting.41 After 3–5 days of illness, a macular, maculopapular, or even vesicular rash appears.41 An eschar at the site of an ectoparasite bite, with local or generalized lymphadenopathy may also occur (Figure 2).2 The eschar may be the only clinical sign that differentiates scrub typhus and tick-borne SFG Rickettsia from other infectious diseases, including murine typhus.28,41 Travelers with imported rickettsial diseases often become sick before or within a few days of returning from a disease-endemic region. Because the incubation period for most rickettsial infections is 6–20 days, an illness that begins more than 18 days after return from a disease-endemic area is unlikely to be rickettsial in origin.11,41
Figure 2.
Rickettsial eschar. Reproduced with written permission from The Commonwealth of Australia, Department of Defense, Joint Health Command (http://www.defence.gov.au/health/infocentre/journals/adfhj_apr06/adfhealth_7_1_10-13.html).
Because clinical features of rickettsial infections are non-specific, many common febrile illnesses can mimic rickettsioses.46 Differential diagnoses are wide and include viral infections, such as dengue, chikungunya, Epstein-Barr virus, cytomegalovirus, other herpesviruses, enteroviruses, flaviviruses, viral hepatitis, and human immunodeficiency virus; bacterial infections, such as typhoid fever, leptospirosis, disseminated gonococcal infections, and syphilis; and parasitic infections, such as malaria and toxoplasmosis.41,47 Because of the undifferentiated nature of presentation, delay in diagnosis is common.
A case-control study along the Thailand–Myanmar border identified jungle excursion (odds ratio [OR] = 5.3), history of arthropod bites and/or rash (OR = 22.9), thrombocytopenia (OR = 3.4), and an increased level of alanine aminotransferase (OR = 3.0) as clinical predictors for diagnosing rickettsial infections in patients with undifferentiated fever.46 Presence of an eschar was further noted to be a strong predictor (OR = 21.1, 95% confidence interval = 5.4–82.4).46 A small comparative case series study in febrile returned travelers also found that an increased level of C-reactive protein and absence of neutropenia may help differentiate rickettsial infections from dengue.48 Although a thorough history and clinical examination may assign a pre-test probability of rickettsial infections, no single factor reliably separates rickettsioses from other infections in either the local populace or returned travelers.
Because the diagnosis of rickettsial infections relies on serologic or molecular tests, often with considerable delay, some clinicians advocate a trial of doxycycline therapy in patients with ongoing undifferentiated fever that exceeds the expected duration of common viral illnesses, or in patients in whom the illness is rapidly progressive. Defervescence within 48–72 hours after initiation of doxycycline is often taken as a marker of rickettsial infections.41 Up front administration of doxycycline has also been tested in a resource-limited country (Laos) for treatment of patients with undifferentiated acute hepatitis where doxycycline responsive infections such as rickettsioses and leptospirosis made up 7.3% and 6.8% of acute hepatitis presentations.49
Endemic (murine) typhus.
Endemic (murine) typhus caused by R. typhi occurs in tropical and temperate climates.11 It is carried by rat flea X. cheopis and typically thrives in markets, grain stores, breweries, and garbage depots where rats (mainly R. rattus or R. norvegicus) serve as the main reservoir. However, other rodent species and cats have also been implicated as reservoirs and cats fleas can also transmit the infection.12,32,33,50,51
Overcrowding, poor public health and sanitation measures provide ideal conditions for transmission of murine typhus. In addition, clearing of land for urban development and construction enable rodent populations to increase and expose humans to the zoonotic life cycle, thus leading to increasing seroprevalence observed in some of the rapidly developing countries, such as Malaysia.19 More aggressive infections have also been reported in refugee camps,2 and this finding may be significant for refugee populations along the Thailand–Myanmar border. It is important to note that imported murine typhus can be acquired after travel to exclusively urban areas. Infections have been reported among city dwellers in more affluent countries such as Singapore, and immigrant workers living in poor and unhygienic conditions seemed to be at a higher risk.11,12,16,52,53 However, no associated seasonal or festive variation with murine typhus has been observed.16,19,28,54
Flea bites and contamination of excoriated skin or respiratory tract with flea feces cause infections, and R. typhi can remain infectious in dried flea feces for ≥ 100 days.11,12,41 the incubation period is usually approximately 8–16 days.41 Infection usually produces a mild or self-limiting illness that lasts 3–7 days; symptoms include headache, rash, and arthralgia.12 Rash can be of variable frequency, ranging from 20–80%, and is described as non-pruritic, macular or maculopapular spreading from center to periphery and occurring a few days after the onset of fever.12 Murine typhus is frequently misdiagnosed in cases where rash is absent or fleeting and atypical symptoms, such as gastrointestinal manifestations, are prominent.11 Severe complications appear rare, but where present, can cause meningoencephalitis, pneumonia, shock, renal failure, myocarditis, endocarditis, and splenic rupture.12,28,41,54,55 Reported mortality ranges from 1% to 4%, and higher mortality is associated with lack of antibiotics.12
Imported murine typhus has been reported in travelers returning from Bali and Lombok regions in Indonesia55–57 and also from southern Vietnam (Cu Chi) where murine typhus has not been reported since the 1960s, but probably occurs sporadically among the local population.58 A severe case of imported murine typhus from northern Thailand causing septic shock and acute respiratory failure has also been described.59 Murine typhus complicated by dengue hemorrhagic fever has also been reported in a returned traveler from Brunei.60
Scrub typhus.
O. tsutsugamushi is acquired from the bite of the larval stage of infected trombiculid mites (chiggers) living on the waist-high grass growing in previously cleared jungle around villages and plantations.2,61 Chiggers have also been found on many rodent vectors.29 Cases were acquired during land clearing, logging, road building, and military operations.42 Active rice fields are also an important and underappreciated sources of infection.62 Travelers with eco-tourism activities, such as trekking, camping, or rafting in rural areas, are at most risk.47,52,61 Outbreaks have also been reported mainly in rural agricultural areas,29 but cases have been seen in urban centers of Southeast Asia.22,30,63 Unlike murine typhus, seasonal variations can be found with scrub typhus; incidence increases towards the end of the rainy season and the beginning of winter months (July–November) in Southeast Asia.27,28
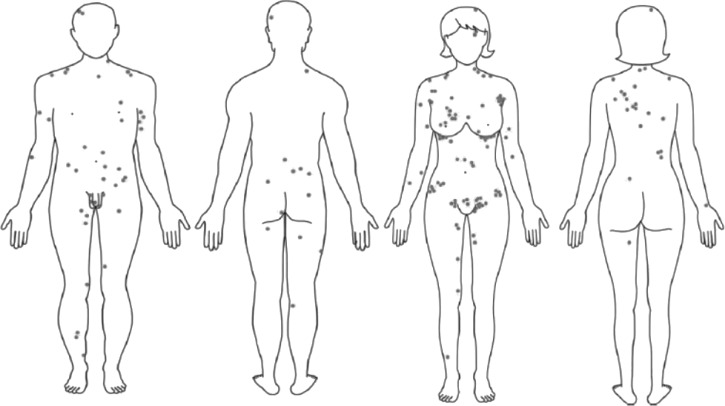
Most scrub typhus cases are mild and characterized by acute onset fever, myalgia, generalized lymphadenopathy, maculopapular rash, and splenomegaly. Eschar is variably seen in 20–90% of cases, typically on torso, axilla, or groin, and may be diagnostic (Figure 3).2,28,52,64,65 Conjunctival suffusion can also occur.41 Of all rickettsial diseases, scrub typhus is more likely to present with a severe clinical picture because of a more prominent inflammatory response,42 which causes multiorgan dysfunction, meningoencephalitis, coma, and persistent neurologic deficits.11,66 Pulmonary involvement occurs in up to 50% of patients28,65 but clinical manifestation of acute respiratory distress syndrome is rare.67–70 Mortality rates can be up to 25% and scrub typhus could be potentially mistaken for melioidosis or leptospirosis, which are more common in the region.69,70 Atypical clinical features and absence of eschar may result in delayed diagnosis, complications, or death.47,66–68 Laboratory abnormalities of scrub typhus may include leukopenia or mild leukocytosis and thrombocytopenia. Hepatic dysfunction is common and transaminitis was noted in 90% of patients in a cases series.28 Overall mortality rates for scrub typhus range from 2.6% to 15%.28,41 Most case reports for imported scrub typhus were from Thailand or the Thailand–Myanmar border.66,67
Figure 3.
Typical distribution (grey dots) of scrub typhus eschar on body sites.64
Spotted fever group rickettsiae.
The SFGR contain a large number of species transmitted from rodents, dogs, and wild animals by ticks and sometimes by lice. Clinical presentation may vary between species and geographic regions. R. honei, R. conorii subsp. indica (Indian strain), R. helvetica, R. japonica, and R. felis have been reported to cause human infections in Southeast Asia on the basis of serodiagnostic studies (Supplemental Table). Some authors considered R. felis to be part of a transitional group of rickettsiae. However, the presence of the ompA gene confirms its membership in the SFGR.12,71 It is also important to note that with SFGR, given the limitations of immunofluorescence serologic tests, antigenically cross-reacting but phylogenetically distinct SFGR species, which are yet to be identified by culture or molecular methods, may be present in Southeast Asia.
R. honei, formerly known as Thai tick typhus TT-118, was first isolated from Ixodes (particularly I. granulatus) and Rhipicephalus species of ticks in Chiang Mai Province, Thailand in 1962.72 R. honei is now considered to be one of the SFGR endemic to Thailand (and possibly the remainder of Southeast Asia) that causes human disease73,74 and may be used as a reference species in serologic tests for SFGR in this region (Supplemental Table). Infections likely occur in rural areas throughout Southeast Asia, where human and animal interactions are more common, although one clinical case from urban Bangkok, Thailand, without any antecedent exposure to rural setting has been reported.75 Infections usually cause mild disease with fever, headache, and myalgia. Lymphadenopathy, maculopapular rash, or rarely, petechial rash may be present and an eschar may be seen.
R. felis is transmitted mainly by the cat flea C. felis (the rat flea and other arthropods can also transmit it), and is an emerging infection globally.71 In Southeast Asia, two serologically confirmed clinical cases have been reported from the Thailand–Myanmar border and Vientiane, Laos (Supplemental Table).3,63 The presence of R. felis in this region was further supported by rodent and entomologic studies. R. felis infections produce a clinical syndrome similar to that of murine typhus.35 Given the clinical similarities, some authors believe that previously reported outbreaks of murine typhus infections may have been caused by the more recently recognized R. felis.12
R. helvetica is transmitted by Ixodes spp., and clinical cases of infection have been reported in Thailand, along the Thailand–Myanmar border, and Laos.3,63,76 Infection with R. helvetica presents as a mild disease, especially in warm seasons, with fever, headache, and myalgia but no rash.76 Human infection with SFGR species closely related to R. japonica acquired from camping at Khao Yai National Park in Thailand has also been noted.77,78
Diagnosis
Historically, the Weil-Felix agglutination test has been used to diagnose rickettsial infections on the basis of the observation that cross-reactivity occurs between antibodies to Rickettsia and phylogenetically unrelated bacteria of the genus Proteus (strains OX2, OX19, and OXK).79,80 Although this test lacks sensitivity and specificity and thus is no longer recommended,79–81 it may still provide cheap and rapid diagnostic option for rickettsial infections in many resource-limited areas of Southeast Asia.
Serologic diagnosis using indirect immunofluorescence assays (IFAs) is the gold standard serologic test for diagnosing rickettsial infections.12,79 The IFA serologic test uses whole rickettsial cells and a positive antigen–antibody reaction is visualized as fluorescence by using an ultraviolet microscope.79 The IFA can also differentiate between different antibody classes (IgM, IgG, and IgA), and antibody concentration is reported as a titer, the highest serum dilution that shows a positive result.79
The IFA often identifies the rickettsial species causing infection by measuring microimmunofluorescence assay titers of serum against different rickettsial species.80 Antigens against the Wilmington strain of R. typhi are commonly used for diagnosing murine typhus, and three serotypes (Karp, Kato, and Gilliam) of O. tsutsugamushi are usually used for diagnosing scrub typhus. Usually, homologous antibody titers are higher than heterologous antibody titers.80 However, among members of the same biogroup, heterologous titers may be as high as homologous titers and cross-absorption studies may help differentiate between the two results, as for identification of the SFGR species.80
Conversely, the IFA is expensive, requires a fluorescence microscope, and also requires training.82 There is also little consensus on the choice of IFA method (i.e., antigenic strains used and antibody isotypes measured) and titers selected as positive may vary for diagnostic and epidemiologic studies.20 At low dilutions, patient serum may also cross-react with other related bacteria such as Anaplasma phagocytophilum, Ehrlichia chaffeensis, and even unrelated Coxiella burnetti.3,80,83
The indirect immunoperoxidase test has been accepted as an alternative standard assay by the World Health Organization to diagnose rickettsial diseases (particularly for scrub typhus) without the need for an ultraviolet microscope by replacing fluorescein with peroxidase, thus reducing laboratory staff training.80,82,84 The performance of this test has been validated by comparing it against IFA on clinical samples in Thailand.85
There are several pitfalls for all serologic methods. First, paired serum samples obtained at least 10 days apart are usually required for diagnosis (≥ 4-fold increase between acute- phase and convalescent-phase antibody titers). Thus, where access is limited and resources are scarce, it may not be a practical approach in many parts of Southeast Asia. Second, host antibody response may be abrogated by early antibiotic treatment or inability (genetic and acquired) to mount an antibody response.79 This finding is especially important in situations in which empiric doxycycline is given or in parts of Southeast Asia where human immunodeficiency virus prevalence is high. Third, sensitivity and specificity of tests vary,12,79 and positive and negative predictive values for any assay also vary with the population being examined.
Nucleic acid amplification tests (NAATs) are fairly recent developments in rickettsial diagnostics, and may enable early detection and diagnosis of infection before seroconversion, but they are not yet commercially available and remain restricted mainly to research settings and developed countries.79 In the NAAT, the citrate synthase gene and 17-kDa antigen gene can be amplified to detect all Rickettsia spp. because these genes are present in all species.79 However, detection of the ompA gene is specific to SFGR.79 The 47-kDa outer membrane protein antigen gene has been shown to be highly specific for O. tsutsugamushi.86 Such PCR methods can be used for eschar and blood (eschar is more sensitive than blood).82 Conversely, cell cultures for Rickettsia are still a research tool.79 Cell culture is necessary to obtain a rickettsial isolate, although NAAT can often identify the rickettsial species involved in an infection. Serologic analysis is the best tool for determining prevalence.
Unfortunately, at the present time, in many parts of Southeast Asia where rickettsial infections are rampant, and where the local populace would benefit from rapid PCR tests83 or serologic methods, they remain unavailable and presumptive clinical diagnosis by trial of doxycycline remains the standard practice.
Treatment and Prevention
Doxycycline (100 mg, twice a day) is the mainstay of treatment of murine typhus for adults and children (weighing ≥ 45 kg), and should be continued for a minimum of five days in total, or three days after the resolution of symptoms.12 For children weighing < 45 kg, doxycycline at a dose of 0.9 mg/kg/day in two divided doses is recommended. Doxycycline is considered safe in children < 9 years of age, has limited risk of dental staining with short courses, and fever resolution is seen within 48 hours in most.54 Chloramphenicol (250–500 mg every 6 hours) can be used to treat women in first or second trimester of pregnancy, and quinolones may be an alternative.12 However, murine typhus may show variable responses to quinolones, and treatment failure has been reported with ciprofloxacin in a returned traveler from Thailand.87
Doxycycline (100 mg, twice a day) or chloramphenicol (250–500 mg every 6 hours) can be used to treat scrub typhus.88 The O. tsutsugamushi Kato strain has been reported to be intrinsically resistant to quinolones.89 Reduced susceptibility to doxycycline and chloramphenicol has also been reported from Chiangrai in northern Thailand.90 A randomized controlled trial from the same area showed that treatment with rifampicin (300–450 mg, twice a day) for one week was superior to doxycycline in terms of time to defervescence and relapse rates.91 However, combination therapy with rifampicin and doxycycline did not have any added efficacy.91
In areas where reduced susceptibility to tetracyclines is present, a prospective, open-label, randomized trial showed that a single dose of azithromycin (500 mg) may be equally effective as a seven-day course of doxycycline (100% versus 93.5% cure; P = 0.12) with similar median time to defervescence (21 hours versus 29 hours; P = 0.97).92 A randomized control trial in northern Thailand involving 57 patients with scrub typhus, 14 patients with murine typhus, and 11 patients with leptospirosis comparing a three-day course of azithromycin to a seven-day course of doxycycline showed that fever clearance was similar (48 hours, range = 8–336 hours versus 48 hours, range = 8–118 hours; P = 0.57), although more adverse effects were found with doxycycline treatment.93 Azithromycin is also considered safe in pregnant women. A five-day regimen of 800 mg/day of telithromycin may have efficacy similar to that of doxycycline in treatment of mild-to-moderate scrub typhus.94 Optimal duration of treatment with doxycycline is uncertain, and courses as short as three days have been studied.95
Doxycycline (100 mg, twice a day for 5–7 days) is recommended for treatment of all SFGR infections. Once a week doses of doxycycline (200 mg) can prevent scrub typhus,96,97 but there have been no studies on prophylaxis therapy for other rickettsioses. The use of insect repellents, such as N,N-diethyl-3-methylbenzamide (DEET) and permethrin-impregnated clothing and bedding, may also form part of preventive measures against scrub typhus in travelers to disease-endemic areas in Southeast Asia.
Conclusions
Rickettsial infections are a major cause of non-malarial febrile illnesses among the residents of Southeast Asia and remain an important differential diagnosis for febrile returned travelers from that region. However, many challenges remain in the diagnosis and management of rickettsial infections. The regional epidemiology needs to be established to determine the true burden of rickettsial diseases compared with other tropical illnesses in Southeast Asia. To achieve this epidemiology, improved diagnostic measures and facilities need to be made accessible to many areas within Southeast Asia, especially to rural and remote settings, where the disease burden may be higher. A collaborative effort between nations in Southeast Asia may be needed to address rickettsial epidemiology, diagnostics, and management. From an individual physician's perspective, rickettsial infections remain a diagnostic challenge in resource-poor settings. Therefore, it is important to recognize the clinical features promptly, include rickettsioses in the differential diagnoses, and consider early initiation of appropriate treatment.
Supplementary Material
Footnotes
Authors' addresses: Ar Kar Aung, Department of Infectious Diseases and Microbiology, The Alfred Hospital, Melbourne, Australia, E-mail: a.aung@alfred.org.au. Denis W. Spelman, Department of Infectious Diseases and Microbiology, The Alfred Hospital and Microbiology Unit, Monash University, Melbourne, Victoria, Australia, E-mail: d.spelman@alfred.org.au. Ronan J. Murray, Department of Infectious Diseases and Microbiology and Pathwest Laboratory Medicine, Sir Charles Gairdner Hospital, Perth, Western Australia, Australia, and School of Pathology and Laboratory Medicine, University of Western Australia, Perth, Western Australia, Australia, E-mail: ronan.murray@health.wa.gov.au. Stephen Graves, Australian Rickettsial Reference Laboratory Foundation, Geelong Hospital, Geelong, Victoria, Australia, and New South Wales Health Pathology, University of Newcastle, Newcastle, New South Wales, Australia, E-mail: stephen.graves@hnehealth.nsw.gov.au.
References
- 1.Acestor N, Cooksey R, Newton PN, Menard D, Guerin PJ, Nakagawa J, Christophel E, Gonzalez IJ, Bell D. Mapping the aetiology of non-malarial febrile illness in Southeast Asia through a systematic review: terra incognita impairing treatment policies. PLoS ONE. 2012;7:e44269. doi: 10.1371/journal.pone.0044269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowan G. Rickettsial diseases: the typhus group of fevers: a review. Postgrad Med J. 2000;76:269–272. doi: 10.1136/pmj.76.895.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parola P, Miller RS, McDaniel P, Telford III, Sr., Rolain J-M, Wongsrichanalai C, Raoult D. Emerging rickettsioses of the Thai-Myanmar border. Emerg Infect Dis. 2003;9:592–595. doi: 10.3201/eid0905.020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGready R, Ashley EA, Wuthiekanun V, Tan SO, Pimanpanarak M, Viladpai-Nguen SJ, Jesadapanpong W, Blacksell SD, Peacock SJ, Paris DH. Arthropod borne disease: the leading cause of fever in pregnancy on the Thai-Burmese border. PLoS Negl Trop Dis. 2010;4:e888. doi: 10.1371/journal.pntd.0000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottieau E, Clerinx J, Schrooten W, Van den Enden E, Wouters R, Van Esbroeck M, Vervoort T, Demey H, Colebunders R, Van Gompel A. Etiology and outcome of fever after a stay in the tropics. Arch Intern Med. 2006;166:1642–1648. doi: 10.1001/archinte.166.15.1642. [DOI] [PubMed] [Google Scholar]
- 6.Wilson ME, Weld LH, Boggild A, Keystone JS, Kain KC, von Sonnenburg F, Schwartz E. Fever in returned travelers: results from the GeoSentinel Surveillance Network. Clin Infect Dis. 2007;44:1560–1568. doi: 10.1086/518173. [DOI] [PubMed] [Google Scholar]
- 7.O'Brien D, Tobin S, Brown GV, Torresi J. Fever in returned travelers: review of hospital admissions for a 3-year period. Clin Infect Dis. 2001;33:603–609. doi: 10.1086/322602. [DOI] [PubMed] [Google Scholar]
- 8.Freedman DO, Weld LH, Kozarsky PE, Fisk T, Robins R, von Sonnenburg F, Keystone JS, Pandey P, Cetron MS. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med. 2006;354:119–130. doi: 10.1056/NEJMoa051331. [DOI] [PubMed] [Google Scholar]
- 9.Phommasone K, Paris DH, Anantatat T, Castonguay-Vanier J, Keomany S, Souvannasing P, Blacksell SD, Mayxay M, Newton PN. Concurrent infection with murine typhus and scrub typhus in southern Laos: the mixed and the unmixed. PLoS Negl Trop Dis. 2013;7:e2163. doi: 10.1371/journal.pntd.0002163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tay S, Rohani M. The use of the indirect immunoperoxidase test for the serodiagnosis of rickettsial diseases in Malaysia. Southeast Asian J Trop Med Public Health. 2002;33:314–320. [PubMed] [Google Scholar]
- 11.Hendershot EF, Sexton DJ. Scrub typhus and rickettsial diseases in international travelers: a review. Curr Infect Dis Rep. 2009;11:66–72. doi: 10.1007/s11908-009-0010-x. [DOI] [PubMed] [Google Scholar]
- 12.Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913–918. doi: 10.1086/527443. [DOI] [PubMed] [Google Scholar]
- 13.Izzard L, Fuller A, Blacksell SD, Paris DH, Richards AL, Aukkanit N, Nguyen C, Jiang J, Fenwick S, Day NP. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48:4404–4409. doi: 10.1128/JCM.01526-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensenius M, Davis X, von Sonnenburg F, Schwartz E, Keystone JS, Leder K, Lopéz-Véléz R, Caumes E, Cramer JP, Chen L. Multicenter GeoSentinel analysis of rickettsial diseases in international travelers, 1996–2008. Emerg Infect Dis. 2009;15:1791–1798. doi: 10.3201/eid1511.090677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensenius M, Han PV, Schlagenhauf P, Schwartz E, Parola P, Castelli F, von Sonnenburg F, Loutan L, Leder K, Freedman DO. Acute and potentially life-threatening tropical diseases in western travelers: a GeoSentinel Multicenter Study, 1996–2011. Am J Trop Med Hyg. 2013;88:397–404. doi: 10.4269/ajtmh.12-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong A, Tambyah P, Ooi S, Kumarasinghe G, Chow C. Endemic typhus in Singapore: a re-emerging infectious disease? Singapore Med J. 2001;42:549–552. [PubMed] [Google Scholar]
- 17.Chen M, Chua J, Lee C, Leo Y, Kumarasinghe G. Epidemiological, clinical and laboratory characteristics of 19 serologically confirmed rickettsial disease in Singapore. Singapore Med J. 2001;42:553–558. [PubMed] [Google Scholar]
- 18.Wong S, Lam M. Rickettsioses: the new and old diseases. Singapore Med J. 2001;42:546–548. [PubMed] [Google Scholar]
- 19.Sekhar W, Devi S. The increasing prevalence of endemic typhus in Kuala Lumpur and an evaluation of a diagnostic ELISA dot test for the detection of antibodies to Rickettsia typhi. Singapore Med J. 2000;41:226–231. [PubMed] [Google Scholar]
- 20.Blacksell SD, Bryant NJ, Paris DH, Doust JA, Sakoda Y, Day NP. Scrub typhus serologic testing with the indirect immunofluorescence method as a diagnostic gold standard: a lack of consensus leads to a lot of confusion. Clin Infect Dis. 2007;44:391–401. doi: 10.1086/510585. [DOI] [PubMed] [Google Scholar]
- 21.Tay S, Ho T, Rohani M, Devi S. Antibodies to Orientia tsutsugamushi, Rickettsia typhi and spotted fever group rickettsiae among febrile patients in rural areas of Malaysia. Trans R Soc Trop Med Hyg. 2000;94:280–284. doi: 10.1016/s0035-9203(00)90322-5. [DOI] [PubMed] [Google Scholar]
- 22.Tay S, Kamalanathan M, Rohani M. Antibody prevalence of Orientia tsutsugamushi, Rickettsia typhi and TT118 spotted fever group rickettsiae among Malaysian blood donors and febrile patients in the urban areas. Southeast Asian J Trop Med Public Health. 2003;34:165–170. [PubMed] [Google Scholar]
- 23.Tay S, Rohani M, Ho T, Devi S. Isolation and PCR detection of rickettsiae from clinical and rodent samples in Malaysia. Southeast Asian J Trop Med Public Health. 2002;33:772–779. [PubMed] [Google Scholar]
- 24.Tay ST, Kamalanathan M, Suan KA, Chun SS, Ming HT, Yasin RM, Devi S. Seroepidemiologic survey of Orientia tsutsugamushi, Rickettsia typhi, and TT118 spotted fever group rickettsiae in rubber estate workers in Malaysia. Am J Trop Med Hyg. 1999;61:73–77. doi: 10.4269/ajtmh.1999.61.73. [DOI] [PubMed] [Google Scholar]
- 25.Vallée J, Thaojaikong T, Moore CE, Phetsouvanh R, Richards AL, Souris M, Fournet F, Salem G, Gonzalez J-PJ, Newton PN. Contrasting spatial distribution and risk factors for past infection with scrub typhus and murine typhus in Vientiane City, Lao PDR. PLoS Negl Trop Dis. 2010;4:e909. doi: 10.1371/journal.pntd.0000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thitivichianlert S, Suthee Panichkul M, Boonmee P, Ketupanya A. Incidence of rickettsial infection in patients with acute fever in provincial Thai Army hospitals. J Med Assoc Thai. 2009;92:S39–S46. [PubMed] [Google Scholar]
- 27.Suttinont C, Losuwanaluk K, Niwatayakul K, Hoontrakul S, Intaranongpai W, Silpasakorn S, Suwancharoen D, Panlar P, Saisongkorh W, Rolain J. Causes of acute, undifferentiated, febrile illness in rural Thailand: results of a prospective observational study. Ann Trop Med Parasitol. 2006;100:363–370. doi: 10.1179/136485906X112158. [DOI] [PubMed] [Google Scholar]
- 28.Suputtamongkol Y, Suttinont C, Niwatayakul K, Hoontrakul S, Limpaiboon R, Chierakul W, Losuwanaluk K, Saisongkork W. Epidemiology and clinical aspects of rickettsioses in Thailand. Ann N Y Acad Sci. 2009;1166:172–179. doi: 10.1111/j.1749-6632.2009.04514.x. [DOI] [PubMed] [Google Scholar]
- 29.Rodkvamtook W, Gaywee J, Kanjanavanit S, Ruangareerate T, Richards AL, Sangjun N, Jeamwattanalert P, Sirisopana N. Scrub typhus outbreak, northern Thailand, 2006–2007. Emerg Infect Dis. 2013;19:774–777. doi: 10.3201/eid1905.121445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richards AL, Ratiwayanto S, Rahardjo E, Kelly DJ, Dasch GA, Fryauff DJ, Bangs MJ. Serologic evidence of infection with ehrlichiae and spotted fever group rickettsiae among residents of Gag Island, Indonesia. Am J Trop Med Hyg. 2003;68:480–484. [PubMed] [Google Scholar]
- 31.Brown G, Robinson D, Huxsoll D, Ng T, Lim K, Sannasey G. Scrub typhus: a common cause of illness in indigenous populations. Trans R Soc Trop Med Hyg. 1976;70:444–448. doi: 10.1016/0035-9203(76)90127-9. [DOI] [PubMed] [Google Scholar]
- 32.Ibrahim IN, Okabayashi T, Lestari EW, Yanase T, Muramatsu Y, Ueno H, Morita C. Serosurvey of wild rodents for rickettsioses (spotted fever, murine typhus and Q fever) in Java Island, Indonesia. Eur J Epidemiol. 1999;15:89–93. doi: 10.1023/a:1007547721171. [DOI] [PubMed] [Google Scholar]
- 33.Richards AL, Rahardjo E, Rusjdi AF, Kelly DJ, Dasch GA, Church CJ, Bangs MJ. Evidence of Rickettsia typhi and the potential for murine typhus in Jayapura, Irian Jaya, Indonesia. Am J Trop Med Hyg. 2002;66:431–434. doi: 10.4269/ajtmh.2002.66.431. [DOI] [PubMed] [Google Scholar]
- 34.Okabayashi T, Tsutiya K, Muramatsu Y, Ueno H, Morita C. Serological survey of spotted fever group Rickettsia in wild rats in Thailand in the 1970s. Microbiol Immunol. 1996;40:895–898. doi: 10.1111/j.1348-0421.1996.tb01157.x. [DOI] [PubMed] [Google Scholar]
- 35.Jiang J, Soeatmadji DW, Henry KM, Ratiwayanto S, Bangs MJ, Richards AL. Rickettsia felis in Xenopsylla cheopis, Java, Indonesia. Emerg Infect Dis. 2006;12:1281–1283. doi: 10.3201/eid1208.060327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kernif T, Socolovschi C, Wells K, Lakim MB, Inthalad S, Slesak G, Boudebouch N, Beaucournu J-C, Newton PN, Raoult D. Bartonella and Rickettsia in arthropods from the Lao PDR and from Borneo, Malaysia. Comp Immunol Microbiol Infect Dis. 2012;35:51–57. doi: 10.1016/j.cimid.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbara KA, Farzeli A, Ibrahim IN, Antonjaya U, Yunianto A, Winoto I, Perwitasari D, Widjaya S, Richards AL, Williams M. Rickettsial infections of fleas collected from small mammals on four islands in Indonesia. J Med Entomol. 2010;47:1173–1178. doi: 10.1603/me10064. [DOI] [PubMed] [Google Scholar]
- 38.Mokhtar AS, Tay ST. Molecular detection of Rickettsia felis, Bartonella henselae, and B. clarridgeiae in fleas from domestic dogs and cats in Malaysia. Am J Trop Med Hyg. 2011;85:931–933. doi: 10.4269/ajtmh.2011.10-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirunkanokpun S, Kittayapong P, Cornet J-P, Gonzalez J-P. Molecular evidence for novel tick-associated spotted fever group rickettsiae from Thailand. J Med Entomol. 2003;40:230–237. doi: 10.1603/0022-2585-40.2.230. [DOI] [PubMed] [Google Scholar]
- 40.Walker DH. Rickettsiae and rickettsial infections: the current state of knowledge. Clin Infect Dis. 2007;45:S39–S44. doi: 10.1086/518145. [DOI] [PubMed] [Google Scholar]
- 41.Walker DH. Rickettsial diseases in travelers. Travel Med Infect Dis. 2003;1:35–40. doi: 10.1016/S1477-8939(03)00025-5. [DOI] [PubMed] [Google Scholar]
- 42.Paris D, Chansamouth V, Nawtaisong P, Löwenberg E, Phetsouvanh R, Blacksell S, Lee S, Dondorp A, van der Poll T, Newton P. Coagulation and inflammation in scrub typhus and murine typhus: a prospective comparative study from Laos. Clin Microbiol Infect. 2012;18:1221–1228. doi: 10.1111/j.1469-0691.2011.03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chierakul W, de Fost M, Suputtamongkol Y, Limpaiboon R, Dondorp A, White NJ, van der Poll T. Differential expression of interferon-γ and interferon-γ-inducing cytokines in Thai patients with scrub typhus or leptospirosis. Clin Immunol. 2004;113:140–144. doi: 10.1016/j.clim.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Feng H-M, Whitworth T, Popov V, Walker DH. Effect of antibody on the Rickettsia-host cell interaction. Infect Immun. 2004;72:3524–3530. doi: 10.1128/IAI.72.6.3524-3530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng H-M, Whitworth T, Olano JP, Popov VL, Walker DH. Fc-dependent polyclonal antibodies and antibodies to outer membrane proteins A and B, but not to lipopolysaccharide, protect SCID mice against fatal Rickettsia conorii infection. Infect Immun. 2004;72:2222–2228. doi: 10.1128/IAI.72.4.2222-2228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pickard AL, McDaniel P, Miller RS, Uthaimongkol N, Buathong N, Murray CK, Telford SR, Parola P, Wongsrichanalai C. A study of febrile illnesses on the Thai-Myanmar border: predictive factors of rickettsioses. Southeast Asian J Trop Med Public Health. 2004;35:657–663. [PubMed] [Google Scholar]
- 47.Nachega JB, Bottieau E, Zech F, Van Gompel A. Travel-acquired scrub typhus: emphasis on the differential diagnosis, treatment, and prevention strategies. J Travel Med. 2007;14:352–355. doi: 10.1111/j.1708-8305.2007.00151.x. [DOI] [PubMed] [Google Scholar]
- 48.Raby E, Dyer JR. Endemic (murine) typhus in returned travelers from Asia, a case series: clues to early diagnosis and comparison with dengue. Am J Trop Med Hyg. 2013;88:701–703. doi: 10.4269/ajtmh.12-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Syhavong B, Rasachack B, Smythe L, Rolain J-M, Roque-Afonso A-M, Jenjaroen K, Soukkhaserm V, Phongmany S, Phetsouvanh R, Soukkhaserm S. The infective causes of hepatitis and jaundice amongst hospitalised patients in Vientiane, Laos. Trans R Soc Trop Med Hyg. 2010;104:475–483. doi: 10.1016/j.trstmh.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Azad AF, Radulovic S, Higgins JA, Noden B, Troyer JM. Flea-borne rickettsioses: ecologic considerations. Emerg Infect Dis. 1997;3:319–327. doi: 10.3201/eid0303.970308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azad AF, Sacci JB, Nelson WM, Dasch GA, Schmidtmann ET, Carl M. Genetic characterization and transovarial transmission of a typhus-like Rickettsia found in cat fleas. Proc Natl Acad Sci USA. 1992;89:43–46. doi: 10.1073/pnas.89.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ericsson CD, Jensenius M, Fournier P-E, Raoult D. Rickettsioses and the international traveler. Clin Infect Dis. 2004;39:1493–1499. doi: 10.1086/425365. [DOI] [PubMed] [Google Scholar]
- 53.Loh K, Leo Y, Heng M, Goh B. Murine typhus: a forgotten cause of febrile illness in Singapore. Singapore Med J. 1996;37:39–43. [PubMed] [Google Scholar]
- 54.Silpapojakul K, Chayakul P, Krisanapan S. Murine typhus in Thailand: clinical features, diagnosis and treatment. QJM. 1993;86:43–47. [PubMed] [Google Scholar]
- 55.Stockdale AJ, Weekes MP, Kiely B, Lever AM. Case report: severe typhus group rickettsiosis complicated by pulmonary edema in a returning traveler from Indonesia. Am J Trop Med Hyg. 2011;85:1121–1123. doi: 10.4269/ajtmh.2011.11-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parola P, Vogelaers D, Roure C, Janbon F, Raoult D. Murine typhus in travelers returning from Indonesia. Emerg Infect Dis. 1998;4:677–680. doi: 10.3201/eid0404.980423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeshita N, Imoto K, Ando S, Yanagisawa K, Ohji G, Kato Y, Sakata A, Hosokawa N, Kishimoto T. Murine typhus in two travelers returning from Bali, Indonesia: an underdiagnosed disease. J Travel Med. 2010;17:356–358. doi: 10.1111/j.1708-8305.2010.00438.x. [DOI] [PubMed] [Google Scholar]
- 58.Azuma M, Nishioka Y, Ogawa M, Takasaki T, Sone S, Uchiyama T. Murine typhus from Vietnam, imported into Japan. Emerg Infect Dis. 2006;12:1466–1468. doi: 10.3201/eid1209.060071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakamoto N, Nakamura-Uchiyama F, Kobayashi K, Takasaki T, Ogasawara Y, Ando S, Iwabuchi S, Ohnishi K. Severe murine typhus with shock and acute respiratory failure in a Japanese traveler after returning From Thailand. J Travel Med. 2013;20:50–53. doi: 10.1111/j.1708-8305.2012.00678.x. [DOI] [PubMed] [Google Scholar]
- 60.Kueh Y, Ti T, Heptonstall J. Murine typhus infection complicated by dengue haemorrhagic fever. Ann Acad Med Singapore. 1988;17:595–599. [PubMed] [Google Scholar]
- 61.McDonald JC, MacLean JD, McDade JE. Imported rickettsial disease: clinical and epidemiologic features. Am J Med. 1988;85:799–805. doi: 10.1016/s0002-9343(88)80024-x. [DOI] [PubMed] [Google Scholar]
- 62.Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16:429–436. doi: 10.1097/00001432-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 63.Phongmany S, Rolain J-M, Phetsouvanh R, Blacksell SD, Soukkhaseum V, Rasachack B, Phiasakha K, Soukkhaseum S, Frichithavong K, Chu V. Rickettsial infections and fever, Vientiane, Laos. Emerg Infect Dis. 2006;12:256–262. doi: 10.3201/eid1202.050900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim D-m, Won KJ, Park CY, Yu KD, Kim HS, Yang TY, Lee JH, Kim HK, Song H-J, Lee S-H. Distribution of eschars on the body of scrub typhus patients: a prospective study. Am J Trop Med Hyg. 2007;76:806–809. [PubMed] [Google Scholar]
- 65.Sirisanthana V, Puthanakit T, Sirisanthana T. Epidemiologic, clinical and laboratory features of scrub typhus in thirty Thai children. Pediatr Infect Dis J. 2003;22:341–345. doi: 10.1097/01.inf.0000059400.23448.57. [DOI] [PubMed] [Google Scholar]
- 66.Watt G, Strickman D. Life-threatening scrub typhus in a traveler returning from Thailand. Clin Infect Dis. 1994;18:624–626. doi: 10.1093/clinids/18.4.624. [DOI] [PubMed] [Google Scholar]
- 67.Cracco C, Delafosse C, Baril L, Lefort Y, Morelot C, Derenne J-P, Bricaire F, Similowski T. Multiple organ failure complicating probable scrub typhus. Clin Infect Dis. 2000;31:191–192. doi: 10.1086/313906. [DOI] [PubMed] [Google Scholar]
- 68.Kurup A, Issac A, Jimmy LJP, Lee TB, Chua R, Bist P, Chao C-C, Lewis M, Gubler DJ, Ching WM. Scrub typhus with sepsis and acute respiratory distress syndrome: a case report. J Clin Microbiol. 2013;51:2787–2790. doi: 10.1128/JCM.00463-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang C-C, Liu S-F, Liu J-W, Chung Y-H, Su M-C, Lin M-C. Acute respiratory distress syndrome in scrub typhus. Am J Trop Med Hyg. 2007;76:1148–1152. [PubMed] [Google Scholar]
- 70.Tsay RW, Chang FY. Acute respiratory distress syndrome in scrub typhus. QJM. 2002;95:126–128. doi: 10.1093/qjmed/95.2.126. [DOI] [PubMed] [Google Scholar]
- 71.Pérez-Osorio CE, Zavala-Velázquez JE, León JJA, Zavala-Castro JE. Rickettsia felis as emergent global threat for humans. Emerg Infect Dis. 2008;14:1019–1023. doi: 10.3201/eid1407.071656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robertson RG, Wisseman CL. Tick-borne rickettsiae of the spotted fever group in west Pakistan ii. serological classification of isolates from west pakistanan and thailand: evidence for two new species. Am J Epidemiol. 1973;97:55–64. doi: 10.1093/oxfordjournals.aje.a121485. [DOI] [PubMed] [Google Scholar]
- 73.Graves S, Stenos J. (Rickettsia honei).Ann N Y Acad Sci. 2003;990:62–66. doi: 10.1111/j.1749-6632.2003.tb07338.x. [DOI] [PubMed] [Google Scholar]
- 74.Jiang J, Sangkasuwan V, Lerdthusnee K, Sukwit S, Chuenchitra T, Rozmajzl PJ, Eamsila C, Jones JW, Richards AL. Human infection with Rickettsia honei, Thailand. Emerg Infect Dis. 2005;11:1473–1475. doi: 10.3201/eid1109.050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sangkasuwan V, Chatyingmongkol T, Sukwit S, Eamsila C, Chuenchitra T, Rodkvamtook W, Jiang J, Richards AL, Lerdthusnee K, Jones JW. Description of the first reported human case of spotted fever group rickettsiosis in urban Bangkok. Am J Trop Med Hyg. 2007;77:891–892. [PubMed] [Google Scholar]
- 76.Fournier P-E, Allombert C, Supputamongkol Y, Caruso G, Brouqui P, Raoult D. Aneruptive fever associated with antibodies to Rickettsia helvetica in Europe and Thailand. J Clin Microbiol. 2004;42:816–818. doi: 10.1128/JCM.42.2.816-818.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gaywee J, Sunyakumthorn P, Rodkvamtook W, Ruang-areerate T, Mason CJ, Sirisopana N. Human infection with Rickettsia sp. related to R. japonica, Thailand. Emerg Infect Dis. 2007;13:671–673. doi: 10.3201/eid1304.060585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takada N, Fujita H, Kawabata H, Ando S, Sakata A, Takano A, Chaithong U. Spotted fever group Rickettsia sp. Closely related to R. japonica, Thailand. Emerg Infect Dis. 2009;15:610–611. doi: 10.3201/eid1504.081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Graves S, Stenos J, Unsworth N, Nguyen C. Laboratory diagnosis of rickettsial infection. Aust J Med Sci. 2006;27:39–44. [Google Scholar]
- 80.La Scola B, Raoult D. Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. J Clin Microbiol. 1997;35:2715–2727. doi: 10.1128/jcm.35.11.2715-2727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tay S, Kamalanathan M, Rohani M. Detection of rickettsial antibodies using Weil-Felix (OXK and OX 19) antigens and the indirect immunoperoxidase assay. Southeast Asian J Trop Med Public Health. 2003;34:171–174. [PubMed] [Google Scholar]
- 82.Koh GC, Maude RJ, Paris DH, Newton PN, Blacksell SD. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368–370. doi: 10.4269/ajtmh.2010.09-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilde H, Suankratay C. There is need for antigen-based rapid diagnostic tests to identify common acute tropical illnesses. J Travel Med. 2007;14:254–258. doi: 10.1111/j.1708-8305.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- 84.Kelly DJ, Wong P, Gan E, Lewis G., Jr Comparative evaluation of the indirect immunoperoxidase test for the serodiagnosis of rickettsial disease. Am J Trop Med Hyg. 1988;38:400–406. doi: 10.4269/ajtmh.1988.38.400. [DOI] [PubMed] [Google Scholar]
- 85.Pradutkanchana J, Silpapojakul K, Paxton H, Pradutkanchana S, Kelly DJ, Strickman D. Comparative evaluation of four serodiagnostic tests for scrub typhus in Thailand. Trans R Soc Trop Med Hyg. 1997;91:425–428. doi: 10.1016/s0035-9203(97)90266-2. [DOI] [PubMed] [Google Scholar]
- 86.Jiang J, Chan T-C, Temenak JJ, Dasch GA, Ching W-M, Richards AL. Development of a quantitative real-time polymerase chain reaction assay specific for Orientia tsutsugamushi. Am J Trop Med Hyg. 2004;70:351–356. [PubMed] [Google Scholar]
- 87.Laferl H, Fournier PE, Seiberl G, Pichler H, Raoult D. Murine typhus poorly responsive to ciprofloxacin: a case report. J Travel Med. 2002;9:103–104. doi: 10.2310/7060.2002.21970. [DOI] [PubMed] [Google Scholar]
- 88.Panpanich R, Garner P. Antibiotics for treating scrub typhus. Cochrane Database Syst Rev. 2002;3:CD002150. doi: 10.1002/14651858.CD002150. [DOI] [PubMed] [Google Scholar]
- 89.Tantibhedhyangkul W, Angelakis E, Tongyoo N, Newton PN, Moore CE, Phetsouvanh R, Raoult D, Rolain J-M. Intrinsic fluoroquinolone resistance in Orientia tsutsugamushi. Int J Antimicrob Agents. 2010;35:338–341. doi: 10.1016/j.ijantimicag.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Watt G, Chouriyagune C, Ruangweerayud R, Watcharapichat P, Phulsuksombati D, Jongsakul K, Teja-Isavadharm P, Bhodhidatta D, Corcoran KD, Dasch GA. Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet. 1996;348:86–89. doi: 10.1016/s0140-6736(96)02501-9. [DOI] [PubMed] [Google Scholar]
- 91.Watt G, Kantipong P, Jongsakul K, Watcharapichat P, Phulsuksombati D, Strickman D. Doxycycline and rifampicin for mild scrub-typhus infections in northern Thailand: a randomised trial. Lancet. 2000;356:1057–1061. doi: 10.1016/S0140-6736(00)02728-8. [DOI] [PubMed] [Google Scholar]
- 92.Kim Y-S, Yun H-J, Shim SK, Koo SH, Kim SY, Kim S. A comparative trial of a single dose of azithromycin versus doxycycline for the treatment of mild scrub typhus. Clin Infect Dis. 2004;39:1329–1335. doi: 10.1086/425008. [DOI] [PubMed] [Google Scholar]
- 93.Phimda K, Hoontrakul S, Suttinont C, Chareonwat S, Losuwanaluk K, Chueasuwanchai S, Chierakul W, Suwancharoen D, Silpasakorn S, Saisongkorh W. Doxycycline versus azithromycin for treatment of leptospirosis and scrub typhus. Antimicrob Agents Chemother. 2007;51:3259–3263. doi: 10.1128/AAC.00508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim D-M, Yu KD, Lee JH, Kim HK, Lee S-H. Controlled trial of a 5-day course of telithromycin versus doxycycline for treatment of mild to moderate scrub typhus. Antimicrob Agents Chemother. 2007;51:2011–2015. doi: 10.1128/AAC.01460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Song J-H, Lee C, Chang WH, Choi SW, Choi JE, Kim YS, Cho SR, Ryu J, Pai CH. Short-course doxycycline treatment versus conventional tetracycline therapy for scrub typhus: a multicenter randomized trial. Clin Infect Dis. 1995;21:506–510. doi: 10.1093/clinids/21.3.506. [DOI] [PubMed] [Google Scholar]
- 96.Olson JG, Bourgeois AL, Fang RC, Coolbaugh JC, Dennis DT. Prevention of scrub typhus. Prophylactic administration of doxycycline in a randomized double blind trial. Am J Trop Med Hyg. 1980;29:989–997. [PubMed] [Google Scholar]
- 97.Twartz JC, Shirai A, Selvaraju G, Saunders JP, Huxsoll DL, Groves M. Doxycycline prophylaxis for human scrub typhus. J Infect Dis. 1982;146:811–818. doi: 10.1093/infdis/146.6.811. [DOI] [PubMed] [Google Scholar]
- 98.Kasper MR, Blair PJ, Touch S, Sokhal B, Yasuda CY, Williams M, Richards AL, Burgess TH, Wierzba TF, Putnam SD. Infectious etiologies of acute febrile illness among patients seeking health care in south-central Cambodia. Am J Trop Med Hyg. 2012;86:246–253. doi: 10.4269/ajtmh.2012.11-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gasem MH, Wagenaar JF, Goris MG, Adi MS, Isbandrio BB, Hartskeerl RA, Rolain J-M, Raoult D, van Gorp EC. Murine typhus and leptospirosis as causes of acute undifferentiated fever, Indonesia. Emerg Infect Dis. 2009;15:975–977. doi: 10.3201/eid1506.081405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mayxay M, Castonguay-Vanier J, Chansamouth V, Dubot-Pérès A, Paris DH, Phetsouvanh R, Tangkhabuanbutra J, Douangdala P, Inthalath S, Souvannasing P. Causes of non-malarial fever in Laos: a prospective study. The Lancet Global Health. 2013;1:e46–e54. doi: 10.1016/S2214-109X(13)70008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sagin DD, Ismail G, Nasian LM, Jok JJ, Pang E. Rickettsial infection in five remote Orang Ulu villages in upper Rejang River, Sarawak, Malaysia. Southeast Asian J Trop Med Public Health. 2000;31:733–735. [PubMed] [Google Scholar]
- 102.Pradutkanchana J, Pradutkanchana S, Kemapanmanus M, Wuthipum N, Silpapojakul K. The etiology of acute pyrexia of unknown origin in children after a flood. Southeast Asian J Trop Med Public Health. 2003;34:175–178. [PubMed] [Google Scholar]
- 103.Leelarasamee A, Chupaprawan C, Chenchittikul M, Udompanthurat S. Etiologies of acute undifferentiated febrile illness in Thailand. J Med Assoc Thai. 2004;87:464–472. [PubMed] [Google Scholar]
- 104.Ellis RD, Fukuda MM, McDaniel P, Welch K, Nisalak A, Murray CK, Gray MR, Uthaimongkol N, Buathong N, Sriwichai S. Causes of fever in adults on the Thai-Myanmar border. Am J Trop Med Hyg. 2006;74:108–113. [PubMed] [Google Scholar]
- 105.Camer GA, Alejandria M, Amor M, Satoh H, Muramatsu Y, Ueno H, Morita C. Detection of antibodies against spotted fever group Rickettsia (SFGR), typhus group Rickettsia (TGR), and Coxiella burnetii in human febrile patients in the Philippines. Jpn J Infect Dis. 2003;56:26–28. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.