Abstract
Cyclospora cayetanensis, a coccidian parasite, can cause gastrointestinal illness in humans and is characterized by watery and persistent diarrhea and abdominal pain. Cyclosporiasis has been associated with traveler's diarrhea. The infection is acquired through food and waterborne transmission, particularly by consumption of contaminated fresh fruits and vegetables. In the present study, stool samples from 8,877 children were examined for ova and parasites at the Pediatric Hospital of Morelia in Michoacán, Mexico, during 2000–2009. Sixty children (0.67%) had Cyclospora in their stools. Diarrhea (45.8%), abdominal pain (39.6%), and vomiting (18.8%) were the most frequent symptoms of cases with cyclosporiasis. Most of the cases (93.3%) were observed during June–August, the rainy season. In 45 children, Cyclospora was the only parasitic pathogen detected (75%); 15 children were co-infected with commensal, pathogenic, or both groups of parasites. Our findings suggest that C. cayetanensis is endemic to Michoacán and shows characteristically temporal patterns.
Introduction
Cyclospora cayetanensis is a coccidian parasite1 that causes watery and persistent diarrhea in immunocompetent and immunocompromised persons.2 It has been associated with traveler's diarrhea3 and with foodborne and waterborne outbreaks of cyclosporiasis. The most widely known waterborne outbreak occurred in 1994 in Nepal where 12 of 14 soldiers and dependents developed diarrheal illness. Contaminated chlorinated water was believed to be the most likely source of infection.4 Cyclosporiasis can also be acquired by the ingestion of contaminated uncooked fruits and vegetables,5–7 particularly lettuce,8 basil,9,10 snow peas,11 and berries.12–14
Cyclospora infections have been described in tropical and subtropical areas, and are considered endemic to certain regions in developing countries, such as Peru, Guatemala, Haiti, and Nepal.7,15–19 A few reports have described cyclosporiasis in travelers returning from Mexico20,21 or in Mexican nationals without prior travel history.7,22–24 Trace-back investigations of an outbreak of Cyclospora infections in the United States were linked to consumption of basil grown in Mexico.10
This study reports the frequency, distribution, clinical presentation, and seasonality of Cyclospora alone and or in the presence of other parasites in children seeking medical attention at the Pediatric Hospital of Morelia in Michoacán, Mexico.
Materials and Methods
Children ≤ 15 years of age seeking medical attention at the Pediatric Hospital submitted stools samples for standard ova and parasite examination. Stool samples were examined by direct smear, and by concentration (Faust and Ritchie standard methods). Positive fecal smears were stained by using the Kinyoun and Gomori trichrome techniques. Confirmation of Cyclospora oocysts included morphometric characteristics and successful sporulation (i.e., identification of two sporocysts within the oocyst) in 2.5% potassium dichromate at 20 ± 2°C and by polymerase chain reaction (PCR),25 followed by sequencing. All routine copro-parasitologic tests and clinical evaluations were performed at the Microbiology and Parasitology Research Laboratory of the Pediatric Hospital of Morelia during January 2000–December 2009. Necessary ethics approvals were obtained from the Institutional Review Board at the Hospital. De-identified samples were submitted for confirmation (sporulation, autofluorescence, and PCR) to the University of Georgia.
Results
Ninety percent of the 8,877 stool samples from children seeking medical attention were collected at the outpatient clinic and the rest from hospitalized patients. Sixty cases (0.67%) of C. cayetanensis infections were detected. Cyclospora was most frequently identified in boys of school age (Table 1). Data for symptoms were available from 48 cases with cyclosporiasis (Table 2). Six children (10%) were admitted to the hospital but for complications not related to Cyclospora infection: three had neoplastic conditions, one had sepsis, and two had pre-existing rheumatoid diseases. Nine of 17 children with cyclosporiasis who had differential cell blood counts results (53%) also had eosinophilia (4–8%).
Table 1.
Cyclospora distribution in children by age and sex, Pediatric Hospital of Morelia, Michoacán, Mexico, 2000–2009
| Age (years) | Female, no. (%) | Male, no. (%) | Total, no. (%) |
|---|---|---|---|
| > 1–2 | 5 (8.3) | 10 (16.7) | 15 (25) |
| 3–5 | 6 (10.0) | 12 (20.0) | 18 (30) |
| 6–11 | 9 (15.0) | 13 (21.7) | 22 (36.7) |
| 12–15 | 2 (3.3) | 3 (5.0) | 5 (8.3) |
| Total | 22 (36.6) | 38 (63.4) | 60 (100.0) |
Table 2.
Clinical presentation of 48 of 60 children with Cyclospora infection seeking medical attention at the Pediatric Hospital of Morelia, Pediatric Hospital of Morelia, Michoacán, Mexico, 2000–2009
| Symptom | No. | % |
|---|---|---|
| Asymptomatic | 2 | 4.2 |
| Diarrhea | 22 | 45.8 |
| Abdominal pain | 19 | 39.6 |
| Vomiting | 9 | 18.8 |
| Nausea | 7 | 14.6 |
| Abdominal discomfort | 7 | 14.6 |
| Headache | 7 | 14.6 |
| Gas | 7 | 14.6 |
| Hyporexia | 5 | 10.4 |
| Fever | 4 | 8.3 |
| Constipation | 3 | 6.3 |
| Anorexia | 3 | 6.3 |
| Malnutrition | 1 | 2.1 |
| Chills | 1 | 2.1 |
| Increased peristalsis | 1 | 2.1 |
| Anal pruritus | 1 | 2.1 |
| General malaise | 1 | 2.1 |
| Tenesmus | 1 | 2.1 |
| Postprandrial fullness | 1 | 2.1 |
Infection with Cyclospora in the absence of other parasites was identified in 75% of the cases; 13% were co-infected with commensal parasites and 10% were infected with pathogenic parasites (Table 3). Blastocystis was the parasite most frequently identified in fecal samples containing Cyclospora oocysts. Hymenolepis nana and Trichuris trichiura were the only helminth eggs found in samples positive for Cyclospora.
Table 3.
Co-infection platform of Cyclospora with other parasites, Pediatric Hospital of Morelia, Michoacán, Mexico, 2000–2009*
| Association | No. (%) |
|---|---|
| Cyclospora alone | 45 (75.0) |
| Co-infections with commensal | 1 (1.7) |
| Endolimax nana | 1 (1.7) |
| Co-infections with pathogenic parasites | 6 (10.0) |
| Blastocystis sp. | 3 (5.0) |
| Giardia lamblia | 2 (3.3) |
| Trichuris trichiura, Hymenolepis nana | 1 (1.7) |
| Co-infections with commensal and pathogenic parasites | 8 (13.3) |
| E. coli, Blastocystis sp. | 4 (6.5) |
| E. nana, Blastocystis sp. | 1 (1.7) |
| E. coli, E. nana, Blastocystis sp. | 1 (1.7) |
| G. lamblia, E. nana, Blastocystis sp. | 1 (1.7) |
| E. coli, E. hartmanni, Blastocystis sp., H. nana | 1 (1.7) |
| Total | 60 (100.0) |
Bacteriologic testing was conducted for 16 children. Escherichia coli was identified in 12 children. No other bacterial enteric pathogens were isolated. Testing for enteroviruses was not conducted for these children.
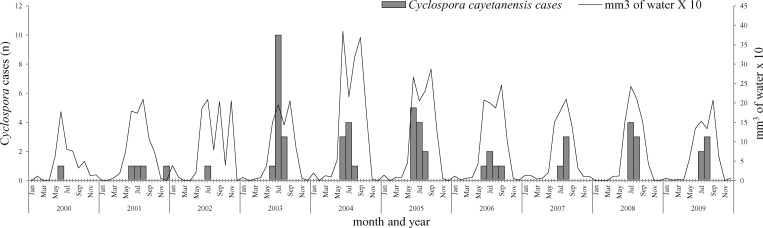
Cases of Cyclospora infections were consistently identified in the rainy season (June–August) in all years during 2000–2009 (Figure 1). Ambient temperatures ranged from a daily minimum of 12°C to a daily maximum of 27–29°C during the months of high incidence of infections.
Figure 1.
Seasonality of Cyclospora in children seeking medical attention at the Pediatric Hospital of Morelia, Michoacán, Mexico, 2000–2009.
Discussion
This study identified Cyclospora in 0.67% of 8,877 children seeking medical assistance at the pediatric hospital of Morelia in Michoacán, Mexico. In Cuba, Cyclospora was identified in 0.2% of 7,956 fecal samples collected for 18 months at a pediatric hospital26 and, in a two-year study at a pediatric hospital in Peru, less than 1% of 381 in-patient children with diarrhea and moderate to severe dehydration had Cyclospora infection.27 In Nigeria, Cyclospora was identified in 11 (0.99%) of 1,109 stool samples analyzed at a laboratory hospital (mean ± SD age of persons = 30 ± 13 years). Of the positive cases, four were seropositive for human immunodeficiency virus.28 In Turkey, 7% of the children visiting the Kars Maternal and Children's Hospital had Cyclospora infection.29 In a hospital in Brazil, the prevalence of Cyclospora cases was 0.3%; 71.4% were females and case-patients had a median age of 34 years.30 In Nepal, 6,562 stools samples from five medical units were examined for Cyclospora. These units included a pediatric hospital, a rural health center, a health clinic (expatriate clientele), and a tropical disease research center. Overall, Cyclospora was identified in 29.7% of 2,123 cases during 1995–1998 and 22.2% of 4,439 during 1999–2000. In the pediatric hospital, 30.3% cases during 1995–1998 and 25.7% during 1999–2000 had Cyclospora infection.19
The clinical presentation most frequently associated with cyclosporiasis includes diarrhea, weight loss, abdominal distension, nausea, vomiting, abdominal pain, flatulence, and fever.3,31,32 In the present study, diarrhea was most commonly reported, which is consistent with other studies worldwide.16,19,26,31–33
Eosinophilia (4–8%) was observed in 53% of 17 cases of cyclosporiasis in our study. This condition has not been reported previously, although in histopathologic studies, eosinophils and lymphocytic infiltration have been observed in duodenal biopsy specimens of patients with cyclosporiasis.34,35 Immunodeficiency does not seem to be a factor for high incidence of Cyclospora in children32,41 and, in the present study, 90% of the infected children were immunocompetent.
In the present study, elementary school age children (6–11 years old) and younger children (3–5 years old) were most frequently infected with Cyclospora. In Guatemala, infection was most common among children 1.5–9 years old.16 Cyclospora was more frequently identified in children 3–11 years old19 in Nepal, and in an endemic community in Peru Cyclospora was present among children 1–9 years old.33
Of the 60 patients infected with Cyclospora identified in this study, 63.4% were male and 36.6% were female (P = 0.042). In Cuba, of 20 reported patients with cyclosporiasis, 60% were male and 40% were female.26 In Colombia, of 56 patients, 73.2% were female and 26.8% were male. However, these patients were associated with an outbreak of cyclosporiasis and presumably all were adults.5 In Guatemala and Peru, the overall rate of infection in children was not significantly different by sex.16,33
In our study, 93.3% of the cases were reported during the months of June–August, which coincides with the rainy season in Morelia.36 In other reported disease-endemic areas, Cyclospora infection was also present during the rainy season.37,38 In Guatemala, infection is more frequently observed during the early rainy season (21–22°C),16 whereas in Peru it occurs during the summer (16–19°C) in the coastal desert areas where precipitation is low.33 In Haiti, prevalence is highest during January–April, which are the coolest (27–29°C) and driest months of the year.18 In Katmandu, Nepal, the high infection season occurs during the summer and the beginning of the rainy season during months of June and July (average temperature = 19–29°C.19
In 75% of the cases of Cyclospora infections identified, it was the only pathogenic parasite associated with gastrointestinal illness. Of the 15 stool samples found to contain Cyclospora and another infectious agent, Blastocystis sp. was most frequent (73.3%), followed by Entamoeba coli (40%) and Endolimax nana (26.7%). Of the helminths, Hymenolepis nana (13.3%) was most frequent. In another study in a native community in Venezuela, co-infections with Cyclospora and Entamoeba histolytica were most common, but co-infections with Blastocystis were observed in only 10.5% of the cases.39 Tests for the presence of bacterial and viral infections were not conducted in the present study, but in a limited number of samples, Escherichia coli was the most frequently identified bacterium.
Testing for C. cayetanensis in patients with gastrointestinal illness is not routinely conducted. Thus, adequate diagnosis and treatment are not always conducted promptly. In addition, the lack of epidemiologic information for Cyclospora makes it difficult to include its differential diagnosis in some locations. Daily and consistent oocyst excretion was not observed in 50% of the cases in our study, suggesting the need for more sensitive assays to diagnose Cyclospora infections. Molecular testing such as nested PCR may aid by increasing the sensitivity and specificity for detecting Cyclospora.40
In conclusion, Cyclospora was identified in children with symptoms of illness at the Pediatric Hospital of Morelia, Mexico during 2000–2009. Only 45.8% of the children had diarrhea, which suggested prior exposures. Further epidemiologic studies are needed to determine the prevalence of Cyclospora infection in Mexico and Central America, and if eosinophilia is a significant characteristic of cyclosporiasis.
ACKNOWLEDGMENTS
We thank the staff of the parasitology division of the pediatric hospital, particularly Cecilia García Ruiz de Chávez, Lilia Altamirano Rojas, Guadalupe Bolaños Monroy, and Dr. José Luis Martínez Toledo for processing of samples.
Footnotes
Authors' addresses: Guadalupe E. Orozco-Mosqueda and Orlando A. Martínez-Loya, Laboratorio de Investigacion en Microbiologia y Parasitologia, Hospital Infantil de Morelia Eva Samano de Lopez Mateos, Justo Mendoza S/N Colonia Centro, CP 58000 Morelia, Michoacan, Mexico, E-mail: erendira4@hotmail.com. Ynes R. Ortega, Center for Food Safety, University of Georgia, Griffin, GA, E-mail: ortega@uga.edu
References
- 1.Ortega YR, Sterling CR, Gilman RH, Cama VA, Diaz F. Cyclospora species: a new protozoan pathogen of humans. N Engl J Med. 1993;328:1308–1312. doi: 10.1056/NEJM199305063281804. [DOI] [PubMed] [Google Scholar]
- 2.Agudelo P, Restrepo M, Galvis MT, Botero D. Infeccion por Cyclospora sp en tres pacientes inmunocompetentes. Biomedica. 2000;20:25–32. [Google Scholar]
- 3.Puente S, Morente A, Garcia-Benayas T, Subirats M, Gascon J, Gonzalez-Lahoz JM. Cyclosporiasis: a point source outbreak acquired in Guatemala. J Travel Med. 2006;13:334–337. doi: 10.1111/j.1708-8305.2006.00059.x. [DOI] [PubMed] [Google Scholar]
- 4.Rabold JG, Hoge CW, Shlim DR, Kefford C, Rajah R, Echeverria P. Cyclospora outbreak associated with chlorinated drinking water. Lancet. 1994;344:1360–1361. doi: 10.1016/s0140-6736(94)90716-1. [DOI] [PubMed] [Google Scholar]
- 5.Botero-Garces J, Montoya-Palacio MN, Barguil JI, Castano-Gonzalez A. An outbreak of Cyclospora cayetanensis in Medellin, Colombia. Rev Salud Publica (Bogota) 2006;8:258–268. doi: 10.1590/s0124-00642006000300011. [DOI] [PubMed] [Google Scholar]
- 6.Shields JM, Olson BH. Cyclospora cayetanensis: a review of an emerging parasitic coccidian. Int J Parasitol. 2003;33:371–391. doi: 10.1016/s0020-7519(02)00268-0. [DOI] [PubMed] [Google Scholar]
- 7.Ortega YR, Sanchez R. Update on Cyclospora cayetanensis, a food-borne and waterborne parasite. Clin Microbiol Rev. 2010;23:218–234. doi: 10.1128/CMR.00026-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doller PC, Dietrich K, Filipp N, Brockmann S, Dreweck C, Vonthein R, Wagner-Wiening C, Wiedenmann A. Cyclosporiasis outbreak in Germany associated with the consumption of salad. Emerg Infect Dis. 2002;8:992–994. doi: 10.3201/eid0809.10.3201/eid0809.010517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoang LM, Fyfe M, Ong C, Harb J, Champagne S, Dixon B, Isaac-Renton J. Outbreak of cyclosporiasis in British Columbia associated with imported Thai basil. Epidemiol Infect. 2005;133:23–27. doi: 10.1017/s0950268804003176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez AS, Dodson DR, Arrowood MJ, Orlandi PA, Jr, da Silva AJ, Bier JW, Hanauer SD, Kuster RL, Oltman S, Baldwin MS, Won KY, Nace EM, Eberhard ML, Herwaldt BL. Outbreak of cyclosporiasis associated with basil in Missouri in 1999. Clin Infect Dis. 2001;32:1010–1017. doi: 10.1086/319597. [DOI] [PubMed] [Google Scholar]
- 11.Outbreak of cyclosporiasis associated with snow peas–Pennsylvania, 2004. MMWR Morb Mortal Wkly Rep. 2004;53:876–878. [PubMed] [Google Scholar]
- 12.Caceres VM, Ball RT, Somerfeldt SA, Mackey RL, Nichols SE, MacKenzie WR, Herwaldt BL. A foodborne outbreak of cyclosporiasis caused by imported raspberries. J Fam Pract. 1998;47:231–234. [PubMed] [Google Scholar]
- 13.Ho AY, Lopez AS, Eberhart MG, Levenson R, Finkel BS, da Silva AJ, Roberts JM, Orlandi PA, Johnson CC, Herwaldt BL. Outbreak of cyclosporiasis associated with imported raspberries, Philadelphia, Pennsylvania, 2000. Emerg Infect Dis. 2002;8:783–788. doi: 10.3201/eid0808.020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz D, Kumar S, Malecki J, Lowdermilk M, Koumans EH, Hopkins R. Cyclosporiasis associated with imported raspberries, Florida, 1996. Public Health Rep. 1999;114:427–438. doi: 10.1093/phr/114.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bern C, Ortega Y, Checkley W, Roberts JM, Lescano AG, Cabrera L, Verastegui M, Black RE, Sterling C, Gilman RH. Epidemiologic differences between cyclosporiasis and cryptosporidiosis in Peruvian children. Emerg Infect Dis. 2002;8:581–585. doi: 10.3201/eid0806.01-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bern C, Hernandez B, Lopez MB, Arrowood MJ, de Mejia MA, De Merida AM, Hightower AW, Venczel L, Herwaldt BL, Klein RE. Epidemiologic studies of Cyclospora cayetanensis in Guatemala. Emerg Infect Dis. 1999;5:766–774. doi: 10.3201/eid0506.990604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eberhard ML, Nace EK, Freeman AR. Survey for Cyclospora cayetanensis in domestic animals in an endemic area in Haiti. J Parasitol. 1999;85:562–563. [PubMed] [Google Scholar]
- 18.Lopez AS, Bendik JM, Alliance JY, Roberts JM, da Silva AJ, Moura IN, Arrowood MJ, Eberhard ML, Herwaldt BL. Epidemiology of Cyclospora cayetanensis and other intestinal parasites in a community in Haiti. J Clin Microbiol. 2003;41:2047–2054. doi: 10.1128/JCM.41.5.2047-2054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherchand JB, Cross JH. Emerging pathogen Cyclospora cayetanensis infection in Nepal. Southeast Asian J Trop Med Public Health. 2001;32((Suppl 2)):143–150. [PubMed] [Google Scholar]
- 20.Berlin OG, Novak SM, Porschen RK, Long EG, Stelma GN, Schaeffer FW ., III Recovery of Cyclospora organisms from patients with prolonged diarrhea. Clin Infect Dis. 1994;18:606–609. doi: 10.1093/clinids/18.4.606. [DOI] [PubMed] [Google Scholar]
- 21.Scaglia M, Gatti S, Bassi P, Viale PL, Novati S, Ranieri S. Intestinal co-infection by Cyclospora sp. and Cryptosporidium parvum: first report in an AIDS patient. Parasite. 1994;1:387–390. doi: 10.1051/parasite/1994014387. [DOI] [PubMed] [Google Scholar]
- 22.Diaz E, Mondragon J, Ramirez E, Bernal R. Epidemiology and control of intestinal parasites with nitazoxanide in children in Mexico. Am J Trop Med Hyg. 2003;68:384–385. [PubMed] [Google Scholar]
- 23.Ponce-Macotela M, Cob-Sosa C, Martinez-Gordillo MN. Cyclospora in 2 Mexican children. Rev Invest Clin. 1996;48:461–463. [PubMed] [Google Scholar]
- 24.Sifuentes-Osornio J, Porras-Cortes G, Bendall RP, Morales-Villarreal F, Reyes-Teran G, Ruiz-Palacios GM. Cyclospora cayetanensis infection in patients with and without AIDS: biliary disease as another clinical manifestation. Clin Infect Dis. 1995;21:1092–1097. doi: 10.1093/clinids/21.5.1092. [DOI] [PubMed] [Google Scholar]
- 25.Li G, Xiao S, Zhou R, Li W, Wadeh H. Molecular characterization of Cyclospora-like organism from dairy cattle. Parasitol Res. 2007;100:955–961. doi: 10.1007/s00436-006-0380-z. [DOI] [PubMed] [Google Scholar]
- 26.Martinez SI, Ayllon VL, Benitez PX. Cyclospora cayetanensis: presentacion de 20 casos. Rev Cubana Pediatr. 2002;74:178–181. [Google Scholar]
- 27.Cama RI, Parashar UD, Taylor DN, Hickey T, Figueroa D, Ortega YR, Romero S, Perez J, Sterling CR, Gentsch JR, Gilman RH, Glass RI. Enteropathogens and other factors associated with severe disease in children with acute watery diarrhea in Lima, Peru. J Infect Dis. 1999;179:1139–1144. doi: 10.1086/314701. [DOI] [PubMed] [Google Scholar]
- 28.Alakpa G, Fagbenro-Beyioku AF, Clarke SC. Cyclospora cayetanensis in stools submitted to hospitals in Lagos, Nigeria. Int J Infect Dis. 2002;6:314–318. doi: 10.1016/s1201-9712(02)90167-0. [DOI] [PubMed] [Google Scholar]
- 29.Arslan MO, Sari B, Kulu B, Mor N. The prevalence of intestinal parasites in children brought to the Kars Maternal and Children's Hospital with complaints of gastrointestinal symptoms [in Turkish] Turkiye Parazitol Derg. 2008;32:253–256. [PubMed] [Google Scholar]
- 30.Goncalves EM, Uemura IH, Castilho VL, Corbett CE. Retrospective study of the occurrence of Cyclospora cayetanensis at Clinical Hospital of the University of Sao Paulo Medical School, SP. Rev Soc Bras Med Trop. 2005;38:326–330. doi: 10.1590/s0037-86822005000400009. [DOI] [PubMed] [Google Scholar]
- 31.Dekker E, Karger PA. Prolonged diarrhea and weight loss after a biking trip from Tibet to Nepal: infection with Cyclospora [in Dutch] Ned Tijdschr Geneeskd. 2002;146:1502–1504. [PubMed] [Google Scholar]
- 32.Sancak B, Akyon Y, Erguven S. Cyclospora infection in five immunocompetent patients in a Turkish university hospital. J Med Microbiol. 2006;55:459–462. doi: 10.1099/jmm.0.46279-0. [DOI] [PubMed] [Google Scholar]
- 33.Madico G, McDonald J, Gilman RH, Cabrera L, Sterling CR. Epidemiology and treatment of Cyclospora cayetanensis infection in Peruvian children. Clin Infect Dis. 1997;24:977–981. doi: 10.1093/clinids/24.5.977. [DOI] [PubMed] [Google Scholar]
- 34.Ortega YR, Nagle R, Gilman RH, Watanabe J, Miyagui J, Quispe H, Kanagusuku P, Roxas C, Sterling CR. Pathologic and clinical findings in patients with cyclosporiasis and a description of intracellular parasite life-cycle stages. J Infect Dis. 1997;176:1584–1589. doi: 10.1086/514158. [DOI] [PubMed] [Google Scholar]
- 35.Connor BA, Reidy J, Soave R. Cyclosporiasis: clinical and histopathologic correlates. Clin Infect Dis. 1999;28:1216–1222. doi: 10.1086/514780. [DOI] [PubMed] [Google Scholar]
- 36.Masucci L, Graffeo R, Siciliano M, Franceschelli A, Bugli F, Fadda G. First Italian case of cyclosporiasis in an immunocompetent woman: local acquired infection. New Microbiol. 2008;31:281–284. [PubMed] [Google Scholar]
- 37.Servicio Metereologico Nacional http://smn.cna.gob.mx/ Available at. Accessed December 1, 2009.
- 38.Herwaldt BL. Cyclospora cayetanensis: a review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clin Infect Dis. 2000;31:1040–1057. doi: 10.1086/314051. [DOI] [PubMed] [Google Scholar]
- 39.Nimri LF. Cyclospora cayetanensis and other intestinal parasites associated with diarrhea in a rural area of Jordan. Int Microbiol. 2003;6:131–135. doi: 10.1007/s10123-003-0121-4. [DOI] [PubMed] [Google Scholar]
- 40.Devera R, Blanco Y, Cabello E. High prevalence of Cyclospora cayetanensis among indigenous people in Bolivar State, Venezuela. Cad Saude Publica. 2005;21:1778–1784. doi: 10.1590/s0102-311x2005000600025. [DOI] [PubMed] [Google Scholar]
- 41.Helmy MM, Rashed LA, Abdel-Fattah HS. Co-infection with Cryptosporidium parvum and Cyclospora cayetanensis in immunocompromised patients. J Egypt Soc Parasitol. 2006;36:613–627. [PubMed] [Google Scholar]