Abstract
A chest-wall lesion of an immunocompetent patient was initially suspicious for a malignant tumor. Histopathological and polymerase chain reaction examinations revealed an infection with the larval stage of the tapeworm Taenia crassiceps. Curative resection of the tumorous lesion was performed. Treatment options for immunocompromised patients and patients without known immune defect are discussed, because most of the infections occur in immunocompromised individuals.
Introduction
In the recent years, several human infections with the larval stage of the tapeworm Taenia crassiceps have emerged,1 most often in immunosuppressed individuals.2–7 The tapeworm is maintained in a sylvatic cycle involving foxes as natural final hosts and rodents as intermediate hosts. The parasite is present in North America, Russia, and Europe. Humans can serve as accidental intermediate hosts, and infection occurs after the ingestion of parasite ova. In immunosuppressed individuals, the larval stages (metacestodes) develop subcutaneously or in muscular tissue,5–8 causing T. crassiceps cysticercosis. Acquired immunodeficiency syndrome (AIDS)2–4,6,7 and non-Hodgkin's lymphoma5 were regarded as pre-disposition. Rarely, the parasite has been found in the eye and the cerebellum; these findings were in patients without known immune defects.1,9–11
Here, we describe a severe subcutaneous infection with T. crassiceps in a patient without any obvious immunodeficiency. The pathogen was diagnosed histopathologically and by molecular techniques. The patient was successfully treated surgically without any additional antihelminthic chemotherapy, leading to a long-term complete cure.
Case Report
A 42-year-old female German patient was admitted to our department of thoracic surgery in October of 2012 with a tumorous soft-tissue swelling in the right upper chest wall that had developed 3 days before. There was no recent history of injury, previous malignancy, or immunosuppression. The patient reported an insect bite in this area during a vacation with outdoor activities in southern Germany 3 months before. A severe and possibly allergic skin reaction with swelling and inflammation had developed, which had spontaneously resolved after a few days. The patient was on no regular medication and worked as an executive assistant. She had been keeping a pet dog and cats for several years.
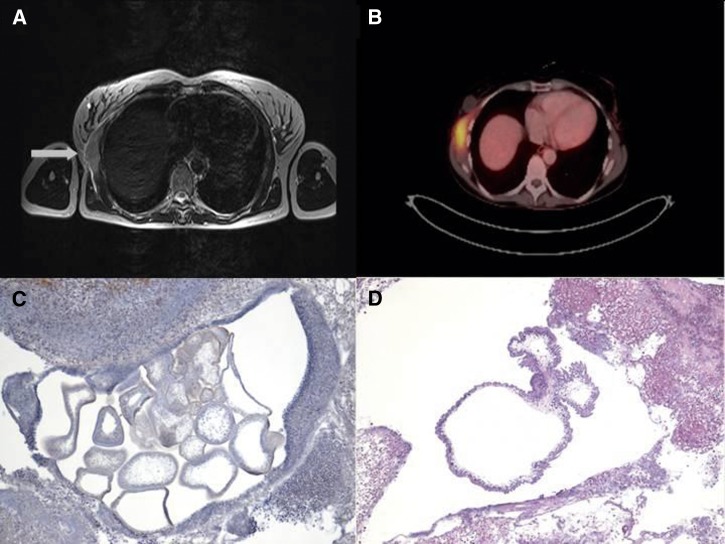
Physical examination showed a tumor with no clinical signs of infection. The axillary lymph nodes were enlarged and painless, and the patient had no fever. Leukocyte count and C-reactive protein levels were normal. A routinely performed screening for human immunodeficiency virus (HIV) infection and hepatitis B and C was negative. Mammography was performed to exclude breast cancer. The patient underwent magnetic resonance imaging (MRI) of the chest, which showed a chest-wall tumor measuring 7 × 2.3 × 8.4 cm that extended to the right mammary gland. The tumor was accompanied by enlarged axillary and infraclavicular lymph nodes, which were suspicious for malignancy (Figure 1A). Ultrasonography of the chest wall showed subcutaneous and muscular edema. With the impression of soft-tissue malignancy, a positron emission tomography scan (PET-CT) was performed. The tumor showed an increased 18F-fluorodeoxyglucose uptake within the anterior serratus muscle with a standardized uptake value (SUV)max of 7.5 and an increased uptake in the axillary lymph nodes (Figure 1B).
Figure 1.
(A) MRI of the thorax showing the prominent tumor (arrow) at the right upper chest wall located within the chest-wall muscles. T2-weighted image. (B) Positron emission tomography scan showing a pathologic 18F-fluorodeoxyglucose uptake of the tumor. An uptake was also seen in the surrounding lymph nodes (not shown). (C) Histopathological examination of the resected severely inflamed tumor. Multiple sections through several cystic cestode larvae are visible. Original magnification, ×40 (hematoxylin stain). (D) In the center of the inflammatory lesion, a section through the cystic body of a cestode larva is clearly visible. The parasite's tegument appears ruffled and surrounds a spongy stroma. Protruding from the larvae's body, two buds characteristic for T. crassiceps are seen. Differential diagnoses for T. crassiceps cysticercosis include cysticercosis caused by T. solium (single cyst without buds), coenurosis caused by T. multiceps/T. serialis (single cyst with many protoscoleces), and alveolar echinococcosis (multiple cysts but with strong outer laminated layer). Original magnification, ×40 (hematoxylin and eosin stain).
To clarify the etiology of the tumorous lesion, an incisional biopsy was performed. A soft and well-encapsulated tumor between the latissimus dorsi muscle and the serratus anterior muscle was found that was biopsied. Histopathological examination from the fresh-frozen specimen showed severe inflammation of the soft tissue with densely packed lymphocytes initially suspicious for a lymphoma. Lymphocytic infiltrates within the walls of vessels, eosinophils, histiocytes, and plasma cells were also seen. Immunohistochemistry revealed a lesion rich in CD4-positive T lymphocytes and CD56-positive natural killer cells. Seven days later, the remaining tumor was resected. Histopathology showed sections with several helminth parasites (Figure 1C) surrounded by the severe inflammatory infiltrate already seen before. Additional examination revealed an outer ruffled tegument and an inner spongy matrix containing calcareous corpuscles, typical for a larval cestode. In some larvae, bud-like structures protruding from the main body were visible (Figure 1D). Polymerase chain reactions (PCRs) were performed from formalin-fixed, paraffin-embedded histopathological slides for cestode nicotinamide adenine dinucleotide (NADH) dehydrogenase12 and cytochrome oxidase13 subunit I genes after removal of the coverslip. Sequencing of the amplicons showed 99% and 100% identity, respectively, with T. crassiceps. Serology for alveolar and cystic echinococcosis (indirect hemagglutionation, enzyme-linked immunosorbent assay [ELISA], and immunoblot) and T. solium cysticercosis (ELISA and immunoblot) was negative.
The post-surgical course was uncomplicated. No antihelminthic drug therapy was administered. The patient was discharged 3 days after surgery, and a 16-month follow-up period was uneventful.
Discussion
T. crassiceps is a tapeworm in the Holarctic region that circulates commonly between canids and rodents. Unlike T. solium and Echinococcus multilocularis diseases, T. crassiceps infections in humans occur very rarely. However, an increasing number of human cases have been reported in the recent years (current overview in ref. 1 and an additional case reported in ref. 14). Of the infected patients, including our patient, 47% were immunocompromised (7 of 15 patients) because of either AIDS or hematological malignancy. Our patient had been clinically healthy previously without any signs of immunosuppression or malignancy. Human cysticercosis caused by T. crassiceps, similar to T. solium infections, often involves the subcutis and muscles (67%; 10 of 15 cases). Except for four cases, including our case, subcutaneous infections occurred in immunocompromised patients. In at least 4 previously published cases of 10 subcutaneous infections, the parasitic infection developed after a trauma or hematoma at the respective body site.3–5,14 Our patient did not report such incidents but did repot an insect bite leading to inflammatory tissue damage at the later site of parasite lodging. Thus, hematoma or traumatically altered or inflamed tissue seems to be a predilection site for an infection. The reason for this finding is unclear. In the remaining five human non-subcutaneous infections (33%), the eye or as recently reported, the cerebellum was infected. All these patients were not immunosuppressed.
Transmission to intermediate hosts occurs by infective ova. In one case, an eye infection could be traced back to a pet dog.9,11 Several other infected patients were also dog keepers,1,5,14 including our patient. However, the route of infection in the currently described case is unknown. Other than the dog, the patient owns two cats. The pets were regularly dewormed, except for one cat imported from the Greek island of Karpathos. The animal was imported 4 months before disease onset. Deworming therapy was carried out later, however, without a prior stool examination. In another German case described in 2006,5 the infected patient owned an imported stray dog from the Greek isle of Crete. The parasite is prevalent in Greece15; however, despite these parallels, most human infections were described from central Europe (Germany, N = 4; France, N = 3; Switzerland, N = 3; Austria, N = 1). It is, thus, more likely that the parasite is a local strain, also taking into account that the cestode is more often associated with canids than felids.16 Prevalence of the parasite in European dogs and cats is very low (< 1%). It is much higher in foxes, the natural hosts (7.8% in Switzerland, 24% in Germany, and 26.4% in Lithuania1,5,14).
For diagnosis of human infections, no commercial serological tests are available. Serological cross-reactions occur extensively between T. solium and T. crassiceps.17 However, such cross-reactions may be weak in central nervous system infections1 or even absent in subcutaneous disease in immunocompromised individuals.5 In our case of a subcutaneous infection, no antibodies against larval cestodes could be shown. In the majority of cases, diagnosis was achieved after resection of the parasite larvae followed by parasitological inspection and histopathology.4,5 Larvae can even be cultivated in cell culture after resection, showing posterior budding of the metacestodes,5 a characteristic feature that was also seen histopathologically in our case. Similar to our report, PCR has been successfully used in two recent human infections.1,14 Imaging results of soft-tissue infections are inconclusive of the etiology, showing enhancement in T2-weighted MRI images (this report and ref. 5). In the recently described case of a cerebellar infection, the cystic appearance of the larvae was clearly visible by MRI.1 To the best of our knowledge, our study is the first PET-CT investigation in a T. crassiceps infection. Similar to other infections, including alveolar echinococcosis, a pathological uptake presumably by inflammatory cells is visible.
The larval stage of T. crassiceps (Cysticercus longicollis) is unique among the taeniids in the way that the larvae proliferate by posterior budding, making complete resections of parasite larvae difficult. Little experience exists regarding the appropriate treatment of the infection; however, surgical treatment should primarily be sought in any case. Here, a complete resection of the tumorous mass was performed under the impression of malignancy, which led to a complete cure. The effectiveness of conservative medical treatment with antihelminthic chemotherapy for this organism is uncertain. Experiences with immunocompromised patients showed that relapses have occurred in HIV-infected patients. despite a combined antiparasitic chemotherapy with albendazole and praziquantel after surgery.4,8 Presumably, not all parasite larvae had been resected in these cases. General recommendations concerning long-term antihelminthic therapy to prevent relapses do not exist. Our immunocompetent patient did not receive antiparasitic chemotherapy and has not shown disease recurrence for an extended period. We, thus, assume that additional chemotherapy in immunocompetent individuals with evidence of a complete parasite resection might not be necessary. In contrast, in patients without clear evidence of a complete parasite resection and especially individuals with immunosuppression or parasite location in the eye or brain, an additional antihelminthic chemotherapy with albendazole and praziquantel seems reasonable. The latter patient group should be monitored closely for disease recurrence.
Footnotes
Authors' addresses: Christian Roesel, Stefan Welter, and Georgios Stamatis, Department of Thoracic Surgery and Thoracic Endoscopy, Ruhrlandklinik, West German Lung Center, University of Duisburg-Essen, Essen, Germany, E-mails: Christian.Roesel@ruhrlandklinik.uk-essen.de, Stefan.Welter@ruhrlandklinik.uk-essen.de, and georgios.stamatis@ruhrlandklinik.uk-essen.de. Dirk Theegarten, Institute of Pathology, University Hospital Essen, University of Duisburg-Essen, Essen, Germany, E-mail: dirk.theegarten@uk-essen.de. Dennis Tappe, Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany, E-mail: tappe@bnitm.de.
References
- 1.Ntoukas V, Tappe D, Pfütze D, Simon M, Holzmann T. Cerebellar cysticercosis caused by larval Taenia crassiceps tapeworm in immunocompetent woman, Germany. Emerg Infect Dis. 2013;19:2008–2011. doi: 10.3201/eid1912.130284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.François A, Favennec L, Cambon-Michot C, Gueit I, Biga N, Tron F, Brasseur P, Hemet J. Taenia crassiceps invasive cysticercosis: a new human pathogen in acquired immunodeficiency syndrome? Am J Surg Pathol. 1998;22:488–492. doi: 10.1097/00000478-199804000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Maillard H, Marionneau J, Prophette B, Boyer E, Célerier P. Taenia crassiceps cysticercosis and AIDS. AIDS. 1998;20:1551–1552. doi: 10.1097/00002030-199812000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Klinker H, Tintelnot K, Joeres R, Müller J, Gross U, Schmidt-Rotte H, Landwehr P, Richter E. Taenia crassiceps infection in AIDS. Dtsch Med Wochenschr. 1992;117:133–138. doi: 10.1055/s-2008-1062291. [DOI] [PubMed] [Google Scholar]
- 5.Heldwein K, Biedermann HG, Hamperl WD, Bretzel G, Löscher T, Laregina D, Frosch M, Büttner DW, Tappe D. Subcutaneous Taenia crassiceps infection in a patient with non-Hodgkin's lymphoma. Am J Trop Med Hyg. 2006;75:108–111. [PubMed] [Google Scholar]
- 6.Chermette R, Bussiéras J, Marionneau J, Boyer E, Roubin C, Prophette B, Maillard H, Fabiani B. Invasive cysticercosis due to Taenia crassiceps in an AIDS patient. Bull Acad Natl Med. 1995;179:777–780. [PubMed] [Google Scholar]
- 7.Flammer Anikpeh Y, Grimm F, Lindenblatt N, Zinkernagel A. It isn't always caviar. BMJ Case Rep. 2014;1 doi: 10.1136/bcr-2013-200078. pii:bcr2013200078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goesseringer N, Lindenblatt N, Mihic-Probst D, Grimm F, Giovanoli P. Taenia crassiceps upper limb fasciitis in a patient with untreated acquired immunodeficiency syndrome and chronic hepatitis C infection–the role of surgical debridement. J Plast Reconstr Aesthet Surg. 2011;64:e174–e176. doi: 10.1016/j.bjps.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Arocker-Mettinger E, Huber-Spitzy V, Auer H, Grabner G, Stur M. Taenia crassiceps in the anterior chamber of the human eye. A case report. Klin Monbl Augenheilkd. 1992;201:34–37. doi: 10.1055/s-2008-1045865. [DOI] [PubMed] [Google Scholar]
- 10.Shea M, Maberley AL, Walters J, Freeman RS, Fallis AM. Intraocular Taenia crassiceps (Cestoda) Trans Am Acad Ophthalmol Otolaryngol. 1973;77:778–783. [PubMed] [Google Scholar]
- 11.Chuck RS, Olk RJ, Weil GJ, Akduman L, Benenson IL, Smith ME, Kaplan HJ. Surgical removal of a subretinal proliferating cysticercus of taeniaeformis crassiceps. Arch Ophthalmol. 1997;11:562–563. doi: 10.1001/archopht.1997.01100150564028. [DOI] [PubMed] [Google Scholar]
- 12.Bowles J, McManus DP. NADH dehydrogenase 1 gene sequences compared for species and strains of the genus Echinococcus. Int J Parasitol. 1993;23:969–972. doi: 10.1016/0020-7519(93)90065-7. [DOI] [PubMed] [Google Scholar]
- 13.Bowles J, Blair D, McManus DP. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol Biochem Parasitol. 1992;54:165–173. doi: 10.1016/0166-6851(92)90109-w. [DOI] [PubMed] [Google Scholar]
- 14.Schmid S, Grimm F, Huber M, Beck B, Custer P, Bode B. Taenia crassiceps infection - an unusual presentation of a tapeworm diagnosed by FNA cytology and PCR. Cytopathology. 2013 doi: 10.1111/cyt.12092. 10.1111/cyt.12092. [DOI] [PubMed] [Google Scholar]
- 15.Papadopoulos H, Himonas C, Papazahariadou M, Antoniadou-Sotiriadou K. Helminths of foxes and other wild carnivores from rural areas in Greece. J Helminthol. 1997;71:227–232. doi: 10.1017/s0022149x00015960. [DOI] [PubMed] [Google Scholar]
- 16.Wünschmann A, Garlie V, Averbeck G, Kurtz H, Hoberg EP. Cerebral cysticercosis by Taenia crassiceps in a domestic cat. J Vet Diagn Invest. 2003;15:484–488. doi: 10.1177/104063870301500517. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki LA, Arruda GC, Quagliato EM, Rossi QL. Evaluation of Taenia solium and Taenia crassiceps cysticercal antigens for immunodiagnosis of neurocysticercosis using ELISA on cerebrospinal fluid samples. Rev Soc Bras Med Trop. 2007;40:152–155. doi: 10.1590/s0037-86822007000200002. [DOI] [PubMed] [Google Scholar]