Abstract
The progress of the integrated control policy for schistosomiasis implemented since 2005 in China, which is aiming at reducing the roles of bovines and humans as infection sources, may be challenged by persistent presence of infected snails in lake and marshland areas. Based on annual parasitologic data for schistosomiasis during 2004–2011 in Xingzi County, a spatio-temporal kriging model was used to investigate the spatio-temporal pattern of schistosomiasis risk. Results showed that environmental factors related to snail habitats can explain the spatio-temporal variation of schistosomiasis. Predictive maps of schistosomiasis risk illustrated that clusters of the disease fluctuated during 2004–2008; there was an extensive outbreak in 2008 and attenuated disease occurrences afterwards. An area with an annually constant cluster of schistosomiasis was identified. Our study suggests that targeting snail habitats located within high-risk areas for schistosomiasis would be an economic and sustainable way of schistosomiasis control in the future.
Introduction
Chronic schistosomiasis, which caused by the parasitic blood flukes Schistosoma haematobium, S. mansoni, S. intercalatum, S. mekongi, or S. japonicum, is among the most common human parasitic diseases; more than 230 million persons are affected worldwide.1 In China, zoonotic schistosomiasis japonica is associated with chronic liver and intestinal fibrosis. It is estimated that more than 50 million persons in China are at risk; approximately one million persons and several hundred thousand livestock are infected.2 Major foci of endemicity are found in marsh and lake areas (Poyang Lake and Dongting Lake) along the Yangtze River basin, where elimination of transmission has proven difficult.3,4
China was one of the first countries in eastern Asia to initiate a national schistosomiasis control program, and achievements in schistosomiasis control have reduced by more than 90% the peak estimates of human prevalence in the mid 1950s,5 which ranged between 10.5 and 11.8 million.6 In a renewed attempt to reduce the burden of schistosomiasis in China, a 10-year World Bank Loan Project (WBLP) was launched in 1992.7 It used large-scale chemotherapy and additional interventions, such as health education, chemical control of snails, and other environmental exposure modifications, to reduce exposure. However, studies suggested that schistosomiasis re-emerged shortly after the WBLP.8,9 Because of high rates of re-infection, progress toward further reduction in prevalence or disease elimination was difficult.10
To reach the aggressive control target that the rate be less than 5% and 1% by 2008 and 2015,11 respectively, a national control program with a revised strategy to control schistosomiasis using integrated measures was implemented in 2005, which is aiming at reducing the roles of bovines and humans as infection sources.12 Unfortunately, infected Oncomelania hupensis snails were still found recently in certain locations along the Yangtze River,13 and there is a concern that they might spread again, possibly resulting in extensive re-emergence of infections among humans and domestic animals. Therefore, a study assessing the progress of the integrated control strategy within those regions where infected snails were found is needed, but longitudinal data for re-emergence are sparse.14
There has been progress in modeling burden of disease during recent decades by using geographic information systems, remote sensing, and geostatistics. These methods have been applied to a range of diseases, such as, malaria,15,16 schistosomiasis,9,17 and Loa loa filariasis.18 However, much of the work used modeling aiming to investigate patterns of those diseases across space, as well as time separately. Recent advances in space-time statistics19,20 enabled us to analyze such patterns not only in separate temporal and spatial domains, but as a whole. Investigating spatio-temporal (ST) patterns of epidemics could help to generate new information for further etiologic studies;21 construct hypotheses on the different mechanisms of transmission involved in disease spread;22 and identify risk areas in which to focus surveillance and allocate resources (e.g., antibiotics, rapid diagnostic tests).23
ST kriging, an extension to (purely spatial) kriging,24 offers a variety of techniques to make optimal use of measurement information for interpolating variables in space and time. Over the past decade, progress has been made in building ST geostatistical models in several scientific disciplines (e.g., environmental science,25 meteorology,26 and soil science27). In this study, we applied the ST kriging method to model the ST pattern of schistosome infections in Xingzi, China, a typical schistosomiasis-endemic area of lake and marshland, by using annual parasitologic data obtained through standardized surveys during 2004–2011 and assessing the effect of snail habitats on the ST pattern of schistosomiasis.
Materials and Methods
Study area.
Xingzi County, located on the northwest corner of Poyang Lake (Figure 1), is one of the areas in Jiangxi Province to which schistosomiasis is highly endemic. Ten of 12 townships are in schistosomiasis-endemic areas, including 56 villages and communities.28 Physical environments around the Poyang Lake region make Xingzi one of the major schistosomiasis-endemic areas in China. Poyang Lake is connected to five rivers (Ganjiang, Huhe, Xinjiang, Raohe, and Xiuhe) and the Yangtze River through a narrow passage at Hukou. During spring (April–June), the lake fills up with water from these five rivers and covers all lowland marshlands. During summer (July–September), water levels in the lake reach their peak by back flowing of flooding from the Yangtze River. During winter (November–March), water levels decrease and vast grass-covered marshlands emerge. These changes form a special scenery of land in winter and water in summer in the lake. In addition, there are numerous water bodies in the region, such as Beng Lake, Liaohuachi Lake, and Shixia Lake (Figure 1). The annual average temperature is 16–18°C, and annual rainfall ranges from 1,340 mm to 1,780 mm.29 Thus, climate, geographic environment, and marshland characteristics in the lake area are ideal for snail growth and reproduction.
Figure 1.
Location of sample villages during 2004 in Xingzi, Jiangxi, China. The geographic layer of a water body was overlaid. The map was created by using AcrGIS software version 10.0 (ESRI Inc., Redlands, CA).
Parasitologic data.
Schistosoma japonicum infection data for 2004–2011were provided by the local anti-schistosomiasis station in Xingzi. For each year, the database consisted of a random sample of villages (range = 30–42), but with human prevalence data in each village included. These data were collected through village-based field surveys by using a two-pronged diagnostic approach (all residents 5–65 years of age were screened by using a serologic test and then confirmed by using a fecal parasitologic test [Kato-Katz technique]).30 Additional details describing the process of data collection have been reported.31
Ethics statement.
Approval for oral consent and other aspects of this survey was granted by the Ethics Committee of Fudan University (ID: IRB#2011-03-0295). Written informed consent was also obtained from all participants.
Environmental data.
The location of snail habitats was recorded by using a handheld Global Positioning System instrument (MobileMapper™; Thales Navigation Inc., San Dimas, CA). Water body data, including lake and wetland, were extracted from the World Wildlife Fund's Conservation Science Data Sets (http://worldwildlife.org). For each sample village, the Euclidian distances to nearest snail habitat and the nearest water body were calculated by using AcrGIS software version 10.0 (ESRI Inc., Redlands, CA).
Statistical analysis.
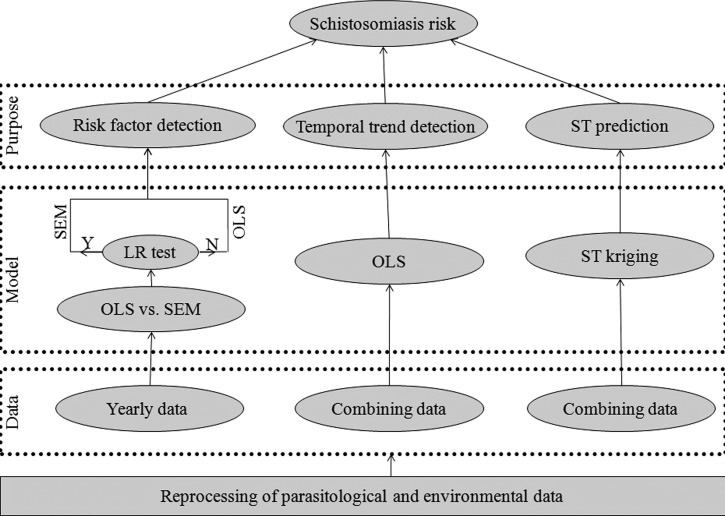
First, a spatial error model (SEM) for each year implemented in GeoDA 1.4.5 was used to measure the relationships between the human prevalence of schistosomiasis and environmental determinants (i.e., distance to snail habitat and distance to a water body). In this model, spatial neighbors of a village were defined as those within 5 km around the village, and elements in the weight matrix were given a value of 1 if two villages were neighbors and 0 otherwise. An ordinary least squares (OLS) regression model was also used to fit the data, and a likelihood ratio (LR) test was then conducted to compare the OLS model with the SEM. If this test shows no significant difference, the OLS model was chosen; otherwise, the SEM was used. To detect temporal variation of the disease, we took time into account as a covariate in the OLS model by combining data from all years and using time as an ordinal variable. Collinearity diagnostics was conducted before regression, and indicator of variance inflation factor for the both covariates showed that no collinearity exists (variance inflation factor = 1.03). A conceptual framework of the data analysis is shown in Figure 2.
Figure 2.
Workflow of data analysis. ST = spatio-temporal; SEM = spatial error model; Y = significantly different; LR = likelihood ratio; N = non-significantly different; OLS = ordinary least squares.
To obtain the ST pattern of schistosomiasis, a ST kriging model was used. The equations for kriging in the ST domain are exactly the same as the standard spatial kriging equations. Kriging is a method of interpolation that predicts unknown values from data observed at known locations.32 This method uses variogram to express the spatial variation, and it minimizes the error of predicted values, which are estimated by spatial distribution of the predicted values. Variogram is defined as the variance of the difference between field values at two locations across realizations of a spatial random field. Three parameters are used to describe variograms: nugget, the height of the jump of the variogram at the discontinuity at the origin; sill, the limit of the variogram tending to infinity lag distances, and range, the distance at which the difference of the variogram from the sill becomes negligible.
Ordinary kriging, one type of kriging, filters the mean from the simple kriging estimator by requiring that the kriging weights sum to one.33 This feature results in the following ordinary kriging estimator:
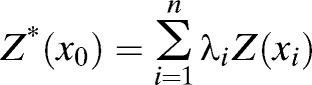 |
where Z* (x0) is the predicted value spatially located at x0, λi is the weight, and Z (xi) is the observed value located at xi. The standard error (SE) of predicted value equals
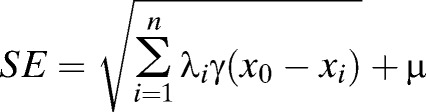 |
where γ(x0 − xi) is the variogram between x0 and xi, and μ is the Lagrange multiplier.
One should be aware of the consequences of kriging in the T domain. Future measurements influence present predictions, just as much as past measurements, because they are both weighed using the same variogram. At the ST domain, the variogram is given by
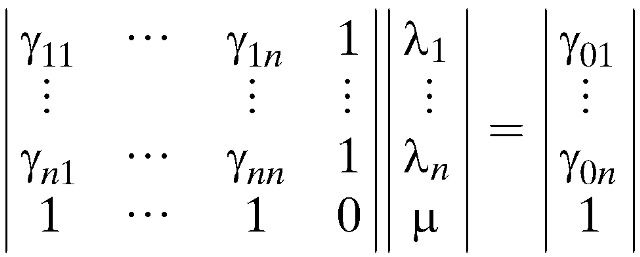 |
where  is the same variogram as in equation (2). For the ST variogram structure, we choose a product-sum covariance model34 as follows:
is the same variogram as in equation (2). For the ST variogram structure, we choose a product-sum covariance model34 as follows:
where γs(hs) and γt(ht) are valid variogram functions in .. (spatial domain: D) and ℜ (time domain: T), respectively, and k is a real coefficient. For prediction, the study area was divided at the level of 1-km resolution. A Box-Cox transformation35 of the crude prevalence was performed to apply the Gaussian model before implementing ST kriging. The precision of the kriging interpolation would be poor if the spatial stratification is strong and spatial autocorrelation is weak.36 Therefore, we also checked the spatial heterogeneity (defined as an attribute whose statistical properties, e.g., mean and standard deviation, change in space) of the yearly prevalence of schistosomiasis, using an indicator of power of determinant (PD) in the geographic detector.37 The main idea of the geographic detector is that the disease will exhibit a spatial distribution similar to that of an environmental factor if the environment contributes to disease transmission and PD is used to quantify this similarity. The value of PD ranges from 0 to 1, where closer to 1 means the disease has more similar spatial stratification with that of the factor and closer to 0 means the spatial stratification of the two are different. The spatial distribution of distance to snail habitat and distance to a water body can be characterized by buffer zones, which are classified into four buffers: 0–5 km, 5–10 km, 10–20 km, and > 20 km.
The performance of the ST kriging was evaluated by using leave-one-out cross-validation. In the process of this cross-validation, one sample village was retained as the validation data for testing the ST kriging model, and the remaining sample villages were used as training data to construct the model. This procedure was repeated such that each sample village is used once as the validation data. An indicator of root-mean-square error was used to quantify the accuracy of the model. PD values were calculated by using software available at http://www.sssampling.org/Excel-GeoDetector/. ST kriging and cross-validation were implemented in the R package gstat,38 and mapping of predicted prevalence of schistosomiasis and its corresponding SE were performed by using AcrGIS software version 10.0 (ESRI Inc.).
Results
As shown in Table 1, the mean observed prevalence of S. japonicum infection generally increased from 0.15% in 2004 to a peak of 1.48% in 2008 (with an abrupt increase in 2005) and then decreased to 0.12% in 2011. The Kruskal-Wallis test showed that the mean prevalence differed significantly by year (χ2 [7, n = 298] = 108.00, P < 0.01). The median prevalence of 0.08% in 2005 and 1.07% in 2008, combined with the corresponding mean prevalence (of 0.93% in 2005 and 1.48% in 2011), suggests a more severe burden of schistosomiasis compared with the remaining years.
Table 1.
Human prevalence (%) of schistosomiasis in sample villages in Xingzi, Jiangxi, China, 2004–2011
| Year | No. sample villages | Minimum | Mean | Median | Maximum | SD |
|---|---|---|---|---|---|---|
| 2004 | 30 | 0 | 0.15 | 0 | 3.90 | 0.01 |
| 2005 | 37 | 0 | 0.93 | 0.08 | 7.20 | 0.02 |
| 2006 | 35 | 0 | 0.29 | 0 | 6.96 | 0.01 |
| 2007 | 36 | 0 | 0.56 | 0 | 13.10 | 0.02 |
| 2008 | 42 | 0 | 1.48 | 1.07 | 9.12 | 0.01 |
| 2009 | 36 | 0 | 0.33 | 0 | 2.86 | 0.01 |
| 2010 | 35 | 0 | 0.05 | 0 | 1.02 | 0.002 |
| 2011 | 42 | 0 | 0.12 | 0 | 1.92 | 0.004 |
Results from a model for each year separately are shown in Table 2. The LR test showed that there was no significant difference between the OLS and the SEM in fitting the data for each year except 2011. Therefore, OLS was used to fit the data for 2004–2010, and the spatial autoregressive error model was used for 2011. The spatial regression results show that the distance to snail habitats was significantly negatively associated with schistosomiasis (P < 0.05), and the distance to a water body also showed a negative association with schistosomiasis, except in 2008, when there was a positive association.
Table 2.
Model estimations for the human prevalence of schistosomiasis in Xingzi, Jiangxi, China, 2004–2011*
| Year | Distance to snail habitat | Distance to water body | Spatial error coefficient | Likelihood ratio test |
|---|---|---|---|---|
| 2004 | −0.92 × 10–2 (0.27) | −0.70 × 10–2 (0.92) | 0.81 × 10–3 (0.98) | |
| 2005 | −0.56 × 10–2 (< 0.01) | −0.18 × 10–2 (0.16) | 0.68 (0.41) | |
| 2006 | −0.15 × 10–2 (< 0.01) | −0.03 × 10–2 (0.78) | 0.45 (0.52) | |
| 2007 | −0.44 × 10–2 (0.02) | −0.10 × 10–2 (0.63) | 0.02 (0.88) | |
| 2008 | −0.32 × 10–2 (0.01) | 0.26 × 10–2 (0.36) | 2.06 (0.15) | |
| 2009 | −0.91 × 10–2 (< 0.01) | −0.89 × 10–2 (0.86) | 3.41 (0.06) | |
| 2010 | −0.30 × 10–2 (< 0.01) | −0.41 × 10–2 (0.81) | 0.03 (0.86) | |
| 2011 | −0.41 × 10–2 (0.01) | −0.12 × 10–2 (0.72) | 0.64 (< 0.01) | 8.61 (< 0.01) |
Values in parentheses are corresponding P values. Coefficients in bold are significant at the 5% level. An ordinary least square regression model was used to fit data for 2004–2010, and a spatial error model was used to fit data for 2011.
The OLS model estimates for all years combined, including time as a covariate, are shown in Table 3. There was a significant correlation between schistosomiasis and distance to snail habitat (P < 0.01), and no statistical association of disease with distance to a water body was found (P = 0.27). The negative temporal effect indicates that the prevalence of infection was decreasing with time although the effect was not statistically significant (P = 0.16).
Table 3.
Ordinary least square regression model estimations for schistosomiasis in Xingzi, Jiangxi, China, 2004–2011*
| Parameter | Estimate | Standard error | t | P |
|---|---|---|---|---|
| Distance to snail habitat | −0.21 × 10–2 | 0.05 × 10–2 | −4.81 | < 0.01 |
| Distance to water body | −0.53 × 10–2 | 0.48 × 10–2 | −1.11 | 0.27 |
| Time | −0.44 × 10–1 | 0.32 × 10–1 | −1.41 | 0.16 |
Time (i.e., 2004–2011) was used as ordinal variable in the model.
The PD values of the two environmental factors for each year are shown in Table 4. These values help to check spatial heterogeneity of the prevalence of schistosomiasis. The PD values of distance to snail habitat did not vary significantly, ranging from 0.17 to 0.22 (mean = 0.20), and the PD values of distance to a water body changed slightly, ranging from 0.12 to 0.16 (mean = 0.14).
Table 4.
Power of determinant values of the environmental factors for each year in Xingzi, Jiangxi, China, 2004–2011
| Factor | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 |
|---|---|---|---|---|---|---|---|---|
| Distance to snail habitat | 0.21 | 0.18 | 0.18 | 0.17 | 0.22 | 0.21 | 0.20 | 0.19 |
| Distance to water body | 0.14 | 0.16 | 0.13 | 0.14 | 0.16 | 0.15 | 0.12 | 0.12 |
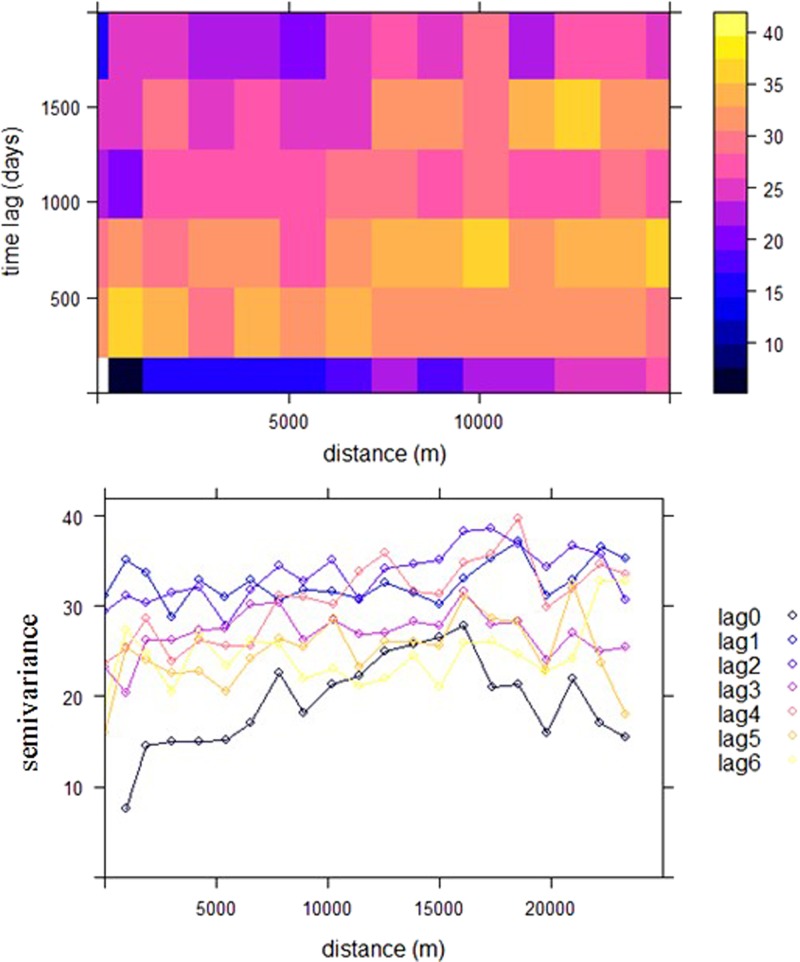
The empirical ST variogram of the human prevalence of schistosomiasis is shown in Figure 3. The increasing trend at different time lags indicates that ST correlation is present although it is not strong at higher time lags. The range of spatial dependency and temporal dependency was 10 km and 1.64 years (i.e., 600 days), respectively. Cross-validation confirmed that the ST kriging model has a good predictive ability (root-mean-square error = 0.98).
Figure 3.
Empirical spatio-temporal (ST) variogram of transformed prevalence of human schistosomiasis in each sample village, Xingzi, Jiangxi, China. Top: ST sample variogram map; bottom: sample variograms for each time lag. The unit of time lag is year. The figure was produced by using the R package gstat.38
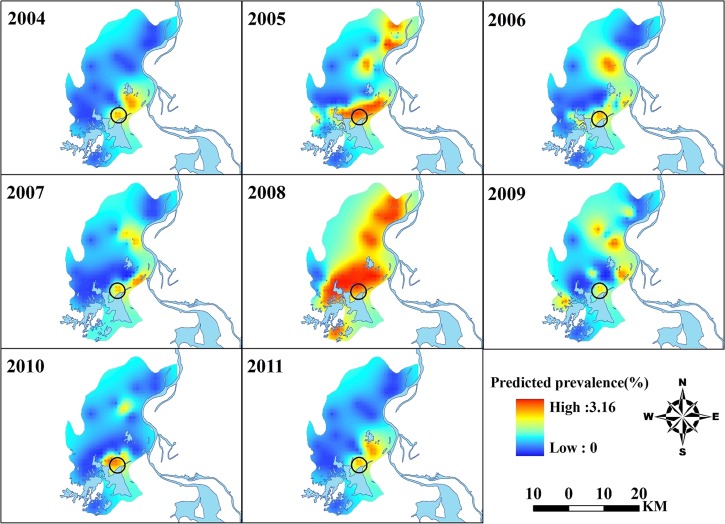
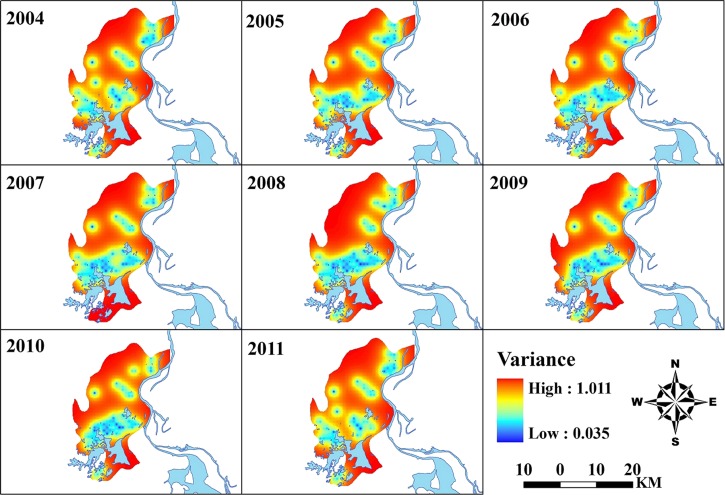
The annual map of predicted prevalence for S. japonicum infection is shown in Figure 4. Prevalence of infection showed a focal spatial pattern, and that there was a fluctuation in the extent of areas with relative high endemicity (shaded with yellow-to-red color) temporally, with a peak (the largest spatial extent) in 2008, and then contracting gradually until 2011. Moreover, for each year, clusters of relatively high prevalence occurred mostly in the middle-eastern part of the county (i.e., near Poyang Lake), and a constant focus (denoted by the black circle in each panel in Figure 2) was observed near Beng Lake each year. Corresponding estimates of the variance of the predictions are shown in Figure 5. As expected, a lower level of uncertainty was apparent in locations near sampled villages and a higher level of uncertainty was apparent in locations distant from sampled villages. However, the prediction uncertainty was generally low over the study area.
Figure 4.
Annual predicted prevalence of the predictions of schistosomiasis during 2004–2011 in Xingzi, Jiangxi, China. The black circle in each panel delineates a cluster of infection; the geographic layer of the water body was overlaid. The maps were created by using AcrGIS software version 10.0 (ESRI Inc., Redlands, CA).
Figure 5.
Annual uncertainties of the predictions of schistosomiasis during 2004–2011 in Xingzi, Jiangxi, China. The geographical layer of the water body was overlaid. The maps were created by using AcrGIS software version 10.0 (ESRI Inc., Redlands, CA).
Discussion
In this study, we have applied a ST kriging method to assess the changing patterns in the burden of S. japonicum infection risks in Xingzi, China. The OLS and spatial autoregressive error model identified the important role of environmental determinants related to snail habitats in explaining the geographic variation of infection. The ST kriging model was used here to map the annual prevalence of schistosomiasis not only spatially but also temporally. With the improvement of epidemic surveillance system, it is more likely to obtain longitudinal data. Thus, ST models, such as ST kriging, are needed to obtain more accurate and comprehensive assessment of epidemics.
No significant difference between the OLS and the SEM was observed during 2004–2010, as shown by LR test (Table 2), which indicated that almost no spatial trend existed for the residual of OLS model, or alternatively, that distance to snail habitats and distance to a water body can adequately explain the spatial variation of schistosomiasis risk; the SEM was significantly different from the OLS in 2011, which indicated that some unmeasured factors responsible for the spatial pattern of the disease were captured by the model. These unmeasured factors are possibly related to socioeconomic, demographic, and environmental factors. It is impossible to consider all the potential risk factors related with schistosomiasis because either the information is unavailable or disease mechanisms are unclear.
The association of the two snail-related factors with schistosomiasis is interpretable according to the epidemiology of infection and known biology of freshwater snails. As mentioned, marshlands in Xingzi are ideal places for formation of snail habitats. With frequent flooding of the Poyang Lake, snails in these habitats were dispersed and subsequently deposited widely in various localities, such as lakes and wetlands. Persons living on or near the shore are more likely to contact snails as a result of swimming, agricultural activities, and fishing, thus increasing their exposure to S. japonicum. The distances to snail habitats and water bodies can be regarded as the indicators of exposure because persons tend to contact these environments more frequently if they live nearby. This finding is consistent with those of previous studies, but the constantly significant association of snail habitats with schistosomiasis reminds us to adjust our focus on the snail habitats.
The negative temporal effect, as shown in Table 3, suggested that prevalence of the disease had a tendency of decreasing generally, although the effect was not statistically significant. Although causality cannot be unambiguously attributed to the national program for schistosomiasis control implemented in 2005, we believe it is reasonable to conclude that these efforts may have been responsible for such reductions in disease burden. Conversely, the spatial pattern of the infection varied annually. The extensive outbreak in 2008 indicated fluctuations in flooding areas that were related to abrupt variation of precipitation and might also be related to an increasing trend of infection rate of snails during that period.39 The contracted extent of the disease since 2009 might be caused by persistently low water levels and the significant decrease of snail densities.40 Although the schistosomiasis situation in Xingzi County has been improved, studies showed that the infected snails were still found,13 and this finding might potentially affect the risk of schistosomiasis transmission, which was shown in our study.
Ranges of spatial and temporal dependency can characterize the spatial and temporal variation of schistosomiasis. The long spatial range (i.e., 10 km) suggested that the spatial correlation become negligible after 10 km, and such distance implied that transmission occurred between villages rather than within and around them and could be attributed to distribution of snail habits located between villages. Conversely, the small temporal correlation (i.e., 1.63 years) indicated that temporal correlation become negligible after less than two years, and such a short period indicated that the burden of the disease varied significantly and quickly and could relate to implementation of control strategies.
Kriging is based on spatial or spatiotemporal autocorrelation. The precision of the kriging interpolation would be poor if the spatial stratification is strong and spatial autocorrelation is weak. The mean PD value of 0.20 for distance to snail habitat indicated that buffers of distance to snail habitat explained 20% of the variation of schistosomiasis, and the mean PD value of 0.14 for distance to a water body suggested that buffers of distance to snail habitat explained only 14% of the variation of the disease. These findings implied that prevalence of schistosomiasis showed week spatial heterogeneity within the buffers. Combined with ST correlation, this week spatial heterogeneity of the disease justified use of ST kriging.
The predicted ST pattern of schistosomiasis risk also provided an empirical basis for identifying prior areas when implementing schistosomiasis controls in local regions. As shown in Figure 4, a focus area of relatively high risk (with prevalence of 2.0–3.0%) near Beng Lake compared with other areas, occurred constantly over the years despite implementation of the revised control strategy, and this area would definitely be a priority for targeting schistosomiasis control in the future.
In addition to replacement of cows with machines, the integrated program included such strategies as the treatment of night soil and the safety of the water supply,41 breeding of domestic animals in barns,42 change of snail habitats by use of a water conservancy project,42 flourishing forests aimed at reducing snail populations, in addition to chemotherapy and health education.43 This program assumes that a reduction in transmission of the disease from humans and bovines to the intermediate Oncomelania snail host would eventually block the life cycle of this parasite, but transmission of S. japonicum is relatively complicated and more than 40 species of mammalians can serve as potential zoonotic reservoirs.44 Furthermore, this complex program is time- and money- consuming in terms of implementation. The budget for schistosomiasis control might be reduced in foreseeable future. Therefore, how to choose an economic, effective, and sustainable strategy is of great concern. Our analysis gives a clue: snail habitats located within areas at high risk for schistosomiasis, as shown in Figure 4, should be key control targets.
The limitations of the current study should also be recognized. First, we were unable to unambiguously attribute changes in schistosomiasis prevalence to the revised control strategy implemented in 2005 because we did not have control areas with which to compare ST patterns of disease burden. Second, when analyzing the dynamic pattern of schistosomiasis, we only considered the effect of a water body and snail habitat; other factors, such as climatic conditions and land use, were not included in the study. We assumed these changed little over the study area at each year because the climatic conditions tend to be homogeneous at such a small scale (village level in our study);45 the climatic conditions in the past decade have not changed substantially;46 and the land cover in Xingzi County is fairly homogeneous; mainly rice paddies and a scarcity of grassland around Poyang Lake.47 Third, the sensitivity and specificity of the serologic and stool tests are not perfect.48
In summary, this study investigated the ST pattern of schistosomiasis during 2004–2011 in Xingzi, China, a region where infected snails were still found. Results indicated that clusters of S. japonicum infection occurred mostly near water bodies, but the precise location varied with time and an outbreak of the disease occurred in 2008. The ST pattern of schistosomiasis presented in our study suggested that the revised control policy, with more focus on snails, should be further strengthened. Snail habitats located within areas at high risk for schistosomiasis should be given a priority when schistosomiasis control programs are implemented.
ACKNOWLEDGMENTS
We thank the staff at the Anti-Schistosomiasis Station of Xingzi for their kind collaboration and the reviewers for their comments and suggestions.
Footnotes
Financial support: This study was supported by grants 81102167, 81172609, and J1210041 from National Natural Science Foundation of China to Zhijie Zhang and Qingwu Jiang; the Specialized Research Fund for the Doctoral Program of Higher Education (grant 20110071120040); a Foundation for the Author of National Excellent Doctoral Dissertation of the People's Republic of China (201186); the National S&T Major Program (2012ZX10004220 and 2008ZX10004-011); the Ecological Environment and Humanities/Social Sciences Interdisciplinary Research Project of Tyndall Center of Fudan University (FTC98503A09); the Talent Programs for Fostering Outstanding Youth of Shanghai (XYQ2013071); and the Independent Research Project of Fudan University (20520133105).
Authors' addresses: Yi Hu, Liqian Sun, and Qingwu Jiang, Department of Epidemiology and Biostatistics, School of Public Health, Fudan University, Shanghai, China, E-mails: huy@lreis.ac.cn, 13211020063@fudan.edu.cn, and jiangqw@fudan.edu.cn. Jie Gao, Shandong Institute of Prevention and Control for Endemic Disease, Jinan, China, E-mail: agao1224@163.com. Meina Chi, Shanghai Institute of Occupational Disease for Chemical Industry, Shanghai, China, E-mail: 1039021808@qq.com. Can Luo, Department of Environmental Art and Architecture, Changsha Environmental Protection Vocational Technical College, Changsha, China, E-mail: cherry12204@163.com. Henry Lynn, Biomedical Statistical Center, Fudan University, Shanghai, China, E-mail: hslynn@shmu.edu.cn. Bo Tao, Xingzi Station for Schitosomiasis Prevention and Control, Jiangxi Province, China, E-mail: xzxuefangzhan@163.com. Decheng Wang, Medical Science College, China Three Gorges University, Yichang, China, E-mail: 13580573@qq.com. Zhijie Zhang, Key Laboratory of Public Health Safety, Ministry of Education, Shanghai, China, E-mail: epistat@gmail.com.
References
- 1.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:11–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 2.McManus DP, Gray DJ, Li Y, Feng Z, Williams GM, Stewart D, Rey-Ladino J, Ross AG. Schistosomasis in the People's Republic of China: the era of the Three Gorges Dam. Clin Microbiol Rev. 2010;23:442–466. doi: 10.1128/CMR.00044-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Jiang Q, Wang L, Zhao G, Zhao Q, Gu Y. Schistosomiasis situation in the People's public of China in 2001 [in Chinese] Chin J Schisto Control. 2002;14:241–243. [Google Scholar]
- 4.Zhou XN, Wang LY, Chen MG, Wu XH, Jiang QW, Chen XY, Zheng J, Utzinger J. The public health significance and control of schistosomiasis in China–then and now. Acta Trop. 2005;96:97–105. doi: 10.1016/j.actatropica.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Utzinger J, Zhou XN, Chen MG, Bergquist R. Conquering schistosomiasis in China: the long march. Acta Trop. 2005;96:69–96. doi: 10.1016/j.actatropica.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Mao SP, Shao BR. Schistosomiasis control in the People's Republic of China. Am J Trop Med Hyg. 1982;1:92–99. [PubMed] [Google Scholar]
- 7.Yuan H, Jiagang G, Bergquist R, Tanner M, Xianyi C. The 1992–1999 World Bank schistosomiasis research initiative in China: outcome and prospectives. Parasitol Int. 2005;49:195–207. doi: 10.1016/s1383-5769(00)00045-3. [DOI] [PubMed] [Google Scholar]
- 8.Utzinger J, Bergquist R, Shu-Hua X, Singer BH, Tanner M. Sustainable schistosomiasis control–the way forward. Lancet. 2003;362:1932–1934. doi: 10.1016/S0140-6736(03)14968-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Zhu R, Ward MP, Xu W, Zhang L, Guo J, Zhao F, Jiang Q. Long-term impact of the World Bank Loan Project for schistosomiasis control: a comparison of the spatial distribution of schistosomiasis risk in China. PLoS Negl Trop Dis. 2012;6:e1620. doi: 10.1371/journal.pntd.0001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seto EY, Remais JV, Carlton EJ, Wang S, Liang S, Brindley PJ, Qiu D, Spear RC, Wang LD, Wang TP, Chen HG, Dong XQ, Wang LY, Hao Y, Bergquist R, Zhou XN. Toward sustainable and comprehensive control of schistosomiasis in China: lessons from Sichuan. PLoS Negl Trop Dis. 2011;5:e1372. doi: 10.1371/journal.pntd.0001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Chen H, Guo J, Zeng X, Hong X. A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med. 2009;360:121–128. doi: 10.1056/NEJMoa0800135. [DOI] [PubMed] [Google Scholar]
- 12.Li SZ, Luz A, Wang XH, Xu LL, Wang Q, Qian YJ, Wu XH, Guo JG, Xia G, Wang LY, Zhou XN. Schistosomiasis in China: acute infections during 2005–2008. Chin Med J (Engl) 2009;122:1009–1014. [PubMed] [Google Scholar]
- 13.Yang K, Li W, Sun LP, Huang YX, Zhang JF, Wu F, Hang DR, Steinmann P, Liang YS. Spatio-temporal analysis to identify determinants of Oncomelania hupensis infection with Schistosoma japonicum in Jiangsu province, China. Parasit Vectors. 2013;6:138. doi: 10.1186/1756-3305-6-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang S, Seto EY, Remais JV, Zhong B, Yang C, Hubbard A, Davis GM, Gu X, Qiu D, Spear RC. Environmental effects on parasitic disease transmission exemplified by schistosomiasis in western China. Proc Natl Acad Sci USA. 2007;104:7110–7115. doi: 10.1073/pnas.0701878104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gosoniu L, Vounatsou P, Sogoba N, Smith T. Bayesian modeling of geostatistical malaria risk data. Geospat Health. 2006;1:127–139. doi: 10.4081/gh.2006.287. [DOI] [PubMed] [Google Scholar]
- 16.Reid H, Haque U, Clements AC, Tatem AJ, Vallely A, Ahmed SM, Islam A, Haque R. Mapping malaria risk in Bangladesh using Bayesian geostatistical models. Am J Trop Med Hyg. 2010;83:861–867. doi: 10.4269/ajtmh.2010.10-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Carpenter TE, Chen Y, Clark AB, Lynn HS, Peng W, Zhou Y, Zhao G, Jiang Q. Identifying high-risk regions for schistosomiasis in Guichi, China: a spatial analysis. Acta Trop. 2008;107:217–223. doi: 10.1016/j.actatropica.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 18.Diggle PJ, Thomson MC, Christensen OF, Rowlingson B, Obsomer V, Gardon J, Wanji S, Takougang I, Enyong P, Kamgno J, Remme JH, Boussinesq M, Molyneux DH. Spatial modelling and the prediction of Loa loa risk: decision making under uncertainty. Ann Trop Med Parasitol. 2007;101:499–509. doi: 10.1179/136485913X13789813917463. [DOI] [PubMed] [Google Scholar]
- 19.Cressie N, Wikle C. Statistics for Spatio-Temporal Data. New York: John Wiley; 2011. [Google Scholar]
- 20.Le ND, Zidek JV. Statistical Analysis of Environmental Space-Time Processes. New York: Springer; 2006. [Google Scholar]
- 21.Baker RD. Identifying space–time disease clusters. Acta Trop. 2004;91:291–299. doi: 10.1016/j.actatropica.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Metras R, Porphyre T, Pfeiffer DU, Kemp A, Thompson PN, Collins LM, White RG. Exploratory space-time analyses of Rift Valley Fever in South Africa in 2008–2011. PLoS Negl Trop Dis. 2012;6:e1808. doi: 10.1371/journal.pntd.0001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paireau J, Girond F, Collard JM, Mainassara HB, Jusot JF. Analysing spatio-temporal clustering of meningococcal meningitis outbreaks in Niger reveals opportunities for improved disease control. PLoS Negl Trop Dis. 2012;6:e1577. doi: 10.1371/journal.pntd.0001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isaaks E, Srivastava R. Applied Geostatistics: Oxford, United Kingdom: Oxford University Press; 1989. [Google Scholar]
- 25.Kyriakidis PC, Journel AG. Stochastic modeling of atmospheric pollution: a spatial time-series framework: part I. Methodology. Atmos Environ. 2001;35:2331–2337. [Google Scholar]
- 26.Bechini L, Ducco G, Donatelli M, Stein A. Modelling, interpolation and stochastic simulation in space and time of global solar radiation. Agric Ecosyst Environ. 2000;81:29–42. [Google Scholar]
- 27.Snepvangers JJ, Heuvelink GB, Huisman JA. Soil water content interpolation using spatio-temporal kriging with external drift. Geoderma. 2003;112:253–271. [Google Scholar]
- 28.Tao P, Jiang QL, Luo CJ, Yin ZH, Wang JM. Endemic situation of schistosomiasis in Xingzi County. Chin J Schisto Control. 2009;21:62–63. [Google Scholar]
- 29.Chen H, Lin D. The prevalence and control of schistosomiasis in Poyang Lake region, China. Parasitol Int. 2004;53:115–125. doi: 10.1016/j.parint.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Yu JM, de Vlas SJ, Jiang QW, Gryseels B. Comparison of the Kato-Katz technique, hatching test and indirect hemagglutination assay (IHA) for the diagnosis of Schistosoma japonicum infection in China. Parasitol Int. 2007;56:45–49. doi: 10.1016/j.parint.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Hu Y, Zhang Z, Chen Y, Wang Z, Gao J, Tao B, Jiang Q, Jiang Q. Spatial pattern of schistosomiasis in Xingzi, Jiangxi Province, China: the effects of environmental factors. Parasit Vectors. 2013;6:214. doi: 10.1186/1756-3305-6-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Journel AG, Huijbregts CJ. Mining Geostatistics. London: Academic Press; 1978. [Google Scholar]
- 33.Deutsch CV, Journel AG. GSLIB: Geostatistical Software Library and User's Guide. New York: Oxford University Press; [Google Scholar]
- 34.De Cesare L, Myers DE, Posa D. Product-sum covariance for space-time modeling: an environmental application. Environmetrics. 2001;12:11–23. [Google Scholar]
- 35.Box GE, Cox DR. An analysis of transformations (with discussion) J R Stat Soc B. 1964;26:211–252. [Google Scholar]
- 36.Fischer MM, Wang JF. Spatial Data Analysis: Models, Methods and Techniques. New York: Springer; 2011. [Google Scholar]
- 37.Wang JF, Li XH, Christakos G, Liao YL, Zhang T, Gu X, Zheng XY. Geographical detectors-based health risk assessment and its application in the neural tube defects study of the Heshun region, China. Int J Geogr Inf Sci. 2010;24:107–127. [Google Scholar]
- 38.Pebesma E. Spatio-Temporal Geostatistics using gstat. Package Vignettes. Munster, Germany: University of Munster; 2011. [Google Scholar]
- 39.Hao Y, Wang LY, Zhou XN, Chen HG, Huang XB, Liang YS. Causes and risks of schistosomiasis transmission in Poyang Lake region of Jiangxi Province, China. Chin J Schisto Control. 2009;21:345–349. [Google Scholar]
- 40.Zhou YS, Peng GH, Hu ZH, Zhu R, Yu Q, Cao CL, Hu GH, Guo JG. Influencing factors of changes of Oncomelania snail densities in Poyang Lake region, Nanchang City, 2011. Chin J Schisto Control. 2012;3:315–317. [PubMed] [Google Scholar]
- 41.Zhang S, Wang T, Tao C, Chen G, Chen J, Xu H. Observation on comprehensive measures of safe treatment of night-soil and water supply, replacement of bovine with machine for schistosomiasis control. Chin J Schisto Control. 2005;17:437–442. [Google Scholar]
- 42.Chen XY, Wang LY, Cai JM, Zhou XN, Zheng J, Guo JG, Wu XH, Engels D, Chen MG. Schistosomiasis control in China: the impact of a 10-year World Bank Loan Project (1992–2001) Bull World Health Organ. 2005;83:43–48. [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L. Management of human and animal feces is a key element for effective control of pidemic of endemic schistosomiasis in China. Chin J Epidemiol. 2005;26:929–930. [PubMed] [Google Scholar]
- 44.Zhou YB, Liang S, Jiang QW. Factors impacting on progress towards elimination of transmission of schistosomiasis japonica in China. Parasit Vectors. 2012;5:275. doi: 10.1186/1756-3305-5-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brooker S, Clements AC, Bundy DA. Global epidemiology, ecology and control of soil-transmitted helminth infections. Adv Parasitol. 2006;62:221–261. doi: 10.1016/S0065-308X(05)62007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guemas V, Doblas-Reyes F, Andreu-Burillo I, Asif M. Retrospective prediction of the global warming slowdown in the past decade. Nat Clim Change. 2013;3:649–653. [Google Scholar]
- 47.Yan HM, Huang HQ, Xiao XM, Jiang LG. Spatio-temporal distribution of multiple cropping systems in the Poyang Lake. Acta Ecol Sin. 2008;28:4517–4523. [Google Scholar]
- 48.Wang XH, Zhou XN, Vounatsou P, Chen Z, Utzinger J, Yang K, Steinmann P, Wu XH. Bayesian spatio-temporal modeling of Schistosoma japonicum prevalence data in the absence of a diagnostic ‘gold’ standard. PLoS Negl Trop Dis. 2008;2:e250. doi: 10.1371/journal.pntd.0000250. [DOI] [PMC free article] [PubMed] [Google Scholar]