Summary
The malaria parasite Plasmodium falciparum infects humans and first targets the liver where liver-stage parasites undergo pre-erythrocytic replication. Liver-stage antigen-1 (LSA-1) is currently the only identified P. falciparum protein for which expression is restricted to liver stages. Yet, the importance of LSA-1 for liver-stage parasite development remains unknown. Here we deleted LSA-1 in the NF54 strain of P. falciparum and analysed the lsa-1− parasites throughout their life cycle. lsa-1− sporozoites had normal gliding motility and invasion into hepatocytes. Six days after infection of a hepatocytic cell line, lsa-1− parasites exhibited a moderate phenotype with an ∼50% reduction of late liver-stage forms when compared with wild type. Strikingly, lsa-1− parasites growing in SCID/Alb-uPA mice with humanized livers showed a severe defect in late liver-stage differentiation and exo-erythrocytic merozoite formation 7 days after infection, a time point when wild-type parasites develop into mature merozoites. The lsa-1− parasites also showed aberrant liver-stage expression of key parasite proteins apical membrane antigen-1 and circumsporozoite protein. Our data show that LSA-1 plays a critical role during late liver-stage schizogony and is thus important in the parasite transition from the liver to blood. LSA-1 is the first P. falciparum protein identified to be required for this transitional stage of the parasite life cycle.
Introduction
Plasmodia are obligatory intracellular parasites transmitted to humans and other mammals by the bite of parasite-infected Anopheline mosquitoes. In mammals Plasmodium parasites have two distinct intracellular phases: the liver stage and the blood stage. The liver stage starts when motile sporozoites released from an infected mosquito during a bite into the skin make their way to the liver and invade hepatocytes. In the hepatocytes, sporozoites differentiate into liver stages, also known as exoerythrocytic forms (EEFs), which are ensconced in a parasitophorous vacuole (PV) (Prudencio et al., 2006). After days of growth and differentiation, mature liver stages release tens of thousands of red blood cell infectious exo-erythrocytic merozoites that initiate the intraerythrocytic phase of the life cycle. Although work on rodent malaria parasite liver stages, which can be analysed in mice, has revealed some of the cellular and molecular processes that control parasite amplification in hepatocytes, this part of the life cycle is still poorly understood (Vaughan et al., 2008). Even less is known about the liver-stage biology of human malaria parasites due to the difficulty of studying liver stages in humans and the paucity of small animal models. Consequently, parasite proteins expressed in human liver stages remain largely unknown with little understanding of how they are involved in the development of this clinically silent aspect of the life cycle.
Interest in studying liver stages has been enhanced recently due to results showing that immunization with attenuated sporozoites, which can infect hepatocytes but developmentally arrest and fail to cause blood-stage infection, confers effective protection against subsequent infection (Hoffman et al., 2002; Vaughan et al., 2010). This suggests the importance of liver-stage antigens as the target of protective immunity and has led to the search for pre-erythrocytic vaccine candidates.
Historically, the first liver-stage antigens were discovered using antisera of individuals living in malaria-endemic areas to screen parasite-expressed proteins (Guerin-Marchand et al., 1987). These sera specifically recognized Plasmodium falciparum liver stages in infected livers of Cebus apella monkeys (Druilhe et al., 1984). Subsequently, it was shown that the sera reacted with a 200 kDa protein accumulating as a flocculent in the PV lumen of liver stages matrix during exo-erythrocytic schizogony (Fidock et al., 1994). The importance of LSA-1 as an antigen was elucidated by further studies demonstrating that immune responses against LSA-1 are associated with protection against malaria in naturally exposed individuals (John et al., 2004; 2008). However, another study did not confirm this association (John et al., 2002). Furthermore, LSA-1 contains a T-cell epitope that has been associated with protection against malaria in people harbouring the HLA-B53 haplotype (Hill et al., 1992). The predicted structure of LSA-1 exhibits highly inter-strain-conserved N- and C-terminal non-repeat regions containing B- and T-cell epitopes flanked by a central region with 86 copies of a 17-amino-acid repeat (Zhu and Hollingdale, 1991; Fidock et al., 1994). In spite of the relatively high conservation of LSA-1 sequences across P. falciparum strains (Fidock et al., 1994; Yang et al., 1995), which suggest a crucial role for parasite growth and survival, the function of the protein and its importance for liver-stage development remains unknown. LSA-1 is unique to P. falciparum, having no orthologues in rodent or non-human primate Plasmodium species and it therefore cannot be analysed in these malaria models. Efforts to identify an LSA-1 orthologue by synteny in composite contigs generated from genomic sequences of three closely related rodent Plasmodium species also did not identify a satisfactory candidate (Kooij et al., 2005).
To gain a better understanding of the role of LSA-1 during P. falciparum liver-stage development, we deleted the LSA-1 locus in the NF54 strain by homologous recombination. The resulting knockout line was analysed for liver-stage defects in a hepatocytic cell line and a humanized mouse model carrying human hepatocytes (Sacci et al., 2006; Sattabongkot et al., 2006). As expected from a gene that is expressed only in liver-stage development, the deletion of LSA-1 had no observable effect on the parasites ability to infect and proliferate in erythrocytes. It also showed no defect during growth in mosquitoes. Importantly, analysis of lsa-1− parasite liver infections in SCID/Alb-uPA mice with humanized livers showed that late liver stages suffered a severe defect in differentiation and exo-erythrocytic merozoite formation accompanied by protein expression abnormalities after 7 days of growth. This defect could result in a severe attenuation and impair the transition of the parasite from the liver stage to the blood stage.
Results
Deletion of the P. falciparum LSA-1 locus
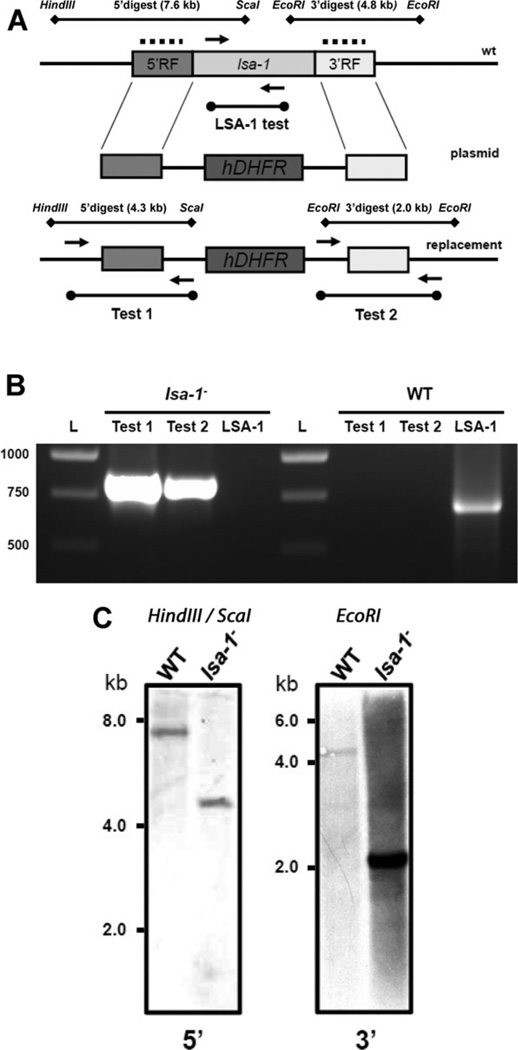
To delete LSA-1 from the NF54 P. falciparum genome we used the pCC-1 plasmid, which allows for a positive-negative selection strategy as described before (Maier et al., 2006; 2008) (Fig. 1A). The NF54 strain was used as a recipient because it readily produces gametocytes and allows for robust mosquito infections (VanBuskirk et al., 2009). The double-cross-over homologous recombination between the targeting sequences allowed us to truncate and replace the majority of the LSA-1 locus with the human dihydrofolate reductase (hdhfr) selectable marker. Asexual parasites were transfected with the targeting construct and subjected to positive selection with WR92210. After a stable, drug-resistant parasite population was established, negative selection against cytosine deaminase-uracil phosphoribosyl transferase with 5-fluorocytosine was performed. The resulting recombinant parasite line was analysed for successful gene deletion and plasmid-specific integration by PCR genotyping and Southern blotting (Fig. 1B and C). No residual wild-type (WT) LSA-1 was detected in the selected parasite population by PCR. We decided to use a parental uncloned lsa-1− parasite population in all the subsequent phenotypic evaluation experiments to avoid clone-to-clone variability, which could bias results. To confirm that the lsa-1− parasite culture was still free of WT contamination, cultures were retested for WT by PCR as described in Fig. 1B after several rounds of blood-stage propagation and gametocyte production. We did not detect any WT genomic DNA in the lsa-1− culture confirming its genetic homogeneity (data not shown).
Fig. 1.
Deletion of the P. falciparum LSA-1 locus.
A. Schematic representation of LSA-1 gene deletion in the NF54 strain of P. falciparum (wt – wild-type gene locus; plasmid – linearized replacement plasmid; replacement – replacement of LSA-1 gene with the plasmid containing an hdhfr cassette) and genomic DNA digestion strategy for Southern blot analysis (dotted lines – location of probe hybridization).
B. Gene deletion analysis was performed on LSA-1-deficient (lsa-1−) and NF54 genomic DNA with primers pairs as outlined in (A). ‘Test 1’ and ‘Test 2’ confirmed LSA-1 gene-specific replacement by double homologous recombination. The lack of the LSA-1 open reading frame in genomic DNA was confirmed by ‘LSA-1 test’ in the lsa-1− parasites; L – DNA ladder.
C. Probes specific to the 5′ and 3′ targeting flanks of LSA-1 locus were hybridized to genomic DNA fragments from lsa-1− parasites and the WT NF54 parasite line. Analysis of the lsa-1− parasite line showed specific restriction fragments recognized by the probes at predicted sizes indicating successful deletion of the locus. The restriction fragment recognized by the 5′ probe (left panel) is 7.6 kilobases (kb) for the WT locus and 4.3 kb for the lsa-1− locus. The fragment recognized by the 3′ probe (right panel) is 4.8 kb for the WT locus and 2.0 kb for the lsa-1− locus.
lsa-1− parasites produce biologically active sporozoites
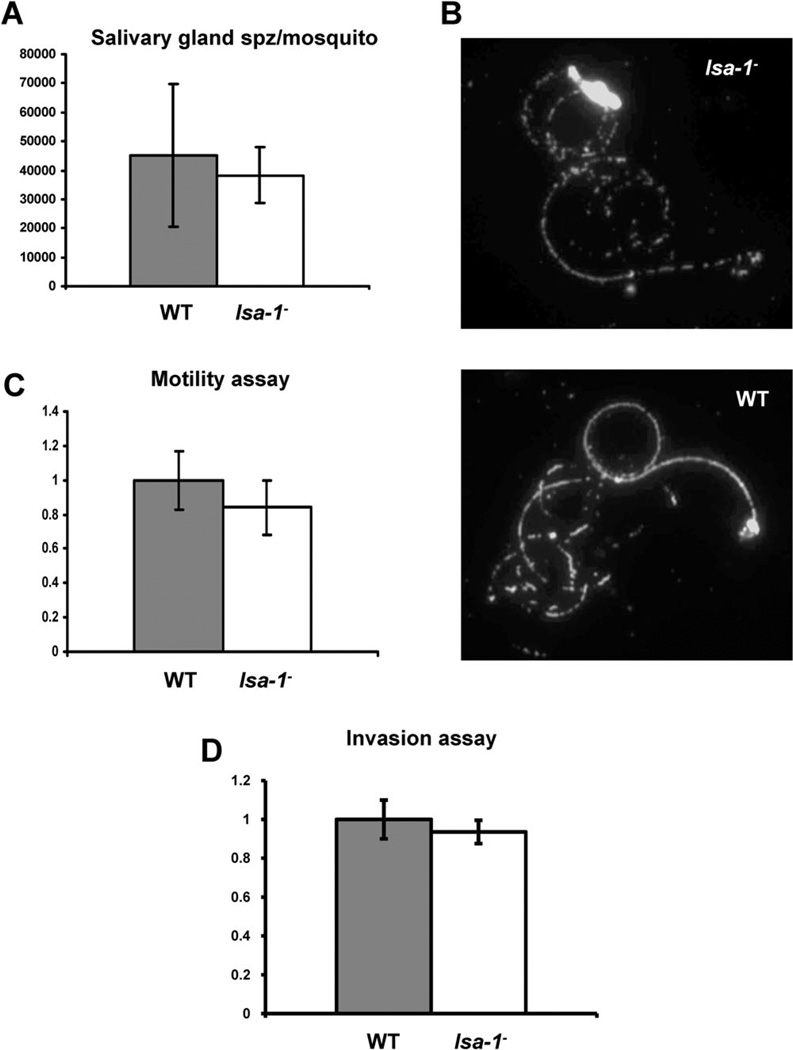
LSA-1 is specifically expressed in liver stages (Fidock et al., 1994). Consequently, deletion of LSA-1 in the erythrocytic stages was possible and did not result in any noticeable defect during blood-stage asexual replication or gametocyte differentiation (data not shown). This observation confirms that LSA-1 has no apparent function during these stages of the parasite life cycle. Next, gametocyte cultures were produced and fed to Anopheles stephensi mosquitoes by membrane feeding. Evaluation of infected mosquito midguts showed no differences in quantity or quality of oocysts between the lsa-1− and WT NF54 parasites (data not shown). Furthermore, we found comparable numbers of lsa-1− and WT sporozoites in the salivary glands of infected mosquitoes (P = 0.22) (Fig. 2A). When lsa-1− sporozoites were dissected from the salivary glands, they were biologically active and exhibited normal gliding motility (P = 0.16) in comparison with WT NF54 parasites, as determined by their ability to shed circumsporozoite (CS) protein in trails (Fig. 2B) on a solid glass substrate (Fig. 2C, Table S1).
Fig. 2.
Analysis of P. falciparum lsa-1− sporozoites.
A. lsa-1− sporozoites show normal invasion of the mosquito salivary glands as compared with WT NF54 parasites. The experiment was performed on three independent occasions.
B. lsa-1− sporozoites show normal CS protein-shedding and circular gliding activity on a solid glass surface when compared with NF54 sporozoites suggesting no motility defects for the lsa-1− parasite.
C. Gliding activity of lsa-1− sporozoites is similar to the gliding activity observed for WT parasites. The gliding assay was performed in triplicate.
D. lsa-1− and WT parasites have comparable invasion rates; the experiment was performed in triplicate.
lsa-1− parasites infect host cells in vitro and exhibit normal early liver-stage development but show a subsequent developmental defect
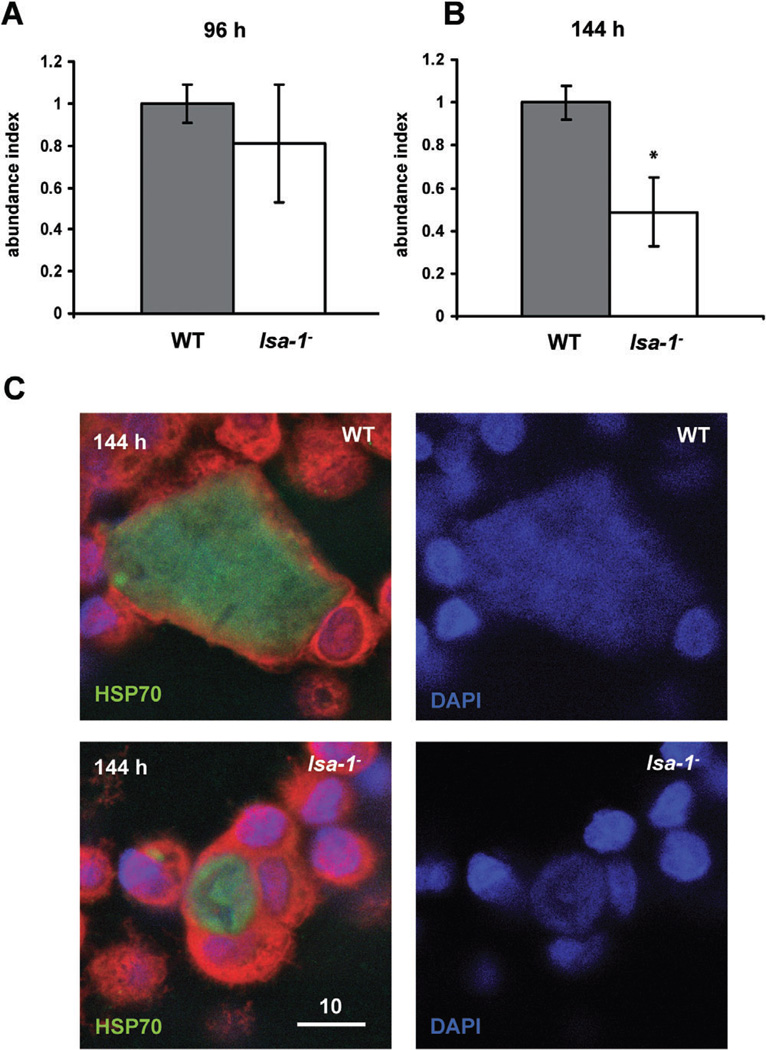
We investigated the ability of lsa-1− sporozoites to invade host cells in vitro using the HC-04 cell line, which has been developed to study the pre-erythrocytic biology of human malaria parasites (Sattabongkot et al., 2006). Invasion was assessed microscopically by counting the number of intracellular sporozoites (Hollingdale et al., 1983). There were no observable differences in invasion rates between lsa-1− and WT sporozoites (P = 0.28) (Fig. 2D, Table S2). Next, we tested intracellular development of lsa-1− parasites. LSA-1 is expressed in the late phase of liver-stage development; therefore, we were interested to compare lsa-1− and WT parasite development in HC-04 cells at 96 h and 144 h after sporozoite infection. The earlier liver-stage development time point (96 h) appeared unaffected by the lack of LSA-1 as we could detect comparable numbers of liver stages in the in vitro developmental assay (P = 0.73) (Fig. 3A, Table S3). Interestingly, we observed a significant decrease in lsa-1− liver-stage numbers when compared with WT parasites at 144 h after sporozoite infection (P = 0.0019) (Fig. 3B, Table S3). The microscopic evaluation of the liver stages in HC-04 also revealed an apparent developmental retardation of lsa-1− parasites (Fig. 3C).
Fig. 3.
lsa-1− parasites appear normal in in vitro early liver-stage development but show a late liver-stage defect.
A. The lsa-1− liver-stage parasite appears to have no defect in early development (96 h post sporozoite infection) as lsa-1− numbers and developmental maturation of liver stages are comparable to WT parasites in the HC-04 development assay.
B. Late liver-stage development (144 h post sporozoite infection) is abnormal in lsa-1− parasites. lsa1− liver stages showed an ∼50% reduction in numbers when compared with WT and also appeared to suffer developmental retardation. The experiment was performed in triplicate.
*Significant difference as evaluated by the Mann–Whitney U-test at the 95% confidence level.
C. WT and lsa-1− liver-stage parasites in HC-04 hepatoma cells at 144 h post sporozoites infection were stained with HSP70 antibodies. Scale bar – 10 µm.
lsa-1− liver stages show a late differentiation defect and lack of exo-erythrocytic merozoite formation in a humanized mouse infection
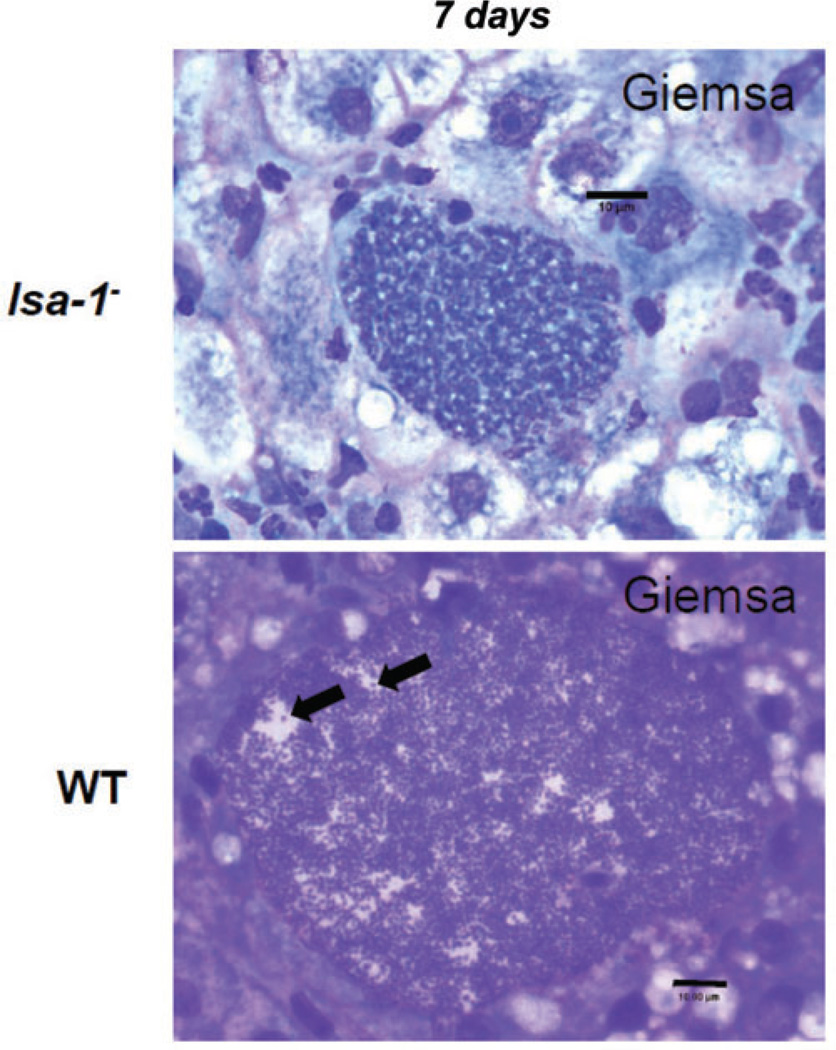
Complete development of P. falciparum late liver stages is exceedingly difficult to analyse in HC-04 cells because parasites need to be cultured for more than 6 days. To further evaluate any possible defects in late liver-stage development of the lsa-1− parasites, we decided to utilize a humanized mouse model where mouse hepatocytes in the mouse liver are replaced with human hepatocytes (Sacci et al., 2006). Immunodeficient mice homozygous for the urokinase-type plasminogen activator transgene (SCID Alb-uPA) and engrafted with primary human hepatocytes (SCID Alb-uPA hu Hep) were infected intravenously (IV) with 1.5 × 106 lsa-1− or WT sporozoites. The infection was allowed to continue for 7 days at which time the mice were euthanized and livers removed for analysis. To detect late liver-stage developmental defects in the humanized mouse model, we used Giemsa staining on tissue sections (Fig. 4). WT NF54 liver stages showed a high degree of terminal differentiation and maturation with numerous exo-erythrocytic schizonts undergoing merozoite formation resulting in the accumulation of thousands of merozoites within the infected hepatocyte [at least half of the observed NF54 liver-stage parasites (n = 50 from non-serial sections) contained matured merozoites] (Fig. 4). In striking contrast, merozoite maturation was not observed in 7-day-old lsa-1− liver stages (n = 50 from non-serial sections). The lsa-1− parasites exhibited extensive nuclear proliferation and cellular differentiation but no clear merozoite development.
Fig. 4.
lsa-1− liver stages show defective in vivo exo-erythrocytic merozoite maturation at day seven post sporozoite infection. P. falciparum LSA-1-deficient and WT NF54 parasites are detected in the livers of immunodeficient mice homozygous for the urokinase-type plasminogen activator transgene (SCID Alb-uPA) with engrafted primary human hepatocytes (SCID Alb-uPA hu Hep) at 7 days post sporozoite infection. lsa-1− liver stages, however, show defective exo-erythrocytic merozoite formation (top) when compared with wild-type NF54 liver stages at the same developmental time point (bottom). Black arrows point individual merozoites detected in the late WT NF54 liver stage, which were not observed in the late lsa− liver stages. Liver sections were stained with Giemsa. Scale bar – 10 µm.
lsa-1− liver stages show protein expression abnormalities in late liver-stage development
We next evaluated protein expression profiles by probing infected liver sections with antisera to proteins for which expression in liver stages has been established (Sacci et al., 2006). lsa-1− and WT liver stages were compared at day five and day seven after sporozoite inoculation (Table 1). Staining profiles for 5-day-old lsa-1− and WT liver stages were similar for the proteins assayed. LSA-1 staining, which was not detected in lsa-1− parasites at day five or day seven, confirmed the deletion of the lsa-1 gene in the knockout line (Fig. 5). Seven-day-old lsa-1− liver stages were positive for erythrocyte binding antigen 175 (EBA175) and merozoite surface protein 1 (MSP-1), blood-stage proteins, which are detected in the late liver-stage development (Gruner et al., 2001) (Sacci et al., 2006). MSP-2, MSP-4, MSP-5 and heat shock protein 70 (HSP70) expression was also positive in NF54 and lsa-1− parasites (Table 1). Thrombospondin-related anonymous protein (TRAP), a protein expressed in Plasmodium sporozoites but not in the growing liver stages, was not detected in both WT and LSA-1-deficient liver stages for day five and day seven, as expected.
Table 1.
Protein expression profile of wild-type (WT) NF54 and lsa-1− liver stages at 5 and 7 days after infection.
| Liver-stage IFA |
||||
|---|---|---|---|---|
| NF54 WT Day 5 |
lsa-1− Day 5 |
NF54 WT Day 7 |
lsa-1− Day 7 |
|
| CS protein | pos | pos | neg | pos |
| TRAP | pos | pos | neg | neg |
| HSP70 | pos | pos | pos | pos |
| LSA-1 | pos | neg | pos | neg |
| AMA-1 | neg | neg | pos | neg |
| EBA-175 | neg | neg | pos | pos |
| MSP-1 | pos | pos | pos | pos |
| MSP-2 | pos | pos | pos | pos |
| MSP4 | pos | pos | pos | pos |
| MSP-5 | pos | pos | pos | pos |
Bold entries represent differences between expression in WT and lsa-1− parasites.
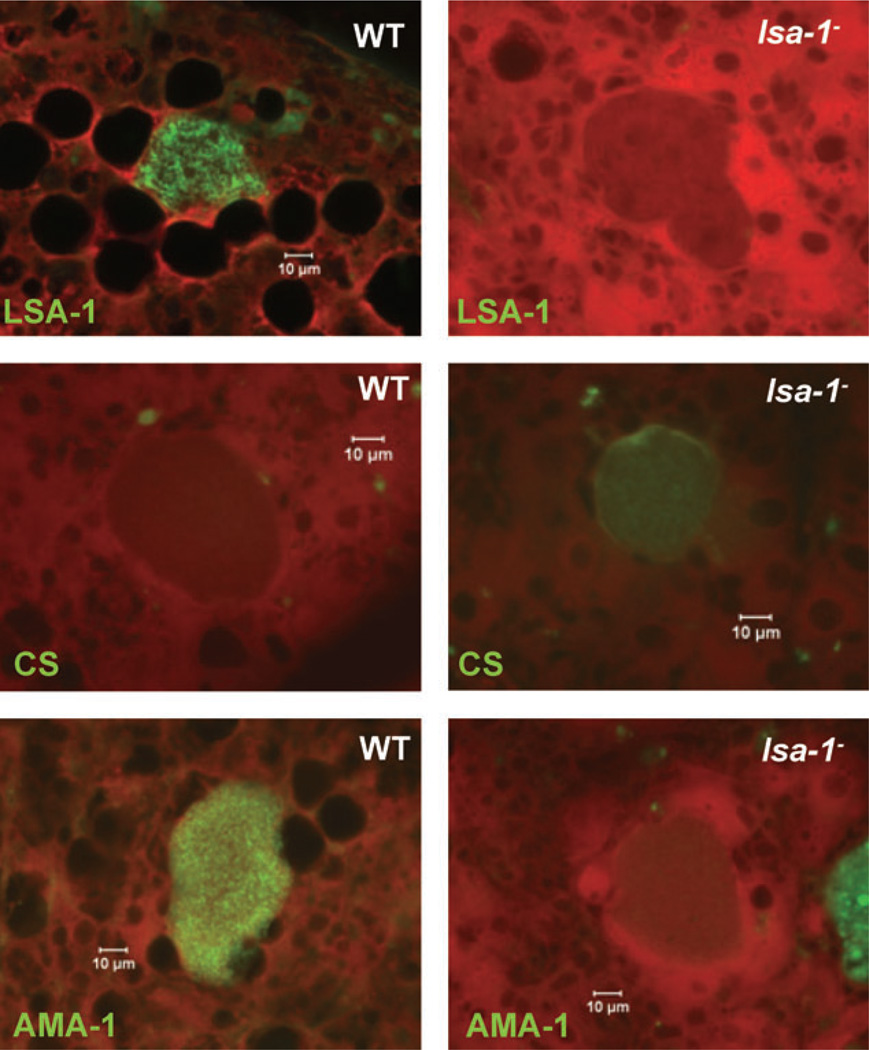
Fig. 5.
lsa-1− late liver stages show protein expression abnormalities. Immunofluorescent micrographs of chimeric SCID/Alb-uPA liver cryosections containing 7-day-old WT or lsa-1− liver stages were stained with parasite-specific antibodies. The micrographs in the top row show that LSA-1-specific antibodies do not bind to lsa-1− liver stages, confirming successful gene deletion. lsa-1− liver stages show abnormal expression of CS protein (middle row micrographs) and AMA-1 protein (bottom row micrographs) when compared with WT liver stages. Scale bar – 10 µm.
Surprisingly, the CS protein, a sporozoite and early liver-stage-expressed protein (Nardin et al., 1982; Sacci et al., 2006), showed expression in 7-day-old lsa-1− liver stages but not WT. This was an unexpected result because late liver stages do not normally express CS protein (Fig. 5). Furthermore, apical membrane antigen 1 (AMA-1)-specific staining was dramatically reduced in lsa-1− liver stages on day seven when compared with WT parasites (Fig. 5). The intensity of AMA-1 staining was further assessed using ImageJ on non-serial tissue sections confirming the difference in AMA-1 expression between 7-day-old lsa-1− and WT liver stages (lsa-1−: 32 ± 7, NF-54: 115 ± 14).
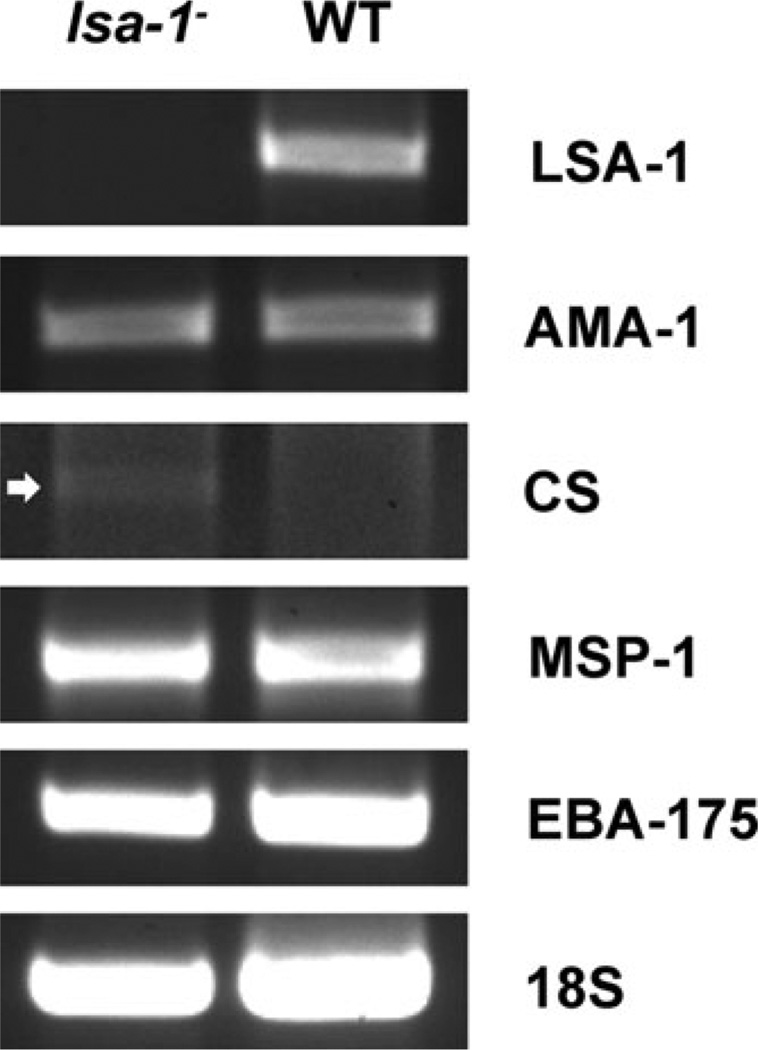
To determine if the aberrant expression of proteins was due to abnormal gene transcription, we next assayed for the presence of select messenger RNAs in the mouse livers infected with lsa-1− or WT parasites at day seven post infection. Reverse transcriptase PCR (RT-PCR) detected mRNA for AMA-1 in lsa-1− and WT liver stages indicating that the locus is transcribed. As expected, we also detected mRNA for MSP-1 and EBA-175 (Fig. 6). Interestingly, we did observe a difference in the transcription of the CS gene: day seven lsa-1− liver stages were transcribing the CS gene but WT liver stages were not.
Fig. 6.
RT-PCR analysis of WT and lsa-1− liver stages at day seven post sporozoite infection. Total RNA was isolated from liver fragments of SCID Alb-uPA hu Hep mice that were infected with lsa-1− or WT NF54 sporozoites 7 days prior to harvest. Total RNA was used to produce cDNA. Gene-specific primers were used for PCR amplification of cDNA normalized for 18S ribosomal RNA expression; white arrow points to a weak CS protein gene amplicon in the lsa-1− parasite-infected liver.
Discussion
LSA-1 remains the only known protein exclusively expressed in liver stages of the P. falciparum life cycle and yet, its function remains unknown. Due to the fact that there is no clear LSA-1 orthologue in other Plasmodium species infecting rodents or monkeys, as well as the technical challenges surrounding propagation of P. falciparum liver stages, elucidating the importance of this protein has been difficult.
To gain insight into the role of LSA-1 during the parasite life cycle, we targeted the LSA-1 locus in P. falciparum for deletion via homologous recombination. The successful recombination resulted in a parasite population that did not express LSA-1 protein. lsa-1− parasites showed normal development throughout most of their life cycle including robust mosquito salivary gland infection, normal sporozoite gliding motility and infection of hepatocytes. This was an anticipated finding as it has been shown that LSA-1 is only expressed in liver stages starting at day three of development (Fidock et al., 1994). The liver stage-specific expression pattern of LSA-1 thus suggests a function restricted to this life cycle stage. Despite the absence of LSA-1 protein expression in lsa-1− parasites, liver stages developed normally throughout days in vitro and in vivo and lsa-1− liver stages were still observed at day seven of development in the SCID Alb-uPA hu Hep mouse model. However, it was in the final phase of liver-stage development at day seven when a developmental defect phenotype became apparent. The lsa-1− liver stages exhibited extensive growth, nuclear proliferation and cellular differentiation typically seen in late liver stages, but they did not differentiate into exo-erythrocytic merozoites. This is in striking contrast to WT NF54 parasites, where approximately 50% of the liver stages examined exhibited merozoite formation at day seven of growth in the SCID Alb-uPA hu Hep mouse model. This deficiency in exo-erythrocytic merozoite formation is a never before seen phenotype of a P. falciparum knockout parasite. The only other previously studied P. falciparum gene knockout liver-stage phenotype was observed early in development. Parasites that are p52− or p52−/p36− show a severe defect in early hepatocyte infection and do not grow beyond the trophozoite stage (van Schaijk et al., 2008; VanBuskirk et al., 2009).
Interestingly, a late liver-stage phenotype was recently observed in Plasmodium yoelii that lacked type II fatty acid synthesis (FAS II) (Vaughan et al., 2009; Pei et al., 2010). The deletion of the genes encoding FAS II enzymes, FabB/F, FabZ and the pyruvate dehydrogenase (PDH) subunits PDH e1a and PDH e3 allowed for substantial liver-stage development but blocked the ability of knockout parasites to form exo-erythrocytic merozoites and initiate blood-stage infection in mice. It is not likely however that LSA-1 has a role in the biosynthesis of parasite fatty acids. Some information on LSA-1’s spatial and temporal distribution during liver schizogony is available. LSA-1 localizes to the PV lumen starting on day three of liver-stage development and accumulates as flocculent material infiltrating the membrane invaginations (cytomeres) that precede exo-erythrocytic merozoite formation (Hollingdale et al., 1990; Fidock et al., 1994; Nicoll et al., 2011). In the final stages of schizogony, LSA-1 forms a matrix that embeds maturing exo-erythrocytic merozoites and thus it function is likely associated with merozoite morphogenesis.
Although late lsa-1− liver stages showed normal expression of a number of proteins assayed including MSP-1, expression of two other proteins, CS protein and AMA-1, was found to be abnormal. CS protein was still detectable in lsa-1− liver stages at 7 days post infection. This differs from expression typically observed for WT NF54 parasites for which CS protein expression at the same time point is lacking as shown here and previously observed (Sacci et al., 2006). CS protein is a major protein expressed during the sporozoite stages with a number of functions including sporozoite morphogenesis and hepatocyte infection (Aly et al., 2009). The expression of CS protein and lack of expression of proteins associated with blood-stage parasites (e.g. MSP-1 and EBA-175) is indicative of an early phase of liver-stage development (Sacci et al., 2006). Thus, expression of CS protein concurrently with MSP-1 and EBA-175 seen in lsa-1− liver stages implies a developmental dysregulation of gene expression, which could interfere with normal development. Conversely, AMA-1 expression was dramatically reduced in lsa-1− late liver stages at 7 days post infection but robust expression was detected in WT parasites. Interestingly we detected the transcript for AMA-1 in the lsa-1− liver stages at 7 days post infection indicating that the lack of AMA-1 protein is likely due to a translational problem. AMA-1 is a secreted parasite ligand involved in host cell invasion and it is expressed in sporozoites and merozoites (Silvie et al., 2004). The expression of AMA-1 occurs in late liver schizogony and coincides with the formation of exo-erythrocytic merozoites (Silvie et al., 2004). Thus, the lack of merozoite formation and the lack of AMA-1 expression in late lsa-1−liver stages imply a stalling of the final differentiation and maturation during exo-erythrocytic schizogony. It is possible that the expression of other proteins is affected in late lsa-1− liver stages. A more comprehensive proteome and transcriptome analysis might reveal further abnormalities but such an analysis is currently hindered by the scarcity of liver-stage material obtained from humanized mouse infections.
It remains to be investigated if the observed phenotype for the late lsa-1− liver stages constitutes a developmental delay or a developmental block in merozoite maturation. To determine which of these fates the late lsa-1− liver-stage succumbs to will require growing lsa-1− liver stages in SCID Alb-uPA hu Hep mice beyond 7 days. Further work could also be carried out to reconstitute the mice with human red blood cells in order to examine the possibility that lsa-1− liver stages can form functional exo-erythrocytic merozoites (Tsuji et al., 1995). Another experimental avenue is the analysis of lsa-1− liver stages in non-human primates that are susceptible to P. falciparum sporozoite infection (Collins et al., 2006). Thus, if the lsa-1− liver stages can differentiate into exo-erythrocytic merozoites, these merozoites should be capable of emerging from hepatocytes and infecting red blood cells to establish a blood-stage infection.
It has been shown that LSA-1 is highly conserved in P. falciparum strains (Fidock et al., 1994), which suggests an important function of LSA-1 during liver-stage development and our data now demonstrates such a role. Based on strain comparisons between LSA-1 sequence conservation, expression profile (Fidock et al., 1994) and its association with protection against malaria in endemic areas (Hill et al., 1992), LSA-1 was considered an attractive pre-erythrocytic vaccine candidate. However, a recent clinical trial that tested a recombinant LSA-1 protein vaccine in human volunteers did not detect any protection against sporozoite challenge (Cummings et al., 2009). However, protection against malaria infection engendered by an immune response against an intrahepatocytic parasite antigen will likely be mediated by CD8 T cells, which are not effectively elicited by recombinant protein vaccines (Bruder et al., 2010; Liu, 2010). Virally vectored vaccination could be a better choice for immunization studies with an LSA-1 subunit vaccine and the data presented here give new impetus to test virally vectored LSA-1 in future clinical studies.
Experimental procedures
Design and production of gene targeting constructs
Targeting sequences for P. falciparum LSA-1 were cloned into plasmid pCC1 (Maier et al., 2008) to facilitate positive–negative selection (Maier et al., 2006). Restriction sites Sacll/Spel for the 5′ flank and Avrll/Sfol for the 3′ flank were used. Sequencing was performed to confirm and test specificity of the inserts. Primers used for amplification of the flanks were as follows:
5′ flank: 5′-ATCCGCGGATGAAACATATTTTGTACATATC-3′ and 5′-ATTGATCACTAGATCTTCTTGTCTGTTT-3′
3′ flank: 5′-ATCCTAGGCAGCTATAGAACTTCCATCA-3′ and 5′-ATGGCGCCGATAATTCTTCTGATGATT-3′
Transfection of P. falciparum with targeting constructs
Plasmid DNA was extracted with the Maxi Prep Kit (QIAGEN). Two days prior to transfection, NF54 P. falciparum parasites (Walter Reed Army Institute for Research) were synchronized at ring stage with sorbitol. Transfection of P. falciparum ring stages with 100 mg of DNA was performed by electroporation at 0.31 kV and 950 µF with a Bio-Rad Gene Pulser (Bio-Rad, La Jolla, CA). Cultures were placed on the positive selection drug (WR99210; Jacobus Pharmaceuticals, Princeton, NJ) 6 h post transfection and maintained as described (Trager and Jenson, 1978; Crabb et al., 2004). Negative selection utilized the cytosine deaminase/uracil phosphoribosyl transferase gene product with the substrate 5-fluorocytosine and was performed in order to obtain a parental line with double-cross-over homologous recombination.
PCR analysis and Southern blot
To ensure the appropriate homologous recombination of the pCC-1 plasmid containing the 5′ and 3′ LSA-1-flanking regions spaced by the hdhfr gene with the endogenous LSA-1 locus, a standard genotyping PCR was performed using the following primer pairs:
Test 1 – 5′-GAGAGACTTGCTAAAGAAAAGTT-3′ and 5′-AAACCAATAGATAAAATTTGTAGAG-3′
Test 2 – 5′-GGATGAGTTATCTGAAGATATAAC-3′ and 5′-CCTAATCATGTAAATCTTAA-3′
LSA-1 WT test – 5′-ATGAAACATATTTTGTACATATC-3′ and 5′-AAGTCTCTCGTTCGTTCTAA-3′
Genomic DNA from WT NF54 and lsa-1− blood-stage parasites was digested for 16 h with the following enzymes: 5′ test; HindIII/Scal and 3′ test; EcoRI. Digested DNA was run on a 1% TAE agarose gel at 15 V for 18 h and transferred to Hybond-N membrane (Amersham) overnight at room temperature, UV cross-linked and pre-hybridized with herring sperm DNA for 2.5 h. A digoxygenin-labelled probe was prepared by PCR per supplier protocol (Roche) using the same primers used to create the 5′ and 3′ knockout flanks. Hybridization was carried out for 18 h at 55°C. The blot was exposed to film for 10–60 min and developed per standard protocol.
Sporozoite production
Gametocyte cultures of WT and lsa-1− parasites were cultured in vitro using pooled human A+ sera (Interstate Blood Bank, Memphis, TN), RPMI-Hepes (Life Technologies/Gibco), hypoxanthine (Sigma) and washed, type O+ erythrocytes. Medium was changed daily and exflagellation was observed at room temperature by phase-contrast microscopy at 400× magnification beginning 12–13 days after the cultures were initiated. Parasites from the cultures were fed to the mosquitoes when the majority of the gametocytes were morphologically mature and vigorous exflagellation was observed. Anopheles stephensi mosquitoes aged 4–7 days were pre-starved for 2–4 h and then fed for a minimum of 30 min on a 37°C culture using a membrane feeder apparatus with bandruche membrane (Joseph Long, Belleville, NJ). One cage of 250–300 mosquitoes was exposed to concentrated erythrocytes from a 30 ml gametocyte culture mixed with an equal volume of fresh erythrocytes and two volumes of serum. Mosquitoes were incubated at 27°C, 80% humidity and sporozoites were harvested at 16–22 days post infection.
Sporozoite counts and motility assays
Twenty thousand sporozoites were seeded per well on 12-well glass slides previously coated with 3% BSA in RPMI-1640. The slides were incubated at 37°C for 1 h. The sporozoites were then fixed for 10 min with 4% paraformaldehyde at room temperature, and washed with 1% FBS in 1× PBS. Slides were blocked with 10% FBS/PBS overnight at 4°C. Sporozoite trails were visualized by incubation with an anti-P. falciparum CS protein monoclonal antibody for 45 min at 37°C and washed with 1% FBS/PBS. Slides were incubated with (1:200) anti-mouse IgG Alexa Fluor 488 (Molecular Probes) for 45 min at 37°C and washed with 1% FBS/PBS. Slides were mounted using Vectashield mounting medium (Vector Labs, Burlingame, CA) and evaluated at 400× magnification by epifluorescence microscopy (Olympus BX 50 microscope). Quantification was performed by direct microscopic counting of triplicate wells.
In vitro invasion and development assays
To assess the invasion capability of lsa-1− parasites, 25 000 sporozoites were added per well to a HC-04 cell monolayer on eight-well glass Labtek chamber slides and incubated for 3 h at 37°C and 5% CO2. To visualize invasion, slides were washed with PBS and fixed with cold methanol. After 30 min of incubation with anti-CS protein mAb at room temperature, slides were washed again with PBS and blocked with 0.1% BSA in PBS followed by the addition of 1:200 HRP goat anti-mouse IgG (Kirkegaard and Perry, Gaithersburg, MD) as secondary antibody for 30 min at room temperature. Slides were mounted using Permount (Fisher Scientific). Intracellular sporozoites were counted using microscopy at 400× magnification in triplicate wells (Hollingdale et al., 1983). Due to traversal activity of sporozoites, counting of some invading sporozoites may be omitted using this method. Development assays were performed by adding 60 000 sporozoites per well to HC-04 cells monolayers in eight-well Permanox Labtek chamber slides. Excess sporozoites were removed and cells washed after a 3 h incubation at 37°C and 5% CO2. Cultures were maintained with daily medium changes for 72, 96 and 144 h. Chamber slides were methanol fixed and stained using a mAb against HSP70 (mAb 4C9) (Tsuji et al., 1994), EXP-1 and EBA175 as primary antibody and Alexa Fluor 488 anti-mouse IgG (Molecular Probes) as the secondary antibody diluted in 0.1% Evans blue/PBS in a similar manner as described above. Slides were mounted using Vectashield plus DAPI (Vector Labs, Burlingame, CA). The total number of liver stages per well was counted in triplicate wells in three independent experiments. Parasites were observed by epi-fluorescence microscopy at 400× magnification using an Olympus BX 50 epifluorescent microscope. Photographs were taken using a Bio-Rad Radiance 2100 Confocal microscope.
In vivo assessment of infection in the SCID Alb-uPA hu Hep mouse
Five- to 14-day-old SCID mice homozygous for the urokinase type plasminogen activator transgene (SCID Alb-uPA) received an inoculation, by intrasplenic injection, of 106 human hepatocytes that had been isolated (with informed consent) from surgically resected liver specimens by collagenase digestion and Percoll gradient centrifugation (Mercer et al., 2001). After engraftment, chimeric mice received an intravenous tail vein injection of 1.6 × 106 P. falciparum WT or lsa-1− sporozoites and were then euthanized by CO2 overdose at 5 or 7 days post infection and their livers removed for cryosectioning or RNA extraction. Livers were rinsed in PBS and the lobes cut into separate pieces. Selected lobes were embedded in Tissue-Tek O.C.T. compound (Miles Scientific, Naperville, IL) and tissue sections (7 µm) were cut on a Leica CM1900 (Leica Microsystems, Deerfield, IL), fixed in absolute methanol and stored at −80°C until used. Diluted antisera (anti-CSP, HSP70, LSA-1, EBA-175, MSP-1, MSP-2, MSP-4, MSP-5, AMA-1) were then applied to the tissue section (in a volume sufficient to cover the tissue) and the slides were incubated for 30 min at 37°C in a humidity chamber. We use isotypic antibodies for control staining. Liver section slides were placed in a staining dish and washed three times for 5 min with PBS. A fluorescein-conjugated IgG (Kirkegaard and Perry, Gaithersburg, MD) was used as the secondary antibody. The secondary antibody was diluted 1:40 into PBS containing 0.02% Evan’s blue. The Evan’s blue was added to act as a counterstain and to suppress any autofluorescence in the tissue. The diluted secondary antibody was added and the slides placed in a humidity chamber, in the dark, and incubated at 37°C for 30 min. Tissue sections were then washed and the slides mounted, using Vectashield mounting medium (Vector Labs, Burlingame, CA). The stained slides were screened with a Nikon Eclipse E600 epifluorescent microscope and digital images collected with a SPOT digital camera (Diagnostic Instruments, Sterling Hgts, MI). The intensity of AMA-1 staining was assessed using ImageJ, an image analysis program originally developed at NIH. Schizonts to be analysed were initially defined and scored using a drawing tool and pixel intensity within the area. The overall intensity range was 0–255. The intensities in the table are the means and SD of at least three separate images analysed from tissue sections stained at the same time under identical conditions.
RT-PCR analysis
Total RNA was isolated from liver fragments of SCID Alb-uPA hu Hep mice which were infected with lsa-1− and WT P. falciparum sporozoites 7 days prior to harvest. Briefly, total RNA was extracted using Trizol reagent (Invitrogen). RNA was treated with DNase I (Invitrogen). Oligonucleotide primer sequences used in the RT-PCR analysis were:
AMA-1 forward primer (F) 5′-TACTGCGTATTATTATTGAGCG CCTTTG-3′, reverse primer (R) 5′-CCTTTACCAAATACTGGACA TTTCCCTG-3′; CS F 5′-ATGATGAGAAAATTAGCTATT-3′, R 5′-TCCATTACCTTGATTGTTTT-3′
MSP-1 F 5′-AGAAGAAATTACTACAAAAGGTGCAAGTGC-3′, R 5′-CCGAACACAAGTTTTTTAAGTACGTCTA-3′
EBA-175 F 5′-GGATTTTGGAGGTTATTCAACTAAGG-3′, R 5′-CGAGAGTAGTAGTATGTTTACAATCACA-3′
18S F 5′-AACCTGGTTGATCTTGCCAGTAGTCATATG-3′, R 5′-CCAAAAATTGGCCTTGCATTGTTAT-3′
LSA-1 F 5′-ATGAAACATATTTTGTACATATC-3′, R 5′-AAGTCT CTCGTTCGTTCTAA-3′
PCR conditions used: 94°C for 5 min then 94°C for 30 s, 55°C for 30 s; 60°C for 1 min (40 cycles); 60°C for 10 min.
Statistical analysis
Quantitative differences in salivary gland sporozoite numbers, gliding motility, hepatocyte invasion and liver-stage development between the WT and the lsa-1− parasite line were evaluated by the Mann–Whitney U-test at the 95% confidence level.
Supplementary Material
Acknowledgements
This work was supported by the Bill and Melinda Gates Foundation through the Foundation at the National Institutes of Health Grand Challenges in Global Health Initiative and the US Department of Defense.
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article:
Table S1. Sporozoite gliding assay (20 000 spz per well).
Table S2. Sporozoite invasion assay (25 000 spz per well).
Table S3. Liver-stage development assay (60 000 spz per well).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Aly AS, Vaughan AM, Kappe SH. Malaria parasite development in the mosquito and infection of the mammalian host. Annu Rev Microbiol. 2009;63:195–221. doi: 10.1146/annurev.micro.091208.073403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder JT, Angov E, Limbach KJ, Richie TL. Molecular vaccines for malaria. Hum Vaccin. 2010;6:54–77. doi: 10.4161/hv.6.1.10463. [DOI] [PubMed] [Google Scholar]
- Collins WE, Sullivan JS, Williams A, Nace D, Williams T, Galland GG, Barnwell JW. Aotus nancy-maae as a potential model for the testing of anti-sporozoite and liver stage vaccines against Plasmodium falciparum . Am J Trop Med Hyg. 2006;74:422–424. [PubMed] [Google Scholar]
- Crabb BS, Rug M, Gilberger TW, Thompson JK, Triglia T, Maier AG, Cowman AF. Transfection of the human malaria parasite Plasmodium falciparum . Methods Mol Biol. 2004;270:263–276. doi: 10.1385/1-59259-793-9:263. [DOI] [PubMed] [Google Scholar]
- Cummings JF, Spring MD, Schwenk RJ, Ockenhouse CF, Kester KE, Polhemus ME, et al. Recombinant Liver Stage Antigen-1 (LSA-1) formulated with AS01 or AS02 is safe, elicits high titer antibody and induces IFN-gamma/IL-2 CD4+ T cells but does not protect against experimental Plasmodium falciparum infection. Vaccine. 2009;28:5135–5144. doi: 10.1016/j.vaccine.2009.08.046. [DOI] [PubMed] [Google Scholar]
- Druilhe P, Puebla RM, Miltgen F, Perrin L, Gentilini M. Species- and stage-specific antigens in exo-erythrocytic stages of Plasmodium falciparum . Am J Trop Med Hyg. 1984;33:336–341. doi: 10.4269/ajtmh.1984.33.336. [DOI] [PubMed] [Google Scholar]
- Fidock DA, Gras-Masse H, Lepers JP, Brahimi K, Benmohamed L, Mellouk S, et al. Plasmodium falciparum liver stage antigen-1 is well conserved and contains potent B and T cell determinants. J Immunol. 1994;153:190–204. [PubMed] [Google Scholar]; Published erratum appears in. J Immunol. 1994;153:5347. [Google Scholar]
- Gruner AC, Brahimi K, Letourneur F, Renia L, Eling W, Snounou G, Druilhe P. Expression of the erythrocyte-binding antigen 175 in sporozoites and in liver stages of Plasmodium falciparum . J Infect Dis. 2001;184:892–897. doi: 10.1086/323394. [DOI] [PubMed] [Google Scholar]
- Guerin-Marchand C, Druilhe P, Galey B, Londono A, Patarapotikul J, Beaudoin RL, et al. A liver-stage-specific antigen of Plasmodium falciparum characterized by gene cloning. Nature. 1987;329:164–167. doi: 10.1038/329164a0. [DOI] [PubMed] [Google Scholar]
- Hill AV, Elvin J, Willis AC, Aidoo M, Allsopp CE, Gotch FM, et al. Molecular analysis of the association of HLA-B53 and resistance to severe malaria. Nature. 1992;360:434–439. doi: 10.1038/360434a0. [DOI] [PubMed] [Google Scholar]
- Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- Hollingdale MR, Leland P, Leef JL, Schwartz AL. Entry of Plasmodium berghei sporozoites into cultured cells, and their transformation into trophozoites. Am J Trop Med Hyg. 1983;32:685–690. doi: 10.4269/ajtmh.1983.32.685. [DOI] [PubMed] [Google Scholar]
- Hollingdale MR, Aikawa M, Atkinson CT, Ballou WR, Chen GX, Li J, et al. Non-CS pre-erythrocytic protective antigens. Immunol Lett. 1990;25:71–76. doi: 10.1016/0165-2478(90)90094-7. [DOI] [PubMed] [Google Scholar]
- John CC, Ouma JH, Sumba PO, Hollingdale MR, Kazura JW, King CL. Lymphocyte proliferation and antibody responses to Plasmodium falciparum liver-stage antigen-1 in a highland area of Kenya with seasonal variation in malaria transmission. Am J Trop Med Hyg. 2002;66:372–378. doi: 10.4269/ajtmh.2002.66.372. [DOI] [PubMed] [Google Scholar]
- John CC, Moormann AM, Sumba PO, Ofulla AV, Pregibon DC, Kazura JW. Gamma interferon responses to Plasmodium falciparum liver-stage antigen 1 and thrombospondin-related adhesive protein and their relationship to age, transmission intensity, and protection against malaria. Infect Immun. 2004;72:5135–5142. doi: 10.1128/IAI.72.9.5135-5142.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CC, Tande AJ, Moormann AM, Sumba PO, Lanar DE, Min XM, Kazura JW. Antibodies to pre-erythrocytic Plasmodium falciparum antigens and risk of clinical malaria in Kenyan children. J Infect Dis. 2008;197:519–526. doi: 10.1086/526787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij TW, Carlton JM, Bidwell SL, Hall N, Ramesar J, Janse CJ, Waters AP. A Plasmodium whole-genome synteny map: indels and synteny breakpoints as foci for species-specific genes. PLoS Pathog. 2005;1:e44. doi: 10.1371/journal.ppat.0010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MA. Immunologic basis of vaccine vectors. Immunity. 2010;33:504–515. doi: 10.1016/j.immuni.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Maier AG, Braks JA, Waters AP, Cowman AF. Negative selection using yeast cytosine deaminase/uracil phosphoribosyl transferase in Plasmodium falciparum for targeted gene deletion by double crossover recombination. Mol Biochem Parasitol. 2006;150:118–121. doi: 10.1016/j.molbiopara.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Maier AG, Rug M, O’Neill MT, Brown M, Chakravorty S, Szestak T, et al. Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell. 2008;134:48–61. doi: 10.1016/j.cell.2008.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, et al. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–933. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- Nardin EH, Nussenzweig V, Nussenzweig RS, Collins WE, Harinasuta KT, Tapchaisri P, Chomcharn Y. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax . J Exp Med. 1982;156:20–30. doi: 10.1084/jem.156.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll WS, Sacci JB, Rodolfo C, Di Giacomo G, Piacentini M, Holland ZJM, et al. Plasmodium falciparum liver stage antigen-1 is cross-linked by tissue transglutaminase. Malaria J. 2011;10:14. doi: 10.1186/1475-2875-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Tarun AS, Vaughan AM, Herman RW, Soliman JM, Erickson-Wayman A, Kappe SH. Plasmodium pyruvate dehydrogenase activity is only essential for the parasite’s progression from liver infection to blood infection. Mol Microbiol. 2010;75:957–971. doi: 10.1111/j.1365-2958.2009.07034.x. [DOI] [PubMed] [Google Scholar]
- Prudencio M, Rodriguez A, Mota MM. The silent path to thousands of merozoites: the Plasmodium liver stage. Nat Rev Microbiol. 2006;4:849–856. doi: 10.1038/nrmicro1529. [DOI] [PubMed] [Google Scholar]
- Sacci JB, Jr, Alam U, Douglas D, Lewis J, Tyrrell DL, Azad AF, Kneteman NM. Plasmodium falciparum infection and exoerythrocytic development in mice with chimeric human livers. Int J Parasitol. 2006;36:353–360. doi: 10.1016/j.ijpara.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Sattabongkot J, Yimamnuaychoke N, Leelaudomlipi S, Rasameesoraj M, Jenwithisuk R, Coleman RE, et al. Establishment of a human hepatocyte line that supports in vitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and P. vivax . Am J Trop Med Hyg. 2006;74:708–715. [PubMed] [Google Scholar]
- van Schaijk BC, Janse CJ, van Gemert GJ, van Dijk MR, Gego A, Franetich JF, et al. Gene disruption of Plasmodium falciparum p52 results in attenuation of malaria liver stage development in cultured primary human hepatocytes. PLoS ONE. 2008;3:e3549. doi: 10.1371/journal.pone.0003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvie O, Franetich JF, Charrin S, Mueller MS, Siau A, Bodescot M, et al. A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. J Biol Chem. 2004;279:9490–9496. doi: 10.1074/jbc.M311331200. [DOI] [PubMed] [Google Scholar]
- Trager W, Jenson JB. Cultivation of malarial parasites. Nature. 1978;273:621–622. doi: 10.1038/273621a0. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Mattei D, Nussenzweig RS, Eichinger D, Zavala F. Demonstration of heat-shock protein 70 in the sporozoite stage of malaria parasites. Parasitol Res. 1994;80:16–21. doi: 10.1007/BF00932618. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Ishihara C, Arai S, Hiratai R, Azuma I. Establishment of a SCID mouse model having circulating human red blood cells and a possible growth of Plasmodium falciparum in the mouse. Vaccine. 1995;13:1389–1392. doi: 10.1016/0264-410x(95)00081-b. [DOI] [PubMed] [Google Scholar]
- VanBuskirk KM, O’Neill MT, De La Vega P, Maier AG, Krzych U, Williams J, et al. Preerythrocytic, live-attenuated Plasmodium falciparum vaccine candidates by design. Proc Natl Acad Sci USA. 2009;106:13004–13009. doi: 10.1073/pnas.0906387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AM, Aly AS, Kappe SH. Malaria parasite pre-erythrocytic stage infection: gliding and hiding. Cell Host Microbe. 2008;4:209–218. doi: 10.1016/j.chom.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AM, O’Neill MT, Tarun AS, Camargo N, Phuong TM, Aly AS, et al. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell Microbiol. 2009;11:506–520. doi: 10.1111/j.1462-5822.2008.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AM, Wang R, Kappe SH. Genetically engineered, attenuated whole-cell vaccine approaches for malaria. Hum Vaccin. 2010;6:107–113. doi: 10.4161/hv.6.1.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Shi YP, Udhayakumar V, Alpers MP, Povoa MM, Hawley WA, et al. Sequence variations in the non-repetitive regions of the liver stage-specific antigen-1 (LSA-1) of Plasmodium falciparum from field isolates. Mol Biochem Parasitol. 1995;71:291–294. doi: 10.1016/0166-6851(95)00069-d. [DOI] [PubMed] [Google Scholar]
- Zhu J, Hollingdale MR. Structure of Plasmodium falciparum liver stage antigen-1. Mol Biochem Parasitol. 1991;48:223–226. doi: 10.1016/0166-6851(91)90117-o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.