Summary
Activation of the phosphoinositide 3-kinase (PI3K) pathway occurs frequently in breast cancer. However, clinical results of single-agent PI3K inhibitors have been modest to date. A combinatorial drug screen on multiple PIK3CA mutant cancers with decreased sensitivity to PI3K inhibitors revealed that combined CDK 4/6/ PI3K inhibition synergistically reduces cell viability. Laboratory studies revealed that sensitive cancers suppress RB phosphorylation upon treatment with single-agent PI3K inhibitors, but cancers with reduced sensitivity fail to do so. Similarly, patients' tumors that responded to the PI3K inhibitor BYL719 demonstrated suppression of pRB, while nonresponding tumors showed sustained or increased levels of pRB. Importantly, the combination of PI3K and CDK 4/6 inhibitors overcomes intrinsic and adaptive resistance leading to tumor regressions in PIK3CA mutant xenografts.
Introduction
The phosphatidylinositol 3-kinase (PI3K) pathway is a key regulator of growth, survival, and metabolism in both normal and malignant cells (Engelman et al., 2006; Hanker et al., 2013; Katso et al., 2001; Miller et al., 2010; Wang et al., 2012; Yuan and Cantley, 2008; Zhao and Vogt, 2008). Over 70% of breast cancers have activation of the PI3K pathway through mechanisms such as HER2 amplification, deletion of the tumor suppressor PTEN, or oncogenic mutations in PIK3CA (Garcia-Martinez and Alessi, 2008). Inhibition of PI3K therefore represents a potentially attractive strategy for treatment of breast cancer, and a number of agents have entered clinical trials (Bachman et al., 2004; Bendell et al., 2012; Mahadevan et al., 2012; NCT00876109; NCT00620594; NCT01219699).
Laboratory studies and these early clinical trials indicate that several of the PI3K inhibitors (PI3Ki) demonstrate preferential inhibition of tumors with PIK3CA mutations (Bendell et al., 2012; O'Brien et al., 2010). However, while long term stabilization and partial tumor responses have been observed in PIK3CA breast cancers treated with PI3Ki (NCT01219699), the majority of PIK3CA mutant cancers still do not experience substantial regressions.
We recently identified a strategy to overcome both de novo and adaptive resistance to PI3Ki through combined inhibition of PI3K and mTORC (Elkabets et al., 2013). Thus far, dual PI3K and mTOR inhibitors such as BEZ235 and GDC-0980 have made their way into clinical trials (Markman et al., 2012), though the therapeutic window for these agents is limited due to treatment related toxicities. Our present study sought to identify additional strategies that may increase the efficacy of PI3Ki, by both improving initial responses and overcoming adaptive resistance.
Results
PI3Ki resistant PIK3CA mutant breast cancer cell lines fail to undergo growth arrest and maintain higher levels of pS6
Despite oncogenic activation of the PI3K pathway, PI3Ki are not as effective as single agents as was initially hoped (Maira, 2011, NCT01219699). In order to determine ways to improve response to PI3K inhibition, we studied three PIK3CA mutant breast cancer cell line models that had adapted to PI3Ki after chronic exposure to the drug. Two of the cell lines, T47D and MDA-MB-453 (453), were treated with the p110α-isoform specific inhibitor BYL719 whereas the third cell line, MCF7, was treated with the pan-isoform inhibitor GDC-0941.
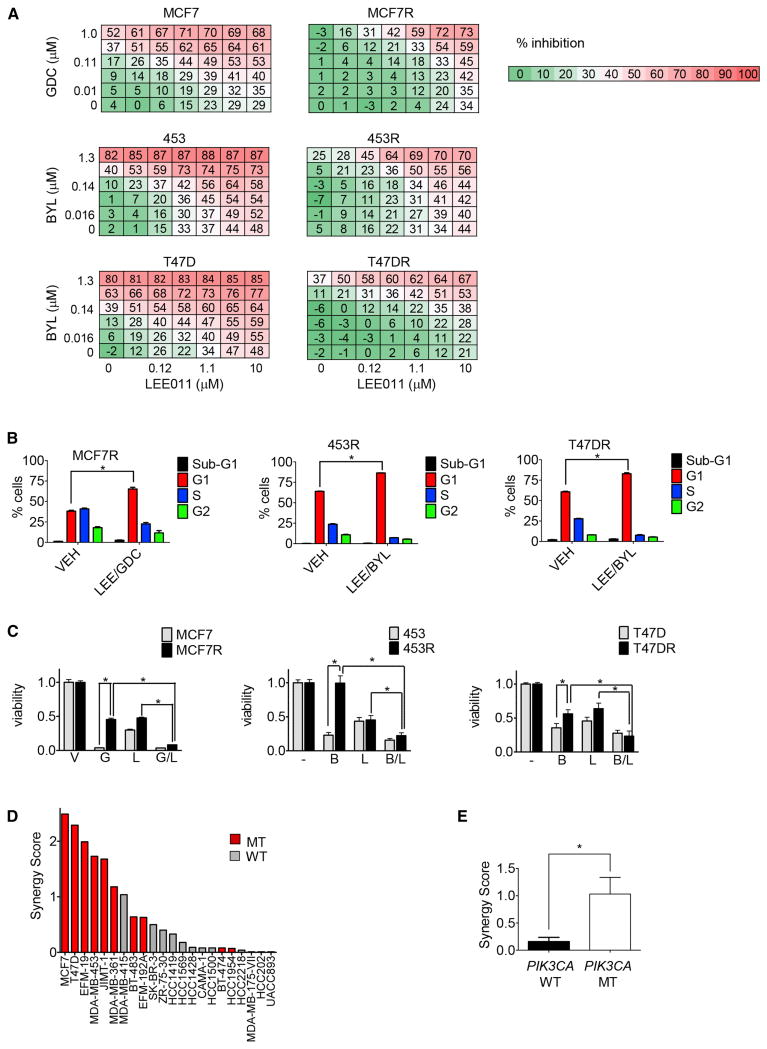
The chronically exposed cells were more resistant to PI3Ki than the treatment naïve (i.e., parental) cells. They demonstrated increased viability in the presence of 1 μM of PI3Ki (BYL719 or GDC-0941, as indicated, Figure 1A) and a rightward shift in the dose response curve (Figure 1B). Consistent with this finding, the chronically exposed cells exhibited less cell cycle arrest in response to the indicated PI3Ki, with significantly more cells remaining in S phase relative to parental cells (Figure 1C). Of note, PI3Ki fail to induce substantial apoptosis in the parental and resistant cells (Figure S1A). Both parental and resistant cell lines exhibited suppression of Akt phosphorylation upon treatment with PI3Ki. However, phosphorylation of S6 was maintained to a greater extent in resistant cells, as we recently reported (Elkabets et al., 2013) (Figure 1D). These results suggest that sensitivity to PI3Ki in these PIK3CA models may be dependent on the ability to suppress mTOR signaling and modulate cell cycle progression.
Figure 1. Viability, cell cycle profiles, and signaling in PIK3CA mutant breast cancer cell lines with acquired resistance to PI3Ki.
A) Parental and resistant MCF7 were treated with either vehicle or GDC-0941 1 μM. Parental and resistant 453 and T47D cell lines were treated with either vehicle or BYL719 1 μM. When vehicle treated cells grew to confluence, all cells were fixed and stained for nucleic acid with Syto-60 and absorbance was quantified. Representative plates are shown. Data are mean of three independent experiments performed in triplicate. *indicates p<0.05 by student's t test.
B) Cells were treated with escalating doses of either BYL719 or GDC-0941 as indicated for 72 hours. Viability was assessed using Cell-Titer Glo as described by the manufacturer. Data represent mean of 6 replicates.
C) Cell lines were treated as indicated for 24 hours after which cell cycle analysis was performed using propidium iodide staining followed by flow cytometry. The percentage decrease in S phase relative to vehicle controls is depicted. Data are mean of three independent experiments performed in triplicate. *indicates p<0.05 by student's t test.
D) MCF7, 453, and T47D cells with their corresponding resistant lines were treated for 24 hours with vehicle or the specified agent and dose, lysed and probed with the indicated antibodies.
All error bars in this figure represent +/- SEM.
See also Figure S1 and Table S1.
These resistant cells also displayed cross-resistance to other PI3Ki. For example, the T47DR and 453R cell lines were less sensitive to GDC-0941 than the corresponding parental lines, and the MCF7R cell line was also less sensitive to BYL719 (Figure S1B-C). The difference between parental and resistant lines was less pronounced to the dual PI3K/mTORC inhibitor BEZ235 (Figure S1D) as might be expected since maintenance of mTORC activity promotes resistance in these cells. In general, the parental and resistant lines were similarly sensitive to other targeted therapies and the chemotherapeutic agent paclitaxel (Figure S1E, Table S1), suggesting that resistance is specific to inhibitors of the PI3K pathway.
Combination drug screen identifies inhibitors of CDK 4/6, mTORC, and Akt as sensitizers to PI3Ki in resistant cell lines
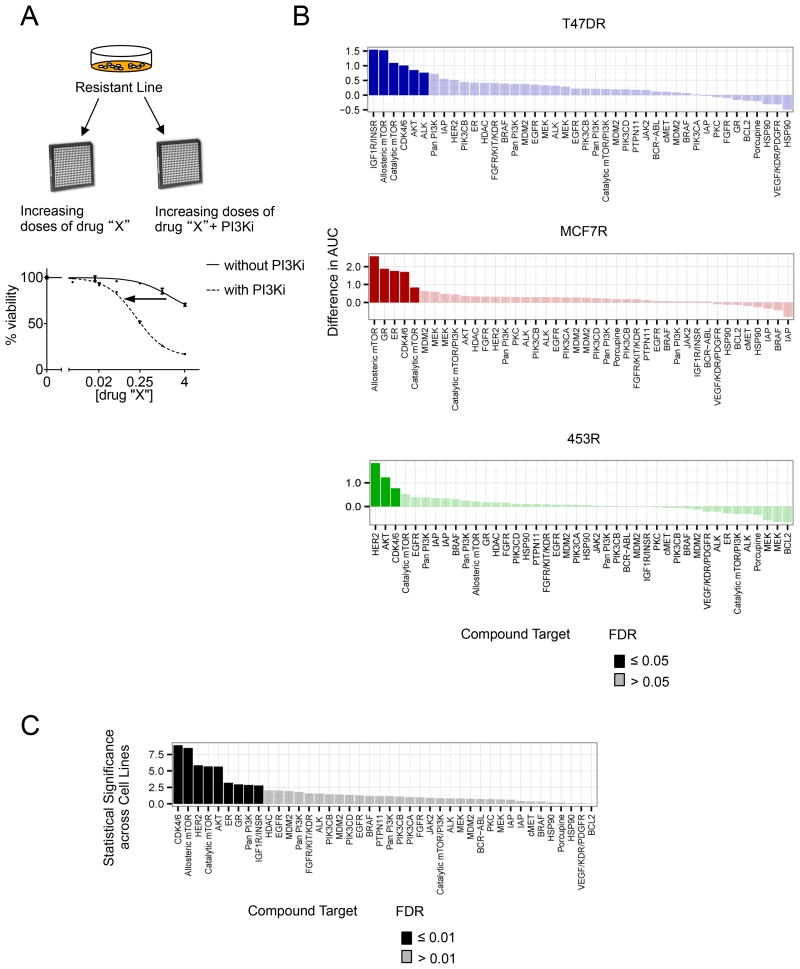
To identify potential therapeutic strategies to overcome resistance to PI3Ki, we performed a combinatorial drug screen using 42 agents that inhibit pathways involved in growth, metabolism, and apoptosis (Figure 2A). Cell proliferation was assessed after treatment with increasing doses of candidate drugs in the presence and absence of PI3Ki, and agents were ranked according to the greatest shift in dose response that occurred with the addition of PI3Ki. Compounds with false discovery rate (FDR) values ≤ 0.05 were considered sensitizers for each individual cell line (Figure 2B, Figure S2A).
Figure 2. Combinatorial Drug Screen to identify sensitizers to PI3K inhibition in resistant PIK3CA mutant cell lines.
A) An outline of the drug screening protocol. Resistant cells were seeded in 96 well plates and treated in triplicate with escalating doses of each of the 42 compounds in the drug screen, either singly or in combination with 1 μM dose of PI3Ki.
B) For each of the resistant cell lines, dose response curves to the targeted agents were generated, in the absence or presence of 1 μM of BYL719 for T47DR and 453R or GDC-0941 for MCF7R. The agents were ranked according to greatest difference in area under the curve (AUC) between these two dose response curves for each cell line. Targets of each agent, ranked by difference in AUC, are depicted for each cell line. Darkened values indicate statistically significant sensitizers (FDR<0.05).
C) Data from all three resistant cell lines were pooled and ranked by statistical significance (-log (p-value)). Darkened values indicate statistically significant sensitizers (FDR<0.01).
See also Figure S2 and Table S2.
In a composite analysis of all cell lines, LEE011 emerged as the strongest sensitizer across all three resistant models (Figure 2C). LEE011 is a highly specific inhibitor of CDK 4/6 (Figure S2B-C). Additionally, the allosteric mTORC inhibitor Rad001, catalytic mTORC inhibitor AZD8055, and the Akt inhibitor MK2206 were sensitizers in two resistant lines. Five other agents sensitized just one of the three resistant lines (Table S2). These findings suggest that targeting downstream nodes in the PI3K signaling pathway may sensitize PI3Ki resistant cancers.
Akt substrate phosphorylation and Phosphatidylinositol (3,4,5)-triphosphate (PIP3) levels correlate with sensitization by Akt inhibitor
The finding that Akt inhibitors overcame resistance to PI3Ki in two of the PI3Ki resistant lines was initially surprising because Akt phosphorylation appeared to be suppressed by PI3Ki in all of the resistant lines (Figure 1D). To determine if certain resistant cells might retain a low level of PI3K pathway activity in the presence of PI3Ki, we examined levels of phosphorylated Akt (pAkt) and Akt substrates upon treatment with the PI3Ki to which each cell line was made resistant. Because both 453R and T47DR cells were re-sensitized to PI3Ki by an Akt inhibitor whereas MCF7R cells were not, we compared 453R and T47DR to MCF7R cells. Upon treatment with the respective PI3Ki, we observed that phosphorylation of the Akt substrates, ATP citrate lyase and PRAS40 (pATPCL and pPRAS, respectively), were higher in 453R and T47DR, than in MCF7R cells (Figure 3A). Furthermore, PIP3, which accumulates upon phosphorylation of phosphatidylinositol 4,5-bisphosphate (PIP2) by PI3K and therefore serves as a more direct measure of PI3K activity, was higher in 453R and T47DR cells relative to their parental counterparts, but not in MCF7R cells relative to MCF7 (Figure 3B). It appears therefore that residual PI3K activity may contribute to resistance to PI3Ki in 453R and T47DR cells, but not in MCF7R cells. These results reinforce the notion that there is low-level persistence of Akt signaling in some of the resistant lines that is not appreciated simply by measuring pAkt.
Figure 3. The correlation between post-treatment PIP3 levels and the capacity of Akt inhibition to re-sensitize resistant cells to PI3Ki.
A) Each resistant line was treated with escalating doses of the agent to which it was made resistant for 24 hours (BYL719 for 453R and T47DR, and GDC-0941 for MCF7R). Lysates were prepared and were probed with the indicated antibodies.
B) Phospholipids in parental and resistant cells were isolated after 24 hr treatment with vehicle or the indicated PI3Ki (for parental lines) or continuous treatment with PI3Ki (for resistant lines. PIP3 and PIP2 levels were measured and the data shown as mean of three independent experiments. *indicates p<0.05 by student's t test.
C) Viability was assessed in parental and resistant 453 and T47D lines treated with escalating doses of MK2206 for 5 days. Resistant lines were also treated with escalating doses of MK2206 in the presence of 1 μM BYL719, and dose response curves were generated. Viability values for each curve were normalized to the measured inhibition value at zero dose of MK2206 (-/+ BYL719 1 μM for 453R and T47DR). Data represent mean of 3 replicates.
D) MCF7 and MCF7R cells were treated with escalating doses of MK2206. MCF7R was also treated with MK2206 in the presence of 1 μM dose of GDC-0941 or BYL719. Viability values for each curve were normalized to the measured inhibition value at zero dose of MK2206 (-/+ GDC-0941 1 μM for MCF7R). Data represent mean of 3 replicates.
E-F) Parental and resistant 453, T47D (E), and MCF7 (F) cells were treated for 24 hours with the specified agents. Lysates were made after 24 hours, and probed with the indicated antibodies.
All error bars in this figure represent +/- SEM.
See also Figure S3.
Consistent with this, and confirming the results of the drug screen, the Akt inhibitor MK2206 re-sensitized the 453R and T47DR cell lines to 1 μM BYL719 (Figure 3C). However, MK2206 did not re-sensitize the MCF7R cells to PI3Ki (either GDC-0941 or BYL719 1 μM) (Figure 3D). This is consistent with the finding that PI3Ki alone was sufficient to suppress PIP3, pAkt, and phosphorylation of Akt substrates in the MCF7R cells (Figure 3A-B). In the 453R and T47DR cells, treatment with BYL719 partially reduced Akt substrate and S6 phosphorylation levels, and addition of MK2206 enhanced suppression of these downstream nodes of the PI3K pathway (Figure 3E). The effect appeared to be cell line rather than inhibitor specific, as the 453R and T47DR lines were also sensitized by MK2206 to 1 μM GDC-0941 with similar effects on pAkt and its substrates' phosphorylation (Figure S3A-B). By contrast, in MCF7R, addition of MK2206 had no effect on proliferation (Figure 3D), phosphorylation of Akt substrates (which were largely suppressed by PI3Ki alone), or pS6 (Figure 3F), regardless of whether BYL719 or GDC-0941 was used as the PI3Ki. Thus incomplete suppression of PIP3 production and subsequent residual Akt signaling may contribute to resistance to PI3Ki in some contexts and may be overcome by combination of PI3K and Akt inhibition.
Of note, all three resistant cell lines displayed cross-resistance to single agent MK2206, both in regards to viability and signaling (Figure 3C-F). In the MCF7R cells, it appears that Akt does not regulate mTORC1 activity. In the 453R and T47DR cells, it is likely that elevated PIP3, pAkt and AKT signaling at baseline account for incomplete inhibition of this pathway with Akt inhibitors alone. In T47DR (which have elevated PIP3 levels), it is also possible that PIP3 effectors other than Akt contribute to sustained mTORC1 signaling. Indeed others have reported the importance of Akt independent effectors of PI3K in PIK3CA mutant breast cancers (Vasudevan et al., 2009).
We next evaluated the top sensitizer in the 453R line, lapatinib, and confirmed that it combined with PI3Ki to further suppress proliferation (Figure S3C), and phosphorylation of Akt substrates and S6 (Figure S3D). This was not surprising as 453 cells have both HER2 amplification and PIK3CA mutation, and both are contributing to the activation of PI3K signaling in these cells. As discussed above, the baseline absolute levels of pAkt and PIP3 are elevated in 453R (Figures 1D and 3B) relative to the parental cell line. Because of the higher basal levels of these signaling molecules, treatment with a given dose of BYL719 results in higher residual levels of pAkt and PIP3 in the resistant line compared to the parental line. However, suppression of HER2 phosphorylation with lapatinib combines with BYL719 to fully inhibit this pathway (Fig. S3D). These findings reinforce the notion that residual PI3K activity contributes to the relative resistance of certain cell lines, and that suppression of this remaining PI3K activity reverses resistance.
CDK 4/6 inhibition sensitizes cells with acquired and intrinsic resistance to PI3K inhibition
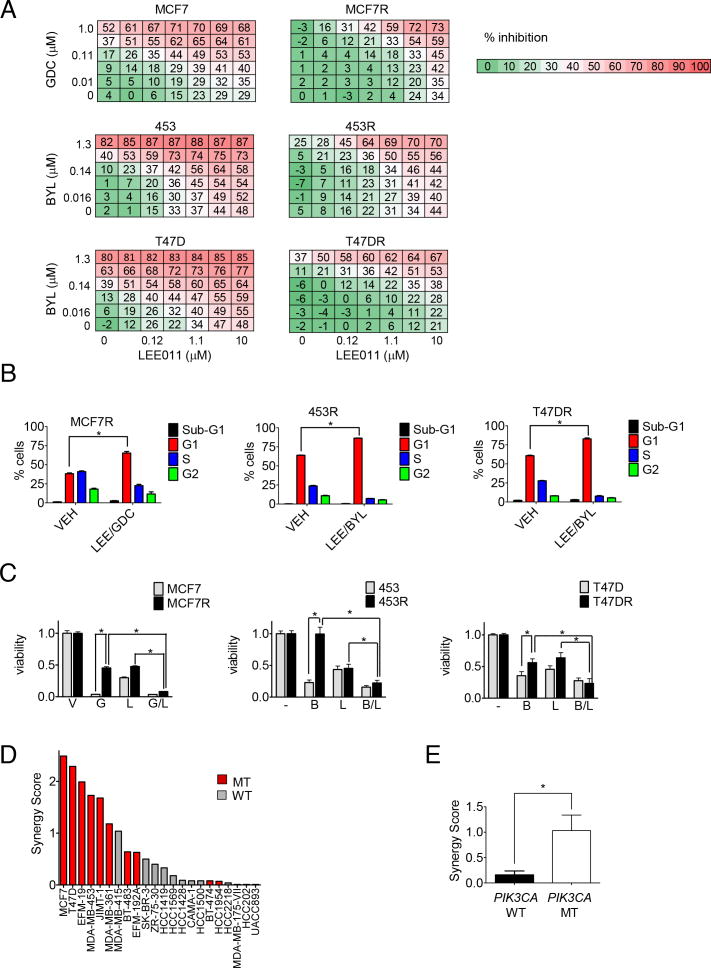
In the present study, the highest scoring drug in the composite analysis of the drug screen was LEE011, a highly specific CDK 4/6 inhibitor currently under clinical development (Figure S2B-C). To confirm that the combination of CDK 4/6 and PI3Ki exhibit synergistic activity against PIK3CA mutant breast cancer cell lines, we treated each of the parental and resistant cell lines with increasing concentrations of PI3Ki and LEE011 alone and in combination. As observed previously, PI3K inhibition alone was relatively ineffective at inhibiting proliferation of resistant cells (Figure 4A). However, for all three resistant cell lines, and, to a lesser extent, parental lines, we observed a synergistic interaction between LEE011 and PI3Ki in suppressing cell proliferation (Figure 4A, Figure S4A). The combination effect in the resistant lines appeared to rely on cell cycle arrest, rather than apoptosis, as an increase in the population of cells in G1, but not sub-G1 was observed (Figure 4B). Cell cycle arrest ultimately translated into decreased long-term cell viability (Figure 4C).
Figure 4. Combined CDK 4/6 and PI3K inhibition in the treatment of resistant PIK3CA breast cancer lines.
A) A dose matrix of PI3Ki, either GDC-0941 (GDC) or BYL719 (BYL) as indicated, and CDK 4/6 inhibitor LEE011 was created in all 3 pairs of parental and resistant cell lines. Viability was assessed after 5 days. Percent inhibition at each dose of drug is presented.
B) Cell lines were treated with vehicle (VEH) or GDC0941 (GDC) and LEE011 (LEE), in the case of MCF7R, or BYL719 (BYL) and LEE011, in the case of 453R and T47DR, at 1 μM doses for 24 hours. Cell cycle analysis was then performed using propidium iodide staining followed by flow cytometry and percentage of cells in subG1, G1, S, and G2 phases were quantified. Data are mean of three replicates. * indicates p<0.05 by student's t test.
C) Parental and resistant cell lines were treated with media containing vehicle (V) or the following drugs, as indicated at 1 μM doses: GDC-0941 (G), LEE011 (L), or BYL719 (B) or the indicated combinations. Fresh drug and media were applied every 72 hours until the vehicle control treated wells were confluent, at which point all the wells were fixed and stained with Syto-60. Absorbance was measured using an infrared imager and quantified. Data depicted are mean of three independent experiments performed in triplicate. *indicates p<0.05 by student's t test.
D) 12 PIK3CA mutant (MT) and 10 PIK3CA wild-type (WT) breast cancer cell lines were treated with BYL719 and LEE011 in dose matrices across 7 concentrations for each agent, and assessed for proliferation after 5 days. The response of each cell line to the combination was scored for synergy (Supplemental Experimental Procedures), and ranked by synergy score.
E) Median synergy scores between the PIK3CA mutants and wild-type lines were compared. * indicates p<0.05 by ANOVA.
All error bars in this figure represent +/- SEM.
See also Figure S4.
The chemically unrelated CDK 4/6 inhibitor PD-0332991 also sensitized the resistant lines to PI3K inhibition (Figure S4B-C), indicating that the observed effects of LEE011 are likely on target. Similar results were obtained using siRNA to CCND1 (which encodes for Cyclin D1) or CDK4 and CDK6 (Figure S4D).
The initial screen was performed in PIK3CA mutant breast cancer cells that had developed resistance after chronic exposure to PI3Ki. However, we also aimed to determine if this combination is effective in PIK3CA mutant cancers that were intrinsically resistant to single-agent PI3Ki. Importantly, adding LEE011 increased the efficacy of PI3Ki in five different PI3KCA mutant breast cancer cell lines with intrinsic resistance to PI3Ki (Figure S4E). In all but one line (MFM223), the effect of the combination was greater than the single-agents alone.
The BYL719/LEE011 combination was examined more broadly in a panel of 22 breast cancer cell lines (Figure 4D-E). This panel included twelve PIK3CA mutant and ten PIK3CA wild-type lines (Barretina et al., 2012). Combination effects were scored relative to Loewe dose-additivity (see Supplemental Experimental Procedures) using a weighted “Synergy Score” calculation (Lehar et al., 2009). Comparison of the combination effects between the two subsets of cell lines demonstrated stronger synergy (p = 0.012, ANOVA) in the PIK3CA mutants, suggesting that PIK3CA mutant breast cancers may be particularly susceptible to this combination.
RB phosphorylation is maintained in the setting of PI3Ki resistance, but suppressed by either mTORC or CDK 4/6 inhibitors
Recently, it was reported that suppression of TORC1 signaling is necessary for sensitivity of PIK3CA mutant cancers to PI3Ki, and combined PI3K and TORC1 inhibition is effective in PIK3CA mutant cancers in which PI3Ki do not fully inhibit TORC1 (Elkabets et al., 2013). Consistent with this finding, the combination of TORC1 plus PI3K inhibition scored highly in our drug screen. However, it was not known whether specific TORC1 outputs exist whose inhibition is critical for response to PI3Ki. That CDK 4/6 emerged as the strongest sensitizer to PI3Ki was therefore particularly interesting to us given that CDK 4/6 exerts its activity by binding and activating Cyclin D1, whose expression is often regulated by TORC1 (Averous et al., 2008; Takuwa et al., 1999). The CDK 4/6-Cyclin D1 complex phosphorylates and inactivates the tumor suppressor RB, and other pocket proteins. In turn, RB, when phosphorylated, releases E2F transcription factors, thereby promoting cell cycle progression from G1 to S phase. We therefore questioned whether RB remains phosphorylated, i.e., inactive, in the setting of resistance to PI3Ki, and whether sensitization to PI3K inhibition by CDK 4/6 inhibitors occurred via de-phosphorylation, i.e., activation, of RB.
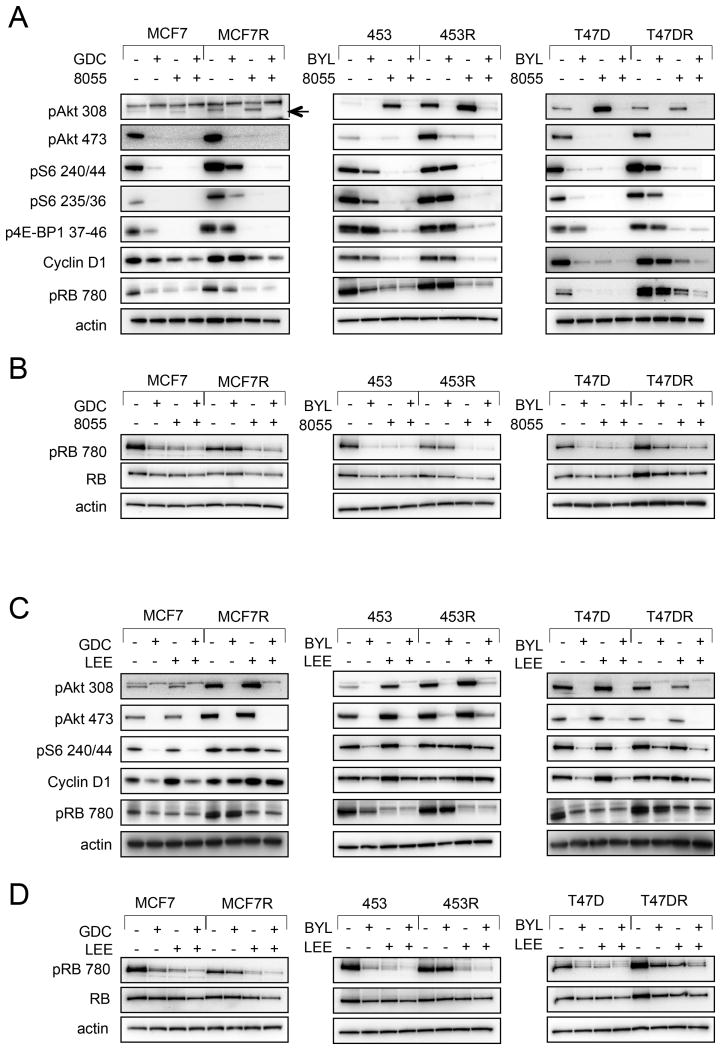
Consistent with this hypothesis, we noted that while PI3Ki monotherapy led to suppression of pAkt and pRB in treatment naïve, sensitive PIK3CA mutant breast cancer cell lines, it was insufficient to suppress pRB or mTORC1 substrates in the resistant lines (Figure 5A). Addition of mTORC inhibition, however, led to Cyclin D1 downregulation and suppression of pRB in these resistant lines (Figure 5A-B). Thus, maintenance of RB phosphorylation in the resistant lines was due to a failure to suppress mTORC signaling.
Figure 5. Comparison of RB phosphorylation between resistant lines and sensitive lines following PI3K inhibition, PI3K/mTORC inhibition, and PI3K/CDK inhibition.
A) Parental and resistant cells were seeded and treated with either AZD-8055 (8055) 500 nM or the indicated PI3Ki 1 μM either alone or in combination. Lysates were made after 24 hours of treatment and probed with the indicated antibodies.
B) Cells were prepared and treated as in (A), but lysed with buffer containing 0.1% SDS.
C) Cells were treated with PI3Ki 1μM or LEE011 1μM alone or in combination and cell lysates were prepared and probed as in (A).
D) Cells were prepared and treated as per C, but lysed with buffer containing 0.1% SDS.
See also Figure S5.
We also observed that CDK 4/6 inhibition suppressed pRB in the acquired resistant lines (Figure 5C-D), and that siRNA directed against CCND1 or CDK4 and CDK6 yielded the same effect (Figure S4D). Thus, in the resistant cells, it appeared that PI3K inhibition suppressed Akt phosphorylation, but failed to suppress CDK 4/6 activity, as measured by RB phosphorylation. By adding either an mTORC or CDK 4/6 inhibitor to a PI3Ki in the resistant cells, RB phosphorylation was suppressed as well as Akt, recapitulating the effect of single-agent PI3Ki in the sensitive cells. RB knockdown in parental MCF7 and 453 cell lines conferred resistance to LEE011 (Figure S5A), moreover, RB knockdown in resistant lines led to resistance to the combination of LEE011 and PI3Ki (Figure S5B), further supporting that the effects of CDK 4/6 inhibition on these cells are mediated, at least in part, via RB activation.
Investigations were undertaken to determine if restoration of RB function was necessary for the growth arrest induced by PI3Ki in sensitive cells. CCND1 overexpression limited suppression of pRB by PI3K inhibition but only partially abrogated the response to PI3Ki (Figure S5C-D). Perhaps this should not be surprising since PI3K inhibition still suppressed growth signals such as Akt and mTORC1 (Figure S5D). Similarly, knockdown of RB was insufficient to cause resistance to PI3Ki (Figure S5E-F). However, additionally, in the setting of RB knockdown experiments, increased expression of other pocket proteins (such as p107) may have compensated for RB loss in cells treated with PI3Ki (Figure S5F). Indeed, a subset of E2F target genes was still suppressed by PI3K inhibition in the RB knockdown cells (Figure S5G). Furthermore, both mTORC1 and Akt were still suppressed in by PI3Ki in the RB knockdown cells (Figure S5F). In total, these experiments suggest that inhibition of Cyclin D/ CDK 4/6 activity is not the sole mediator of growth arrest in response to PI3Ki, at least when Akt and mTORC1 are still suppressed. However, when combined with PI3Ki in a resistant line, both Akt and Cyclin D/ CDK 4/6 are inhibited, leading to impairment of cell cycle progression (Fig. 4B).
Maintenance of pRB may serve as a clinical biomarker of both acquired and de novo resistance to single-agent PI3K inhibition
The findings noted above suggest that suppression of pRB may serve as a biomarker for response to PI3K inhibition. Indeed, there was a good correlation between the doses of PI3Ki required to inhibit cell proliferation and RB phosphorylation in sensitive parental lines (Figure S6A and Figure 1B), and resistant cells failed to suppress RB phosphorylation in response to PI3Ki. Thus, we determined if suppression of RB phosphorylation among treatment naïve PIK3CA mutant breast cancers correlated with sensitivity to single-agent PI3Ki. As shown in Figure 6A, in a panel of 10 PIK3CA mutated breast cancer cell lines, suppression of pRB correlated with sensitivity to BYL719. The effect appeared to be unrelated to the PI3Ki that was used: in the intrinsically resistant CAL51 cells, addition of either BYL719 or GDC-0941 led to a reduction of pAkt but did not suppress pRB unless LEE011 was added (Figure S6B).
Figure 6. Correlation between post-treatment pRB levels and sensitivity to single-agent PI3Ki in vitro and in patient biopsy specimens.
A) 5 sensitive PIK3CA mutant cell lines (IC50≤400nM to BYL719) and 5 de novo resistant PIK3CA cell lines (IC50≥800nM to BYL719) were treated with media or with BYL719 1 μM (BYL) for 24 hours, and lysates were probed with the indicated antibodies. The fraction of remaining pRB (relative to actin) was calculated for both the sensitive and resistant lines and a student's t test was performed. *indicates p<0.05 by student's t test.
B) Eight patients enrolled in early clinical trials of BYL719 were classified as responders or nonresponders as previously described (Elkabets et al., 2013) (n=4 in each group). Biopsy specimens were collected within 2 weeks prior to start of treatment and while on treatment. Slides were prepared, stained and scored for pRB (see Supplemental Experimental Procedures). The percentage change relative to pretreatment levels of pRB for both the responders and the nonresponders is depicted. * indicates p<0.05 by students t-test.
C) Representative sections from a tumor that initially responded to BYL719, stained by IHC for pRB 780 in paired biopsies prior to treatment, day 56 of BYL719 (during response), and at progression, with quantitative H-score.
D) Representative sections from a nonresponsive tumor to BYL719, stained by IHC for pRB 780 in paired biopsies prior to treatment and at day 56 of BYL719, with quantitative H-score.
All error bars in this figure represent +/- SEM.
See also Figure S6.
To determine if analysis of changes in pRB would similarly discriminate sensitive from resistant cancers in patients, we assessed 8 paired biopsy specimens of patients enrolled in clinical trials of BYL719. Patients were classified as responders or nonresponders as previously described (Elkabets et al., 2013). Patients with response to PI3Ki had initial suppression of pRB on treatment relative to baseline. Meanwhile, nonresponders had maintained or increased levels of pRB on treatment relative to baseline (Figure 6B-D, Figure S6C). Moreover, three patients who initially responded ultimately progressed, and had biopsies at that time. Although the responding cancer had suppression of pRB, when it became resistant, pRB levels were restored. These data demonstrate that suppression of pRB may be a marker of response to PI3Ki therapy in the clinic and reinforce the notion that combined treatment with a CDK 4/6 inhibitor may have increased clinical anti-tumor activity.
Combining PI3Ki and LEE011 overcomes intrinsic and acquired resistance in vivo
The efficacy of this combination was next examined in vivo. The activity of this combination was initially examined in vivo using tumor xenografts derived from the MCF7R line, and we observed a non-significant trend towards improved efficacy of the combination over either single-agent therapy (Figure S7A). Although this activity confirmed our in vitro studies, we turned our attention to studying treatment naïve PIK3CA mutant breast cancer models. Despite the sensitivity of some PIK3CA mutant breast cancer cell lines in vitro, we and others have observed that single-agent activity of PI3Ki in vivo, even in the most sensitive models in vitro, is modest (Elkabets et al., 2013; Jamieson et al., 2011; Liu et al., 2013; Ma et al., 2013; Wong et al., 2013; Yuan et al., 2011). This is highly consistent with the clinical trial results to date, in which there has been limited clinical activity of PI3Ki as single-agents in PIK3CA mutant breast cancers. Thus, we speculated that the PIK3CA mutant cancers that were sensitive in vitro take on more resistant features in vivo, perhaps maintaining CDK 4/6 activity.
We first examined the MCF7 xenograft tumor models and noted that single agent GDC-0941 slowed tumor growth, but failed to induce regressions (Figure 7A). While Akt suppression by GDC-0941 was noted, RB phosphorylation was unaffected (Figure 7B). While the reason for the relative discordance between in vivo and in vitro sensitivity remains unclear, importantly, the correlation with resistance and maintenance of RB phosphorylation was retained in vivo, and was effectively overcome by addition of a CDK 4/6 inhibitor. We also examined the efficacy of the combination of LEE011 and GDC-0941 in the xenograft model of the CAL51 cells, a PIK3CA mutant breast cancer line that had maintained RB phosphorylation and was resistant in vitro (Figure S4E). Single-agent GDC-0941 slowed CAL51 tumor growth, but progression still occurred (Figure 7C). Pharmacodynamic studies demonstrated that single-agent GDC-0941 partially suppressed pAkt but failed to suppress RB phosphorylation (Figure 7D). However, the combination with LEE011 led to concomitant suppression of RB phosphorylation and suppression of tumor growth (Figure 7C-D).
Figure 7. PI3K/ CDK 4/6 inhibitor combination in PIK3CA mutant breast cancer xenografts in vivo.
A) MCF7 xenografts were treated with LEE011 75 mg/kg/d, GDC-0941 100 mg/kg/d or the combination. At day 28, three MCF7 xenografts that had progressed on GDC-0941 monotherapy had LEE011 75 mk/kg/d added to their regimen. *indicates p<0.05 by one way ANOVA Kruskal-Wallis test, with Dunn's multiple group comparison test.
B) MCF7 xenografts were treated with the indicated drugs for 72 hours, at which point tumors were harvested and snap frozen. Lysates were prepared and probed with the indicated antibodies.
C) CAL51 xenografts were treated with vehicle, GDC-0941 100 mg/kg/d (GDC), LEE011 75 mg/kg/d (LEE) or the combination. *indicates p<0.05 by one way ANOVA Kruskal-Wallis test, with Dunn's multiple group comparison test.
D) CAL51 xenografts were treated with the indicated drugs for 72 hours, at which point tumors were harvested and snap frozen. Lysates were prepared and probed with the indicated antibodies.
E) T47D xenografts were treated with vehicle, BYL719 25 mg/kg/d (BYL), LEE011 75 mg/kg/d, or the combination. *indicates p<0.05 by one way ANOVA Kruskal-Wallis test, with Dunn's multiple group comparison test.
F) T47D xenografts were treated with the indicated drugs for 72 hours, at which point tumors were harvested and snap frozen. Lysates were prepared and probed with the indicated antibodies.
G) 453 xenografts were treated with vehicle, BYL719 10 mg/kg/d, LEE011 75 mg/kg/d, or the combination. *indicates p<0.05 by one way ANOVA Kruskal-Wallis test, with Dunn's multiple group comparison test.
Tumor measurements in this figure were performed as described in the Supplemental Experimental Procedures. All error bars in this figure represent +/- SEM.
See also Figure S7.
In T47D xenografts, we also observed a significantly improved effect from combination therapy compared to either single agent BYL719 or LEE011 (Figure 7E). Similar to the MCF7 xenograft tumors, single-agent PI3Ki led only to tumor stasis and failed to potently suppress RB phosphorylation, despite suppression of pAkt (Figure 7F). However, mice treated with the combination experienced suppression of both pAkt and pRB (Figure 7F). We also treated 453 xenografts with BYL719 and LEE011, alone or in combination. In this model, regressions were noted with single agent LEE011 therapy, but the combination of agents of BYL719 and LEE011 led to complete regressions (Figure 7G). Furthermore, tumors were measured after cessation of treatment on day 21, and while the LEE011 monotherapy cohort experienced tumor growth roughly two weeks later, mice treated with the combination of agents had not exhibited tumor recurrence at the completion of the study, 5 weeks following treatment cessation.
While our primary objective was to determine whether combined CDK 4/6 and PI3Ki would be a useful strategy as the first line PI3Ki based therapy, we did also examine the efficacy of adding LEE011 after the clear development of resistance to single-agent GDC-0941 in the MCF7 xenografts. Three of the MCF7 xenografts with tumor growth on GDC-0941 monotherapy had LEE011 75 mg/kg added at day 28 and experienced regressions over the subsequent three weeks (Figure 7A).
Thus, the combination of PI3Ki, pan or p110α-specific, with LEE011 was highly effective in four different models of PIK3CA mutant breast cancer with good tolerability as determined by stability of animal weight on treatment (Figure S7B-C). These data together suggest that the combination of PI3K and CDK 4/6 inhibitors have substantial anti-tumor activity in PIK3CA mutant breast cancers.
Discussion
PI3Ki have led to tumor stabilization and some disease responses in PIK3CA mutant breast cancer. However, dramatic tumor regressions are not typical. Multiple recent reports have described mechanisms of resistance to PI3Ki, including MYC overexpression and amplification (Ilic et al., 2011; Liu et al., 2011), matrix associated resistance (Muranen et al., 2012), activity of RSK 3/4 (Serra et al., 2013), and mTORC activation (Elkabets et al., 2013). The goal of the present study was to identify ways to improve efficacy of PI3Ki based therapy, by overcoming adaptive resistance but, importantly, also by improving initial responses to PI3Ki based treatment.
By studying experimentally generated PI3Ki resistant cell line models in this study, we identified two distinct models of resistance to PI3K inhibition (Figure 8). In the first model (453R and T47DR), residual Akt signaling allows for sustained activity of mTORC and its downstream effectors. Fully suppressing Akt signaling with an Akt inhibitor suppressed phosphorylation of downstream nodes such as PRAS40 and mTORC and synergized with PI3K inhibition. In the second model of resistance (MCF7R), PI3K remains potently inhibited, as evidenced by suppressed PIP3 levels and Akt substrates, but mTORC activity is sustained, likely through input from other pathways. In this model, as expected, there is no added benefit from Akt inhibition in combination with PI3K inhibition. Importantly, targeting downstream at CDK 4/6 sensitized both types of resistance models to PI3Ki, highlighting the potential clinical utility of this combination across cancers that fail to respond to PI3Ki due to diverse mechanisms.
Figure 8. Inhibition at vertical nodes within the PI3K pathway improves efficacy of PI3Ki.
Combined inhibition of PI3K and Akt (indicated by the dashed bracket) is synergistic only in resistant models that maintain PIP3 and phosphorylation of Akt substrates (453R, T47DR). Combined inhibition of PI3K and mTOR or PI3K and CDK 4/6 (indicated by the solid brackets) is synergistic in all resistant models; including those that maintain PIP3 and phosphorylation of Akt substrates (453R, T47DR) and those that do not (MCF7R). * indicates that some resistant models maintain low level flux through the PI3K and Akt pathways.
The CDK 4/6 pathway has been implicated in a broad spectrum of cancers including breast cancer, colon cancer, prostate cancer, and hematologic malignancies (Arima et al., 2008; Choi et al., 2012; Gazzeri et al., 1998; Gouyer et al., 1998; Grewe et al., 1999; Myklebust et al., 2012; Nakamura et al., 2012; Nielsen et al., 1998; Petrovic et al., 2013). Notably, targeting this pathway has already shown efficacy in breast cancer (NCT00721409). In a phase 2 trial in patients with hormone receptor positive disease, the addition of the CDK 4/6 inhibitor PD-0332991 to letrozole as frontline therapy caused a statistically significant improvement in progression free survival from 7.5 to 26.2 months. The data provided in this manuscript point to the potential potency of using these agents in combination with PI3Ki in PIK3CA mutant cancers.
This study reveals that insensitivity to PI3Ki is evident by persistent RB phosphorylation and can be effectively overcome by combining a CDK inhibitor with a PI3Ki. In vitro, we noted that PIK3CA breast cancer cell lines with both de novo and acquired resistance maintained RB phosphorylation with PI3Ki treatment. Moreover, in samples from patients treated with BYL719, there was a tight correlation between patient response to BYL719 and suppression of pRB, supporting the notion that failure to suppress RB phosphorylation is associated with resistance to PI3Ki. In studies of xenograft tumor models in vivo, PI3Ki led to slower growth, but not frank regression, mimicking responses typically observed in the clinical setting with PI3K monotherapy. It is notable that this includes cell lines (e.g., MCF7 and T47D cells) whose proliferation was strongly inhibited in vitro. However, RB phosphorylation was not inhibited in vivo by single-agent PI3Ki in these models. It may be that the maintenance of RB phosphorylation contributed to the reduced efficacy of PI3Ki in vivo. While the reason for the differences between in vitro and in vivo sensitivity is not immediately apparent, importantly, persistent RB phosphorylation continued to correlate well with resistance in vivo and in patient samples. Since addition of a CDK 4/6 inhibitor led to loss of pRB and tumor regressions in the in vivo models, we are optimistic that a similar benefit will be observed in patients.
Although maintenance of pRB was associated with resistance, we observed that loss of RB alone was not sufficient to cause resistance to PI3K inhibition. The reasons for this observation may be twofold 1) Firstly, there may be contribution from other pocket proteins with overlapping functions to RB, which may compensate for RB loss. This phenomenon has been observed, for example, in Rb-/- mouse fibroblasts in which there is a compensatory increase in p107 protein levels (Sage et al., 2003). Indeed we observed that p107 expression was higher in RB knockdown cells relative to control cells upon treatment with PI3Ki. 2) Secondly, loss of RB alone may be insufficient to cause resistance in cells where PI3K inhibition continues to potently suppress multiple other effectors, such as Akt and mTORC.
Previous laboratory studies have demonstrated that PIK3CA mutant breast cancers are amongst the most sensitive to single-agent PI3Ki (Beaver et al., 2013; O'Brien et al., 2010), and several clinical development plans for PI3Ki exist in this genetically defined subset of breast cancer. Although not all PIK3CA mutant cell lines that we examined exhibited synergy with the combination, it is also notable that the PI3K/CDK 4/6 inhibitor combination is generally more synergistic in PIK3CA mutant breast cancers than the wild-type counterparts. For these reasons, we favor initial clinical development of this combination in breast cancers harboring PIK3CA mutant breast cancers. However, the studies herein do not fully demonstrate that this combination will be ineffective in other subsets of breast cancer.
While we have previously reported an effective strategy to overcome resistance to PI3Ki, namely combined mTORC-PI3K inhibition (Elkabets et al., 2013), CDK 4/6 and PI3K inhibition is an alternative therapeutic strategy for PIK3CA mutant breast cancer. Given that the most common side effects of PI3K inhibition are hyperglycemia, rash, and gastrointestinal toxicity and those of CDK 4/6 inhibition are primarily hematologic, we are encouraged that the non-overlapping toxicity profile between the two agents may be well tolerated in patients, as they were in the in vivo studies. Indeed, it is likely that therapeutic index in the clinical setting will strongly impact which combination strategy will be more useful for treating PIK3CA mutated breast cancer. In summary, our findings suggest that resistance to PI3K inhibition can be reversed by activation of RB using CDK 4/6 inhibition, and further study of this combination in the clinical setting is warranted.
Experimental Procedures: For full details please see Supplemental Experimental Procedures
Cell lines
Cell lines were verified by SNP or STR analysis and maintained at 37 degrees in a 5% CO2 incubator. To generate resistant lines, the parental cell lines were treated with increasing concentrations of the kinase inhibitor (starting at the GI10), until a target concentration of 1 μM of PI3Ki was achieved.
Western Blotting
Unless otherwise indicated, resistant cells were removed from the drug in which they were cultured for 72 hours prior to experiments. Cells were plated and treated the following day with the indicated agent for 24 hours, after which cells lysed. Proteins were resolved using the NuPAGE Novex Midi Gel system (Invitrogen).
Flow cytometry
Cells were plated and treated the following day with the indicated agents. For cell cycle analyses, treatments were for 24 hours after which cells were washed, permeabilized with triton, treated with RNAse A, and stained with propidium iodide. Fluorescence-activated cell sorting (FACS) analysis was performed on an LSR II flow cytometer (BD Biosciences).
Viability Studies
Long-term viability assays were performed by plating and treating the following day with the indicated agent, with drug and media replenished every 3 days until vehicle treated cells reached confluence (8-12 days). Cells were fixed and stained with Syto60 nucleic acid stain.
Combinatorial Drug Screen
Resistant lines were plated in 96 well plates and treated with escalating doses of each of the 42 agents in the drug screen, in the absence or presence of PI3Ki. Drugs were used at doses that reflected their single agent activity in a broad spectrum of cancer cell lines (Novartis internal data). At the end of 5 days, proliferation was assessed using Cell Titer Glo. Growth inhibition values were normalized to the measured inhibition value at zero dose of the index compound, with or without PI3Ki. Differences in area under the curve (AUC) with and without PI3Ki for each compound were calculated.
Patients
Patients were enrolled in the phase 1 clinical study of BYL719 (NCT01219699) and response was assessed per RECIST criteria. Staging scans were performed prior to treatment and every 2 cycles. Biopsies were obtained within 2 weeks of starting the study agent, at the end of cycle 2 and at progression. Response was defined as previously described (Elkabets et al., 2013). On treatment and progression specimens were collected between 4 and 6 hours of the last treatment dose. All human studies were approved by the Massachusetts General Hospital Institutional Review Board, and informed consent to study was obtained as per protocol from all patients. Please see Supplemental Experimental Procedures for discussion of immunohistochemistry procedures.
Mouse Xenograft Studies
Female nude mice were utilized for MCF7, T47D, and CAL51 xenograft models and SCID mice were utilized for MD-AMB-453 xenografts. For MCF7 and T47D xenografts, estrogen pellet implantation was performed. Tumors were monitored until they reached an average size of 300 mm3, roughly 2-3 weeks, at which point treatments were begun. All mice were euthanized using CO2 inhalation per institutional guidelines at Massachusetts General Hospital. Experiments were approved by the Institutional Animal Care and Use Committee at Massachusetts General Hospital.
Statistical Analyses
Unless otherwise specified, student t tests were performed for statistical analyses and p values <0.05 were considered significant. Statistical comparison amongst groups in xenograft studies was carried out with one way ANOVA Kruskal-Wallis test, with Dunn's multiple group comparison test (GraphPad Prism)
Supplementary Material
Figure S1 (related to Figure 1): The sensitivity of PI3Ki resistant cells to other pathway inhibitors and cytotoxics.
(A) Parental and resistant cell lines were treated with the indicated PI3K inhibitor for 72 hours, after which they were stained with annexin V and propidium iodide. Percentage of apoptotic cells was measured using flow cytometry.
(B) Cell lines were treated as indicated for 24 hours after which cell cycle analysis was performed using propidium iodide staining followed by flow cytometry. The percentage decrease in S phase relative to vehicle controls is depicted. *indicates p<0.05 by student's t test.
(C-D) Cells were treated with escalating doses of either BYL719 or GDC-0941, as indicated (C) or BEZ235 (D) as indicated for 72 hours. Viability was assessed using Cell Titer Glo as described by the manufacturer.
(E) Cells were treated with escalating doses of the indicated agents for 120 hours. Viability was assessed using Cell Titer Glo as described by the manufacturer.
For all experiments data shown are mean of triplicates, with error bars representing -/+ SEM.
Figure S2 (related to Figure 2): Viability curves of sensitizers from combinatorial drug screen.
(A) Resistant cell lines were treated with escalating doses of each of 42 targeted agents, in the absence or presence of a fixed dose of PI3K inhibitor. Proliferation was assessed after 5 days using Cell Titer Glo. Viability values for each curve were normalized to the measured inhibition value at zero dose of the respective targeted agent without or with 1 μM PI3Ki. The agents were scored based on difference in AUC between the two curves (minus and plus PI3K inhibitor) and ranked by greatest difference. Agents with FDR values ≤0.05 were considered sensitizers in the drug screen for individual cell lines. The viability curves for the sensitizers in each of the resistant cell lines are depicted as means of triplicates -/+ SEM.
(B) Structure of CDK 4/6 inhibitor, LEE011.
(C) Biochemical Assay of LEE011 to determine relative IC50 against CDK 4 and other CDKs.
Figure S3 (related to Figure 3): Combination of PI3K inhibitors with MK2206 or lapatinib
(A) Proliferation was assessed by Cell Titer Glo in parental and resistant 453 and T47D lines treated with escalating doses of MK2206 for 5 days. Resistant lines were also treated with escalating doses of MK2206 in the presence of 1 μM GDC-0941, and dose response curves were generated. Values were normalized to the measured inhibition value at zero dose of MK2206 (without or with GDC-0941 1 μM for resistant lines).
(B) Parental and resistant 453 and T47D cells were treated for 24 hours with escalating doses of MK2206. 453R and T47DR cells were also treated with increasing doses of MK2206 in the presence of GDC0941 1 μM. Lysates were prepared at 24 hours and probed with the indicated antibodies.
(C) Long-term viability assays were performed on parental and resistant 453 lines with BYL719 1 μM, lapatinib 1 μM, or the combination. Media was changed every 72 hours. When the untreated controls grew to confluence, the cells in both the vehicle and drug treated wells were fixed and stained for nucleic acid with Syto-60. To assess viability, quantification of absorbance in the Syto-60 stained cells was performed using infrared imaging. *indicates p<0.05 by student's t test.
(D) Parental and resistant 453 cells were treated for 24 hours with escalating doses of lapatinib. 453R cells were also treated with increasing doses of lapatinib in the presence of BYL719 1 μM. Lysates were prepared at 24 hours and probed with the indicated antibodies.
Error bars in this figure represent mean +/-SEM of triplicates.
Figure S4 (related to Figure 4): Synergy scores between PI3K and CDK4/6 inhibition in sensitive and resistant PIK3CA mutant cell lines.
(A) A 6×7 dose matrix of either GDC-0941 (GDC) or BYL719 (BYL) and LEE011 (LEE) was performed and cell viability was measured at the end of 5 days using Cell Titer Glo. Loewe Excess Inhibition values were calculated as a measure of synergy and are displayed. Values greater than 10% were considered to be suggestive of synergy. Calculation of Loewe Excess Inhibition is described in Supplemental Experimental Procedures.
(B) PI3Ki resistant cells were treated with escalating doses of PD-0332991 (PD) in the absence or presence of a fixed 1 μM dose of PI3Ki. Proliferation was assessed after 5 days using Cell Titer Glo. Viability values for each curve were normalized to the measured inhibition value at zero dose of PD (without or with PI3Ki for the resistant lines).
(C) Long-term viability assays were performed on parental and resistant lines with BYL719 or GDC-0941 1 μM (BYL or GDC as indicated), PD0332991 1 μM (PD), or the indicated combination. Media was changed every 72 hours. When the untreated controls grew to confluence, the cells in both the vehicle and drug treated wells were fixed and stained for nucleic acid with Syto-60. To assess viability, quantification of absorbance in the Syto-60 stained cells was performed using infrared imaging. *indicates p<0.05 by student's t test.
(D) Transient knockdown using scrambled control siRNA (scr), or siRNA directed against CCND1 or CDK4 and CDK6 was performed in MCF7R, and knockdown was assessed by Western blotting using lysates prepared 48 hours post transfection. Long-term viability assays were performed on knockdown cells, with drug and media changes occurring every 72 hours until 9 days, at which point cells were fixed and stained for nucleic acid using Syto-60 stain. * indicates p<0.05 by student's t-test.
(E) Long-term viability assays with the indicated agents BYL719 1 μM (BYL), GDC-0941 1 μM (GDC), LEE011 1 μM (LEE), or the indicated combination were performed in PIK3CA mutant cell lines with de novo resistance to PI3K inhibition. Media was changed every 72 hours. When the untreated controls grew to confluence, the cells in both the vehicle and drug treated wells were fixed and stained for nucleic acid with Syto-60. To assess viability, quantification of absorbance in the Syto-60 stained cells was performed using infrared imaging. *indicates p<0.05 by student's t test.
Error bars indicate +/- SEM of triplicates for all experiments in this figure.
Figure S5 (related to Figure 5): The effect of RB knockdown on sensitivity to CDK 4/6i, PI3Ki, and combined PI3Ki/CDK4/6i.
(A) MCF7 and 453 shGFP and shRB cells were treated with escalating doses of LEE011 and proliferation was assessed after 5 days using Cell Titer Glo. Data represent mean of triplicates. Knockdown was assessed by probing lysates, prepared 48 hours after transfection, with the indicated antibodies.
(B) 453R and MCF7R shGFP and shRB cells were treated with escalating doses of LEE011 in the absence or presence of BYL719 or GDC-0941 1 μM, respectively. Proliferation was assessed after 5 days using Cell Titer Glo as described by the manufacturer. Data represent mean of triplicates. Knockdown and effect of PI3Ki on RB levels were assessed in cells seeded 24 hours after transfection and treated for 24 hours with vehicle (-) or the indicated PI3K inhibitor, BYL719 1 μM (BYL) or GDC-0941 1 μM (GDC).
(C) Stable overexpression of the housekeeping gene β-glucoronidase (GUS) and CCND1 was achieved by lentiviral infection of MCF7. Long-term viability assays were performed on infected cells using the indicated agents: Vehicle (V), GDC-0941 1 μM (G), LEE011 1 μM (L), AZD8055 500 nM (A), or the specified combinations. Media was changed every 72 hours. When the untreated controls grew to confluence, the cells in both the vehicle and drug treated wells were fixed and stained for nucleic acid with Syto-60. To assess viability, quantification of absorbance in the Syto-60 stained cells was performed using infrared imaging. Data represent mean of triplicates from three independent experiments. *indicates p<0.05 by student's t test.
(D) Lysates from either GUS or CCND1 overexpressing MCF7 cells were collected after 24 -hour treatment with vehicle or the indicated agents at doses specified above and probed with the indicated antibodies.
(E) MCF7 and 453 shGFP and shRB cells were treated with escalating doses of the indicated PI3Ki, GDC-0941 (GDC) or BYL719 (BYL), and proliferation was assessed after 5 days with Cell Titer Glo. Data represent mean of triplicates.
(F) Knockdown and effect of PI3Ki on RB levels and downstream signaling were assessed in cells seeded 24 hours after transfection and treated for 24 hours with vehicle (-) or the indicated PI3K inhibitor, BYL719 1 μM (BYL) or GDC-0941 1 μM (GDC). Lysates made with 0.1% SDS were probed with the indicated antibodies.
(G) MCF7 shGFP (M shGFP), MCF7 shRB (M shRB), and MCF7R shGFP (MR shGFP) cells were treated with vehicle or GDC-0941 1 μM for 24 hours at which point RNA was isolated and cDNA was synthesized. qPCR was performed for each of the indicated genes. Levels were normalized to actin and represented as percent change relative to vehicle treated samples. Data is represented as mean of three independent experiments performed in triplicate.
Error bars represent -/+SEM for all experiments in this figure.
Figure S6 (related to Figure 6): Correlation between post-treatment RB phosphorylation and response to PI3K inhibition.
(A) MCF7, 453, and T47D cells with their corresponding resistant lines were treated for 24 hours with vehicle or escalating doses of BYL719 (BYL) or GDC0941 (GDC) as indicated, lysed and probed with the indicated antibodies.
(B) CAL51 were treated with vehicle, BYL719 1μM (BYL), GDC-0941 1 μM (GDC), LEE011 1 μM (LEE) or the indicated combination for 24 hours, at which point lysates were made and probed with the indicated antibodies.
(C) Quantitative H scores of biopsy specimens stained with pRB 780 from 4 patients with PIK3CA mutations who responded to treatment with BYL719 (3 of whom later progressed) and from 4 patients who had initial progression. Biopsies were collectedprior to treatment, at day 56, and at progression for patients who experienced initial responses.
Figure S7 (related to Figure 7): Efficacy and tolerability of combination of LEE011 and PI3K inhibitor in laboratory mouse xenograft models
(A) MCF7R xenografts were treated with vehicle, LEE011 150 mg/kg/d, GDC-0941 100 mg/kg/d or the combination. Tumor measurements were performed as described in the Supplemental Experimental Procedures. Percent change at day 21 relative to day 0 is depicted for each animal. *indicates p<0.05 by one way ANOVA Kruskal-Wallis test, with Dunn's multiple group comparison test.
(B-C) Weights for MCF7, CAL51, and 453 xenograft models were measured twice weekly while on treatment. Percentage change compared to day 0 are depicted for A) day 28 and B) end of treatment from all xenograft models treated with the indicated therapies. Data are represented as mean -/+ SEM for all experiments in this figure.
Table S1 (related to Figure 1): Sensitivity of parental and PI3Ki resistant lines to other pathway inhibitors.
Table S2 (related to Figure 2): Sensitizers from Drug Screen.
Highlights.
Synergy between inhibitions of CDK4/6 and PI3K in PIK3CA mutant breast cancer
CDK4/6-PI3K inhibition is effective in several PIK3CA mutant xenograft tumor models
Failure to suppress pRB correlates with resistance to PI3K inhibitors in patients
Significance.
Activating mutations in the PIK3CA gene, which encodes the catalytically active p110α subunit, occur in 30% of breast cancers. Thus, there have been intense efforts to develop PI3K inhibitors to treat these cancers. However, responses to these agents have been underwhelming to date. Our study suggests that concomitant CDK 4/6 inhibition improves initial responses to PI3K inhibitors and overcomes acquired resistance in PIK3CA mutated breast cancers. We observed that maintenance of pRB is a biomarker for intrinsic and acquired PI3K inhibitor resistance in vitro as well as in patients treated with BYL719. These findings provide rationale for study of this drug combination in PIK3CA mutated breast cancer.
Acknowledgments
We thank Drs. Cyril Benes, Jose Baselga, Nicholas Dyson, and Mauri Scaltriti for scientific guidance. We also thank Drs. Matthew Niederst, Anthony Faber, Adam Crystal and Andreas Heilmann for helpful scientific discussions. This work was funded by R01CA137008 (to JAE) and Novartis Institutes for BioMedical Research (NIBR). We would also like to thank Stand Up To Cancer (SUTC) for their generous donation of GDC-0941 for in vivo use and NIBR combination working group for experimental assistance. SV is supported by an American Association for Cancer Research-BioOncology Fellowship, a Terry Brodeur Breast Cancer Foundation research grant, and a DF/HCC Breast Cancer Spore Career Development Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arima Y, Inoue Y, Shibata T, Hayashi H, Nagano O, Saya H, Taya Y. Rb depletion results in deregulation of E-cadherin and induction of cellular phenotypic changes that are characteristic of the epithelial-to-mesenchymal transition. Cancer research. 2008;68:5104–5112. doi: 10.1158/0008-5472.CAN-07-5680. [DOI] [PubMed] [Google Scholar]
- Averous J, Fonseca BD, Proud CG. Regulation of cyclin D1 expression by mTORC1 signaling requires eukaryotic initiation factor 4E-binding protein 1. Oncogene. 2008;27:1106–1113. doi: 10.1038/sj.onc.1210715. [DOI] [PubMed] [Google Scholar]
- Bachman KE, Argani P, Samuels Y, Silliman N, Ptak J, Szabo S, Konishi H, Karakas B, Blair BG, Lin C, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer biology & therapy. 2004;3:772–775. doi: 10.4161/cbt.3.8.994. [DOI] [PubMed] [Google Scholar]
- Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehar J, Kryukov GV, Sonkin D, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JA, Gustin JP, Yi KH, Rajpurohit A, Thomas M, Gilbert SF, Rosen DM, Ho Park B, Lauring J. PIK3CA and AKT1 mutations have distinct effects on sensitivity to targeted pathway inhibitors in an isogenic luminal breast cancer model system. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:5413–5422. doi: 10.1158/1078-0432.CCR-13-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendell JC, Rodon J, Burris HA, de Jonge M, Verweij J, Birle D, Demanse D, De Buck SS, Ru QC, Peters M, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Li X, Hydbring P, Sanda T, Stefano J, Christie AL, Signoretti S, Look AT, Kung AL, von Boehmer H, Sicinski P. The requirement for cyclin d function in tumor maintenance. Cancer Cell. 2012;22:438–451. doi: 10.1016/j.ccr.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkabets M, Vora S, Juric D, Morse N, Mino-Kenudson M, Muranen T, Tao J, Campos AB, Rodon J, Ibrahim YH, et al. mTORC1 Inhibition Is Required for Sensitivity to PI3K p110alpha Inhibitors in PIK3CA-Mutant Breast Cancer. Science translational medicine. 2013;5:196ra199. doi: 10.1126/scitranslmed.3005747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nature reviews Genetics. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) The Biochemical journal. 2008;416:375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- Gazzeri S, Gouyer V, Vour'ch C, Brambilla C, Brambilla E. Mechanisms of p16INK4A inactivation in non small-cell lung cancers. Oncogene. 1998;16:497–504. doi: 10.1038/sj.onc.1201559. [DOI] [PubMed] [Google Scholar]
- Gouyer V, Gazzeri S, Bolon I, Drevet C, Brambilla C, Brambilla E. Mechanism of retinoblastoma gene inactivation in the spectrum of neuroendocrine lung tumors. American journal of respiratory cell and molecular biology. 1998;18:188–196. doi: 10.1165/ajrcmb.18.2.3008. [DOI] [PubMed] [Google Scholar]
- Grewe M, Gansauge F, Schmid RM, Adler G, Seufferlein T. Regulation of cell growth and cyclin D1 expression by the constitutively active FRAP-p70s6K pathway in human pancreatic cancer cells. Cancer Res. 1999;59:3581–3587. [PubMed] [Google Scholar]
- Hanker AB, Pfefferle AD, Balko JM, Kuba MG, Young CD, Sanchez V, Sutton CR, Cheng H, Perou CM, Zhao JJ, et al. Mutant PIK3CA accelerates HER2-driven transgenic mammary tumors and induces resistance to combinations of anti-HER2 therapies. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:14372–14377. doi: 10.1073/pnas.1303204110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic N, Utermark T, Widlund HR, Roberts TM. PI3K-targeted therapy can be evaded by gene amplification along the MYC-eukaryotic translation initiation factor 4E (eIF4E) axis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E699–708. doi: 10.1073/pnas.1108237108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson S, Flanagan JU, Kolekar S, Buchanan C, Kendall JD, Lee WJ, Rewcastle GW, Denny WA, Singh R, Dickson J, et al. A drug targeting only p110alpha can block phosphoinositide 3-kinase signalling and tumour growth in certain cell types. The Biochemical journal. 2011;438:53–62. doi: 10.1042/BJ20110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annual review of cell and developmental biology. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- Lehar J, Krueger AS, Avery W, Heilbut AM, Johansen LM, Price ER, Rickles RJ, Short GF, 3rd, Staunton JE, Jin X, et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nature biotechnology. 2009;27:659–666. doi: 10.1038/nbt.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Rowley BR, Bull CO, Schneider C, Haegebarth A, Schatz CA, Fracasso PR, Wilkie DP, Hentemann M, Wilhelm SM, et al. BAY 80-6946 Is a Highly Selective Intravenous PI3K Inhibitor with Potent p110alpha and p110delta Activities in Tumor Cell Lines and Xenograft Models. Molecular cancer therapeutics. 2013;12:2319–2330. doi: 10.1158/1535-7163.MCT-12-0993-T. [DOI] [PubMed] [Google Scholar]
- Liu P, Cheng H, Santiago S, Raeder M, Zhang F, Isabella A, Yang J, Semaan DJ, Chen C, Fox EA, et al. Oncogenic PIK3CA-driven mammary tumors frequently recur via PI3K pathway-dependent and PI3K pathway-independent mechanisms. Nature medicine. 2011;17:1116–1120. doi: 10.1038/nm.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma BB, Lui VW, Hui CW, Lau CP, Wong CH, Hui EP, Ng MH, Cheng SH, Tsao SW, Tsang CM, et al. Preclinical evaluation of the mTOR-PI3K inhibitor BEZ235 in nasopharyngeal cancer models. Cancer letters. 2013 doi: 10.1016/j.canlet.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Mahadevan D, Chiorean EG, Harris WB, Von Hoff DD, Stejskal-Barnett A, Qi W, Anthony SP, Younger AE, Rensvold DM, Cordova F, et al. Phase I pharmacokinetic and pharmacodynamic study of the pan-PI3K/mTORC vascular targeted pro-drug SF1126 in patients with advanced solid tumours and B-cell malignancies. European journal of cancer. 2012;48:3319–3327. doi: 10.1016/j.ejca.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maira SM. PI3K inhibitors for cancer treatment: five years of preclinical and clinical research after BEZ235. Molecular cancer therapeutics. 2011;10:2016. doi: 10.1158/1535-7163.MCT-11-0792. [DOI] [PubMed] [Google Scholar]
- Markman B, Tabernero J, Krop I, Shapiro GI, Siu L, Chen LC, Mita M, Melendez Cuero M, Stutvoet S, Birle D, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of the oral phosphatidylinositol-3-kinase and mTOR inhibitor BGT226 in patients with advanced solid tumors. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:2399–2408. doi: 10.1093/annonc/mds011. [DOI] [PubMed] [Google Scholar]
- Miller TW, Hennessy BT, Gonzalez-Angulo AM, Fox EM, Mills GB, Chen H, Higham C, Garcia-Echeverria C, Shyr Y, Arteaga CL. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. The Journal of clinical investigation. 2010;120:2406–2413. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranen T, Selfors LM, Worster DT, Iwanicki MP, Song L, Morales FC, Gao S, Mills GB, Brugge JS. Inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer cell. 2012;21:227–239. doi: 10.1016/j.ccr.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myklebust MP, Li Z, Tran TH, Rui H, Knudsen ES, Elsaleh H, Fluge O, Vonen B, Myrvold HE, Leh S, et al. Expression of cyclin D1a and D1b as predictive factors for treatment response in colorectal cancer. British journal of cancer. 2012;107 doi: 10.1038/bjc.2012.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Felizola SJ, Kurotaki Y, Fujishima F, McNamara KM, Suzuki T, Arai Y, Sasano H. Cyclin D1 (CCND1) expression is involved in estrogen receptor beta (ERbeta) in human prostate cancer. The Prostate. 2012 doi: 10.1002/pros.22599. [DOI] [PubMed] [Google Scholar]
- Nielsen GP, Burns KL, Rosenberg AE, Louis DN. CDKN2A gene deletions and loss of p16 expression occur in osteosarcomas that lack RB alterations. The American journal of pathology. 1998;153:159–163. doi: 10.1016/S0002-9440(10)65556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien C, Wallin JJ, Sampath D, GuhaThakurta D, Savage H, Punnoose EA, Guan J, Berry L, Prior WW, Amler LC, et al. Predictive biomarkers of sensitivity to the phosphatidylinositol 3′ kinase inhibitor GDC-0941 in breast cancer preclinical models. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:3670–3683. doi: 10.1158/1078-0432.CCR-09-2828. [DOI] [PubMed] [Google Scholar]
- Petrovic V, Malin D, Cryns VL. alphaB-crystallin promotes oncogenic transformation and inhibits caspase activation in cells primed for apoptosis by Rb inactivation. Breast cancer research and treatment. 2013;138:415–425. doi: 10.1007/s10549-013-2465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage J, Miller AL, Perez-Mancera PA, Wysocki JM, Jacks T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature. 2003;424:223–228. doi: 10.1038/nature01764. [DOI] [PubMed] [Google Scholar]
- Serra V, Eichhorn PJ, Garcia-Garcia C, Ibrahim YH, Prudkin L, Sanchez G, Rodriguez O, Anton P, Parra JL, Marlow S, et al. RSK3/4 mediate resistance to PI3K pathway inhibitors in breast cancer. The Journal of clinical investigation. 2013;123:2551–2563. doi: 10.1172/JCI66343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa N, Fukui Y, Takuwa Y. Cyclin D1 expression mediated by phosphatidylinositol 3-kinase through mTOR-p70(S6K)-independent signaling in growth factor-stimulated NIH 3T3 fibroblasts. Molecular and cellular biology. 1999;19:1346–1358. doi: 10.1128/mcb.19.2.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ, Hennessy BT, Tseng H, Pochanard P, Kim SY, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer cell. 2009;16:21–32. doi: 10.1016/j.ccr.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LE, Ma H, Hale KS, Yin M, Meyer LA, Liu H, Li J, Lu KH, Hennessy BT, Li X, et al. Roles of genetic variants in the PI3K and RAS/RAF pathways in susceptibility to endometrial cancer and clinical outcomes. Journal of cancer research and clinical oncology. 2012;138:377–385. doi: 10.1007/s00432-011-1103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Loong HH, Hui CW, Lau CP, Hui EP, Ma BB, Chan AT. Preclinical evaluation of the PI3K-mTOR dual inhibitor PF-04691502 as a novel therapeutic drug in nasopharyngeal carcinoma. Investigational new drugs. 2013;31:1399–1408. doi: 10.1007/s10637-013-0007-z. [DOI] [PubMed] [Google Scholar]
- Yuan J, Mehta PP, Yin MJ, Sun S, Zou A, Chen J, Rafidi K, Feng Z, Nickel J, Engebretsen J, et al. PF-04691502, a potent and selective oral inhibitor of PI3K and mTOR kinases with antitumor activity. Molecular cancer therapeutics. 2011;10:2189–2199. doi: 10.1158/1535-7163.MCT-11-0185. [DOI] [PubMed] [Google Scholar]
- Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27:5486–5496. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 (related to Figure 1): The sensitivity of PI3Ki resistant cells to other pathway inhibitors and cytotoxics.
(A) Parental and resistant cell lines were treated with the indicated PI3K inhibitor for 72 hours, after which they were stained with annexin V and propidium iodide. Percentage of apoptotic cells was measured using flow cytometry.
(B) Cell lines were treated as indicated for 24 hours after which cell cycle analysis was performed using propidium iodide staining followed by flow cytometry. The percentage decrease in S phase relative to vehicle controls is depicted. *indicates p<0.05 by student's t test.
(C-D) Cells were treated with escalating doses of either BYL719 or GDC-0941, as indicated (C) or BEZ235 (D) as indicated for 72 hours. Viability was assessed using Cell Titer Glo as described by the manufacturer.
(E) Cells were treated with escalating doses of the indicated agents for 120 hours. Viability was assessed using Cell Titer Glo as described by the manufacturer.
For all experiments data shown are mean of triplicates, with error bars representing -/+ SEM.
Figure S2 (related to Figure 2): Viability curves of sensitizers from combinatorial drug screen.
(A) Resistant cell lines were treated with escalating doses of each of 42 targeted agents, in the absence or presence of a fixed dose of PI3K inhibitor. Proliferation was assessed after 5 days using Cell Titer Glo. Viability values for each curve were normalized to the measured inhibition value at zero dose of the respective targeted agent without or with 1 μM PI3Ki. The agents were scored based on difference in AUC between the two curves (minus and plus PI3K inhibitor) and ranked by greatest difference. Agents with FDR values ≤0.05 were considered sensitizers in the drug screen for individual cell lines. The viability curves for the sensitizers in each of the resistant cell lines are depicted as means of triplicates -/+ SEM.
(B) Structure of CDK 4/6 inhibitor, LEE011.
(C) Biochemical Assay of LEE011 to determine relative IC50 against CDK 4 and other CDKs.
Figure S3 (related to Figure 3): Combination of PI3K inhibitors with MK2206 or lapatinib
(A) Proliferation was assessed by Cell Titer Glo in parental and resistant 453 and T47D lines treated with escalating doses of MK2206 for 5 days. Resistant lines were also treated with escalating doses of MK2206 in the presence of 1 μM GDC-0941, and dose response curves were generated. Values were normalized to the measured inhibition value at zero dose of MK2206 (without or with GDC-0941 1 μM for resistant lines).
(B) Parental and resistant 453 and T47D cells were treated for 24 hours with escalating doses of MK2206. 453R and T47DR cells were also treated with increasing doses of MK2206 in the presence of GDC0941 1 μM. Lysates were prepared at 24 hours and probed with the indicated antibodies.
(C) Long-term viability assays were performed on parental and resistant 453 lines with BYL719 1 μM, lapatinib 1 μM, or the combination. Media was changed every 72 hours. When the untreated controls grew to confluence, the cells in both the vehicle and drug treated wells were fixed and stained for nucleic acid with Syto-60. To assess viability, quantification of absorbance in the Syto-60 stained cells was performed using infrared imaging. *indicates p<0.05 by student's t test.
(D) Parental and resistant 453 cells were treated for 24 hours with escalating doses of lapatinib. 453R cells were also treated with increasing doses of lapatinib in the presence of BYL719 1 μM. Lysates were prepared at 24 hours and probed with the indicated antibodies.
Error bars in this figure represent mean +/-SEM of triplicates.
Figure S4 (related to Figure 4): Synergy scores between PI3K and CDK4/6 inhibition in sensitive and resistant PIK3CA mutant cell lines.
(A) A 6×7 dose matrix of either GDC-0941 (GDC) or BYL719 (BYL) and LEE011 (LEE) was performed and cell viability was measured at the end of 5 days using Cell Titer Glo. Loewe Excess Inhibition values were calculated as a measure of synergy and are displayed. Values greater than 10% were considered to be suggestive of synergy. Calculation of Loewe Excess Inhibition is described in Supplemental Experimental Procedures.
(B) PI3Ki resistant cells were treated with escalating doses of PD-0332991 (PD) in the absence or presence of a fixed 1 μM dose of PI3Ki. Proliferation was assessed after 5 days using Cell Titer Glo. Viability values for each curve were normalized to the measured inhibition value at zero dose of PD (without or with PI3Ki for the resistant lines).
(C) Long-term viability assays were performed on parental and resistant lines with BYL719 or GDC-0941 1 μM (BYL or GDC as indicated), PD0332991 1 μM (PD), or the indicated combination. Media was changed every 72 hours. When the untreated controls grew to confluence, the cells in both the vehicle and drug treated wells were fixed and stained for nucleic acid with Syto-60. To assess viability, quantification of absorbance in the Syto-60 stained cells was performed using infrared imaging. *indicates p<0.05 by student's t test.
(D) Transient knockdown using scrambled control siRNA (scr), or siRNA directed against CCND1 or CDK4 and CDK6 was performed in MCF7R, and knockdown was assessed by Western blotting using lysates prepared 48 hours post transfection. Long-term viability assays were performed on knockdown cells, with drug and media changes occurring every 72 hours until 9 days, at which point cells were fixed and stained for nucleic acid using Syto-60 stain. * indicates p<0.05 by student's t-test.
(E) Long-term viability assays with the indicated agents BYL719 1 μM (BYL), GDC-0941 1 μM (GDC), LEE011 1 μM (LEE), or the indicated combination were performed in PIK3CA mutant cell lines with de novo resistance to PI3K inhibition. Media was changed every 72 hours. When the untreated controls grew to confluence, the cells in both the vehicle and drug treated wells were fixed and stained for nucleic acid with Syto-60. To assess viability, quantification of absorbance in the Syto-60 stained cells was performed using infrared imaging. *indicates p<0.05 by student's t test.
Error bars indicate +/- SEM of triplicates for all experiments in this figure.
Figure S5 (related to Figure 5): The effect of RB knockdown on sensitivity to CDK 4/6i, PI3Ki, and combined PI3Ki/CDK4/6i.
(A) MCF7 and 453 shGFP and shRB cells were treated with escalating doses of LEE011 and proliferation was assessed after 5 days using Cell Titer Glo. Data represent mean of triplicates. Knockdown was assessed by probing lysates, prepared 48 hours after transfection, with the indicated antibodies.
(B) 453R and MCF7R shGFP and shRB cells were treated with escalating doses of LEE011 in the absence or presence of BYL719 or GDC-0941 1 μM, respectively. Proliferation was assessed after 5 days using Cell Titer Glo as described by the manufacturer. Data represent mean of triplicates. Knockdown and effect of PI3Ki on RB levels were assessed in cells seeded 24 hours after transfection and treated for 24 hours with vehicle (-) or the indicated PI3K inhibitor, BYL719 1 μM (BYL) or GDC-0941 1 μM (GDC).
(C) Stable overexpression of the housekeeping gene β-glucoronidase (GUS) and CCND1 was achieved by lentiviral infection of MCF7. Long-term viability assays were performed on infected cells using the indicated agents: Vehicle (V), GDC-0941 1 μM (G), LEE011 1 μM (L), AZD8055 500 nM (A), or the specified combinations. Media was changed every 72 hours. When the untreated controls grew to confluence, the cells in both the vehicle and drug treated wells were fixed and stained for nucleic acid with Syto-60. To assess viability, quantification of absorbance in the Syto-60 stained cells was performed using infrared imaging. Data represent mean of triplicates from three independent experiments. *indicates p<0.05 by student's t test.
(D) Lysates from either GUS or CCND1 overexpressing MCF7 cells were collected after 24 -hour treatment with vehicle or the indicated agents at doses specified above and probed with the indicated antibodies.
(E) MCF7 and 453 shGFP and shRB cells were treated with escalating doses of the indicated PI3Ki, GDC-0941 (GDC) or BYL719 (BYL), and proliferation was assessed after 5 days with Cell Titer Glo. Data represent mean of triplicates.
(F) Knockdown and effect of PI3Ki on RB levels and downstream signaling were assessed in cells seeded 24 hours after transfection and treated for 24 hours with vehicle (-) or the indicated PI3K inhibitor, BYL719 1 μM (BYL) or GDC-0941 1 μM (GDC). Lysates made with 0.1% SDS were probed with the indicated antibodies.
(G) MCF7 shGFP (M shGFP), MCF7 shRB (M shRB), and MCF7R shGFP (MR shGFP) cells were treated with vehicle or GDC-0941 1 μM for 24 hours at which point RNA was isolated and cDNA was synthesized. qPCR was performed for each of the indicated genes. Levels were normalized to actin and represented as percent change relative to vehicle treated samples. Data is represented as mean of three independent experiments performed in triplicate.
Error bars represent -/+SEM for all experiments in this figure.
Figure S6 (related to Figure 6): Correlation between post-treatment RB phosphorylation and response to PI3K inhibition.
(A) MCF7, 453, and T47D cells with their corresponding resistant lines were treated for 24 hours with vehicle or escalating doses of BYL719 (BYL) or GDC0941 (GDC) as indicated, lysed and probed with the indicated antibodies.
(B) CAL51 were treated with vehicle, BYL719 1μM (BYL), GDC-0941 1 μM (GDC), LEE011 1 μM (LEE) or the indicated combination for 24 hours, at which point lysates were made and probed with the indicated antibodies.
(C) Quantitative H scores of biopsy specimens stained with pRB 780 from 4 patients with PIK3CA mutations who responded to treatment with BYL719 (3 of whom later progressed) and from 4 patients who had initial progression. Biopsies were collectedprior to treatment, at day 56, and at progression for patients who experienced initial responses.
Figure S7 (related to Figure 7): Efficacy and tolerability of combination of LEE011 and PI3K inhibitor in laboratory mouse xenograft models
(A) MCF7R xenografts were treated with vehicle, LEE011 150 mg/kg/d, GDC-0941 100 mg/kg/d or the combination. Tumor measurements were performed as described in the Supplemental Experimental Procedures. Percent change at day 21 relative to day 0 is depicted for each animal. *indicates p<0.05 by one way ANOVA Kruskal-Wallis test, with Dunn's multiple group comparison test.
(B-C) Weights for MCF7, CAL51, and 453 xenograft models were measured twice weekly while on treatment. Percentage change compared to day 0 are depicted for A) day 28 and B) end of treatment from all xenograft models treated with the indicated therapies. Data are represented as mean -/+ SEM for all experiments in this figure.
Table S1 (related to Figure 1): Sensitivity of parental and PI3Ki resistant lines to other pathway inhibitors.
Table S2 (related to Figure 2): Sensitizers from Drug Screen.