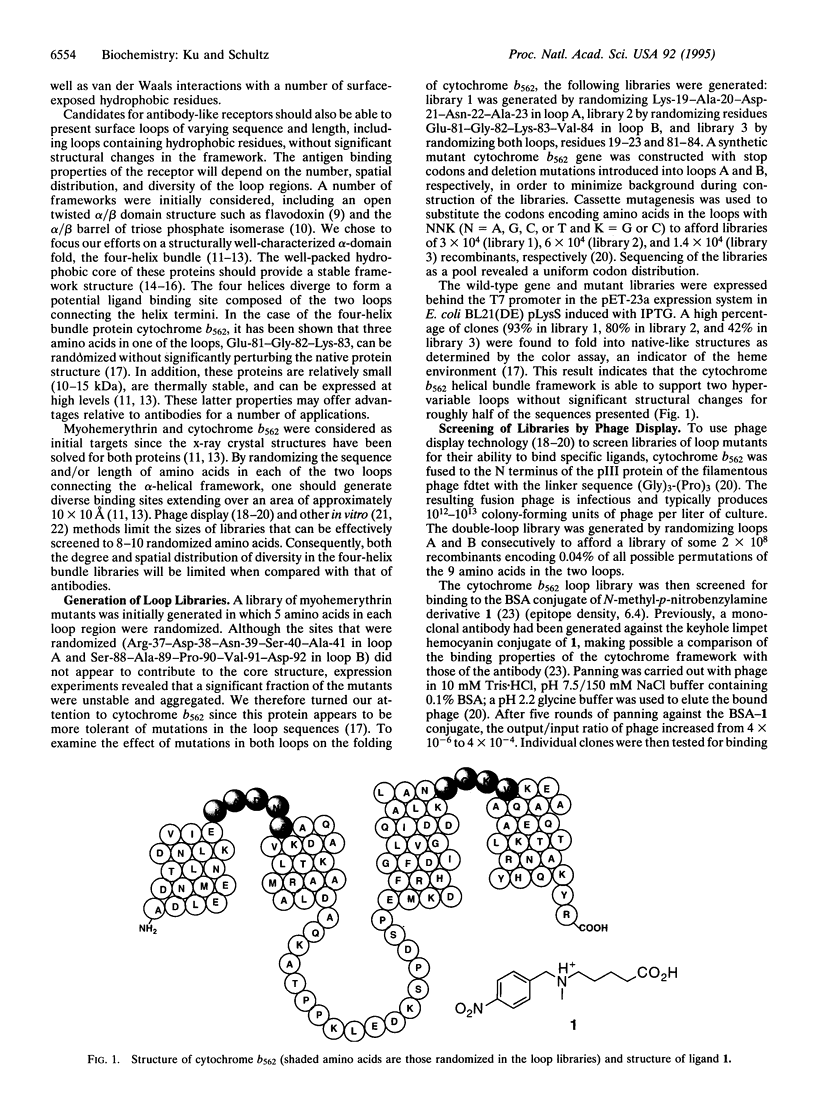
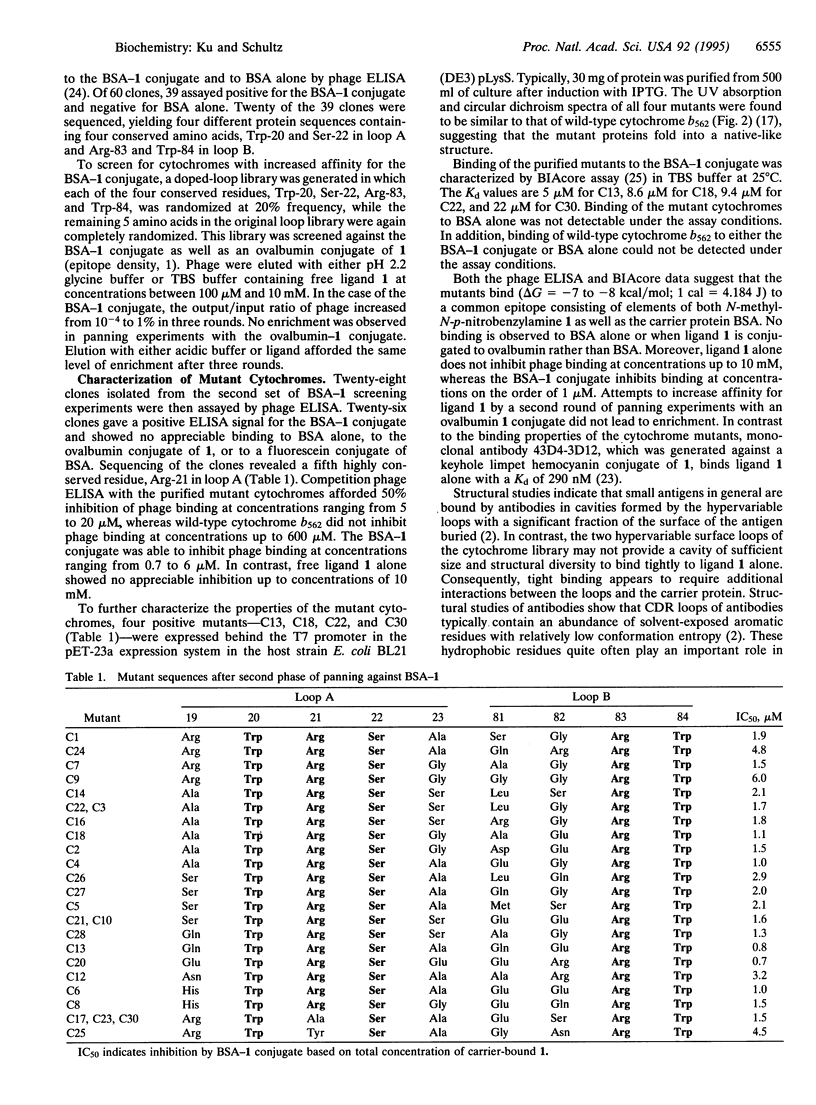
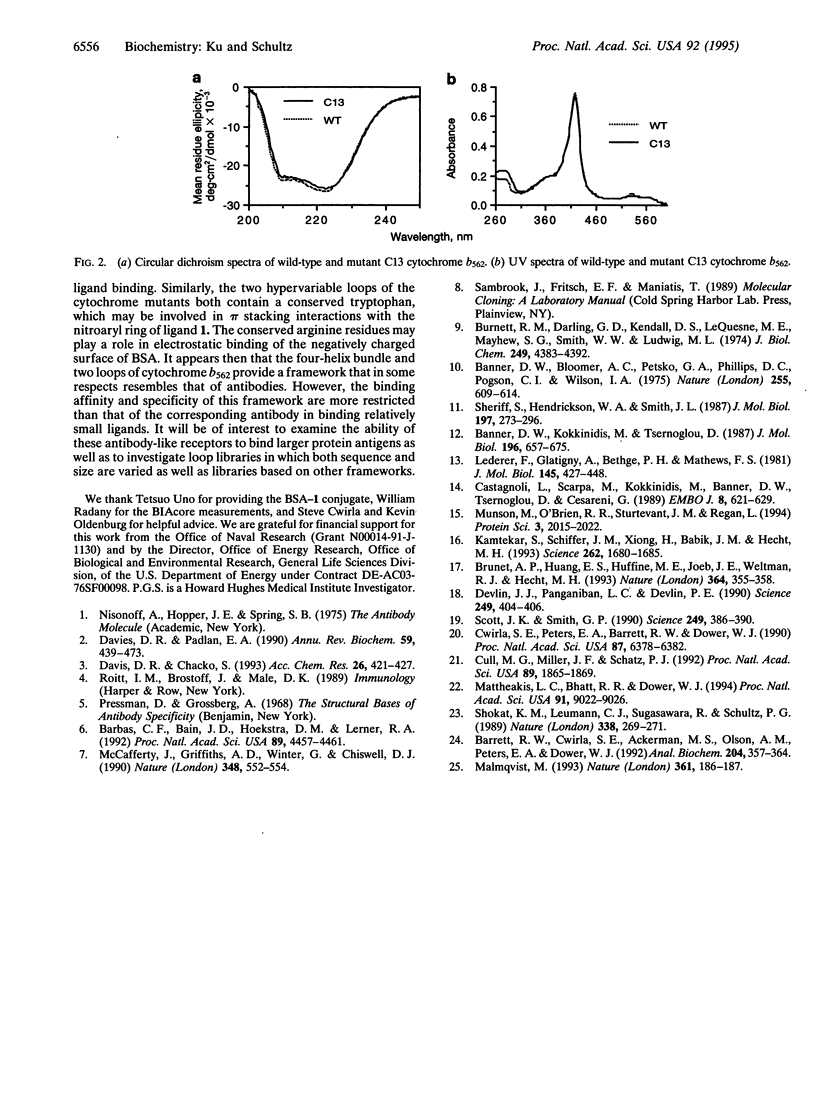
Abstract
In an effort to determine whether proteins with structures other than the immunoglobulin fold can be used to mimic the ligand binding properties of antibodies, we generated a library from the four-helix bundle protein cytochrome b562 in which the two loops were randomized. Panning of this library against the bovine serum albumin (BSA) conjugate of N-methyl-p-nitrobenzylamine derivative 1 by phage display methods yielded cytochromes in which residues Trp-20, Arg-21, and Ser-22 in loop A and Arg-83 and Trp-84 in loop B were conserved. The individual mutants, which fold into native-like structure, bind selectively to the BSA-1 conjugate with micromolar dissociation constants (Kd), in comparison to a monoclonal antibody that binds selectively to 1 with a Kd of 290 nM. These and other antibody-like receptors may prove useful as therapeutic agents or as reagents for both intra- and extracellular studies.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banner D. W., Bloomer A. C., Petsko G. A., Phillips D. C., Pogson C. I., Wilson I. A., Corran P. H., Furth A. J., Milman J. D., Offord R. E. Structure of chicken muscle triose phosphate isomerase determined crystallographically at 2.5 angstrom resolution using amino acid sequence data. Nature. 1975 Jun 19;255(5510):609–614. doi: 10.1038/255609a0. [DOI] [PubMed] [Google Scholar]
- Banner D. W., Kokkinidis M., Tsernoglou D. Structure of the ColE1 rop protein at 1.7 A resolution. J Mol Biol. 1987 Aug 5;196(3):657–675. doi: 10.1016/0022-2836(87)90039-8. [DOI] [PubMed] [Google Scholar]
- Barbas C. F., 3rd, Bain J. D., Hoekstra D. M., Lerner R. A. Semisynthetic combinatorial antibody libraries: a chemical solution to the diversity problem. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4457–4461. doi: 10.1073/pnas.89.10.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett R. W., Cwirla S. E., Ackerman M. S., Olson A. M., Peters E. A., Dower W. J. Selective enrichment and characterization of high affinity ligands from collections of random peptides on filamentous phage. Anal Biochem. 1992 Aug 1;204(2):357–364. doi: 10.1016/0003-2697(92)90252-3. [DOI] [PubMed] [Google Scholar]
- Brunet A. P., Huang E. S., Huffine M. E., Loeb J. E., Weltman R. J., Hecht M. H. The role of turns in the structure of an alpha-helical protein. Nature. 1993 Jul 22;364(6435):355–358. doi: 10.1038/364355a0. [DOI] [PubMed] [Google Scholar]
- Burnett R. M., Darling G. D., Kendall D. S., LeQuesne M. E., Mayhew S. G., Smith W. W., Ludwig M. L. The structure of the oxidized form of clostridial flavodoxin at 1.9-A resolution. J Biol Chem. 1974 Jul 25;249(14):4383–4392. [PubMed] [Google Scholar]
- Castagnoli L., Scarpa M., Kokkinidis M., Banner D. W., Tsernoglou D., Cesareni G. Genetic and structural analysis of the ColE1 Rop (Rom) protein. EMBO J. 1989 Feb;8(2):621–629. doi: 10.1002/j.1460-2075.1989.tb03417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull M. G., Miller J. F., Schatz P. J. Screening for receptor ligands using large libraries of peptides linked to the C terminus of the lac repressor. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1865–1869. doi: 10.1073/pnas.89.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwirla S. E., Peters E. A., Barrett R. W., Dower W. J. Peptides on phage: a vast library of peptides for identifying ligands. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6378–6382. doi: 10.1073/pnas.87.16.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. R., Padlan E. A., Sheriff S. Antibody-antigen complexes. Annu Rev Biochem. 1990;59:439–473. doi: 10.1146/annurev.bi.59.070190.002255. [DOI] [PubMed] [Google Scholar]
- Devlin J. J., Panganiban L. C., Devlin P. E. Random peptide libraries: a source of specific protein binding molecules. Science. 1990 Jul 27;249(4967):404–406. doi: 10.1126/science.2143033. [DOI] [PubMed] [Google Scholar]
- Kamtekar S., Schiffer J. M., Xiong H., Babik J. M., Hecht M. H. Protein design by binary patterning of polar and nonpolar amino acids. Science. 1993 Dec 10;262(5140):1680–1685. doi: 10.1126/science.8259512. [DOI] [PubMed] [Google Scholar]
- Lederer F., Glatigny A., Bethge P. H., Bellamy H. D., Matthew F. S. Improvement of the 2.5 A resolution model of cytochrome b562 by redetermining the primary structure and using molecular graphics. J Mol Biol. 1981 Jun 5;148(4):427–448. doi: 10.1016/0022-2836(81)90185-6. [DOI] [PubMed] [Google Scholar]
- Malmqvist M. Biospecific interaction analysis using biosensor technology. Nature. 1993 Jan 14;361(6408):186–187. doi: 10.1038/361186a0. [DOI] [PubMed] [Google Scholar]
- Mattheakis L. C., Bhatt R. R., Dower W. J. An in vitro polysome display system for identifying ligands from very large peptide libraries. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9022–9026. doi: 10.1073/pnas.91.19.9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCafferty J., Griffiths A. D., Winter G., Chiswell D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990 Dec 6;348(6301):552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- Munson M., O'Brien R., Sturtevant J. M., Regan L. Redesigning the hydrophobic core of a four-helix-bundle protein. Protein Sci. 1994 Nov;3(11):2015–2022. doi: 10.1002/pro.5560031114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. K., Smith G. P. Searching for peptide ligands with an epitope library. Science. 1990 Jul 27;249(4967):386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- Sheriff S., Hendrickson W. A., Smith J. L. Structure of myohemerythrin in the azidomet state at 1.7/1.3 A resolution. J Mol Biol. 1987 Sep 20;197(2):273–296. doi: 10.1016/0022-2836(87)90124-0. [DOI] [PubMed] [Google Scholar]
- Shokat K. M., Leumann C. J., Sugasawara R., Schultz P. G. A new strategy for the generation of catalytic antibodies. Nature. 1989 Mar 16;338(6212):269–271. doi: 10.1038/338269a0. [DOI] [PubMed] [Google Scholar]