Abstract
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) comprises a family of five transcription factors that form distinct protein complexes, which bind to consensus DNA sequences at promoter regions of responsive genes regulating cellular processes. The past three decades have witnessed the remarkable progress in understanding the NF-κB signaling pathway in physiologic and pathologic conditions. The role of NF-κB in human cancer initiation, development, metastasis, and resistance to treatment has drawn particular attention. A significant number of human cancers have constitutive NF-κB activity due to the inflammatory microenvironment and various oncogenic mutations. NF-κB activity not only promotes tumor cells proliferation, suppresses apoptosis, and attracts angiogenesis, but it also induces epithelialmesenchymal transition, which facilitates distant metastasis. In certain circumstances, NF-κB activation may also remodel local metabolism and anergize the immune system to favor tumor growth. Suppression of NF-κB in myeloid cells or tumor cells usually leads to tumor regression, which makes the NF-κB pathway a promising therapeutic target. However, due to its vital role in various biological activities, components of the NF-κB pathway need to be carefully selected and evaluated to design targeted therapies.
Introduction
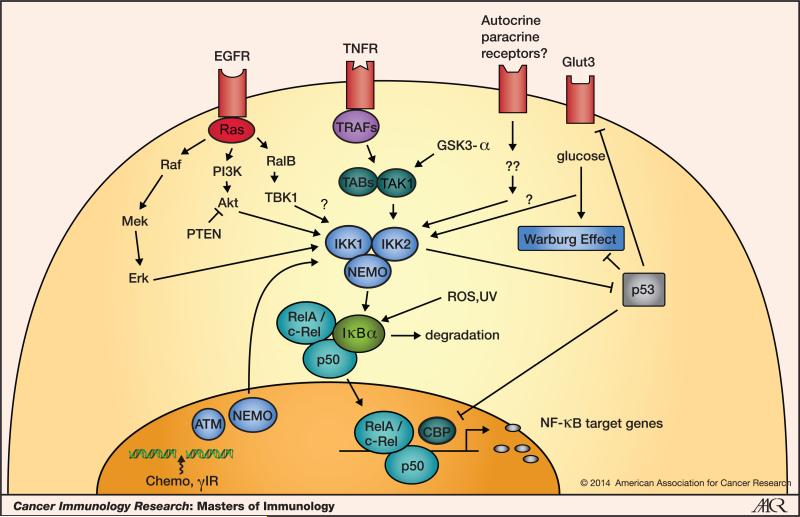
Nuclear factor-κB (NF-κB) is a family of five master transcription factors, including NF-κB1/p105, NF-κB2/p100, RelA/p65, RelB and c-Rel, which can form various heterodimers or homodimers and bind to consensus DNA sequences at promoter regions of responsive genes. Figure 1 shows some of the pathways and mediators involved in NF-κB activation in tumor cells. Originally identified as a regulator of immunoglobulin κ-light chain expression in B lymphocytes (1), its role has since been explored in inflammation, immunity, and in almost all aspects of cellular activities. NF-κB can be activated by various stimuli, such as cytokines (TNF-α, IL-1β), growth factors [epidermal growth factor (EGF)], bacterial and viral products [lipopolysaccharide (LPS), dsRNA], UV and ionizing radiation, reactive oxygen species (ROS), and DNA damage and oncogenic stress from inside the cells. Through a so-called “canonical pathway”, almost all the stimuli eventually lead to the activation of a large cytoplasmic protein complex, the inhibitor of κB (IκB) kinase (IKK) complex. The precise nature of this complex remains to be elucidated but it contains IKK1/IKKα, IKK2/IKKβ, and NEMO/IKKγ as the three seminal components. The activated IKK complex is responsible for the phosphorylation of IκB, marking it for degradation through the β-transducin repeat-containing protein (β-TrCP)-dependent E3 ubiquitin ligase-mediated proteasomal degradation machinery (2, 3). Thus, the free NF-κB dimers can translocate from the cytoplasm to the nucleus, bind to DNA and regulate gene transcription.
Figure 1. NF-κB activation in tumor cells.
At tumor sites, NF-κB is activated by various stimuli, including cytokines, growth factors, cellular and environmental stresses, and DNA damage. Tumor suppressor p53 antagonizes NF-κB function at different levels.
Stringent regulation of NF-κB is indispensable for the integrity of cellular functions, which requires both its prompt activation and termination. Dysregulation of this well-choreographed pathway has been observed in many diseases, including cancer. The study of NF-κB in cancer development started when several members of the NF-κB protein family were found to be mutated in certain types of cancers, especially those with hematopoietic origins. For example, v-Rel, the transforming gene of avian reticuloendotheliosis virus and the mutated form of its cellular homolog c-Rel can induce lymphoid malignancies in both chickens and mammals (4). Amplification and rearrangement of c-Rel are often detected in various non-Hodgkin's B-cell lymphomas (5). Similarly, NF-κB2/p100 is frequently activated through chromosomal translocations in lymphoma as well as in leukemia (6). Direct NF-κB-activating mutations are extremely rare in solid tumors compared to hematopoietic tumors. One example is the deletion of the NFKBIA gene (IκBα) in patients with non-classical glioblastoma (7). This deletion seems to be mutually exclusive to EGFR gene amplification, which suggests that NF-κB activation may substitute for aberrant EGF signaling in certain contexts. IKK1, IKK2, and IKKε mutations have been reported in genomic sequencing studies of breast and prostate cancers (8-10); however, a direct link of these mutations and constitutive NF-κB activation in tumor cells has not been well established. Thus, constitutive NF-κB activation in a variety of solid tumors may be influenced by the microenvironment rather than by genetic mutations within the pathway.
Chronic inflammation, NF-κB, and cancer
Inflammation is a key defense mechanism in innate immunity that fights against bacterial and viral infections, maintains tissue homeostasis, and facilitates wound healing. Inflammation progression can be categorized at the acute, adaptive, and resolution stages (11). Prolonged chronic inflammation may lead to tissue damage, autoimmune diseases, degenerative diseases, and cancers of multiple types by enhancing cellular stress, recruiting inflammatory factors, and accumulating DNA damage. Furthermore, chronic inflammation also promotes tumorigenesis by altering genetic sequences and epigenetic states of the damaged tissue and its microenvironment. Thus, “avoiding immune destruction” and “tumor-promoting inflammation” have been described as two emerging hallmarks of cancer (12). Inflammation plays a two-prong role in tumor formation: it disables the immune system from attacking tumor cells; and it induces cell proliferation and genetic instability that leads to oncogenic mutations.
NF-κB, the master regulator, mediates a crosstalk between inflammation and cancer at multiple levels. In tumorous tissues with elevated NF-κB activity, the accumulation of pro-inflammatory cytokines at the tumor site directly contributes to the pro-tumorigenic microenvironment. In patients with inflammatory bowel disease (IBD), for example, immune cells infiltrating the gastrointestinal mucosa secrete pro-tumorigenic cytokines such as TNF-α, IL-1, and IL-17 to elevate NF-κB activity, and increasing the risk of colon cancer (13). Through epigenetic mechanisms, these inflammatory signals may also fine-tune the level of the let-7 family of tumor suppressor microRNAs and modulate IL-6/STAT3 signaling to establish a positive feed-back loop that results in uncontrolled cell proliferation and cancer initiation (14). Similarly, the cholestatic hepatitis that spontaneously develops in Mdr2-knockout (Mdr2 KO) mice triggers NF-κB activity in a TNF-α-dependent manner. Although this NF-κB activation has little effect on the hepatitis or tumor initiation, it is indispensable for later tumor development (15). As a corollary, the frequent up-regulation of the NF-κB signaling pathway in multiple forms of carcinoma establishes a permissive microenvironment, which is critical for either tumor initiation or tumor development, or both.
On the other hand, a chronic inflammatory microenvironment may lead to immunosuppression and favor tumor escape from immunosurveillance. Myeloid-derived suppressor cells (MDSC) expressing elevated levels of arginase I, inducible nitric oxide synthases (iNOS), and GR1, are recruited to the tumor site to suppress antitumor T-cell functions (16). Meanwhile, MDSCs also promote the development of FOXP3+ regulatory T cells (Treg) in the presence of IFNγ and IL-10. NF-κB directly modulates Treg development by regulating FOXP3 expression at its enhancer region (17). Treg infiltration into the tumor not only leads to tumor immune escape, but also promotes angiogenesis through the release of various chemokines (TGF-β, IL-10, and IL-35) (18). Furthermore, an inflammatory tumor microenvironment often induces tumor-infiltrating macrophages (TAM) to switch from a M1- to M2-polarized state (with low tumoricidal activity, more angiogenesis, and tissue remodeling). Reciprocally, the inhibition of NF-κB in TAMs can revert them to a M1-polarized state (19). Consistent with this idea, NF-κB protein p50 has been shown to suppress M1- and induce M2-polarization of macrophage, thus favoring the immunosuppressive microenvironment (20).
Chronic inflammation can lead to genomic instability and genetic mutations that favor tumor initiation and development (21, 22). ROS are typically released by neutrophils and macrophages at the site of inflammation, and can cause DNA damage. ROS and cytokines released by immune cells also can activate the NF-κB pathway and form a positive feedback loop to enhance NF-κB activity in different types of cells at the site of inflammation. Interestingly, NF-κB activation has been shown to induce the expression of activation-induced cytidine deaminase (AID), an enzyme that introduces mutations in p53, Myc and other cellular genes (23). Furthermore, inflammatory mediators including cytokines, prostaglandin E2 (PGE2), and ROS can suppress the DNA mismatch repair machinery through different mechanisms leading to the accumulation of more genetic mutations (21).
Beyond inflammation
Although a major contribution of the NF-κB pathway in cancer development is through inducing and maintaining a chronic inflammatory microenvironment, other effects are of equal importance. Constitutive NF-κB activity in cancer cells promotes tumor initiation and development, perhaps through the following four mechanisms.
First, NF-κB stimulates cell proliferation and prevents apoptosis
NF-κB induces the expression of anti-apoptotic genes such as the caspase-8 inhibitor FLIP, the inhibitor of apoptosis proteins c-IAP1/2 and XIAP, and members of the Bcl2 family of apoptosis regulators (see http://www.bu.edu/nf-kb/ for a growing list of NF-κB target genes, maintained by Gilmore). Mouse embryos devoid of RelA, IKK2, or NEMO die between E12.5 and E15, mainly due to TNF-α-induced hepatocyte toxicity (see reference 24 for a review of NF-κB knockout and transgenic mice). These observations support the idea that tumor cells may also rely on the NF-κB pathway to escape from apoptosis, which has been identified as one of the essential hallmarks of cancer (12). However, in vivo evidence of the role of NF-κB has not been established for most cancer types. In Kras-induced lung adenocarcinoma, for example, inhibition of the NF-κB pathway in tumor cells by either overexpressing the IκBαM super-repressor or deleting RelA or IKK2 reduced tumor size in general, but alterations in apoptotic pathways were not detected (25-27). Interestingly, data from our lung cancer model indicate that tumor cell proliferation was significantly impaired when IKK2 was deleted. We have also identified a positive feedback loop, Kras—Erk—NF-κB—Timp1—CD63—FAK—Erk, which could be blocked when NF-κB activity is inhibited (26). Based on this result, it is possible that the role of NF-κB in certain types of cancer and at certain stages of cancer development is mainly through promoting cell proliferation rather than inhibiting apoptosis.
Second, NF-κB regulates tumor angiogenesis
One of the most studied angiogenic factors is vascular-endothelial growth factor (VEGF), whose expression is strongly regulated by HIF-1α in hypoxic conditions, and by numerous other stimuli such as cytokines and oncogenes (28), which are also critical mediators of NF-κB activation. It has been shown that inhibition of NF-κB abolishes VEGF production and angiogenesis in a variety of conditions. Furthermore, basic fibroblast growth factor (bFGF), IL-8, matrix metalloproteinase-9 (MMP-9), and other NF-κB target genes are involved in multiple steps of angiogenesis (29). It is worth noting that matrix metalloproteinases including MMP-2, -3, and -9 degrade the basement membrane and remodel the extracellular matrix, which facilitates cell migration and favors either angiogenesis (endothelial cells) or metastasis (cancer cells) in different microenvironment (30).
Third, NF-κB promotes tumor metastasis at different levels
Besides regulating the expression of MMPs as discussed above, NF-κB also plays a significant role in many other aspects of metastasis. Epithelial-mesenchymal transition (EMT) is an early event in metastasis (31). Twist1, one of the key transcription factors modulating EMT, is an NF-κB target in breast cancer cells upon TNF-α stimulation (32). Snail, a zinc-finger transcription repressor, on the other hand, is stabilized by the COP9 signalosome 2 (CSN2), a protein complex in the ubiquitin-proteasome pathway that contributes to NF-κB activation in inflammation-induced cell migration and invasion (33). Cell adhesion molecules such as selectins, integrins and their ligands are largely regulated by the NF-κB pathway (34), and are important in promoting cancer cell extravasation and colonization at distant sites, although the mechanistic details remain elusive (35). In addition to a role in the primary tumor, NF-κB signaling in the pre-metastatic niche may help create a suitable environment for the seeding of primary tumor cells. In the premetastatic lung, inflammation mediator serum amyloid A3 (SAA3)-TLR4 signaling induces NF-κB activity in both lung epithelial cells and myeloid cells, which has been shown to help establish an inflammatory state that facilitates metastasis (36).
Fourth, NF-κB directly remodels tumor metabolism
Reprogramming energy metabolism has been identified as another emerging hallmark of cancer (12). Direct regulation of cell metabolism by the NF-κB pathway has long been speculated and recently addressed by several studies. Tanaka and colleagues showed that NF-κB activation in p53−/− mouse embryonic fibroblasts (MEF) can increase glucose uptake by upregulating the expression of glucose transporter 3 (GLUT3) and maintaining a high glycolytic flux (37). These investigators showed that high levels of glycolysis in the transformed cells activate the NF-κB pathway via an O-linked N-acetylglucosamine (O-GlcNAc) modification of IKK2, thus forming a positive feedback loop (38). However, most of these observations are in immortalized MEFs, and the importance of this positive feedback loop in tumor cells in vivo remains to be clarified. NF-κB also modulates mitochondrial respiration by regulating cytochrome c oxidase (SCO2), a critical subunit of the mitochondrial respiratory complex. NF-κB regulation of SCO2 is mediated by p53; in the absence of p53, NF-κB translocates to the nucleus and blocks mitochondrial oxidative phosphorylation, thus enhancing the “Warburg effect” in cancer cells (39). Regulation of cellular metabolism by NF-κB depends on the status of p53 in cells. This is one of the many aspects of the crucial crosstalk between NF-κB and p53.
NF-κB, Kras, and p53
Many oncogenic mutations, such as those in EGFR, Ras, PI3K and p53, contribute to NF-κB activation in tumor cells. Kras and p53 mutations have been found in 20-25% and in ~50% of all cancers, respectively, and the mutation rates are especially high in pancreatic, colorectal and lung cancers. The molecular mechanism by which Kras activates the NF-κB pathway has been studied extensively. Jacks and colleagues showed in a mouse lung adenocarcinoma model that Kras mutation and p53 deficiency cooperate to activate the NF-κB pathway, which is essential for the survival of tumor cells (25). Results from our laboratory and from the Baldwin laboratory indicate that Kras mutation alone is sufficient to activate NF-κB both in vitro and in the mouse (26, 27). Nevertheless, studies from all three laboratories demonstrated the importance of NF-κB activation in Kras-induced lung cancer. Inhibition of NF-κB either by knocking out RelA or IKK2, or by overexpressing a dominant negative form of IκBα, significantly reduced tumor volume, lowered tumor grade, and prolonged mouse survival. In addition to the canonical pathway that requires IKK2, activation of NF-κB by a non-canonical IκB kinase, TBK1, has been identified in a synthetic lethality screen of Kras mutant tumors (40). c-Rel and Bcl-XL are two essential elements for tumor cell survival downstream of TBK1. While the mechanism of how TBK1 is activated in Kras mutant tumors remains unknown, knockdown of RalB, a component downstream of the Ras effector–RalGDS, selectively kills Kras-dependent tumor cell lines. This observation is consistent with the earlier report that RalB-activated TBK1 signaling is required for cancer cell survival and Kras-induced transformation (41). Furthermore, molecules critical for the survival of the tumors harboring mutated Kras also are involved in the NF-κB pathway. Glycogen synthase kinase 3α (GSK-3α), for example, has been reported to be upregulated in mutated Kras-induced pancreatic cancer. Pharmacologic inhibition of GSK-3α suppresses the growth of human pancreatic tumor explants in mice (42). Interestingly, GSK-3α not only promotes canonical IKK activity by stabilizing the TAK1/TAB complex downstream of Kras, it also promotes the non-canonical NF-κB pathway by controlling the level of NF-κB2 (p100) in the nucleus. Indeed, TAK1 inhibition has also been shown to promote apoptosis in Kras-dependent colon cancers (43). p62 appears to be another critical adaptor linking Kras and NF-κB activation. p62-deficient mice are resistant to Kras-induced lung adenocarcinomas. Mechanistically, Kras increases p62 expression, facilitating TRAF6 polyubiquitination and IKK activation, and thereby protects cells from ROS-induced cell death (44). Furthermore, p62 is an NF-κB target gene with two NF-κB binding sites within its promoter region, and thus it forms a positive-feedback loop to sustain NF-κB activation downstream of Kras (45). Similar feed-forward loops are established with IL-1α and Timp-1 (26).
The crosstalk between p53 and NF-κB has drawn much attention in the cancer research community. As described above, wild-type p53 antagonizes NF-κB function and suppresses tumorigenesis; about 50% of human cancers acquire p53 mutations (or lose the wild-type allele) and thus release the brake on the NF-κB pathway during tumor development.
The first layer of crosstalk has been suggested by Perkins and others to be the competition for a limited pool of transcription co-factor CBP, which binds the cAMP-response element-binding protein (CREB). This suggestion was based on the observation that binding of CBP to p53 or NF-κB decides a cell's fate for apoptosis or survival (46). However, many other transcription factors, besides p53 and NF-κB, use the same pool of CBP/p300 family members as co-factors to activate target gene transcription, so that the crosstalk may be more complicated than passive competition. For example, upon encountering certain stimuli IKK1 can directly phosphorylate CBP and increases its binding to RelA (47). Consistent with this finding, IKK1−/− cells have more CBP bound to CREB, so it is possible that CBP phosphorylation by IKK1 switches its binding affinity for different transcription factors. Similarly, upon LPS stimulation, GSK-3β has been shown to reduce nuclear phosphorylated CREB and their binding to CBP, making CBP more accessible to NF-κB (48). In contrast, phosphorylated CREB facilitates the recruitment of CBP to p53 through the KIX domain on CBP, which favors p53 target-gene expression(49). These studies indicate the complexity of competing for CBP between NF-κB and p53.
The second layer of crosstalk involves the direct regulation of signal pathway components. IKK2 is the essential kinase in the canonical IKK complex, but it also mediates NF-κB-independent functions through phosphorylation of other substrates (2). p53 has been identified as one of the IKK2 substrates, based on a consensus phosphorylation motif search. p53 harbors in its C-terminus (Ser362 and Ser366) a (D/A)S(G/L/D/R){G/D/R}sXS motif found in most of the IKK2 substrates including IκBα. This motif is readily phosphorylated by IKK2 upon DNA damage induced by doxorubicin, and followed by β-TrCP-mediated poly-ubiquitination and proteasomal degradation (50). Interestingly, this regulation only occurs upon doxorubicin treatment but not after treatment with TNF-α, another potent NF-κB activator. This observation suggests that a prerequisite modification on p53 by a particular stimulus is needed, which might be finely tuned in the tumor microenvironment. Furthermore, the NF-κB pathway is also involved in the transcription of Mdm2, a key ubiquitin E3 ligase of p53, thus indirectly regulating p53 protein stability (51).
On the other hand, as we have discussed earlier, wild-type p53 may suppress glucose intake and glycolysis by reducing GLUT3 expression on the cell membrane (37). Low levels of glycolysis result in impaired O-GlcNAc modification of IKK2 and thereby diminished kinase activity (38). This may be one of the mechanisms by which wild-type p53 suppresses the NF-κB pathway to a basal level in untransformed cells. In sharp contrast, p53 mutations prolong NF-κB activation in the presence of inflammatory stimuli. For example, a recent study from the Oren laboratory examined the correlation between nuclear p65 staining and p53 mutation status in multiple head and neck squamous cell carcinomas and non-small cell lung cancers (NSCLC). They found that mutant p53 overexpression correlates with increased NF-κB activity and reduced apoptosis, while tumors harboring wild-type p53 have much less nuclear p65 staining (52). Furthermore, mice harboring a germline p53 mutation develop more severe chronic inflammation and persistent tissue damage in the dextran sulfate sodium (DSS)-induced mouse colon cancer model. These mice are much more prone to inflammation-associated colon cancer when compared to their p53 wild-type counterparts.
Pro- and anti-tumorigenesis in different human organs
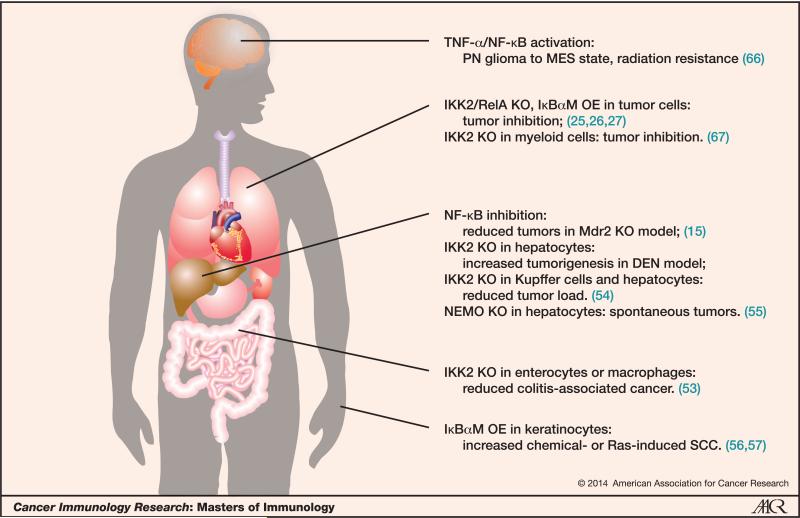
As we have discussed, in most cases NF-κB plays a role as a tumor promoter, especially in the chronic inflammation-related cancers. In a mouse model of colitis-associated colon cancer, selective ablation of IKK2 in enterocytes significantly decreased tumor incidence (53). Similarly, in an Mdr2 knockout (KO)-induced hepatocarcinoma model, overexpression (OE) of the IκBα super-repressor in liver cells blocked tumor development significantly (15). Furthermore, recent studies from many groups, including our own, showed that inhibition of NF-κB in mutated Kras-induced lung cancer and pancreatic cancer greatly reduced tumor initiation and progression (25-27). Figure 2 shows some of the antitumor or protumor effects involving the NF-κB pathways in various human cancers.
Figure 2. Protumor and antitumor effects of NF-κB in different human organs and related cancers.
Abbreviations: PN=proneural; MES=mesenchymal; KO=knockout; OE=overexpression; Mdr2=P-glycoprotein; DEN=diethylnitrosamine; NEMO (IKK-g)=regulatory subunit of the IKK complex; IκBαM=IκBα mutant; SCC=squamous cell carcinoma.
However, every coin has two sides. In several specific cases NF-κB appears to be a tumor suppressor, with the liver being one prime example. Although NF-κB has a tumor promoting role in the Mdr2−/− hepatocellular carcinoma (HCC) model, studies in the diethylnitrosamine(DEN)-induced HCC model yielded opposite results: IKK2 targeted-deletion in hepatocytes strongly enhanced tumorigenesis (54). These IKK2-deficient hepatocytes underwent cell death upon DEN treatment, and the compensatory liver regeneration eventually resulted in HCC. Similarly, NEMO deletion in hepatocytes triggered liver damage, hepatosteatosis, hepatitis, fibrosis, and finally HCC (55). The skin is another special site, since inhibition of NF-κB in keratinocytes led to increased squamous cell carcinoma (SCC) in both the DMBA/TPA- and Ras-induced models (56, 57). These results suggest that suppression of NF-κB in keratinocytes might impair cell-cycle arrest upon DNA damage or oncogenic stress. Interestingly, NF-κB and JNK are two of the major signaling pathways downstream of TNFR1 that counter-regulate one another. Upon NF-κB inhibition, JNK signaling is unleashed leading to excessive oxidative stress and DNA damage (58, 59). This could be one of the mechanisms by which NF-κB acts as a tumor suppressor in both chemically-induced skin cancer and liver cancer.
Importantly, although NF-κB inhibition in different organs has varying effects on tumorigenesis, the inhibition of NF-κB in inflammatory or myeloid cells consistently suppresses tumor development in the models described above. NF-κB activation in myeloid cells typically enhances inflammation in the tumor microenvironment by increasing the secretion of pro-inflammatory cytokines such as TNF-α and IL-6, which eventually leads to rapid proliferation of tumor cells (13).
Prospects: NF-κB in cancer therapy
Given that NF-κB has such an important role in both tumor cells and the tumor microenvironment, targeting NF-κB as a cancer therapy has been explored extensively in the past decades. Hundreds of natural and synthetic compounds have been reported as NF-κB inhibitors, however their clinical application to date has shown little efficacy, except for certain types of lymphoma and leukemia (60). One of the major concerns is immunosuppression after long-term systemic administration of NF-κB inhibitors, since the NF-κB pathway mediates pleiotropic functions in the innate and adaptive immune responses (3). Furthermore, the NF-κB pathway has also been shown to regulate pro-IL-1β processing and secretion. The selective deletion in myeloid cells or the pharmacologic suppression of IKK2 in mice increased endotoxin susceptibility with elevated plasma IL-1β levels (61). With these considerations, the dose, schedule and delivery strategy should be carefully evaluated when applying NF-κB inhibitors to treat human malignancies. One possible future direction is to design inhibitors targeting molecules that are only vulnerable in cancer cells, such as TBK1 that was identified in Kras synthetic lethality screening (40), to avoid systemic toxicity. Another concern is the rapidly-gained resistance to NF-κB inhibitors. In the mouse NSCLC model induced by Kras and p53 compound mutations, treatment with various NF-κB inhibitors prolonged mouse survival (26, 62), however resistant tumors appeared within several weeks. Interestingly, these resistant tumors did not show noticeable elevation of basal NF-κB activity or increased expression of NF-κB target genes (62). Mechanisms that led to this resistance remain to be clarified. Nevertheless, NF-κB inhibitors still appear attractive in combination with other chemotherapies. Many anticancer agents can activate the NF-κB pathway through induction of TNF-α, ROS, and other cellular stresses, or directly by generating DNA double-strand breaks that are sensed by the ATM-NEMO-dependent pathway from inside the nucleus (63). Activation of the NF-κB pathway usually protects cancer cells from apoptosis through antagonizing the p53 pathway or through direct upregulation of a group of anti-apoptotic genes. For example, the adenoviral-mediated delivery of IκBαM into tumor cells in a xenograft model enhanced sensitivity to various chemotherapies (64). The proteasome inhibitor Bortezomib, which blocks IκB degradation, showed similar effects (65). However, these results still need to be verified in human patients with an intact tumor microenvironment. The bottom-line is that NF-κB activation can be an important biomarker for chemo-radiation therapies. A recent study showed that NF-κB activation is linked to mesenchymal differentiation of glioblastoma, and enhanced resistance to radiation therapy (66). Clinical trials are currently investigating the activation of NF-κB in response to treatment with external beam radiotherapy and chemotherapy on rectal carcinomas (NCT00280761) and stage II/III gastric cancers (NCT01905969), and its association with therapeutic outcomes; the results of these studies will be of great interest.
It has been nearly three decades since NF-κB was identified. Since then, many researchers have published thousands of papers delineating components of pathways leading to the activation of NF-κB. A large number of NF-κB inducible genes have been identified in response to a wide variety of stimuli. NF-κB is a central player in innate and adaptive immune responses of the host. Yet it has been a challenge to tame or manipulate the activity of this family of transcription factors because they are pleiotropic. Perhaps acute inhibition of NF-κB may be more therapeutically manageable for beneficial outcome. NF-κB remains a fascinating but elusive target!
Acknowledgements
I.M.V is an American Cancer Society Professor of Molecular Biology, and holds the Irwin and Joan Jacobs Chair in Exemplary Life Science. This work was supported in part by grants from the NIH (R01-AI048034 from the National Institute of Allergy and Infectious Diseases and P30CA014195 from the National Cancer Institute), IpsenBiomeasure, the H.N. and Frances C. Berger Foundation, and the Leona M. and Harry B. Helmsley Charitable Trust grant #2012-PG-MED002.The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
References
- 1.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–16. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- 2.Liu F, Xia Y, Parker AS, Verma IM. IKK biology. Immunol Rev. 2012;246:239–53. doi: 10.1111/j.1600-065X.2012.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–34. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 4.Gilmore TD. Multiple mutations contribute to the oncogenicity of the retroviral oncoprotein v-Rel. Oncogene. 1999;18:6925–37. doi: 10.1038/sj.onc.1203222. [DOI] [PubMed] [Google Scholar]
- 5.Gilmore TD, Kalaitzidis D, Liang MC, Starczynowski DT. The c-Rel transcription factor and B-cell proliferation: a deal with the devil. Oncogene. 2004;23:2275–86. doi: 10.1038/sj.onc.1207410. [DOI] [PubMed] [Google Scholar]
- 6.Neri A, Chang CC, Lombardi L, Salina M, Corradini P, Maiolo AT, et al. B cell lymphoma-associated chromosomal translocation involves candidate oncogene lyt-10, homologous to NF-kappa B p50. Cell. 1991;67:1075–87. doi: 10.1016/0092-8674(91)90285-7. [DOI] [PubMed] [Google Scholar]
- 7.Bredel M, Scholtens DM, Yadav AK, Alvarez AA, Renfrow JJ, Chandler JP, et al. NFKBIA deletion in glioblastomas. N Engl J Med. 2011;364:627–37. doi: 10.1056/NEJMoa1006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–8. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pflueger D, Terry S, Sboner A, Habegger L, Esgueva R, Lin PC, et al. Discovery of non-ETS gene fusions in human prostate cancer using next-generation RNA sequencing. Genome Res. 2011;21:56–67. doi: 10.1101/gr.110684.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehm JS, Zhao JJ, Yao J, Kim SY, Firestein R, Dunn IF, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129:1065–79. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 11.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–14. e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 14.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 16.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31:921–31. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72:2162–71. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, et al. “Reeducating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205:1261–8. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Di Liberto D, et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci U S A. 2009;106:14978–83. doi: 10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 22.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–71. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto Y, Marusawa H, Kinoshita K, Endo Y, Kou T, Morisawa T, et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470–6. doi: 10.1038/nm1566. [DOI] [PubMed] [Google Scholar]
- 24.Gerondakis S, Grumont R, Gugasyan R, Wong L, Isomura I, Ho W, et al. Unravelling the complexities of the NF-kappaB signalling pathway using mouse knockout and transgenic models. Oncogene. 2006;25:6781–99. doi: 10.1038/sj.onc.1209944. [DOI] [PubMed] [Google Scholar]
- 25.Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, Ouyang C, et al. Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature. 2009;462:104–7. doi: 10.1038/nature08462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia Y, Yeddula N, Leblanc M, Ke E, Zhang Y, Oldfield E, et al. Reduced cell proliferation by IKK2 depletion in a mouse lung-cancer model. Nat Cell Biol. 2012;14:257–65. doi: 10.1038/ncb2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basseres DS, Ebbs A, Levantini E, Baldwin AS. Requirement of the NF-kappaB subunit p65/RelA for K-Ras-induced lung tumorigenesis. Cancer Res. 2010;70:3537–46. doi: 10.1158/0008-5472.CAN-09-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–97. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 30.John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res. 2001;7:14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- 31.Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–9. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Li CW, Xia W, Huo L, Lim SO, Wu Y, Hsu JL, et al. Epithelial-mesenchymal transition induced by TNF-alpha requires NF-kappaB-mediated transcriptional upregulation of Twist1. Cancer Res. 2012;72:1290–300. doi: 10.1158/0008-5472.CAN-11-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–28. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 35.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 36.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–75. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 37.Kawauchi K, Araki K, Tobiume K, Tanaka N. p53 regulates glucose metabolism through an IKK-NF-kappaB pathway and inhibits cell transformation. Nat Cell Biol. 2008;10:611–8. doi: 10.1038/ncb1724. [DOI] [PubMed] [Google Scholar]
- 38.Kawauchi K, Araki K, Tobiume K, Tanaka N. Loss of p53 enhances catalytic activity of IKKbeta through O-linked beta-N-acetyl glucosamine modification. Proc Natl Acad Sci U S A. 2009;106:3431–6. doi: 10.1073/pnas.0813210106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mauro C, Leow SC, Anso E, Rocha S, Thotakura AK, Tornatore L, et al. NF-kappaB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat Cell Biol. 2011;13:1272–9. doi: 10.1038/ncb2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462:108–12. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chien Y, Kim S, Bumeister R, Loo YM, Kwon SW, Johnson CL, et al. RalB GTPase-mediated activation of the IkappaB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell. 2006;127:157–70. doi: 10.1016/j.cell.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 42.Bang D, Wilson W, Ryan M, Yeh JJ, Baldwin AS. GSK-3alpha promotes oncogenic KRAS function in pancreatic cancer via TAK1-TAB stabilization and regulation of noncanonical NF-kappaB. Cancer Discov. 2013;3:690–703. doi: 10.1158/2159-8290.CD-12-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh A, Sweeney MF, Yu M, Burger A, Greninger P, Benes C, et al. TAK1 inhibition promotes apoptosis in KRAS-dependent colon cancers. Cell. 2012;148:639–50. doi: 10.1016/j.cell.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duran A, Linares JF, Galvez AS, Wikenheiser K, Flores JM, Diaz-Meco MT, et al. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell. 2008;13:343–54. doi: 10.1016/j.ccr.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Ling J, Kang Y, Zhao R, Xia Q, Lee DF, Chang Z, et al. KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:105–20. doi: 10.1016/j.ccr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webster GA, Perkins ND. Transcriptional cross talk between NF-kappaB and p53. Mol Cell Biol. 1999;19:3485–95. doi: 10.1128/mcb.19.5.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang WC, Ju TK, Hung MC, Chen CC. Phosphorylation of CBP by IKKalpha promotes cell growth by switching the binding preference of CBP from p53 to NF-kappaB. Mol Cell. 2007;26:75–87. doi: 10.1016/j.molcel.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–84. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giebler HA, Lemasson I, Nyborg JK. p53 recruitment of CREB binding protein mediated through phosphorylated CREB: a novel pathway of tumor suppressor regulation. Mol Cell Biol. 2000;20:4849–58. doi: 10.1128/mcb.20.13.4849-4858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia Y, Padre RC, De Mendoza TH, Bottero V, Tergaonkar VB, Verma IM. Phosphorylation of p53 by IkappaB kinase 2 promotes its degradation by beta-TrCP. Proc Natl Acad Sci U S A. 2009;106:2629–34. doi: 10.1073/pnas.0812256106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tergaonkar V, Pando M, Vafa O, Wahl G, Verma I. p53 stabilization is decreased upon NFkappaB activation: a role for NFkappaB in acquisition of resistance to chemotherapy. Cancer Cell. 2002;1:493–503. doi: 10.1016/s1535-6108(02)00068-5. [DOI] [PubMed] [Google Scholar]
- 52.Cooks T, Pateras IS, Tarcic O, Solomon H, Schetter AJ, Wilder S, et al. Mutant p53 prolongs NF-kappaB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell. 2013;23:634–46. doi: 10.1016/j.ccr.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 54.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–90. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 55.Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–32. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 56.Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, et al. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421:639–43. doi: 10.1038/nature01283. [DOI] [PubMed] [Google Scholar]
- 57.van Hogerlinden M, Rozell BL, Ahrlund-Richter L, Toftgard R. Squamous cell carcinomas and increased apoptosis in skin with inhibited Rel/nuclear factor-kappaB signaling. Cancer Res. 1999;59:3299–303. [PubMed] [Google Scholar]
- 58.Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–42. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 59.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–61. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 60.Keutgens A, Robert I, Viatour P, Chariot A. Deregulated NF-kappaB activity in haematological malignancies. Biochem Pharmacol. 2006;72:1069–80. doi: 10.1016/j.bcp.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–31. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xue W, Meylan E, Oliver TG, Feldser DM, Winslow MM, Bronson R, et al. Response and resistance to NF-kappaB inhibitors in mouse models of lung adenocarcinoma. Cancer Discov. 2011;1:236–47. doi: 10.1158/2159-8290.CD-11-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science. 2006;311:1141–6. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- 64.Wang CY, Cusack JC, Jr., Liu R, Baldwin AS., Jr. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med. 1999;5:412–7. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 65.Cusack JC, Jr., Liu R, Houston M, Abendroth K, Elliott PJ, Adams J, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res. 2001;61:3535–40. [PubMed] [Google Scholar]
- 66.Bhat KP, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, et al. Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer Cell. 2013;24:331–46. doi: 10.1016/j.ccr.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell. 2010;17:89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]