Abstract
Self-monitoring of blood glucose (SMBG) is clearly correlated with increased life expectancy and quality of life in type 2 diabetic patients.
Objective
The objective of our study was to record and assess the errors patients make in preparing, performing, and processing self-monitoring of blood glucose (SMBG). Furthermore, the study aimed to determine to what extent a single standardized SMBG instruction session in a community pharmacy might reduce the number of patients making errors or the number of errors per patient.
Methods
Between May and October 2005, SMBG of 462 randomly selected patients with type 2 diabetes was monitored in 32 pharmacies specialized in diabetes care. The patients performed blood glucose self-tests using their own blood glucose meters. Self-testing was monitored using a standardized documentation sheet on which any error made during the performance of the test was recorded. If necessary, patients were instructed in the accurate operation of their meter and the use of the necessary equipment. Additionally, patients obtained written instructions. Six weeks later, assessment of the quality of patient’s SMBG was repeated.
Results
During the first observation, 383 patients (83%) made at least one mistake performing SMBG. By the time of the second observation, this frequency had fallen to 189 (41%) (p<0.001). The average number of mistakes fell from 3.1 to 0.8 per patient. Mistakes that may potentially have led to inaccurate readings were initially recorded for 283 (61%) and at study end for 110 (24%) patients (p<0.001).
Conclusion
It is important to periodically instruct type 2 diabetic patients in the proper SMBG technique in order to ensure accurate measurements. In this study it was shown that community pharmacies specialized in diabetes care can provide this service effectively.
Keywords: Diabetes mellitus, Blood glucose self- monitoring, Patient education, Community pharmacy services, Germany
INTRODUCTION
An important goal in the treatment of diabetes is to achieve and maintain blood glucose levels as close to normal as possible. That is why it is essential to train patients in how to effectively self-manage their diabetes,1,2,3,4,5 not only to improve their treatment but also to improve their quality of life. The development in the late 1970s of methods to self-monitor blood glucose levels6,7 was an indispensable prerequisite for this.
Only through regular self-monitoring of blood glucose levels (SMBG) it has become possible to coordinate drug therapy as well as food intake and exercise so that a good metabolic control can be achieved.8,9,10 Furthermore, it has become easier to identify asymptomatic hypo- and hyperglycemias and blood glucose fluctuations.
According to recommendations of the German Diabetes Society (DDG), regular monitoring of blood glucose should be conducted by all persons with type 1 diabetes after they have undergone specific training. The benefit of regular SMBG in this therapy concept is evidence-based.11
For patients with type 2 diabetes the situation is more differentiated. For this group of patients, the DDG recommends blood glucose measurements when therapeutic consequences result from the readings.12 The benefit of SMBG is not disputed for intensified insulin treatment. This is different for patients who are treated only with a diabetic nutrition plan, with oral antidiabetic drugs (OAD), or with conventional insulin treatment. Until now for them SMBG was only indicated under certain circumstances e.g., for an abnormal renal threshold or pregnancy. The benefit of regular blood glucose testing for this patient group has been the subject of controversial discussion for decades.8,9,10,13,14,15,16,17,18 The ROSSO study provided recently clear evidence on the benefits of SMBG for type 2 diabetic patients, for the first time. For the SMBG group, the risk of developing cardiovascular diseases was about one third lower than for the group without self- monitoring, and the mortality rate was even 50% lower. Also for the subgroup of patients not receiving insulin, the morbidity risk was about a third lower and the mortality risk was about 40% lower when compared to the control group not performing SMBG.19 The ROSSO study concludes that people with type 2 diabetes benefit from SMBG, regardless of their kind of treatment.
The quality of SMBG depends on the quality of the blood glucose meter and how well the user prepares and carries out the test and performs the follow-up steps.
Most commonly used meters cover the blood glucose range of 0.6 – 33.3 mmol/L (10-600 mg/dL).
For laboratory testing, according to German Medical Association guidelines on the quality assurance of quantitative tests in medical laboratories, maximal deviations of the individual levels of ± 15% are allowed.20 For test strips and sensors for blood glucose meters at least 95% of all results must be within a ± 20% deviation from the true value.21
Due to the progress achieved in recent years in the development of new systems for blood glucose self- monitoring, the meters currently available on the market are easy to use. Nevertheless, this user- friendliness does not ensure that the readings are error-free. Different studies on the evaluation of blood glucose self-monitoring in individuals with diabetes have shown that a number of errors occur during the self-monitoring.22,23,24,25,26,27,28,29,30,31,32 The American Diabetes Association (ADA) assumed in a consensus report published in 1990 that up to 50% of the self-monitored blood glucose readings have more than 20% deviation from the true values.33 However, more recent studies found the percentage of deviation to be less. Alto et al. found deviations of over 20% in 16% of the study participants22 and Kabadi et al. found greater deviations in 25% of the participants.24 In the studies of Bergenstal and Schrot et al., 19% and 31%, respectively, of the readings deviated more than 15% from the control reading.27,30
To reduce this error rate, the ADA recommends regular evaluations of the patients’ SMBG by healthcare professionals.34
The aims of this study were to determine the quality of SMBG performed by individuals with type 2 diabetes and to reduce the number of patients who make errors or the number of errors per patient. These were to be accomplished by offering a structured training session in self-monitoring in community pharmacies. The purpose of the study was not to test the accuracy of the readings or to validate the blood glucose meters. Taking venous blood samples is not allowed in pharmacies in Germany, and therefore no exact quality-controlled laboratory readings of the blood glucose concentrations could be provided.
METHODS
The study was conducted in the pharmacies between May and October 2005.
The project was designed as a prospective multi- center intervention study.
Since several studies have shown that patient education tends to increase the quality of
SMBG25,26,29,30 we decided to make a before-and-after comparison. To achieve this, the number of patients who made errors in performing the self- monitoring as well as the average number of errors were compared at the beginning of the study and 6 weeks later. The purpose was to assess whether the one-time patient education session in SMBG had any effect on how well the patients performed the self-monitoring.
Since this study was not a clinical trial in the sense of Germany’s Pharmaceutical Product Act or Medical Devices Act, no approval was needed by an ethic committee. Further training courses within the framework of the study were not required for the participating pharmacies, because on the basis of the defined inclusion criteria it could be assumed that a high level of counseling competence already existed with respect to conducting SMBG. This competence was documented by:
successful completion of the certified continuing education program in pharmaceutical care for patients with diabetes, in accordance with the joint recommendations of the German Diabetes Society (DDG) and the Federal Chamber of Pharmacists (BAK),35 i.e., a 36-hour curriculum, 3-day internship in a doctor’s office or clinic specialized in diabetes care and final examination. Germany-wide this curriculum includes a uniform 8-hour module on “Devices and Test Methods”,
or participation in a special intensive seminar on diabetes, which was conducted four times in 2004; certification of completion of the DDG/BAK advanced training course was a prerequisite, or
active participation in a diabetes-oriented intra-or inter-professional quality circle.
An additional criterion for the pharmacies was the existence of a customer data file with at least 50 type 2 diabetic patients that fulfilled the inclusion criteria.
For eligible patients, the following inclusion criteria were defined:
diabetes mellitus type 2
performs self-monitoring of blood glucose levels
age at least 18 years
speaks German
capable of interactive cooperation
submits a qualified declaration of consent
willingness to participate in the study
Further inclusion criteria for the patients were not defined in order to depict the day-by-day diabetes care as realistically as possible.
Procedure
Thirty-two pharmacies took part in the study. Seven more pharmacies which were originally registered to participate withdrew from the study due to lack of time or because of illness of the responsible pharmacist. In each pharmacy a special contact person for the study center (ABDA) was appointed. The study center randomly selected the patients for the study out of the pseudonymized pharmacy customer data files. Each pharmacy participating in the study sent a pseudonymized list extracted from the customer file of all type 2 diabetic patients who fulfilled the inclusion criteria to the study center. Using a random generator, 20 patients were selected for each pharmacy to receive an offer to participate in the study. Potential replacements for the selected patients in case they would not participate were also determined.
In a first step, the selected individuals with type 2 diabetes were informed in writing about the study and were offered the opportunity to participate. In a second step they were contacted by telephone. To minimize the effort for the participating pharmacies, it was decided not to document the patients’ reactions to being contacted.
For each study participant, two individual appointments were made in the respective study pharmacy: at study entry = t1 and 6 ± 2 weeks post = t2. At baseline (t1), a standardized interview was first conducted to record basic data. Next, the patients measured their blood glucose levels independently using their own meters. With the aid of a standardized documentation sheet all of the individual steps in performing the measurement were assessed (checklist). This was followed by personalized SMBG exercises and instructions to empower the patients to take the measurements without making any mistakes. Written instructions were provided as a supplement.
Pharmacies that offer blood glucose testing have a separate testing and counseling area, since requirements with respect to hygiene and discretion must be met. The evaluation of the patients’ self- monitoring took place in this separate area, as did the setting of appointments for the study participants, ensuring that the activities of the study were clearly separated from the routine operation of the pharmacies.
Sample Size
The calculation of the sample size was carried out with the program nQuery Advisor® 5.0. The basis of the calculation was an “SMBG campaign” that was carried out in a community pharmacy in Krefeld, Germany, in 2002.36 Fifty diabetic individuals were monitored for potential errors in performing SMBG. In 23 (46%) of the individuals, relevant errors were determined. This number was used as baseline value for the computation of the sample size (p=0.54). The objective was defined as a 50% increase in the number of patients who perform the measurements without making any mistake. The McNemar test in the exact version (binomial distribution) was used for the calculation. Alpha was defined as 0.05 and beta as 0.8. Furthermore, an assumed dropout rate was conservatively taken into account, as this was observed in another pharmacy- based project which was a lot more effort- and time- consuming for the patients (128 of 183 patients completed the one-year study).37 The resulting minimal number of cases per subgroup amounted to n=69, based on these assumptions. The kind of treatment regimen (only insulin, insulin and OAD, OAD only) and previous attendance at a training session for conducting SMBG were viewed as relevant stratifying variables. Thus, the number was n=138 per treatment group with the same distribution. With respect to stratification dependent on the administered treatment, a theoretically required total case number N=414 resulted with the same subgroup allocation, which, however, was not to be assumed initially. Accordingly, this was merely defined as minimum number of cases.
Error Categories
In principle, every single step of preparing, performing, and processing a blood glucose test as well as the set-up and condition of the utilized technical devices was evaluated as “correct” or “incorrect/false”. Individual aspects, which due to special device features could not be faulty, e.g. that the date and time were already preset or that manual coding was not required, were automatically evaluated as “correct”. To assess such device- specific features, the pharmacies participating in the study were provided with a detailed device list with a presentation of study-specific content as work material, in order to standardize the error evaluation.
For the evaluation, an error classification system was developed containing a weighting and interpretation of the errors and the resulting consequences. Errors were classified in the categories F1 to F5 (table 1). For the individual errors see table 3.
Table 1.
Classification of potential errors in conducting blood glucose tests
| Error Code | Error Category | Evaluation |
|---|---|---|
| F1 | Errors that make a reading useless (due to the error, it is not possible to anticipate whether the measured blood glucose level is higher or lower than the true value and how great the deviation is). | If only one of these errors is made, the result cannot be interpreted. The test must be repeated (correctly). |
| F2 | Errors that can lead to a false reading (or a false interpretation) in individual cases. | The significance is dependent on the exact situation or the consequence that is drawn. |
| F3 | Errors that can have a negative effect on compliance. | These errors have no impact on the measuring results, but possibly affect the willingness of the patient to perform the self-monitoring |
| F4 | Errors that can make conducting the test more difficult or prevent it entirely (device components). | Either the handling is unsatisfactory or the functionality is impaired. |
| F5 | Errors which make a consequential action / interpretation (by the patient, doctor, or pharmacist) more difficult. | Readings not available for evaluation over the middle term. |
Table 3.
Frequency of the occurrence of individual errors in N = 462 patients at t1 and t2, respectively, and the correlation to the different error classes
| Possible sources of error | Error class | t1 | T2 | ||
|---|---|---|---|---|---|
| Number | Percent | Number | Percent | ||
| [n] | [%] | [n] | [%] | ||
| Squeezing out the blood | F1 | 216 | 46.8 | 77 | 16.7 |
| Settings (date, time) | F5 | 209 | 45.2 | 65 | 14.1 |
| Sideways pricking of the finger pad | F3 | 157 | 34.0 | 36 | 7.8 |
| Hand-washing | F1 | 99 | 21.4 | 20 | 4.3 |
| Inserting / changing the lancet (skill) | F3 | 83 | 18.0 | 22 | 4.8 |
| Stimulating circulation (if needed) | F3 | 81 | 17.5 | 13 | 2.8 |
| Drying the hands | F1 | 71 | 15.4 | 6 | 1.3 |
| Adjusting the prick depth | F3 | 63 | 13.6 | 8 | 1.7 |
| Closing the test strip container after taking one out | F2 | 59 | 12.8 | 15 | 3.3 |
| Documentation / saving the result | F5 | 55 | 11.9 | 11 | 2.4 |
| If disinfected, was the skin dry? | F1 | 52 | 11.3 | 10 | 2.2 |
| Checking the coding | F1 | 41 | 8.9 | 8 | 1.7 |
| Cleanliness of device and measuring cell | F2 | 37 | 8.0 | 14 | 3.0 |
| Sufficiently large drop of blood extracted | F2 | 26 | 5.6 | 6 | 1.3 |
| Applying the blood sample / absorption of the test field | F2 | 25 | 5.4 | 6 | 1.3 |
| Quantity of the applied blood | F2 | 25 | 5.4 | 6 | 1.3 |
| Changing the coding (if required) | F1 | 21 | 4.6 | 4 | 0.9 |
| Loading the lancet | F4 | 18 | 3.9 | 3 | 0.7 |
| Expiration date of the test strips | F1 | 15 | 3.3 | 5 | 1.1 |
| Storage conditions of the test strips | F1 | 14 | 3.0 | 0 | 0 |
| Inserting the test strip / inserting the disk | F4 | 14 | 3.0 | 5 | 1.1 |
| Check with the glucose control solution | F1 | 13 | 2.8 | 3 | 0.7 |
| Condition of the battery | F4 | 12 | 2.5 | 8 | 1.7 |
| Basic handling of the measuring device | F4 | 4 | 0.9 | 0 | 0 |
| Choice of the desired unit (mg/dL, mmol/L) | F2 | 2 | 0.4 | 2 | 0.4 |
| Use of correct test strips | F1 | 0 | 0 | 0 | 0 |
Statistical Evaluation
The primary effect variable was the reduction of the number of individuals with type 2 diabetes who make errors when self-testing their blood glucose levels. Analyses were carried out regardless of the kind of error or the error subgroup. The secondary effect variable was the reduction of the average number of errors per study participant.
The chi-squared test according to McNemar was performed to measure the effect variables if the data were dichotomous. With metric data, the t-test was performed for paired random samples, since due to the selected before-after comparison a dependence of the data was given. As a matter of principle, an error probability of less than 5% was demanded (p<0.05). Testing was always performed two sided.
RESULTS
Patient Characteristics
Altogether, 478 patients were included in the study. This was on average 15 patients per pharmacy. Nine patients were excluded from the evaluation, because a change to another blood glucose meter occurred after the questionnaire data were recorded. Four patients did not show up for their t2 appointment and three were excluded from the evaluation due to incomplete data. In total, the data of 462 individuals with type 2 diabetes were considered in the evaluation.
The percentage of women (54%) among the participants was higher than the percentage of men. The average age of the participants was 67 years. (SD=10.3; range=27-89). There were no sex- specific differences with regard to age, diabetes duration, participation in a disease management program (DMP) and how long the patient had been performing SMBG. However, it was significantly more frequent that female patients had less school education than the male patients (p<0.05). The average diabetes duration was 13.1 years (SD=9.4; range=0-46) and the average duration of performing SMBG was 7 years (SD=6.2; range=0-43). 37% of the patients (n=171) stated at study begin that they were taking or had taken part in a DMP “type 2 diabetes mellitus”. Additional socio-demographical data are presented in table 2.
Table 2.
Description of the study population (N=462)
| Age (years) | Number | % |
|---|---|---|
| Under 50 | 28 | 6.1 % |
| 50 – 59 | 73 | 15.8 % |
| 60 – 69 | 159 | 34.4 % |
| 70 – 75 | 108 | 23.4 % |
| 76 and older | 94 | 20.3 % |
| Years of school | ||
| Under 8 | 9 | 2.0 % |
| 8 - 9 | 298 | 64.5 % |
| 10 – 11 | 100 | 21.6 % |
| 12 – 13 | 43 | 9.3 % |
| More than 13 | 5 | 1.1 % |
| No data available | 7 | 1.5 % |
| Duration of diabetes (years) | ||
| 1 or less | 27 | 5.8 % |
| 2 – 5 | 87 | 18.8 % |
| 6 – 10 | 110 | 23.8 % |
| 11 – 15 | 83 | 18.0 % |
| 16 or more | 152 | 33.0 % |
| No data available | 3 | 0.6 % |
Drug Therapy
Of the 462 study participants, about 70% (n=325) stated that they administered insulin. Of these, 193 study participants stated that they use insulin only, and the remaining 132 took an OAD additionally (figure 1). Of the 325 insulin users, 190 (58%) stated that they adjusted their dosage depending on the individual blood glucose level that was measured.
Figure 1.
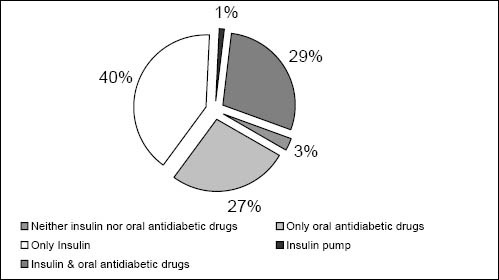
Therapy scheme of study population (n=460; no data available for two patients)
336 study participants (73%) stated that they measured their blood glucose at least once daily or more often. 30% stated that they measured their blood glucose four times a day or more often (n=139), just as many measured between two or three times a day. Only 11% measured once a week or less often. Over 61% of the diabetic patients (n=283) had already attended one or two instruction sessions on how to perform SMBG, 17% had already attended three or four sessions and 8% even five or more. More than 13% (n=61) stated they had never received an introduction into SMBG. The study participants had most often demonstrations (63%) and oral instructions (58%)
274 patients stated that they were educated in managing their diabetes in a doctor’s office, 161 in a hospital and 86 in a pharmacy. Other individual patients stated that they received diabetes management education from acquaintances, medical insurance companies or nursing services.
Error Description
Pressing the finger tip during blood extraction was the error most frequently documented. This error was observed with 216 patients (49%). The second most frequent error occurred during adjustment of the settings (date, time) and was observed with 209 patients. This error has no influence on the integrity of the reading, but it can make an interpretation of the measurements more difficult later. All other errors corresponding to their frequency observed at t1 are presented in table 3.
Apart from the errors that were listed last (irrelevant due to the observed frequency), all other errors could be reduced significantly and on a relevant scale for the day-to-day self-monitoring, within the scope of this study (table 3).
Measurement Quality
At study entry (t1), 79 of 462 patients (17%) carried out the measurement without making any mistakes. At t2 the proportion was 272 or 59% of the study participants (p<0.001). Thus, the percentage of faulty measurements could be reduced by more than half, from 83% at the beginning to 41% at t2. Moreover, the average number of errors per patient could be reduced from 3.1 to 0.8 (p<0.001).
The greatest improvement was in error group F3 (errors that can have a negative impact on compliance) with a reduction of around 74%. Since this error group contains parameters that directly influence the amount of pain felt, this observation is plausible. The errors in category F1 are of special significance because they can render a reading useless, making it impossible to anticipate whether the measured blood glucose level is higher or lower than the true value and how great the deviation is. These errors could be reduced from initially 283 patients with measurement errors to 111 patients with such mistakes. This corresponds to a reduction of 60%. All achieved differences between t1 and t2 were statistically significant.
Subgroup Analyses
Subgroup analyses were performed depending on the different drug therapy options, i.e. only insulin, insulin and OAD, and OAD only.
The average number of errors of the patients who were treated solely with OAD dropped over the period of the study from 4.1 errors to 1 error per patient. In the group of patients that in addition to OAD also received insulin, the average number of errors dropped from 2.7 to 0.7. In the participants who received insulin therapy exclusively, the initial number of errors dropped from 2.6 to 0.7. All of the improvements were statistically significant.
In addition, due to the special relevance, the subgroup of patients with insulin therapy who adjust their insulin dose on the basis of their self-measured blood glucose readings was analyzed (n=190). In total at t1, 142 of these 190 study participants (75%) made at least one error while measuring. Errors from category F1, which make a measurement useless, were documented for 100 of these 190 diabetic patients (53%). The number of patients who at the end of the study could not perform the measurements without making mistakes was reduced to 66 (35%). In relation to error category F1 at t2, 41 patients (22%) still made such errors. These changes were also statistically significant.
Table 4 shows the number of patients with faulty measurements in total as well as separately for the individual error categories.
Table 4.
Number of errors of the patients (N = 462) in blood glucose self-testing at t1 and t2, respectively, in total and according to the different error categories.
| Error category | Patients at t1 with > 1 errors | Average number of errors | Patients at t2 with > 1 errors | Average number of errors | p-value (t-test) | ||
|---|---|---|---|---|---|---|---|
| [n] | [%] | [n] | [%] | ||||
| Total | 383 | 82.9 | 3.1 | 190 | 41.1 | 0.8 | < 0.001 |
| F1 | 283 | 61.3 | 1.5 | 111 | 24.0 | 0.4 | < 0.001 |
| F2 | 123 | 26.6 | 0.8 | 42 | 9.1 | 0.2 | < 0.001 |
| F3 | 240 | 52.0 | 0.8 | 62 | 13.4 | 0.2 | < 0.001 |
| F4 | 42 | 9.1 | 0.1 | 16 | 3.5 | 0.0 | = 0.002 |
| F5 | 225 | 48.7 | 0.6 | 74 | 16.0 | 0.2 | < 0.001 |
| F1 or F2 | 310 | 67.1 | 1.5 | 126 | 27.3 | 0.4 | < 0.001 |
DISCUSSION
This study shows that the majority of individuals with type 2 diabetes (83%) make at least one mistake in carrying out the measurement of blood glucose levels with their own device. The study revealed two kinds of errors that were quite frequent: errors that falsify the measurement reading as well as errors that can have a negative effect on patient compliance. In the reference literature there is a consensus that individuals with diabetes make numerous mistakes in the self- monitoring of their blood glucose levels and that remedial training sessions are required.22,23,24,25,26,27,28,29,30,33,34,38,39 But there is little data on how much impact to expect from these remedial training sessions for type 2 diabetic patients. One problem is that there is no standard method for remedying the errors, so that the kind of error assessment is variable at the time of our study. A validated documentation sheet was not available. The documentation sheets used in various studies22 are designed to record 13 to 45 sources of error.31 Thus, no comparability exists among the various studies. The main difference in the evaluations consisted in whether the components of the SMBG were summarized or recorded in a very detailed way. Common to all of them, however, is that it could be shown in principle that diabetes management education sessions instructing how to carry out blood glucose self- testing are both necessary and effective, even if no general statement could be made about the extent of the success.25,29,30
The kinds of mistakes observed in the studies, however, were very similar. In other studies, too, the main mistakes were in cleaning of the hands, making adjustments to the settings of the meter and problems with coding.23,30,31
The study presented here was able to show that a one-time, standardized intervention in community pharmacies specialized in diabetes care is able to more than triple the number of patients who carried out the self-monitoring without making any errors: initially 17% compared to 59% at the end of the study. However, a selection bias in the patient population of the study cannot be fully excluded, since such offers are probably accepted more frequently by motivated patients rather than by unmotivated patients.
Altogether, the pharmacy setting is suited for carrying out such evaluations along with giving corresponding instructions on how to correctly perform SMBG. Such an intervention comprised verbal instructions as well as practical exercises and took on average about 20 to 30 minutes, including the study documentation.
One reservation regarding our study is that any SMBG training sessions that may have taken place outside the pharmacy in the period of six weeks between t1 and t2 were not recorded, but this would have only distorted the result false-positively. However, this error is probably negligible with respect to the size of the effect found.
A further limitation of the study is due to the fact that the chosen design did not allow for checking the accuracy of the blood glucose readings, since the required extraction of venous blood for a comparative measurement is not possible in the setting of a community pharmacy in Germany. Thus, no assertions can be made about the extent the readings deviated from a comparative lab control reading due to the observed errors.
Whether the positive effect will be sustained past the trial period cannot be answered with the present study. Presumably, it would be necessary to repeat the diabetes management education sessions to ensure the most accurate measurements possible over the long term.
Another topic that must be discussed is that the results show that such one-time interventions are not sufficient for all patients to learn how to perform error-free measurements. Additional follow-up instructions and exercises would probably increase the number of type 2 diabetic patients who could monitor their blood glucose levels without making any mistakes. However, even then, probably not all patients would be capable of performing error-free measurements. For this group of individuals it might be a good option to integrate another person, e.g. the spouse or partner, into the measuring of the blood glucose levels.
CONCLUSIONS
Through a one-time intervention as described here, the number of patients making mistakes in SMBG was reduced by half over a period of six weeks. In view of this background, the results of the present study are practice relevant. A concept like in figure 2 could possibly show how an evaluation of SMBG in pharmacies can be integrated into the given care process of individuals with type 2 diabetes. Accordingly, such an evaluation could take place following the physician’s diabetes management education program. Patients who measure without making any mistakes should repeat this process once a year or when they switch to another blood glucose meter. Patients who make mistakes in carrying out their SMBG should receive instruction in the pharmacy once. If this does not lead to error- free self-testing, remedial training should be given by the physician and his diabetes team.
Figure 2.
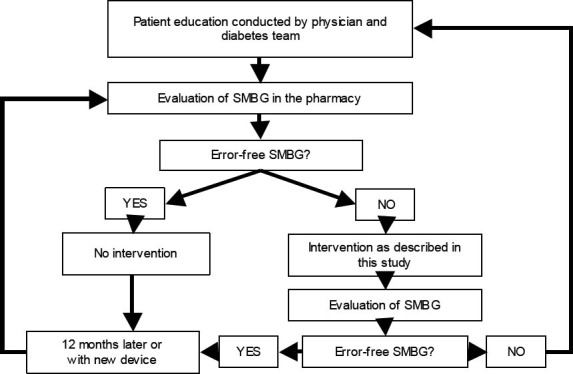
Suggestion for an implementation scheme to integrate the evaluation of SMBG in pharmacies in the healthcare process of individuals with type 2 diabetes
In community pharmacies this approach should be integrated into the existing pharmaceutical care concepts for patients with diabetes, which in our case were worked out in detail by a commission of the German Diabetes Society (DDG) and the
Federal Chamber of Pharmacists (BAK) and published under the title “Integration of Pharmacists into Diabetic Care”.35,40,41 The prerequisite for providing such concerted and qualified care would include the following elements: (i) DDG/BAK-certified advanced training diabetes program, which until now more than 5,500 pharmacists across Germany have completed. (ii) working in compliance with the consensus agreements regarding the competences of the different healthcare professions,35 and (iii) working with quality assurance instruments such as Standard Operating Procedures and checklists, e.g. with instructions for SMBG.42 Through regular monitoring of how well the patient performs the measurements, the patient’s skill and security in dealing with the self-test can be increased. Moreover, through device function checks, the reliability of the blood glucose meter can be ensured. The study also showed a high number of patients complying with special instructions about how to extract capillary blood less painfully, since they benefit directly.
It should be investigated whether such instruction sessions in the pharmacy can reduce superfluous self-testing to a certain extent and thus make a contribution to a more efficient use of blood glucose test strips.
ACKNOWLEDGEMENTS
We wish to thank all patients involved in the study for their active participation. Furthermore, we would like to thank all participating pharmacies for their outstanding commitment. Additional thanks go to Bayer Vital GmbH Diabetes Care for sponsoring the project and Dominique Wecker (mc-consult, Hamburg) for her critical analysis of the data.
Contributor Information
Uta Müller, Center for Drug Information and Pharmacy Practice, ABDA - Federal Union of German Associations of Pharmacists. Berlin (Germany).
Andrea Hämmerlein, Center for Drug Information and Pharmacy Practice, ABDA, Berlin (Germany).
Annette Casper, Bayer Vital GmbH Diabetes Care, Leverkusen (Germany).
Martin Schulz, Adjunct Professor University of Frankfurt, Frankfurt (Germany), and Head, Center for Drug Information and Pharmacy Practice, ABDA, Berlin (Germany).
References
- 1.Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24(3):561–87. doi: 10.2337/diacare.24.3.561. [DOI] [PubMed] [Google Scholar]
- 2.Panja S, Starr B, Colleran KM. Patient knowledge improves glycemic control: is it time to go back to the classroom? J Investig Med. 2005;53(5):264–6. doi: 10.2310/6650.2005.53509. [DOI] [PubMed] [Google Scholar]
- 3.Hauner H. [Evicende based therapy of obesity]. Internist. 2006;47(2):159–70. doi: 10.1007/s00108-005-1558-7. [DOI] [PubMed] [Google Scholar]
- 4.Keers JC, Bouma J, Links TP, et al. One-year follow-up effects of diabetes rehabilitation for patients with prolonged selfmanagement difficulties. Patient Educ Couns. 2006;60(1):16–23. doi: 10.1016/j.pec.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Schiel R, Voigt U, Ross IS, Braun A, Rillig A, Hunger-Dathe W, Stein G, Muller WA. Structured diabetes therapy and education improves the outcome of patients with insulin treated diabetes mellitus. The 10 year follow-up of a prospective, population-based survey on the quality of diabetes care (the JEVIN Trial) Exp Clin Endocrinol Diabetes. 2006;114(1):18–27. doi: 10.1055/s-2005-873079. [DOI] [PubMed] [Google Scholar]
- 6.Sonksen PH, Judd SL, Lowy C. Home monitoring of blood-glucose. Method for improving diabetic control. Lancet. 1978;1(8067):729–32. doi: 10.1016/s0140-6736(78)90854-1. [DOI] [PubMed] [Google Scholar]
- 7.Walford S, Gale EA, Allison SP, Tattersall RB. Self-monitoring of blood-glucose. Improvement of diabetic control. Lancet. 1978;1(8067):732–5. doi: 10.1016/s0140-6736(78)90855-3. [DOI] [PubMed] [Google Scholar]
- 8.Karter AJ, Ackerson LM, Darbinian JA, D’Agostino RB, Jr, Ferrara A, Liu J, Selby JV. Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes registry. Am J Med. 2001;111(1):1–9. doi: 10.1016/s0002-9343(01)00742-2. [DOI] [PubMed] [Google Scholar]
- 9.Schiel R, Muller UA, Rauchfub J, Sprott H, Muller R. Blood-glucose self-monitoring in insulin treated type 2 diabetes mellitus a cross-sectional study with an intervention group. Diabetes Metab. 1999;25(4):334–40. [PubMed] [Google Scholar]
- 10.Schwedes U, Siebolds M, Mertes G. Meal-related structured self-monitoring of blood glucose: effect on diabetes control in non-insulin-treated type 2 diabetic patients. Diabetes Care. 2002;25(11):1928–32. doi: 10.2337/diacare.25.11.1928. [DOI] [PubMed] [Google Scholar]
- 11.Dreyer M, Kiess W, Lüdecke H, Redaélli M, Schatz H, Waldhäusl W. Therapie des Diabetes mellitus Typ 1 Evidenzbasierte Diabetes-Leitlinie DDG. Scherbaum WA, Landgraf R, editors. Diabetes und Stoffwechsel. 2003;12(Suppl. 2) [Google Scholar]
- 12.Häring H, Joost H, Laube H, Matthaei S, Meissner HP, Panten U, Schernthaner G. Antihyperglykämische Therapie des Diabetes mellitus Typ 2. Evidenzbasierte Diabetes-Leitlinie DDG. Scherbaum WA, Landgraf R, editors. Diabetes und Stoffwechsel. 2003;12(Suppl. 2):13–31. [Google Scholar]
- 13.Faas A, Schellevis FG, Van Eijk JT. The efficacy of self-monitoring of blood glucose in NIDDM subjects. A criteria-based literature review. Diabetes Care. 1997;20(9):1482–6. doi: 10.2337/diacare.20.9.1482. [DOI] [PubMed] [Google Scholar]
- 14.Fontbonne A, Billault B, Acosta M, Percheron C, Varene P, Besse A, Eschwege E, Monnier L, Slama G, Passa P. Is glucose self-monitoring beneficial in non-insulin-treated diabetic patients?Results of a randomized comparative trial. Diabete Metab. 1989;15(5):255–60. [PubMed] [Google Scholar]
- 15.Franciosi M, Pellegrini F, De Berardis G, Belfiglio M, Cavaliere D, Di Nardo B, Greenfield S, Kaplan SH, Sacco M, Tognoni G, Valentini M, Nicolucci A QuED Study Group. The impact of blood glucose self-monitoring on metabolic control and quality of life in type 2 diabetic patients: an urgent need for better educational strategies. Diabetes Care. 2001;24(11):1870–7. doi: 10.2337/diacare.24.11.1870. [DOI] [PubMed] [Google Scholar]
- 16.Harris MI. Frequency of blood glucose monitoring in relation to glycemic control in patients with type 2 diabetes. Diabetes Care. 2001;24(6):979–82. doi: 10.2337/diacare.24.6.979. [DOI] [PubMed] [Google Scholar]
- 17.Muchmore DB, Springer J, Miller M. Self-monitoring of blood glucose in overweight type 2 diabetic patients. Acta Diabetol. 1994;31(4):215–9. doi: 10.1007/BF00571954. [DOI] [PubMed] [Google Scholar]
- 18.Rindone JP, Austin M, Luchesi J. Effect of home blood glucose monitoring on the management of patients with noninsulin dependent diabetes mellitus in the primary care setting. Am J Manag Care. 1997;3(9):1335–8. [PubMed] [Google Scholar]
- 19.Martin S, Schneider B, Heinemann L, Lodwig V, Kurth HJ, Kolb H, Scherbaum WA. Self-monitoring of blood glucose in type 2 diabetes and long-term outcome: an epidemiological cohort study. Diabetologia. 2006;49(2):271–8. doi: 10.1007/s00125-005-0083-5. [DOI] [PubMed] [Google Scholar]
- 20.Bundesärztekammer. Richtlinie der Bundesärztekammer (BÄK) zur Qualitätssicherung quantitativer laboratoriumsmedizinischer Untersuchungen. Dtsch Ärztebl. 2001;98:A 2747–59. [Google Scholar]
- 21.Thomas A, Hasche H. Selbstkontrolle bei Diabetes. Vol. 2003. Mainz: Kirchheim-Verlag; Anforderungen an die Blutzuckermessgeräte; pp. 39–42. [Google Scholar]
- 22.Alto WA, Meyer D, Schneid J, Bryson P, Kindig J. Assuring the accuracy of home glucose monitoring. J Am Board Fam Pract. 2002;15(1):1–6. [PubMed] [Google Scholar]
- 23.Delamater AM, Davis SG, Bubb J, Santiago JV, Smith JA, White NH. Self-monitoring of blood glucose by adolescents with diabetes: technical skills and utilization of data. Diabetes Educ. 1989;15(1):56–61. doi: 10.1177/014572178901500115. [DOI] [PubMed] [Google Scholar]
- 24.Kabadi UM, O’Connell KM, Johnson J, Kabadi M. The effect of recurrent practice at home on the acceptability of capillary blood glucose readings. Accuracy of self blood glucose testing. Diabetes Care. 1994;17(10):1110–23. doi: 10.2337/diacare.17.10.1110. [DOI] [PubMed] [Google Scholar]
- 25.Kristensen GB, Nerhus K, Thue G, Sandberg S. Standardized evaluation of instruments for self-monitoring of blood glucose by patients and a technologist. Clin Chem. 2004;50(6):1068–71. doi: 10.1373/clinchem.2004.031575. [DOI] [PubMed] [Google Scholar]
- 26.Kristensen GB, Nerhus K, Thue G, Sandberg S. Results and feasibility of an external quality assessment scheme for self-monitoring of blood glucose. Clin Chem. 2006;52(7):1311–7. doi: 10.1373/clinchem.2006.068114. [DOI] [PubMed] [Google Scholar]
- 27.Schrot RJ, Foulis PR, Morrison AD, Farese RV. A computerized model for home glucose monitoring proficiency testing: efficacy of an innovative testing program. Diabetes Educ. 1999;25(1):48–55. doi: 10.1177/014572179902500107. [DOI] [PubMed] [Google Scholar]
- 28.Skeie S, Thue G, Nerhus K, Sandberg S. Instruments for self-monitoring of blood glucose: comparisons of testing quality achieved by patients and a technician. Clin Chem. 2002;48(7):994–1003. [PubMed] [Google Scholar]
- 29.Ward WK, Haas LB, Beard JC. A randomized, controlled comparison of instruction by a diabetes educator versus selfinstruction in self-monitoring of blood glucose. Diabetes Care. 1985;8(3):284–6. doi: 10.2337/diacare.8.3.284. [DOI] [PubMed] [Google Scholar]
- 30.Bergenstal R, Pearson J, Cembrowski GS, Bina D, Davidson J, List S. Identifying variables associated with inaccurate self-monitoring of blood glucose: proposed guidelines to improve accuracy. Diabetes Educ. 2000;26(6):981–9. doi: 10.1177/014572170002600610. [DOI] [PubMed] [Google Scholar]
- 31.Dorchy H, Van Vlaenderen C, Roggemans MP. Sources of errors in blood glucose self-monitoring in 100 young diabetics. Rev Med Brux. 2003;24(2):77–81. [PubMed] [Google Scholar]
- 32.Perwien AR, Johnson SB, Dymtrow D, Silverstein J. Blood glucose monitoring skills in children with Type I diabetes. Clin Pediatr. 2000;39(6):351–7. doi: 10.1177/000992280003900605. [DOI] [PubMed] [Google Scholar]
- 33.American Diabetes Association. Self-monitoring of blood glucose. Diabetes Care. 1990;13(Supplement 1):S62–6. [Google Scholar]
- 34.American Diabetes Association. Tests of glycemia in diabetes. Diabetes Care. 2004;27(Supplement):S91–3. doi: 10.2337/diacare.27.2007.s91. [DOI] [PubMed] [Google Scholar]
- 35.Eickhoff C, Schulz M. Einbindung der Apotheker in die Diabetikerversorgung. Pharm Ztg. 2000;145(7):512–4. [Google Scholar]
- 36.Krüger M. MessTest-Aktion. Personal communication. 2002 [Google Scholar]
- 37.Mangiapane S, Schulz M, Muhlig S, Ihle P, Schubert I, Waldmann H C. Community pharmacy-based pharmaceutical care for asthma patients. Ann Pharmacother. 2005;39(12):1817–22. doi: 10.1345/aph.1G180. [DOI] [PubMed] [Google Scholar]
- 38.Meadows S. Improving blood glucose monitoring for diabetes. FDA Consum. 1990 May [Google Scholar]
- 39.Steel LG. Identifying technique errors. Self-monitoring of blood glucose in the home setting. J Gerontol Nurs. 1994;20(2):9–12. doi: 10.3928/0098-9134-19940201-04. [DOI] [PubMed] [Google Scholar]
- 40.Gerdemann A, Müller U, Schulz M. Akzeptanz und Evaluation der zertifizierten Diabetes-Fortbildung. Pharm Ztg. 2004;149(46):4052–4. [Google Scholar]
- 41.Krüger M. Stufenkonzept: Diabetische Patienten qualifizierter betreuen. Pharm Ztg. 2000;145(45):3812–4. [Google Scholar]
- 42.Müller U, Hämmerlein A, Schulz M. Blutzucker fehlerfrei selbst bestimmen. Pharm Ztg. 2005;150(38):3396–7. [Google Scholar]


