Abstract
We seek to provide a background of the current state of pediatric urologic surgery including a brief history, procedural outcomes, cost considerations, future directions, and the state of robotic surgery in India. Pediatric robotic urology has been shown to be safe and effective in cases ranging from pyeloplasty to bladder augmentation with continent urinary diversion. Complication rates are in line with other methods of performing the same procedures. The cost of robotic surgery continues to decrease, but setting up pediatric robotic urology programs can be costly in terms of both monetary investment and the training of robotic surgeons. The future directions of robot surgery include instrument and system refinements, augmented reality and haptics, and telesurgery. Given the large number of children in India, there is huge potential for growth of pediatric robotic urology in India. Pediatric robotic urologic surgery has been established as safe and effective, and it will be an important tool in the future of pediatric urologic surgery worldwide.
KEY WORDS: Pediatric urology, robotic surgery, robotic training
INTRODUCTION
At the turn of the millennium, robotic surgery seemed more like science fiction than a commonly used tool with wide application throughout most fields of surgery. First approved by the United States (US) Food and Drug Administration in 2000, the da Vinci (Intuitive Surgical, Inc., Mountain View, Ca) robotic surgical platform was quickly embraced by surgeons worldwide. The three-dimensional vision (augmented with high-definition video) and 7° of freedom of movement combine intricate and dexterous movement with gentle tissue handling to allow for minimally invasive surgery in areas of the body previously considered too difficult or even impossible. In addition, the tremor filtering and motion scale have proven immensely beneficial when performing the most intricate parts of surgery. Pediatric robotic urologic surgery has undergone tremendous growth since its first application in 2002.
PROCEDURAL APPLICATIONS AND OUTCOMES
The first pediatric robotic procedure performed at most institutions was the robotic assisted laparoscopic pyeloplasty. It has since become increasingly commonly performed, accounting for 11-12.6% of pyeloplasties performed in the US by 2009.[1] The relatively high-incidence of ureteropelvic junction obstruction combined with surgeon familiarity with laparoscopic pyeloplasty made it a natural first robotic procedure. Soon, ureteral reimplantation robotic-assisted laparoscopic ureteral reimplantation[2] and uretero-ureterostomy[3] were described and disseminated into widespread clinical practice. While, extirpative procedures were described, they never became popular with a robotic approach likely due to the fact that they were relatively easy to master with a pure laparoscopic approach.
Select centers with highly specialized pediatric robotic urologists continued to push the envelope with robotics. Rather than focusing on the relatively straightforward pyeloplasties and ureteral reimplantations, complex reconstructive procedures have been described, including continent catheterizable channels,[4] robotic bladder augmentation with appendicovesicostomy robotic-assisted laparoscopic augmentation ileocystoplasty and Mitrofanoff appendicovesicostomy (RALIMA) or without (RALI),[5] bladder neck reconstruction,[6] and retroperitoneal lymph node dissection.[7] Large-scale series and long-term outcomes regarding these procedures have not yet been published, but early data are promising. At the very least, pediatric urologists have provided proof of principle that the most complex reconstructive surgeries can be performed robotically.
Renal procedures
Pyeloplasty
Robotic pyeloplasty has been shown to have very good outcomes. A 2011 report from Minnillo et al. have reported a 96% success rate at a median follow-up of 31.7 months.[8] Given the success of robotic pyeloplasty in the general pediatric population, we have begun performing robotic pyeloplasty on infants. We found that, when compared with open pyeloplasty in infants, robotic pyeloplasty was associated with longer operative time, but nonsignificant differences in estimated blood loss, hospital length of stay, days to regular diet, or duration of catheterization.[9] More recent meta-analyses and reports have indicated that robotic pyeloplasty is the same as open with consideration to postoperative urinary leaks, hospital readmission rate, and operative time,[10] and robotic pyeloplasty may result in higher overall rates of resolution of hydronephrosis.[11] With 2 year's follow-up, only 5% of patients who underwent robotic pyeloplasty require a secondary procedure, when compared with 13% who underwent a pure laparoscopic pyeloplasty.[12] Other technique papers have indicated that redopyeloplasty is a feasible procedure,[13] and robotic ureterocalycostomy can be considered when a redo pyeloplasty is not possible.[14]
Duplex kidney heminephrectomy and ureteroureterostomy
We have previously reported on the use of robotics for management of renal duplication anomalies.[15] This approach provides excellent exposure for identifications, delineation, and handling of the normal moiety as well as identification of the diseased moiety and its vasculature. Other centers have reported that robotic heminephrectomy can be safely performed for the nonfunctioning moiety[16] and that ureteroureterostomy has been performed with equivalent success.[17]
Pyelolithotomy
Pyelolithotomy has been shown to be effective with a robotic approach,[18] although long-term data are lacking. In areas with high-incidence of nephrolithiasis, robotic pyelolithotomy may help decrease the necessity for percutaneous procedures in children. This could potentially have the benefits of decreased pain, shorter procedural length, and improvement in clearing of stones from the collecting system.
Pelvic procedures
Ureteral reimplantation
Robotic ureteral reimplantation has become an increasingly popular technique for treatment of vesicoureteral reflux. While initial success rates were low,[19] more recent reports indicate success rates of 97-99%,[20,21] when compared with open surgery, robotic ureteral reimplantation was associated with shorter duration of urethral catheterization, fewer bladder spasms, and shorter hospitalizations,[22,23] there is decreased incidence of postoperative urinary retention with this approach.[24]
Bladder neck reconstruction and retroperitoneal lymph node dissection
Pediatric urologists have also applied the robotic approach to bladder neck reconstruction and retroperitoneal lymph node dissection. Bladder neck reconstruction with appendicovesicostomy has been safely performed,[6] although long-term continence data have not yet been published. In addition, robotic retroperitoneal lymphadenectomy for paratesticular rhabdomyosarcoma and testicular germ cell tumors have been performed with good functional and oncologic outcomes.[7]
Augmentation cystoplasty and appendicovesicostomy
The field of pediatric robotic urology has matured to the point where surgeons are performing the most complex cases robotically, including augmentation cystoplasty with appendicovesicostomy. In our experience with RALIMA, we were able to complete 86% of cases robotically.[5] Length of stay ranged from 5 to 7 days, and all patients were continent with catheterizable channels at first report.[5] Outcomes from robotic appendicovesicostomy in terms of continence and stomal stenosis are similar to those of published open series,[4] but no direct comparisons have been published to date.
Are we justified performing robotic surgery?
Morbidity
In terms of morbidity and surgical outcomes, we have seen advantages in a variety of procedures in terms of decreased length of hospitalization and patient pain without compromising surgical outcomes,[10,22,23] It has also been shown that robotic pyeloplasty is associated with less narcotic requirement than open pyeloplasty.[25] Much of this is likely due to the improved visualization and tissue handling provided by the robotic platform as well as the decreased incision size required as compared with open surgery.
Cost and financial considerations
Despite the many advantages outlined about regarding the surgical robot, it does have one major downside-cost. These include purchase of the system, service contracts, and disposables for each case, and instruments that can only be used for a set number of cases before needing to be replaced. While there are cost advantages to laparoscopy as compared with open surgery, the same cannot yet be said when comparing pure laparoscopic surgery and robotic surgery. Until date, a single-institution retrospective review found that direct costs were actually lower for robotic surgery when compared with open surgery, but the indirect costs of robot purchase and maintenance made the overall cost of robotic surgery higher than open surgery.[26] In many ways, comparing open and robotic surgery is fairer than comparing robotic surgery with laparoscopic surgery because robotic surgery allows for a minimally invasive approach in complex cases that could not be achieved with pure laparoscopy.
There is currently only one surgical robot manufacturer on the market, intuitive surgical, leading to a monopoly not only on the robot, but also on all disposables and instruments used with the robot. More robotic manufacturers and expiring patents on disposables and instruments will increase competition in the market, which should drive down costs.
In addition, there are other methods of making the robot financially viable for an institution during this early phase of robotic surgery. Shrewd marketing campaigns can highlight the availability of robotic surgery, which may draw patients to an institution who would otherwise have sought care elsewhere. The “halo effect” of increased patient numbers and use of ancillary services (e.g., radiology) helps to offset the increased costs of robotic surgery in the operating room. In addition, increased utilization of the robot by other subspecialties will result in decreased percase indirect costs, which may help to even the costs between open and robotic surgery. Philanthropy is also a possible option for institutions to acquire a robot.
Parental capital gains
Left out of the of the cost-benefit analysis of robotic pediatric surgery to this point is a consideration of the patient and family perceptions regarding robotic surgery and the financial implication that robotic surgery can have on families outside of the hospital bill. Both patients and their families prefer the cosmesis of robotic scars to open surgical scars for the same procedures.[27] Furthermore, shorter hospitalization and convalescence for children allows for their parents to take less time off work, decreasing lost wages for the family and lost productivity for society as a whole.[28] Less quantifiable monetarily, but no less important overall, is the fact that parents report higher satisfaction with “overall life” after choosing robotic surgery for their children.[29]
Undetermined benefits
Until date, published outcomes regarding pediatric urology have focused on classical measures such as operative time, blood loss, length of hospitalization, and standard outcomes. However, much is still unknown about the basic physiology of robotic surgery when compared with open surgery, and we still do not know what we do not know. For example, we have shown in a porcine model that there are decreased numbers and complexity of adhesions with robotic ileocystoplasty over a conventional open approach.[30] Given that many spina bifida patients require multiple abdominal surgeries over the course of their lives, decreased adhesions may result in decreased complications in the long run. There may be many more benefits that have yet to be quantified or manifested given the relatively short time course of robotic surgery to date.
Safety and complications
We must also be aware of potential safety issues associated with the surgical robot. A recent single-institution study reported a low complication rate for pediatric robotic procedures.[31] In the US, a review of the Food and Drug Administration manufacturer and user facility device experience database reported on a significant, and likely underreported, number of robotic arm failures.[32] Ultimately, it will be incumbent upon surgeons to recognize and openly manage robotic complications to maintain patient trust in the system.[33] The technology is neutral; ultimately it depends on the operator to become successful and safe.
Surgeons' role and establishing robotics programs
A compassionate lead surgeon with the drive to establish a successful robotics program is the first prerequisite for success. If the surgeon can find a partner, this is more ideal, and collaboration from other specialties that will use the robot is critical to develop the initial 5-year business plan.
Currently, individual institutions define individual credentialing procedures to define who is qualified to perform pediatric robotic surgery. This is in contrast to having well-defined curricula and credentialing programs established by professional organizations with the intent of maximizing the quality of procedures and patient safety. One key organization in the US working toward a standardized curriculum is the Fundamentals of Robotic Surgery working group.[34] Certification should follow the steps as we think of case observation, simulation, performing cases with proctor supervision, and then slowly graduating from simple cases to more complex ones as the surgeon's comfort level increases [Figure 1].
Figure 1.
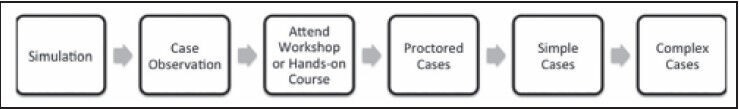
Our suggested training model for pediatric robotic urological surgery
Improved surgical simulation will be key to the training of future robotic pediatric surgeons. Current simulators have been shown to improve comfort with the robotic platform,[35] but the higher quality of wet labs is associated with much higher costs.[36] Even after training, these simulations are valuable, as “warming up” prior to surgery may improve outcomes and help surgeons maintain their skills.[37]
Transfer of skills to trainees
Of course, current residents in training should be trained on robotic surgery as an integral part of their training. At our institution, we adhere to the protocol illustrated in Figure 2.[38]
Figure 2.
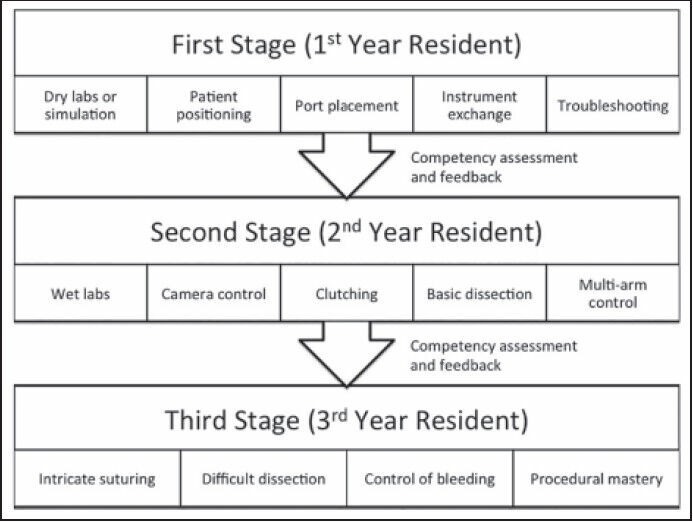
Transfer of skills to trainees
Future refinements in robotic surgery
Robot technology
When thinking about pediatric urologic applications of robotic surgery, miniaturization of robotic arms, especially in the form of table-mounted systems, will have increased dexterity. Miniaturization of instruments is an obvious area for improvement as well. Five mm instruments have been introduced, but they have not proven useful because the pulley mechanism in these instruments does not provide the same level of control that the articulating arms of the 8-mm instruments. The lack of specialized instruments for pediatric cases may affect the ability of pediatric surgeons to maximize efficiency in the operating room. As more companies bring additional robots and instruments onto the market in coming years, we can hope that there will be improvements in miniaturization of robotic instruments that will allow for optimal working conditions in the limited space children present.
Certain soft- and hard-ware modifications could help to minimize future complications. In particular, systems to prevent overshoot of instruments (that is, being able to place instruments beyond the field of view into possibly critical structures) could be critically important for pediatric cases given the limited working space in children. Traditionally, tactile feedback has been a critical part of open surgery as surgeons relied on feel to assess tissue properties, dissect planes, and properly tension sutures. The lack of force feedback when operating robotically presents a major limitation to the device. The solution to this problem will be either through force-feedback mechanisms translated from the robot to the console or through on-screen display that given an indication as to the force being applied to tissue. Augmented reality is the next frontier for compensating haptics.[37] One major potential advantage offered by robotic surgery is telesurgery, or the ability for an expert surgeon to operate on a patient who may be a great distance away. Telesurgery has already been successfully performed transatlantically,[39] and there is even a fully viable remote surgery service in existence in Canada.[40] Telesurgery will be most exciting in locations and situations where expert surgeons are in short supply and must provide services for patients in a large geographical area in order to greatly improve access to cutting edge care.
India and other developing countries
While the majority of robotic surgery to date has been performed in North America and Europe, the robotic platform will certainly have huge applications in India and the remainder of the developing world if only due to the sheer volume of young children (nearly 360 million children 0-14 in India[41] as compared with nearly 62 million in the US).[42] There are currently 24 robots being used in India (intuitive data), and India represents a major growth sector for robotic pediatric urology given the large population and current relative lack of robotics.[43]
Since 2013, two pediatric centers at New Delhi and Chennai at the Apollo hospitals have spearheaded the pediatric robotic surgery program in India. Over the last 12 months, increasingly complex surgeries have been performed, including pyeloplasty, ureteric reimplantation, heminephroureterectomies, urteroureterostomy, and even ileocystoplasty. The early experience is very encouraging, and the program is almost ready to enter the maturation phase in both these centers (personal communication and Sujit Chowdhary).
The high price of the da Vinci platform as well as the associated costs (disposables, service contract, etc.) have prevented greater uptake of robotic surgery worldwide. Realistically, the manufacturers may have to consider subsidizing the system and come up with less consumption of disposables to make it accessible and affordable worldwide. This humanitarian effort on the part of manufacturers will increase the availability of the robot as well as allow families to better afford such surgeries. Surgeons in the developing world will also have to band together to form referral centers of excellence to maximize the utilization of these robots. These referral centers also provide ideal locations to serve as training centers, and affiliations with other centers in the world will develop future refinements.
CONCLUSION
Pediatric urological surgery is in the midst of a major paradigm shift in the delivery of care [Figure 3]. Where large incisions for open surgery were once required, even the most complex of cases can be formed in a minimally invasive fashion because of robotic technology. The next decade of progress will involve further validation of robotic procedures now that we are past the learning curve and dissemination of robotic technology worldwide to ensure all have access to this modern medical tool. Currently, we have shown most procedures can be performed with comparable outcomes-now is the time to have large clinical trials and refine the technology to be available universally. This is the beginning platform of the digital age of the 21st century with a promising future.
Figure 3.
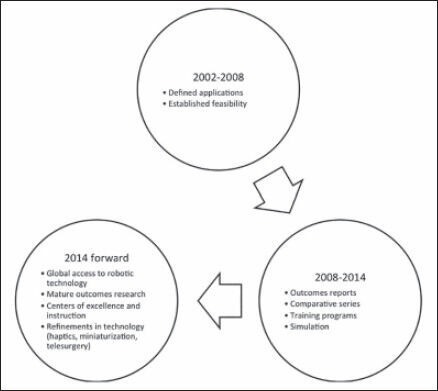
Timeline of robotic surgery
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sukumar S, Roghmann F, Sood A, Abdo A, Menon M, Sammon JD, et al. Correction of ureteropelvic junction obstruction in children: National trends and comparative effectiveness in operative outcomes. J Endourol. 2014;28:592–8. doi: 10.1089/end.2013.0618. [DOI] [PubMed] [Google Scholar]
- 2.Peters CA, Woo R. Intravesical robotically assisted bilateral ureteral reimplantation. J Endourol. 2005;19:618–21. doi: 10.1089/end.2005.19.618. [DOI] [PubMed] [Google Scholar]
- 3.Corbett ST, Burris MB, Herndon CD. Pediatric robotic-assisted laparoscopic ipsilateral ureteroureterostomy in a duplicated collecting system. J Pediatr Urol. 2013;9:1239.e1–2. doi: 10.1016/j.jpurol.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Famakinwa OJ, Rosen AM, Gundeti MS. Robot-assisted laparoscopic Mitrofanoff appendicovesicostomy technique and outcomes of extravesical and intravesical approaches. Eur Urol. 2013;64:831–6. doi: 10.1016/j.eururo.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Gundeti MS, Acharya SS, Zagaja GP, Shalhav AL. Paediatric robotic-assisted laparoscopic augmentation ileocystoplasty and Mitrofanoff appendicovesicostomy (RALIMA): Feasibility of and initial experience with the University of Chicago technique. BJU Int. 2011;107:962–9. doi: 10.1111/j.1464-410X.2010.09706.x. [DOI] [PubMed] [Google Scholar]
- 6.Bagrodia A, Gargollo P. Robot-assisted bladder neck reconstruction, bladder neck sling, and appendicovesicostomy in children: description of technique and initial results. J Endourol. 2011;25:1299–305. doi: 10.1089/end.2011.0031. [DOI] [PubMed] [Google Scholar]
- 7.Cost NG, DaJusta DG, Granberg CF, Cooksey RM, Laborde CE, Wickiser JE, et al. Robot-assisted laparoscopic retroperitoneal lymph node dissection in an adolescent population. J Endourol. 2012;26:635–40. doi: 10.1089/end.2011.0214. [DOI] [PubMed] [Google Scholar]
- 8.Minnillo BJ1, Cruz JA, Sayao RH, Passerotti CC, Houck CS, Meier PM, et al. Long-term experience and outcomes of robotic assisted laparoscopic pyeloplasty in children and young adults. J Urol. 2011;185:1455–60. doi: 10.1016/j.juro.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 9.Dangle PP, Kearns J, Anderson B, Gundeti MS. Outcomes of infants undergoing robot-assisted laparoscopic pyeloplasty compared to open repair. J Urol. 2013;190:2221–6. doi: 10.1016/j.juro.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 10.Braga LH, Pace K, DeMaria J, Lorenzo AJ. Systematic review and meta-analysis of robotic-assisted versus conventional laparoscopic pyeloplasty for patients with ureteropelvic junction obstruction: effect on operative time, length of hospital stay, postoperative complications, and success rate. Eur Urol. 2009;56:848–57. doi: 10.1016/j.eururo.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 11.Barbosa JA, Kowal A, Onal B, Gouveia E, Walters M, Newcomer J, et al. Comparative evaluation of the resolution of hydronephrosis in children who underwent open and robotic-assisted laparoscopic pyeloplasty. J Pediatr Urol. 2013;9:199–205. doi: 10.1016/j.jpurol.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Lucas SM, Sundaram CP, Wolf JS, Jr, Leveillee RJ, Bird VG, Aziz M, et al. Factors that impact the outcome of minimally invasive pyeloplasty: Results of the Multi-institutional Laparoscopic and Robotic Pyeloplasty Collaborative Group. J Urol. 2012;187:522–7. doi: 10.1016/j.juro.2011.09.158. [DOI] [PubMed] [Google Scholar]
- 13.Thiel DD. Navigating the difficult robotic assisted pyeloplasty. ISRN Urol. 2012;2012:291235. doi: 10.5402/2012/291235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindgren BW, Hagerty J, Meyer T, Cheng EY. Robot-assisted laparoscopic reoperative repair for failed pyeloplasty in children: A safe and highly effective treatment option. J Urol. 2012;188:932–7. doi: 10.1016/j.juro.2012.04.118. [DOI] [PubMed] [Google Scholar]
- 15.Gundeti M. The role of robotics in the management of renal duplication anomalies. AUA News. 2012;17:1, 7–8. [Google Scholar]
- 16.Mason MD, Anthony Herndon CD, Smith-Harrison LI, Peters CA, Corbett ST. Robotic-assisted partial nephrectomy in duplicated collecting systems in the pediatric population: Techniques and outcomes. J Pediatr Urol. 2014;10:374–9. doi: 10.1016/j.jpurol.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Traxel EJ, Minevich EA, Noh PH. A review: The application of minimally invasive surgery to pediatric urology: Lower urinary tract reconstructive procedures. Urology. 2010;76:115–20. doi: 10.1016/j.urology.2009.11.073. [DOI] [PubMed] [Google Scholar]
- 18.Lee RS, Passerotti CC, Cendron M, Estrada CR, Borer JG, Peters CA. Early results of robot assisted laparoscopic lithotomy in adolescents. J Urol. 2007;177:2306–9. doi: 10.1016/j.juro.2007.01.178. [DOI] [PubMed] [Google Scholar]
- 19.Lendvay T. Robotic-assisted laparoscopic management of vesicoureteral reflux. Adv Urol. 2008:732942. doi: 10.1155/2008/732942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith RP, Oliver JL, Peters CA. Pediatric robotic extravesical ureteral reimplantation: comparison with open surgery. J Urol. 2011;185:1876–81. doi: 10.1016/j.juro.2010.12.072. [DOI] [PubMed] [Google Scholar]
- 21.Kasturi S, Sehgal SS, Christman MS, Lambert SM, Casale P. Prospective long-term analysis of nerve-sparing extravesical robotic-assisted laparoscopic ureteral reimplantation. Urology. 2012;79:680–3. doi: 10.1016/j.urology.2011.10.052. [DOI] [PubMed] [Google Scholar]
- 22.Marchini GS, Hong YK, Minnillo BJ, Diamond DA, Houck CS, Meier PM, et al. Robotic assisted laparoscopic ureteral reimplantation in children: Case matched comparative study with open surgical approach. J Urol. 2011;185:1870–5. doi: 10.1016/j.juro.2010.12.069. [DOI] [PubMed] [Google Scholar]
- 23.Gundeti MS, Kojima Y, Haga N, Kiriluk K. Robotic-assisted laparoscopic reconstructive surgery in the lower urinary tract. Curr Urol Rep. 2013;14:333–41. doi: 10.1007/s11934-013-0328-7. [DOI] [PubMed] [Google Scholar]
- 24.Casale P, Patel RP, Kolon TF. Nerve sparing robotic extravesical ureteral reimplantation. J Urol. 2008;179:1987–9. doi: 10.1016/j.juro.2008.01.062. [DOI] [PubMed] [Google Scholar]
- 25.Lee RS, Retik AB, Borer JG, Peters CA. Pediatric robot assisted laparoscopic dismembered pyeloplasty: Comparison with a cohort of open surgery. J Urol. 2006;175:683–7. doi: 10.1016/S0022-5347(05)00183-7. [DOI] [PubMed] [Google Scholar]
- 26.Rowe CK, Pierce MW, Tecci KC, Houck CS, Mandell J, Retik AB, et al. A comparative direct cost analysis of pediatric urologic robot-assisted laparoscopic surgery versus open surgery: Could robot-assisted surgery be less expensive? J Endourol. 2012;26:871–7. doi: 10.1089/end.2011.0584. [DOI] [PubMed] [Google Scholar]
- 27.Barbosa JA, Barayan G, Gridley CM, Sanchez DC, Passerotti CC, Houck CS, et al. Parent and patient perceptions of robotic vs open urological surgery scars in children. J Urol. 2013;190:244–50. doi: 10.1016/j.juro.2012.12.060. [DOI] [PubMed] [Google Scholar]
- 28.Behan JW, Kim SS, Dorey F, De Filippo RE, Chang AY, Hardy BE, et al. Human capital gains associated with robotic assisted laparoscopic pyeloplasty in children compared to open pyeloplasty. J Urol. 2011;186:1663–7. doi: 10.1016/j.juro.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 29.Freilich DA, Penna FJ, Nelson CP, Retik AB, Nguyen HT. Parental satisfaction after open versus robot assisted laparoscopic pyeloplasty: Results from modified Glasgow Children's Benefit Inventory Survey. J Urol. 2010;183:704–8. doi: 10.1016/j.juro.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 30.Razmaria AA, Marchetti PE, Prasad SM, Shalhav AL, Gundeti MS. Does robot-assisted laparoscopic ileocystoplasty (RALI) reduce peritoneal adhesions compared with open surgery? BJU Int. 2014;113:468–75. doi: 10.1111/bju.12284. [DOI] [PubMed] [Google Scholar]
- 31.Bansal D, Defoor WR, Jr, Reddy PP, Minevich EA, Noh PH. Complications of robotic surgery in pediatric urology: a single institution experience. Urology. 2013;82:917–20. doi: 10.1016/j.urology.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 32.Friedman DC, Lendvay TS, Hannaford B. Instrument failures for the da Vinci surgical system: A Food and Drug Administration MAUDE Database Study. Surg Endosc. 2013;27:1503–8. doi: 10.1007/s00464-012-2659-8. [DOI] [PubMed] [Google Scholar]
- 33.Kearns J, Gundeti MS. Are there safety concerns with robotic surgery in pediatrics. AUA News. 2014;19:23. [Google Scholar]
- 34.Smith R, Patel V, Satava R. Fundamentals of robotic surgery: A course of basic robotic surgery skills based upon a 14-society consensus template of outcomes measures and curriculum development. Int J Med Robot. 2013 doi: 10.1002/rcs.1559. [DOI] [PubMed] [Google Scholar]
- 35.Lendvay TS, Casale P, Sweet R, Peters C. VR robotic surgery: Randomized blinded study of the dV-Trainer robotic simulator. Stud Health Technol Inform. 2008;132:242–4. [PubMed] [Google Scholar]
- 36.Liss MA, McDougall EM. Robotic surgical simulation. Cancer J. 2013;19:124–9. doi: 10.1097/PPO.0b013e3182885d79. [DOI] [PubMed] [Google Scholar]
- 37.Lendvay TS. Surgical simulation in pediatric urologic education. Curr Urol Rep. 2011;12:137–43. doi: 10.1007/s11934-011-0170-8. [DOI] [PubMed] [Google Scholar]
- 38.Gundeti MS, Kearns JT. Pediatric Robotic Urological Surgery: A Decade of Progress and Future Directions. Submitted for Publication. 2014 [Google Scholar]
- 39.Marescaux J, Leroy J, Gagner M, Rubino F, Mutter D, Vix M, et al. Transatlantic robot-assisted telesurgery. Nature. 2001;413:379–80. doi: 10.1038/35096636. [DOI] [PubMed] [Google Scholar]
- 40.Anvari M, McKinley C, Stein H. Establishment of the world's first telerobotic remote surgical service: For provision of advanced laparoscopic surgery in a rural community. Ann Surg. 2005;241:460–4. doi: 10.1097/01.sla.0000154456.69815.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demographics of India. Wikipedia, the free encyclopedia. 2014. [Last cited on 2014 Apr 07]. Available from: http://www.en.wikipedia.org/w/index.php?title=Demographics_of_India&oldid=603045485 .
- 42.Demographics of the United States. Wikipedia, the free encyclopedia. 2014. [Last cited on 2014 Apr 07]. Available from: http://www.en.wikipedia.org/w/index.php?title=Demographics_of_the_United_States&oldid=603093948 .
- 43.Chowdhary S. Pediatric Robotic and Reconstructive Urology: A Comprehensive Guide. 1st ed. Oxford, UK: Wiley-Blackwell; 2012. Asian continents: is it ready for new technology. Indian perspective? p. 364. [Google Scholar]


