Background: Low molecular mass hyaluronan (LMHA) is proinflammatory, but the role of the N-acetyl moieties is unknown.
Results: Chemical reacetylation of LMHA results in maximal proinflammatory cytokine production by human macrophages, compared with other N-acylations. Partial N-butyrylation blocks cytokine stimulation.
Conclusion: The N-acetyl moieties of glucosamine are critical for LMHA proinflammatory properties.
Significance: N-Acetylation and butyrylation of LMHA modulate proinflammatory cytokine production.
Keywords: CD44, Cytokine, Glycobiology, Hyaluronate, Inflammation, Macrophage, Toll-like Receptor (TLR), Hyaluronan, N-Acylation, Polymers
Abstract
Low molecular mass hyaluronans are known to induce inflammation. To determine the role of the acetyl groups of low molecular mass hyaluronan in stimulating the production of proinflammatory cytokines, partial N-deacetylation was carried out by hydrazinolysis. This resulted in 19.7 ± 3.5% free NH2 functional groups, which were then acylated by reacting with an acyl anhydride, including acetic anhydride. Hydrazinolysis resulted in bond cleavage of the hyaluronan chain causing a reduction of the molecular mass to 30–214 kDa. The total NH2 and N-acetyl moieties in the reacetylated hyaluronan were 0% and 98.7 ± 1.5% respectively, whereas for butyrylated hyaluronan, the total NH2, N-acetyl, and N-butyryl moieties were 0, 82.2 ± 4.6, and 22.7 ± 3.8%, respectively, based on 1H NMR. We studied the effect of these polymers on cytokine production by cultured human macrophages (THP-1 cells). The reacetylated hyaluronan stimulated proinflammatory cytokine production to levels similar to LPS, whereas partially deacetylated hyaluronan had no stimulatory effect, indicating the critical role of the N-acetyl groups in the stimulation of proinflammatory cytokine production. Butyrylated hyaluronan significantly reduced the stimulatory effect on cytokine production by the reacetylated hyaluronan or LPS but had no stimulatory effect of its own. The other partially N-acylated hyaluronan derivatives tested showed smaller stimulatory effects than reacetylated hyaluronan. Antibody and antagonist experiments suggest that the acetylated and partially butyrylated lower molecular mass hyaluronans exert their effects through the TLR-4 receptor system. Selectively N-butyrylated lower molecular mass hyaluronan shows promise as an example of a novel semisynthetic anti-inflammatory molecule.
Introduction
Hyaluronan (HA)2 is a major component of synovial fluid and cartilage extracellular matrix, protecting articular cartilage from damage and playing a central role in joint lubrication (1, 2). HA is composed of repeating GlcNAc and d-glucuronic acid (GlcUA) units (1, 3, 4) linked via an alternating β-1,4- and β-1,3-glycosidic bonds (5, 6). Under physiological conditions, the molecular mass and concentration of HA in synovial fluid are between 2150 and 4960 kDa and less than 4 mg/ml, respectively (3, 7). In certain pathological conditions such as osteoarthritis and rheumatoid arthritis, HA is degraded into polydisperse lower molecular mass HA (LMHA) by either oxidative hydrolysis or by hyaluronidase-mediated degradation (4, 8). Low molecular mass HA binds to certain cell surface receptors such as Toll-like receptors (TLRs) and the cluster of differentiation (CD44) (9–13) to activate the downstream signaling molecules that stimulate overproduction of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 (9, 14). These cytokines induce proteoglycan degradation and matrix metalloproteinase production, leading to further joint destruction. Therefore, the pathological generation of LMHA can be considered as a danger signal, which could activate both innate and acquired immunity, even in the absence of microbial stimuli (10, 15). Intra-articular injection of high molecular mass HA (HMHA) is used mostly in the treatment of osteoarthritis, but may also have applications in rheumatoid arthritis (12, 16), and is thought to work through restoration of viscoelastic properties. However, HMHA also prevents the interaction of certain cell surface receptors such as TLR-4 and CD44 with LMHA with a consequent contribution to resolution of inflammation and to tissue repair (9, 12, 17).
It is not known whether the inflammatory response initiated by LMHA can be modified by replacing the N-acetyl group in the GlcNAc moieties of HA with longer acyl chains. Thus, the objective of this work is to replace the N-acetyl group in the GlcNAc moieties of HA with longer acyl chain and then investigate the inflammatory responses of the human macrophage cell line (THP-1) when cultured with these chemically modified HA compounds. Our second objective is to identify the cell surface receptor responsible for these responses.
EXPERIMENTAL PROCEDURES
Materials
IL-1β (CL76121K), IL-8 (CL76128K), IL-6 (CL76126K), TNF-α (430207) ELISA kits, the polyclonal antibody to TLR-4 (pab-hstlr4), the TLR-4 antagonist, LPS-RS Ultrapure (tlrl-prslps), the THP-1 cell line (ATCC line TIB-202), and RPMI 1640 medium (30–2001) were from Cedarlane. XTT sodium salt bioreagent, phenazine methosulfate (PMS), sodium salt of HA from Streptococcus equi (1500–1800 kDa), LPS from Escherichia coli 0111:B4C, L4391, phorbol 12-myristate 13-acetate (P1585) (PMA), acetic anhydride, propanoic anhydride, butyric anhydride, valeric anhydride, isovaleric anhydride, and Select-HA LoLadder were purchased from Sigma-Aldrich. CD44 antibodies (555476, clone G44-26, and 550988, clone 514, designated Ab1 and Ab2, respectively, in this study), which are different IgG κ, were from Becton Dickinson. The TLR-2 agonist (PG-PS 10S) is from Thermo Fisher Scientific. Vivaspin polyethersulfone gel filtration spin columns were purchased from Thermo Fisher Scientific. Spectra/Por® dialysis membrane with molecular mass cutoff of 6,000–8,000 was from Spectrum Laboratories, Inc. All other chemicals were from Sigma-Aldrich or Thermo Fisher Scientific.
Partial Deacetylation of HA
To form the partially deacetylated HA (DHA), using the hydrazinolysis reaction, 3 g of HA was dissolved in 150 ml of hydrazine monohydrate and 1.5 g of hydrazine sulfate at 55 °C for 6, 24, 48, 72, and 96 h (18, 19). The polymeric product was precipitated, washed with ethanol, and dried at 25 °C for 24 h. The sample was dissolved in a mixture of 50 ml of 5% (v/v) acetic acid and 30 ml of 0.5 m iodic acid and kept in a bath at 4 °C for at least 1 h (18, 19). 8.75 ml of aqueous 57% (v/v) CH3I was added and stirred for 15 min. The deep violet color of the solution was removed via liquid-liquid extraction with ethyl ether. The pH of the aqueous layer, containing the deacetylated HA, was adjusted to 7.0–7.5 and precipitated with ethanol. The polymer was dissolved in water, dialyzed against double-distilled H2O, and freeze-dried.
For comparison with HA deacetylated for 72 h using the hydrazinolysis reaction (DHA), HA was also deacetylated using NaOH as the deacetylating agent (DHA-NaOH) according to the method used by Wada et al. (20). 2 g of HA was dissolved in 200 ml of 2.5 n NaOH, and the solution was stirred at room temperature for 72 h. After the reaction, 140 ml of water was added to the reaction mixture, and the reaction was stirred in an ice bath, whereas the pH of the solution was adjusted to 10.0 with concentrated HCl. The solution was poured into 800 ml of a 7:1 acetone-H2O mixture at 0 °C. The white fibrous precipitate formed was recovered by filtration. The precipitate was dissolved in water, and the pH was adjusted to 7.0. Acetone was evaporated using a rotary evaporator, and the solution was lyophilized. The polymer was redissolved in water, dissolved salt was removed by dialysis, and the sample was relyophilized.
Reacylation of DHA or DHA-NaOH
Reacylation of DHA and DHA-NaOH to form AHA or AHA-NaOH was done according to the method used for N-propionylation of the glucosamine monomer (21), and recently used by Zhang et al. (22), with some modifications. Briefly, 0.1 g of DHA or DHA-NaOH was dissolved in 30 ml of distilled water, and 6 ml of saturated NaHCO3 was added. Acetic or butyric anhydride was added to absolute alcohol at concentration of 0.05, 0.5, 2.5, 5.0, and 10.0% (v/v) and added to the reaction mixture. The reaction mixture was stirred for 10 min and then quenched in a boiling water bath for 5 min. Residual ethanol was evaporated by using the rotary evaporator, and then the sample was lyophilized to remove the water. The lyophilized sample was dialyzed against distilled water and relyophilized. 6,000 Da molecular mass cutoff dialysis tubing was used to ensure that the LMHA preparations did not contain oligosaccharides.
Digestion of HA
As control, HA was digested with hyaluronidase from bovine testes to obtain low molecular mass HA with similar molecular mass with the DHA and AHA. 3 mg/ml of HA was also digested with 10 units/ml hyaluronidase from bovine testes in PBS (pH 7.2) at 37 °C for 30 min (HA-digested) (23). The reaction was stopped by boiling for 5 min. The sample was lyophilized, and dissolved salt and oligosaccharides were removed by dialysis (6000-Da molecular mass cut off) and then relyophillized. Digested HA was separated into fractions smaller and larger than 30 kDa using a polyethersulfone gel filtration spin column.
1H NMR
To confirm the structure and purity of the polymers and to provide preliminary measurements of the degree of deacetylation, 1H NMR spectra of the polymers were recorded at 348 K in D2O at 600 MHz. The resulting peaks were compared with the solvent peaks relative to tetramethylsilane as reference. 1H spectra assignment was performed by two-dimensional NMR, i.e. 1H-H COSY and total correlation spectroscopy and 1H-13C heteronuclear multiple-bond correlation spectroscopy and heteronuclear single-quantum correlation spectroscopy. The degree of deacetylation was estimated from the 1H NMR spectrum according to the method used by Crescenzi et al. (18, 19). In the native polymer repeating unit, there are three methyl protons in the GlcNAc unit for every two anomeric protons from the GlcNAc and GlcUA unit, and the ratio of the signal for these protons is 1.5 (18, 19). From the 1H NMR spectra of the DHA obtained in this study, we calculated the ratio of the integration of the peaks (Y) for three methyl protons in the GlcNAc unit observed at (2.4–2.5 ppm) and the anomeric protons of the GlcNAc, GlcUA and glucosamine units observed at 4.9–5.3. The deacetylation degree, defined as the molar ratio of glucosamine to glucosamine + GlcNAc unit was calculated according to equation deacetylation degree = (1.0 − (Y/1.5))100) (18, 19).
Colorimetric Assays
To further confirm the 1H NMR data, colorimetric quantification of the glucosamine and glucuronic content of the polymer were determined. The glucosamine content was quantified according to the method used previously, for chitosan (24), which is based on the derivatization reaction of its primary amino group with o-phthaldehyde and the thiol group of N-acetyl-l-cysteine. The glucuronic content was quantified according to the carbazole reaction method used by Cesaretti et al. (25). The results are expressed as the molar ratios of glucosamine to glucuronic acid.
Molecular Mass Estimation
The molecular mass range of the samples was estimated by electrophoresis of HA on a 0.75% (w/v) agarose gel cast and run in TAE buffer (pH 8.0) at 100 V for 90 min. The bromphenol blue tracking dye migrated close to the end of the gel during this time period. Immediately after the run, the gel was placed in ∼100 ml of solution containing 0.005%w/v Stains-All in 50% (v/v) ethanol overnight in the dark at room temperature. For destaining, the gel was transferred to 10% (v/v) ethanol solution and stored in the dark for 1 day with two or more changes of destaining solution (26).
Viscosity of Polymers
Steady shear viscosity of the polymers were conducted with a TA Instruments AR 2000TM rheometer (TA Instruments, New Castle, DE) equipped with a cone and plate fixture consisting of a 0.5 degrees, 4-cm-diameter stainless steel cone, in the steady shear mode. Approximately 300 μl of fluid sample was required, and the temperature was controlled at 37 °C. The concentration of HA and the HA derivatives used was 5 mg/ml.
Mass Spectrometry
For HA, 3 ml of 5 mg/ml of HA was digested with 1 ml of 800–2000 units/ml of bovine testes hyaluronidase incubated at 37 °C for 24 h. For HA derivatives, 500 μl of 30 mg/ml were digested with 1 ml of 800–2000 units/ml of bovine testicular hyaluronidase. The enzyme was precipitated by boiling for 5 min and centrifuged at 2050 rpm for 10 min. The supernatant was collected and separated with a micron centrifugal filter device (Millipore Corporation) with a molecular mass cutoff of 3000 Da. The filtrate obtained from this digestion was lyophilized and used for the MS analyses. MS and MS/MS analyses were performed on a QSTAR XL hybrid quadrupole/TOF tandem mass spectrometer (Applied Biosystems/MDS SCIEX) equipped with electrospray ionization source and was further confirmed using Thermo LTQ Orbitrap Velos Pro with heated electrospray ionization source under negative mode. For electrospray ionization analysis under negative mode, the following source conditions were used: a curtain gas setting of 25, ion spray voltage of −4000 V, Q0 declustering potential of −90 V, and focusing potential −350 V. Nitrogen was used as the collision gas (CAD setting 5) for both TOF MS and MS/MS scans.
Proinflammatory Cytokine Response to Reacylated HA (AHA)
Prior to the study, the polymers were placed into 1.5-ml Eppendorf tubes and were sterilized by exposing to germicidal UV source (Phillips; G30T8; 253.7 nm) for 30 min in the laminar flow hood. The human suspension monocyte cell line (THP-1) was maintained in RPMI 1640 medium, supplemented with 10% fetal bovine serum at 37 °C in humidified atmosphere with 5% CO2. To investigate the proinflammatory cytokine response to our compounds, the THP-1 cells were seeded at 5 × 105 cell/ml in RPMI1640, 10% fetal bovine serum, 50 μm β-mercaptoethanol, and 100 nm PMA, at 37 °C, in humidified atmosphere, with 5% CO2 in 24- or 96-well plates. For the induction of monocyte-macrophage differentiation, THP-1 cells were seeded with 100 nm PMA (27). After 72 h, nonattached cells were removed by aspiration and washed three times with serum-free medium. The medium of the adherent macrophages was replaced with RPMI 1640, 1% fetal bovine serum, 100 nm PMA, with and without HA derivatives, TLR agonists and antagonists, or neutralizing antibodies. After 24 h, media were removed, and the proinflammatory cytokine concentrations were determined using enzyme-linked immunosorbent assay kits for TNF-α, IL-1β, IL-6, and IL-8 from Cedarlane. The absorbance was measured on a microplate spectrometer (μQuant; Bio-Tek Instruments, Inc.) at a single wavelength of 450 nm. According to the manufacturer's instructions, a curve of absorbance versus log concentration of individual cytokines in the standard wells was constructed and used to determine the concentrations of cytokines in the media. The standard curve was plotted using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, A). A measure of the attached THP-1 macrophage cell numbers was determined using the XTT/PMS assay (28). The TLR-2 agonist is purified peptidoglycan polysaccharide polymer from the cell wall of Streptococcus pyogenes group A, D58 strain. LPS.EC (L4391) is lipopolysaccharide from E. coli 0111:B4. The TLR-4 antagonist, LPS-RS Ultrapure (tlrl-prslps), has been isolated from the bacterium Rhodobacter sphaeroides. This ultrapure grade has only TLR-4 antagonist activity, as opposed to the standard grade, which has TLR-4 antagonist and TLR-2 agonist activities.
The size range of the polymer and the molar ratio of its free amino groups of glucosamine to glucuronic acid was determined, as described below. The degree of AHA acetylation necessary for inflammatory activity was determined by the biological assay. To do this, DHA was treated with increasing concentrations of acetic anhydride in absolute ethanol. The inflammatory activity of these differently acetylated HA derivatives was assayed by measuring the IL-1β secretion, as described. DHA was fractionated on the basis of size using polyethersulfone gel filtration spin columns with molecular mass cutoffs of 30,000, 50,000, 100,000, or 300,000 Da. The filtrates were freeze-dried and resuspended with 0.01 n HCl, and the glucosamine and glucuronic acid concentrations were determined using the o-phthalaldehyde and carbazole colorimetric assays, respectively (24, 25).
RESULTS
Deacetylation of Polymers
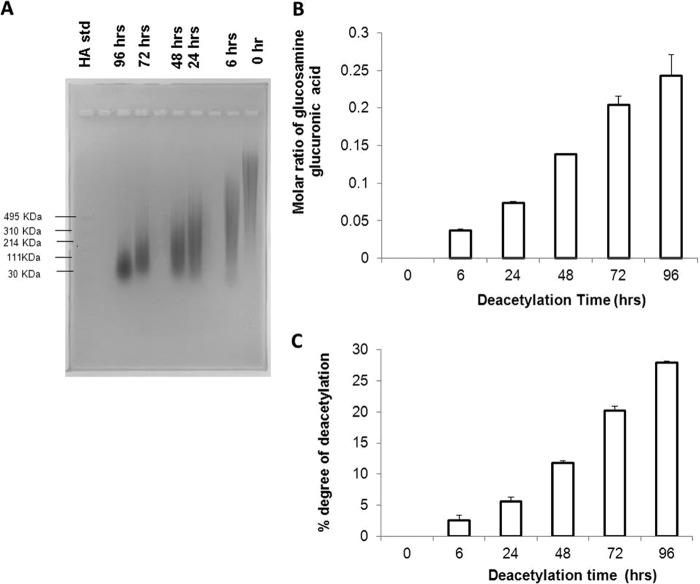
Some of the GlcNAc units of HA were converted to glucosamine via the hydrazinolysis reaction at 55 °C for 6, 24, 48, 72, and 96 h. With increasing reaction time, the molecular mass range of the polymer decreased (Fig. 1A), whereas the molar ratio of glucosamine to glucuronic acid increased (Fig. 1, B and C). The anomeric protons of GlcNAc and GlcUA in HA were observed by NMR at 4.9–5.1 ppm. As the reaction proceeds with time, there is an increasing appearance of peaks at 5.15–5.25 ppm, which was assigned to the anomeric protons of the generated glucosamine unit and the GlcUA unit linked via the β1,4-glycosidic bond from the one- and two-dimensional 1H NMR. Fig. 2 shows the chemical structure of the repeating units of HA and its derivatives. The hydrazinloysis reaction done at 72 h (DHA) generated HA within the molecular mass range that is known to generate an inflammatory response and was therefore used for further studies (9, 17, 29). In this sample (DHA), the ratio of the integration of the NMR peaks for the three methyl protons in the GlcNAc unit and the anomeric protons of the GlcNAc, GlcUA, and glucosamine was calculated to be 1.24 ± 0.05. From this ratio, the deacetylation degree was calculated to be 19.7 ± 3.5%. HA was also deacetylated by reacting with NaOH (DHA-NaOH) and by digesting with hyaluronidase (HA-digested) as described under “Experimental Procedures.” The reaction conditions used in the two methods of deacetylation for making DHA, DHA-NaOH, and DHA-digested yielded LM HA of similar size range and IL-1β stimulation, as indicated Figs. 3B and 4D, respectively.
FIGURE 1.
Characterization of HA and HA deacetylated for 6, 24, 48, 72, and 96 h. A, HA and HA deacetylated for 6, 24, 48, 72, and 96 h and Select-HA LoLadder were run on a 0. 75% (w/v) agarose gel in TAE running buffer, at a constant voltage of 100 V for 90 min and then visualized with 0.005% (w/v) Stains-All. B, the molar ratio of glucosamine to glucuronic acid for HA and HA deacetylated for 6, 24, 48, 72, and 96 h is illustrated. This was obtained using the o-phthalaldehyde and carbazole colorimetric assays, respectively. C, the degree of deacetylation of polymers deacetylated for 6, 24, 48, 72, and 96 h obtained from the 1H NMR peaks recorded at 348 K in D2O at 600 MHz is illustrated. The resulting peaks were compared with the solvent peaks relative to tetramethylsilane as reference, because in the native polymer repeat unit, there are three methyl protons (acetyl group) for every two anomeric protons, and the ratio of the signal for these protons is 1.5 (18). The degree of deacetylation of the deacetylated samples was estimated by using the following equation: deacetylation degree = (1 − (X/Y)/1.5) × 100 (18). X is the integration of the acetyl group signal in the spectra at 2.4–2.5 ppm, and Y is the integration of the two anomeric protons signals at 4.9–5.3 ppm.
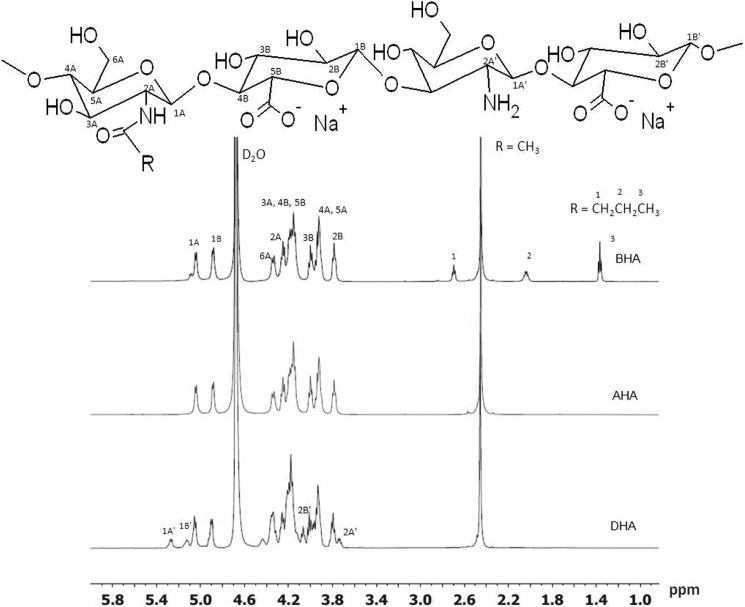
FIGURE 2.
1H NMR of DHA, AHA and BHA in D2O at 349 K. 25 mg/ml of the polymers were dissolved in deuteriated water (D2O).1H NMR spectra of the polymers were recorded at 348 K in D2O at 600 MHz. The resulting peaks were compared with the solvent peaks relative to tetramethylsilane as reference, because in the native polymer repeat unit, there are three methyl protons (acetyl group) for every two anomeric protons, and the ratio of the signal for these protons is 1.5 (18). The degree of deacetylation in our sample DHA was estimated by using the following equation: deacetylation degree = (1 − (X/Y)/1.5) × 100 (18). X is the integration of the acetyl group signal in DHA at 2.4–2.5 ppm, and Y is the integration of the two anomeric protons signals at 4.9–5.3 ppm. Their ratio is ∼1.24 ± 0.05. Therefore, the percentage of glucosamine was estimated to be 19.7 ± 3.5%. R = COCH3 in the structure shown for HA, R = H, COCH3 in DHA, R = H, COCH3 in AHA, and R = H, COCH3, and COCH2CH2CH3 in BHA.
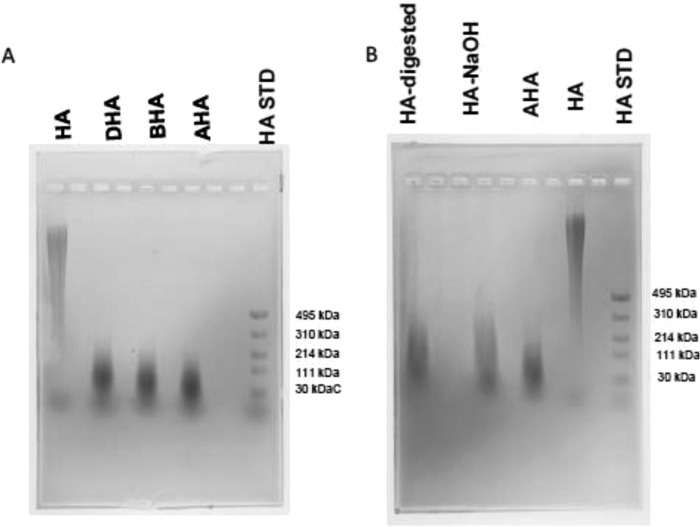
FIGURE 3.
Electrophoretic separation of HA derivatives. A, HA, DHA, BHA, AHA, and Select-HA LoLadder (HA STD) were run on a 0.75% (w/v) agarose gel in TAE running buffer at a constant voltage of 100 V for 1 h and then visualized with 0.005% (w/v) Stains-All. B, HA-digested, AHA-NaOH, AHA, HA, and Select-HA LoLadder were run on a 0.75% (w/v) agarose gel in TAE running buffer, at a constant voltage of 100 V for 1.5 h and then visualized with 0.005% (w/v) Stains-All.
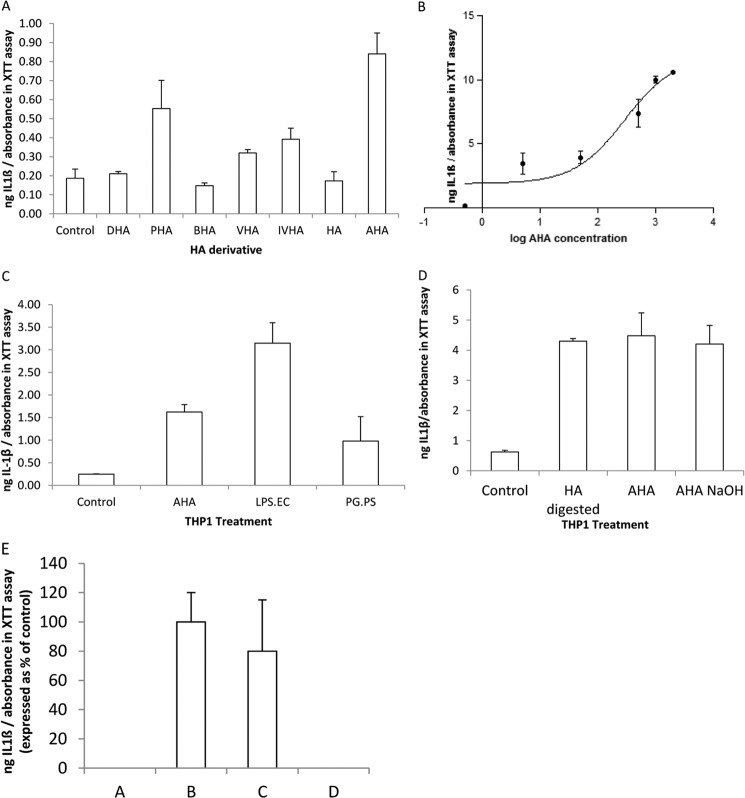
FIGURE 4.
Stimulation of the inflammatory response in THP-1 macrophages. THP-1 macrophages are treated for 24 h with hyaluronan (HA) derivatives and Toll-like receptor (TLR) agonists, and then the media were assayed for cytokines using ELISA, and a measure of the attached cell numbers was determined using the XTT/PMS cell proliferation assay. In this and subsequent figures, the total ng of cytokines secreted per well, determined by ELISA, is divided by the absorbance measured at 450 nm using the XTT/PMS cell proliferation assay. This calculation gives an estimate of the ng of cytokines secreted per cell. The data are means ± S.D. (n = 3). A, THP-1 IL-1β secretion in the presence of different derivatives of hyaluronic acid. THP-1 cells were treated with 500 μg/ml HA derivatives for 24 h. PHA, propionylated HA; VHA, valerylated HA; IVHA, isovalerylated HA. The increase in IL-1β secretion for THP-1 treated with reacetylated HA (AHA) was significantly greater than the control (no HA derivative) (p = 0.0486, Student's t test). B, THP-1 IL-1β secretion in the presence of increasing concentrations of AHA. The dose-dependent response of IL-1β secretion with increasing concentrations of AHA is illustrated. The EC50 is 292 μg/ml. C, THP-1 IL-1β secretion in the presence and absence of AHA, TLR-4 agonist (LPS.EC), or TLR-2 agonist (PG.PS). To investigate the cell surface receptor response for the stimulation of IL-1β by AHA, THP-1 was treated with 500 μg/ml of AHA or 250 ng/ml of TLR-4 agonist (LPS.EC) or 250 ng/ml TLR-2 agonist (PG.PS). The TLR-2 agonist are purified peptidoglycan polysaccharide polymers from the cell wall of S. pyogenes group A, D58 strain. LPS.EC (L4391) are lipopolysaccharides from E. coli 0111:B4. The control is THP-1 that is not treated with AHA, LPS-EC, or PG.PS. For AHA and LPS.EC, this increase in IL-1β secretion over control is statistically significant (p < 0.05, Student's t test). D, THP-1 IL-1β secretion in the presence of acetylated low molecular mass HA. Starting with the hyaluronic acid sodium salt from S. equi (HA) (1500–1800 kDa), different protocols were used to generate acetylated low molecular mass HA. THP-1 cells were then treated with 500 μg/ml of these acetylated low molecular mass HA derivatives for 24 h. Abbreviations for the acetylated low molecular mass HA compounds were HA deacetylated via hydrazinolysis reaction and reacetylated with acetic anhydride (AHA), HA partially digested with bovine testicular hyaluronidase (HA digested), and HA deacetylated with NaOH and reacetylated with acetic anhydride (AHA NaOH). Control is THP-1 that is not treated with any low molecular mass HA compound. E, THP-1 IL-1β secretion in the presence of fractionated and unfractionated hyaluronidase digests of HA. HA was digested for 30 min with testicular hyaluronidase to generate low molecular mass HA. THP-1 cells were treated for 24 h with either total/unfractionated low molecular mass HA or size-fractionated low molecular mass HA. Total/unfractionated low molecular mass HA (column B) or low molecular mass HA >30 kDa (column C) was used at 500 μg/ml. Low molecular mass HA fragments of <30 kDa (column D) were used at 12.5 μg/ml, because this is its percentage of total low molecular mass HA. Control is THP-1 that is not treated with any low molecular mass HA compound (column A).
Reacylation of Polymers
For HA deacetylated via the hydrazinolysis reaction, several N-acyl derivatives were synthesized using the appropriate anhydride. The 1H NMR spectra of AHA, BHA, and DHA in D2O confirmed the expected major peaks associated with the backbones of the polymers (Fig. 2). Table 1 shows the percentages of glucosamine, N-acetyl-glucosamine, and N-butyryl glucosamine composition of AHA, BHA, and DHA as calculated from 1H NMR spectrum according to the method used by Crescenzi et al. (18, 19). Table 1 also shows the ratio of the free amino group to the glucuronic acid group. Reacylation with acetic anhydride or butyric anhydride increased with increases in concentration of the acyl anhydride used.
TABLE 1.
The percentage of −NH2, N-acetyl, and N-butyryl moieties in DHA, AHA, and BHA as calculated from 1H NMR spectra
| Sample | −NH2/GlcUA | Glucosamine | N-Acetyl-glucosamine | N-Butyryl-glucosamine |
|---|---|---|---|---|
| molar ratio | % | % | % | |
| DHA | 0.2200 ± 0.0100 | 19.7 ± 3.5 | 82.8 ± 3.1 | |
| AHA | 0.0044 ± 0.0005 | 98.7 ± 1.5 | ||
| BHA | 0.0040 ± 0.0010 | 82.2 ± 4.6 | 22.7 ± 3.8 |
Molecular Mass Range Estimation
There was glycosidic bond cleavage of the initial HA chain (molecular mass of 1500–1800 kDa) causing a significant reduction of the molecular mass to 30–214 kDa, as estimated by agarose gel electrophoresis (Fig. 3A). The HA LoLadder standard in the molecular mass range of 30–495 kDa was used to estimate the molecular mass of the polymers. The reacylation reaction had no significant effect on the molecular mass range of the polymers. The molecular mass range of AHA is similar to the molecular mass of HA-digested and AHA-NaOH (Fig. 3B) at the conditions used.
Viscosity of Polymer
5 mg/ml of HA exhibited a non-Newtonian behavior with higher viscosity at low shear rate and shear thinning as the shear rate increases. Similar concentrations of AHA, BHA, and DHA exhibited Newtonian behavior with much lower viscosity compared with HA but approximately three times higher than that of water.
Mass Spectrometry
Digestion of HA and its derivatives by testicular hyaluronidase, an endo-β-N-acetylhexosaminidase yielded a homologous series of oligosaccharides having repeating units of (4GlcUAβ1–3GlcNAcβ1). The oligosaccharides obtained were separated with micron centrifugal filter device with a molecular mass cutoff of 3000 Da. As shown in Table 2, the observed m/z is very similar to the predicted m/z as shown. The ion spray mass spectra of the filtrate produced singly charged disaccharides (396.1159) and singly (797.2095) and doubly charged (387.1104) tetrassaccharides as a result of sodium abstraction. The number of charged ions observed corresponds to the number of glucuronic acid sodium salt moieties contained in the oligosaccharides. In addition to the molecular ions generated by the enzymatic degradation of AHA and DHA, the spectra generated by BHA showed additional molecular ions that coincide with singly charged disaccharides of GlcNBU and GlcUA (424.1473), singly (853.2743) and doubly (415.1405) charged tetrassaccharides of GlcNBU and GlcUA, and singly (825.2433) and doubly (401.1260) charged tetrassaccharides of GlcNBU, GlcNAc, and GlcUA (4GlcUAβ1–3GlcNAcβ1-4GlcUAβ1–3GlcNBUβ1). This further confirms, from the 1H NMR spectra, that the glucosamine moieties in DHA were successfully N-butyrylated (BHA). Because the doubly charged tetrassaccharides of GlcNAc and GlcUA were the most abundant in all the samples, it was used as the target peak, and the intensities of the other relevant peaks were calculated based on the target peak as shown in Table 2.
TABLE 2.
Observed molecular ion species in hyaluronidase digests of AHA, BHA, and DHA and their relative intensities
| Molecular ions | Charge | Predicted m/z | Observed m/z |
||
|---|---|---|---|---|---|
| AHA | BHA | DHA | |||
| Dissaccharide of GlcNAc and GlcUA | −1 | 396.1142 | 396.1158 (31) | 396.1159 (28) | 396.1156 (28) |
| Tetrasaccharide of GlcNAc and GlcUA | −1 | 797.2076 | 797.2092 (22) | 797.2095 (12) | 797.2098 (9) |
| Tetrasaccharide of GlcNAc and GlcUA | −2 | 387.1095 | 387.1104 (100) | 387.1104 (100) | 387.1102 (100) |
| Dissaccharide of GlcNBU and GlcUA | −1 | 424.1455 | 424.1473 (2) | ||
| Tetrasaccharide of GlcNBu and GlcUA | −1 | 853.2708 | 853.2743 (0.3) | ||
| Tetrasaccharide of GlcNBu and GlcUA | −2 | 415.1408 | 415.1419 (3) | ||
| Tetrasaccharide of GlcNAc, GlcNBU, and GlcUA | −1 | 825.2395 | 825.2433 (2) | ||
| Tetrasaccharide of GlcNAc, GlcNBU, and GlcUA | −2 | 401.1251 | 401.1259 (36) | ||
Proinflammatory Cytokine Response to Reacylated HA Derivatives
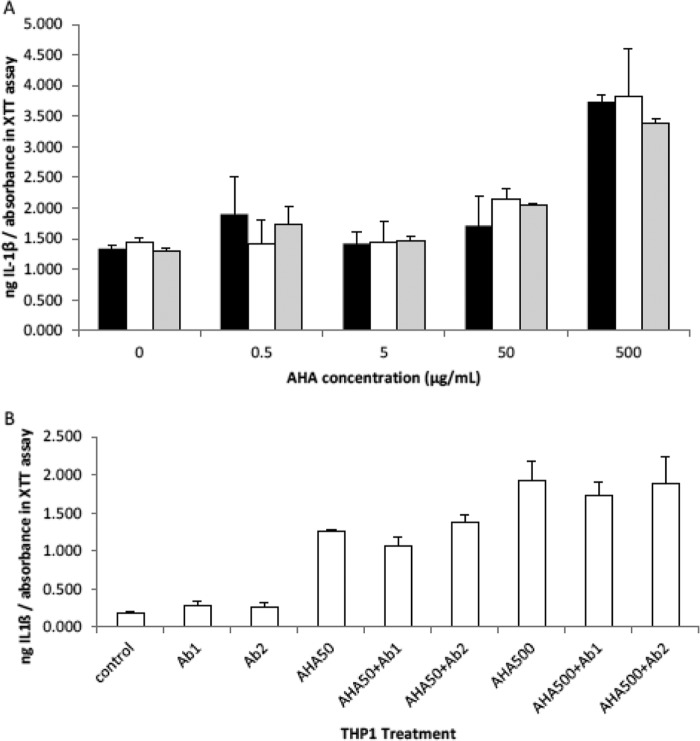
In line with previously reported studies, cytokine elaboration was negligible in differentiated macrophages after PMA treatment (2, 3). Exposure of the adherent THP-1 macrophages to 500 μg/ml of AHA resulted in enhanced secretion of IL-1β compared with control without the addition of an HA derivative. This increase in IL-1β secretion by AHA represented the largest response of all our reacylated derivatives shown in Fig. 4A (partially propionylated HA, BHA, partially valerylated HA, and partially isovalerylated HA). BHA, DHA, and HA had levels of IL-1β secretion comparable with control (Fig. 4A). It is noteworthy that only the N-acetyl group shows a significant increase in IL-1β secretion, compared with control. Partially propionylated HA, partially valerylated HA, and partially isovalerylated HA demonstrate smaller nonsignificant increases at 500 μg/ml (Fig. 4A) or other concentrations (data not shown). DHA, BHA, and HA itself show no effect whatsoever in stimulating IL-1β secretion.
Proinflammatory Cytokine Response and Cell Surface Receptor for AHA
AHA causes increased secretion of IL-1β by THP-1 cells in a dose-dependent manner with an EC50 of 292 μg/ml (Fig. 4B). The maximal IL-1β secretion was compared with that seen with TLR agonists known to stimulate IL-1β secretion. THP-1 was treated with 500 μg/ml of AHA or 250 ng/ml of TLR-4 agonist (LPS.EC) or 250 ng/ml TLR-2 agonist (PG.PS). The proinflammatory cytokine response is stimulated in the THP-1 cells by AHA as well as the TLR-4 agonist, LPS.EC, and the TLR-2 agonist, PG.PS (Fig. 4C). The stimulation of IL-1β secretion was LPS.EC > AHA > PG.PS > control (Fig. 4C). The degree of IL-1β stimulation by AHA is comparable with that seen by LMHA obtained by digesting HA with bovine testis hyaluronidase (HA-digested) and LMHA obtained by deacetylating HA with NaOH and reacetylating with acetic anhydride (AHA-NaOH) (Fig. 4D). The HA-digested (500 μg/ml) greater than 30 kDa accounted for the total increased THP-1 IL-1β secretion over control (Fig. 4E). When used at the concentration present in the unfractionated HA digest (12.5 μg/ml), HA-digested smaller than 30 kDa does not stimulate THP-1 IL-1β secretion over control (Fig. 3E). We wished to determine whether AHA produced its proinflammatory cytokine effect through the TLR-4 cell surface receptor, and if by blocking the TLR-4 receptor, with specific antibody, the AHA effect would be abrogated. THP-1 was treated with 50 or 500 μg/ml of AHA in the presence or absence of 5 μg/ml of neutralizing antibody to TLR-4. The presence of antibody to TLR-4 inhibited the secretion of IL-1β by THP-1 incubated with AHA (Fig. 5A). We reasoned that if AHA produced its stimulatory effect on proinflammatory production through the TLR-4 cell surface receptor, then blocking the TLR-4 receptor, with a TLR-4 antagonist, should diminish this effect. To verify this, THP-1 cells were treated with 50 μg/ml of AHA in the presence or absence of 5 μg/ml of a TLR-4 antagonist (LPS.RS Ultrapure). The presence of the TLR-4 antagonist inhibited the secretion of IL-1β by THP-1 cells coincubated with AHA in a dose-dependent manner (Fig. 5B). This suggests that AHA exerts its stimulatory effect on proinflammatory cytokine production by interacting with the TLR-4 cell surface receptor. It has been reported that small molecular mass fragments of HA can exert their inflammatory response either through the TLR-4 receptor or the CD44 cell surface receptor (9, 12, 17). To determine whether AHA also exerts its inflammatory response through the CD44 cell surface receptor, THP-1 macrophages were treated with AHA with or without the CD44 agonist, HA, or antibodies to CD44. When the cells were preincubated with increasing concentrations of 1500–1800 kDa HA, AHA-stimulated IL-1β production by THP-1 cells was not diminished (Fig. 6A). Also, coincubation of antibodies to CD44 with AHA does not diminish the effect of AHA on increasing IL-1β secretion by THP-1 cells (Fig. 6B). Also, HA does not stimulate the IL-1β secretion by THP-1 (Fig. 4A). In combination, these results suggest that AHA does not exert its inflammatory effect by interacting with the CD44 cell surface receptor.
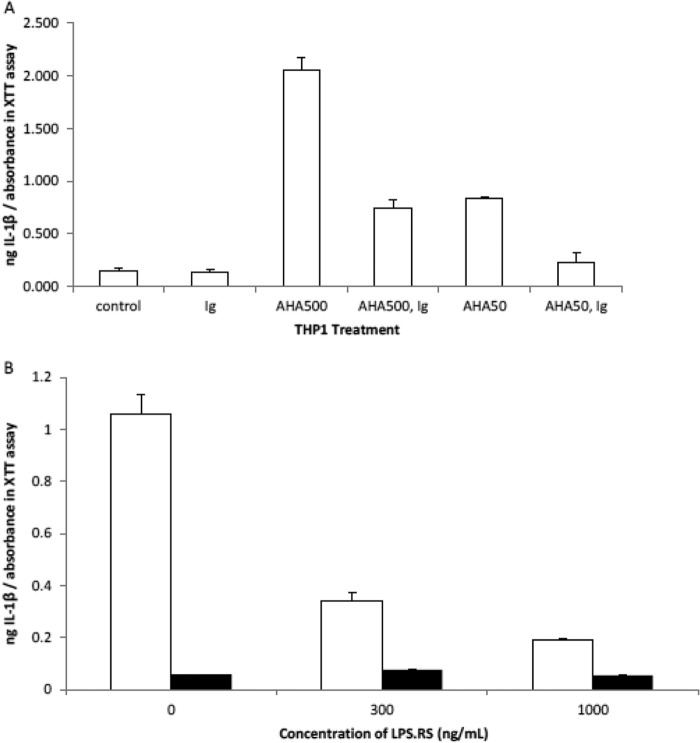
FIGURE 5.
AHA exerts its inflammatory effect through the TLR-4 receptor. THP-1 macrophages are treated for 24 h with AHA in the presence and absence of TLR-4 antagonists or neutralizing antibody, and then the media were assayed for cytokines using ELISA, and a measure of the attached cell numbers was determined using the XTT/PMS cell proliferation assay. The data are means ± S.D. (n = 3). A, IL-1β secretion by THP-1 in the presence and absence of antibody to TLR-4 and AHA. Control is THP-1 that is not treated with AHA or antibody to TLR-4 (Ig). AHA is used at either 50 or 500 μg/ml and Ig at 5 μg/ml. The increase in IL-1β secretion over control for THP-1 treated with 50 and 500 μg/ml AHA is very significant (p < 0.01). When the THP-1 is coincubated with Ig and either 50 or 500 μg/ml AHA, there is a significant (p < 0.05) decrease in this IL-1β secretion compared with AHA in the absence of Ig. B, THP-1 IL-1β secretion with increasing concentrations of the TLR4 antagonist, LPS.RS, in the presence and absence of AHA.THP-1 incubated with increasing concentrations of TLR-4 antagonist (LPS.RS) in the presence (white bars) and absence (black bars) of 50 μg/ml AHA. In the absence of LPS.RS, the increase in IL-1β secretion over the control (no AHA, no LPS.RS) is very significant (p = 0.0057, Student's t test) for AHA-treated cells. Compared with AHA in the absence of LPS.RS, the decrease in IL-1β secretion seen when coincubated with 300 and 1000 ng/ml LPS.RS is significant (p = 0.0131) and very significant (p = 0.0076), respectively.
FIGURE 6.
AHA probably does not exert its inflammatory effect through CD44. THP-1 macrophages are treated for 24 h with AHA with and without the CD44 agonist, HA (1500–1800 kDa from S. equi), or antibodies to CD44, and then the media were assayed for cytokines using ELISA, and a measure of the attached cell numbers was determined using the XTT/PMS cell proliferation assay. The data are the means ± S.D. (A, THP-1 secretion of IL-1β in the presence of increasing concentrations of AHA in the presence and absence of HA.THP-1 are incubated with increasing concentrations of AHA in the presence of 50 μg/ml HA (gray bars), 5 μg/ml HA (white bars), or no HA (black bars). B, THP-1 secretion of IL-1β in the presence and absence of AHA and CD44 antibodies (Ab1 and Ab2). Control is THP-1 that is not treated with AHA or antibody to CD44. AHA is used at either 50 or 500 μg/ml and antibody at 5 μg/ml.
Anti-inflammatory Response and Cell Surface Receptor for BHA
THP-1 macrophages were treated with increasing concentrations of the TLR-4 agonist LPS-EC with or without 500 μg/ml BHA. When the cells were preincubated with increasing concentration of LPS.EC, the IL-1β production by THP-1 cells was inhibited when coincubated with 500 μg/ml of BHA (Fig. 7A). The treatment of THP-1 with 500 μg/ml of AHA stimulated the secretion of IL-1β, IL-6, IL-8, and TNF-α, which was inhibited when coincubated with 500 μg/ml of BHA (Fig. 7B). However, stimulation of IL-1β production by THP-1 cells was not inhibited when the cells were coincubated with increasing concentration of the TLR-2 agonist, PG.PS, and 500 μg/ml of BHA. This suggests that BHA does not compete with the TLR-2 agonist, PG.PS (Fig. 7C). The inhibition of AHA and LPS.EC-mediated secretion of IL-1β seems to be specific to BHA and is not shown by other derivatives of HA (data not shown). Overall, the data suggest that BHA exerts its anti-inflammatory effect through TLR-4.
FIGURE 7.
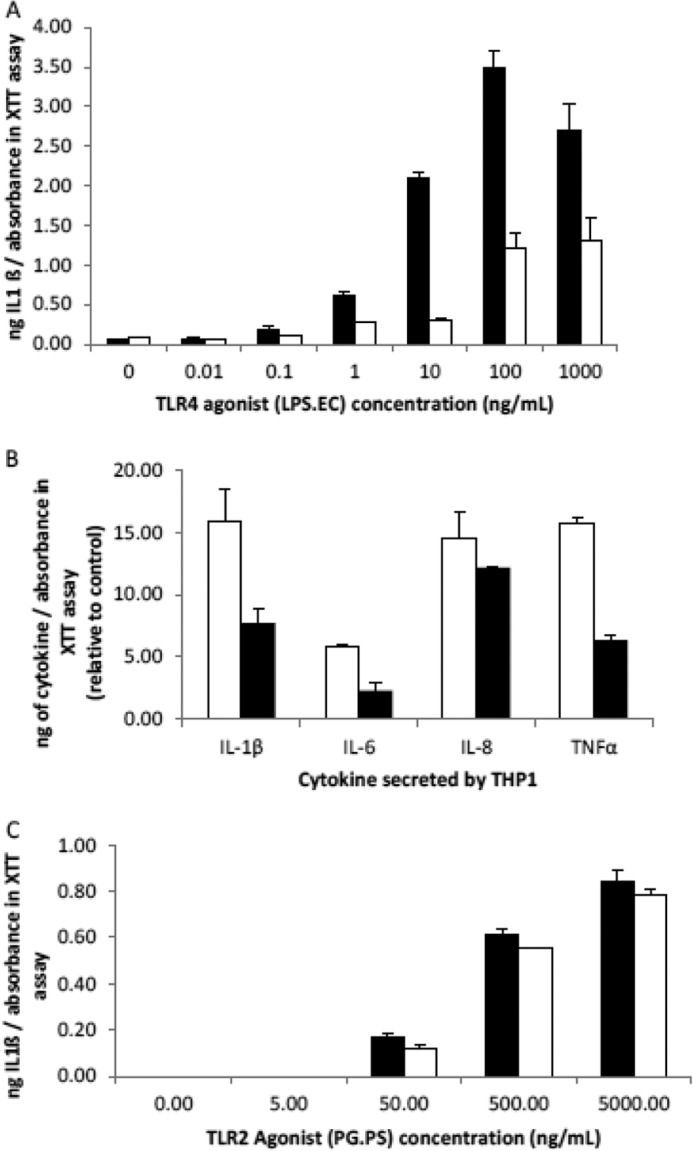
BHA exerts its anti-inflammatory effect through the TLR-4 receptor. THP-1 macrophages are treated for 24 h with 500 μg/ml of BHA, AHA, or the TLR-4 agonist, LPS.EC, and then the media were assayed for cytokines using ELISA, and a measure of the attached cell numbers was determined using the XTT/PMS cell proliferation assay. The data are the means ± S.D. (n = 3). A, THP-1 IL-1β secretion when incubated with increasing concentrations of LPS.EC in the presence and absence of 500 μg/ml BHA. THP-1 are incubated with increasing concentrations of the TLR-4 agonist, LPS.EC, in the presence (white bars) and absence (black bars) of 500 μg/ml BHA. This BHA inhibition of the AHA-dependent stimulation of IL-1β secretion was present between 0.1 and 1000 ng/ml (p < 0.05, Student's t test). B, THP-1 cytokine secretion when treated with AHA in the presence and absence of BHA. THP-1 cells are untreated or treated with either 500 μg/ml AHA only (white bars) or 500 μg/ml BHA with 500 μg/ml AHA (black bars). The cytokine levels are expressed relative to untreated (THP-1 that is not treated with either AHA or BHA). C, BHA probably does not act through the TLR-2 receptor. THP-1 are incubated with increasing concentrations of the TLR-2 agonist, PG.PS, in the presence (white bars) and absence (black bars) of 500 μg/ml BHA.
Degree of Reacetylation and Pro-cytokine Activity
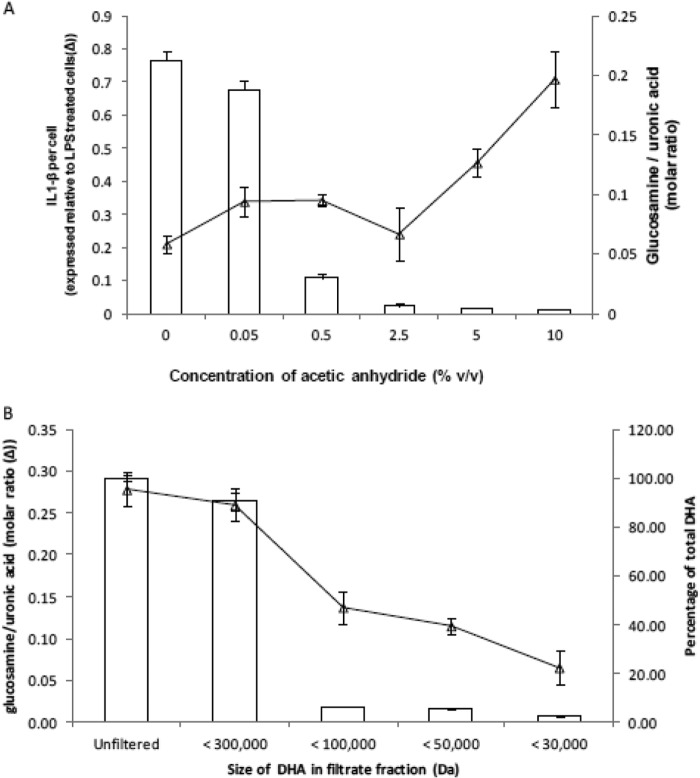
We wished to determine the extent of reacetylation that was necessary for HA derivatives to display inflammatory activity. To this end, we supplemented THP-1 cells with 500 μg/ml of DHA treated with increasing concentrations of acetic anhydride. The greater the degree of reacetylation, the greater the inflammatory response with the inflection point being a molar ratio of glucosamine to GlcUA of 0.007 ± 0.002 (Fig. 8A), shown by the height of the open bars. This molar ratio is inversely correlated with the degree of reacetylation of DHA. Gel filtration of the AHA starting substrate, DHA, indicated that the molecular mass range of AHA with proinflammatory cytokine activity was between 100,000 and 300,000 Da (Fig. 8B). Combining this with the agarose gel data (Fig. 3A), the size of this fraction can be more narrowly defined between 100 and 214 kDa. Importantly, the glucosamine to glucuronic acid ratio was much higher in the 100–300 kDa range, compared with the smaller fractions (Fig. 8B).
FIGURE 8.
The molar ratio of primary amino to uronic acid groups in HA derivatives and the degree of DHA re-N-acetylation necessary for inflammatory activity. The primary amino group and uronic acid concentrations were determined using the o-phthalaldehyde and carbazole colorimetric assays, respectively. A, DHA was treated with increasing concentrations of acetic anhydride in absolute ethanol. The ratio of glucosamine to GlcUA was determined and is displayed as white bars. The inflammatory activity of these differently acetylated HA derivatives was determined by measuring the IL-1β secretion of THP-1 cells treated with 500 μg/ml of each of these differently acetylated HA derivatives (▵). B, DHA was size-fractionated using Vivaspin polyethersulfone gel filtration spin columns with molecular mass cutoffs of 30,000, 50,000, 100,000, or 300,000 Da. The filtrates were freeze-dried and resuspended with 0.01 n HCl, and the primary amino group and uronic acid concentrations were determined. The amount of DHA for each size range is expressed as a percentage of the unfiltered (total) DHA and displayed as white bars. The molar ratio of glucosamine to uronic acid for the different size ranges of DHA is shown as a line graph (▵).
DISCUSSION
Synthesis and Viscosity of the N-Acyl Derivatives
The HA deacetylation process results in a polysaccharide with GlcUA, GlcNAc, and glucosamine. In general, the deacetylation of glycosaminoglycans by hydrazinolysis has been shown to occur simultaneously with glycosidic bond cleavage (18, 19, 30, 31). This results in reduction of the molecular mass and provides HA polymers that can be modified further for a variety of applications. At high temperature, the deacetylating agent, hydrazine monohydrate, in the presence of the catalyst hydrazine sulfate, converts carboxylic groups of the d-glucuronic acid residue to carboxylic hydrazides. This may, to a limited extent, produce C-5 epimerization and elimination at the C-4 position (18, 19, 30, 31). Any traces of carboxylic hydrazides that may form are converted back to the glucuronic residue by treating with iodic acid as previously suggested (18, 30, 31). Also, because the formation of carboxylic hydrazides and glycosidic bond cleavage increases with increases in reaction time and temperature (30), the hydrazinolysis reaction in this study was done at a milder temperature of 55 °C for 72 h as reported by Crescenzi et al. (18, 19). From our 1H NMR structure, there is no evidence of a 4,5 unsaturated residue (a structure with a double bond between the C-4 and C-5 of a sugar unit) as a result of the elimination at the C-4 position. This indicates that the deacetylation procedure leads to partially deacetylated HA without secondary products, but with glycosidic bond cleavage, which resulted in reduced molecular mass. The degree of deacetylation of HA in our study was 19.7 ± 3.5%, which is close to the 17 and 23% reported by Crescenzi et al. (18, 19) using a similar procedure.
The average molecular mass of HA used in this study was 1500–1800 kDa. The viscosity decreases with shear rate because of molecular disentanglement and alignment in the direction of shear (32). Similar concentrations of AHA, BHA, and DHA with molecular masses less than 214 kDa exhibited a Newtonian behavior that was much lower than HA but approximately three times higher than that of water at 5 mg/ml. As shown by the molecular mass range and the viscosity of the polymers, reacylation of DHA to form AHA or BHA had no significant effect on the molecular mass range and rheological properties of AHA and BHA. This suggests that the bond cleavage observed occurred only as a result of the N-deacetylation reaction process and not during the N-acylation step.
Biological Mechanisms of Action of HA
Under normal conditions, native high molecular mass HA (4, 15) is produced on the inner surface of the plasma membrane and extruded into the extracellular environment (9, 10, 13). HA in synovial fluid is between 2150 and 4960 kDa (3, 7), whereas in the lungs and skin it is 6000 kDa (15) and between 600 and 1000 kDa, respectively (33). Physiological HA maintains homeostasis and potentially down-regulates inflammation (9, 12, 17) by binding to CD44 (1, 11, 14, 34) and to the intercellular adhesion molecule-1 (ICAM-1) (2, 3). HA is known to contain multiple CD44 cell surface receptor binding sites because of the repetitive structure (GlcNAc β1–4 GlcUA)n and could interact simultaneously with many cell surface receptors to induce CD44 clustering (4, 35). CD44 clears the degraded HA to the endosomes and subsequently to the lysosomes where hyaluronidases such as hyaluronidase-1 and hyaluronidase-2 further degrade it (5, 36).
In certain pathological conditions, the native HA is extensively degraded into small fragments (8, 12) that are able to stimulate the TLR-4 and CD44 receptors to activate inflammation by two different pathways: The adaptor molecule myeloid differentiation primary response protein 88 (Myd88) (12, 15, 17, 37) and PKC (9, 38). Both pathways result in the activation of NF-κB (9, 38). Activated NF-κB primes the transcription of several proinflammatory mediators such as TNF-α, IL-1β, IL-6, etc. (9, 11).
CD44 removes the small HA from the inflammatory environment (36). HMHA inhibits the PKC pathway and therefore limits the NF-κB activation, which eventually leads to the resolution of inflammation and tissue repair. Monocytes and macrophages are an essential cellular component of the inflammatory response (8). The THP-1 cells used in our study differentiate from monocyte to macrophage by seeding with 100 nm PMA (27), making them an immunocompetent cell type. IL-1β, IL-6, and TNF-α are expressed very early on and play major roles in the extent of inflammation (14, 39). In our study, we utilized the elaboration of IL-1β as our main routine proinflammatory cytokine assay.
As shown in the results, our low molecular mass reacetylated HA (AHA) is polydisperse, with a molecular mass range of 30–214 kDa (Fig. 3). AHA stimulated THP-1 cells to secrete more IL-1β compared with the negative control. In contrast, the HMHA control, of average molecular mass between 1500 and 1800 kDa, neither stimulated pro-cytokine production nor decreased the stimulation by AHA (Fig. 6A). Our findings are in line with previous results that LMHA induced proinflammatory biological activities in LPS-stimulated (3, 10, 11) and nonstimulated cells (10, 15, 17, 33). Previous studies have shown that the inhibitory effect of HA on LPS stimulated chondrocytes (3, 10) and U937 macrophages (3) is largely dependent on the molecular mass of HA. Campo et al. (9) found that HA with molecular mass of 50 kDa induced the expression of TLR-4 receptors of murine chondrocytes and other inflammatory mediators such as MyD88 and TRAF6, whereas HA with molecular mass 1000 kDa and 5000 kDa had no effect. In murine bone marrow-derived dendritic cells, HA fragments of tetra- and hexasaccharide size, but not of intermediate size (80–200 KDa) or high molecular mass HA (600–1,000 KDa), up-regulated the production of IL-1β and TNF-α production in a dose-dependent manner, similar to the effect induced by LPS alone (33). The inhibitory effect of HA in LPS-stimulated macrophages (3, 17) and chondrocytes (10) on the production of inflammatory cytokines diminished with decreasing molecular mass of HA from 2,700 to 800 kDa (3, 17) and 5000–1000 kDa (10).
In mouse chondrocytes (10), the high molecular mass HA (5000 kDa) did not stimulate inflammatory cytokines. In our studies, our 1500–1800 kDa HA itself did not stimulate proinflammatory cytokine production and did not reduce it when tested in combination with AHA.
Previous studies have shown that TLR-4 and CD44 were predominantly expressed in macrophages (17) and chondrocytes (9, 11) treated with low molecular mass HA fragments. For example, 100–150-kDa hyaluronan induced IL-6 and MCP-1 production in peripheral blood mononuclear cells via the CD44 and TLR-4 cell surface receptor (17). Soluble hyaluronan degradation products (135 kDa) generated during lung injury were shown to stimulate peritoneal macrophages via the TLR-4 and TLR-2 pathway (37). TLR-2 and not TLR-4 or CD44 is the sole receptor for 200-kDa HA when exposed to mouse alveolar macrophage cell line (29). However, the stimulatory effects of low molecular mass HA (12–16 disaccharides) on dendritic cells were mediated by components of the signaling pathway activated by TLR-4 and not TLR-2 (40).
Receptors for AHA and BHA
Our results demonstrate that LMHA could stimulate proinflammatory cytokine production by interacting with TLR-4 because the partial block of the TLR-4 receptor with antibody to TLR-4 or the TLR-4 antagonist significantly affected the production of inflammatory cytokines (Fig. 5 A and B). It should be noted that the TLR-4 antagonist, LPS-RS, is the ultrapure form with no TLR-2 activity. However, we did not demonstrate that our LMHA interacted with CD44 because the CD44 agonist and antibodies to CD44 did not significantly affect the elaboration of cytokines (Fig. 6, A and 6B). The reason for the participation of different receptor molecules in different studies might be the size distribution of HA, as well as the cell type under investigation.
Butyrylation of the glucosamine in LMHA mitigates the increased cytokine production by macrophages, stimulated by bacterial lipopolysaccharide or reacetylated LMHA (Fig. 7A). The IL-1β secretion in response to our N-butyrylated HA derivative (BHA) at 500 μg/ml, which has very similar molecular mass and viscosity to AHA, was negligible and was similar to the response elicited in our control differentiated macrophages (Fig. 4A). In addition, coincubation of 500 μg/ml of BHA significantly reduced the THP-1 IL-1β, IL-6, IL-8, and TNF-α secretion in response to AHA or LPS.EC in a concentration-dependent manner. Also, BHA competes with LPS.EC and AHA for binding to the TLR-4 receptor but does not appear to compete with the TLR-2 agonist, PG.PS (Fig. 7C). It is possible that BHA binding to TLR-4 receptors prevents the binding of proinflammatory mediators, such as LPS.EC and AHA. As a result, it would appear that BHA also exerts its anti-inflammatory action through the TLR-4 receptor and not through the TLR-2 cell surface receptor.
Proinflammatory Role of N-acetylation and Possible “Block” Structures
It is clear that AHA has a major stimulatory effect on proinflammatory cytokine production by THP-1 cells, whereas DHA has no stimulatory effect (Fig. 4A). AHA and the other N-acylated HA compounds were synthesized from DHA. However, it is only AHA that shows significant stimulation of proinflammatory cytokine production (Fig. 4A). This suggests that charge alone cannot account for the difference in proinflammatory activity between the different N-acylated HA compounds and that there is a requirement for the N-acetyl group to achieve maximal activity. Also, proinflammatory cytokine production increases as reacetylation of DHA increases, and only a small proportion of the glucosamine moieties need to be nonacetylated (molar ratio of glucosamine to GlcUA of 0.007) for the proinflammatory effect to be abolished (Fig. 8A). There is some specificity to the N-acetyl moiety, because N-acetylation, compared with other N-acylations, has the highest degree of pro-cytokine stimulation (Fig. 4A). These experiments establish the requirement of N-acetylation in LMHA for pro-cytokine stimulation, at least for IL-1β production by THP-1 macrophages.
However, the topographical distribution of the N-acetyl groups within the LMHA is also of interest. The fractionation experiments by gel filtration show much higher ratios of free glucosamine in the 100,000- and 300,000-Da fraction (Fig. 8B), compared with lower molecular masses, as indicated above. This suggests that the deacetylation occurs mainly in a block structure and is not random. The proinflammatory cytokine stimulatory activity of AHA can be inferred to reside largely in a block structure that can be more narrowly defined between 100 and 214 kDa. We found no evidence of biological activity in fractions less than 30 kDa, which would be expected to contain oligosaccharides. However, it is not possible to say whether the reacylation reactions, occurring within a block, might in some way be ordered or are random.
Butyrylation likely follows a similar pattern. From 1H NMR, the degree of N-acetylation was 98.7 ± 1.5% for AHA; the degrees of N-acetylation and N-butyrylation were 82.2 ± 4.6 and 22.7 ± 3.8%, respectively, for BHA. This suggests that the reacylation reactions also occur in the block preceded by the deacetylation, because almost all free glucosamine has been re-N-acylated by butyryl groups.
This work was supported by National Sciences and Engineering Research Council of Canada (NSERC) Strategic Grant 381564-09.
- HA
- hyaluronan
- AHA
- reacetylated HA
- BHA
- partially butyrylated HA
- DHA
- partially N-deacetylated HA
- TAE
- Tris-acetate-EDTA
- GlcUA
- N-glucuronic acid
- TLR
- Toll-like receptor
- CD44
- cluster determinant 44
- LMHA
- low molecular mass HA
- HMHA
- high molecular mass HA
- PMA
- phorbol 12-myristate 13-acetate
- PMS
- phenazine methosulfate
- GlcNBU
- N-butyryl glucosamine.
REFERENCES
- 1. Shimizu M., Yasuda T., Nakagawa T., Yamashita E., Julovi S. M., Hiramitsu T., Nakamura T. (2003) Hyaluronan inhibits matrix metalloproteinase-1 production by rheumatoid synovial fibroblasts stimulated by proinflammatory cytokines. J. Rheumatol. 30, 1164–1172 [PubMed] [Google Scholar]
- 2. Yasuda T. (2011) Hyaluronan inhibits Akt, leading to nuclear factor-κB down-regulation in lipopolysaccharide-stimulated U937 macrophages. J. Pharmacol. Sci. 115, 509–515 [DOI] [PubMed] [Google Scholar]
- 3. Yasuda T. (2007) Hyaluronan inhibits cytokine production by lipopolysaccharide-stimulated U937 macrophages through down-regulation of NF-κB via ICAM-1. Inflamm. Res. 56, 246–253 [DOI] [PubMed] [Google Scholar]
- 4. Yang C., Cao M., Liu H., He Y., Xu J., Du Y., Liu Y., Wang W., Cui L., Hu J., Gao F. (2012) The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J. Biol. Chem. 287, 43094–43107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sadowitz B., Seymour K., Gahtan V., Maier K. G. (2012) The role of hyaluronic acid in atherosclerosis and intimal hyperplasia. J. Surg. Res. 173, e63–e72 [DOI] [PubMed] [Google Scholar]
- 6. Stern R., Asari A. A., Sugahara K. N. (2006) Hyaluronan fragments: an information-rich system. Eur. J. Cell Biol. 85, 699–715 [DOI] [PubMed] [Google Scholar]
- 7. Dahl L. B., Dahl I. M., Engström-Laurent A., Granath K. (1985) Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann. Rheum. Dis. 44, 817–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Noble P. (2002) Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 21, 25–29 [DOI] [PubMed] [Google Scholar]
- 9. Campo G. M., Avenoso A., Campo S., D'Ascola A., Nastasi G., Calatroni A. (2010) Small hyaluronan oligosaccharides induce inflammation by engaging both Toll-like-4 and CD44 receptors in human chondrocytes. Biochem. Pharmacol. 80, 480–490 [DOI] [PubMed] [Google Scholar]
- 10. Campo G. M., Avenoso A., Campo S., D'Ascola A., Nastasi G., Calatroni A. (2010) Molecular size hyaluronan differently modulates Toll-like receptor-4 in LPS-induced inflammation in mouse chondrocytes. Biochimie 92, 204–215 [DOI] [PubMed] [Google Scholar]
- 11. Campo G. M., Avenoso A., D'Ascola A., Scuruchi M., Prestipino V., Calatroni A., Campo S. (2012) Hyaluronan in part mediates IL-1β-induced inflammation in mouse chondrocytes by up-regulating CD44 receptors. Gene 494, 24–35 [DOI] [PubMed] [Google Scholar]
- 12. Campo G. M., Avenoso A., Nastasi G., Micali A., Prestipino V., Vaccaro M., D'Ascola A., Calatroni A., Campo S. (2011) Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression. Biochim. Biophys. Acta 1812, 1170–1181 [DOI] [PubMed] [Google Scholar]
- 13. Taylor K. R., Yamasaki K., Radek K. A., Di Nardo A., Goodarzi H., Golenbock D., Beutler B., Gallo R. L. (2007) Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J. Biol. Chem. 282, 18265–18275 [DOI] [PubMed] [Google Scholar]
- 14. Noble P. W., Lake F. R., Henson P. M., Riches D. W. (1993) Hyaluronate activation of CD44 induces insulin-like growth factor-I expression by a tumor necrosis factor-α dependent mechanism in murine macrophages. J. Clin. Invest. 91, 2368–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Black K. E., Collins S. L., Hagan R. S., Hamblin M. J., Chan-Li Y., Hallowell R. W., Powell J. D., Horton M. R. (2013) Hyaluronan fragments induce IFNβ via a novel TLR4-TRIF-TBK1-IRF3-dependent pathway. J. Inflamm. (Lond.) 10, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsuno H., Yudoh K., Kondo M., Goto M., Kimura T. (1999) Biochemical effect of intra-articular injections of high molecular weight hyaluronate in rheumatoid arthritis patients. Inflamm. Res. 48, 154–159 [DOI] [PubMed] [Google Scholar]
- 17. Yamawaki H., Hirohata S., Miyoshi T., Takahashi K., Ogawa H., Shinohata R., Demircan K., Kusachi S., Yamamoto K., Ninomiya Y. (2009) Hyaluronan receptors involved in cytokine induction in monocytes. Glycobiology 19, 83–92 [DOI] [PubMed] [Google Scholar]
- 18. Crescenzi V., Francescangeli A., Renier D., Bellini D. (2002) New cross-linked and sulfated derivatives of partially deacetylated hyaluronan: synthesis and preliminary characterization. Biopolymers 64, 86–94 [DOI] [PubMed] [Google Scholar]
- 19. Crescenzi V., Francescangeli A., Segre A., Capitani D., Mannina L., Renier D., et al. (2002) NMR structural study of hydrogels based on partially deacetylated hyaluronan. Macromol. Biosci. 2, 272–279 [Google Scholar]
- 20. Wada T., Chirachanchai S., Izawa N., Inaki Y., Takemoto K. (1994) Synthesis and properties of hyaluronic-acid conjugated nucleic-acid analogs: 1. synthesis of deacetylhyaluronan and introduction of nucleic-acid bases. J. Bioact. Compatible Polym. 9, 429–447 [Google Scholar]
- 21. Anastassiades T. P. (1973) Effect of a synthetic hexosamine derivative on mucopolysaccharide synthesis by human capsule and synovium. Biochem. Pharmacol. 22, 3013–3023 [DOI] [PubMed] [Google Scholar]
- 22. Zhang W., Mu H., Zhang A., Cui G., Chen H., Duan J., Wang S. (2013) A decrease in moisture absorption–retention capacity of N-deacetylation of hyaluronic acid. Glycoconj. J. 30, 577–583 [DOI] [PubMed] [Google Scholar]
- 23. Maharjan A. S., Pilling D., Gomer R. H. (2011) High and low molecular weight hyaluronic acid differentially regulate human fibrocyte differentiation. PLoS One 6, e26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Larionova N. I., Zubaerova D. K., Guranda D. T., Pechyonkin M. A., Balabushevich N. G. (2009) Colorimetric assay of chitosan in presence of proteins and polyelectrolytes by using o-phthalaldehyde. Carbohydr. Polym. 75, 724–727 [Google Scholar]
- 25. Cesaretti M., Luppi E., Maccari F., Volpi N. (2003) A 96-well assay for uronic acid carbazole reaction. Carbohydr. Polym. 54, 59–61 [Google Scholar]
- 26. Cowman M. K., Chen C. C., Pandya M., Yuan H., Ramkishun D., LoBello J., Bhilocha S., Russell-Puleri S., Skendaj E., Mijovic J., Jing W. (2011) Improved agarose gel electrophoresis method and molecular mass calculation for high molecular mass hyaluronan. Anal. Biochem. 417, 50–56 [DOI] [PubMed] [Google Scholar]
- 27. Weldon S., Mitchell S., Kelleher D., Gibney M. J., Roche H. M. (2004) Conjugated linoleic acid and atherosclerosis: no effect on molecular markers of cholesterol homeostasis in THP-1 macrophages. Atherosclerosis 174, 261–273 [DOI] [PubMed] [Google Scholar]
- 28. Roehm N. W., Rodgers G. H., Hatfield S. M., Glasebrook A. L. (1991) An improved colorimetric assay for cell-proliferation and viability utilizing the tetrazolium salt Xtt. J. Immunol. Methods 142, 257–265 [DOI] [PubMed] [Google Scholar]
- 29. Scheibner K. A., Lutz M. A., Boodoo S., Fenton M. J., Powell J. D., Horton M. R. (2006) Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J. Immunol. 177, 1272–1281 [DOI] [PubMed] [Google Scholar]
- 30. Dahl L. B., Laurent T. C., Smedsrød B. (1988) Preparation of biologically intact radioiodinated hyaluronan of high specific radioactivity: coupling of I-125 tyramine-cellobiose to amino groups after partial N-deacetylation. Anal. Biochem. 175, 397–407 [DOI] [PubMed] [Google Scholar]
- 31. Shaklee P. N., Conrad H. E. (1984) Hydrazinolysis of heparin and other glycosaminoglycans. Biochem. J. 217, 187–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fam H., Kontopoulou M., Bryant J. T. (2009) Effect of concentration and molecular weight on the rheology of hyaluronic acid/bovine calf serum solutions. Biorheology 46, 31–43 [DOI] [PubMed] [Google Scholar]
- 33. Termeer C. C., Hennies J., Voith U., Ahrens T., Weiss J. M., Prehm P., Simon J. C. (2000) Oligosaccharides of hyaluronan are potent activators of dendritic cells. J. Immunol. 165, 1863–1870 [DOI] [PubMed] [Google Scholar]
- 34. Julovi S. M., Yasuda T., Shimizu M., Hiramitsu T., Nakamura T. (2004) Inhibition of interleukin-1β-stimulated production of matrix metalloproteinases by hyaluronan via CD44 in human articular cartilage. Arthritis Rheum. 50, 516–525 [DOI] [PubMed] [Google Scholar]
- 35. Lesley J., Hascall V. C., Tammi M., Hyman R. (2000) Hyaluronan binding by cell surface CD44. J. Biol. Chem. 275, 26967–26975 [DOI] [PubMed] [Google Scholar]
- 36. Teder P., Vandivier R. W., Jiang D., Liang J., Cohn L., Puré E., Henson P. M., Noble P. W. (2002) Resolution of lung inflammation by CD44. Science 296, 155–158 [DOI] [PubMed] [Google Scholar]
- 37. Jiang D., Liang J., Fan J., Yu S., Chen S., Luo Y., Prestwich G. D., Mascarenhas M. M., Garg H. G., Quinn D. A., Homer R. J., Goldstein D. R., Bucala R., Lee P. J., Medzhitov R., Noble P. W. (2005) Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med. 11, 1173–1179 [DOI] [PubMed] [Google Scholar]
- 38. Fitzgerald K. A., Bowie A. G., Skeffington B. S., O'Neill L. A. (2000) Ras, protein kinase C zeta, and I kappa B kinases 1 and 2 are downstream effecters of CD44 during the activation of NF-κB by hyaluronic acid fragments in T-24 carcinoma cells. J. Immunol. 164, 2053–2063 [DOI] [PubMed] [Google Scholar]
- 39. Ferraccioli G., Bracci-Laudiero L., Alivernini S., Gremese E., Tolusso B., De Benedetti F. (2010) Interleukin-1β and interleukin-6 in arthritis animal models: roles in the early phase of transition from acute to chronic inflammation and relevance for human rheumatoid arthritis. Mol. Med. 16, 552–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Termeer C., Benedix F., Sleeman J., Fieber C., Voith U., Ahrens T., Miyake K., Freudenberg M., Galanos C., Simon J. C. (2002) Oligosaccharides of hyaluronan activate dendritic cells via Toll-like receptor 4. J. Exp. Med. 195, 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]