Background: Cyanobacteriochrome photoreceptors have diverse spectral properties and regulate photosynthetic antenna composition and motility of cyanobacteria.
Results: A blue/green-responsive cyanobacteriochrome SesA is a blue light-dependent diguanylate cyclase and induces cell aggregation of Thermosynechococcus under blue light.
Conclusion: SesA regulates sessility in a light-dependent way likely via cyclic diguanylate signaling.
Significance: SesA is the first cyanobacteriochrome photoreceptor that regulates sessility.
Keywords: Bacterial Signal Transduction, Cyanobacteria, Cyclic di-GMP (c-di-GMP), Microbiology, Photoreceptor, Thermosynechococcus, Cell Aggregation, Cyanobacteriochrome, Sessility
Abstract
Cyanobacteria have unique photoreceptors, cyanobacteriochromes, that show diverse spectral properties to sense near-UV/visible lights. Certain cyanobacteriochromes have been shown to regulate cellular phototaxis or chromatic acclimation of photosynthetic pigments. Some cyanobacteriochromes have output domains involved in bacterial signaling using a second messenger cyclic dimeric GMP (c-di-GMP), but its role in cyanobacteria remains elusive. Here, we characterize the recombinant Tlr0924 from a thermophilic cyanobacterium Thermosynechococcus elongatus, which was expressed in a cyanobacterial system. The protein reversibly photoconverts between blue- and green-absorbing forms, which is consistent with the protein prepared from Escherichia coli, and has diguanylate cyclase activity, which is enhanced 38-fold by blue light compared with green light. Therefore, Tlr0924 is a blue light-activated diguanylate cyclase. The protein's relatively low affinity (10.5 mm) for Mg2+, which is essential for diguanylate cyclase activity, suggests that Mg2+ might also regulate c-di-GMP signaling. Finally, we show that blue light irradiation under low temperature is responsible for Thermosynechococcus vulcanus cell aggregation, which is abolished when tlr0924 is disrupted, suggesting that Tlr0924 mediates blue light-induced cell aggregation by producing c-di-GMP. Given our results, we propose the name “sesA (sessility-A)” for tlr0924. This is the first report for cyanobacteriochrome-dependent regulation of a sessile/planktonic lifestyle in cyanobacteria via c-di-GMP.
Introduction
Light is a fundamental environmental factor especially for photosynthetic organisms, which employ various kinds of photoreceptors to sense and then adapt to their light environments (1). Cyanobacteriochromes (CBCRs)3 are a class of photoreceptors (2, 3). CBCRs covalently bind a linear tetrapyrrole (bilin) chromophore in the cGMP phosphodiesterase/adenylyl cyclase/FhlA (GAF) domain and respond to a wide spectral range of light from near-UV to red. CBCRs are distantly related to phytochromes that also bind a bilin chromophore, although the phytochromes respond mainly to red and far-red light (3–5). CBCRs are widely distributed in cyanobacteria, and a variety of CBCRs has been found, especially in freshwater species. Phytochromes are also found in some bacteria, fungi, algae, and plants but retain their red/far-red type spectral properties. The marked difference between these two classes of photoreceptors can be ascribed to the domain architectures of their photosensory modules as follows: CBCRs contain only the chromophore-binding GAF domain, and phytochromes usually require a tandem arrangement of PAS (Per/ARNT/Sim), GAF, and phytochrome-specific domains for a fully functional photocycle (3, 4). Crystal structures of CBCR photosensory GAF domains have recently been solved, giving us additional insight into their intra-protein signaling mechanisms (6–8).
Although the photochemistry of the various CBCRs has been extensively studied (9, 10), their output activities and physiological roles have not been fully elucidated. Several signaling output domains are associated with the CBCR-type GAF domain, including the histidine kinase domain, the methyl-accepting chemotaxis domain, and the GGDEF/EAL domains, although other CBCRs do not harbor any known output domain. The best characterized output domain is the histidine kinase domain in CcaS of Synechocystis and Nostoc punctiforme. CcaS is a green light sensor, which induces autophosphorylation and phosphotransfer to its cognate response regulator, which then induces expression of phycobilisome-related genes (11, 12). Another protein containing a histidine kinase domain is RcaE, which is a red light sensor that autophosphorylates and eventually induces expression of many phycobilisome-related genes in Fremyella diplosiphon (13, 14). PixA/NirS is probably a UV sensor that induces gene expression involved in the negative phototaxis in Synechocystis, although the output activity of its histidine kinase-like domain has not been clearly demonstrated (15, 16). SyPixJ1 is likely a blue light sensor that induces the suppression of positive phototaxis (17, 18), although the output activity of its methyl-accepting chemotaxis domain has not been studied. Most CBCRs with a GGDEF/EAL domain have not been characterized yet except for that of a fragment of the phytochrome-CBCR composite protein Cph2, which regulates motility in Synechocystis (19).
The universal bacterial second messenger c-di-GMP generally induces sessile multicellular lifestyles and represses planktonic single cellular ones (20). The GGDEF domain contains a diguanylate cyclase activity for the synthesis of c-di-GMP (21), whereas the EAL domain degrades c-di-GMP via its phosphodiesterase activity (22). Generally, many GGDEF/EAL domains are encoded in cyanobacterial genomes (20) (e.g. up to 58 genes in Acaryochloris marina), suggesting that c-di-GMP signaling is important in cyanobacteria (19, 23, 24). Some of these encoded cyanobacterial proteins also contain a CBCR-type GAF domain. The thermophilic cyanobacterium Thermosynechococcus elongatus strain BP-1 harbors three such genes (tlr0924, tlr1999, and tlr0911) in the genome (25), and a close relative Thermosynechococcus vulcanus strain RKN is known for its cellulose-dependent cell aggregation under irradiation at low temperature conditions (at 31 °C compared with optimum temperature at 45 °C) (26, 27). These observations prompted us to study light-responsive c-di-GMP signaling using Thermosynechococcus as the model cyanobacterium. Tlr0924 possesses one CBCR-GAF domain and one GGDEF domain (Fig. 1A), whereas Tlr1999 and Tlr0911 possess both GGDEF and EAL domains. Given its simple domain composition, we chose Tlr0924 as the initial target for the study of CBCR-mediated c-di-GMP signaling in cyanobacteria.
FIGURE 1.
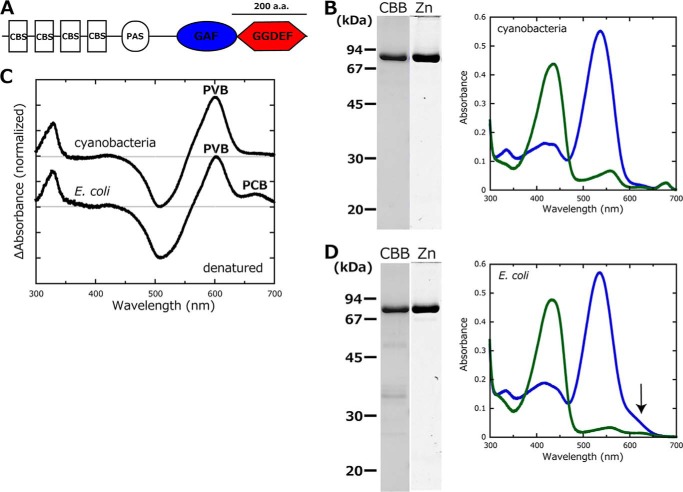
Properties of SesA prepared from the cyanobacterial expression system and E. coli expression system. A, domain composition of SesA deduced by SMART (58) and InterProScan (59). CBS, cystathionine-β-synthase; PAS, Per/ARNT/Sim; GGDEF, diguanylate cyclase domain. B, left, SDS-polyacrylamide gels of Coomassie Brilliant Blue (CBB) (CBB)-stained and Zn2+-enhanced fluorescence (Zn) of SesA expressed in the cyanobacterial system. Right, absorption spectra of native SesA expressed in the cyanobacterial system. When irradiated with blue light, SesA photoconverts to the Pg form (blue line). When irradiated with green light, SesA photoconverts to the Pb form (green line). C, chromophore composition. Light-induced difference spectra of the chromophore (C15-Z minus C15-E) for the acid/urea-denatured Pg form of SesA. Top spectrum, that of SesA expressed in the cyanobacterial system; bottom spectrum, that of SesA expressed in the E. coli system. D, left, SDS-polyacrylamide gels of Coomassie Brilliant Blue (CBB)-stained and Zn2+-enhanced fluorescence (Zn) of SesA expressed in the E. coli system. Right, absorption spectra of native SesA expressed in the E. coli system. When irradiated with blue light, SesA photoconverts to the Pg form (blue line). When irradiated with green light, SesA photoconverts to the Pb form (green line).
Herein, we show that Tlr0924 is a blue light-activated diguanylate cyclase that induces cell aggregation in Thermosynechococcus. Given our data, we propose and use the name “sesA (SesA; Sessility-A)” for tlr0924 (Tlr0924). This study reveals that the CBCR modulates the sessile/planktonic lifestyles of cyanobacteria by c-di-GMP regulation.
EXPERIMENTAL PROCEDURES
Protein Preparation
Plasmid construction and His-tagged protein expression/purification using the cyanobacterial and Escherichia coli expression systems were performed as described (28, 29). In both cases, the full-length sesA was amplified from the T. elongatus BP-1 genome, with the primers 5′-CCCATATGGAGCAGTTGAGTTCAG-3′ and 5′-CCCGGATCCTCACGATAGCTCCCTAGC-3′, and cloned into the slr2031-based Synechocystis expression system (29) and pET28a (Novagen, Madison, WI).
SDS-PAGE and Spectroscopy
SDS-PAGE with Coomassie Brilliant Blue or the zinc-enhanced fluorescent staining were performed as described (30). Absorption spectra were recorded at room temperature using a UV-2400PC spectrophotometer (Shimadzu). For blue light irradiation, light-emitting diodes with λmax = 460 nm (full-width at half-maximum (FWHM) = 23 nm, EIL53-3B, Toyoda Gosei) were used. For green light irradiation, light-emitting diodes with λmax = 515 nm (FWHM = 28 nm, EIL53-3G, Toyoda Gosei) were used. For acid/urea denaturation, proteins of Pg form (C15-E) were denatured in 8 m urea, pH 2.0, at room temperature in the dark and then converted almost irreversibly to C15-Z by irradiation with white light (28). For native proteins, the peak wavelengths were obtained from the difference spectra (the Pb form − the Pg form) to avoid the effect of contaminating chlorophyll when prepared in a cyanobacterial expression system.
Diguanylate Cyclase Activity Assays
The product of SesA catalysis was identified using an HPLC-based assay in conjunction with mass spectrometry (MS). Each reaction (50 μl of 50 mm Tris-HCl, pH 7.5, 10 mm MgCl2, 40 μm GTP, 1.0 μm SesA) was incubated at 25 °C for 10 min. The reactions were stopped by sequentially adding 0.5 m EDTA (25 μl) and 1 m triethylammonium formate (25 μl). Each sample was chromatographed through a C18 column (150 × 4.6 mm inner diameter., YMC Pack Pro C18, YMC Co.) under the control of a Shimadzu LC-10Ai HPLC system. The following buffers served as the eluents: buffer A (25 mm triethylammonium formate, 2% (v/v) methanol, pH 6.0) and buffer B (25 mm triethylammonium formate, 90% (v/v) methanol, pH 6.0). The gradient program (where the values are in minutes and percentage of buffer B, respectively) was as follows: 0, 0; 13, 0; 20, 5; 45, 30; 50, 100; 55, 100; and 60, 0 at a flow rate of 0.4 ml min−1. Nucleotide absorbance was detected at 256 nm.
For MS, the peak at 39 min was dried in a centrifugal evaporator and dissolved in 10 μl of water. One μl of the solution was spotted onto a MALDI plate. The sample was dried, and 0.5 μl of 10 mg/ml 9-aminoacridine hemihydrate (Acros Organics) in 90% (v/v) methanol was spotted onto the sample. For MS, a MALDI-TOF mass spectrometer (AXIMA CFRplus, Shimadzu) was used, and for tandem MS (MS/MS and MS/MS/MS), a MALDI-quadrupole ion-trap TOF mass spectrometer (AXIMA QIT, Shimadzu) was used.
For the kinetic measurements, the amount of inorganic pyrophosphate, a by-product of c-di-GMP synthesis, was monitored at 360 nm by a UV-2400PC spectrophotometer using EnzChek pyrophosphate assay kit reagents (Invitrogen) (31). The kit includes inorganic pyrophosphatase, which catalyzes conversion of inorganic pyrophosphate into 2 eq of inorganic phosphate. In the presence of inorganic phosphate, the substrate 2-amino-6-mercapto-7-methylpurine ribonucleoside was enzymatically converted to ribose 1-phosphate and 2-amino-6-mercapto-7-methylpurine, which shows the absorption at 360 nm. Each reaction contained 50 mm Tris-HCl, pH 7.5, 10 mm MgCl2, 100 μm GTP, and 0.5 μm SesA. The reactions were incubated at 25 °C. As a control, 100 μm ATP was substituted for GTP, with all other conditions kept the same.
Strains and Cultures
The thermophilic cyanobacterium T. vulcanus strain RKN was cultured at 45 °C (normal temperature) or 31 °C (low temperature) in BG11 medium (32) under continuous illumination provided by white fluorescent lamps (30 μmol photons m−2 s−1), and bubbled with air containing 1.0% (v/v) CO2. Culture absorbance was monitored at 730 nm.
Cell Aggregation
Cells grown at 45 °C were diluted to give a culture OD730 nm of ∼0.3 and then cultured at 31 °C under photosynthetic red light (light-emitting diodes; λmax = 639 nm, FWHM = 13 nm, L645-04V, Epitex Japan, 30 μmol photons m−2 s−1). The cells were irradiated with blue or green light (5 μmol photons m−2 s−1) in certain cases. The cellulose-dependent cell aggregation was evaluated as the aggregation index (26). Briefly, cell suspensions were thoroughly mixed, and aliquots were transferred to cuvettes. Aggregated cells had mostly precipitated to the bottom of the cuvette after standing for 30 min at room temperature. The OD730 of the remaining supernatant was measured as ODNA (i.e. OD730 of nonaggregated cells). Cellulase was added to the suspension, and the mixture was incubated for 20 min at 37 °C to disperse the aggregated cells completely. The OD730 of the resulting cell suspension without aggregation, ODtotal, was measured. ODtotal was also used for analyses of cell growth. The aggregation index was defined as: aggregation index (%) = ((ODtotal − ODNA)/ODtotal) × 100.
Gene Disruption
The T. elongatus genomic region, including sesA (tlr0924), was PCR-amplified (AmpliTaq Gold DNA Polymerase; Applied Biosystems). The primers were 5′-GTCAGCCAAGGTAGGATGATG-3′ and 5′-CCCGGATCCTCACGATAGCTCCCTAGC-3′. The PCR product was cloned into a pT7Blue T-vector (Novagen), and sesA in the plasmid was interrupted at the EcoRV site with a chloramphenicol resistance cassette.
Because T. vulcanus and T. elongatus are very closely related, the disrupted T. elongatus sesA construct was efficiently introduced into T. vulcanus according to Ref. 33. Chloramphenicol (3.4 μg ml−1) was added into the medium to maintain the mutant cells. Gene disruption with full segregation in multiple copies of the genome was confirmed by PCR (data not shown).
The DNA construct for the disruption of tll0007 cellulose synthase was made using In-Fusion system (TaKaRa, Ohtsu, Japan). PCR-Script was amplified by PCR with the primers (5′-GGGCTAGAGCGGCCGCCA-3′ and 5′-GGGCGGATCCCCCGGGCT-3′) and PCR-Script vector (Agilent Technologies, La Jolla, CA). A chloramphenicol resistance cassette was amplified by PCR with the primers (5′-AGCGCTGATGTCCGGCGG-3′ and 5′-AGGCCTGCTAACCGTTTT-3′) and a chloramphenicol-resistant vector (33). For amplifying the upstream and the downstream region of tll0007 by PCR, we used the primers 5′-CCGGGGGATCCGCCCGGCGGCTTAGCGGGGCAA-3′, 5′-CCGGACATCAGCGCTCGGCAGGGACCCTAAAAA-3′ and 5′-ACGGTTAGCAGGCCTCCCATGGGCTCATGGCTG-3′, 5′-CGGCCGCTCTAGCCCGTATTATTCGCACATGGC-3′, respectively.
The DNA construct for the truncation of the PilZ domain of tll0007 was also made using In-Fusion system. PCR-Script was amplified as described above. A chloramphenicol resistance cassette was amplified by PCR with the primers 5′-GCCGTAAAGCGCTGATGTCCGGCGG-3′ and 5′-CCCATGGGAGGcctGCTAACCGTTTT-3′ and a chloramphenicol-resistant vector. For amplifying the upstream and the downstream region of the PilZ domain of tll0007 by PCR, we used the primers 5′-CCGGGGGATCCGCCCTTCATTGTCATTCTTTTT-3′, 5′-TCAGCGCTTTACGGCTGGTCAATAGACGA-3′ and 5′-CAGGCCTCCCATGGGCTCATGGCTG-3′, 5′-CGGCCGCTCTAGCCCGTATTATTCGCACATGGC-3′, respectively.
RESULTS AND DISCUSSION
Spectral Properties
We prepared full-length T. elongatus BP-1 SesA in a cyanobacterial expression system (Fig. 1, A and B). The holoprotein reversibly photoconverted between a blue light-absorbing form (Pb; λmax = 436 nm) and a green light-absorbing form (Pg; λmax = 538 nm; Fig. 1B). We could not observe dark reversion at ambient temperature, showing that the Pb and Pg are stable in the dark. We can deduce the chromophore species and configurations according to the light-induced difference spectrum of the acid/urea-denatured protein by removing any modifications with the protein (28). The SesA chromophore was homogeneously phycoviolobilin (PVB) (Fig. 1C). We also prepared SesA in an E. coli expression system and that protein photoconverted in a very similar manner as the one from the cyanobacterial system (Fig. 1D). Unlike SesA produced in the cyanobacterial system, the E. coli-produced SesA contained a small amount of phycocyanobilin (PCB) (Fig. 1, C and D, in which an arrow identifies the PCB peak in the spectrum of native SesA from E. coli) (34). The spectral properties of SesA from E. coli are almost identical to those reported previously (34–36). Chromophore heterogeneity is common for PVB-type CBCRs produced in E. coli (30, 34, 37). During assembly of the holoprotein, PCB is isomerized to PVB after PCB is covalently linked to the apoprotein (37). The chromophore heterogeneity found for SesA from E. coli suggests that isomerization is not efficient in E. coli. There may be a chromophore maturation factor in cyanobacterial cells. Another drawback associated with the E. coli system is that small amounts of other proteins were co-purified with SesA (Fig. 1D), which did not occur when the cyanobacterial system was used.
Diguanylate Cyclase Activity
Because SesA contains a GGDEF domain, which is implicated in c-di-GMP synthesis, we characterized the ability of SesA to convert GTP into c-di-GMP. We used an HPLC/MS-based assay for this purpose. SesA prepared from the cyanobacterial system converted GTP to a second compound (retention time, 39 min; Fig. 2), and this conversion occurred to a greater extent under blue light irradiation (the Pg form) than under green light irradiation (the Pb form; Fig. 2). The combination of MS, MS/MS, and MS/MS/MS definitively identified this peak as c-di-GMP (supplemental Fig. S1). Therefore, SesA is a blue light-activated diguanylate cyclase.
FIGURE 2.
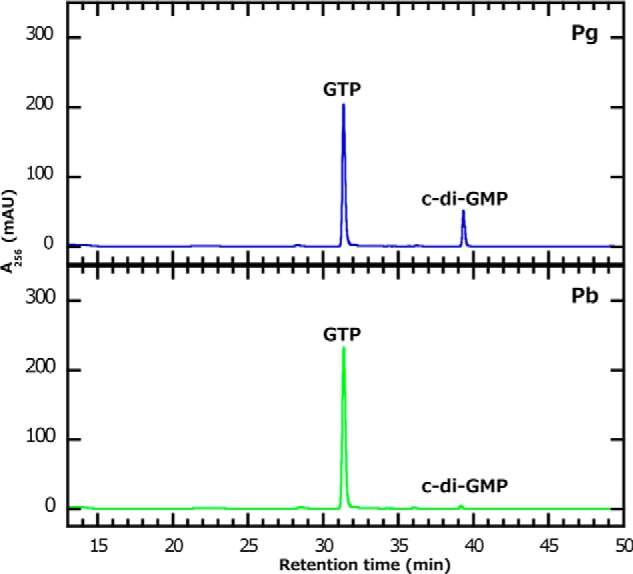
Characterization of SesA as a diguanylate cyclase. SesA was prepared from the cyanobacterial expression system. The reactions included the Pb (bottom panel) or Pg (top panel) forms of SesA, which were incubated for 10 min with GTP. GTP and the product c-di-GMP were separated by HPLC. The mass spectra that proved the second peak to be c-di-GTP are shown in supplemental Fig. S1.
We also measured the reaction rate for the diguanylate cyclase activity of SesA using an assay that measures the amount of pyrophosphate, which is a by-product of diguanylate cyclase reaction (31). SesA had a reaction rate of 1.89 ± 0.09 μmol PPi·μmol protein−1·min−1 when blue light-irradiated, although it was much less active (the reaction rate, 0.05 ± 0.01 μmol PPi·μmol protein−1·min−1) when green light-irradiated (Table 1 and Fig. 3). The activity of the light-activated SesA is comparable with the typical diguanylate cyclases (38). Pyrophosphate was not produced when ATP was supplied instead of GTP.
TABLE 1.
SesA activity as measured by pyrophosphate production rate
| Expression system | Pg | Pb |
|---|---|---|
| μmol PPi·μmol protein−1·min−1 | ||
| Cyanobacteria | 1.89 ± 0.09a | 0.05 ± 0.01 |
| E. coli | 1.99 ± 0.16 | 0.17 ± 0.01 |
a Standard deviation is shown (n = 3).
FIGURE 3.
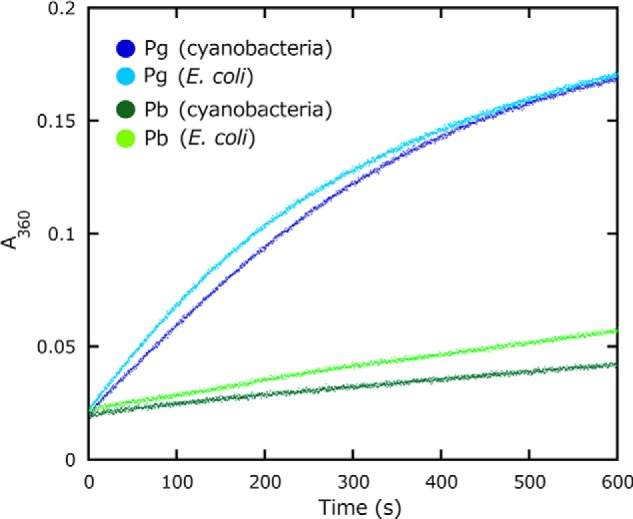
SesA diguanylate cyclase activity. Activity was measured as pyrophosphate production monitored by the change in A360 nm. Each reaction was irradiated with blue light (blue) or green light (green) prior to the assay. SesA was prepared from the cyanobacterial (dark blue and green lines) or the E. coli (light blue and green lines) expression system.
The level of the diguanylate cyclase activity for SesA prepared from the cyanobacterial system was strictly dependent on the wavelength of incident light (∼38-fold difference between the blue and green light-induced activities), whereas that for SesA from E. coli was less dependent (∼12-fold difference; Table 1 and Fig. 3). Given that the SesA preparation from E. coli contains both PCB and PVB forms of SesA, the diguanylate cyclase activity of the PCB form may be less tightly regulated by the spectral wavelength than is the PVB form. Some PCB population may be impaired due to misfolding because PCB is not native chromophore for SesA. Alternatively, the major PVB population might also be partially disordered in E. coli cells, to exhibit less tight regulation. Taken together, the evidence indicated that the cyanobacterial system is the better one for production of SesA than the E. coli system.
The SesA diguanylate cyclase activity is much more strictly light-regulated than are the activities of other light-regulated GGDEF- and EAL-containing proteins (e.g. SL2, 2-fold difference (39); BlrP1, 4-fold difference (40); and the C-terminal fragment of Cph2, 2-fold difference (19)), suggesting that SesA is suitable for the study of light regulation mechanisms and as an optogenetic tool (41). Recently obtained crystal structures of CBCR-GAF domains suggest that they form a helical bundle by dimerization and transduce their signals to their C termini via these helices (6, 8). Dimerization of GGDEF domain proteins is necessary for their diguanylate cyclase activities (31, 42). A structural study on SesA should reveal how light controls its diguanylate cyclase activity.
Generally, Mg2+ is essential for diguanylate cyclase activity. The Kd for SesA/Mg2+ dissociation was 10.5 mm (Fig. 4), which is a rather large value and may be similar to the physiological cellular concentration of Mg2+ (43). We previously reported that Mg2+ suppresses the σ factor SigE activity by promoting the interaction of SigE and the subunit of the magnesium-chelatase ChlH in Synechocystis (44). The availability of external Mg2+ affects the c-di-GMP signaling pathway in Vibrio fischeri (45). We anticipate that Mg2+ fluctuations may influence c-di-GMP signaling in vivo in this cyanobacterium, although we cannot rule out the possibility that another divalent cation may be a native cofactor.
FIGURE 4.
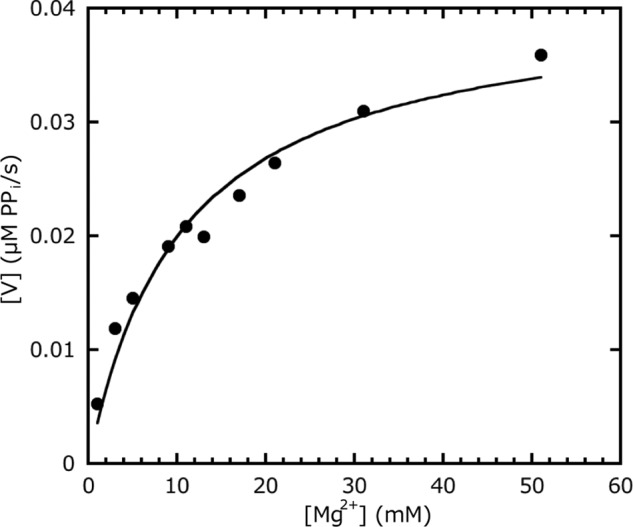
Mg2+ dependence of the SesA diguanylate cyclase activity. The data are fitted to Michaelis-Menten kinetics using the KaleidaGraph fitting program (Synergy Software). The activity (V) is measured as the rate of pyrophosphate production.
Phenotype Analysis
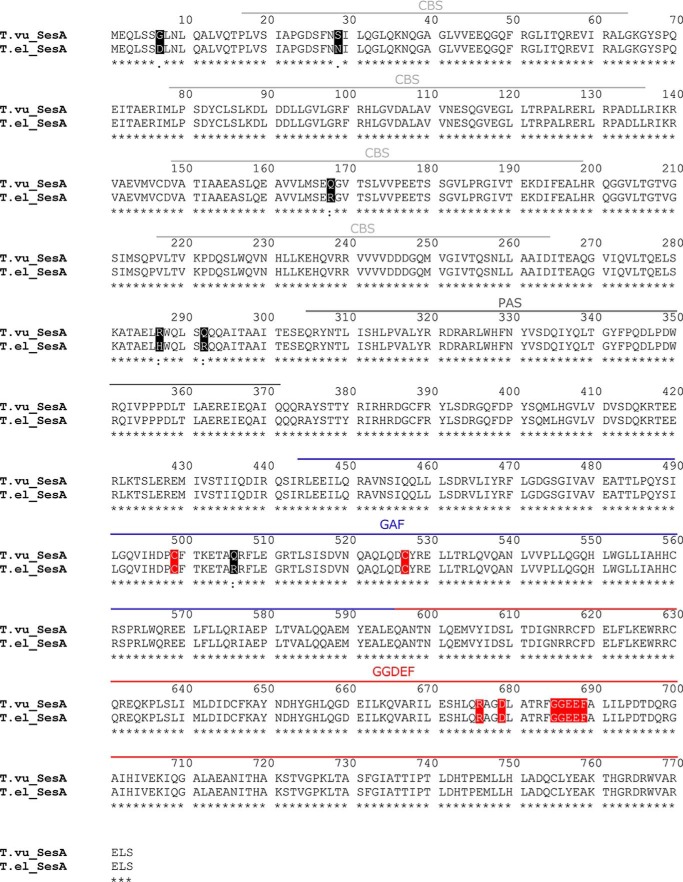
c-di-GMP is generally implicated in multicellular processes, e.g. biofilm formation (20). Although T. elongatus is not known to form a biofilm-related structure, T. vulcanus, a close relative, shows cell aggregation in a cellulose-dependent manner at relatively low temperatures of 31 °C under white light (26). Sequence analysis revealed that SesA is indeed highly conserved between T. elongatus and T. vulcanus (Fig. 5); the GGDEF domain for diguanylate cyclase is identical, and the photosensory GAF domain has a single amino acid substitution, which is located in a peripheral region far apart from the chromophore and the signaling helices according to the crystal structure of TePixJ (6). Thus, we assume that SesA of T. vulcanus is also the blue light-activated diguanylate cyclase, and therefore, we assessed its role for light-induced cell aggregation in T. vulcanus at 31 °C.
FIGURE 5.
Amino acid sequence alignment for T. elongatus and T. vulcanus SesA. The residues that differ in the sequences of the two species are shaded in black. The functionally crucial residues (Cys-499 and Cys-527 in the GAF chromophore-binding domain, Arg-676 and Asp-679 in the GGDEF primary inhibition site, and Gly-685–Phe-689 in the GGDEF active site) are all conserved and are shaded in red. Domains are identified by the bars above the alignment.
We previously reported that the cell aggregation is a slow biological process that requires photosynthesis and cellulose production at low temperature (26, 27). To support such background reactions, we irradiated cells with photosynthetic red light (30 μmol photons m−2 s−1) and signaling blue or green light (5 μmol photons m−2 s−1). Irradiation of wild type with only red light did not cause cell aggregation (Fig. 6). However, cell aggregation was induced under a combination of red and blue light but not under red and green light. Complete aggregation occurred after culturing the irradiated cells for ∼24 h under red/blue light at 31 °C. The cell aggregates were quickly (<20 min) and completely dispersed by cellulase treatment as reported (26). Only blue light or green light did not induce the cell aggregation. Taken together, the blue light signal was responsible for cellulose-dependent cell aggregation. For the ΔsesA mutant, however, blue light irradiation did not cause cell aggregation (Fig. 6), which suggests that SesA mediates the blue light-induced cell aggregation.
FIGURE 6.
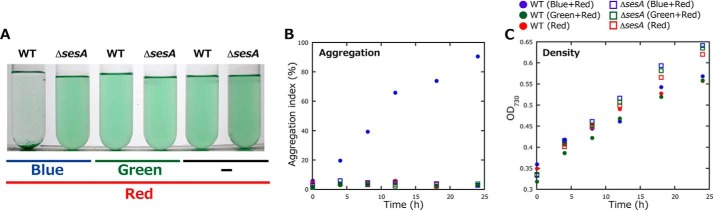
Effects of light color on T. vulcanus cell aggregation at a relatively low temperature of 31 °C. Data are representative of replicated experiments (n = 4). A, photographs of wild-type (WT) T. vulcanus and its ΔsesA mutant (ΔsesA) cultured at 31 °C for 24 h and then incubated for 30 min without bubbling in the dark. The cells were cultured with irradiation with blue or green light (5 μmol photons m−2 s−1) in addition to red light (30 μmol photons m−2 s−1), which was needed for photosynthetic growth. Cell aggregation (B) and cell density (C) were reported as the culture OD730 nm as a function of culture time. The definition of the aggregation index refers to “Experimental Procedures.”
Cellulose accumulation via cellulose synthase Tll0007 is essential for cell aggregation under low temperature/light conditions (26). Tll0007 has a conserved PilZ domain, which is a c-di-GMP receptor module (46). Notably, the key residues that are involved in the c-di-GMP binding are conserved in the PilZ domain of Tll0007. When this PilZ domain was truncated from Tll0007, the blue light-induced cell aggregation was completely abolished (Fig. 7). Knowledge of the functions of SesA and Tll0007 allows us to propose a c-di-GMP signaling pathway in Thermosynechococcus (Fig. 8) as follows. Step 1, blue light activates SesA to produce c-di-GMP. Step 2, c-di-GMP binds to the PilZ domain of Tll0007 to activate its cellulose synthase, as is known for the bacterial cellulose synthase BcsA (47, 48). Step 3, cellulose, which accumulates extracellularly, triggers cell aggregation. This description is the first example of a light-responsive c-di-GMP metabolism, and downstream c-di-GMP signaling to be fully modeled, in contrast with other studies on light-responsive c-di-GMP signaling in bacteria (39, 40, 49, 50).
FIGURE 7.
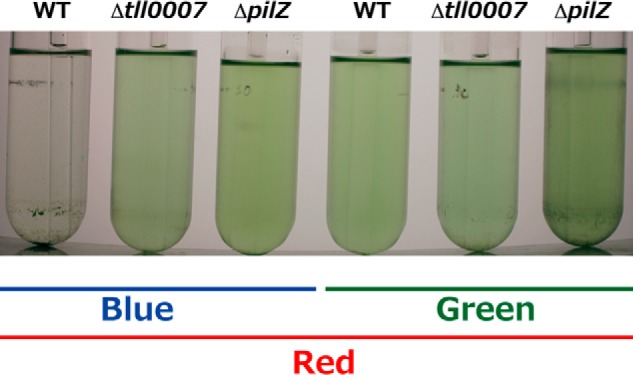
Effects of deletion of the PilZ domain of the cellulose synthase Tll0007 on T. vulcanus cell aggregation at a relatively low temperature of 31 °C. Photographs of wild-type (WT) T. vulcanus, its Δtll0007 mutant (Δtll0007), and the mutant whose tll0007 lacks its PilZ domain (ΔpilZ) cultured at 31 °C for 3 days and then incubated for 30 min without bubbling in the dark. The cells were cultured with irradiation with blue or green light (5 μmol photons m−2 s−1) in addition to red light (30 μmol photons m−2 s−1), which was needed for photosynthetic growth.
FIGURE 8.
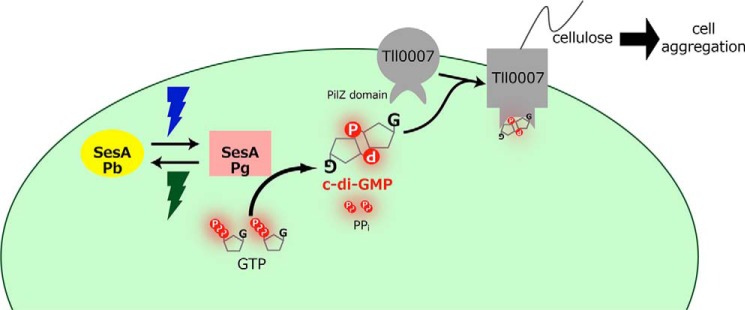
Proposed signaling pathway for SesA. Blue light irradiation photoconverts the Pb form of SesA into the Pg form, which catalyzes the formation of c-di-GMP from two GTP molecules via its diguanylate cyclase activity. When Tll007 binds c-di-GMP at its PilZ domain, it then acts as a cellulose synthase. Extracellular cellulose accumulation results in cell aggregation.
Many bacteria show blue light-activated surface attachment (51), biofilm formation (49), biofilm maturation (52), or blue light-activated inhibition of biofilm formation (53), suggesting that a blue light-dependent lifestyle change is a prevalent phenomenon among bacteria (54). Photoreceptors that are involved in the above examples belong to light-oxygen-voltage (LOV), blue light sensing using FAD (BLUF), or photoactive yellow protein (PYP) families and are activated by blue light and revert to their inactive state in the dark (1). They sense light and dark as signals for environmental acclimation. Conversely, Thermosynechococcus SesA senses two different wavelengths of light, i.e. it is up-regulated by blue light and down-regulated by green light. Photosynthesis is driven by visible light, but blue light also damages the manganese cluster of the oxygen-evolving complex of photosystem II in plants and cyanobacteria (55). SesA seems to serve not as a light/dark sensor but as a spectral color sensor for a better phototrophic life. The cell aggregation is known to be effective in protection against photoinhibition by self-shading (27).
The Pfam database (56) contains many PilZ domain proteins for which sequences have been deduced from various cyanobacterial genomic sequences. Mostly, these protein sequences are considered to be of cellulose synthases, although only Tll0007 has been experimentally investigated (26). This evidence strongly suggests that c-di-GMP signaling mediates cell aggregation and biofilm formation via activation of a PilZ domain of a cellulose synthase that then produces cellulose or a related extracellular polysaccharide. Many reports have indicated that naturally occurring cyanobacteria produce various extracellular polysaccharides for the formation of sheaths or cellular mats (57). Our results may provide the impetus for pioneering work that dissects the molecular mechanism for such properties. Biotechnological engineering of these regulatory systems would also be useful for production and harvesting of the photosynthetic biomass.
In conclusion, we identified that CBCRs regulate a sessile/planktonic lifestyle transition besides chromatic acclimation and motility. We demonstrated that the CBCR SesA, acting as a diguanylate cyclase, induces Thermosynechococcus cell aggregation under the control of blue light and at a relatively low temperature. Two other CBCRs that have GGDEF/EAL domains (Tlr1999 and Tlr0911) have been identified in the genome of Thermosynechococcus. We reported that Tlr1999 photoconverts between blue- and teal-absorbing forms (30). This evidence suggests that the ambient light signal should be independently perceived by the three CBCRs and then integrated into the c-di-GMP signaling pathway(s). Our data revealed an important role for SesA under our experimental conditions. Additional studies on these CBCRs should provide insight into how they work together in the natural habitat(s) of Thermosynechococcus.
Supplementary Material
Acknowledgments
We thank Drs. Yu Kanesaki and Hirofumi Yoshikawa (Genome Research Center, NODAI Research Institute, Tokyo University of Agriculture, 1-1-1 Sakuragaoka, Setagaya-ku, Tokyo 156-8502, Japan) for providing the amino acid sequence of T. vulcanus SesA.
This work was supported by scientific research grants (to M. I. and R. N.), the GCOE Program “From the Earth to Earths” from the Ministry of Education and Science (to M. I.), by PRESTO, Japan Science and Technology Agency (to R. N.), and by CREST, Japan Science and Technology Agency (to M. I.).
This article was selected as a Paper of the Week.

This article contains supplemental Fig. S1.
- CBCR
- cyanobacteriochrome
- c-di-GMP
- cyclic dimeric GMP
- GAF
- cGMP phosphodiesterase/adenylyl cyclase/FhlA
- FWHM
- full-width at half-maximum
- PVB
- phycoviolobilin
- PCB
- phycocyanobilin
- Pg
- green light-absorbing form
- Pb
- blue light-absorbing form.
REFERENCES
- 1. Möglich A., Yang X., Ayers R. A., Moffat K. (2010) Structure and function of plant photoreceptors. Annu. Rev. Plant Biol. 61, 21–47 [DOI] [PubMed] [Google Scholar]
- 2. Ikeuchi M., Ishizuka T. (2008) Cyanobacteriochromes: a new superfamily of tetrapyrrole-binding photoreceptors in cyanobacteria. Photochem. Photobiol. Sci. 7, 1159–1167 [DOI] [PubMed] [Google Scholar]
- 3. Rockwell N. C., Lagarias J. C. (2010) A brief history of phytochromes. Chemphyschem. 11, 1172–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Auldridge M. E., Forest K. T. (2011) Bacterial phytochromes: More than meets the light. Crit. Rev. Biochem. Mol. Biol. 46, 67–88 [DOI] [PubMed] [Google Scholar]
- 5. Rockwell N. C., Martin S. S., Feoktistova K., Lagarias J. C. (2011) Diverse two-cysteine photocycles in phytochromes and cyanobacteriochromes. Proc. Natl. Acad. Sci. U.S.A. 108, 11854–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Narikawa R., Ishizuka T., Muraki N., Shiba T., Kurisu G., Ikeuchi M. (2013) Structures of cyanobacteriochromes from phototaxis regulators AnPixJ and TePixJ reveal general and specific photoconversion mechanism. Proc. Natl. Acad. Sci. U.S.A. 110, 918–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burgie E. S., Walker J. M., Phillips G. N., Jr., Vierstra R. D. (2013) A photo-labile thioether linkage to phycoviolobilin provides the foundation for the blue/green photocycles in DXCF-cyanobacteriochromes. Structure 21, 88–97 [DOI] [PubMed] [Google Scholar]
- 8. Rockwell N. C., Ohlendorf R., Möglich A. (2013) Cyanobacteriochromes in full color and three dimensions. Proc. Natl. Acad. Sci. U.S.A. 110, 806–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fukushima Y., Iwaki M., Narikawa R., Ikeuchi M., Tomita Y., Itoh S. (2011) Photoconversion mechanism of a green/red photosensory cyanobacteriochrome AnPixJ: time-resolved optical spectroscopy and FTIR analysis of the AnPixJ-GAF2 domain. Biochemistry 50, 6328–6339 [DOI] [PubMed] [Google Scholar]
- 10. Freer L. H., Kim P. W., Corley S. C., Rockwell N. C., Zhao L., Thibert A. J., Lagarias J. C., Larsen D. S. (2012) Chemical inhomogeneity in the ultrafast dynamics of the DXCF cyanobacteriochrome Tlr0924. J. Phys. Chem. B 116, 10571–10581 [DOI] [PubMed] [Google Scholar]
- 11. Hirose Y., Narikawa R., Katayama M., Ikeuchi M. (2010) Cyanobacteriochrome CcaS regulates phycoerythrin accumulation in Nostoc punctiforme, a group II chromatic adapter. Proc. Natl. Acad. Sci. U.S.A. 107, 8854–8859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirose Y., Shimada T., Narikawa R., Katayama M., Ikeuchi M. (2008) Cyanobacteriochrome CcaS is the green light receptor that induces the expression of phycobilisome linker protein. Proc. Natl. Acad. Sci. U.S.A. 105, 9528–9533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hirose Y., Rockwell N. C., Nishiyama K., Narikawa R., Ukaji Y., Inomata K., Lagarias J. C., Ikeuchi M. (2013) Green/red cyanobacteriochromes regulate complementary chromatic acclimation via a protochromic photocycle. Proc. Natl. Acad. Sci. U.S.A. 110, 4974–4979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kehoe D. M., Gutu A. (2006) Responding to color: the regulation of complementary chromatic adaptation. Annu. Rev. Plant Biol. 57, 127–150 [DOI] [PubMed] [Google Scholar]
- 15. Narikawa R., Suzuki F., Yoshihara S., Higashi S., Watanabe M., Ikeuchi M. (2011) Novel photosensory two-component system (PixA-NixB-NixC) involved in the regulation of positive and negative phototaxis of cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 52, 2214–2224 [DOI] [PubMed] [Google Scholar]
- 16. Song J. Y., Cho H. S., Cho J. I., Jeon J. S., Lagarias J. C., Park Y. I. (2011) Near-UV cyanobacteriochrome signaling system elicits negative phototaxis in the cyanobacterium Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. U.S.A. 108, 10780–10785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshihara S., Suzuki F., Fujita H., Geng X. X., Ikeuchi M. (2000) Novel putative photoreceptor and regulatory genes required for the positive phototactic movement of the unicellular motile cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 41, 1299–1304 [DOI] [PubMed] [Google Scholar]
- 18. Yoshihara S., Katayama M., Geng X., Ikeuchi M. (2004) Cyanobacterial phytochrome-like PixJ1 holoprotein shows novel reversible photoconversion between blue- and green-absorbing forms. Plant Cell Physiol. 45, 1729–1737 [DOI] [PubMed] [Google Scholar]
- 19. Savakis P., De Causmaecker S., Angerer V., Ruppert U., Anders K., Essen L. O., Wilde A. (2012) Light-induced alteration of c-di-GMP level controls motility of Synechocystis sp. PCC 6803. Mol. Microbiol. 85, 239–251 [DOI] [PubMed] [Google Scholar]
- 20. Römling U., Galperin M. Y., Gomelsky M. (2013) Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 77, 1–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paul R., Weiser S., Amiot N. C., Chan C., Schirmer T., Giese B., Jenal U. (2004) Cell cycle-dependent dynamic localization of a bacterial response regulator with a novel di-guanylate cyclase output domain. Genes Dev. 18, 715–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Christen M., Christen B., Folcher M., Schauerte A., Jenal U. (2005) Identification and characterization of a cyclic di-GMP-specific phosphodiesterase and its allosteric control by GTP. J. Biol. Chem. 280, 30829–30837 [DOI] [PubMed] [Google Scholar]
- 23. Neunuebel M. R., Golden J. W. (2008) The Anabaena sp. strain PCC 7120 gene all2874 encodes a diguanylate cyclase and is required for normal heterocyst development under high-light growth conditions. J. Bacteriol. 190, 6829–6836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agostoni M., Koestler B. J., Waters C. M., Williams B. L., Montgomery B. L. (2013) Occurrence of cyclic di-GMP-modulating output domains in cyanobacteria: an illuminating perspective. mBio 4, e00451-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakamura Y., Kaneko T., Sato S., Ikeuchi M., Katoh H., Sasamoto S., Watanabe A., Iriguchi M., Kawashima K., Kimura T., Kishida Y., Kiyokawa C., Kohara M., Matsumoto M., Matsuno A., Nakazaki N., Shimpo S., Sugimoto M., Takeuchi C., Yamada M., Tabata S. (2002) Complete genome structure of the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1. DNA Res. 9, 123–130 [DOI] [PubMed] [Google Scholar]
- 26. Kawano Y., Saotome T., Ochiai Y., Katayama M., Narikawa R., Ikeuchi M. (2011) Cellulose accumulation and a cellulose synthase gene are responsible for cell aggregation in the cyanobacterium Thermosynechococcus vulcanus RKN. Plant Cell Physiol. 52, 957–966 [DOI] [PubMed] [Google Scholar]
- 27. Hirano A., Kunito S., Inoue Y., Ikeuchi M. (1997) Light and low temperature induced cell flocculation of thermophilic cyanobacterium Synechococcus vulcanus. Plant Cell Physiol. 38, s37 [Google Scholar]
- 28. Ishizuka T., Narikawa R., Kohchi T., Katayama M., Ikeuchi M. (2007) Cyanobacteriochrome TePixJ of Thermosynechococcus elongatus harbors phycoviolobilin as a chromophore. Plant Cell Physiol. 48, 1385–1390 [DOI] [PubMed] [Google Scholar]
- 29. Ishizuka T., Shimada T., Okajima K., Yoshihara S., Ochiai Y., Katayama M., Ikeuchi M. (2006) Characterization of cyanobacteriochrome TePixJ from a thermophilic cyanobacterium Thermosynechococcus elongatus strain BP-1. Plant Cell Physiol. 47, 1251–1261 [DOI] [PubMed] [Google Scholar]
- 30. Enomoto G., Hirose Y., Narikawa R., Ikeuchi M. (2012) Thiol-based photocycle of the blue and teal light-sensing cyanobacteriochrome Tlr1999. Biochemistry 51, 3050–3058 [DOI] [PubMed] [Google Scholar]
- 31. De N., Pirruccello M., Krasteva P. V., Bae N., Raghavan R. V., Sondermann H. (2008) Phosphorylation-independent regulation of the diguanylate cyclase WspR. PLoS Biol. 6, e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 35, 171–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iwai M., Katoh H., Katayama M., Ikeuchi M. (2004) Improved genetic transformation of the thermophilic cyanobacterium, Thermosynechococcus elongatus BP-1. Plant Cell Physiol. 45, 171–175 [DOI] [PubMed] [Google Scholar]
- 34. Rockwell N. C., Martin S. S., Gulevich A. G., Lagarias J. C. (2012) Phycoviolobilin formation and spectral tuning in the DXCF cyanobacteriochrome subfamily. Biochemistry 51, 1449–1463 [DOI] [PubMed] [Google Scholar]
- 35. Rockwell N. C., Njuguna S. L., Roberts L., Castillo E., Parson V. L., Dwojak S., Lagarias J. C., Spiller S. C. (2008) A second conserved GAF domain cysteine is required for the blue/green photoreversibility of cyanobacteriochrome Tlr0924 from Thermosynechococcus elongatus. Biochemistry 47, 7304–7316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rockwell N. C., Martin S. S., Lagarias J. C. (2012) Mechanistic insight into the photosensory versatility of DXCF cyanobacteriochromes. Biochemistry 51, 3576–3585 [DOI] [PubMed] [Google Scholar]
- 37. Ishizuka T., Kamiya A., Suzuki H., Narikawa R., Noguchi T., Kohchi T., Inomata K., Ikeuchi M. (2011) The cyanobacteriochrome, TePixJ, isomerizes its own chromophore by converting phycocyanobilin to phycoviolobilin. Biochemistry 50, 953–961 [DOI] [PubMed] [Google Scholar]
- 38. Boehm A., Steiner S., Zaehringer F., Casanova A., Hamburger F., Ritz D., Keck W., Ackermann M., Schirmer T., Jenal U. (2009) Second messenger signalling governs Escherichia coli biofilm induction upon ribosomal stress. Mol. Microbiol. 72, 1500–1516 [DOI] [PubMed] [Google Scholar]
- 39. Cao Z., Livoti E., Losi A., Gärtner W. (2010) A blue light-inducible phosphodiesterase activity in the cyanobacterium Synechococcus elongatus. Photochem. Photobiol. 86, 606–611 [DOI] [PubMed] [Google Scholar]
- 40. Barends T. R., Hartmann E., Griese J. J., Beitlich T., Kirienko N. V., Ryjenkov D. A., Reinstein J., Shoeman R. L., Gomelsky M., Schlichting I. (2009) Structure and mechanism of a bacterial light-regulated cyclic nucleotide phosphodiesterase. Nature 459, 1015–1018 [DOI] [PubMed] [Google Scholar]
- 41. Möglich A., Moffat K. (2010) Engineered photoreceptors as novel optogenetic tools. Photochem. Photobiol. Sci. 9, 1286–1300 [DOI] [PubMed] [Google Scholar]
- 42. Wassmann P., Chan C., Paul R., Beck A., Heerklotz H., Jenal U., Schirmer T. (2007) Structure of BeF3–modified response regulator PleD: implications for diguanylate cyclase activation, catalysis, and feedback inhibition. Structure 15, 915–927 [DOI] [PubMed] [Google Scholar]
- 43. Kandhavelu M., Lihavainen E., Muthukrishnan A. B., Yli-Harja O., Ribeiro A. S. (2012) Effects of Mg2+ on in vivo transcriptional dynamics of the lar promoter. Biosystems 107, 129–134 [DOI] [PubMed] [Google Scholar]
- 44. Osanai T., Imashimizu M., Seki A., Sato S., Tabata S., Imamura S., Asayama M., Ikeuchi M., Tanaka K. (2009) ChlH, the H subunit of the Mg-chelatase, is an anti-σ factor for SigE in Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. U.S.A. 106, 6860–6865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O'Shea T. M., Klein A. H., Geszvain K., Wolfe A. J., Visick K. L. (2006) Diguanylate cyclases control magnesium-dependent motility of Vibrio fischeri. J. Bacteriol. 188, 8196–8205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Amikam D., Galperin M. Y. (2006) PilZ domain is part of the bacterial c-di-GMP binding protein. Bioinformatics 22, 3–6 [DOI] [PubMed] [Google Scholar]
- 47. Morgan J. L., Strumillo J., Zimmer J. (2013) Crystallographic snapshot of cellulose synthesis and membrane translocation. Nature 493, 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ross P., Weinhouse H., Aloni Y., Michaeli D., Weinberger-Ohana P., Mayer R., Braun S., de Vroom E., van der Marel G. A., van Boom J. H., Benziman M. (1987) Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 325, 279–281 [DOI] [PubMed] [Google Scholar]
- 49. Kanazawa T., Ren S., Maekawa M., Hasegawa K., Arisaka F., Hyodo M., Hayakawa Y., Ohta H., Masuda S. (2010) Biochemical and physiological characterization of a BLUF protein-EAL protein complex involved in blue light-dependent degradation of cyclic diguanylate in the purple bacterium Rhodopseudomonas palustris. Biochemistry 49, 10647–10655 [DOI] [PubMed] [Google Scholar]
- 50. Tarutina M., Ryjenkov D. A., Gomelsky M. (2006) An unorthodox bacteriophytochrome from Rhodobacter sphaeroides involved in turnover of the second messenger c-di-GMP. J. Biol. Chem. 281, 34751–34758 [DOI] [PubMed] [Google Scholar]
- 51. Purcell E. B., Siegal-Gaskins D., Rawling D. C., Fiebig A., Crosson S. (2007) A photosensory two-component system regulates bacterial cell attachment. Proc. Natl. Acad. Sci. U.S.A. 104, 18241–18246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tschowri N., Busse S., Hengge R. (2009) The BLUF-EAL protein YcgF acts as a direct anti-repressor in a blue-light response of Escherichia coli. Genes Dev. 23, 522–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bonomi H. R., Posadas D. M., Paris G., Carrica Mdel C., Frederickson M., Pietrasanta L. I., Bogomolni R. A., Zorreguieta A., Goldbaum F. A. (2012) Light regulates attachment, exopolysaccharide production, and nodulation in Rhizobium leguminosarum through a LOV-histidine kinase photoreceptor. Proc. Natl. Acad. Sci. U.S.A. 109, 12135–12140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gomelsky M., Hoff W. D. (2011) Light helps bacteria make important lifestyle decisions. Trends Microbiol. 19, 441–448 [DOI] [PubMed] [Google Scholar]
- 55. Nishiyama Y., Allakhverdiev S. I., Murata N. (2011) Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. Physiol. Plant. 142, 35–46 [DOI] [PubMed] [Google Scholar]
- 56. Punta M., Coggill P. C., Eberhardt R. Y., Mistry J., Tate J., Boursnell C., Pang N., Forslund K., Ceric G., Clements J., Heger A., Holm L., Sonnhammer E. L., Eddy S. R., Bateman A., Finn R. D. (2012) The Pfam protein families database. Nucleic Acids Res. 40, D290–D301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pereira S., Zille A., Micheletti E., Moradas-Ferreira P., De Philippis R., Tamagnini P. (2009) Complexity of cyanobacterial exopolysaccharides: composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 33, 917–941 [DOI] [PubMed] [Google Scholar]
- 58. Letunic I., Doerks T., Bork P. (2012) SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 40, D302–D305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Quevillon E., Silventoinen V., Pillai S., Harte N., Mulder N., Apweiler R., Lopez R. (2005) InterProScan: protein domains identifier. Nucleic Acids Res. 33, W116–W120 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.