Background: The mechanism whereby arachidonic acid (AA) activates the phagocyte oxidase Nox2 is not well understood, except for the AA-induced conformational change of p47phox, a partner of the Nox2 activator p67phox.
Results: AA also triggers Rac-GTP formation and Nox2 interaction with the p67phox·Rac-GTP complex.
Conclusion: AA regulates Nox2 assembly at multiple steps.
Significance: p67phox-Nox2 interaction is a novel regulatory step.
Keywords: Arachidonic Acid (AA) (ARA), NADPH Oxidase, Rac (Rac GTPase), Reactive Oxygen Species (ROS), Signal Transduction, Superoxide Ion, Nox2, p67phox
Abstract
The phagocyte NADPH oxidase Nox2, heterodimerized with p22phox in the membrane, is dormant in resting cells but becomes activated upon cell stimulation to produce superoxide, a precursor of microbicidal oxidants. Nox2 activation requires two switches to be turned on simultaneously: a conformational change of the cytosolic protein p47phox and GDP/GTP exchange on the small GTPase Rac. These proteins, in an active form, bind to their respective targets, p22phox and p67phox, leading to productive oxidase assembly at the membrane. Although arachidonic acid (AA) efficiently activates Nox2 both in vivo and in vitro, the mechanism has not been fully understood, except that AA induces p47phox conformational change. Here we show that AA elicits GDP-to-GTP exchange on Rac at the cellular level, consistent with its role as a potent Nox2 activator. However, even when constitutively active forms of p47phox and Rac1 are both expressed in HeLa cells, superoxide production by Nox2 is scarcely induced in the absence of AA. These active proteins also fail to effectively activate Nox2 in a cell-free reconstituted system without AA. Without affecting Rac-GTP binding to p67phox, AA induces the direct interaction of Rac-GTP-bound p67phox with the C-terminal cytosolic region of Nox2. p67phox-Rac-Nox2 assembly and superoxide production are both abrogated by alanine substitution for Tyr-198, Leu-199, and Val-204 in the p67phox activation domain that localizes the C-terminal to the Rac-binding domain. Thus the “third” switch (AA-inducible interaction of p67phox·Rac-GTP with Nox2) is required to be turned on at the same time for Nox2 activation.
Introduction
The NADPH oxidase (Nox)2 family enzymes deliberately produce reactive oxygen species and, therefore, contribute to a variety of functions, including host defense, signal transduction, and hormone synthesis (1–5). The Nox oxidases contain two distinct hemes in the N-terminal transmembrane region and FAD- and NADPH-binding sites in the C-terminal cytoplasmic domain and, thus form a complete electron-transporting apparatus from NADPH to O2 via FAD and hemes in a single protein. Nox2 (also known as gp91phox), the original member of the Nox family, is highly expressed in professional phagocytes such as neutrophils. The phagocyte oxidase Nox2 is completely inactive in resting cells but becomes activated during phagocytosis of invading microbes to produce superoxide, a precursor of microbicidal reactive oxygen species (1–5). The significance of this oxidase in host defense is evident because recurrent and life-threatening infections occur in patients with chronic granulomatous disease whose phagocytes fail to kill pathogenic microbes because of the genetic defect in the Nox2-based reactive oxygen species-producing system (6, 7).
Superoxide production by Nox2 is elicited not only during phagocytosis but also in response to soluble stimulants such as arachidonic acid (AA) and phorbol 12-myristate 13-acetate (PMA), a potent activator of PKC (8–11). Upon phagocyte stimulation, AA is released from membrane phospholipids. AA release for Nox2 activation is considered to be catalyzed by phospholipase A2 enzymes such as cPLA2 (12–14) and peroxyredoxin 6 (15, 16). Nox2 is stably dimerized with the membrane-integrated protein p22phox, and the heterodimer assembles into the active complex with the small GTPase Rac and the specialized Nox-activating proteins p47phox and p67phox, which are recruited from the cytosol to the membrane upon cell stimulation (1–5). The assembly of the Nox2-based oxidase requires two switches to be turned on simultaneously: a conformational change of p47phox and GDP-to-GTP exchange on Rac. The stimulus-induced conformational change of p47phox, comprising 390 amino acid residues, allows the bis-SH3 domain of this protein to interact with the C-terminal proline-rich region (PRR) of p22phox, an interaction essential for Nox2 activation (17, 18). The bis-SH3 domain is normally masked via an intramolecular association with the autoinhibitory region (AIR) of amino acid residues 286–340, which locates immediately C-terminal to the second SH3 domain (19–22) (see Fig. 1A). The release of the inhibitory association as a switch can be induced by direct action of AA (22, 23) or by PKC-catalyzed phosphorylation of multiple serine residues in p47phox-AIR (19, 22–24).
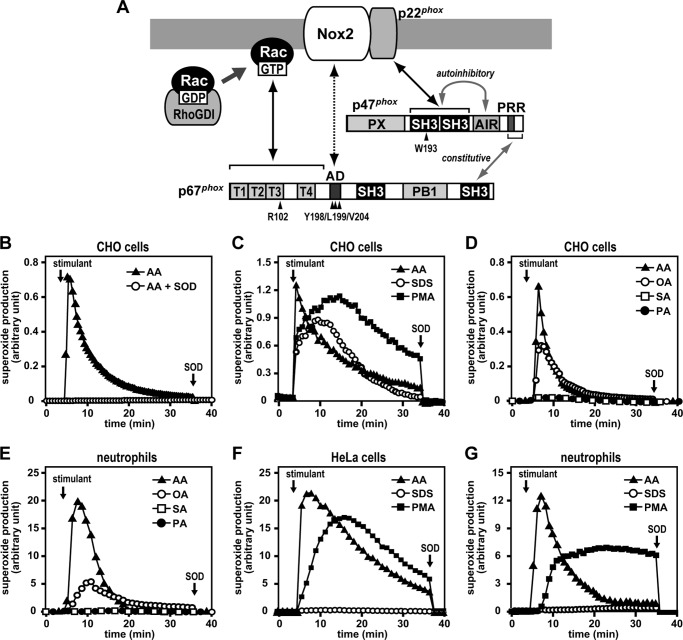
FIGURE 1.
AA- and SDS-induced superoxide production by the Nox2-based NADPH oxidase. A, assembly of the Nox2-based NADPH oxidase. p47phox comprises a phox-homology (PX) domain, two SH3 domains, an AIR, and a PRR. The cytosolic protein p47phox constitutively associates with p67phox via a tail-to-tail interaction between the p47phox PRR and the p67phox C-terminal SH3 domain. The bis-SH3 domain of p47phox is normally masked with an AIR and becomes exposed upon cell stimulation to interact with the membrane protein p22phox. The cytosolic oxidase-activating protein p67phox harbors an N-terminal domain composed of four TPR motifs, followed by an activation domain (AD), two SH3 domains, and a PB1 domain. The TPR domain binds directly to Rac-GTP. Rac localizes to the cytosol in resting cells as a heterodimer with RhoGDI and translocates upon cell stimulation to the membrane to undergo GTP exchange for GDP. The activation domain of p67phox is considered to interact directly with Nox2. Amino acid residues mutated in this study are indicated by arrowheads. B–G, superoxide production by the Nox2-based oxidase reconstituted in CHO or HeLa cells or by the neutrophil NADPH oxidase Nox2. CHO cells (B–D) or HeLa cells (F) were transfected simultaneously with pcDNA3-Nox2, pEF-BOS-p22phox, pEF-BOS-FLAG-p47phox, and pEF-BOS-Myc-p67phox (B–D) or with pcDNA3-Nox2, pEF-BOS-FLAG-p47phox, pEF-BOS-Myc-p67phox, and pEF-BOS-Myc-Rac1 (Q61L) (F), as described under “Experimental Procedures.” Transfected CHO cells were stimulated with AA (50 μm) in the presence or absence of superoxide dismutase (B); with AA (50 μm), SDS (100 μm), or PMA (200 ng/ml) (C); or with 20 μm AA, oleic acid (OA), stearic acid (SA), or palmitic acid (PA) (D). Human neutrophils were stimulated with 20 μm AA, oleic acid, stearic acid, or palmitic acid (E) or with AA (50 μm), SDS (100 μm), or PMA (200 ng/ml) (G). F, transfected HeLa cells were stimulated with AA (50 μm), SDS (100 μm), or PMA (200 ng/ml). The chemiluminescence change was monitored continuously with Diogenes. Superoxide dismutase (SOD, 50 μg/ml) was added where indicated. The experiments were repeated more than three times with similar results.
The PKC activator PMA also elicits GDP-to-GTP exchange on Rac in neutrophils, another event required for Nox2 activation (25), although it has remained unclear whether AA triggers the exchange as well. Rac-GTP, but not Rac-GDP, directly interacts with the N-terminal tetratricopeptide repeat (TPR) domain of p67phox (26–28), whose interaction is required for Nox2 activation. C-terminal to the Rac-binding TPR domain, p67phox (composed of 526 amino acid residues) harbors the activation domain (amino acid residues 190–210), which is followed by two SH3 domains and a PB1 domain that intervenes between them (Fig. 1A). The activation domain is crucial for Nox2 activation but does not seem to participate in p67phox interaction with Rac-GTP (29–31). Little is known about the molecular mechanism by which Rac-GTP-bound p67phox activates Nox2, although Rac-GTP is presumed to induce a conformational change of p67phox, which might direct the activation domain toward Nox2 for superoxide production.
The Nox2-based oxidase can be activated in a cell-free system reconstituted with the Nox2/p22phox-abundant phagocyte membrane, p47phox, p67phox, and Rac-GTP. The activation is elicited with an in vitro activator, commonly represented by an anionic amphiphile such as AA or SDS, but not with PMA (32–34). A target of the amphiphiles is p47phox. AA and SDS are each capable of directly disrupting the AIR-mediated inhibitory association in p47phox to render the bis-SH3 domain in a state accessible to p22phox (22). On the other hand, it has remained unclear whether the conformational change of p47phox is enough to activate Nox2 in the presence of Rac-GTP.
In this study, we show that AA and SDS are each able to trigger GDP-to-GTP exchange on Rac in intact cells. These anionic amphiphiles do not affect binding of Rac-GTP to p67phox, but allow the p67phox·Rac-GTP complex to interact directly with Nox2. This interaction and the subsequent superoxide production are impaired by alanine substitution for Tyr-198, Leu-199, and Val-204 in the activation domain of p67phox. Combined with the previous finding that p47phox is a target of amphiphiles (22, 23), the present findings indicate that AA induces the assembly of the productive Nox2 complex by functioning at multiple steps.
EXPERIMENTAL PROCEDURES
Chemicals
AA, oleic acid, stearic acid, and palmitic acid were purchased from Nacalai Tesque. PMA was purchased from Sigma-Aldrich, and GF109203X was obtained from Biomol Research Laboratories. Other chemicals used were of the highest purity commercially available.
Plasmid Construction
The DNA fragments encoding the following human proteins were prepared as described previously (22, 31, 35, 36): full-length p47phox (amino acid residues 1–390), p47-(SH3)2 (amino acid residues 151–286), full-length p67phox (amino acid residues 1–526), p67phox-(1–212), p67phox-(1–212/R102E), Rac1 (Q61L), full-length Nox2 (amino acid residues 1–570), Nox2-C (amino acid residues 384–570), full-length p22phox (amino acid residues 1–195), p22phox-C (amino acid residues 132–195), p22phox-C (P156Q), and the p21 (Cdc42/Rac)-binding domain (PBD) of the protein kinase Pak (amino acid residues 66–147). The cDNA fragment for p47phox-ΔAIR, lacking the region of amino acid residues 286–340, was amplified by PCR using the cDNA for full-length p47phox as a template. The cDNA for the chimeric protein p67phox-Rac1 (Q61L) that comprises p67phox-(1–212), the 30-serine-residue linker, and Rac1 with the Q61L/C189S substitution were generated by PCR. Mutations leading to the indicated amino acid substitutions were introduced by PCR-mediated site-directed mutagenesis. The DNA fragments were ligated to the following expression vectors: pGEX-6P (GE Healthcare Biosciences) or pMAL (New England Biolabs) for expression of proteins fused to GST or maltose-binding protein in Escherichia coli, respectively; pcDNA3 (Invitrogen) for expression of Nox2 in CHO and HeLa cells; and pEF-BOS (37) for expression of HA-, FLAG-, or Myc-tagged proteins in CHO and HeLa cells. All constructs were sequenced for confirmation of their identities.
Activation of the Nox2-based Oxidase in the Whole-cell System
CHO cells were transfected using FuGENE 6 transfection reagent (Roche Applied Science) with the following plasmids: 0.5 μg of pEF-BOS-Myc-p67phox; 0.5 μg of pEF-BOS-FLAG-p47phox or pEF-BOS-FLAG-p47phox-ΔAIR; 1.0 μg of pcDNA3-Nox2; and 0.1 μg of pEF-BOS-p22phox. Because HeLa cells express endogenous p22phox but require expression of Rac1 (Q61L) for PMA-induced Nox2 activation (35, 36), HeLa cells were transfected using Lipofectamine transfection reagent (Invitrogen) with the following plasmids: 0.1 μg of pEF-BOS-Myc-p67phox, 0.1 μg of pEF-BOS-FLAG-p47phox or pEF-BOS-FLAG-p47phox-ΔAIR, 1.0 μg of pcDNA3-Nox2, and 1.5 μg of pEF-BOS-Myc-Rac1 (Q61L). The transfected cells were cultured for 24 h in Ham's F12 medium (CHO cells) or DMEM (HeLa cells) containing 10% fetal bovine serum. For serum starvation, cells were washed with PBS (137 mm NaCl, 2.68 mm KCl, 8.1 mm Na2HPO4, and 1.47 mm KH2PO4 (pH 7.4)), and cultured for 12 h in Ham's F12 medium (CHO cells) or DMEM (HeLa cells) containing 0.1% serum. Cells were harvested by incubation for 2 min at 37 °C with trypsin/EDTA and washed with PBS. Human neutrophils were prepared from fresh venous blood of healthy volunteers by dextran sedimentation, hypotonic lysis, and Conray/Ficoll method as described previously (22, 25). In the preparation, more than 98% of the cells were neutrophils.
CHO cells, HeLa cells, or human neutrophils were suspended in Hepes-buffered saline (120 mm NaCl, 5 mm KCl, 5 mm glucose, 1 mm MgCl2, 1 mm CaCl2, and 17 mm Hepes (pH 7.4)), and preincubated for 30 min at 37 °C. The superoxide-producing activity was determined by superoxide dismutase-inhibitable chemiluminescence with an enhancer-containing luminol-based detection system (Diogenes, National Diagnostics), as described previously (38). After the addition of the enhanced luminol-based substrate, cells were preincubated for 5 min at 37 °C and subsequently stimulated at the same temperature with the indicated concentrations of PMA, AA, oleic acid, stearic acid, palmitic acid, or SDS. The chemiluminescence change was monitored at 37 °C using a luminometer (Auto Lumat LB953, EG&G Berthold).
For estimation of protein levels of FLAG-p47phox, Myc-p67phox, Myc-Rac1, and p22phox, proteins in cell lysates were subjected to SDS-PAGE, transferred to a polyvinylidene difluoride membrane (Millipore), and probed with an anti-FLAG monoclonal antibody (Sigma-Aldrich), an anti-Myc monoclonal antibody (Roche Applied Science), and an anti-p22phox polyclonal antibody (Santa Cruz Biotechnology), respectively. The blots were developed using ECL Plus (GE Healthcare Biosciences) for visualization of the antibodies.
Preparation of Recombinant Proteins
GST- or maltose-binding protein-tagged proteins were expressed in E. coli BL21 (Stratagene) and purified by glutathione-Sepharose-4B (GE Healthcare Biosciences) or amylose resin (New England Biolabs), respectively, according to the protocols of the manufacturers. For purification of recombinant Rac1 (Q61L) (the GTP-bound, active form of Rac1 carrying the Q61L/C189S substitution), full-length p67phox, p67phox-(1–212), p67phox–Rac1 (Q61L), and p67phox (Y198A/L199A/V204A)-Rac1 (Q61L), GST-tagged proteins were applied to glutathione-Sepharose-4B beads, and bound proteins were eluted from the beads by cleavage with PreScission protease (GE Healthcare Biosciences) according to the protocol of the manufacturer. Proteins were analyzed by SDS-PAGE, followed by staining with Coomassie Brilliant Blue (CBB).
Cell-free Activation of the Phagocyte Oxidase Nox2
The membrane fraction of human neutrophils was prepared as described previously (31, 35, 36). The membranes (3 μg/ml) were mixed with 100 nm wild-type or mutant p47phox, 100 nm p67phox, and 100 nm Rac1 (Q61L) in 100 mm potassium phosphate (pH 7.0), containing 75 μm cytochrome c, 15 μm FAD, 1.0 mm MgCl2, 1.0 mm EGTA, and 1.0 mm NaN3. After incubation for 2.5 min at 25 °C with the indicated concentration of AA or SDS, the reaction was initiated by addition of 1.0 mm NADPH. The NADPH-dependent superoxide-producing activity was measured by determining the rate of superoxide dismutase-inhibitable ferricytochrome c reduction at 550–540 nm using a Hitachi 557 dual wavelength spectrophotometer. The superoxide-producing activity was represented as moles of superoxide produced per second per mole of cytochrome b558 heme.
An in Vitro Binding Assay Using Purified Proteins
For in vitro pull-down assays for p47phox binding to p22phox, 20 μg of GST alone or GST-p47phox and 30 μg of maltose-binding protein-p22phox-C were incubated for 30 min at 4 °C in 300 μl of 100 mm potassium phosphate (pH 7.0). A slurry of glutathione-Sepharose 4B beads was subsequently added, followed by further incubation for 30 min at 4 °C. After washing three times with the buffer above containing 0.5% Triton X-100, the proteins were eluted from the beads with 10 mm glutathione in 200 mm NaCl and 200 mm Tris-HCl (pH 8.0), containing 0.1% Triton X-100. The eluate was subjected to SDS-PAGE, followed by staining with CBB.
In vitro binding of Rac to p67phox was performed as described previously (31, 36). Briefly, 20 μg of GST alone or GST-p67phox-(1–212) with or without the R102E substitution was incubated for 15 min at 4 °C with 30 μg of Rac1 (Q61L) in 400 μl of 100 mm KCl, 100 mm potassium phosphate (pH 7.0) containing 0.005% Triton X-100. For in vitro interaction of p67phox-Rac (Q61L) to Nox2-C, 50 μg of p67phox-Rac (Q61L) and 20 μg of GST-Nox2-C (384–570) were incubated in 200 μl of 100 mm KCl and 100 mm potassium phosphate (pH 7.0) containing 0.005% Triton X-100. A slurry of glutathione-Sepharose 4B beads was added to the incubation mixture, followed by further incubation for 30 min at 4 °C. After washing four times with the buffer above, proteins were eluted from the beads with 20 mm glutathione in 200 mm NaCl and 200 mm Tris-HCl (pH 8.0), containing 0.1% Triton X-100. The eluate was subjected to SDS-PAGE, followed by staining with CBB or by immunoblot analysis with an anti-Rac monoclonal antibody (BD Biosciences).
Estimation of Rac Activation in Intact Cells
Rac activation in intact cells was estimated as described previously (25, 39). Briefly, HeLa cells, CHO cells, or human neutrophils were broken by the addition of the same volume of a lysis buffer (20 mm Tris (pH 7.5), 150 mm NaCl, 0.5% Triton X-100, 2 mm MgCl2, and 5 mm EGTA containing 1% (v/v) protease inhibitor mixture (Sigma-Aldrich)). The lysate was centrifuged for 20 s at 12,000 × g, and the supernatant was incubated for 15 min with GST-Pak-PBD. Proteins were precipitated with glutathione-Sepharose-4B (GE Healthcare Biosciences), and the precipitants were analyzed by immunoblotting with the anti-Rac antibody.
RESULTS
AA Activates Nox2 in a Whole-cell System
It is well known that neutrophils produce superoxide in response to AA (8, 9), an anionic amphiphile that is also capable of activating the phagocyte oxidase Nox2 in vitro (32–34). On the other hand, the PKC activator PMA elicits superoxide production by intact cells but is unable to activate the oxidase in a cell-free system. To know the mechanism for oxidase activation by AA at the cellular level, we reconstituted the Nox2-based oxidase in CHO cells by ectopically expressing Nox2, p22phox, p47phox, and p67phox and tested the effect of AA on superoxide production by these cells. In the whole-cell system, Nox2 was rapidly activated to produce superoxide in response to AA (Fig. 1B) as well as PMA (Fig. 1C). SDS, another anionic amphiphile that can activate the phagocyte oxidase in a cell-free system (33, 34), also triggered superoxide production in Nox2-expressing CHO cells (Fig. 1C). Superoxide production induced by AA terminated earlier than that induced by other stimulants, which seems to result from the fact that exogenous AA is incorporated immediately into membrane phospholipids (40). It is known that PMA is not rapidly metabolized, and, therefore, its action is sustained, leading to prolonged activation of Nox2. By contrast, the endogenous PMA analog diacylglycerol acts transiently because of its fast metabolism (41). Nox2 has been shown to be activated by cis-unsaturated fatty acids but not by trans-unsaturated or saturated ones (8, 42). Indeed, oleic acid activated the Nox2-based oxidase reconstituted in CHO cells (Fig. 1D) and the neutrophil oxidase Nox2 (Fig. 1E), albeit to a lesser extent than that by AA. On the other hand, Nox2 activation did not occur upon neutrophil stimulation with stearic acid or palmitic acid (Fig. 1E). These saturated fatty acids also failed to activate the Nox2-based oxidase reconstituted in CHO cells (Fig. 1D). We also used HeLa cells for analysis of in vivo activation of Nox2. As shown in Fig. 1F, the addition of AA to HeLa cells resulted in superoxide production to the same extent as that induced by stimulation with PMA. Thus AA effectively activates the Nox2-based oxidase in whole-cell reconstituted systems. On the other hand, SDS only marginally activated Nox2 in HeLa cells (Fig. 1F) as well as in neutrophils (Fig. 1G).
AA Induces GDP-to-GTP Exchange on Rac in Intact Cells
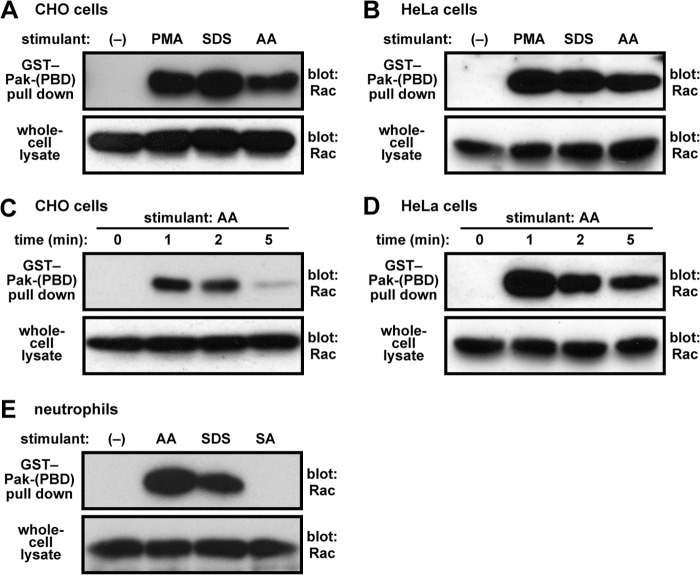
Superoxide production by Nox2 is triggered by turning on the following two switches simultaneously: p47phox conformational change and GDP-to-GTP exchange on Rac (1–5). Although AA is known to induce the conformational change of p47phox (22, 23), the role for AA on Rac activation remains to be elucidated. To test the possibility that AA elicits conversion of Rac to the GTP-bound, active form at the cellular level, we performed a pull-down assay using GST-Pak-PBD (the Cdc42/Rac-binding domain of the protein kinase Pak), followed by immunoblot analysis with the anti-Rac antibody (25, 39). As we have shown previously in human neutrophils (25), PMA induced formation of Rac-GTP in CHO cells (Fig. 2A) and in HeLa cells (Fig. 2B). In both cell types, treatment with AA culminated in a rapid exchange of GTP for GDP on Rac (Fig. 2, C and D). In addition, AA also induced formation of Rac-GTP in human neutrophils, although the formation was not observed in response to the poor oxidase activator stearic acid (Fig. 2E). Furthermore, cell treatment with SDS led to the activation of Rac (Fig. 2, A, B, and E). These findings indicate that AA as well as SDS elicit GDP-to-GTP exchange on Rac, one of the two switches to be turned on for Nox2 activation.
FIGURE 2.
Formation of GTP-bound Rac in cells stimulated with AA or SDS. CHO cells (A and C), HeLa cells (B and D), or human neutrophils (E) were stimulated for 2 min (A, B, and E) or for the indicated times (C and D) with 50 μm AA, 100 μm SDS, 50 μm stearic acid (SA), or 200 ng/ml PMA, followed by cell lysis. Proteins in the lysate were mixed with GST-Pak-PBD and pulled down by glutathione-Sepharose-4B beads. Rac in the precipitant or the whole-cell lysate was estimated by immunoblot analysis with the anti-Rac antibody. The experiments were repeated more than three times with similar results.
Deletion of p47phox-AIR Facilitates Nox2 Activation in a Whole-cell System
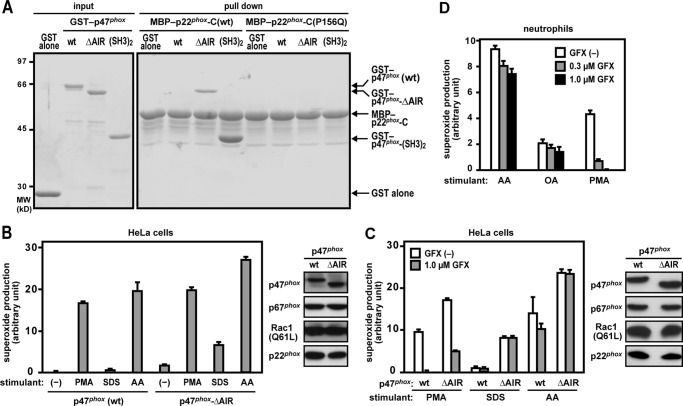
In the resting state, p47phox is folded so that the bis-SH3 domain is inaccessible to its target p22phox-PRR because of the intramolecular interaction with the AIR (19–22) (Fig. 1A). Indeed, a p47phox protein lacking the AIR (p47phox-ΔAIR) did interact with p22phox-PRR under conditions where full-length p47phox was incapable of binding to p22phox (Fig. 3A), suggesting that p47phox-ΔAIR likely serves as an active form. Expression of both the active form of p47phox and the constitutively active, GTP-bound Rac1 (Q61L) is expected to induce Nox2-catalyzed superoxide production even without cell stimulants such as PMA and AA, given that turning on the two switches (the conformational change of p47phox and formation of GTP-liganded Rac) is sufficient to activate the Nox2-based oxidase. Contrary to expectations, simultaneous expression of p47phox-ΔAIR and Rac1 (Q61L) in HeLa cells did not result in superoxide production by Nox2 (Fig. 3B). The finding suggests that turning on the two switches of p47phox and Rac is not sufficient to activate the Nox2-based oxidase in vivo. On the other hand, when HeLa cells were stimulated with SDS, p47phox-ΔAIR was much more effective in activating Nox2 than wild-type p47phox (Fig. 3, B and C), supporting the idea that p47phox-ΔAIR functions as an active form. The finding also suggests that SDS functions as a poor activator of p47phox. On the other hand, when Nox2 was reconstituted with wild-type p47phox in CHO cells, SDS induced superoxide production to almost the same extent as AA and PMA (Fig. 1C), which implies the possibility that p47phox may be more easily activated in CHO cells (see “Discussion”). In addition, although GF109203X, a potent inhibitor of PKC, blocked PMA-induced superoxide production, the inhibitor did not affect Nox2 activation elicited by AA, oleic acid, or SDS (Fig. 3, C and D), suggesting that these anionic amphiphiles function independently of PKC, possibly in a more direct manner.
FIGURE 3.
Role for the p47phox AIR in interaction with p22phox and Nox2 activation. A, effect of p47phox-AIR truncation on binding to p22phox. GST-p47phox (WT), GST-p47phox-ΔAIR, GST-p47phox-(SH3)2 (the bis-SH3 domain, amino acid residues 154–292), or GST alone was incubated with GST-p22phox-C (WT) or GST-p22phox-C (P156Q) and pulled down with amylose resin. The precipitated proteins were applied to SDS-PAGE, followed by staining with CBB, as described under “Experimental Procedures.” Positions for marker proteins are indicated in kilodaltons. The data are representative of results from four independent experiments. B–D, superoxide production by the Nox2-based oxidase reconstituted in HeLa cells or by Nox2 in human neutrophils. HeLa cells were transfected simultaneously with pcDNA3-Nox2, pEF-BOS-p22phox, pEF-BOS-Myc-p67phox, pEF-BOS-Myc-Rac1 (Q61L), and pEF-BOS-FLAG-p47phox (WT) or pEF-BOS-FLAG-p47phox-ΔAIR. After preincubation in the presence (C and D) or absence (B–D) of GF109203X (GFX), the cells were stimulated with AA (50 μm), SDS (100 μm), or PMA (200 ng/ml). The chemiluminescence change was monitored continuously with Diogenes. Each graph represents the mean ± S.D. of the chemiluminescence values integrated for 10 min, which were obtained from three independent transfections. Protein levels of p47phox, p67phox, Rac1, and p22phox were analyzed by immunoblotting as described under “Experimental Procedures.”
Deletion of p47phox-AIR Facilitates Nox2 Activation in a Cell-free System
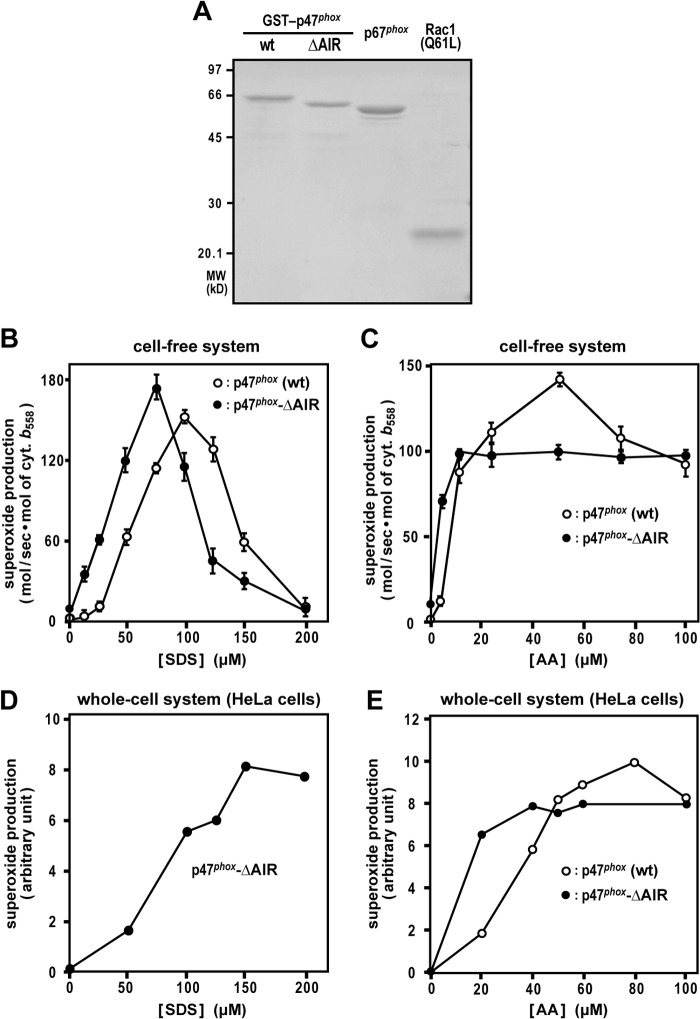
We next studied the effect of the active form of p47phox in a cell-free system for activation of the phagocyte NADPH oxidase. The system was reconstituted with the recombinant proteins p47phox, p67phox, and Rac1 (Q61L) (Fig. 4A) and human neutrophil membranes, in which the Nox2-p22phox heterodimer is highly enriched (35, 36). As shown in Fig. 4B, in the presence of full-length p47phox, the addition of SDS led to superoxide production with maximal activity at a concentration of 100 μm. When p47phox-ΔAIR was used instead of the full-length protein, Nox2 was activated at lower concentrations of SDS (Fig. 4B), indicating that p47phox-ΔAIR also serves as an active form in vitro. In addition, p47phox-ΔAIR supported AA-induced superoxide production at lower concentrations compared with full-length p47phox. At 5 μm AA, the superoxide-producing activity by p47phox-ΔAIR was several times higher than that of the full-length protein, although full-length p47phox was more active than p47phox-ΔAIR at 50 μm AA (Fig. 4C). The concentrations of SDS and AA for maximal activation of Nox2 in HeLa cells (Fig. 4, D and E) were higher than those in the cell-free system (Fig. 4, B and C). In both the whole-cell and cell-free systems, AA fully functioned at concentrations lower than its critical micellar concentration of 73 μm (43), indicating that Nox2 activation by AA is not due to a detergent effect. It should be noted that, even in the presence of both p47phox-ΔAIR and Rac1 (Q61L), only a marginal superoxide-producing activity was detected without stimulation with AA or SDS (Fig. 4, B and C), suggesting that the two switches of p47phox and Rac are insufficient to elicit Nox2 activation in vitro. Taken together with a similar insufficiency in vivo (Fig. 3), it seems likely that a third switch localizes downstream of the formation of activated p47phox or GTP-bound Rac and is required to be turned on for activation of the Nox2-based phagocyte oxidase.
FIGURE 4.
Effect of p47phox-AIR truncation on cell-free and whole-cell activation of the phagocyte oxidase Nox2. A, SDS-PAGE analysis of proteins used in a cell-free activation system for the Nox2-based NADPH oxidase. Purified GST-p47phox (WT), GST-p47phox-ΔAIR, full-length p67phox, and Rac1 (Q61L) were subjected to SDS-PAGE, followed by staining with CBB. Positions for marker proteins are indicated in kilodaltons. B and C, role for p47phox-AIR in a cell-free activation system of the phagocyte oxidase Nox2. p67phox, Rac1 (Q61L), and GST-p47phox (WT) or GST-p47phox-ΔAIR were used for a cell-free system activated with SDS (B) or AA (C) at the indicated concentrations, and the NADPH-dependent superoxide-producing activity was represented by the rate of superoxide dismutase-inhibitable cytochrome c reduction, as described under “Experimental Procedures.” Data are mean ± S.D. from three independent experiments. D and E, HeLa cells were transfected simultaneously with pcDNA3-Nox2, pEF-BOS-p22phox, pEF-BOS-Myc-p67phox, pEF-BOS-Myc-Rac1 (Q61L), and pEF-BOS-FLAG-p47phox (WT), or pEF-BOS-FLAG-p47phox-ΔAIR. The cells were stimulated with the indicated concentration of SDS (D) or AA (E). The chemiluminescence change was monitored continuously with Diogenes. Each graph represents the mean of the chemiluminescence values integrated for 10 min, which were obtained from two independent transfections.
AA Does Not Affect Rac Binding to p67phox
To explore the third switch for Nox2 activation, we focused on steps after formation of Rac-GTP. The GTP-bound, active Rac is known to directly interact with the N-terminal domain of p67phox, comprising four TPR motifs (26–28, Fig. 1A). This interaction is essential for Nox2 activation (26, 44), although Rac-GTP binds to p67phox with a low affinity. Using purified Rac1 (Q61L) and GST-p67phox-(1–212) (Fig. 5A), we performed a GST pull-down assay followed by immunoblot analysis for detecting low-affinity proteins. As shown in Fig. 5B, Rac1 (Q61L) interacted with GST-p67phox-(1–212) in the absence of an anionic amphiphile. This interaction was abrogated by substitution of Glu for Arg-102 in the Rac-binding TPR domain of p67phox, a mutation that completely impairs activation of the Nox2-based oxidase (26, 44). The addition of AA did not affect the interaction between Rac1 (Q61L) and GST-p67phox-(1–212) (Fig. 5B). Similarly, the interaction was not enhanced or disrupted by the addition of SDS (Fig. 5C). These findings indicate that binding of Rac-GTP to p67phox does not function as a switch for activating Nox2, at least in response to anionic amphiphiles such as AA and SDS.
FIGURE 5.
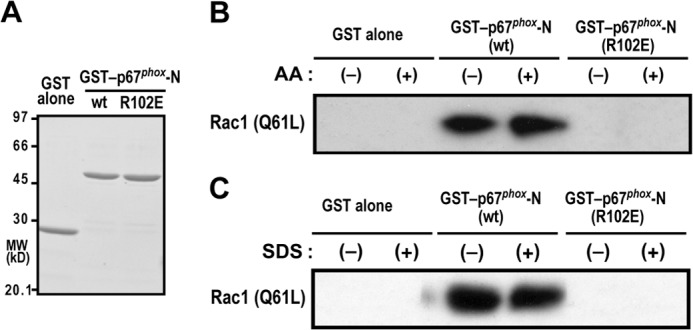
Effect of AA and SDS on the interaction between p67phox and Rac-GTP. A, SDS-PAGE analysis of purified GST fusion proteins that were used in a GST pulldown assay for interaction with Rac. GST alone, GST-fused wild-type (WT) p67phox-(1–212), and GST-p67phox-(1–212/R102E), a mutant protein carrying the R102E substitution, were subjected to SDS-PAGE, followed by staining with CBB. Positions for marker proteins are indicated in kilodaltons. MW, molecular weight. B and C, effect of AA and SDS on the interaction of GST-p67phox and Rac1 (Q61L). GST-p67phox-(1–212) (WT) or GST-p67phox-(1–212/R102E) was incubated with Rac1 (Q61L) in the presence or absence of 50 μm AA (B) or 100 μm SDS (C) and pulled down with glutathione-Sepharose-4B beads. The precipitated proteins were analyzed by immunoblotting with the anti-Rac antibody as described under “Experimental Procedures.” Data are representative of results from four independent experiments.
AA Induces Assembly of Nox2 with the p67phox-Rac Complex
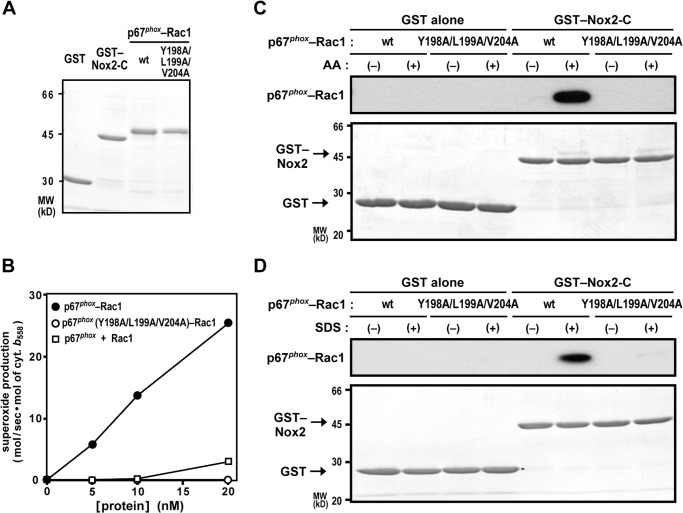
To investigate whether the p67phox·Rac-GTP complex directly interacts with Nox2, we prepared a chimeric protein comprising the N-terminal region of p67phox (amino acid residues 1–212) and Rac1 (Q61L) (Fig. 6A). The N-terminal region of p67phox is known to be as effective as full-length p67phox in activating the Nox2-based oxidase in the presence of Rac1-GTP and p47phox in a cell-free system (22, 26). The p67phox-Rac1 (Q61L) chimera is used due to the fact that p67phox binds to Rac1 (Q61L) with a low affinity, and thus the chimeric protein can activate Nox2 at lower concentrations than those used when p67phox-(1–212) and Rac1 (Q61L) were added separately (45). As shown in Fig. 6B, the present p67phox-Rac1 (Q61L) chimeric protein supported superoxide production by Nox2 in a cell-free system much more effectively than separated p67phox-(1–212) and Rac1 (Q61L). A triple alanine substitution for Tyr-198, Leu-199, and Val-204 in the activation domain of the p67phox moiety resulted in a complete loss of activation of the Nox2-based oxidase (Fig. 6B). This finding is consistent with the previous observation, using separated proteins, that these amino acid residues are not involved in binding to Rac-GTP but required for Nox2 activation both in vivo and in vitro (31).
FIGURE 6.
Effect of AA and SDS on the interaction of the p67phox·Rac-GTP complex with Nox2. A, SDS-PAGE analysis of purified proteins used in this study. GST alone, GST-Nox2-C, the wild-type p67phox (1–212)-Rac1 (Q61L) chimera, and a mutant p67phox-Rac1 (Q61L) chimera carrying the Y198A/L199A/V204A substitution in the activation domain of the p67phox moiety were subjected to SDS-PAGE, followed by staining with CBB. Positions for marker proteins are indicated in kilodaltons. MW, molecular weight. B, effect of the Y198A/L199A/V204A substitution in the activation domain of p67phox on cell-free activation of the phagocyte oxidase Nox2. The wild-type p67phox-Rac1 (Q61L), p67phox (Y198A/L199A/V204A)-Rac1, or the isolated p67phox (WT) and isolated Rac1 (Q61L) was used with p47phox for a cell-free system activated with 50 μm AA, and the NADPH-dependent superoxide-producing activity was represented by the rate of superoxide dismutase-inhibitable cytochrome c reduction, as described under “Experimental Procedures.” Data are mean from three independent experiments. C and D, effect of AA and SDS on the interaction of the p67phox-Rac1 complex with Nox2-C. GST-Nox2-C or GST alone was incubated with the wild-type p67phox-Rac1 or p67phox (Y198A/L199A/V204A)-Rac1 in the presence or absence of 50 μm AA (C) or 100 μm SDS (D) and pulled down with glutathione-Sepharose-4B beads. The precipitated proteins were subjected to SDS-PAGE, followed by staining with CBB (bottom panels) and by immunoblot analysis with the anti-Rac antibody (top panels), as described under “Experimental Procedures.” The data are representative of results from four independent experiments. Positions for marker proteins are indicated in kilodaltons.
We next examined whether the chimeric protein p67phox-Rac1 (Q61L) is capable of directly interacting with Nox2 using purified proteins (Fig. 6A). Because Nox2 in the resting state is unable to bind to the substrate NAPDH and, therefore, Nox2 activation likely involves the increase in affinity for NADPH (1–5), we tested the NADPH-binding region that exists in the Nox2 C terminus (Nox2-C, amino acid residues 384–570) as a target of p67phox-Rac1 (Q61L). In the absence of in vitro Nox2-activating reagents such as AA and SDS, the p67phox-Rac1 (Q61L) chimera failed to associate with Nox2-C. In contrast, the addition of AA culminated in a direct interaction of p67phox-Rac1 (Q61L) with Nox2-C (Fig. 6C). This interaction was completely impaired by the Y198A/L199A/V204A substitution in the p67phox moiety, indicative of a crucial role for the activation domain. In addition, SDS also induced p67phox-Rac1 (Q61L) binding to Nox2-C in a manner dependent on the activation domain of p67phox (Fig. 6D). Therefore, p67phox, in complex with Rac-GTP, appears to interact with Nox2 via the activation domain, whose interaction is induced by the Nox2-activating anionic amphiphiles AA and SDS.
DISCUSSION
The phagocyte oxidase Nox2, heterodimerized with p22phox, becomes activated via its assembly with p47phox, p67phox, and Rac-GTP. The assembly requires two essential events: a conformational change of p47phox for its binding to p22phox and formation of GTP-liganded Rac for its association with p67phox (1–5). AA, a potent activator of the phagocyte oxidase Nox2 both in vivo and in vitro, is known to induce one of the two events, i.e. the conformational change of p47phox (22, 23). The change leads to the SH3-mediated binding of p47phox to p22phox, which is crucial for assembly of the Nox2-p22phox heterodimer at the membrane with the cytosolic activating proteins p47phox and p67phox. p67phox is recruited via a constitutive tail-to-tail interaction with p47phox (1–5) (Fig. 1A). On the other hand, a more detailed molecular mechanism for AA-mediated Nox2 activation remains to be elucidated. Here we demonstrate that AA as well as SDS, another amphiphile activator of Nox2, elicits GTP exchange for GDP on Rac at the cellular level (Fig. 2).
In resting cells, Rac localizes to the cytosol as a heterodimer with Rho GDP dissociation inhibitor (RhoGDI), Rac being in the GDP-bound form. Upon cell stimulation, Rac translocates to the membrane in a manner independent of p47phox and p67phox (1–5). It has been reported that cell treatment with AA or SDS leads to membrane translocation of Rac (46–49) and that AA induces Rac-mediated processes in fibroblasts, although formation of Rac-GTP is not directly estimated (50). Rac activation involves dissociation of Rac-GDP from RhoGDI and GTP exchange for GDP on Rac, which is facilitated by guanine nucleotide exchange factors for Rac (51, 52). It is known that AA and SDS are each capable of dissociating Rac from RhoGDI in vitro (45, 46, 53). The dissociation of Rac and RhoGDI is known to promote Rac activation (51). As shown in the present study, AA by itself induces in vivo formation of GTP-liganded Rac (Fig. 2). This is consistent with the fact that the GTP-bound form of Rac is a prerequisite for Nox2 activation and that the activation can be triggered in vivo by the addition of AA (1–5). Cell treatment with another Nox2-activatable anionic amphiphile, SDS, also leads to GDP/GTP exchange on Rac (Fig. 2). It is known that PKC is known to phosphorylate RhoGDI to accelerate the dissociation of the Rac-RhoGDI complex (51, 52), and, indeed, the PKC activator PMA induces in vivo activation of Rac (Fig. 2) (24). Although AA is capable of activating PKC (54), it is unlikely that the amphiphile functions via PKC in Nox2 activation. This is because AA-induced superoxide production by Nox2 is not affected by GF109203X, a potent inhibitor of PKC (Fig. 3). In addition to AA-induced dissociation of Rac and RhoGDI, AA might further promote Rac activation by directly stimulating the function of Rac guanine nucleotide exchange factors such as Dock2, Vav1, and P-Rex, which are known to contribute to Nox2-catalyzed superoxide production in phagocytes (55–57). The possibility should be addressed in future studies.
In contrast to AA, SDS serves as a very poor stimulant for Nox2 activation in HeLa cells and neutrophils (Fig. 1), although SDS is able to fully induce formation of GTP-bound Rac in these cells (Fig. 2). The precise reason for the difference between SDS and AA at the cellular level remains unclear, but it has been reported that SDS has little effect on p47phox in neutrophils and thus only marginally elicits superoxide production (58). Consistently, the potent Rac activator SDS (Fig. 2) is capable of substantially activating Nox2-based oxidase reconstituted with a constitutively active p47phox (p47phox-ΔAIR) but not with the wild-type protein in HeLa cells (Fig. 3). On the other hand, p47phox may be easily activated in CHO cells (because of an unknown mechanism) because SDS is as active as AA and PMA in activation of the Nox2-based oxidase in CHO cells expressing wild-type p47phox (Fig. 1).
This study also shows that deletion of the p47phox-AIR, responsible for maintaining this protein in an inactive form in resting cells, facilitates superoxide production in cells stimulated with AA, SDS, and PMA (Figs. 3 and 4). Similarly, p47phox-ΔAIR supports cell-free activation of Nox2 at lower concentrations of AA or SDS compared with wild-type p47phox (Fig. 4). However, even when a constitutively active p47phox (p47phox-ΔAIR) and a constitutively active GTP-bound Rac1 (Q61L) are both present, Nox2 activation still requires a cell stimulant, such as AA or PMA, in intact cells (Fig. 3) or an anionic amphiphile, such as AA or SDS, in a cell-free system (Fig. 4). These findings indicate that a heretofore unidentified event serves as the third switch for Nox2 activation. It is well established that Rac-GTP, but not Rac-GDP, interacts with the N-terminal TPR domain of p67phox (26, 27), which plays an essential role in Nox2 activation (26, 39). The interaction occurs in the absence of AA or SDS and is not increased by their presence (Fig. 5). Thus the amphiphile activators of Nox2 do not appear to regulate formation of the binary complex of p67phox and Rac-GTP, an event that immediately follows GDP/GTP exchange on Rac.
The binary complex of p67phox and Rac-GTP has been thought to induce a conformational change of Nox2, leading to superoxide production (59–62). It has been reported previously that Rac directly interacts with the Nox2-p22phox heterodimer via the insert region, a surface-exposed α-helix that is unique to the Rho subfamily among Ras-related small GTPases (63). However, this region appears to be dispensable for Nox2 activation in both cell-free and whole-cell systems (35, 61, 62). On the other hand, it has been considered possible that the activation domain of p67phox participates in interaction with Nox2, although it has remained unclear whether the result of the conformational change in p67phox is to augment the actual binding of p67phox to gp91phox or to endow p67phox with an ability to elicit electron flow in gp91phox. Here we demonstrate that AA and SDS each induce direct binding of Rac-complexed p67phox to the NADPH-binding region of Nox2 (Nox2-C) as the third switch for Nox2 activation (Fig. 6) without affecting p67phox interaction with Rac-GTP (Fig. 5). The role of the Nox2 NADPH-binding region as a target of p67phox-Rac is consistent with a prevailing model hypothesizing that the increase in affinity for NADPH is crucial for Nox2 activation (1–5). Alanine substitution for Tyr-198, Leu-199, and Val-204 in the activation domain of p67phox (amino acid residues 190–210) abrogates both the p67phox-Rac interaction with Nox2 and subsequent Nox2 activation (Fig. 6), suggesting a crucial role for the activation domain. In this context, it should be noted that, although the N terminus of the activation domain is a part of an α-helix (amino acid residues 187–193) in a Rac-free p67phox (28), this domain is flexible or disordered in p67phox complexed with Rac-GTP (27). The Rac-induced flexibility may allow productive interaction of p67phox with Nox2 in the presence of AA. It is also possible that AA may modulate Nox2 conformational states relevant to binding to the p67phox-Rac complex because AA is known to be capable of directly inducing a conformational change of Nox2 (64). Thus the present study shows that AA directly triggers the three crucial events for Nox2 activation: conversion of p47phox into the active conformation; GDP to GTP exchange on Rac; and interaction of the p67phox·Rac-GTP complex with Nox2, i.e. the third switch identified here.
Acknowledgments
We thank Yohko Kage (Kyushu University) and Namiko Kubo (Kyushu University) for technical assistance. We also thank the Research Support Center, Kyushu University Graduate School of Medical Sciences, Fukuoka, Japan for technical support.
This work was supported in part by MEXT (the Ministry of Education, Culture, Sports, Science and Technology) KAKENHI Grant 26111009 and by the Uehara Memorial Foundation.
- Nox
- NADPH oxidase
- AA
- arachidonic acid
- PMA
- phorbol 12-myristate 13-acetate
- PRR
- proline-rich region
- AIR
- autoinhibitory region
- TPR
- tetratricopeptide repeat
- PBD
- p21 (Cdc42/Rac)-binding domain
- CBB
- Coomassie Brilliant Blue.
REFERENCES
- 1. Lambeth J. D. (2004) NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4, 181–189 [DOI] [PubMed] [Google Scholar]
- 2. Nauseef W. M. (2004) Assembly of the phagocyte NADPH oxidase. Histochem. Cell Biol. 122, 277–291 [DOI] [PubMed] [Google Scholar]
- 3. Groemping Y., Rittinger K. (2005) Activation and assembly of the NADPH oxidase: a structural perspective. Biochem. J. 386, 401–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sumimoto H. (2008) Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 275, 3249–3277 [DOI] [PubMed] [Google Scholar]
- 5. Leto T. L., Morand S., Hurt D., Ueyama T. (2009) Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid. Redox Signal. 11, 2607–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heyworth P. G., Cross A. R., Curnutte J. T. (2003) Chronic granulomatous disease. Curr. Opin. Immunol. 15, 578–584 [DOI] [PubMed] [Google Scholar]
- 7. Quinn M. T., Ammons M. C., Deleo F. R. (2006) The expanding role of NADPH oxidases in health and disease: no longer just agents of death and destruction. Clin. Sci. 111, 1–20 [DOI] [PubMed] [Google Scholar]
- 8. Badwey J. A., Curnutte J. T., Karnovsky M. L. (1981) cis-Polyunsaturated fatty acids induce high levels of superoxide production by human neutrophils. J. Biol. Chem. 256, 12640–12643 [PubMed] [Google Scholar]
- 9. Bromberg Y., Pick E. (1983) Unsaturated fatty acids as second messengers of superoxide generation by macrophages. Cell Immunol. 79, 240–252 [DOI] [PubMed] [Google Scholar]
- 10. El Benna J., Faust L. P., Babior B. M. (1994) The phosphorylation of the respiratory burst oxidase component p47phox during neutrophil activation: phosphorylation of sites recognized by protein kinase C and by proline-directed kinases. J. Biol. Chem. 269, 23431–23436 [PubMed] [Google Scholar]
- 11. Inanami O., Johnson J. L., McAdara J. K., Benna J. E., Faust L. R., Newburger P. E., Babior B. M. (1998) Activation of the leukocyte NADPH oxidase by phorbol ester requires the phosphorylation of p47phox on serine 303 or 304. J. Biol. Chem. 273, 9539–9543 [DOI] [PubMed] [Google Scholar]
- 12. Dana R., Leto T. L., Malech H. L., Levy R. (1998) Essential requirement of cytosolic phospholipase A2 for activation of the phagocyte NADPH oxidase. J. Biol. Chem. 273, 441–445 [DOI] [PubMed] [Google Scholar]
- 13. Zhao X., Bey E. A., Wientjes F. B., Cathcart M. K. (2002) Cytosolic phospholipase A2 (cPLA2) regulation of human monocyte NADPH oxidase activity: cPLA2 affects translocation but not phosphorylation of p67phox and p47phox. J. Biol. Chem. 277, 25385–25392 [DOI] [PubMed] [Google Scholar]
- 14. Shmelzer Z., Haddad N., Admon E., Pessach I., Leto T. L., Eitan-Hazan Z., Hershfinkel M., Levy R. (2003) Unique targeting of cytosolic phospholipase A2 to plasma membranes mediated by the NADPH oxidase in phagocytes. J. Cell Biol. 162, 683–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chatterjee S., Feinstein S. I., Dodia C., Sorokina E., Lien Y. C., Nguyen S., Debolt K., Speicher D., Fisher A. B. (2011) Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2 activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages. J. Biol. Chem. 286, 11696–11706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ambruso D. R., Ellison M. A., Thurman G. W., Leto T. L. (2012) Peroxiredoxin 6 translocates to the plasma membrane during neutrophil activation and is required for optimal NADPH oxidase activity. Biochim. Biophys. Acta 1823, 306–315 [DOI] [PubMed] [Google Scholar]
- 17. Sumimoto H., Kage Y., Nunoi H., Sasaki H., Nose T., Fukumaki Y., Ohno M., Minakami S., Takeshige K. (1994) Role of Src homology 3 domains in assembly and activation of the phagocyte NADPH oxidase. Proc. Natl. Acad. Sci. U.S.A. 91, 5345–5349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leto T. L., Adams A. G., de Mendez I. (1994) Assembly of the phagocyte NADPH oxidase: binding of Src homology 3 domains to proline-rich targets. Proc. Natl. Acad. Sci. U.S.A. 91, 10650–10654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ago T., Nunoi H., Ito T., Sumimoto H. (1999) Mechanism for phosphorylation-induced activation of the phagocyte NADPH oxidase protein p47phox: triple replacement of serines 303, 304, and 328 with aspartates disrupts the SH3 domain-mediated intramolecular interaction in p47phox, thereby activating the oxidase. J. Biol. Chem. 274, 33644–33653 [DOI] [PubMed] [Google Scholar]
- 20. Groemping Y., Lapouge K., Smerdon S. J., Rittinger K. (2003) Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell 113, 343–355 [DOI] [PubMed] [Google Scholar]
- 21. Yuzawa S., Suzuki N. N., Fujioka Y., Ogura K., Sumimoto H., Inagaki F. (2004) A molecular mechanism for autoinhibition of the tandem SH3 domains of p47phox, the regulatory subunit of the phagocyte NADPH oxidase. Genes Cells 9, 443–456 [DOI] [PubMed] [Google Scholar]
- 22. Shiose A., Sumimoto H. (2000) Arachidonic acid and phosphorylation synergistically induce a conformational change of p47phox to activate the phagocyte NADPH oxidase. J. Biol. Chem. 275, 13793–13801 [DOI] [PubMed] [Google Scholar]
- 23. Swain S. D., Helgerson S. L., Davis A. R., Nelson L. K., Quinn M. T. (1997) Analysis of activation-induced conformational changes in p47phox using tryptophan fluorescence spectroscopy. J. Biol. Chem. 272, 29502–29510 [DOI] [PubMed] [Google Scholar]
- 24. Ago T., Kuribayashi F., Hiroaki H., Takeya R., Ito T., Kohda D., Sumimoto H. (2003) Phosphorylation of p47phox directs phox homology domain from SH3 domain toward phosphoinositides, leading to phagocyte NADPH oxidase activation. Proc. Natl. Acad. Sci. U.S.A. 100, 4474–4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Akasaki T., Koga H., Sumimoto H. (1999) Phosphoinositide 3-kinase-dependent and -independent activation of the small GTPase Rac2 in human neutrophils. J. Biol. Chem. 274, 18055–18059 [DOI] [PubMed] [Google Scholar]
- 26. Koga H., Terasawa H., Nunoi H., Takeshige K., Inagaki F., Sumimoto H. (1999) Tetratricopeptide repeat (TPR) motifs of p67phox participate in interaction with the small GTPase Rac and activation of the phagocyte NADPH oxidase. J. Biol. Chem. 274, 25051–25060 [DOI] [PubMed] [Google Scholar]
- 27. Lapouge K., Smith S. J., Walker P. A., Gamblin S. J., Smerdon S. J., Rittinger K. (2000) Structure of the TPR domain of p67phox in complex with RacGTP. Mol. Cell 6, 899–907 [DOI] [PubMed] [Google Scholar]
- 28. Grizot S., Fieschi F., Dagher M. C., Pebay-Peyroula E. (2001) The active N-terminal region of p67phox: structure at 1.8 Å resolution and biochemical characterizations of the A128V mutant implicated in chronic granulomatous disease. J. Biol. Chem. 276, 21627–21631 [DOI] [PubMed] [Google Scholar]
- 29. Hata K., Takeshige K., Sumimoto H. (1997) Roles for proline-rich regions of p47phox and p67phox in the phagocyte NADPH oxidase activation in vitro. Biochem. Biophys. Res. Commun. 241, 226–231 [DOI] [PubMed] [Google Scholar]
- 30. Han C.-H., Freeman J. L., Lee T., Motalebi S. A., Lambeth J. D. (1998) Regulation of the neutrophil respiratory burst oxidase: identification of an activation domain in p67phox. J. Biol. Chem. 273, 16663–16668 [DOI] [PubMed] [Google Scholar]
- 31. Maehara Y., Miyano K., Yuzawa S., Akimoto R., Takeya R., Sumimoto H. (2010) A conserved region between the TPR and activation domains of p67phox participates in activation of the phagocyte NADPH oxidase. J. Biol. Chem. 285, 31435–31445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bromberg Y., Pick E. (1984) Unsaturated fatty acids stimulate NADPH-dependent superoxide production by cell-free system derived from macrophages. Cell Immunol. 88, 213–221 [DOI] [PubMed] [Google Scholar]
- 33. Bromberg Y., Pick E. (1985) Activation of NADPH-dependent superoxide production in a cell-free system by sodium dodecyl sulfate. J. Biol. Chem. 260, 13539–13545 [PubMed] [Google Scholar]
- 34. Pick E. (2014) Cell-free NADPH oxidase activation assays: “in vitro veritas.” Methods Mol. Biol. 1124, 339–403 [DOI] [PubMed] [Google Scholar]
- 35. Miyano K., Koga H., Minakami R., Sumimoto H. (2009) The insert region of the Rac GTPases is dispensable for activation of superoxide-producing NADPH oxidases. Biochem. J. 422, 373–382 [DOI] [PubMed] [Google Scholar]
- 36. Miyano K., Sumimoto H. (2012) Assessment of the role for Rho family GTPases in NADPH oxidase activation. Methods Mol. Biol. 827, 195–212 [DOI] [PubMed] [Google Scholar]
- 37. Mizushima S., Nagata S. (1990) pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 18, 5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takeya R., Ueno N., Sumimoto H. (2006) Regulation of superoxide-producing NADPH oxidases in nonphagocytic cells. Methods Enzymol. 406, 456–468 [DOI] [PubMed] [Google Scholar]
- 39. Miyano K., Ueno N., Takeya R., Sumimoto H. (2006) Direct involvement of the small GTPase Rac in activation of the superoxide-producing NADPH oxidase Nox1. J. Biol. Chem. 281, 21857–21868 [DOI] [PubMed] [Google Scholar]
- 40. Pérez-Chacón G., Astudillo A. M., Balgoma D., Balboa M. A., Balsinde J. (2009) Control of free arachidonic acid levels by phospholipases A2 and lysophospholipid acyltransferases. Biochim. Biophys. Acta 1791, 1103–1113 [DOI] [PubMed] [Google Scholar]
- 41. Griner E. M., Kazanietz M. G. (2007) Protein kinase C and other diacylglycerol effectors in cancer. Nat. Rev. Cancer 7, 281–294 [DOI] [PubMed] [Google Scholar]
- 42. Souabni H., Thoma V., Bizouarn T., Chatgilialoglu C., Siafaka-Kapadai A., Baciou L., Ferreri C., Houée-Levin C., Ostuni M. A. (2012) trans Arachidonic acid isomers inhibit NADPH-oxidase activity by direct interaction with enzyme components. Biochim. Biophys. Acta 1818, 2314–2324 [DOI] [PubMed] [Google Scholar]
- 43. Glick J., Santoyo G., Casey P. J. (1996) Arachidonate and related unsaturated fatty acids selectively inactivate the guanine nucleotide-binding regulatory protein, Gz. J. Biol. Chem. 271, 2949–2954 [DOI] [PubMed] [Google Scholar]
- 44. Ueno N., Takeya R., Miyano K., Kikuchi H., Sumimoto H. (2005) The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: its regulation by oxidase organizers and activators. J. Biol. Chem. 280, 23328–23339 [DOI] [PubMed] [Google Scholar]
- 45. Miyano K., Ogasawara S., Han C.-H., Fukuda H., Tamura M. (2001) A fusion protein between rac and p67phox (1–210) reconstitutes NADPH oxidase with higher activity and stability than the individual components. Biochemistry 40, 14089–14097 [DOI] [PubMed] [Google Scholar]
- 46. Sawai T., Asada M., Nunoi H., Matsuda I., Ando S., Sasaki T., Kaibuchi K., Takai Y., Katayama K. (1993) Combination of arachidonic acid and guanosine 5′-O-(3-thiotriphosphate) induce translocation of rac p21s to membrane and activation of NADPH oxidase in a cell-free system. Biochem. Biophys. Res. Commun. 195, 264–269 [DOI] [PubMed] [Google Scholar]
- 47. Abo A., Webb M. R., Grogan A., Segal A. W. (1994) Activation of NADPH oxidase involves the dissociation of p21rac from its inhibitory GDP/GTP exchange protein (rhoGDI) followed by its translocation to the plasma membrane. Biochem. J. 298, 585–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Price M. O., McPhail L. C., Lambeth J. D., Han C. H., Knaus U. G., Dinauer M. C. (2002) Creation of a genetic system for analysis of the phagocyte respiratory burst: high-level reconstitution of the NADPH oxidase in a nonhematopoietic system. Blood 99, 2653–2661 [DOI] [PubMed] [Google Scholar]
- 49. Kim C., Dinauer M. C. (2006) Impaired NADPH oxidase activity in Rac2-deficient murine neutrophils does not result from defective translocation of p47phox and p67phox and can be rescued by exogenous arachidonic acid. J. Leukocyte Biol. 79, 223–234 [DOI] [PubMed] [Google Scholar]
- 50. Shin E. A., Kim K. H., Han S. I., Ha K. S., Kim J. H., Kang K. I., Kim H. D., Kang H. S. (1999) Arachidonic acid induces the activation of the stress-activated protein kinase, membrane ruffling and H2O2 production via a small GTPase Rac1. FEBS Lett. 452, 355–359 [DOI] [PubMed] [Google Scholar]
- 51. DerMardirossian C., Bokoch G. M. (2005) GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 15, 356–363 [DOI] [PubMed] [Google Scholar]
- 52. Garcia-Mata R., Boulter E., Burridge K. (2011) The “invisible hand”: regulation of RHO GTPases by RHOGDIs. Nat. Rev. Mol. Cell Biol. 12, 493–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chuang T. H., Bohl B. P., Bokoch G. M. (1993) Biologically active lipids are regulators of Rac·GDI complexation. J. Biol. Chem. 268, 26206–26211 [PubMed] [Google Scholar]
- 54. Koide H., Ogita K., Kikkawa U., Nishizuka Y. (1992) Isolation and characterization of the ϵ subspecies of protein kinase C from rat brain. Proc. Natl. Acad. Sci. U.S.A. 89, 1149–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kunisaki Y., Nishikimi A., Tanaka Y., Takii R., Noda M., Inayoshi A., Watanabe K., Sanematsu F., Sasazuki T., Sasaki T., Fukui Y. (2006) DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J. Cell Biol. 174, 647–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim C., Marchal C. C., Penninger J., Dinauer M. C. (2003) The hemopoietic Rho/Rac guanine nucleotide exchange factor Vav1 regulates N-formyl-methionyl-leucyl-phenylalanine-activated neutrophil functions. J. Immunol. 171, 4425–4430 [DOI] [PubMed] [Google Scholar]
- 57. Lawson C. D., Donald S., Anderson K. E., Patton D. T., Welch H. C. (2011) P-Rex1 and Vav1 cooperate in the regulation of formyl-methionyl-leucyl-phenylalanine-dependent neutrophil responses. J. Immunol. 186, 1467–1476 [DOI] [PubMed] [Google Scholar]
- 58. Nigorikawa K., Okamura N., Hazeki O. (2004) The effect of anionic amphiphiles on the recruitment of Rac in neutrophils. J. Biochem. 136, 463–470 [DOI] [PubMed] [Google Scholar]
- 59. Nisimoto Y., Motalebi S., Han C.-H., Lambeth J. D. (1999) The p67phox activation domain regulates electron flow from NADPH to flavin in flavocytochrome b558. J. Biol. Chem. 274, 22999–23005 [DOI] [PubMed] [Google Scholar]
- 60. Sarfstein R., Gorzalczany Y., Mizrahi A., Berdichevsky Y., Molshanski-Mor S., Weinbaum C., Hirshberg M., Dagher M. C., Pick E. (2004) Dual role of Rac in the assembly of NADPH oxidase, tethering to the membrane and activation of p67phox: a study based on mutagenesis of p67phox-Rac1 chimeras. J. Biol. Chem. 279, 16007–16016 [DOI] [PubMed] [Google Scholar]
- 61. Berdichevsky Y., Mizrahi A., Ugolev Y., Molshanski-Mor S., Pick E. (2007) Tripartite chimeras comprising functional domains derived from the cytosolic NADPH oxidase components p47phox, p67phox, and Rac1 elicit activator-independent superoxide production by phagocyte membranes: an essential role for anionic membrane phospholipids. J. Biol. Chem. 282, 22122–22139 [DOI] [PubMed] [Google Scholar]
- 62. Mizrahi A., Berdichevsky Y., Casey P. J., Pick E. (2010) A prenylated p47phox-p67phox-Rac1 chimera is a quintessential NADPH oxidase activator: membrane association and functional capacity. J. Biol. Chem. 285, 25485–25499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Diebold B. A., Bokoch G. M. (2001) Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat. Immunol. 2, 211–215 [DOI] [PubMed] [Google Scholar]
- 64. Taylor R. M., Foubert T. R., Burritt J. B., Baniulis D., McPhail L. C., Jesaitis A. J. (2004) Anionic amphiphile and phospholipid-induced conformational changes in human neutrophil flavocytochrome b observed by fluorescence resonance energy transfer. Biochim. Biophys. Acta 1663, 201–213 [DOI] [PubMed] [Google Scholar]