Background: Toll pathway is the innate immune response targeting Plasmodium berghei infection in Anopheles mosquitoes.
Results: Innexin AGAP001476, but not AGAP006241, alters parasite loading in mosquitoes via immune elicitor TEP1.
Conclusion: AGAP001476 influences TEP1-mediated lysis of Plasmodium ookinetes.
Significance: Understanding mosquito immunity against Plasmodium will aid in designing strategies to control malaria transmission.
Keywords: Gap Junction, Host-Pathogen Interaction, Malaria, Microbial Pathogenesis, Plasmodium, Innexin, Transmission Blocking
Abstract
The Toll and IMD pathways are known to be induced upon Plasmodium berghei and Plasmodium falciparum infection, respectively. It is unclear how Plasmodium or other pathogens in the blood meal and their invasion of the midgut epithelium would trigger the innate immune responses in immune cells, in particular hemocytes. Gap junctions, which can mediate both cell-to-cell and cell-to-extracellular communication, may participate in this signal transduction. This study examined whether innexins, gap junction proteins in insects, are involved in anti-Plasmodium responses in Anopheles gambiae. Inhibitor studies using carbenoxolone indicated that blocking innexons resulted in an increase in Plasmodium oocyst number and infection prevalence. This was accompanied by a decline in TEP1 levels in carbenoxolone-treated mosquitoes. Innexin AGAP001476 mRNA levels in midguts were induced during Plasmodium infection and a knockdown of AGAP001476, but not AGAP006241, caused an induction in oocyst number. Silencing AGAP001476 caused a concurrent increase in vitellogenin levels, a TEP1 inhibitor, in addition to a reduced level of TEP1-LRIM1-APL1C complex in hemolymph. Both vitellogenin and TEP1 are regulated by Cactus under the Toll pathway. Simultaneous knockdown of cactus and AGAP001476 failed to reverse the near refractoriness induced by the knockdown of cactus, suggesting that the AGAP001476-mediated anti-Plasmodium response is Cactus-dependent. These data demonstrate a critical role for innexin AGAP001476 in mediating innate immune responses against Plasmodium through Toll pathway in mosquitoes.
Introduction
Malaria, caused by Plasmodium species, is an arthropod-borne disease that results in illness in millions of people and ∼0.66 million deaths each year (1). To control malaria and as part of eradication efforts, researchers are attempting to develop transmission-blocking vaccines or drugs that influence Plasmodium in its arthropod vector. Transmission-blocking agents target the sexual stage of Plasmodium in blood, thereby lowering the proportion of mosquitoes carrying Plasmodium (2), the early stages of Plasmodium development in the mosquito, or the vector itself (3, 4). For instance, bumped kinase inhibitor alters gametocyte exflagellation inside the mosquito hemolymph, thereby interfering with Plasmodium development (5). Due to the long incubation time required for Plasmodium maturation, infected mosquitoes need multiple blood meals between the time of Plasmodium acquisition and subsequent transmission. Mosquitocidal vaccines that kill mosquitoes before Plasmodium develop into infective sporozoites (6) are another strategy for disease prevention.
When Plasmodium gametocytes enter the mosquito midgut following a blood meal, the innate immune response eliminates most Plasmodium parasites (7, 8). Because ookinete migration from hemolymph to midgut epithelium for oocyst formation is a bottleneck in Plasmodium development in the mosquito, it may serve as an intervention target. A number of factors involved in the mosquito anti-Plasmodium response have been identified, including reactive oxygen species generated by microbes in the midgut (9), thioester-containing protein 1 (TEP1)-mediated lysis (10), protein nitration, and midgut permeability to immune elicitors (11). Improving our understanding of anti-Plasmodium immune factors and signaling pathways would help identify targets for transmission intervention in the mosquito vector.
Gap junctions in vertebrates mediate communication between cells of the same or different types and between cells and the extracellular space (12). Innexins are gap junction proteins expressed in invertebrates, and their role in mosquitoes remains largely unexplored. It is possible that innexin-based gap junction channels are involved in the signaling events in anti-Plasmodium responses. There are six predicted innexins in the Anopheles gambiae genome, the major vector for Plasmodium in Africa. In this study, functional studies of innexins during Plasmodium development were performed using carbenoxolone (Cbx),2 a chemical inhibitor of innexin channels, and dsRNA for the knockdown of specific innexin members, to elucidate the role of communicating junctions in Plasmodium survival in A. gambiae.
EXPERIMENTAL PROCEDURES
Ethics Statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The use of animals for the experiments reported herein was approved by the Institutional Animal Care and Use Committee of Yale University with protocol number 2011-07941.
Animals
Swiss-Webster mice were purchased from Charles River Laboratories. Animals were housed and cared for at the Animal Resources Center of Yale University School of Medicine.
Mosquito Rearing and Plasmodium berghei Infection
A. gambiae (4ARR strain) mosquitoes were reared at 27 °C, 80% humidity under a 12-h dark/light cycle at the insectary facility and maintained on 10% w/v sucrose solution during the adult stage (13). 4–6-week-old female Swiss Webster mice were infected with P. berghei (GFP-expressing ANKA strain) via intraperitoneal injection of frozen stock of parasitized blood. Giemsa staining of thin blood smears was performed to validate the parasitemia and presence of gametocytes. Female mosquitoes of about 2–7 days post-emergence were fed on the Plasmodium gametocyte-positive mice. The blood feeding was preceded by starvation of mosquitoes for 12–18 h to improve the overall blood feeding rate. Blood-fed mosquitoes were then kept at 21 °C as high temperatures prohibit Plasmodium early development (14). The Plasmodium infection rate was assessed by counting the number of oocysts in mosquito midguts at 8–10 days post-infection (dpi) under fluorescent stereomicroscope. In the direct feeding assay where the number of oocysts formed would be compared among control and treatment groups, mosquitoes were fed on the same infected host.
Cbx Treatment
Mosquitoes were maintained on 10% sucrose (control group) or 10% sucrose with 500 μm Cbx from 3 days before the Plasmodium-infected blood meal until the dissection of midguts for oocyst examination. In the other treatment group, the Cbx treatment started at 28–32 h post-infection (hpi), at which time Plasmodium ookinetes have evaded the early immune response and reached the midgut epithelium for oocyst development.
RNA Interference
Gene knockdown in mosquitoes was achieved by double-stranded RNA (dsRNA). dsRNAs specifically targeting AGAP001476, AGAP006241, or control firefly luciferase gene (luc) were synthesized using primers listed in Table 1 and MEGAscript RNAi kit (Ambion, Invitrogen). Adult A. gambiae mosquitoes, at 2–5 days post-emergence, received an intrathoracic injection of 138 nl of dsRNA (3 μg/μl) using a Nanoject II Auto-Nanoliter injector (Drummond Scientific) as described (15). Microinjected mosquitoes were allowed to rest for 2–3 days before having an infectious blood meal. Whole mosquitoes and mosquito midguts were harvested to analyze the gene knockdown efficiency and expression levels of various markers during Plasmodium infection.
TABLE 1.
List of primers used in the amplification of specific gene fragments for dsRNA synthesis
S, sense; AS, antisense.
| Target | Sequence (5′ to 3′) | |
|---|---|---|
| AGAP001476 (XM_321648.5) | S | taatacgactcactatagggagGCTGTCATCATCTTTGTGCC |
| AS | taatacgactcactatagggagCGATACGTTTCGCAAGCTCT | |
| AGAP006241 (XM_316309.3) | S | taatacgactcactatagggagCCGGAGTGATCGATCCATAG |
| AS | taatacgactcactatagggagCCTTACAAACTCCAGCATCTCG | |
| Cactus (XM_317542.4) | S | taatacgactcactatagggagGGTTTCTTTCCGGGCATAAC |
| AS | taatacgactcactatagggagCGGACTCTAGCTCCTCGTCT | |
| Firefly luciferase (luc) | S | taatacgactcactatagggagGGTTCCTGGAACAATTGCTT |
| AS | taatacgactcactatagggagCTGCAACTCCGATAAATAAC |
Lowercase letters indicate the T7 promoter sequence. The GenBank accession numbers are listed in parentheses adjacent to the corresponding genes.
Immunofluorescence
Fluorescent microscopy was performed using mosquito midguts on silane-coated slides (Sigma Aldrich). Samples were fixed with 4% formaldehyde and permeabilized with 0.2% Triton X-100. Rabbit anti-TEP1 antibody (Biorbyt) was prepared at a 1:100 dilution in 1% bovine serum albumin and 0.2% Triton X-100. Samples were incubated in primary antibody solution at 4 °C overnight. After washing, samples were incubated for 4 h in secondary antibodies, which were prepared at a 1:300 dilution in 1% bovine serum albumin and 0.2% Triton X-100. After further washing, samples were mounted with ProLong Gold antifade solution (Invitrogen) and stored at 4 °C. Micrographs were acquired using the Zeiss LSM510 fluorescent confocal microscope.
Hemolymph Analysis
To collect hemolymph, the mosquito proboscis was clipped with dissection scissors. The thorax was pressed, and the hemolymph droplet that formed on the proboscis tip was collected in Laemmli sample buffer. Hemolymph samples were resolved by SDS-PAGE, and each lane was loaded with hemolymph collected from 10 mosquitoes. Immunoprecipitation was performed by incubating 50 hemolymph samples, in 200 μl of PBS supplemented with cOmplete protease inhibitor (Roche Applied Science), with 2 μg of rabbit anti-APL1C antibody or normal rabbit IgG (negative control) at 4 °C overnight. On the following day, 20 μl of protein A/G plus agarose beads (Santa Cruz Biotechnology) was added, which was followed by a 4-h incubation at 4 °C. After four washes with 300 μl of Nonidet P-40 lysis buffer, the protein complexes were eluted and resolved by SDS-PAGE. Rabbit anti-TEP1 antibody, rabbit antiserum against PPO6, and guinea pig antiserum against LRIM1-APL1C complex were used at a 1:200, 1:2000, and 1:1000 dilutions, respectively. Immunoprecipitated TEP1 was detected using the Clean-Blot IP detection reagent (Pierce Biotechnology). A Dodeca silver stain kit (Bio-Rad) was used to assess the vitellogenin level. Densitometric analysis was performed using ImageJ (version 1.48i, National Institutes of Health).
Quantitative PCR
Whole mosquito samples were homogenized in a Bullet Blender using 0.5-mm zirconium oxide beads (Next Advance). RNA extraction of homogenized mosquitoes and other tissues was performed using the TRIzol reagent (Invitrogen). RNAs were reverse-transcribed using the iScript cDNA synthesis kit (Bio-Rad). Quantitative PCR (qPCR) was performed using the iQ SYBR Green supermix and CFX96 real-time system (Bio-Rad). Primers (Table 2) were used at 0.35 μm each. Gene expression levels were determined by the relative standard curve method and normalized against the actin level. CFX manager software (version 3.0, Bio-Rad) was used to determine the CT value.
TABLE 2.
List of primers used in qPCR analyses of Anopheles gambiae genes
S, sense; AS, antisense.
| Target | Sequence (5′ to 3′) | GenBankTM accession number | |
|---|---|---|---|
| AGAP001476 | S | CTTCGTGCTGTTCTTCCAGGCGA | XM_321648.5 |
| AS | TTCGCATCAGTCCGCCCTCCA | ||
| AGAP006241 | S | GCGTGCATGTTCTCGGTGCC | XM_316309.3 |
| AS | TCCGGAACGATGGGTGTAGTCAA | ||
| Actin | S | GCATCCACGAGACCACCTACAAC | XM_001230771.2 |
| AS | GTGATCTCCTTCTGCATGCGGT | ||
| TEP1 | S | AAGTGGCAGCAGCGTGTTTC | AF291654.1 |
| AS | TGCTTCGAGGCCAACCAATC | ||
| Vitellogenin | S | TGACCGTAGCTTCGCTATCC | AF281078.1 |
| AS | TCCATCGAAGGTGTTGACGA | ||
| Lipophorin | S | CAGCCAGGATGGTGAGCTTAA | XM_321226.5 |
| AS | CACCAGCACCTTGGCGTT | ||
| LRIM1 | S | CATCCGCGATTGGGATATGT | XM_316370.4 (32) |
| AS | CTTCTTGAGCCGTGCATTTTC | ||
| APL1C | S | GCAAAGAAAGTGACAAGCCGTAT | XM_001688017.2 (32) |
| AS | CGCTCGTCAGGGCAATGTA | ||
| Caspar | S | CACGCGCACGCACGCAATAC | XM_316513.4 |
| AS | GGCCGTTATGCTCTGAAAGTCGG | ||
| Cactus | S | TAACACTGCGCTTCATTTGG | XM_317542.4 |
| AS | GAAGTGTTTCCATGCTGCCA | ||
| FBN9 | S | CCAAGATGTCGGGCAAGTAT | XM_309445.3 |
| AS | TTGTGGTACGTCAGCGAGTC | ||
| SRPN6 | S | CGGTCAGTGGAATCGGTACTACA | XM_319990.3 (33) |
| AS | GCCGTACGCACCATTGGT | ||
| NOS | S | GCTCGAACTATCTGGCCAAC | GU990160.1 (34) |
| AS | CCACTCTTGCCAGAACGAAC | ||
| HPX2 | S | CCGCTTCTACAACACGATGA | XM_319784.4 (11) |
| AS | CGACCAGATGGGCAAGTAT | ||
| Relish 1 | S | TCAACAGATGCCAAAAGAGGAAAT | XM_310177.3 (25) |
| AS | CTGGTTGGAGGGATTGTG | ||
| Relish 2 | S | ACCGATACGGAAAGTGTGCT | XM_308995.4 (35) |
| AS | GTATCGTTGCGTCGGATTG | ||
| AGAP001477 | S | CGCTACTCCTGCACAACAGATCC | XM_321647.5 |
| AS | CAGCCACTCGGTGAAGCGCA | ||
| AGAP001487 | S | AACGCACATGGAGGCTGACC | XM_321635.2 |
| AS | CGTGTCGTTCAACTTCTCCGCT | ||
| AGAP001488 | S | GCTACCGCCAGTGCCAGGAT | XM_001238516.2 |
| AS | TCCTGCGCTATCGGACGACAC | ||
| AGAP004510 | S | GCGCTTTGTGTGCTGGCGT | XM_313810.3 |
| AS | AGCCGGAAGCGACCGCAAGT | ||
| Bacteria 16S | S | TCCTACGGGAGGCAGCAGT | 34 |
| AS | GGACTACCAGGGTATCTAATCCTGTT | ||
| Enterobacteria 16S | S | ATGGCTGTCGTCAGCTCGT | 36 |
| AS | CCTACTTCTTTTGCAACCCACTC |
Statistical Analysis
Prism software (version 6.00, GraphPad Software) was used for statistical analyses. Each experiment was repeated at least three times. Statistical significance was analyzed with the nonparametric Mann-Whitney test, the Kruskal-Wallis test followed by Dunn's multiple comparison test, or two-way ANOVA followed by Bonferroni's multiple comparison. Values in bar charts and line graphs represent means ± S.E. of the mean of at least three independent experiments. Horizontal lines in scatter plots represent the median in a representative experiment.
RESULTS
AGAP001476 Was Induced in the A. gambiae Midgut after P. berghei Infection
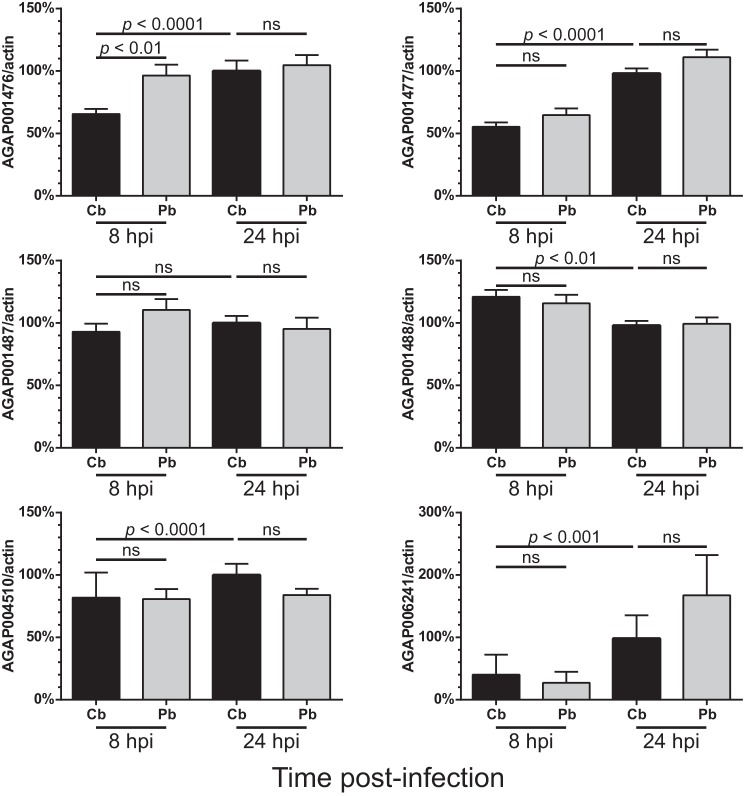
After a blood meal, there are alterations in the microenvironment of the mosquito gut. The expression levels of various innexins in the midgut were investigated to determine whether the levels of these gap junction proteins were associated with these rapid changes. In the A. gambiae genome, there are six annotated innexin genes, namely AGAP001476, AGAP001477, AGAP001487 (innexin shaking-B), AGAP001488, AGAP004510, and AGAP006241. Innexins showed differential responses toward blood feeding (Fig. 1), but only AGAP001476 became significantly induced at 8 hpi in mosquito midguts when compared with the noninfectious blood meal group. A previous translational level study reported that both AGAP001476 and AGAP006241 showed increased translational activities in response to Plasmodium falciparum infection (16). Therefore, it is possible that these two innexins are involved in modulating anti-Plasmodium responses.
FIGURE 1.
AGAP001476 expression is induced in the A. gambiae midgut after ingestion of a P. berghei-infected blood meal. After receiving a noninfectious (Cb) or Plasmodium-infected (Pb) blood meal, mosquitoes were dissected at 8 and 24 hpi to collect midgut tissues. The mRNA levels of various innexins were assessed by qPCR. The expression levels of genes of interest were normalized against actin level and expressed in levels relative to the control group at 24 hpi. Only AGAP001476 became transiently induced at 8 hpi, but its level was not significantly different from the control group at 24 hpi. n = 15. Kruskal-Wallis test was followed by the Dunn's multiple comparison test. ns, no statistical significance. The data represent the means ± S.E. of the mean compiled from three independent experiments.
Cbx Treatment before Blood Meal Results in More Plasmodium Oocysts in the Mosquito Midgut
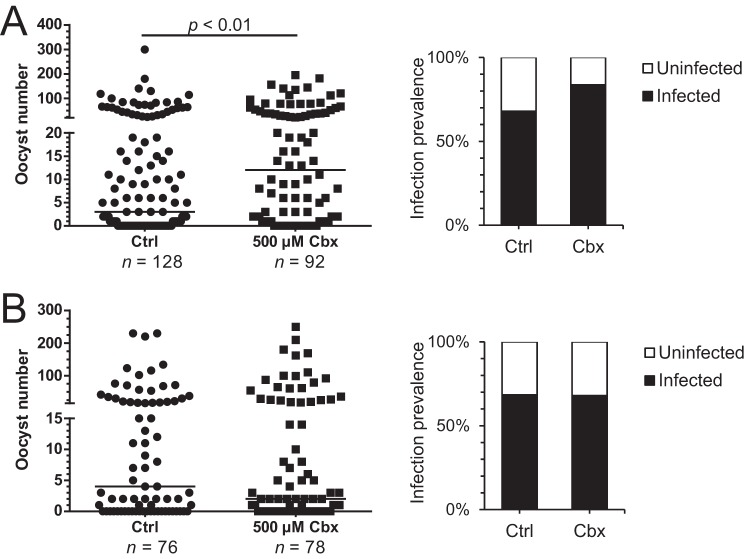
Cbx, a known inhibitor of pannexin and innexin channels (17, 18), was then used to investigate whether inhibition of innexons (innexin hemichannels) would affect Plasmodium development in the mosquito midgut. When mosquitoes received Cbx (500 μm) starting from 3 days before the infectious blood meal (Fig. 2A), there were more oocysts in the treatment group at 8–10 dpi when compared with the control group. In addition to a higher parasite load, an increase in infection prevalence was also observed. Similar results were observed when 200 and 50 μm was used (data not shown). Because of the prolonged treatment time and complex life cycle of Plasmodium, it is important to distinguish whether the actions of Cbx target early Plasmodium development, between the ookinete and oocyst stage, or oocyst development. An experiment with Cbx treatment starting from 28 to 32 hpi, when most ookinetes would have migrated to the basal lamina for further development and evaded the early phase of innate immunity, was therefore performed (Fig. 2B). There were no significant changes in the oocyst number or the infection prevalence in this case. Hence, Cbx treatment and its inhibition of innexons promote early Plasmodium survival in the mosquito midgut.
FIGURE 2.
Blocking of innexons with Cbx prior to blood meal promotes Plasmodium survival in midgut. Mosquitoes ingested a Plasmodium-infected blood meal, and the parasite burden was assessed at 8–10 dpi by counting the GFP-expressing oocysts. Mosquitoes received Cbx treatment 3 days prior to the blood meal in A and 28–32 hpi in B. A significantly higher number of oocysts were observed in the group receiving Cbx prior to the blood meal, and more mosquitoes became infected with Plasmodium. Cbx treatment after blood feeding did not affect the oocyst number. The scatter plots in A and B are the results of a representative experiment. Each data point represents the oocyst number in a single midgut. Three additional independent experiments yielded similar results, and statistical significance was assessed with the Mann-Whitney test. Ctrl, control.
Cbx Reduces Mosquito TEP1 Expression following Plasmodium Infection
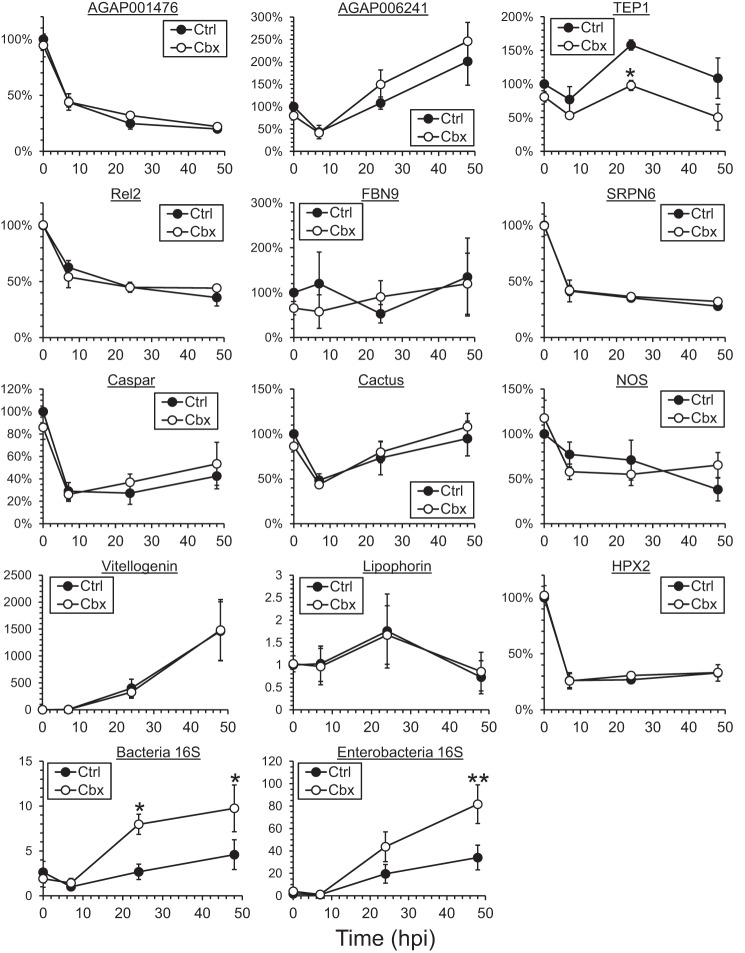
To understand how innexins modulate innate immune responses against Plasmodium in Anopheles mosquitoes, the expression levels of various immune-related molecules were assessed in whole mosquito samples during early Plasmodium infection (Fig. 3). For innexins, Plasmodium infection caused a reduction in AGAP001476 in whole mosquitoes and an induction in AGAP006241 after an initial decline at 7 hpi. These differential responses of innexins probably suggest different functional roles during Plasmodium infection. Their levels were unaffected by Cbx treatment.
FIGURE 3.
Induction of TEP1 expression upon Plasmodium infection is inhibited by Cbx treatment. The expression levels of selected gene markers involved in the innate immune response were assessed in whole mosquitoes that received Cbx treatment before the Plasmodium-infected blood meal. The expression levels of the various genes assessed in this study were not altered by Cbx treatment before blood acquisition. The induction of TEP1 normally observed after blood feeding was abolished in the Cbx treatment group at 24 hpi. The expression levels of other markers in the treatment group remained unchanged from the control. The bacteria level of nascent mosquitoes treated with Cbx did not show significant differences from the control group. The induction of bacteria level after blood meal, including enterobacteria, was further enhanced in the Cbx treatment group at 24 and 48 hpi. The data represent the means ± S.E. of the mean compiled from three independent experiments. *, p < 0.05; **, p < 0.01 by two-way ANOVA and Bonferroni's multiple comparisons test.
Cbx treatment did not affect basal immunity because all marker genes examined remained unchanged before blood meal. The only effector that was transcriptionally affected by Cbx treatment during Plasmodium infection was TEP1, which is responsible for killing microbes including Plasmodium in mosquitoes (10). Besides the complement-like protein TEP1, other effectors known to target Plasmodium were examined in this study, including antimicrobial peptide (fibrinogen 9 (FBN9), serine protease inhibitor 6 (SRPN6) of the melanization response, nitric oxide synthase (NOS), heme peroxidase 2 (HPX2) of the epithelial nitration pathway, Toll and immune deficiency pathway regulators (relish 2, caspar, and cactus), and nutrient transport proteins (vitellogenin and lipophorin) (8, 11, 19–21). However, these genes were not transcriptionally affected by Cbx treatment. A significant induction in the TEP1 expression level in the control group was observed at 24 and 48 hpi, consistent with previous studies (10), and this induction was abolished upon Cbx treatment. As TEP1 is responsible for targeting both bacteria and Plasmodium for phagocytosis (21), an increase in bacteria loading was also observed in Cbx-treated mosquitoes, although their level in nascent mosquitoes was unaffected (Fig. 3). It included an increase in enterobacteria level that was earlier shown to alter Plasmodium survival through generation of reactive oxygen species (9). This inhibition of TEP1 induction and thus parasite killing may contribute to the increase in parasite burden, in terms of oocyst number, in mosquitoes treated with Cbx.
Knockdown of AGAP001476 Increases the Plasmodium Oocyst Numbers and Reduces Functional TEP1 Levels
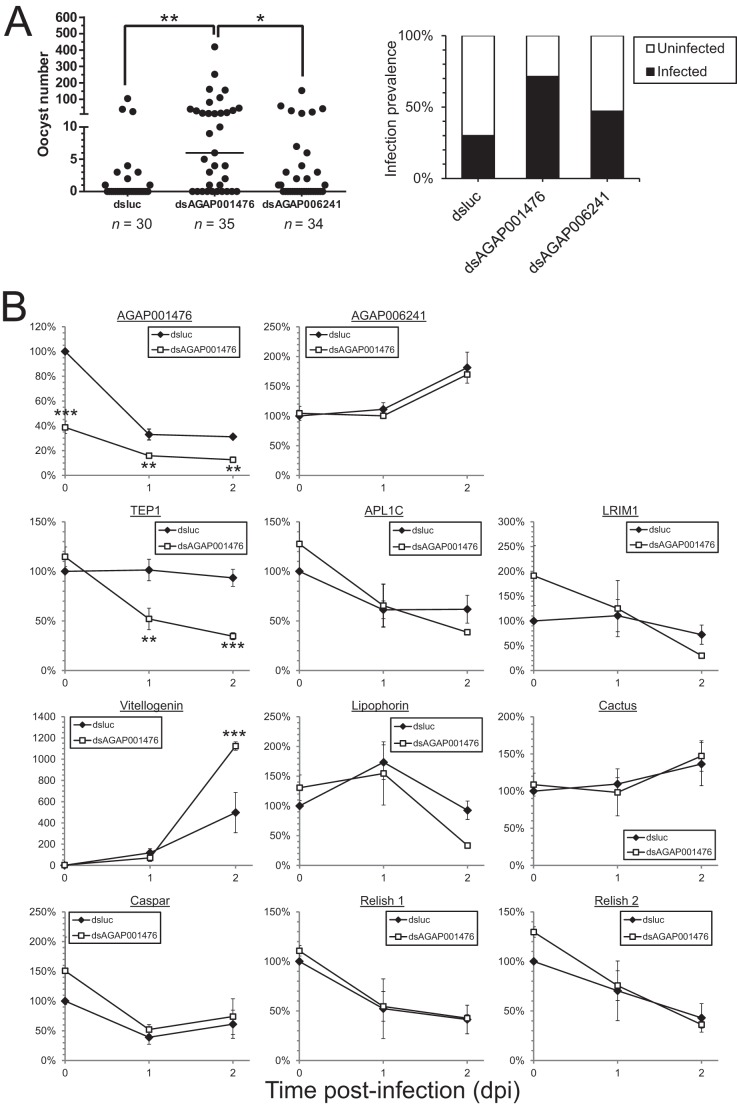
Different innexins would presumably have overlapping yet unique functions, like their vertebrate counterparts, connexins (12). Cbx is a slow acting inhibitor that may act on innexins indirectly (22), and it is also known to inhibit molecules other than gap junction members, such as 11β-hydroxysteroid dehydrogenase. Therefore, it is essential to perform knockdown experiments with dsRNA to verify the results observed in Cbx treatment and to determine the innexin members responsible for such changes. As mentioned earlier, AGAP001476 and AGAP006241 were translationally induced in midguts at 24 hpi during P. falciparum infection (16), and the transcriptional up-regulation of AGAP001476 in midgut was observed in this study (Fig. 1). A knockdown of these two innexins was performed by injecting corresponding dsRNA into the mosquito thorax 2–3 days prior to blood acquisition (Figs. 4 and 5). Knockdown of AGAP001476, but not AGAP006241, resulted in an increase in oocyst number and infection prevalence similar to the Cbx treatment (Fig. 4A). This indicated that innexin channels formed by AGAP001476 in part mediate innate immune responses and affect Plasmodium survival in the mosquito midgut.
FIGURE 4.
Depletion of AGAP001476 specifically increases Plasmodium survival, which is accompanied by a decline in TEP1 and an increase in vitellogenin level. Each mosquito was injected with 0.4 μg of dsluc, dsAGAP001476, or dsAGAP006241. The oocyst number and the percentage of infected mosquitoes are shown in A. Mosquitoes receiving dsAGAP001476 had significantly more oocysts in their midguts than those receiving dsluc or dsAGAP006241. The expression levels of selected genes involved in innate immune responses and the two innexin genes of interest were assessed by qPCR and normalized against actin levels in B. Apart from a reduction in AGAP001476, mosquitoes receiving dsAGAP001476 had less TEP1 at 1 and 2 dpi and more vitellogenin at 2 dpi. Values in A represent the median from a representative experiment that was repeated three times. Values in B represent means ± S.E. of the mean compiled from four independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001 by Kruskal-Wallis test and Dunn's multiple comparisons test in A, and two-way ANOVA and Bonferroni's multiple comparisons test in B.
FIGURE 5.
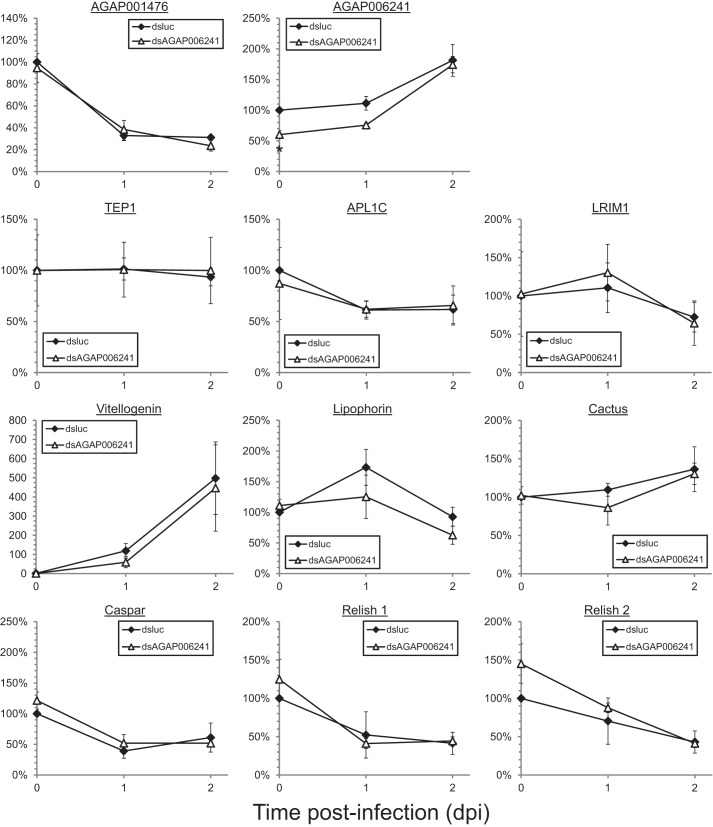
Expression levels of innexins and selected genes involved in innate immunity in mosquitoes receiving dsluc or dsAGAP006241. Whole mosquitoes that received dsRNA injection were collected at 0, 1, and 2 dpi for mRNA analysis. The expression levels of selected genes involved in innate immune responses and the two innexin genes of interest were assessed by qPCR and normalized against actin level. Only AGAP006241 level showed a significant reduction in dsAGAP006241-treated mosquitoes, whereas other selected immune markers remained unchanged. Values represent means ± S.E. of the mean compiled from at least four independent experiments. *, p < 0.05 by two-way ANOVA and Bonferroni's multiple comparisons test.
In dsAGAP001476 mosquitoes, a 60% knockdown in mRNA level was observed. The mRNA studies revealed an inhibition of TEP1 and induction of vitellogenin, a nutrient transport protein involved in oogenesis that can inhibit TEP1 protein function (20) (Fig. 4B). However, the expression levels of its interacting partners, LRIM1 and APL1C, and other TEP1 upstream regulators including cactus and relish 1, remain unaffected.
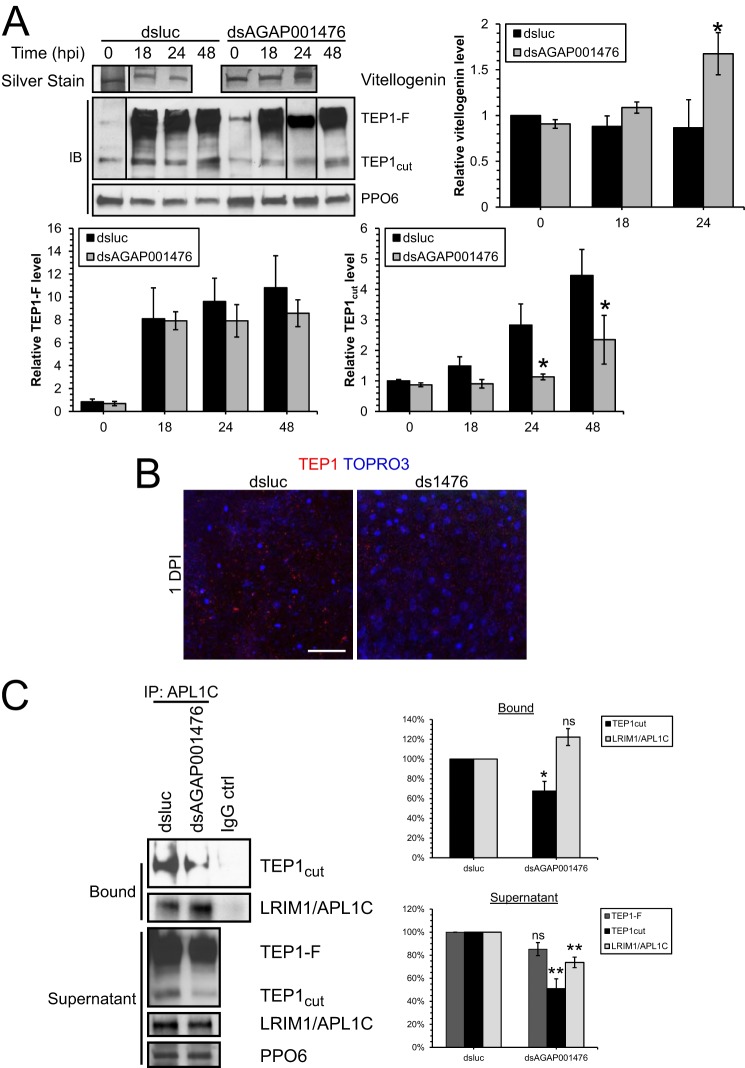
Hemolymph analysis by immunoblotting showed that less TEP1 and more vitellogenin were released from hemocytes and fat body cells, respectively, into the hemolymph in dsAGAP001476 mosquitoes (Fig. 6A). TEP1-F is the full-length proactive form that needs to be cleaved by proteolysis into the functional TEP1cut form (23), which is then stabilized by binding with LRIM1 and APL1C to localize onto the ookinete surface (21, 24). During Plasmodium infection, both TEP1-F and TEP1cut became induced (Fig. 6A) despite the lack of induction in transcript level (Fig. 4B). In dsAGAP001476 mosquitoes, less TEP1cut was present in the hemolymph at 24 and 48 hpi when compared with dsluc mosquitoes, whereas TEP1-F did not show significant changes. This suggests that innexin AGAP001476 may also be involved in regulating the proteolytic cleavage of TEP1-F to TEP1cut or stability of TEP1cut, in addition to transcriptional control. The ingestion of a Plasmodium-infected blood meal induced release of vitellogenin into the hemolymph (Fig. 6A). Silver staining revealed a further induction of vitellogenin levels in hemolymph in dsAGAP001476 mosquitoes at 24 hpi. Immunofluorescence studies of midgut tissues also indicated that less TEP1 attached onto the midgut epithelium in dsAGAP001476 mosquitoes (Fig. 6B). The results indicate that the knockdown of AGAP001476 lowers the bioavailability of complement-like protein TEP1 in both hemocoel and midgut and increases the protein level of its inhibitor, vitellogenin, thus promoting Plasmodium ookinete survival and oocyst development in the midgut.
FIGURE 6.
The TEP1-LRIM1-APL1C complex was reduced in dsAGAP001476 mosquitoes at 1 dpi. A, Western blot (IB) analysis and silver staining of hemolymph collected from mosquitoes treated with dsluc or dsAGAP001476 at 0, 18, 24, and 48 hpi. More TEP1 (both TEP1-F and TEP1cut) was detected in the hemolymph during Plasmodium infection, but TEP1cut was induced to a lesser extent in dsAGAP001476 mosquitoes at 24 and 48 hpi. The induction of Vg protein level in hemolymph during Plasmodium infection was potentiated at 24 hpi in dsAGAP001476 mosquitoes. Each lane represents hemolymph collected from 10 mosquitoes. PPO6 serves as loading control. Data in the bar chart represent means ± S.E. of the mean compiled from three independent experiments. B, immunofluorescence staining of TEP1 in midguts of dsluc or dsAGAP001476 mosquitoes. There was less TEP1 attached onto the midgut epithelium in dsAGAP001476 mosquitoes at 1 dpi. C, TEP1 and LRIM1-APL1C complex was immunoprecipitated from hemolymph collected at 24 hpi using rabbit anti-APL1C antibody to assess the amount of TEP1-LRIM1-APL1C complex. Bound and unbound (supernatant) fractions were resolved by SDS-PAGE. A 30% reduction in TEP1cut-LRIM1-APL1C complex was observed from dsAGAP001476 hemolymph, in addition to a 50% reduction in TEP1cut in the supernatant. Both SDS-PAGE and immunofluorescence had been performed in three independent experiments. (*, p < 0.05; **, p < 0.01 by two-way ANOVA and Bonferroni's multiple comparisons test, or t test.) ns, no statistical significance. Scale bar, 50 μm.
TEP1cut is stabilized by binding with the LRIM1-APL1 complex, forming a functional complex that subsequently tags the Plasmodium parasite surface (24). Immunoprecipitation studies show that in addition to a general decline of TEP1cut levels in the hemolymph, less TEP1cut is complexed with LRIM1-APL1C in dsAGAP001476 mosquitoes at 1 dpi (Fig. 6C). Intriguingly, the APL1C antibody was able to pull out more LRIM1-APL1C complex from dsAGAP001476 hemolymph. Thus, a smaller amount of functional TEP1-LRIM1-APL1C complex was present in dsAGAP001476 mosquitoes to target Plasmodium for parasite killing.
Knockdown of Cactus Abolishes the Effect of the Knockdown of AGAP001476 on P. berghei Development
The Toll pathway of the innate immune response is activated upon P. berghei infection (19). Cactus is a negative regulator of Relish 1 in this pathway, which in turn is the upstream regulator of TEP1 and vitellogenin (20, 25). As the levels of both TEP1 and vitellogenin were modulated in dsAGAP001476 mosquitoes (Figs. 4B and 6), a concomitant knockdown of AGAP001476 and cactus was performed to determine whether AGAP001476 mediates anti-Plasmodium responses through the Toll signaling pathway (Fig. 7). Single knockdown of AGAP001476 results in a higher oocyst number and infection prevalence in the mosquito midgut, whereas knockdown of cactus resulted in inhibition of Plasmodium development, as observed in previous studies (25). In mosquitoes that had a concomitant knockdown of AGAP001476 and cactus, the induction of Plasmodium oocyst number by AGAP001476 was abolished, suggesting that the effect of AGAP001476 on Plasmodium development in the mosquito midgut is Cactus-dependent. Hence, AGAP001476 may be partly responsible for the signal transduction from midgut to hemocytes and fat body cells to induce the Toll pathway for anti-Plasmodium responses (Fig. 8).
FIGURE 7.

Concomitant knockdown of AGAP001476 and cactus could not reverse the refractoriness toward Plasmodium caused by knockdown of cactus. Each mosquito was injected with a total of 0.4 μg of dsluc, dsAGAP001476, or dsCactus. Knockdown of AGAP001476 promoted Plasmodium development as observed earlier, but it did not affect the near refractoriness induced by Cactus depletion. The result suggests that the AGAP001476-mediated anti-Plasmodium response is Cactus-dependent. *, p < 0.05; **, p < 0.01; ***, p < 0.0001 by Kruskal-Wallis test and Dunn's multiple comparisons test.
FIGURE 8.
Schematic diagram showing the possible role of AGAP001476 in anti-Plasmodium response via TEP1-mediated lysis. When Plasmodium is ingested through an infective blood meal, Plasmodium gametocytes undergo sexual reproduction and develop into ookinetes in the mosquito midgut. The ingestion of blood meal and Plasmodium traversal through the midgut epithelial cell induced the Toll pathway of innate immune responses, partly via a yet to be known signal through the innexon hemichannel. The expression of innexin AGAP001476 in midgut increases upon infection. It is uncertain how innexons containing AGAP001476 transduce signal from midgut to hemocytes and fat body. The induction of Toll pathway induces the expression of complement-like protein TEP1 and nutrient transport protein Vg, which inhibits TEP1 function. TEP1 then targets ookinetes that become exposed to the hemolymph in the basal lamina for killing. When innexons are blocked by carbenoxolone or innexin AGAP001476 is reduced by gene silencing, the induction of Toll pathway would become hampered. This leads to a reduced level of TEP1 released from hemocytes and thus formation of functional TEP1-LRIM1-APL1C complex. The elevated level of Vg from fat body cells in turn inhibits the action of the already reduced TEP1. The lowered bioavailability of functional TEP1 thus results in an increase of ookinete survival and oocyst number.
DISCUSSION
Innexin AGAP001476 Modulates Innate Immune Responses against P. berghei Infection
In the innate immune responses of anopheline mosquitoes, the Toll pathway is activated upon P. berghei infection, whereas the IMD pathway is activated upon P. falciparum infection (19). The former pathway involves Relish 1 and Cactus and the latter involves Relish 2 and Caspar (8). Although the expression level of relish 1 and cactus of the Toll pathway remained unchanged in the knockdown group, it is likely that the AGAP001476-modulation of anti-Plasmodium responses is mediated through the Toll pathway that regulates both TEP1 and vitellogenin. The knockdown of cactus is known to give rise to refractory mosquitoes (25), and the double knockdown in this study shows that AGAP001476 cannot reverse the refractoriness toward P. berghei induced by cactus knockdown. Thus, AGAP001476 likely mediates anti-P. berghei responses via the Toll pathway.
The ingestion of a blood meal and Plasmodium traversal through the midgut epithelial cell induced the innate immune responses: in the case of P. berghei, the Toll pathway. The signaling involved in transducing the signal from the midgut to immune cells is unclear. This study reports that it is partially mediated through the innexon hemichannel (Fig. 8). The expression of innexin AGAP001476 in midgut increases upon infection and possibly transduces signals to both hemocytes and fat body cells. Although both hemocytes and fat body cells express AGAP001476, their levels became repressed upon clean and Plasmodium-infected blood meal (data not shown). It is unlikely that the signaling from midgut is transmitted through innexons containing AGAP001476 in the two cell types. The induction of Toll pathway induces the expression of complement-like protein TEP1 and nutrient transport protein vitellogenin (Vg), respectively, which inhibits TEP1 function. TEP1 then targets ookinetes that become exposed to the hemolymph in the basal lamina for killing. When innexons are blocked by Cbx or innexin AGAP001476 is reduced by gene silencing, the induction of the Toll pathway would become hampered. This leads to a reduced level of TEP1 released from hemocytes and an increased level of Vg from fat body cells, inhibiting the action of the already reduced TEP1. Together with a reduced level of TEP1-LRIM1-APL1C complex, the lowered bioavailability of functional TEP1 thus results in an increase of ookinete survival and oocyst number.
TEP1 is an important immune factor that targets various bacteria and pathogens and determines the susceptibility toward Plasmodium in insects (10, 19). In the expression level analysis, Cbx only interfered with TEP1 induction after Plasmodium infection, possibly due to differential effects of innexins on innate immunity. The knockdown of AGAP001476 potentiated the up-regulation of Vg protein level, apart from the reduction in TEP1 proteins. Vitellogenin was previously shown to negatively affect the efficiency of TEP1-mediated lysis of Plasmodium ookinetes, without any effect on its protein level (20). As the knockdown of AGAP001476, but not AGAP006241, caused an increase in Plasmodium oocyst number in the midgut, as observed in the Cbx treatment group, AGAP001476 specifically modulates Plasmodium development via its effect on both the activity and the expression level of complement-like protein TEP1. Because only the transcription level of selected immune factors was investigated in this study, it is possible that there are additional factors involved and that AGAP001476 may also regulate through post-translational modifications including phosphorylation and protein turnover.
Innexin AGAP001476 in Mosquito Innate Immunity
Only a few studies have addressed the role of innexins, the invertebrate homologs of connexins, in mosquitoes and insects. For instance, AGAP006241 is responsible for gonad development in A. gambiae (26). Innexin 2 in Drosophila ovaries is essential for oogenesis (27). Due to the ubiquitous expression of connexins in all mammalian immune cells, gap junctions have been implicated in various aspects of the immune system, including antigen presentation and transfer of apoptotic signals (28), but these are mostly speculative. It is uncertain whether innexins, which can form both gap junction channels and hemichannels (17), would have roles in the mosquito innate immune response. This study shows that innexins, in particular AGAP001476, mediates the anti-Plasmodium responses and is transcriptionally induced during early infection in mosquito midgut. The up-regulation of AGAP001476 mRNA levels in the midgut, despite a general decrease in the whole mosquito, suggests that an induction of AGAP001476 and its signaling in midgut epithelium is required for mediating anti-plasmodial responses, in part by regulating the release of hemocyte-derived factors, including TEP1. Innexin AGAP001476 is also expressed in hemocytes and fat body cells, but it is uncertain whether innexins in these cells participate in regulating the innate immunity.
Connexin channels are permeable to a wide range of cytoplasmic biomolecules less than 1.5 kDa in molecular mass, including inorganic ions, second messengers, siRNA, glucose, and even short peptides (12, 28, 29). Gap junction channels formed by different connexins have various pore sizes and selective permeabilities toward cytoplasmic molecules. In addition, connexins often have different expression patterns and are under alternative regulation, resulting in the overlapping and yet differential functions of connexins channels observed in mammals. Although less is known about the properties and functions of innexins and their channels, they are expected to have different selective permeabilities, gating properties and functions. Further study will be required to delineate the exact signals that pass through AGAP001476-formed innexons to transduce Toll pathway activation in both hemocytes and fat body cells.
AGAP001476 as Potential Target for Transmission Intervention in Mosquito Vector
Transmission blocking of Plasmodium through modulating innate immunity in mosquitoes has been considered one of the strategies in eradicating malaria. In this study, innexin AGAP001476 was shown to modulate the anti-Plasmodium immune response and affect Plasmodium survival in the midgut. Oocyst prevalence from a feeding assay has been reported to be a predictor of mosquito infectivity (30). AGAP001476 may serve as a target for intervening in Plasmodium transmission to human hosts. Although most small molecules currently targeting innexins are inhibitors (22), the development of chemicals that activate innexins specifically may help to reduce Plasmodium burden in mosquitoes and thus transmission when applied along with insecticides.
Antimalarials such as mefloquine and artemisinin have been shown to be able to block connexin channels and pannexin hemichannels (22, 31). Many connexin and pannexin blockers, including chemicals and peptides, are known to cross-react with innexin channels. Due to the induction of Plasmodium survival by blocking innexons, it is also of interest to understand whether residual antimalarial drugs in human blood, when taken up in mosquito blood meal, would affect Plasmodium development and thus transmission in mosquitoes.
Acknowledgments
The following reagents were obtained through the Malaria Research and Reference Reagent Resource Center (MR4) as part of the Biodefense and Emerging Infections Research Resources Repository (BEI Resources), NIAID, National Institutes of Health: A. gambiae 4ARR, MRA-121, deposited by M. Q. Benedict; and Plasmodium berghei (ANKA) GFPcon 259cl2, MRA-865, deposited by C. J. Janse and A. P. Waters. We thank Dr. Hassan Yassine and Prof. George K Christophides for generously providing the antibodies against APL1C, LRIM1-APL1C, and PPO6. We thank Dr. Richard Baxter for the rabbit antibody against TEP1. We thank Kathleen DePonte, Ying Yang, Jingyi Pang, and Shuang Cui for technical support in the maintenance of mosquito colonies.
Footnotes
- Cbx
- carbenoxolone
- Vg
- vitellogenin
- dpi
- days post-infection
- hpi
- hours post-infection
- qPCR
- quantitative PCR
- ANOVA
- analysis of variance.
REFERENCES
- 1. World Health Organization (2012) World Malaria Report 2012, www.who.int/malaria/publications/world_malaria_report_2012/report/en/index.html, World Health Organization, Geneva, Switzerland [Google Scholar]
- 2. Butcher G. A. (1997) Antimalarial drugs and the mosquito transmission of Plasmodium. Int. J. Parasitol. 27, 975–987 [DOI] [PubMed] [Google Scholar]
- 3. Cirimotich C. M., Clayton A. M., Dimopoulos G. (2011) Low- and high-tech approaches to control Plasmodium parasite transmission by Anopheles mosquitoes. J. Trop. Med. 2011, 891342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raghavendra K., Barik T. K., Reddy B. P. N., Sharma P., Dash A. P. (2011) Malaria vector control: from past to future. Parasitol. Res. 108, 757–779 [DOI] [PubMed] [Google Scholar]
- 5. Ojo K. K., Pfander C., Mueller N. R., Burstroem C., Larson E. T., Bryan C. M., Fox A. M. W., Reid M. C., Johnson S. M., Murphy R. C., Kennedy M., Mann H., Leibly D. J., Hewitt S. N., Verlinde C. L. M. J., Kappe S., Merritt E. A., Maly D. J., Billker O., Van Voorhis W. C. (2012) Transmission of malaria to mosquitoes blocked by bumped kinase inhibitors. J. Clin. Invest. 122, 2301–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Billingsley P. F., Foy B., Rasgon J. L. (2008) Mosquitocidal vaccines: a neglected addition to malaria and dengue control strategies. Trends Parasitol. 24, 396–400 [DOI] [PubMed] [Google Scholar]
- 7. Angrisano F., Tan Y.-H., Sturm A., McFadden G. I., Baum J. (2012) Malaria parasite colonisation of the mosquito midgut: placing the Plasmodium ookinete centre stage. Int. J. Parasitol. 42, 519–527 [DOI] [PubMed] [Google Scholar]
- 8. Cirimotich C. M., Dong Y., Garver L. S., Sim S., Dimopoulos G. (2010) Mosquito immune defenses against Plasmodium infection. Dev. Comp. Immunol. 34, 387–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cirimotich C. M., Dong Y., Clayton A. M., Sandiford S. L., Souza-Neto J. A., Mulenga M., Dimopoulos G. (2011) Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 332, 855–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blandin S., Shiao S.-H., Moita L. F., Janse C. J., Waters A. P., Kafatos F. C., Levashina E. A. (2004) Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell 116, 661–670 [DOI] [PubMed] [Google Scholar]
- 11. Oliveira G. de A., Lieberman J., Barillas-Mury C. (2012) Epithelial nitration by a peroxidase/NOX5 system mediates mosquito antiplasmodial immunity. Science 335, 856–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meşe G., Richard G., White T. W. (2007) Gap junctions: basic structure and function. J. Invest. Dermatol. 127, 2516–2524 [DOI] [PubMed] [Google Scholar]
- 13. Malaria Research and Reference Reagent Resource Center (2009) Methods in Anopheles Research Manual, www.mr4.org/Portals/3/Pdfs/Protocol-Book/MethodsAnophelesResearchV4c.pdf, Malaria Research and Reference Reagent Resource Center, Manassas, VA [Google Scholar]
- 14. Vanderberg J. P., Yoeli M. (1966) Effects of temperature on sporogonic development of Plasmodium berghei. J. Parasitol. 52, 559–564 [PubMed] [Google Scholar]
- 15. Garver L., Dimopoulos G. (2007) Protocol for RNAi assays in adult mosquitoes (A. gambiae). J. Vis. Exp. 5, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mead E. A., Li M., Tu Z., Zhu J. (2012) Translational regulation of Anopheles gambiae mRNAs in the midgut during Plasmodium falciparum infection. BMC Genomics 13, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bao L., Samuels S., Locovei S., Macagno E. R., Muller K. J., Dahl G. (2007) Innexins form two types of channels. FEBS Lett. 581, 5703–5708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luo K., Turnbull M. W. (2011) Characterization of nonjunctional hemichannels in caterpillar cells. J. Insect. Sci. 11, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clayton A. M., Dong Y., Dimopoulos G. (2014) The Anopheles innate immune system in the defense against malaria infection. J. Innate Immun. 6, 169–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rono M. K., Whitten M. M. A., Oulad-Abdelghani M., Levashina E. A., Marois E. (2010) The major yolk protein vitellogenin interferes with the anti-Plasmodium response in the malaria mosquito Anopheles gambiae. PLoS Biol. 8, e1000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hillyer J. F. (2010) Mosquito immunity. Adv. Exp. Med. Biol. 708, 218–238 [DOI] [PubMed] [Google Scholar]
- 22. Verselis V. K., Srinivas M. (2013) Connexin channel modulators and their mechanisms of action. Neuropharmacology 75, 517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baxter R. H. G., Steinert S., Chelliah Y., Volohonsky G., Levashina E. A., Deisenhofer J. (2010) A heterodimeric complex of the LRR proteins LRIM1 and APL1C regulates complement-like immunity in Anopheles gambiae. Proc. Natl. Acad. Sci. U.S.A. 107, 16817–16822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Povelones M., Waterhouse R. M., Kafatos F. C., Christophides G. K. (2009) Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science 324, 258–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frolet C., Thoma M., Blandin S., Hoffmann J. A., Levashina E. A. (2006) Boosting NF-κB-dependent basal immunity of Anopheles gambiae aborts development of Plasmodium berghei. Immunity 25, 677–685 [DOI] [PubMed] [Google Scholar]
- 26. Magnusson K., Mendes A. M., Windbichler N., Papathanos P.-A., Nolan T., Dottorini T., Rizzi E., Christophides G. K., Crisanti A. (2011) Transcription regulation of sex-biased genes during ontogeny in the malaria vector Anopheles gambiae. PLoS One 6, e21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bohrmann J., Zimmermann J. (2008) Gap junctions in the ovary of Drosophila melanogaster: localization of innexins 1, 2, 3 and 4 and evidence for intercellular communication via innexin-2 containing channels. BMC Dev. Biol. 8, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neijssen J., Pang B., Neefjes J. (2007) Gap junction-mediated intercellular communication in the immune system. Prog. Biophys. Mol. Biol. 94, 207–218 [DOI] [PubMed] [Google Scholar]
- 29. Li M. W. M., Mruk D. D., Cheng C. Y. (2012) Gap junctions and blood-tissue barriers. Adv. Exp. Med. Biol. 763, 260–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stone W. J. R., Eldering M., van Gemert G.-J., Lanke K. H. W., Grignard L., van de Vegte-Bolmer M. G., Siebelink-Stoter R., Graumans W., Roeffen W. F. G., Drakeley C. J., Sauerwein R. W., Bousema T. (2013) The relevance and applicability of oocyst prevalence as a read-out for mosquito feeding assays. Sci. Rep. 3, 3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dahl G., Qiu F., Wang J. (2013) The bizarre pharmacology of the ATP release channel pannexin1. Neuropharmacology 75, 583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Habtewold T., Povelones M., Blagborough A. M., Christophides G. K. (2008) Transmission blocking immunity in the malaria non-vector mosquito Anopheles quadriannulatus species A. PLoS Pathog. 4, e1000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abraham E. G., Pinto S. B., Ghosh A., Vanlandingham D. L., Budd A., Higgs S., Kafatos F. C., Jacobs-Lorena M., Michel K. (2005) An immune-responsive serpin, SRPN6, mediates mosquito defense against malaria parasites. Proc. Natl. Acad. Sci. U.S.A. 102, 16327–16332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kumar S., Molina-Cruz A., Gupta L., Rodrigues J., Barillas-Mury C. (2010) A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science 327, 1644–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meister S., Kanzok S. M., Zheng X.-L., Luna C., Li T.-R., Hoa N. T., Clayton J. R., White K. P., Kafatos F. C., Christophides G. K., Zheng L. (2005) Immune signaling pathways regulating bacterial and malaria parasite infection of the mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. U.S.A. 102, 11420–11425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Castillo M., Martín-Orúe S. M., Manzanilla E. G., Badiola I., Martín M., Gasa J. (2006) Quantification of total bacteria, enterobacteria and lactobacilli populations in pig digesta by real-time PCR. Vet. Microbiol. 114, 165–170 [DOI] [PubMed] [Google Scholar]