Background: Homer proteins bind multiple Ca2+-signaling proteins to shape the Ca2+ signal by poorly understood mechanisms.
Results: Homer2 regulates PMCA expression and activity in parotid acinar cells.
Conclusion: Homer2 acts as a regulator of PMCA-mediated Ca2+ clearance.
Significance: Inhibition of Ca2+ clearance by Homer2 further clarifies its role in Ca2+ signaling.
Keywords: Calcium ATPase, Calcium Transport, Cell Signaling, Protein-Protein Interaction, Scaffold Protein, Homer Proteins, Plasma Membrane Ca2+-ATPase, Parotid Gland, Proline-rich Motif
Abstract
Homer proteins are scaffold molecules with a domain structure consisting of an N-terminal Ena/VASP homology 1 protein-binding domain and a C-terminal leucine zipper/coiled-coil domain. The Ena/VASP homology 1 domain recognizes proline-rich motifs and binds multiple Ca2+-signaling proteins, including G protein-coupled receptors, inositol 1,4,5-triphosphate receptors, ryanodine receptors, and transient receptor potential channels. However, their role in Ca2+ signaling in nonexcitable cells is not well understood. In this study, we investigated the role of Homer2 on Ca2+ signaling in parotid gland acinar cells using Homer2-deficient (Homer2−/−) mice. Homer2 is localized at the apical pole in acinar cells. Deletion of Homer2 did not affect inositol 1,4,5-triphosphate receptor localization or channel activity and did not affect the expression and activity of sarco/endoplasmic reticulum Ca2+-ATPase pumps. In contrast, Homer2 deletion markedly increased expression of plasma membrane Ca2+-ATPase (PMCA) pumps, in particular PMCA4, at the apical pole. Accordingly, Homer2 deficiency increased Ca2+ extrusion by acinar cells. These findings were supported by co-immunoprecipitation of Homer2 and PMCA in wild-type parotid cells and transfected human embryonic kidney 293 (HEK293) cells. We identified a Homer-binding PPXXF-like motif in the N terminus of PMCA that is required for interaction with Homer2. Mutation of the PPXXF-like motif did not affect the interaction of PMCA with Homer1 but inhibited its interaction with Homer2 and increased Ca2+ clearance by PMCA. These findings reveal an important regulation of PMCA by Homer2 that has a central role on PMCA-mediated Ca2+ signaling in parotid acinar cells.
Introduction
Ca2+ is a common second messenger with roles in fertilization, muscle contraction, neurotransmitter release, exocytosis, learning, and memory. In addition, it regulates critical functions of polarized secretory gland cells (1, 2). Secretory cell Ca2+ signaling is evoked primarily by activation of G protein-coupled receptors (GPCRs),2 which are coupled to Gq or Gi. These cells are an excellent model system to study Ca2+ signaling and the role of scaffolding proteins in the function of various components of the Ca2+ signal, including plasma membrane Ca2+-ATPase (PMCA) and sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pumps, the inositol 1,4,5-triphosphate (IP3) receptors (IP3Rs), Ca2+ release channels in the endoplasmic reticulum (ER), and Ca2+ influx channels in the plasma membrane (3).
Several GPCRs possess binding motifs that associate with members of the Homer family of scaffolding proteins. The Homer family, which includes Homer1, Homer2, and Homer3, as well as several splice variants, was discovered when homer1a was cloned as an immediate early gene product expressed in neurons upon prolonged stimulation (4–7). With the exception of Homer1a, all Homer proteins (i.e. Homer1b/c, 2, and 3; referred to as “long Homers”) are expressed constitutively throughout the central nervous system (CNS). These proteins are composed of an N-terminal Ena/VASP homology 1 protein-binding domain and a C terminus that folds into a coiled-coil multimerization domain and leucine zipper (7, 8). The Ena/VASP homology 1 domain is a protein-protein binding module that recognizes the proline-rich motifs PPXXF, PPXF, and LPSSP (9–12) and binds to several GPCRs, canonical transient receptor potential channels, IP3Rs, ryanodine receptors, and the Shank family of scaffolding proteins (10, 12–16). Homer proteins play a central role in Ca2+ signaling via regulation of neuronal transcription activity and thereby regulate dendritic spine morphogenesis, synapse remodeling, and synaptic clustering of CNS neurons (9, 17–19). Previous research found that Homer1 regulates Ca2+ influx by associating IP3Rs with transient receptor potential channels (12). Homer2 tunes GPCR stimulus intensity by regulating the regulator of G protein signaling proteins and phospholipase Cβ-promoting guanosine triphosphatase by Gα in pancreatic acinar cells. Moreover, Homer2 and Homer3 bind nuclear factor of activated T cells by competing with calcineurin in T lymphocytes (14, 20). However, the role of Homer proteins in Ca2+ signaling in nonexcitable cells remains poorly characterized.
In this study, the role of Homer2 on Ca2+ signaling in parotid gland acinar cells was investigated using Homer2 knock-out (Homer2−/−) mice. We report that Homer2 interacts with PMCA (particularly PMCA isoform 4) in model systems and in vivo and that this interaction regulates PMCA activity. The findings suggest a mechanism by which Homer proteins can regulate PMCA expression and PMCA-mediated Ca2+ efflux in parotid acinar cells. We suggest that by inhibiting transient receptor potential channel-mediated Ca2+ influx (12) and by differentially modulating Ca2+ extrusion by PMCA (present data), the Homers serve to protect the cells from Ca2+ toxicity by facilitating cytosolic Ca2+ clearance to limit the Ca2+ signal duration.
EXPERIMENTAL PROCEDURES
Antibodies and DNA Constructs
Anti-Homer1a (M-13), anti-Homer1 (D-3), anti-Homer2, and anti-PMCA (5F10) antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-IP3R3 antibodies were purchased from BD Transduction Laboratories (San Jose, CA). Anti-PMCA4 (JA9) antibodies were obtained from Affinity BioReagents (Golden, CO). Anti-FLAG M2 antibodies were obtained from Sigma. PMM2-hPMCA1b and PMM2-hPMCA4b were generous gifts from Dr. Emanuel E. Strehler (Mayo Clinic College of Medicine, Rochester, MN). pRK5-HA-Homer1a, pRK5-HA-Homer1c, and pRK5-HA-Homer2 were generously provided by Dr. Paul Worley (The Johns Hopkins University School of Medicine, Baltimore, MD).
Animals and Preparation of Parotid Acinar Cells
Wild-type (WT) and Homer2−/− mice have been described previously (14). The life span of Homer2−/− mice is similar to that of WT littermates. All animal protocols were performed according to institution guidelines. Mice were sacrificed by cervical dislocation. The cells were prepared from the parotids of WT and Homer2−/− mice by limited collagenase digestion as described previously (21). After isolation, the acinar cells were resuspended in an extracellular physiologic salt solution, the composition of which was as follows (in mm): 140 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 HEPES, and 10 glucose, adjusted to pH 7.4 and 310 mosm.
Cell Culture and DNA Transfection
HEK293 cells (Korean Cell Line Bank, South Korea) were maintained in Dulbecco's modified Eagle's medium (Invitrogen) containing 10% fetal bovine serum (Invitrogen) and 100 units/ml penicillin and streptomycin in a 5% CO2 incubator. Approximately 1–5 × 105 cells were seeded onto coverslips in 60-mm dishes and then incubated in antibiotic-free medium. The next day, DNA was mixed with Lipofectamine reagent (Invitrogen) and Opti-MEM, incubated for 20 min at room temperature, and then added to the cell culture media. The transfected cells were assayed at 38–48 h after post-transfection.
Measurement of Intracellular Ca2+ Concentration ([Ca2+]i)
Parotid acinar cells from WT and Homer2−/− mice were loaded with 5 μm Fura-2/AM (Teflabs Inc., Austin, TX) and 0.05% pluronic F-127 for 60 min in physiologic salt solution. Fura-2 fluorescence was measured at the appropriate excitation wavelengths (340/380 nm) and emission at 510 nm wavelengths (ratio = F340/F380) using a Molecular Devices (Downingtown, PA) imaging system. The emitted fluorescence was monitored with a charge-coupled device camera (Photometrics, Tucson, AZ) attached to an inverted microscope. Fluorescence images were obtained at 2-s intervals. All data were analyzed using MetaFluor software (Molecular Devices).
Immunocytochemistry
The immunostaining procedure was described previously (14). In brief, parotid acinar cells from WT and Homer2−/− mice were stained with antibodies against Homer2 (1:50); IP3R1, -2, or -3 (1:100); SERCA2, PMCA, or PMCA4 (1:200). Staining was detected using goat anti-rabbit or anti-mouse IgG conjugated to fluorescein or rhodamine. Images were collected with confocal LSM 510 and LSM 710 laser scanning microscopes (Zeiss, Göttingen, Germany).
Western Blotting
Protein extracts were prepared from parotid, submandibular gland, and pancreatic acini from WT and Homer2−/− mice. Acinar cells were lysed in a buffer containing 150 mm NaCl, 10 mm Tris (pH 7.8 with HCl), 1 mm EDTA, 1% Nonidet P-40, 0.1% SDS, and protease inhibitors (i.e. 2 mm Na3VO4, 10 mm NaF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 10 μg/ml PMSF). The extracts were spun at 13,000 × g for 15 min, and protein concentration was measured using a Bio-Rad protein assay. The samples (50 μg of protein/well) were separated to 6–8% SDS-polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane, blocked with 5% skimmed milk, and incubated overnight with the specific primary antibody, an anti-SERCA2b (1:500), anti-PMCA4 (JA9, 1:500), and anti-PMCA (5F10, 1:500), at 4 °C. The blots were exposed to horseradish peroxidase-conjugated secondary antibodies for 1 h and detected by chemiluminescence (Amersham Biosciences).
Immunoprecipitation
The immunoprecipitation procedure was modified from Shin et al. (22) and Kim et al. (23). In brief, parotid and HEK293 microsomes were prepared by homogenizing a minced parotid and harvested HEK293 in a buffer containing 20 mm Mops (pH 6.7 with KOH), 250 mm sucrose, 1 mm EDTA, 1 mm MgCl2, 10 mm benzamidine, and 0.2 mm PMSF. The homogenized samples were centrifuged at 400 × g for 10 min. The supernatants were collected and centrifuged at 900 × g for 10 min at 4 °C. To avoid protein degradation by digestive enzymes, immunoprecipitation was initiated immediately after completion of microsomal preparation. Microsomes were lysed in a buffer containing 50 mm Tris (pH 6.8 with HCl), 150 mm NaCl, 3 mm EDTA, 2 mm EGTA, and 0.5% Triton X-100 supplemented with protease inhibitors. The lysates were cleared by centrifugation at 14,000 × g for 15 min. About 150 μl of the extract (300 μg of protein/sample) was incubated with 10 μl of anti-PMCA (5F10) or 10 μl of anti-FLAG antibodies for 2 h by rocking at 4 °C. Protein A/G-agarose (Thermo Fisher Scientific Inc., Waltham, MA) was added to each mixture, and rocking was continued overnight at 4 °C. Protein A/G-agarose was pelleted at 1,000 × g for 10 s, and the beads were quickly washed with cold PBS. The immunoprecipitated proteins were separated by SDS-PAGE and probed with anti-FLAG (M2, 1:1,000) by overnight incubation at 4 °C.
Measurement of [Ca2+] Efflux
To measure directly the rate of Ca2+ efflux by PMCA, we measured the appearance of Ca2+ in the external medium using the procedure published by Zhao et al. (24) with slight modifications. Intact parotid acini from WT and Homer2−/− mice were washed once and then suspended in 1 ml of medium containing 140 mm NaCl, 5 mm KCl, 10 mm glucose, and 10 mm HEPES, pH 7.4, with NaOH. 100 μl of cell suspension were added to 1.5 ml of a similar medium containing 2 μm free acid Fura-2 in a cuvette. After initiation of fluorescence recording, 10 μm EGTA was added to reduce the extracellular Ca2+ concentration ([Ca2+]o) to ∼100 nm. After establishing a baseline leak for ∼1 min, the cells were stimulated with 1 mm carbachol. At the end of the experiment, the signals were calibrated by adding 1 mm CaCl2 and then 1 mm MnCl2 to the medium as described previously (24). The design of experiments for PMCA stimulation while measuring cytoplasmic Ca2+ was as follows. The cells were stimulated with 1 mm carbachol and 100 μm CPA for about 15–20 s to release most of the Ca2+ from internal stores and inhibit the SERCA Ca2+ pumps. Efflux by PMCA was initiated by inhibition of the muscarinic receptors with the antagonist 10 μm atropine and removal of external Ca2+ to prevent Ca2+ influx. CPA was maintained to inhibit the SERCA pumps.
Measurement of Ca2+ Uptake and Release from Internal Stores
IP3-mediated Ca2+ release from internal stores was measured in SLO-permeabilized cells as described previously (24). Cells were washed with a high K+ (120 mm KCl, 20 mm NaCl, 10 mm glucose, and 10 mm HEPES, pH 7.4, with NaOH), Chelex-treated medium and added to Chelex-treated medium containing an ATP regeneration system (composed of 3 mm ATP, 5 mm MgCl2, 10 mm creatine phosphate, and 5 units/ml creatine kinase), a mixture of mitochondrial inhibitors, 2 μm Fluo-3, and 3 mg/ml SLO (Difco). After the addition of cells, the concentration of free Ca2+ in this medium was ∼350–400 nm. In this medium, the cells were permeabilized almost instantaneously so that Ca2+ uptake into the ER could be measured immediately. Uptake of Ca2+ into the ER was allowed to continue until [Ca2+] in the medium was stabilized. Then IP3 was added in increasing concentrations to measure the extent of Ca2+ release and the potency of IP3 in mobilizing Ca2+ from the ER. Subsequent addition of 1 mm carbachol was used to monitor the receptor-evoked Ca2+ release.
Data Analysis and Statistics
All numeric values are represented as the mean ± S.E. Statistical significance, determined to be p < 0.05, was calculated using the Student's unpaired t test.
RESULTS
Homer2 Deletion Does Not Affect the Polarized Expression of IP3Rs, IP3-mediated Ca2+ Release, or SERCA Activity
Comparison of the receptor-evoked Ca2+ signaling in wild-type (WT) and Homer2−/− acini revealed the altered signaling in the Homer2−/− acini. To further examine this phenomenon, we analyzed the expression and activity of key Ca2+ transporters. IP3Rs are established binding partners of Homer proteins (10, 12). Therefore, we first examined the localization and expression of Homer2 and IP3Rs in parotid acinar cells from WT and Homer2−/− mice. In WT parotid acinar cells, Homer2, and IP3Rs, positive staining was primarily observed in the apical pole. As expected, Homer2 staining was not detected in Homer2−/− acini, and the expression and localization of all IP3R isoforms remained unchanged in Homer2−/− acini (Fig. 1A).
FIGURE 1.
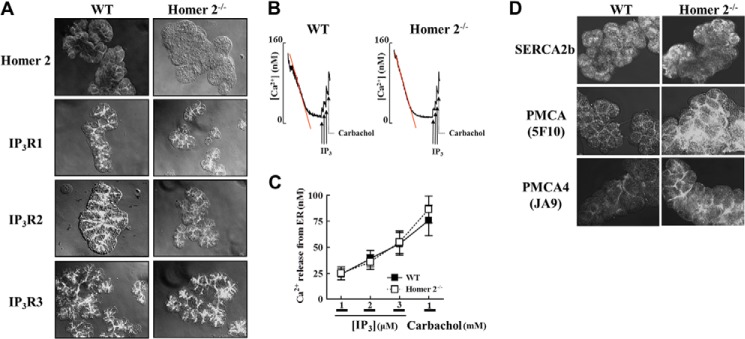
Localization of Homer2, IP3Rs, Ca2+ pumps, and IP3-mediated Ca2+ release in WT and Homer2−/− cells. A, parotid acini from WT and Homer2−/− mice were stained for Homer2, IP3R1, IP3R2, and IP3R3. Deletion of Homer2 did not affect the expression and localization of the IP3Rs. B, cells from WT and Homer2−/− mice were permeabilized with SLO and allowed to reduce [Ca2+]i in the incubation media to approximately 75 nm. Next, Ca2+ release was measured by adding increasing concentrations of IP3 (solid arrows) and 1 mm carbachol (dotted arrows). C, quantitation of the results shown in B. D, parotid acini were stained for SERCA2b, total PMCA, and PMCA isoform 4 (PMCA4). Expression of PMCA increased in the apical membrane of Homer2−/− cells. Data are presented as the mean ± S.E.
We next examined whether Homer2 deletion affects Ca2+ uptake into the ER, as well as the activity of IP3Rs and their response to IP3. Parotid acinar cells from WT and Homer2−/− mice were permeabilized with SLO within 10–15 s, and the [Ca2+] in the incubation media was reduced to 50–80 nm within 2 min at 37 °C by SERCA-mediated Ca2+ uptake into the IP3-mobilizable pool, similar to the method used with pancreatic acinar cells (14). The rate and extent of Ca2+ uptake into the ER was similar in WT and Homer2−/− cells, providing the first indication that, unlike in the pancreas (14), Homer2 deletion did not affect parotid acinar cell SERCA activity. The addition of increasing concentrations of IP3 and the muscarinic agonist carbachol resulted in a similar increase in [Ca2+] due to Ca2+ release from stores of SLO-permeabilized WT and Homer2−/− cells (Fig. 1, B and C). Thus, no compensatory effects in expression, localization, and IP3R activity were observed in Homer2−/− mice.
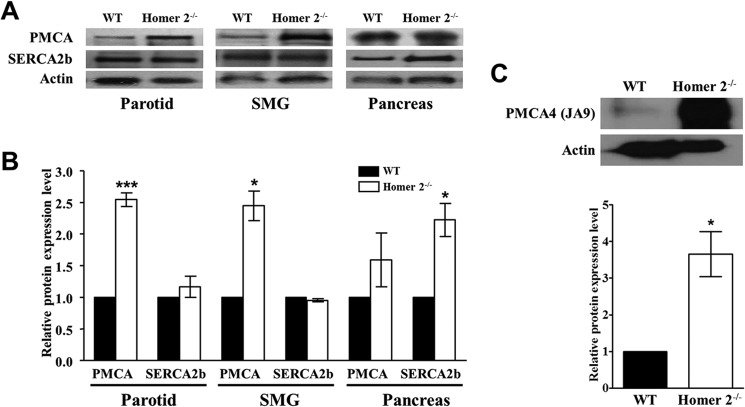
PMCA Expression Is Selectively Increased in Specific Tissues from Homer2−/− Mice
Next, we examined the expression of the SERCA and PMCA pumps. Because both PMCA1 and PMCA4 are expressed in salivary gland cells, we used a pan anti-PMCA antibody, 5F10, which detects both isoforms, to determine PMCA expression. To verify this PMCA expression, we used the anti-PMCA antibody JA9, which is specific for PMCA4 (14, 24). Notably, immunostaining experiments suggested increased PMCA expression in the apical region of Homer2−/− parotid acinar cells, whereas the SERCA2 expression remained unaffected in these mice (Fig. 1D). To further analyze how Ca2+ pump expression was affected by Homer2 deletion, we examined protein expression using Western blotting analyses and parotid membranes prepared from WT and Homer2−/− parotid acinar cells. As shown in Fig. 2, A and B, PMCA expression in parotid acinar cells from Homer2−/− mice increased significantly to 2.5 ± 0.1-fold greater than parotid acinar cells from WT mice (n = 4, p < 0.001). However, the expression of SERCA2b (1.2 ± 0.2-fold of WT, n = 4) remained unchanged in the parotid membranes. Similar results were obtained with submandibular gland membranes. Interestingly, however, opposite results were observed in pancreas membranes, suggesting a tissue-specific adaptive response to Homer2 deletion. Furthermore, the protein levels of the PMCA4 isoform were higher in Homer2−/− parotid membranes compared with WT (3.7 ± 0.6-fold of WT, n = 4, p < 0.05, Fig. 2C).
FIGURE 2.
Expression of Ca2+ pumps in WT and Homer2−/− cells. A, total PMCA and SERCA2b levels were analyzed by Western blot prepared from parotid, submandibular gland, and pancreatic acini from four WT and four Homer2−/− mice. Band intensity was analyzed by densitometry. B, quantitation of Western blot data in A. C, PMCA4 expression increased in the parotid of Homer2−/− mice. Quantitation is shown in the bar graph. Data were normalized to expression levels in WT cells and are presented as the mean ± S.E. *, p < 0.05; ***, p < 0.001 (compared with WT).
Rate of [Ca2+]efflux Is Increased in Homer2−/− Parotid Cells
The major routes for Ca2+ clearance in nonexcitable cells, such as parotid acinar cells, are Ca2+ uptake into the ER by the SERCA pumps and Ca2+ efflux across the plasma membrane by PMCA (25–27). The results shown in Figs. 1B and 2 suggest that Homer2 deletion does not affect SERCA expression and activity but increases the PMCA expression. To determine whether increased PMCA protein expression translates to increased PMCA activity in intact cells, we examined PMCA-mediated Ca2+ clearance in WT and Homer2−/− parotid acini. In the first protocol, cells were stimulated with a high concentration of carbachol and treated with the SERCA inhibitor CPA to release ER Ca2+ and maximally activate Ca2+ influx to cause a large increase in cytoplasmic Ca2+. Ca2+ clearance was then initiated by terminating cell stimulation with atropine while simultaneously inhibiting SERCA activity. Fig. 3A shows that the addition of 10 μm atropine in a Ca2+-free solution resulted in an immediate clearance of Ca2+ primarily by PMCA (Fig. 3A). Comparing the slope of Ca2+ clearance revealed that Ca2+ clearance in Homer2−/− cells is 1.5-fold faster compared with WT cells (1.5 ± 0.3-fold, n = 4, p < 0.05, Fig. 3B). In a second protocol, we assayed PMCA activity by measuring the change in [Ca2+]o in cells incubated in media with low external Ca2+ concentration and stimulated with 1 mm carbachol. As shown in Fig. 3C, [Ca2+]o increased significantly in response to carbachol stimulation in both cell types. Importantly, the change in [Ca2+]o was ∼1.5-fold higher in Homer2−/− cells compared with WT cells (n = 4, p < 0.01, Fig. 3D).
FIGURE 3.
Characterization of Ca2+ signaling in WT and Homer2−/− cells. A, parotid acini from WT and Homer2−/− mice were stimulated with 1 mm carbachol while inhibiting SERCA with 100 μm CPA to elevate [Ca2+]i and prevent Ca2+ uptake by the ER. When [Ca2+]i stabilized at a steady state representing a balance of Ca2+ efflux and influx, Ca2+ clearance was initiated by removing external Ca2+ and adding of 10 μm atropine. B, average rate of Ca2+ clearance was determined from the slope of [Ca2+]i decline as a measure of PMCA activity. Results were normalized to the slope obtained from WT cells and are presented as the mean ± S.E. The rate of Ca2+ clearance increased significantly in Homer2−/− cells. C, WT and Homer2−/− parotid acinar cells in lightly Ca2+-buffered media were exposed to 1 mm carbachol while measuring [Ca2+]o. Homer2−/− cells exhibited an increased rate of [Ca2+]o, indicating a higher rate of PMCA in Homer2−/− than cells from WT mice. D, quantitation of multiple experiments similar to those shown in C. The average rate of increased [Ca2+]o was significantly higher in Homer2−/− cells. Data are depicted as the mean ± S.E. *, p < 0.05; **, p < 0.01 (compared with WT).
Homer2 Interacts with and Regulates PMCA Expression
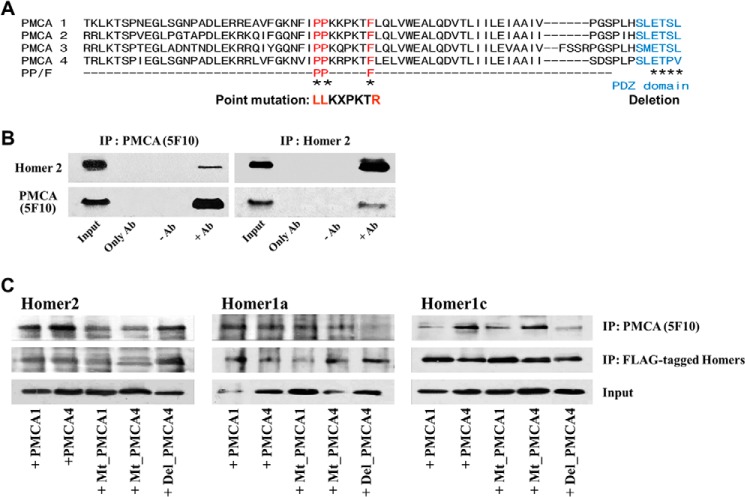
To identify potential interacting sites between PMCA and the Homer proteins, we searched for a PPXXF-like motif in PMCA that may interact with the Ena/VASP homology 1 domain of Homer proteins (16). The only such potential motif is present in the N-terminal 91–98 residues of several PMCA subtypes; however, proline and phenylalanine are separated by more than two residues (Fig. 4A). To determine whether this region functions as a Homer-binding motif to mediate the PMCA-Homer2 interaction, we first examined whether Homer2 selectively binds PMCA in parotid acinar cells from WT mice using co-immunoprecipitation assays. As shown in Fig. 4B, PMCA co-immunoprecipitates with endogenous Homer2 suggesting that endogenous Homer2 associates with PMCA.
FIGURE 4.
Interaction of Homer proteins with the PPXXF-like motif and PDZ-binding domain of PMCAs. A, amino acid sequences of mouse PMCA isoforms were aligned using BLAST. Alignments show the mutations in the PPXXF-like motif (red) and the PDZ-binding motif of PMCA (blue). B, PMCA and Homer2 co-immunoprecipitate from cell lysates prepared from parotid acini of WT mice. In immunoprecipitation (IP) experiments, Input denotes extract samples used for Western blot; Only Ab denotes control IP using antibodies without extract in the IP assay; −Ab denotes control IP using extract without antibodies in the IP assay; and +Ab denotes IP using extract and antibodies in the IP assay. C, co-IP of Homer proteins (FLAG-tagged) and PMCA (wild-type, PPXXF-like motif mutants (Mt_PMCA),or PMCA4 with deleted PDZ-binding motif (Del_PMCA4) co-transfected in HEK293 cells. Extracts prepared from the cells co-transfected with the respective FLAG-Homers and the indicated PMCA constructs were used to IP either PMCA with the 5F10 antibodies or the Homers with anti-FLAG antibodies, and the precipitates were probed with anti-FLAG antibodies to detect the Homers. Note that Mt_PMCAs reduced the interaction of PMCA with Homer2 but not the interaction of PMCA with Homer1a and Homer1c. However, the interaction of PMCA with Homer1a and Homer1c was decreased when the Del_PMCA4 mutant was used.
To examine whether Homer proteins bind PMCA1 and/or PMCA4 in cell lysates and whether mutations in the potential Homer-binding motif of PMCA affect the interaction, we performed co-immunoprecipitation assays using HEK293 cells that transiently express Homer2 and PMCA or PMCA mutants. As shown in Fig. 4C, Homer2 co-immunoprecipitated with PMCA1b and PMCA4b but did not co-immunoprecipitate with the PMCA mutants (Mt_PMCA), which changes the PP/LL and F/R of the PPXXF-like motif. These results indicate that the PPXXF-containing region functions as a Homer-binding motif and mediates Homer2 interaction with PMCAs. To determine whether these mutants interact with other Homer proteins, we examined the association between Homer1 and PMCAs in transfected HEK293 cells. Mutation of PMCA did not affect the interaction with short Homer1a, which lacks the coiled-coil domain, or long Homer1c. This result suggests significant specificity of the Homer-binding motif on PMCA toward Homer2.
PMCA isoforms 2b and 4b have PDZ domain-binding ligands in their C terminus that interact with various scaffolding proteins such as membrane-associated guanylate kinase, NHERF2 (Na+/H+ exchanger regulatory factor-2), NOS-I (nitric-oxide synthase I), and Homer1 (Ania-3) (28–31), some of which interact with the Homers. Therefore, we examined whether mutation of the PDZ ligand of PMCA4 (marked Del_PMCA4) affected the interaction of Homers with PMCA. Co-expression of Homers and Del_PMCA4 showed reduced interaction of Homer1a and Homer1c with Del_PMCA4 compared with their interaction with WT PMCA4. However, interaction of Homer2 was unaffected (Fig. 4C). These results indicate that the PDZ ligand of PMCA4b may function to mediate Homer1 variant interaction with PMCA.
A previous study suggests that exogenous expression of Homer1a, Homer1c, or Homer2a had no effect on endogenous PMCA function, whereas knockdown of Homer1 slowed PMCA-mediated Ca2+ clearance in neuronal cells (44). We re-examined these effects in nonexcitable cells by expressing the Homers in HEK293 cells and measured native PMCA activity. Transfection of Homer1a enhanced native PMCA activity, whereas transfection of the long Homers, Homer1c or Homer2, had no effect (Fig. 5A). These findings suggest that the level of endogenous long Homers are saturating with respect to PMCA in HEK293 cells, and thus further expression had no inhibitory effect. Accordingly, Homer1a likely relieved the tonic inhibition by the long Homers to activate the native PMCA activity. Therefore, to examine the relationship between the Homer-binding motif and PMCA activity, we measured the effect of Homer proteins on Ca2+ extrusion by expressed WT and mutant PMCA pumps. Overexpression of WT PMCAs increased Ca2+ clearance, and expression of the PPXXF-like mutants additionally accelerated Ca2+ clearance (Fig. 5, B and E). Co-transfection of Homer2 and WT PMCA pumps significantly slowed Ca2+ clearance. This inhibitory effect was abolished when Homer2 was co-expressed with the PMCA mutants (Fig. 5, C and E). However, Homer1a did not increase PMCA activity when cells were co-transfected with WT and mutant PMCA pumps compared with the overexpression of Homer1a or WT and mutant PMCA pumps (Fig. 5, D and E). These results indicate that Homer2 interaction with the PPXXF-like motif on PMCA is essential to regulate Ca2+ clearance and Ca2+ signaling in nonexcitable cells.
FIGURE 5.
Effects of the Homers on Ca2+ clearance by PMCA in HEK293 cells. A, overexpression of Homer1a increases the rate of Ca2+ clearance, whereas expression of Homer1c and Homer2 do not (panel a). Panel b, quantitation of the results in (panel a). B, overexpression of WT PMCA (panel a) and Mt_PMCA (panel b) increase the rate of the Ca2+ clearance. C, overexpression of Homer2 significantly decreases the effect of WT PMCA (panel a) but not of Mt_PMCA (panel b) on Ca2+ efflux in co-transfected HEK293 cells. D, overexpression of Homer1a does not activate WT PMCA (panel a) or Mt_PMCA (panel b) in co-transfected cells. E, quantitation of PMCA activity in B–D. Results were normalized to GFP expression levels and are presented as the mean ± S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (compared with GFP).
DISCUSSION
In this study, we demonstrate a novel interaction between Homer2 and PMCA in native parotid acinar cells. Furthermore, we provide evidence in support of a critical role for Homer2 in modulating PMCA activity and thus Ca2+ signaling. Previous reports that focused on the molecular structure of Homer proteins in the CNS indicated that these proteins bind GPCRs, such as mGluR1/5, and IP3Rs, as well as act as scaffolding proteins for assembling Ca2+-signaling complexes in cellular microdomains (8–10, 13, 32). The apical region of exocrine cells is equivalent to the CNS synaptic region, where signaling complexes are clustered to form a “trigger zone” from which all forms of Ca2+ signals, including Ca2+ waves, are initiated (33, 34). Consistently, immunocytochemical studies have demonstrated that all IP3R types are highly enriched in the apical region (14, 35, 36). Accordingly, we found that Homer2 and IP3Rs are expressed in the apical pole of parotid acinar cells. However, Homer2 deletion had no effect on the expression or function of any of the IP3R isoforms, suggesting that Homer2 may have another role on Ca2+ signaling in parotid acinar cells.
Further analyses revealed that Homer2 affected PMCA expression in the apical membranes of parotid acinar cells. Hence, the most interesting finding from our study is the adaptive increase in PMCA protein expression in Homer2-deficient parotid and submandibular gland acinar cells. Like SERCA, the PMCAs have a crucial role in maintaining Ca2+ homeostasis (37). Previous studies suggested that PMCAs are expressed in both the lateral and apical regions of pancreatic, submandibular gland, and parotid cells, as well as in the brain (31, 38–41). In addition, PMCAs co-localize with mGluR1, IP3R1, and Homer proteins, including Homer1a, Homer1b/c, and Homer3, in neurons (31, 40, 41). Here, we discovered that Homer2 interacts with PMCA and decreases the rate of [Ca2+]i clearance in native parotid acinar cells, which is absent in Homer2-deficient mice. These results are similar to previous findings showing up-regulation of specific PMCA isoforms due to adaptations in Ca2+ signaling and Ca2+-dependent cellular functions in pancreatic and submandibular gland acinar cells from serca2-deficient mice (24). Therefore, it appears that PMCA expression and function is particularly sensitive to perturbations in Ca2+ signaling, and cells modify PMCA activity to adjusting the Ca2+ signal.
Adaptations of PMCA expression may be regulated by Ca2+ itself. Several studies have shown that Ca2+ can alter the expression levels of Ca2+-signaling components, such as pumps and channels, thereby maintaining flexibility in Ca2+-signaling remodeling systems (3). In primary cultured cerebellar granule cells, PMCA2, PMCA3, and IP3R1 expression is up-regulated during the activation of Ca2+-dependent events. In contrast, type 2 Na+/Ca2+ exchanger and PMCA4 are rapidly down-regulated (1, 42, 43). An interesting aspect of our findings is that Homer2-deficient parotid cells exhibit increased expression and activity of PMCA but not of SERCA2. However, Homer2-deficient pancreatic acinar cells increase SERCA2 but not PMCA expression, suggesting that PMCA adaptation occurs in a tissue-specific manner, perhaps reflecting specific cellular functions and localization.
PMCAs interact with partner molecules through their PDZ-binding motif in the C terminus (28–30). In this study, we characterized an additional novel binding region (PPXXF-like motif) of PMCA isoforms that participate in the interaction with, and inhibition of, PMCA by Homer2. Thus, mutation of the PPXXF-like motif reduced the interaction of PMCA1 and PMCA4 with Homer2 (Fig. 4C) and prevented inhibition of ectopically expressed PMCA by Homer2 (Fig. 5E). In contrast, the PMCA4 PDZ motif does not appear to be essential for the interaction with Homer2 because its deletion did not prevent the interaction of PMCA4 with Homer2 (Fig. 4C).
Interestingly, the role of the PPXXF-like motif appears to be specific for the interaction of PMCAs with Homer2. Thus, mutation of the PPXXF-like motif does not affect the interaction of PMCAs with a short or long Homer1 (Fig. 4C) or activation of PMCA activity by Homer1a in co-transfected HEK293 cells (Fig. 5, D and E). These results were unexpected in view of the reports that Homer1a (Ania-3) retains binding to the PDZ-binding motif in the C terminus of PMCA isoforms and activates [Ca2+]i clearance by PMCA when expressed with long Homer1 or the N terminus of Homer2 as an analog of Homer1a in neuronal cells (31, 44). Using expression in HEK293 cells, we confirmed interaction of Homer1 with the PDZ motif of PMCA. We found that this interaction is eliminated by deletion of the PDZ motif on PMCA4, as was reported previously (31). However, unlike a previous report, the Ca2+ clearance rate by native PMCA was increased by overexpression of short Homer1a but not by overexpression of long Homers (Fig. 5A) (44). These experiments suggest that the level of long Homers in HEK293 cells (and perhaps other nonexcitable cells) is saturating with respect to PMCA, and thus additional expression of the long Homers caused no further inhibition. A prediction of this interpretation is the deletion of the long Homers, and their inhibition by the short Homer1a should accelerate PMCA activity and Ca2+ clearance. This was indeed the case, in which deletion of Homer2 in mice or expression of Homer1a in HEK293 cells increased the native PMCA activity.
In summary, the key findings of the our study are as follows: 1) Homer2 binding to the PPXXF-like motif of PMCAs inhibited PMCA activity, and 2) expression and binding of Homer1 to the PDZ domain of PMCAs increased their activity. Thus, PMCAs undergo dual regulation by Homer proteins, i.e. inhibition by the Homer2 and stimulation by Homer1. In this manner, Homer proteins can regulate the duration of the Ca2+ signal in parotid acinar cells to either extend or shorten the signal by inhibition or stimulation of PMCA activity, respectively.
Acknowledgments
We thank Dr. Paul F. Worley for sharing the Homer2−/− mice and Boryung Park for excellent technical support for the immunoprecipitation experiment.
This work was authored, in whole or in part, by National Institutes of Health staff. This work was supported by the grant (NRF-2012R1A2A1A01003487) from the Korea government (MSIP).
- GPCR
- G protein-coupled receptor
- SERCA
- sarco/endoplasmic reticulum Ca2+-ATPase
- PMCA
- plasma membrane Ca2+-ATPase
- IP3R
- inositol 1,4,5-triphosphate receptors
- IP3
- inositol 1,4,5-trisphosphate
- ER
- endoplasmic reticulum
- SLO
- streptolysin O
- IP
- immunoprecipitation
- CPA
- cyclopiazonic acid.
REFERENCES
- 1. Berridge M. J., Lipp P., Bootman M. D. (2000) The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21 [DOI] [PubMed] [Google Scholar]
- 2. Petersen O. H., Tepikin A. V. (2008) Polarized calcium signaling in exocrine gland cells. Annu. Rev. Physiol. 70, 273–299 [DOI] [PubMed] [Google Scholar]
- 3. Berridge M. J., Bootman M. D., Roderick H. L. (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]
- 4. Fagni L., Chavis P., Ango F., Bockaert J. (2000) Complex interactions between mGluRs, intracellular Ca2+ stores and ion channels in neurons. Trends Neurosci. 23, 80–88 [DOI] [PubMed] [Google Scholar]
- 5. Szumlinski K. K., Kalivas P. W., Worley P. F. (2006) Homer proteins: implications for neuropsychiatric disorders. Curr. Opin. Neurobiol. 16, 251–257 [DOI] [PubMed] [Google Scholar]
- 6. Kiselyov K., Shin D. M., Muallem S. (2003) Signalling specificity in GPCR-dependent Ca2+ signalling. Cell. Signal. 15, 243–253 [DOI] [PubMed] [Google Scholar]
- 7. Worley P. F., Zeng W., Huang G., Kim J. Y., Shin D. M., Kim M. S., Yuan J. P., Kiselyov K., Muallem S. (2007) Homer proteins in Ca2+ signaling by excitable and non-excitable cells. Cell Calcium 42, 363–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fagni L., Worley P. F., Ango F. (2002) Homer as both a scaffold and transduction molecule. Sci. STKE 2002, re8. [DOI] [PubMed] [Google Scholar]
- 9. Kato A., Ozawa F., Saitoh Y., Fukazawa Y., Sugiyama H., Inokuchi K. (1998) Novel members of the Vesl/Homer family of PDZ proteins that bind metabotropic glutamate receptors. J. Biol. Chem. 273, 23969–23975 [DOI] [PubMed] [Google Scholar]
- 10. Tu J. C., Xiao B., Yuan J. P., Lanahan A. A., Leoffert K., Li M., Linden D. J., Worley P. F. (1998) Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron 21, 717–726 [DOI] [PubMed] [Google Scholar]
- 11. Brakeman P. R., Lanahan A. A., O'Brien R., Roche K., Barnes C. A., Huganir R. L., Worley P. F. (1997) Homer: a protein that selectively binds metabotropic glutamate receptors. Nature 386, 284–288 [DOI] [PubMed] [Google Scholar]
- 12. Yuan J. P., Kiselyov K., Shin D. M., Chen J., Shcheynikov N., Kang S. H., Dehoff M. H., Schwarz M. K., Seeburg P. H., Muallem S., Worley P. F. (2003) Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell 114, 777–789 [DOI] [PubMed] [Google Scholar]
- 13. Xiao B., Tu J. C., Petralia R. S., Yuan J. P., Doan A., Breder C. D., Ruggiero A., Lanahan A. A., Wenthold R. J., Worley P. F. (1998) Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron 21, 707–716 [DOI] [PubMed] [Google Scholar]
- 14. Shin D. M., Dehoff M., Luo X., Kang S. H., Tu J., Nayak S. K., Ross E. M., Worley P. F., Muallem S. (2003) Homer 2 tunes G protein-coupled receptors stimulus intensity by regulating RGS proteins and PLCβ GAP activities. J. Cell Biol. 162, 293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feng W., Tu J., Yang T., Vernon P. S., Allen P. D., Worley P. F., Pessah I. N. (2002) Homer regulates gain of ryanodine receptor type 1 channel complex. J. Biol. Chem. 277, 44722–44730 [DOI] [PubMed] [Google Scholar]
- 16. Tu J. C., Xiao B., Naisbitt S., Yuan J. P., Petralia R. S., Brakeman P., Doan A., Aakalu V. K., Lanahan A. A., Sheng M., Worley P. F. (1999) Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron 23, 583–592 [DOI] [PubMed] [Google Scholar]
- 17. Sala C., Roussignol G., Meldolesi J., Fagni L. (2005) Key role of the postsynaptic density scaffold proteins Shank and Homer in the functional architecture of Ca2+ homeostasis at dendritic spines in hippocampal neurons. J. Neurosci. 25, 4587–4592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gray N. W., Fourgeaud L., Huang B., Chen J., Cao H., Oswald B. J., Hémar A., McNiven M. A. (2003) Dynamin 3 is a component of the postsynapse, where it interacts with mGluR5 and Homer. Curr. Biol. 13, 510–515 [DOI] [PubMed] [Google Scholar]
- 19. Bottai D., Guzowski J. F., Schwarz M. K., Kang S. H., Xiao B., Lanahan A., Worley P. F., Seeburg P. H. (2002) Synaptic activity-induced conversion of intronic to exonic sequence in Homer 1 immediate early gene expression. J. Neurosci. 22, 167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang G. N., Huso D. L., Bouyain S., Tu J., McCorkell K. A., May M. J., Zhu Y., Lutz M., Collins S., Dehoff M., Kang S., Whartenby K., Powell J., Leahy D., Worley P. F. (2008) NFAT binding and regulation of T cell activation by the cytoplasmic scaffolding Homer proteins. Science 319, 476–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zeng W., Lee M. G., Yan M., Diaz J., Benjamin I., Marino C. R., Kopito R., Freedman S., Cotton C., Muallem S., Thomas P. (1997) Immuno and functional characterization of CFTR in submandibular and pancreatic acinar and duct cells. Am. J. Physiol. 273, C442–C455 [DOI] [PubMed] [Google Scholar]
- 22. Shin D. M., Zhao X. S., Zeng W., Mozhayeva M., Muallem S. (2000) The mammalian Sec6/8 complex interacts with Ca2+ signaling complexes and regulates their activity. J. Cell Biol. 150, 1101–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim J. Y., Zeng W., Kiselyov K., Yuan J. P., Dehoff M. H., Mikoshiba K., Worley P. F., Muallem S. (2006) Homer 1 mediates store- and inositol 1,4,5-trisphosphate receptor-dependent translocation and retrieval of TRPC3 to the plasma membrane. J. Biol. Chem. 281, 32540–32549 [DOI] [PubMed] [Google Scholar]
- 24. Zhao X. S., Shin D. M., Liu L. H., Shull G. E., Muallem S. (2001) Plasticity and adaptation of Ca2+ signaling and Ca2+-dependent exocytosis in SERCA2+/− mice. EMBO J. 20, 2680–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bruce J. I., Yule D. I., Shuttleworth T. J. (2002) Ca2+-dependent protein kinase–a modulation of the plasma membrane Ca2+-ATPase in parotid acinar cells. J. Biol. Chem. 277, 48172–48181 [DOI] [PubMed] [Google Scholar]
- 26. Homann V., Kinne-Saffran E., Arnold W. H., Gaengler P., Kinne R. K. (2006) Calcium transport in human salivary glands: a proposed model of calcium secretion into saliva. Histochem. Cell Biol. 125, 583–591 [DOI] [PubMed] [Google Scholar]
- 27. Gorr S. U., Venkatesh S. G., Darling D. S. (2005) Parotid secretory granules: crossroads of secretory pathways and protein storage. J. Dent. Res. 84, 500–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DeMarco S. J., Strehler E. E. (2001) Plasma membrane Ca2+-ATPase isoforms 2b and 4b interact promiscuously and selectively with members of the membrane-associated guanylate kinase family of PDZ (PSD95/Dlg/ZO-1) domain-containing proteins. J. Biol. Chem. 276, 21594–21600 [DOI] [PubMed] [Google Scholar]
- 29. DeMarco S. J., Chicka M. C., Strehler E. E. (2002) Plasma membrane Ca2+ATPase isoform 2b interacts preferentially with Na+/H+ exchanger regulatory factor 2 in apical plasma membranes. J. Biol. Chem. 277, 10506–10511 [DOI] [PubMed] [Google Scholar]
- 30. Schuh K., Uldrijan S., Telkamp M., Rothlein N., Neyses L. (2001) The plasma membrane calmodulin-dependent calcium pump: a major regulator of nitric oxide synthase I. J. Cell Biol. 155, 201–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sgambato-Faure V., Xiong Y., Berke J. D., Hyman S. E., Strehler E. E. (2006) The Homer-1 protein Ania-3 interacts with the plasma membrane calcium pump. Biochem. Biophys. Res. Commun. 343, 630–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kammermeier P. J., Xiao B., Tu J. C., Worley P. F., Ikeda S. R. (2000) Homer proteins regulate coupling of group I metabotropic glutamate receptors to N-type calcium and M-type potassium channels. J. Neurosci. 20, 7238–7245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee M. G., Xu X., Zeng W., Diaz J., Wojcikiewicz R. J., Kuo T. H., Wuytack F., Racymaekers L., Muallem S. (1997) Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. Correlation with initiation and propagation of [Ca2+]i waves. J. Biol. Chem. 272, 15765–15770 [DOI] [PubMed] [Google Scholar]
- 34. Tojyo Y., Tanimura A., Matsumoto Y. (1997) Imaging of intracellular Ca2+ waves induced by muscarinic receptor stimulation in rat parotid acinar cells. Cell Calcium 22, 455–462 [DOI] [PubMed] [Google Scholar]
- 35. Zhang X., Wen J., Bidasee K. R., Besch H. R., Jr., Wojcikiewicz R. J., Lee B., Rubin R. P. (1999) Ryanodine and inositol trisphosphate receptors are differentially distributed and expressed in rat parotid gland. Biochem. J. 340, 519–527 [PMC free article] [PubMed] [Google Scholar]
- 36. Takemura H., Yamashina S., Segawa A. (1999) Millisecond analyses of Ca2+ initiation sites evoked by muscarinic receptor stimulation in exocrine acinar cells. Biochem. Biophys. Res. Commun. 259, 656–660 [DOI] [PubMed] [Google Scholar]
- 37. Floyd R., Wray S. (2007) Calcium transporters and signalling in smooth muscles. Cell Calcium 42, 467–476 [DOI] [PubMed] [Google Scholar]
- 38. Baggaley E., McLarnon S., Demeter I., Varga G., Bruce J. I. (2007) Differential regulation of the apical plasma membrane Ca2+-ATPase by protein kinase A in parotid acinar cells. J. Biol. Chem. 282, 37678–37693 [DOI] [PubMed] [Google Scholar]
- 39. Lee M. G., Xu X., Zeng W., Diaz J., Kuo T. H., Wuytack F., Racymaekers L., Muallem S. (1997) Polarized expression of Ca2+ pumps in pancreatic and salivary gland cells. Role in initiation and propagation of [Ca2+]i waves. J. Biol. Chem. 272, 15771–15776 [DOI] [PubMed] [Google Scholar]
- 40. Kurnellas M. P., Lee A. K., Li H., Deng L., Ehrlich D. J., Elkabes S. (2007) Molecular alterations in the cerebellum of the plasma membrane calcium ATPase 2 (PMCA2)-null mouse indicate abnormalities in Purkinje neurons. Mol. Cell. Neurosci. 34, 178–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sandonà D., Scolari A., Mikoshiba K., Volpe P. (2003) Subcellular distribution of Homer 1b/c in relation to endoplasmic reticulum and plasma membrane proteins in Purkinje neurons. Neurochem. Res. 28, 1151–1158 [DOI] [PubMed] [Google Scholar]
- 42. Genazzani A. A., Carafoli E., Guerini D. (1999) Calcineurin controls inositol 1,4,5-trisphosphate type 1 receptor expression in neurons. Proc. Natl. Acad. Sci. U.S.A. 96, 5797–5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guerini D., García-Martin E., Gerber A., Volbracht C., Leist M., Merino C. G., Carafoli E. (1999) The expression of plasma membrane Ca2+ pump isoforms in cerebellar granule neurons is modulated by Ca2+. J. Biol. Chem. 274, 1667–1676 [DOI] [PubMed] [Google Scholar]
- 44. Salm E. J., Thayer S. A. (2012) Homer proteins accelerate Ca2+ clearance mediated by the plasma membrane Ca2+ pump in hippocampal neurons. Biochem. Biophys. Res. Commun. 424, 76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]