Background: TNFR1 ligation activates NF-κB and JNK signals, regulating cell survival and death.
Results: Tristetraprolin recruited to the TNFR1 is inducibly modified by TRAF2, resulting in prolonged JNK activation.
Conclusion: TTP is involved in the balance of JNK-mediated cell survival versus death.
Significance: TTP appears to be a novel regulatory component of TNFR1 signaling.
Keywords: Apoptosis, c-Jun N-terminal Kinase (JNK), Cell Proliferation, NF-κB, Tumor Necrosis Factor (TNF), Tristetraprolin
Abstract
Binding of TNF to its receptor (TNFR1) elicits the spatiotemporal assembly of two signaling complexes that coordinate the balance between cell survival and cell death. We have shown previously that, following TNF treatment, the mRNA decay protein tristetraprolin (TTP) is Lys-63-polyubiquitinated by TNF receptor-associated factor 2 (TRAF2), suggesting a regulatory role in TNFR signaling. Here we demonstrate that TTP interacts with TNFR1 in a TRAF2-dependent manner, thereby initiating the MEKK1/MKK4-dependent activation of JNK activities. This regulatory function toward JNK activation but not NF-κB activation depends on lysine 105 of TTP, which we identified as the corresponding TRAF2 ubiquitination site. Disabling TTP polyubiquitination results in enhanced TNF-induced apoptosis in cervical cancer cells. Together, we uncover a novel aspect of TNFR1 signaling where TTP, in alliance with TRAF2, acts as a balancer of JNK-mediated cell survival versus death.
Introduction
TNF is a proinflammatory cytokine produced by many cell types under various physiological and pathophysiological conditions and causes a broad range of molecular responses, including inflammation, cellular survival, proliferation, apoptosis, and necroptosis. Therefore, TNF signaling needs to be kept in check to prevent adverse effects such as chronic inflammatory diseases, including cancer-related inflammation (1–4). TNF elicits its function by binding to TNFRs,3 a family of transmembrane proteins consisting of more than 27 members of which TNFR type 1 (TNFR1) has been studied most extensively (5). Binding of TNF to this receptor results in the formation of two temporally and spatially separated signaling complexes, I and II, that crucially affect the fate of a cell. Complex I triggers the expression of antiapoptotic proteins and promotes survival, whereas complex II (or death-inducing complex) initiates processes leading to cell death (6).
Chronologically, after binding of TNF, complex I assembles first and triggers distinct forward signaling cascades leading to the activation of the transcription factors NF-κB and activator protein 1 (AP-1) (7, 8). Therefore, the cytoplasmatic tail of TNFR1 interacts with the adaptor TNF receptor-associated death domain, followed by recruitment of the protein kinase RIP1 and the signal transducer TRAF2. Specifically, signal propagation activating NF-κB, which depends on RIP1 ubiquitination by the linear ubiquitin chain assembly complex and IKK complex activation, results in the transcription of antiapoptotic and antioxidant genes (9–11). The former include the caspase inhibitors cIAP1/2 and the procaspase 8 inhibitory protein cFLIP, which impede proapoptotic signaling brought about by complex II during prolonged stimulations. The latter genes, in turn, aid in the elimination of proapoptotic reactive oxygen species built up in TNF-exposed cells (12–14).
Concomitant to NF-κB activation, JNK is activated dependent on TRAF2 and recruitment of the antiapoptotic proteins cIAP1/2. This part of the cascade, which involves the MAPKs MEKK1 and MKK4/7, regulates processes such as differentiation, proliferation, and apoptosis (15–17). Initially, the TNF-induced TRAF2-dependent JNK activation is rapid and transient and guarantees cell cycle progression by controlling cyclin D expression, which, in turn, entails the activity of the G1/S transitory transcription factor E2F (18). This early activation of JNK is followed by a delayed and persistent activation that is partially dependent on NF-κB inhibition and triggered by reactive oxygen species (19). Contrary to its transient activity, persistent JNK favors the onset of apoptosis. It has been noted that AP1 is crucial for the expression of proapoptotic genes, and it has been shown recently that JNK induces apoptosis by regulating cFLIP turnover, thereby releasing the brake on death-promoting complex II (20).
The formation of TNFR complex II is temporally and spatially separated from complex I and depends on internalization of the ligand-bound receptor. Accordingly, inhibition of its endocytosis results in a resistance to apoptosis (21, 22). Upon internalization, conformational changes within complex I adaptor proteins as well as deubiquitination of RIP1 evoke the recruitment of the Fas-associated death domain, procaspase 8, and RIP3 (6). Subsequently, activated caspase 8 transmits the signal to downstream effectors such as caspase 3 and poly(ADP-ribose) polymerase 1 (PARP-1), finally resulting in apoptotic cell death (23). In contrast, when caspase 8 activities are blocked, RIP1 and RIP3 form the “necroptosome,” resulting in the necrotic demise of the cell (24).
The expression of TTP is induced rapidly by a variety of growth factors and cytokines, including TNF (25–27). The characterization of TTP knockout mice, which display a systemic inflammatory syndrome because of chronic TNF excess, unraveled its role as an mRNA decay factor within the AU-rich element-mediated mRNA decay pathway (28, 29). TTP can bind to various AU-rich element-containing mRNAs encoding, for example, tumor-promoting factors as well as proinflammatory mediators, including TNF (30–33). It has been noted early on that TTP is heavily phosphorylated, for example during serum stimulation (34). The major phosphorylation sites in human TTP have been identified by mass spectrometry, and subsequent biochemical studies suggest that phosphorylation of TTP occurs through a number of kinases such as PKB/AKT, p38 MAPK, MK2, ERK1, MEKK1, and JNK (35–41). However, the effect of phosphorylation in controlling the AU-rich element-binding ability of TTP remains controversial. We showed earlier that MEKK1-mediated phosphorylation serves as a prerequisite for its Lys-63-linked polyubiquitination by TRAF2 (41), pointing toward a role of TTP in TNFR signaling.
Previously, we and others have described a novel role for TTP in NF-κB signaling that occurs independently of its well described mRNA-destabilizing function (42, 43). Here we focused on the specific contribution of TTP in JNK signaling. On the basis of detailed analyses of the TNF-induced signaling kinetics, we were able to correlate JNK activation with different TTP phosphorylation forms. We demonstrate that TTP inducibly associates with TNFR1 and uncover that lysine 105 of TTP, being ubiquitinated by TRAF2, is required for JNK but not NF-κB signaling. Studies of cervical cancer cells suggest that the phosphorylation and ubiquitination status of TTP may be an important feature of malignancy with potential therapeutic applications.
EXPERIMENTAL PROCEDURES
Plasmids and Cloning
To generate FLAGTTPK105R/MycTTPK105R out of pcDNA3.1FLAG-TTP/pCMVMyc-TTP (41, 43), site-directed mutagenesis was performed with K105R forward 5′-GCGCTACAGGACTGAGCTATGTCGGACCTTCTCAGAGAGTGGGC-3′ and K105R reverse 5′-C TCTGAGAAGGTCCGACATAGCTCAGTCCTGTAGCGCGAGGGGGTG-3′ using the Expand High FidelityPLUS PCR system (Roche).
Western Blotting
Cells were resuspended in 2× Laemmli buffer, proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes (Hybond-C, Amersham Biosciences), and then filters were blocked in 5% nonfat dry milk (TBS/0.1% Tween 20) before antibody incubation. Primary antibodies against p-MEKK1, MEKK1, β-actin, TRAF2, cJun, and cyclin D1 were obtained from Santa Cruz Biotechnology (catalog nos. sc-130202, sc-252, sc-1616, sc-876, sc-1694, and sc-450). p-MKK4, MKK4, p-JNK, JNK, p-cJun, as well as the antibody detecting cleaved caspase 3 were from Cell Signaling Technology (catalog nos. 4514, 9152, 9251, 9252, 2361, and 9664). Antibodies against GAPDH and PARP-1 were obtained from Millipore (catalog no. MAB-374) and Enzo (catalog no. BML-SA250), and the TTP antibody was provided by Pavel Kovarik (44).
Cell Culture
WT, TTP−/−, and TRAF2−/− MEFs were provided by P. J. Blackshear and Tak Mak, respectively (44, 45). HEK 293 (catalog no. ACC305), HeLa (catalog no. ACC57), SiHa (catalog no. HTB-35) and C33A (catalog no. HTB-31) cells were obtained from the ATCC and cultured in DMEM (Bio-Whitaker) supplemented with 10% FCS (Sigma), 2 mm l-glutamine (Sigma), penicillin (100 units/ml), and streptomycin (100 μg/ml)). HUVECs were isolated from umbilical cords as described previously (46) and maintained in M199 medium (Lonza) supplemented with 20% FCS (Sigma), 2 mm l-glutamine (Sigma), penicillin (100 units/ml), streptomycin (100 μg/ml), 5 units/ml heparin, and 25 μg/ml endothelial cell growth supplement (Promocell). Human as well as mouse recombinant TNF (210-TA-010 and PMC3016) was used at 10 ng/ml; the IκK inhibitor BMS (BMS-345541) at 25 μm; and the inhibitors for JNK (SP600125, Cell Signaling Technology, catalog no. 1879, batch 6) and caspases (Z-VAD-fmk, Promega, catalog no. G7231) at final concentrations of 10 and 20 μm, respectively. MG132 and cycloheximide were applied at concentrations of 20 μm and 20 μg/ml. Total cell number was determined using a Neubauer improved counting chamber and trypan blue staining.
BrdU-ELISA
A BrdU cell proliferation assay (Millipore) was performed with AdTTP/AdK105R-infected cells (2 × 105 cells/ml, 100 μl/well, 96-well plate). The peroxidase activity of the labeled BrdU antibody was determined by substrate addition and measurement at 450 nm.
FACS
Pellets of AdTTP/AdK105R-infected cells (5 × 104 cells/ml, TNF addition (10 ng/ml) 24 h post-infection for 16 h) were resuspended in binding buffer (PBS with Ca2+ 0.33 g/liter to PBS), double-stained with Annexin V-APC/DAPI (1:10,000 dilution in PBS), and analyzed by flow cytometry (LSRII, BD Biosciences). A first gate was established in a SSC/FSC dot plot for the initial cell population gate. Single cells were gated in an FSC-A, FSC-H plot. Apoptotic cells were identified in the Annexin V-positive, DAPI-negative quadrant. Data were processed with FACS Diva software v6.1.2.
Coimmunoprecipitation
Analysis of the TNFR complex was done as described earlier (47). Briefly, cells were grown in 10-cm dishes to 85–90% confluence, stimulated with 1.4 μg/ml FLAG-TNF for the indicated time points, washed twice with ice-cold PBS containing 10 mm N-ethylmaleimide, collected by scraping, and snap-frozen in liquid nitrogen. Cell extracts were prepared by solubilizing cell pellets in lysis buffer (20 mm Tris-HCl (pH 7.5), 150 mm NaCl, 10% glycerol, 0.5 mm DTT, 0,5% Triton X-100, 0,3% Na-deoxycholate, 10 mm N-ethylmaleimide, 10 nm bortezomib, and 10 μm ZnCl2 supplemented with protease and phosphatase inhibitors) for 10 min on ice, followed by sonication and centrifugation (5000 × g, 10 min, 4 °C). 5% of the cell extract was removed for the detection of total protein expression (“input”), and the TNFR complex was isolated via immunoprecipitation using anti-FLAG-coupled magnetic beads (Sigma) for 2 h at 4 °C and end-over-head rotation. For immunoprecipitation of FLAGTTP/K105R, cells were lysed in PBS containing 0.5% Triton X-100, 0.5% Nonidet P-40, 20 nm bortezomib, and protease and phosphatase inhibitors, and 5% of extracts was utilized for detection of total protein. TTP/K105R was precipitated for 2 h at 4 °C using anti-FLAG-M2 magnetic beads (Sigma) and end-over-head rotation. Immunoprecipitates were denatured by Laemmli buffer and heating for 10 min at 70 °C prior to separation by SDS-PAGE.
Transfection, Reporter, and in Vivo Ubiquitination Assays
HEK293 cells were transfected with CaPO4 with the indicated expression plasmids, and reporter gene analysis as well as nickel-nitrilotriacetic acid-mediated precipitation of ubiquitinated proteins were performed as described previously (41).
Statistical Significance Calculations
Differences between samples were analyzed using paired Student's t test. Two-tailed probability values of <0.05 and <0.01 were considered significant and highly significant, respectively. p values are given in the figure legends.
Adenoviral Transduction
Generation of adenovirus from pCMV-MycTTP/pCMVMycK105R was performed as described previously (41). Cells were infected for 6 h, followed by addition of doxycycline (4 nm) (and TNF (10 ng/ml)/Z-VAD-fmk (20 μm) in the case of long term treatment). TNF kinetics, cell proliferation assays, and Western blot analyses were performed 24 h after infection.
RESULTS
TTP Regulates the Onset of TNF-induced JNK Activation
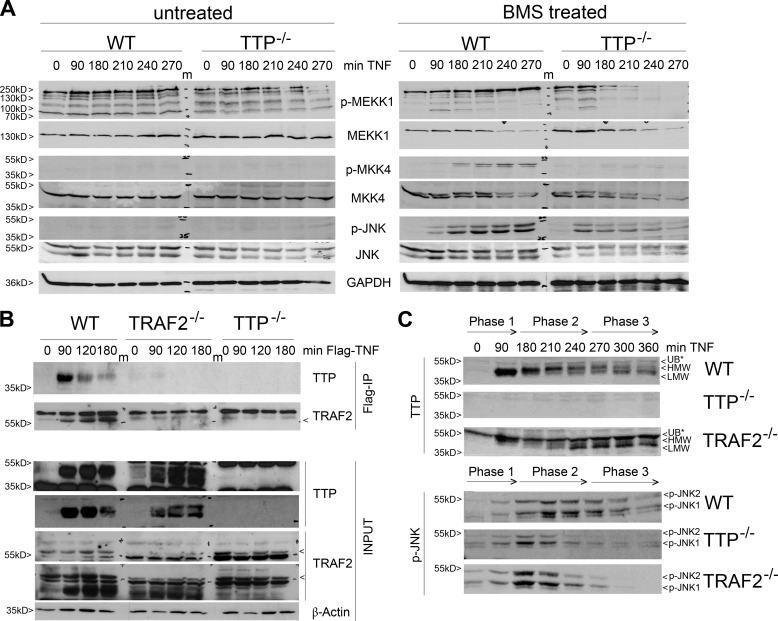
Although sustained JNK activation upon TNF treatment has been observed under certain conditions before, the underlying mechanistic details remain poorly understood. We observed previously (41) that TTP promotes sustained JNK phosphorylation when prolonged TNF-induced IKK2 and NF-κB activation was inhibited. Here we dissected the TNF-induced JNK activation in view of step-by-step kinase activation for a more detailed elucidation. We performed extensive time course experiments and compared the TNF-induced phosphorylation status of MEKK1, MKK4, and JNK in WT and TTP−/− MEFs (Fig. 1A). TNF stimulation alone did not induce JNK activation beyond 90 min, nor did the absence of TTP (Fig. 1A, left panel). To achieve prolonged JNK activation, cells were treated with the IKK inhibitor BMS (48) 30 min after TNF induction, which still allows immediate responses (including TTP synthesis) but impairs the negative feedback regulation of certain NF-κB target genes involved in JNK regulation. Under these conditions, the lack of TTP resulted in severely diminished activation (as measured by phosphorylation) of all investigated kinases (Fig. 1A, right panel).
FIGURE 1.
TTP is involved in TNF-induced JNK activation. A, TTP promotes prolonged JNK activation after inhibition of NF-κB. Western blot analyses of TNF-induced WT and TTP−/− MEFs left untreated (left panel) or treated with the IκK inhibitor BMS. Shown is the activation of the indicated JNK signaling members (p-MEKK1, p-MKK4, and p-JNK) as well as corresponding total protein levels and GAPDH. m, marker lane. B, TTP interacts with the TNF receptor in a time- and TRAF2-dependent manner. TNF immunoprecipitation (IP) was performed with WT, TRAF2−/−, and TTP−/− MEFs treated with FLAG-tagged TNF for the indicated times. Coprecipitated TTP (top panel, top), TRAF2 (top panel, bottom), and total protein levels (input, bottom panel; long and short exposures are shown for TTP and TRAF2) were visualized by Western blotting. C, TTP appearance and JNK activation are altered by lack of TRAF2. WT, TTP−/−, and TRAF2−/− MEFs were treated with TNF and BMS as in A. TTP (top panel) and p-JNK (bottom panel) levels were analyzed by Western blotting. The TNF-induced and time-dependent impact on the appearance of HMW and LMW TTP in WT MEFs was divided into three phases. UB*, nonspecific band.
Importantly, TTP interfered with JNK signaling at the very top level because no phospho-MEKK1 was detectable after 180 min (Fig. 1A, right panel, first row). Correspondingly, MKK4 and JNK activation was severely impaired. Because we observed previously that TTP interacts physically with MEKK1 between 90–180 min, and because the latter is known to interact with the TNFR, we examined whether TTP interferes with the formation of the receptor complex. Treatment of WT MEFs with FLAG-tagged TNF and subsequent immunoprecipitation proved the presence of endogenous TTP in the TNFR complex from 90 min on (Fig. 1B). Of note, TTP present in TNFR immunoprecipitates appeared as a discrete band with increasing molecular weight and decreasing binding behavior over time, suggesting that only specific phosphorylated TTP forms were bound to the receptor. Notably, binding was dependent on the presence of TRAF2, which, in turn, accumulated at the TNFR only when TTP was present, but with different kinetics (Fig. 1B, compare the first and second rows). These observations support a scenario in which, 90 min after TNFR1 ligation, MEKK1-phosphorylated TTP assembles at the receptor in a TRAF2-dependent manner. Moreover, when analyzing TTP expression and JNK activation in TRAF2−/− MEFs, we observed altered TTP phosphorylation patterns. Specifically, the TNF-induced transition of TTP from mainly lower molecular weight (LMW) TTP (phase 1) to higher molecular weight (HMW) TTP (phase 2), and LMW-TTP (phase 3), typically seen in WT MEFs (Fig. 1C, top panel), was altered in favor of LMW-TTP when TRAF2 was absent (Fig. 1C, bottom panel), whereas the latter was found constantly expressed over time in TTP−/− MEFs (data not shown). Furthermore, JNK activation in TRAF2−/− cells at later time points essentially resembled the appearance in TTP−/− cells (Fig. 1C, phase 3, bottom panel), further supporting a functional interdependence of phosphorylated TTP and TRAF2.
TTP Upsets the Balance between JNK-mediated Cell Survival and Death
Downstream of JNK activation, cJun triggers the expression of the cell cycle regulator cyclin D. Comparative studies on respective expression kinetics in WT, TTP−/−, and TRAF2−/− MEFs revealed that cJun activity occurred more rapidly and transiently in the absence of TTP and TRAF2. Also, cyclin D expression was essentially absent after 90–180 min (during the beginning of phase 2) in both knockout cell lines (Fig. 2A). Therefore, HMW-TTP expressed during phase 2 in WT cells but absent in TRAF2−/− MEFs (Fig. 2A, right column) seems to be involved in the timing of cJun activation and subsequent cyclin D expression.
FIGURE 2.
TTP promotes cell cycle regulation. A, TNF-induced cell cycle regulators are comparably altered in TTP and TRAF2−/− MEFs. Shown are Western blot analyses of TNF-induced WT, TTP−/−, and TRAF2−/− MEFs treated with BMS as in Fig. 1A. Activation of cJun (second row, total levels shown below) and expression of cyclin D (third row) were compared with TTP (fourth row) regarding the time phases assigned in Fig. 1C. H, high molecular weight; M, middle molecular weight; L, low molecular weight. B and C, TTP and TRAF2 interdependently activate E2F1 and AP1. Shown are reporter gene assays performed in HEK293 cells transfected with either E2F1-luc (B) or AP1-luc (C) reporter constructs. The influence of increasing TTP levels on luciferase expression was analyzed in the absence (left panels) and presence of coexpressed TRAF2 (center panels) or dominant negative (dn) TRAF2 lacking the RING domain (right panels). Luciferase levels are depicted as mean fold change compared with related controls (ctrl, control without TTP), and error bars represent mean ± S.D. (n = 3). *, p < 0.05; **, p < 0.01; ***; p < 0.001.
The activity of the transcription factors E2F as well as AP1 depends on cJun in an indirect and direct manner, respectively. Interestingly, the respective reporters could not be activated by TTP overexpression unless TRAF2 was coexpressed (Fig. 2, B and C). This interdependent effect was abolished by the TRAF2 RING finger domain deletion mutant (Fig. 2, B and C, right panels), showing that the TRAF2-E3 ligase activity was indispensable for its function toward TTP and JNK activation. Together, two factors appear to be crucial for the activation of JNK downstream events: the specific occurrence of the HMW form of TTP as well as the E3 ligase function of TRAF2.
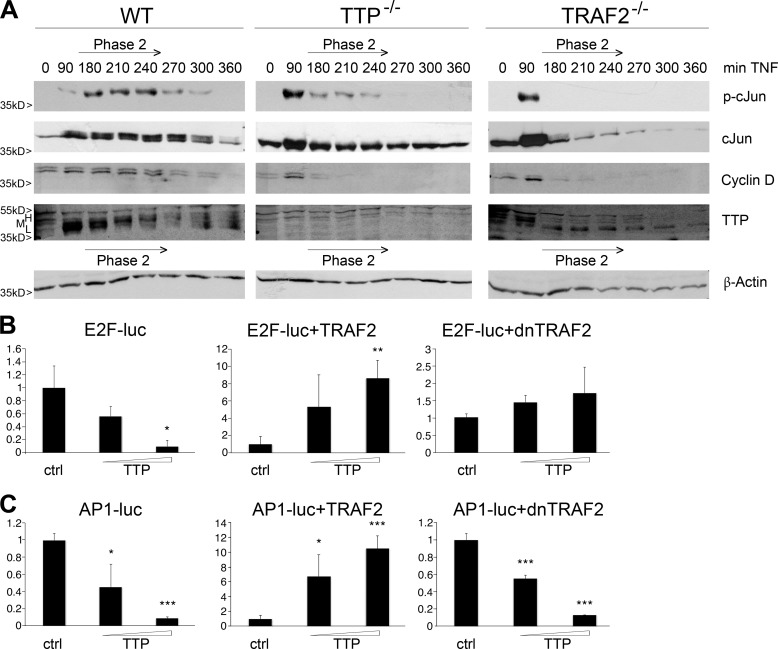
Activation of JNK is a double-edged sword because prolonged activity has been shown to result in cell death. Because of the fact that our cells did not display signs of necrotic cell death during time course experiments, as monitored by microscopic analyses, we investigated the occurrence of apoptotic cell death. In WT MEFs, PARP-1 cleavage occurred by the end of phase 2 (4 h after TNF induction) and correlated with a decrease in HMW-TTP expression (Fig. 3A, left panel, top). In agreement with the impaired cJun activation in TTP−/− and TRAF2−/− MEFs during phase 2 (Fig. 2A, first row), caspase 3 and PARP-1 cleavage occurred around 2 h earlier, at the beginning of phase 2 in these cells (Fig. 3A, top and center panels).
FIGURE 3.
TTP is involved in the regulation of TNF-induced cell death. A, TTP and TRAF2 protect from early cell death. Western blot analysis of WT (left panel), TTP−/− (center panel), and TRAF2−/− (right panel) MEFs after TNF induction (+ BMS as in Fig. 1A) is shown. Cleaved caspase 3 and PARP-1(center panel, top) were compared with TTP expression (center panel, bottom) regarding the time phases defined in Fig. 2A. B, TTP-mediated control of caspase 3 cleavage is JNK-dependent. WT and TTP−/− MEFs were treated with TNF and BMS as in Fig. 1A and analyzed for caspase 3 cleavage in the absence (first row) and presence (second row) of the JNK inhibitor SP600125 by Western blotting. The corresponding time phases are as indicated. GAPDH is shown as a loading control (third and fourth rows). m, marker lane. C, ectopically expressed TTP promotes cell death upon long term TNF treatment. HUVECs were infected with an adenovirus that allows the controlled expression of TTP in the absence of doxycycline (−Dox, left panel, versus +Dox, right panel). After TNF+BMS stimulation as in Fig. 1A, cells were analyzed for cleaved caspase 3 and PARP-1 (first and second rows, respectively) by Western blotting. Ectopic TTP expression as well as endogenous TNF-induced TTP expression are shown in the third rows (note that these represent different exposures for better comparison). The corresponding time phases are as indicated. β-actin represents the loading control.
Further analysis confirmed the involvement of TTP in the regulation of death signaling. TNF-induced cleavage of the upstream caspase 3 reflected PARP-1 behavior not only in WT but also in TTP−/− cells (Fig. 3B). Interestingly, in both cases, treatment with the JNK inhibitor SP600125 led to a delay of cleavage. Because the timing of caspase activation was altered in the absence of TTP, we checked for the opposite effect and could show that TNF treatment of TTP adenovirus-infected primary HUVECs led to caspase 3 cleavage when TTP was expressed (Fig. 3C, left panel). The time point of caspase activation at around 4 h of treatment (beginning of phase 3) was comparable with the situation in WT MEFs. Conversely, when ectopic TTP expression was prevented by the addition of doxycycline, no caspase activation occurred throughout the entire time course in HUVECs (Fig. 3C, right panel). Notably, ectopically expressed and TNF-induced endogenous TTP behaved differently in terms of expression pattern, showing that, in the presence of endogenous HMW-TTP, cells did not activate caspase and PARP-1 cleavage.
TRAF2-mediated TTP Ubiquitination Acts as a Functional Switch for NF-κB versus JNK
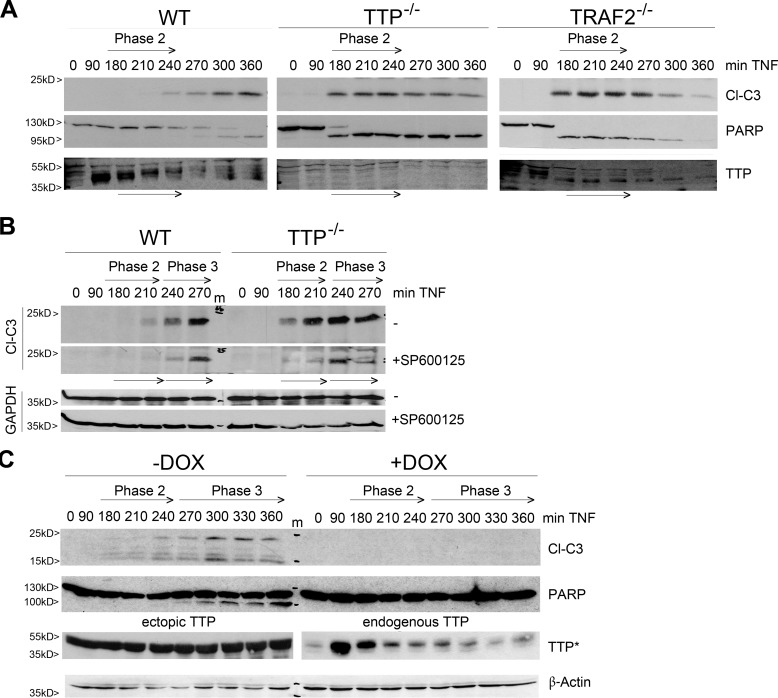
On the basis of the observations above and our earlier finding of MEKK1-dependent, TRAF2-mediated TTP polyubiquitination (41), we bioinformatically analyzed the TTP sequence for potential ubiquitination sites (45). Computational prediction scored Lys-105 (human TTP, corresponding to Lys-97 in mouse TTP, data not shown), which was subsequently mutated (Lys → Arg), subcloned, and tested. Studies in HEK293 cells revealed that TTP K105R degraded faster than WT TTP, but only in the presence of ectopically expressed ubiquitin, suggesting that Lys-105 confers stability and does not act as decay signal (Fig. 4A). Strikingly, the stabilities of TRAF2 and MEKK1 were affected by the integrity of TTP K105 as well. Both proteins degraded faster when the mutant TTP protein was coexpressed in the presence of ubiquitin (Fig. 4A, right panel). In line with this, TTP K105R appeared to be free of polyubiquitination in this setting (Fig. 4B, left panel) but acquired polyubiquitination upon proteasome inhibition (Fig. 4B, right panel). This supports the above finding that Lys-105 confers ubiquitin-dependent protein stability, prohibiting proteasomal degradation. Interestingly, an interaction of TTP and TRAF2 was independent of a functional Lys-105 but depended on the presence of MEKK1 (Fig. 4C). Together, and consistent with our earlier findings that TTP is phosphorylated by MEKK1 prior to Lys-63-linked polyubiquitination by TRAF2 (41), our data support the notion of a TNF induced TTP-TRAF2-MEKK1 “triple complex” assembly whereby the ubiquitination of TTP at Lys-105 stabilizes not only TTP but also TRAF2 and MEKK1 in further consequence. Interestingly, testing the capacity of TTPK105R toward the transcription factors NF-κB, AP1, and E2F uncovered functional differences. Although TTPK105R down-regulated NF-κB activity to the same level as described previously for WT TTP (43), it lost its activating function toward AP1 and E2F (Fig. 4C). Therefore, TRAF2-mediated TTP polyubiquitination appears to be specifically necessary for its function toward JNK activation but not for NF-κB inhibition.
FIGURE 4.
TTP free of TRAF2-mediated ubiquitination is inactive toward E2F1 and AP. A, TTPK105 accounts for TTP-TRAF2-MEKK1 stability. Depicted are Western blot analyses showing the cycloheximide-induced (Chx, 0–8 h) degradation of coexpressed TRAF2 and MEKK1 with either TTP or TTPK105R in the presence (right panel) and absence (left panel) of ubiquitin in HEK293 cells. m, molecular weight marker. B, the TTPK105R mutant acquires degradative polyubiquitination. Left panel, HEK293 cells were transfected with expression vectors for His-tagged ubiquitin, the MEKK1 kinase domain, and TRAF2 together with either TTP or the mutant K105R. Right panel, HEK293 cells were transfected with expression vectors for His-tagged ubiquitin and the TTP mutant K105R together with either TRAF2 alone or a combination of TRAF2 with MEKK1. Cells were treated with the proteasome inhibitor MG132 (20 μm) for 3 h. His-tagged ubiquitin was precipitated using nickel-nitrilotriacetic acid (Ni-NTA)-agarose, and coprecipitated TTP (K105R) was analyzed by Western blotting (left). Total expression levels of transfected proteins are shown in the inputs (right). Note that different parts of the same membrane are shown on the right. C, TTP-TRAF2 interaction depends on the presence of MEKK1. Coimmunoprecipitation (IP) of HEK 293 cells transfected with TTP or its mutant K105R either in the presence of TRAF2 (T2) or of a combination of TRAF2 and the MEKK1 kinase domain (T2/M1) is shown. Pull-down of FLAG-tagged TTP or K105R coprecipitated TRAF2 only in the presence of MEKK1 (top panel, second and third columns). Expression levels of transfected proteins are shown in the input (bottom panel). The experiment was performed in the presence of bortezomib to inhibit protein degradation. Note that different parts of the same membrane are shown. ctrl, control; WB, Western blot; LE*, long exposure. D, TTPK105R fails to activate E2F1 and AP1 but inhibits NF-κB. Shown are reporter gene assays performed in HEK293 cells transfected with either NF-κB-luc (left panel), AP1-luc (center panel), or E2F1-luc (right panel) reporter constructs. The influence of TTP or K105R expression was analyzed either in presence of p65 (NF-κB-luc) or TRAF2 (AP1-luc and E2F-luc). Luciferase levels are depicted as mean fold change compared with related controls (ctrl, control without TTP), and error bars represent mean ± S.D. (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Growth and Survival of Cervical Cancer Cells Is Controlled by TTP in a TNF-dependent Manner
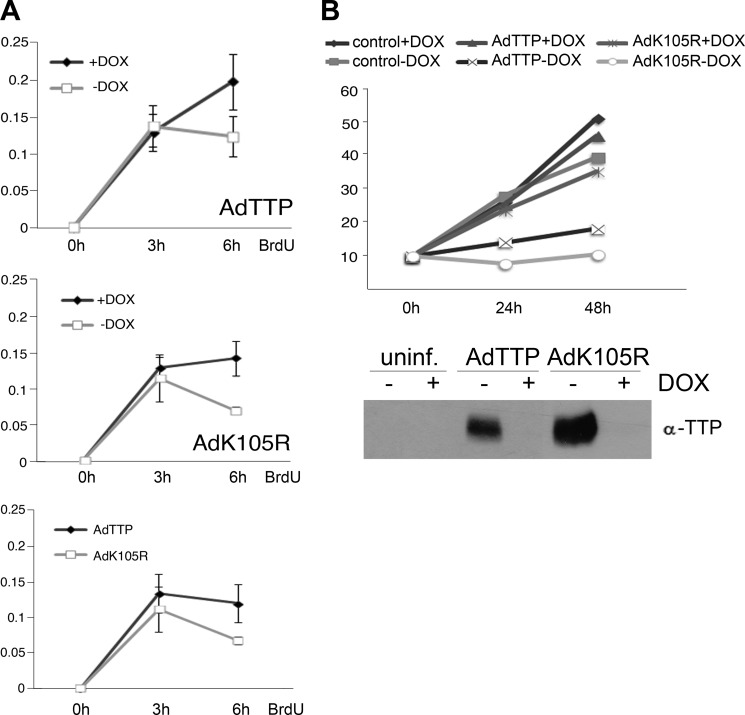
Because the above results indicated that TTP ubiquitination at Lys-105 was required for JNK activation, we analyzed its impact on cell survival and death. SiHa cervical cancer cells were transduced with either TTP or TTPK105R adenovirus and analyzed for proliferation using BrdU incorporation. Both WT TTP and the TTPK105R mutant impaired proliferation, the latter showing a more pronounced effect (Fig. 5A). In agreement, TTP-expressing cells grew slower, whereas cells expressing the TTP mutant stopped growing completely (Fig. 5B).
FIGURE 5.
TTP controls growth of cervical cancer cells in the absence of TNF. A, TTP/K105R affect BrdU incorporation in cancer cells. SiHa cells were infected with adenoviruses for the controlled expression (−Dox; +Dox inhibits expression) of TTP or its mutant K105R (AdTTP, top panel; AdK105R, center panel; and AdTTP versus AdK105R in the absence of Dox, bottom panel). 24 h after infection, a BrdU-ELISA was performed, and incorporation was measured after 3, 6, and 9 h (x axis) at 450 nm (y axis). Error bars represent mean ± S.D. (n = 3). B, TTP slows down and TTP-K105R stops the growth of cancer cells. SiHa cells were either left uninfected (control, uninf.) or transduced with AdTTP/AdK105R, as in A, in the absence (−Dox) or presence of doxycycline (+Dox). Depicted is the total cell number of living cells determined 48 and 72 h after infection. A Western blot analysis of the same samples is shown in the bottom panel.
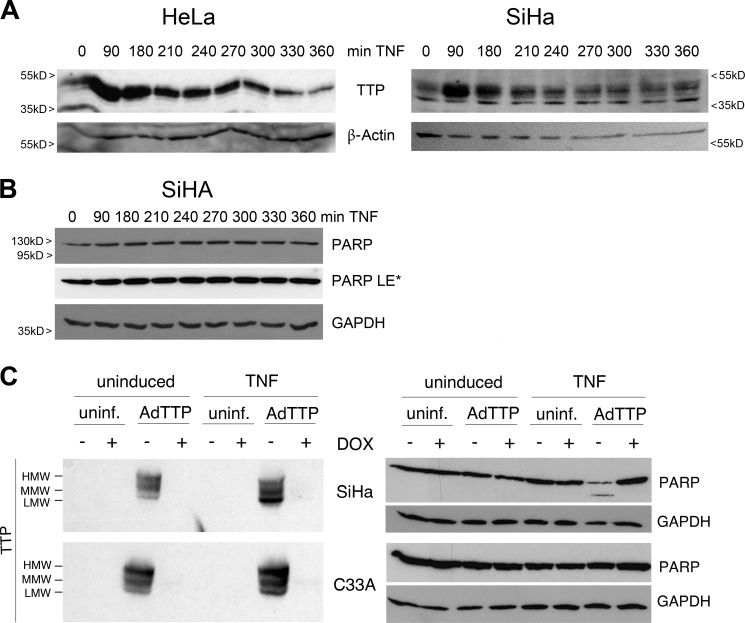
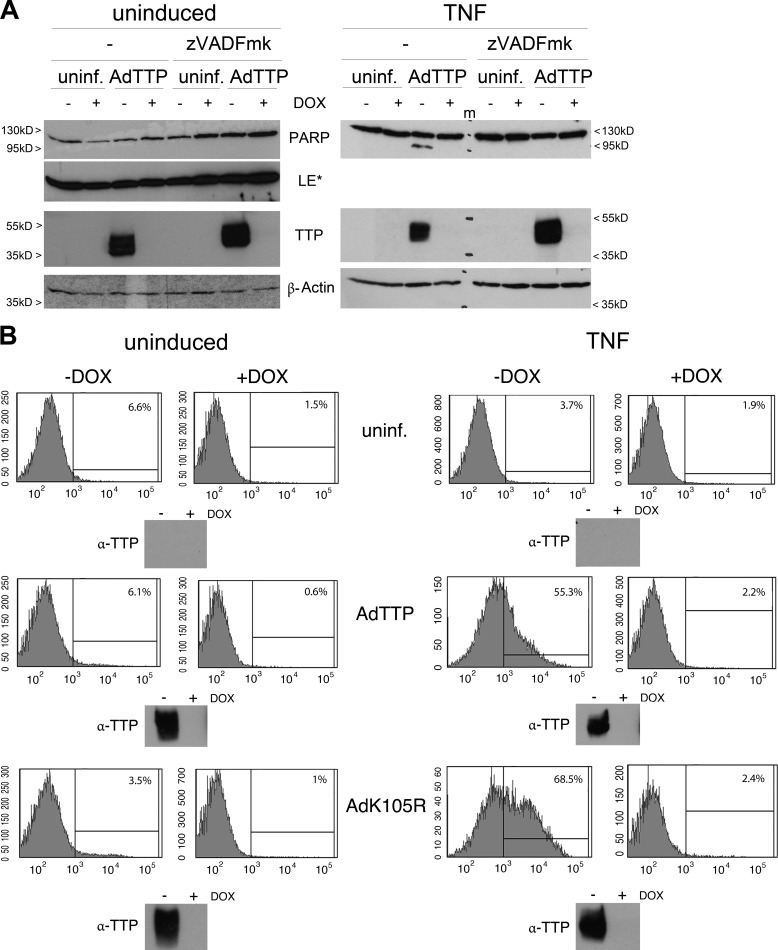
The same adenovirus-transduced cells were further analyzed for TNF-induced TTP expression and PARP-1 cleavage following BMS treatment (Fig. 6, A and B). Especially the fact that no PARP cleavage occurred in the presence of only one TTP form reflected the situation already observed in HUVECs (Fig. 3C). Remarkably, TTP adenovirus-infected cancer cells showed PARP-1 cleavage exclusively when costimulated with TNF (Figs. 6C and 7A). Only one cell line, C33A, showed intact PARP-1 after TNF-TTP treatment (Fig. 6C), and ectopically produced HMW-TTP-levels seemed to override other TTP forms in these cells, supporting the findings above. Moreover, the synergistic effect of TNF and TTP was prevented by the pan-caspase inhibitor Z-VAD-fmk (Fig. 7A). The adenovirus-infected SiHa cells described above were further analyzed for cell death by FACS (Fig. 7B). Uninduced as well as TNF-stimulated cells were sorted for Annexin V-positive/DAPI-negative cells resulting in exclusively apoptotic populations. In line with our previous results, TTP as well as TTPK105R led to apoptosis only after TNF costimulation and TTPK105R expression further enhanced the number of apoptotic cells. Notably, neither TTP or its mutant nor the sole TNF treatment had the ability to force these cancer cells into an apoptotic cell death.
FIGURE 6.
TTP expression and TNF-induced apoptosis are both affected in cervical cancer cells. A, restricted transition of TTP forms in HeLa and SiHa cells. HeLa (right panel) and SiHa (left panel) cells were treated with TNF and analyzed for TTP expression by Western blotting. β-Actin is shown as a loading control. B, TNF treatment does not induce PARP-1 cleavage after NF-κB inhibition in SiHa cells. Cells were induced with TNF+BMS as in Fig. 1A as indicated, and PARP cleavage was analyzed by Western blotting. GAPDH was used as a loading control. LE*, long exposure. C, TNF induces PARP-1 cleavage exclusively in the absence of HMW-TTP. SiHa (top panels) or C33A (bottom panels) cells were left uninfected (uninf.) or infected with an adenovirus for the controlled expression of TTP (AdTTP, −Dox). Ectopic TTP levels (left panels) and PARP cleavage (right panels) were analyzed in uninduced compared with TNF-induced cells by Western blotting. GAPDH is shown as a loading control. MMW, middle molecular weight.
FIGURE 7.
TTP promotes apoptosis of cervical cancer cells in the presence of TNF. A, caspase inhibition abrogates the TTP apoptotic effect. HeLa cells were left uninfected (uninf.) or transduced with AdTTP as in Fig. 5A in the presence (+Dox) or absence of doxycycline (−Dox). PARP-1 cleavage (first row) and TTP expression (third row) were analyzed in the absence (−) and presence of the caspase inhibitor Z-VAD-fmk under uninduced and TNF-induced conditions by Western blotting. β-actin was used as a loading control. LE*, long exposure. m, marker lane. B, TTPK105R augments the apoptotic effect of TTP. SiHA cells were left uninfected or transduced with AdTTP/AdK105R as in Fig. 5A. Apoptosis was analyzed in the absence (−Dox) and presence of doxycycline (+Dox) in uninduced (left panel) and TNF-treated (right panel) samples by FACS. The histograms depict counts (y axis) of Annexin V-positive/DAPI-negative cells (x axis). The percentage of apoptotic cells is given in the top right corner of each histogram. Each sample was analyzed for expression of ectopic TTP/K105R by Western blotting (insets below the histograms).
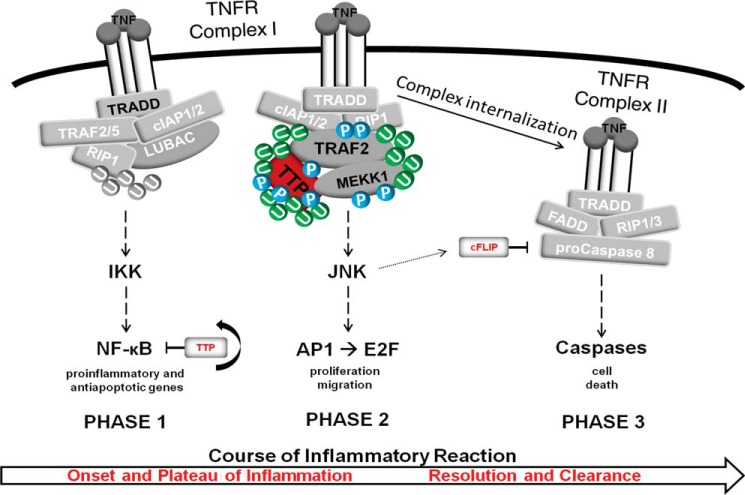
In summary, we provide evidence that, in the context of TNFR 1 signaling, phosphorylated HMW-TTP assembles with TRAF2 within the receptor complex. Subsequent TTP polyubiquitination by TRAF2, in turn, provides the basis for the protection from JNK-initiated, caspase-triggered apoptotic cell death (Fig. 8).
FIGURE 8.
Model of the phases of an inflammatory TNF response. Shown is the immediate response to TNF and assembly of the so-called TNFR complex I. TNF ligation to its receptor induces the membrane proximal assembly of the adaptor TNF receptor-associated death domain, the ubiquitin E3 ligases cIAP1/2, and the linear ubiquitin chain assembly complex, which ubiquitinates and licenses the kinase RIP1 to phosphorylate the IκK complex, leading to the activation of the transcription factor NF-κB. We termed this “phase 1” because the expression of proinflammatory and antiapoptotic genes facilitates the proper onset of inflammation and, concomitantly, impedes phase 2 and phase 3 signaling. Termination of phase 1 is regulated at all levels of signaling, and TTP itself acts as a negative feedback inhibitor at the transcription factor level (43). We hypothesize that the transition to phase 2 signaling is characterized by the rearrangement of complex I, whereby TRAF2, being phosphorylated and ubiquitinated, acts as main player in the signal transduction to downstream MAPKs. We speculate that TTP, following phosphorylation by MEKK1, is recruited to TRAF2 to become polyubiquitinated, which, in turn, stabilizes the TRAF2-MEKK1-TTP interaction. Subsequently, interdependent ubiquitination and phosphorylation events within this triple complex facilitate signal transmission toward JNK and the related transcription factors, such as AP1 or E2F. This most likely overlaps with phase 1 signaling, thereby controlling mainly cell proliferation and migration, which partly represent the plateau phase of inflammation. The change to phase 3 signaling is characterized by internalization of the ligand-receptor complex and the formation of the cytoplasmic complex II, the so-called TNF receptosome. Signaling by complex II results in cell death and is incipiently inhibited by cFLIP, an inhibitory protein whose expression is induced by phase 1-activated NF-κB and degraded later, during phase 2, by activated JNK1. We hypothesize that the transition from phase 2 to phase 3 depends on TTP-TRAF2-MEKK1 triple complex reorganization. A reduction of polyubiquitinated TTP (HMW-TTP) might lead to changes in triple complex stability, finally facilitating JNK1-mediated activation of apoptotic signaling. This might be accomplished by the specificity of MEKK1, which has been shown to protect and promote apoptotic cell death. From a physiologic point of view, this would contribute to the resolution phase of inflammation, constituting a fundamental step in the prevention of chronic inflammation as well as continuous cell proliferation, resulting in cancer development. U, ubiquitination; P, phosphorylation.
DISCUSSION
TTP was originally termed G0/G1 switch regulatory protein 24, long before an increased proliferation rate in TTP−/− MEFs was described. However, the molecular mechanism(s) remained unknown. The fact that TTP is involved in the regulation of cell proliferation is underscored by our observations of cJun and cyclin D dysregulation in knockout MEF as well as by the TTP-dependent decrease of cervical cancer cell growth. Interestingly, the earlier and more rapid appearance of activated JNK and the increased sensitivity to TNF-induced apoptotic cell death recapitulated the phenotype of TRAF2−/− MEFs (45). The balance between JNK1 and JNK2 is crucial for the regulation of cJun phosphorylation and cell cycle progression, whereas JNK1 is solely responsible for the activation of caspases in TNF-induced apoptosis (20, 49, 50). Accordingly, in the absence of TTP, the early appearance of excessive JNK1 was attended by caspase 3 and PARP-1 cleavage. Of note, the inhibition of JNK activities did not completely abolish apoptotic signaling but, rather, delayed its activation. Moreover, both the lack of TTP and TRAF2 altered the kinetics of JNK signaling similarly, but differences could be observed in expression levels. cJun levels appeared to be higher in TTP−/− than in TRAF2−/− MEFs over time (Fig. 2A). In addition, TNF-induced JNK activation appeared to be differentially affected by TTP and TRAF2. TRAF2−/− MEFs displayed reduced activation of JNK over time in comparison with TTP−/− MEFs. More specifically, JNK1 activation was sustained in TTP−/− MEFs, whereas JNK2 activation appeared to be higher in TRAF2−/− MEFs (Fig. 1C). These findings are supported by several earlier studies where, for example, Liu et al. (50) showed that JNK1, but not JNK2, is essential for TNF-induced c-Jun activation and its autoregulated expression, which is in line with our observation of pronounced cJun levels in TTP−/− MEFs. In addition, Yeh et al. (45) described a reduced TNF-induced JNK activity toward cJun in TRAF2−/− MEFs. Moreover, it has been demonstrated recently (51) that TRAF2 phosphorylation is essential for maximal TNF-induced JNK activation and c-Jun activities. Therefore, the lack of TTP as well as TRAF2 alters JNK upstream signals, leading to differences in JNK1/2 abundance, p-cJun levels, and autoregulated cJun transcription in knockout MEFs. When compared, mainly the timing of JNK activation was affected in TTP−/− MEFs. Therefore, we speculate that TRAF2 acts as the main “signal transducer,” whereas TTP functions as a “timer” in the onset of JNK signaling.
Another important aspect in the course of the TNF response concerns the modification of TTP itself. As shown earlier, it became inducibly hyperphosphorylated over time in WT MEFs. After the first appearance as so-termed LMW protein in phase 1 (until 90 min), higher molecular weight species accumulated during phase 2 (until around 4 h) before they returned to LMW forms and finally disappeared. TTP hyperphosphorylation has been, until now, mainly connected to protein stability and subcellular localization, whereas the impact on its described mRNA-degrading function still remains controversial. In this context, our results provide evidence that it is lysine 105 of TTP that confers protein stability in an ubiquitin-dependent manner. In comparison with WT TTP, the K105R mutant acquired ubiquitination only upon proteasome inhibition, which uncovered the novel findings that TTP is decorated with degradative, Lys-48-linked ubiquitin chains dependent on phosphorylation but independent of TRAF2 and K105, that mutation of Lys-105 prohibits Lys-63-linked polyubiquitination, and that degradative TTP ubiquitination appears secondary to Lys-63-linked stabilizing ubiquitination. Moreover, we found that TTP K105 influenced not only the stability of TTP but affected the half-life of MEKK1 and TRAF2 as well. This, together with our earlier findings (41), supports our notion that, at first, a hyperphosphorylated HMW-TTP is produced that then becomes polyubiquitinated by TRAF2 in a Lys-63-linked regulatory manner. We hypothesize that this is a prerequisite for the composition of a stable TTP-MEKK1-TRAF2 triple complex and facilitates the JNK-mediated activation of the transcription factors as AP-1 and E2F (Fig. 8). In this regard, it seems important that apoptotic signaling was induced by the end of phase 2 in WT MEFs, exactly at the same time when HMW-TTP started to disappear. In agreement with this, we observed that not only the lack of TTP, but also the occurrence of TTP forms other than HMW, as in TRAF2−/− cells, promoted early cell death instead of proliferation. Vice versa, the stabilization of HMW-TTP, as found in TNF/BMS treated HUVEC, completely abolished caspase activation and cell death. Strikingly, we found a comparable HMW-TTP stabilization in three different cervical cancer cell lines, which resisted apoptosis following TNF/BMS treatment. Furthermore, cancer cells appeared preactivated in terms of TTP expression because basal levels could be detected already under unstimulated conditions.
The indication that it was the HMW form of TTP that protected from apoptosis was further supported by ectopic TTP expression studies in cancer cells, which displayed not only HMW and LMW TTP but also middle molecular weight TTP. Remarkably, only cancer cells expressing predominantly middle molecular weight TTP and LMW-TTP could be driven into TNF-induced apoptosis, although one cell line, C33A (Fig. 6C), which repeatedly produced predominantly HMW-TTP, did not appear to be apoptotic under the same experimental conditions. Therefore, we uncovered a new function of TTP that depends, at least partly, on the balance and time-dependent occurrence of different TTP modifications and affects the switch between cell cycle regulation and onset of apoptosis.
On the basis of the fact that the activation kinetics of the proliferative as well as the apoptotic JNK response were comparable in TTP−/− and TRAF2−/− MEFs and that integrating our earlier findings showed that TRAF2 polyubiquitinates HMW-TTP (41), it seemed likely that the molecular functions of TTP and TRAF2 regarding JNK signaling are highly interconnected. Furthermore, the major cascades, such as NF-κB and JNK, which are known to be activated by TRAF2, were also affected by TTP. Transcription factors such as AP1 or E2F were up-regulated by TTP only in the presence of a functional TRAF2 E3 ligase domain, indicating that TTP-mediated JNK activation was dependent on its TRAF2-mediated polyubiquitination. Although it has been shown before that TNF-induced TRAF2 ubiquitination is required for selective JNK activation (52), we believe that its molecular interplay with TTP at the receptor is essentially required for sustained JNK activation because we could demonstrate that TTP polyubiquitination was indispensable for JNK activation and protection against apoptosis. Importantly, this function of TTP appeared to be specific for JNK signaling because the ubiquitin mutant was not able to abrogate the inhibitory function of TTP toward NF-κB.
Together, our findings underscore the value of TRAF2-mediated TTP ubiquitination in the specific balanced control of JNK-mediated survival versus death signaling (Fig. 8). We provide novel mechanistic insights into the regulation of TNFR1 signaling and identify TTP as a contributor to the spatiotemporal regulation of TNF-induced signaling events.
Acknowledgments
We thank Tak Mak for the TRAF2−/− MEFs and Rudi Beyaert for the FLAG-tagged TNF.
This work was supported by the Austrian Science Foundation (FWF) Project T511-B20 (to Y. M. H. S.).
- TNFR
- TNF receptor
- PARP
- poly(ADP-ribose) polymerase
- MEF
- mouse embryonic fibroblast
- HUVEC
- human umbilical cord endothelial cell
- Z-VAD-fmk
- benzyloxycarbonyl-VAD-fluoromethyl ketone
- BMS
- BMS-345541
- LMW
- low molecular weight
- HMW
- high molecular weight
- luc
- luciferase
- Dox
- doxycycline.
REFERENCES
- 1. Tracey K. J., Cerami A. (1994) Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu. Rev. Med. 45, 491–503 [DOI] [PubMed] [Google Scholar]
- 2. Tracey K. J., Cerami A. (1993) Tumor necrosis factor, other cytokines and disease. Annu. Rev. Cell Biol. 9, 317–343 [DOI] [PubMed] [Google Scholar]
- 3. Wajant H., Pfizenmaier K., Scheurich P. (2003) Tumor necrosis factor signaling. Cell Death Differ. 10, 45–65 [DOI] [PubMed] [Google Scholar]
- 4. Holler N., Zaru R., Micheau O., Thome M., Attinger A., Valitutti S., Bodmer J. L., Schneider P., Seed B., Tschopp J. (2000) Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1, 489–495 [DOI] [PubMed] [Google Scholar]
- 5. Darnay B. G., Aggarwal B. B. (1999) Signal transduction by tumour necrosis factor and tumour necrosis factor related ligands and their receptors. Ann. Rheum. Dis. 58, I2–I13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Micheau O., Tschopp J. (2003) Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 [DOI] [PubMed] [Google Scholar]
- 7. Hsu H., Huang J., Shu H. B., Baichwal V., Goeddel D. V. (1996) TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4, 387–396 [DOI] [PubMed] [Google Scholar]
- 8. Shu H. B., Takeuchi M., Goeddel D. V. (1996) The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex. Proc. Natl. Acad. Sci. U.S.A. 93, 13973–13978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haas T. L., Emmerich C. H., Gerlach B., Schmukle A. C., Cordier S. M., Rieser E., Feltham R., Vince J., Warnken U., Wenger T., Koschny R., Komander D., Silke J., Walczak H. (2009) Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol. Cell 36, 831–844 [DOI] [PubMed] [Google Scholar]
- 10. Emmerich C. H., Schmukle A. C., Haas T. L., Gerlach B., Cordier S. M., Rieser E., Walczak H. (2011) The linear ubiquitin chain assembly complex forms part of the TNF-R1 signalling complex and is required for effective TNF-induced gene induction and prevents TNF-induced apoptosis. Adv. Exp. Med. Biol. 691, 115–126 [DOI] [PubMed] [Google Scholar]
- 11. Ikeda F., Deribe Y. L., Skånland S. S., Stieglitz B., Grabbe C., Franz-Wachtel M., van Wijk S. J., Goswami P., Nagy V., Terzic J., Tokunaga F., Androulidaki A., Nakagawa T., Pasparakis M., Iwai K., Sundberg J. P., Schaefer L., Rittinger K., Macek B., Dikic I. (2011) SHARPIN forms a linear ubiquitin ligase complex regulating NF-κB activity and apoptosis. Nature 471, 637–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Delhalle S., Deregowski V., Benoit V., Merville M. P., Bours V. (2002) NF-κB-dependent MnSOD expression protects adenocarcinoma cells from TNF-α-induced apoptosis. Oncogene 21, 3917–3924 [DOI] [PubMed] [Google Scholar]
- 13. Pham C. G., Bubici C., Zazzeroni F., Papa S., Jones J., Alvarez K., Jayawardena S., De Smaele E., Cong R., Beaumont C., Torti F. M., Torti S. V., Franzoso G. (2004) Ferritin heavy chain upregulation by NF-κB inhibits TNFα-induced apoptosis by suppressing reactive oxygen species. Cell 119, 529–542 [DOI] [PubMed] [Google Scholar]
- 14. Resch U., Schichl Y. M., Sattler S., de Martin R. (2008) XIAP regulates intracellular ROS by enhancing antioxidant gene expression. Biochem. Biophys. Res. Commun. 375, 156–161 [DOI] [PubMed] [Google Scholar]
- 15. Xia Y., Makris C., Su B., Li E., Yang J., Nemerow G. R., Karin M. (2000) MEK kinase 1 is critically required for c-Jun N-terminal kinase activation by proinflammatory stimuli and growth factor-induced cell migration. Proc. Natl. Acad. Sci. U.S.A. 97, 5243–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fanger G. R., Gerwins P., Widmann C., Jarpe M. B., Johnson G. L. (1997) MEKKs, GCKs, MLKs, PAKs, TAKs, and tpls: upstream regulators of the c-Jun amino-terminal kinases? Curr. Opin. Genet. Dev. 7, 67–74 [DOI] [PubMed] [Google Scholar]
- 17. Johnson G. L., Nakamura K. (2007) The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim. Biophys. Acta 1773, 1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwabe R. F., Bradham C. A., Uehara T., Hatano E., Bennett B. L., Schoonhoven R., Brenner D. A. (2003) c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology 37, 824–832 [DOI] [PubMed] [Google Scholar]
- 19. Kamata H., Honda S., Maeda S., Chang L., Hirata H., Karin M. (2005) Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120, 649–661 [DOI] [PubMed] [Google Scholar]
- 20. Chang L., Kamata H., Solinas G., Luo J. L., Maeda S., Venuprasad K., Liu Y. C., Karin M. (2006) The E3 ubiquitin ligase itch couples JNK activation to TNFα-induced cell death by inducing c-FLIP(L) turnover. Cell 124, 601–613 [DOI] [PubMed] [Google Scholar]
- 21. Schneider-Brachert W., Tchikov V., Neumeyer J., Jakob M., Winoto-Morbach S., Held-Feindt J., Heinrich M., Merkel O., Ehrenschwender M., Adam D., Mentlein R., Kabelitz D., Schütze S. (2004) Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity 21, 415–428 [DOI] [PubMed] [Google Scholar]
- 22. Schneider-Brachert W., Tchikov V., Merkel O., Jakob M., Hallas C., Kruse M. L., Groitl P., Lehn A., Hildt E., Held-Feindt J., Dobner T., Kabelitz D., Krönke M., Schütze S. (2006) Inhibition of TNF receptor 1 internalization by adenovirus 14.7K as a novel immune escape mechanism. J. Clin. Invest. 116, 2901–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Los M., Mozoluk M., Ferrari D., Stepczynska A., Stroh C., Renz A., Herceg Z., Wang Z. Q., Schulze-Osthoff K. (2002) Activation and caspase-mediated inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol. Biol. Cell 13, 978–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vercammen D., Brouckaert G., Denecker G., Van de Craen M., Declercq W., Fiers W., Vandenabeele P. (1998) Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J. Exp. Med. 188, 919–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DuBois R. N., McLane M. W., Ryder K., Lau L. F., Nathans D. (1990) A growth factor-inducible nuclear protein with a novel cysteine/histidine repetitive sequence. J. Biol. Chem. 265, 19185–19191 [PubMed] [Google Scholar]
- 26. Lai W. S., Stumpo D. J., Blackshear P. J. (1990) Rapid insulin-stimulated accumulation of an mRNA encoding a proline-rich protein. J. Biol. Chem. 265, 16556–16563 [PubMed] [Google Scholar]
- 27. Gomperts M., Pascall J. C., Brown K. D. (1990) The nucleotide sequence of a cDNA encoding an EGF-inducible gene indicates the existence of a new family of mitogen-induced genes. Oncogene 5, 1081–1083 [PubMed] [Google Scholar]
- 28. Carballo E., Lai W. S., Blackshear P. J. (1998) Feedback inhibition of macrophage tumor necrosis factor-α production by tristetraprolin. Science 281, 1001–1005 [DOI] [PubMed] [Google Scholar]
- 29. Taylor G. A., Carballo E., Lee D. M., Lai W. S., Thompson M. J., Patel D. D., Schenkman D. I., Gilkeson G. S., Broxmeyer H. E., Haynes B. F., Blackshear P. J. (1996) A pathogenetic role for TNF α in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity 4, 445–454 [DOI] [PubMed] [Google Scholar]
- 30. Blackshear P. J., Lai W. S., Kennington E. A., Brewer G., Wilson G. M., Guan X., Zhou P. (2003) Characteristics of the interaction of a synthetic human tristetraprolin tandem zinc finger peptide with AU-rich element-containing RNA substrates. J. Biol. Chem. 278, 19947–19955 [DOI] [PubMed] [Google Scholar]
- 31. Brewer B. Y., Malicka J., Blackshear P. J., Wilson G. M. (2004) RNA sequence elements required for high affinity binding by the zinc finger domain of tristetraprolin: conformational changes coupled to the bipartite nature of Au-rich MRNA-destabilizing motifs. J. Biol. Chem. 279, 27870–27877 [DOI] [PubMed] [Google Scholar]
- 32. Lai W. S., Carballo E., Thorn J. M., Kennington E. A., Blackshear P. J. (2000) Interactions of CCCH zinc finger proteins with mRNA: binding of tristetraprolin-related zinc finger proteins to Au-rich elements and destabilization of mRNA. J. Biol. Chem. 275, 17827–17837 [DOI] [PubMed] [Google Scholar]
- 33. Worthington M. T., Pelo J. W., Sachedina M. A., Applegate J. L., Arseneau K. O., Pizarro T. T. (2002) RNA binding properties of the AU-rich element-binding recombinant Nup475/TIS11/tristetraprolin protein. J. Biol. Chem. 277, 48558–48564 [DOI] [PubMed] [Google Scholar]
- 34. Taylor G. A., Thompson M. J., Lai W. S., Blackshear P. J. (1995) Phosphorylation of tristetraprolin, a potential zinc finger transcription factor, by mitogen stimulation in intact cells and by mitogen-activated protein kinase in vitro. J. Biol. Chem. 270, 13341–13347 [DOI] [PubMed] [Google Scholar]
- 35. Cao H., Deterding L. J., Venable J. D., Kennington E. A., Yates J. R., 3rd, Tomer K. B., Blackshear P. J. (2006) Identification of the anti-inflammatory protein tristetraprolin as a hyperphosphorylated protein by mass spectrometry and site-directed mutagenesis. Biochem. J. 394, 285–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cao H., Deterding L. J., Blackshear P. J. (2007) Phosphorylation site analysis of the anti-inflammatory and mRNA-destabilizing protein tristetraprolin. Expert Rev. Proteomics 4, 711–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cao H., Dzineku F., Blackshear P. J. (2003) Expression and purification of recombinant tristetraprolin that can bind to tumor necrosis factor-α mRNA and serve as a substrate for mitogen-activated protein kinases. Arch. Biochem. Biophys. 412, 106–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carballo E., Cao H., Lai W. S., Kennington E. A., Campbell D., Blackshear P. J. (2001) Decreased sensitivity of tristetraprolin-deficient cells to p38 inhibitors suggests the involvement of tristetraprolin in the p38 signaling pathway. J. Biol. Chem. 276, 42580–42587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chrestensen C. A., Schroeder M. J., Shabanowitz J., Hunt D. F., Pelo J. W., Worthington M. T., Sturgill T. W. (2004) MAPKAP kinase 2 phosphorylates tristetraprolin on in vivo sites including Ser178, a site required for 14–3-3 binding. J. Biol. Chem. 279, 10176–10184 [DOI] [PubMed] [Google Scholar]
- 40. Mahtani K. R., Brook M., Dean J. L., Sully G., Saklatvala J., Clark A. R. (2001) Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor α mRNA stability. Mol. Cell. Biol. 21, 6461–6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schichl Y. M., Resch U., Lemberger C. E., Stichlberger D., de Martin R. (2011) Novel phosphorylation-dependent ubiquitination of tristetraprolin by mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase 1 (MEKK1) and tumor necrosis factor receptor-associated factor 2 (TRAF2). J. Biol. Chem. 286, 38466–38477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liang J., Lei T., Song Y., Yanes N., Qi Y., Fu M. (2009) RNA-destabilizing factor tristetraprolin negatively regulates NF-κB signaling. J. Biol. Chem. 284, 29383–29390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schichl Y. M., Resch U., Hofer-Warbinek R., de Martin R. (2009) Tristetraprolin impairs NF-κB/p65 nuclear translocation. J. Biol. Chem. 284, 29571–29581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schaljo B., Kratochvill F., Gratz N., Sadzak I., Sauer I., Hammer M., Vogl C., Strobl B., Müller M., Blackshear P. J., Poli V., Lang R., Murray P. J., Kovarik P. (2009) Tristetraprolin is required for full anti-inflammatory response of murine macrophages to IL-10. J. Immunol. 183, 1197–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yeh W. C., Shahinian A., Speiser D., Kraunus J., Billia F., Wakeham A., de la Pompa J. L., Ferrick D., Hum B., Iscove N., Ohashi P., Rothe M., Goeddel D. V., Mak T. W. (1997) Early lethality, functional NF-κB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity 7, 715–725 [DOI] [PubMed] [Google Scholar]
- 46. Zhang W. J., Wojta J., Binder B. R. (1997) Notoginsenoside R1 counteracts endotoxin-induced activation of endothelial cells in vitro and endotoxin-induced lethality in mice in vivo. Arterioscler. Thromb. Vasc. Biol. 17, 465–474 [DOI] [PubMed] [Google Scholar]
- 47. Verhelst K., Carpentier I., Kreike M., Meloni L., Verstrepen L., Kensche T., Dikic I., Beyaert R. (2012) A20 inhibits LUBAC-mediated NF-κB activation by binding linear polyubiquitin chains via its zinc finger 7. EMBO J. 31, 3845–3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Burke J. R., Pattoli M. A., Gregor K. R., Brassil P. J., MacMaster J. F., McIntyre K. W., Yang X., Iotzova V. S., Clarke W., Strnad J., Qiu Y., Zusi F. C. (2003) BMS-345541 is a highly selective inhibitor of I κ B kinase that binds at an allosteric site of the enzyme and blocks NF-κ B-dependent transcription in mice. J. Biol. Chem. 278, 1450–1456 [DOI] [PubMed] [Google Scholar]
- 49. Sabapathy K., Hochedlinger K., Nam S. Y., Bauer A., Karin M., Wagner E. F. (2004) Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol. Cell 15, 713–725 [DOI] [PubMed] [Google Scholar]
- 50. Liu J., Minemoto Y., Lin A. (2004) c-Jun N-terminal protein kinase 1 (JNK1), but not JNK2, is essential for tumor necrosis factor α-induced c-Jun kinase activation and apoptosis. Mol. Cell. Biol. 24, 10844–10856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Blackwell K., Zhang L., Thomas G. S., Sun S., Nakano H., Habelhah H. (2009) TRAF2 phosphorylation modulates tumor necrosis factor α-induced gene expression and cell resistance to apoptosis. Mol. Cell. Biol. 29, 303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Habelhah H., Takahashi S., Cho S. G., Kadoya T., Watanabe T., Ronai Z. (2004) Ubiquitination and translocation of TRAF2 is required for activation of JNK but not of p38 or NF-κB. EMBO J. 23, 322–332 [DOI] [PMC free article] [PubMed] [Google Scholar]