Abstract
Background:
We assessed the effects of low-dose IV ketamine-midazolam versus morphine on pain control in patients with closed limb fracture(s); and also compared the incidence of adverse events (cardio-pulmonary) between two groups.
Materials and Methods:
This prospective, single-blind, non-inferiority trial randomized consecutive emergency department (ED) patients aged 18-60 years to two groups: Receiving 300-500 mcg/kg ketamine plus 0.03 mg/kg midazolam, or 0.05-0.1 mg/kg morphine. Visual analogue score (VAS) and adverse events were verified during an interval of 30 minutes.
Results:
Two hundred and thirty — six patients were selected, among whom 207 were males (87.3%). The average age was 29 ± 2, (range, 18-60 years). The VAS score at T30 (i.e., 30 minutes after initial analgesic dose) was significantly decreased compared with VAS score at T0, in both groups. No statistically significant difference, however, was observed between the two groups (–6.1 ± 1.1 versus –6.2 ± 1.0; P = 0.16). With regard to systolic blood pressure and respiratory rate, however, a meaningful difference was noted between the two groups (1.5 ± 6.4 versus –2.1 ± 6.6; P = 0.000 for SBP, and –0.2 ± 1.1 versus –1.1 ± 6.1; P = 0.048 for RR).
Conclusion:
Low-dose intravenous ketamine plus midazolam has the same analgesic effects as morphine on pain control in trauma patients with closed limb fracture(s), in addition to less respiratory adverse events.
Keywords: Closed limb fracture, ketamine, midazolam, morphine
INTRODUCTION
Limb traumas are among the most painful and stressful events, that anybody may experience during his or her life. So long as pain control is concerned, morphine remains the gold standard for analgesia. It has been the standard analgesic for many years, and the effects of newer analgesics are often expressed in comparison with the effect of morphine. The search for good analgesics, however, should not only focus on analgesia. As far as analgesia is concerned, morphine is an excellent drug; but morphine lacks quality of an ideal analgesia due to its side effects. These side effects include sedation, respiratory suppression, nausea and vomiting and pruritus, which may be avoided by substituting other drugs or combinations with the same effects and lower or no adverse effects such as ketamine with or without midazolam.[1,2,3,4] First synthesized in 1963 during the search for the “ideal” anesthetic, ket-amine was so named because it is a “keto” derivative of an amine. And compared with the R (-) isomer, the S (+) isomer has a fourfold greater affinity for the NMDA receptor, twice the analgesic potency, and fewer psychomimetic effects.[3,4,5,6,32]
Ketamine is useful as a general anesthetic for trauma patients, because it preserves sympathetic reflexes that help support blood pressure in patients who have lost blood. Because it does not interfere with respiratory drive, it is also widely used in resource-poor set-tings where intubation and intraoperative mechanical ventilation are unfeasible. The analgesic action of low, subanesthetic doses of ketamine predominantly de-rives from its activity-dependent, noncompetitive blockade of the glutaminergic NMDA-receptor channel complex, through binding at phen-cyclidine (PCP)-binding sites in the ion channel. Experimental and clinical evidence indicates that ketamine reduces opioid-induced tolerance and hyperalgesia; however, the mechanisms involved are only partially understood. Low-dose ketamine decreased morphine-resistant pain, reduced dosage requirements in opioid-tolerant patients, decreased hyperalgesia after remifentanil infusion, and reduced hyperalgesia and allodynia along surgical incisions.[6,7,8,9]
Benzodiazepines are widely used as anxiolytics, sedatives, hypnotics, and anticonvulsants. Traditionally, they have been considered to lack analgesic action; however, benzodiazepines reduce the minimum alveolar anesthetic concentration of inhaled anesthetics. This is of interest that the neurophysiological mechanisms of the effects of benzodiazepines (i.e. midazolam) on nociception have been studied in animals, but the conclusions often are conflicting.[10] According to these studies midazolam may suppress pain pathways of the spinal cord by its specific action and that effect is not due to the action of the drug on supraspinal systems.[11]
In this study we had two main objectives: (1) to compare the efficacy of two analgesic regimens regarding pain control in trauma patients (closed fractures: Defined as fractures with intact skin and soft tissue above the fracture site); (2) to compare the incidence of adverse events, categorized as respiratory, nausea, and vomiting, and agitation in conjunction with hemodynamic alterations between groups receiving low dose IV ketamine plus midazolam versus IV morphine.
MATERIALS AND METHODS
Study procedures and outcome measure
Study design and Patients
We performed a prospective, randomized, single-blind, non-inferiority clinical trial in the emergency department (ED) of a university medical center from December 2011 until February 2013 (ClinicalTrials.gov Identifier: NCT01807429). The main reason for which we considered this study as a single blind was the possibility of a physician bias resulting from ketamine-induced nystagmus. The study was approved by the hospital ethics committee, and written informed consent was obtained from each patient.
The sample size was determined about 236 patients; considering type 1 error rate as 0.05, statistical power as 80% and common variance as 1.8 for detecting δ = 1 as the fixed non-inferiority margin (pain score in terms of visual analogue score (VAS)).
Two hundred and thirty-six patients of both genders sustaining closed fracture of the extremities (i.e., long bones) who were between the ages of 18 and 60 years, and in good health or with only mild systemic diseases (American Society of Anesthesiologists grade 1 or 2) were recruited for this study as a convenience sample because the availability of such a population in the ED. Patients were eligible for inclusion if they presented a trauma with a severe acute pain defined as a VAS score of at least 60/100 (or 6);[1,2,3,4,5,6,7,8,9,12,13,14,15,16,17,18,19,20,21] were aged between 18 and 60 years; and were without acute respiratory, hemodynamic, or neurologic compromise (respiratory distress signs, systolic blood pressure <90 mmHg, Glasgow Coma Score <15). The patients with a psychiatric history; chronic respiratory, and acute pulmonary infection; renal, or hepatic failure; known ketamine sensitivity; known opioid allergies; treatment of chronic pain or treatment with opioids; incapacity to understand the VAS; the use of drugs that affect the central nervous system; chemical substance abuse; chronic pain; pregnancy; morbid obesity; increased intracranial pressure; cardiovascular, hepatic, renal or ocular pathology; thyroid disease, and pregnancy were not included in the study. Patients, who had already received an opioid analgesic (either by self-administration or by another attending physician or emergency medicine service (EMS)), were also not included in our study.
A table of random numbers determined the randomization sequence, using a restricted randomization scheme to ensure roughly equal numbers in each group. Group assignments were sealed in opaque envelopes and opened sequentially by the investigators. The threshold for administration of analgesics in this study was severe acute pain, defined as a VAS of at least 6. Eligible patients were randomly allocated to receive either morphine (0.05-0.1 mg/kg) followed by additional doses of 3 mg every 5-10 minutes or ketamine-midazolam (300-500 mcg/kg ketamine and 0.03 mg/kg midazolam). The latter regimen was also repeated as needed, until pain relief was obtained as defined by a VAS score not exceeding 30/100.[11,22,23,24,25,26] The study protocol is shown in Figure 1.
Figure 1.
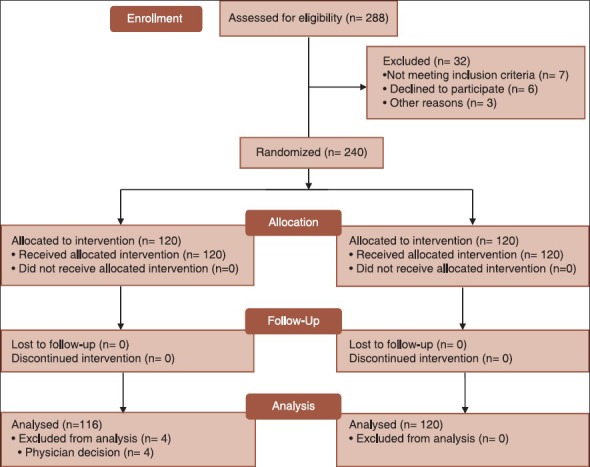
The consort flow diagram
The patients were assessed at the time of arrival at the ED and every 10 minutes for a total interval of 30 minutes, and the following variables were carefully monitored: Visual analogue pain score (VAPS), pulse oxymetry (SPO2), respiratory rate (RR), blood pressure (SBP, DBP), heart rate (HR), nausea and vomiting, screaming, cursing, nightmares, and unpleasant hallucinations.
Pain intensity was verified using a 10 cm (100 mm) VAS, anchored by “no pain” at one end and by “worst possible pain” at the opposite end. The VAS, after teaching the patient as to determining their pain score visually or numerically, was used to evaluate pain, with the patient attributing a value that corresponded to the level of his or her pain. The threshold for efficient analgesia was defined as a 13-mm decline from the base line VAS. The statistically significant analgesia, however, was held to be as a VAS score of 30/100 or lower. Respiratory adverse events were defined as oxygen desaturation (SPO2 < 90%), apnea (a minimum 20 seconds transient cessation of breathing), or laryngospasm. Airway management equipment, flumazenil, and naloxone were always available at the bedside.
All VAS scores were recorded at T0, T10, T20, and T30. Thirty minutes after the first injection, patients, physicians, and nurses were queried about their level of satisfaction, from 1 (least satisfied) to 5 (most satisfied), with a Likert scale. The responses were categorized as satisfied (Likert scale score of 4 or 5) and not satisfied (score of 1, 2, or 3).
Primary outcome measure was the percentage of patients with pain relief (with a VAS score of 30/100 or lower) 30 minutes (T30) after the first injection (T0). Secondary outcomes included pain score comparisons every 10 minutes within the first 30 minutes and comparison of adverse events as mentioned above.
Thirty minutes after initial analgesic dose, if the patient still did not reach a Visual Analogue Score of 30 mm or less, he or she was excluded from the study. When the patient was asleep, no attempt was made at arousal. In this situation the patient was considered as having adequate pain relief and was assigned a VAS score of 0. When pain was initially too severe to obtain a VAS (patient refusal), it was scored 100.
Study analysis
Quantitative variables were expressed as mean ± SD and qualitative variables as number (percentage). Two independent samples t-test was used as the main statistical method for comparing the main outcomes. For all main outcomes the percentage changes from baseline values were calculated and compared between two groups. Within group comparisons was conducted using paired t-test. Chi-square test also was used for comparing the qualitative variables between two studied groups. We analyzed our data by SPSS software version 16.
RESULTS
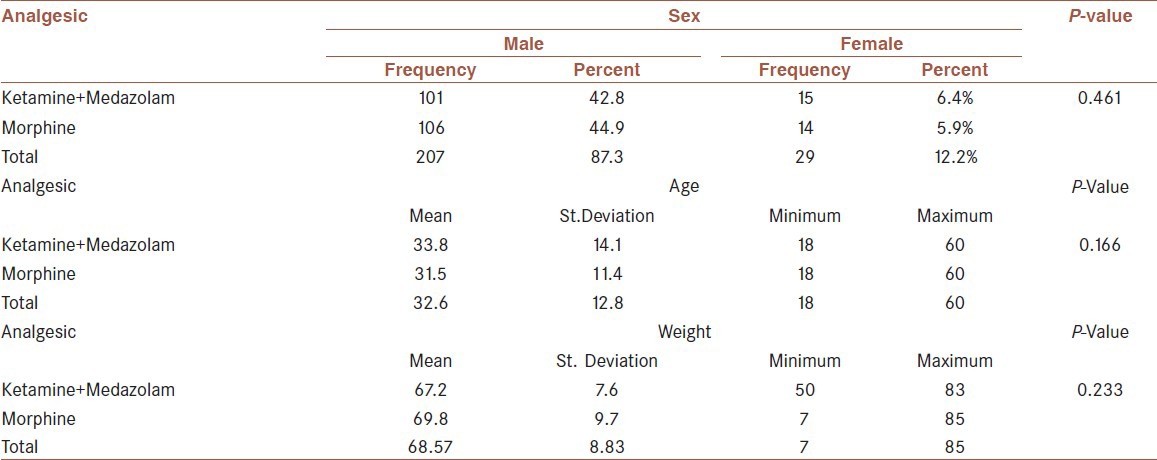
Two hundred and thirty-six patients were selected, among whom were 207 males (87.3%), and 29 females (12.2%). The average age was 32.6 ± 12.8 with extremes of 60 years and 18 years. The patients were divided into two groups: G1: 116 patients receiving ketamine-midazolam (33.8 ± 14.1) and G2: 120 patients receiving morphine alone (31.5 ± 11.4). The median for body weight among G1 patients was 69 kg, and among G2 patients it was 70 kg [Table 1].
Table 1.
Demographic characteristics of two studied groups
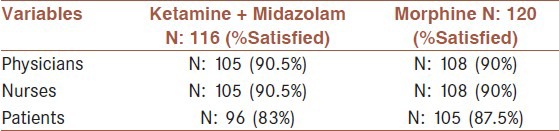
The VAS score at T30 was significantly decreased compared with VAS score at T0, in both groups (P < 0.0001). No statistically significant difference, however, was observed between two groups (P < 0.16). The mean value of the VAPS between two groups showed no meaningful difference; in other words the efficacy of both regimens was the same in terms of pain control. The mean value of DBP revealed no significant difference in both groups; whereas regarding the mean value of RR and SPo2 a meaningful difference was encountered between two groups. Furthermore, the mean value of the SBP and HR depicted a meaningful difference; this means that SBP and HR mean values increased 30 minutes after taking ketamine-midazolam but with morphine these values were decreased [Table 2]. Nausea, vomiting, agitation, or hallucination was not noted in both groups. With reference to satisfaction from pain control process, no significant difference was seen between two groups [Table 3].
Table 2.
Comparing the mean of analgesic in two groups in terms of main studied outcome variables
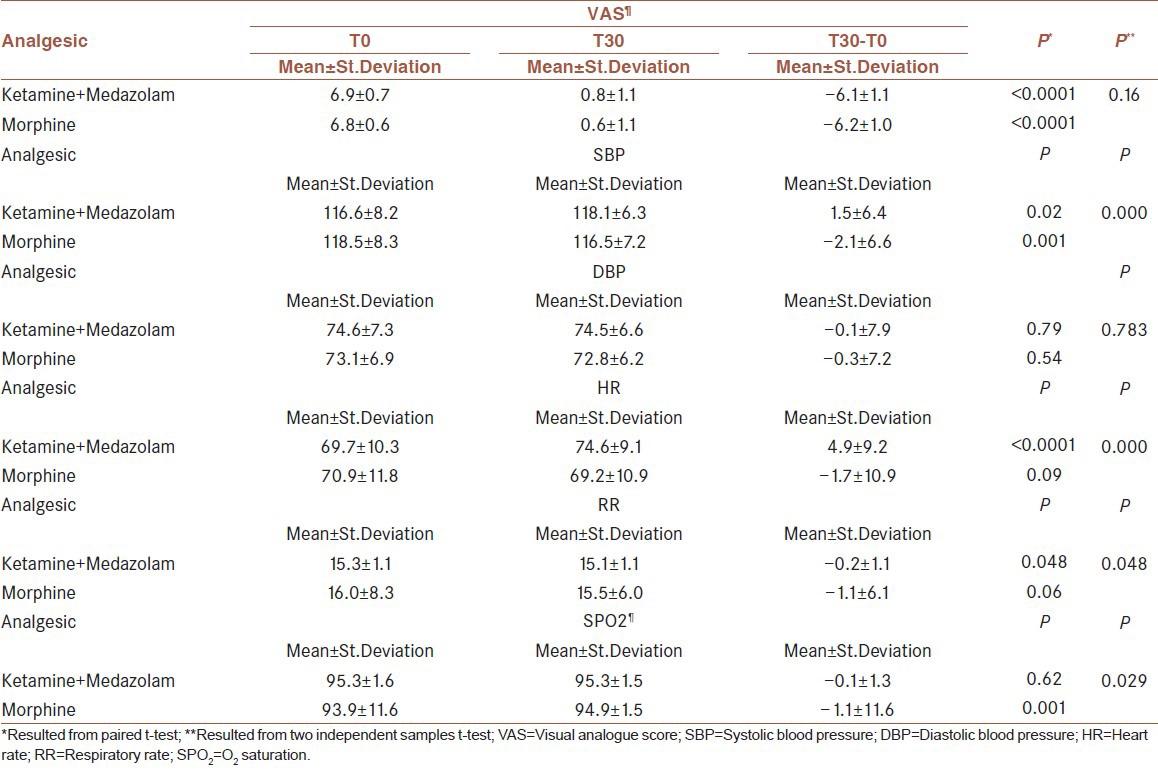
Table 3.
Satisfaction rates among physicians, nurses, and patients
DISCUSSION AND CONCLUSIONS
The most significant finding achieved in this study is that there is no meaningful difference between low-dose ketamine-midazolam and morphine with respect to pain control in patients sustaining closed limb fracture(s). We found that hypoventilation and increased heart rate/blood pressure were more prominent in group morphine and group ketamine plus midazolam, respectively. The changes noted in cardiopulmonary system, however, were congruent with what we already knew according to the previous evidence-based researches.
The emergency atmosphere and ED are somehow stressful per se; so ketamine-midazolam regimen could be a better choice in terms of pain control in limb trauma patients, while it imposes a minimal risk on cardiopulmonary systems (like respiratory depression and apnea). It also makes itself a reasonable choice in opium-addicted patients, in group ketamine-midazolam inflicted with drug tolerance because of its morphine-sparing effects.[10,25,25]
Regarding the effects of ketamine and morphine on pain on the one hand and their interaction, on the other, so many studies have been yet accomplished. In 2003, FJ et al published an article as to comparing the quality of intravenous patient controlled analgesia (PCIA) of low-dose morphine plus ketamine with morphine[11,25] in a group of patients scheduled for elective abdominal hysterectomy. Contrary to our research, this study compared morphine consumption and morphine sparing effect of ketamine. They concluded that morphine plus ketamine PCIA, in doses used in this study (7 mg/kg morphine plus 14 mg/kg ketamine as a bolus) provided analgesia inferior to that of morphine PCIA, but may improve the respiratory side effect profile of morphine. One reason for this conclusion, of course, could be the ultra-low dose of ketamine.
Laeben et al reported a study about low-dose ketamine for analgesia in the emergency department (ED). However, there was concern about the patient group in this study which may interfere with their conclusion when applied to the general population. Compared to our study, the majority of the patients in this research had the record of chronic pain medication use or illicit drug abuse. Tolerance or dependence of opioids or opioid-like agents was frequently seen in these patients, which make them become a “drug seeker” in the ED, and thus, analgesia or so-called improvement of pain for them is more complicated.[10,13,27] In our study, however, anybody with history of chronic drug abuse was excluded.
Galinski assessed management of severe acute pain in emergency settings in an article titled as “ketamine reduces morphine consumption”. The aim of the study was to compare in emergency settings two analgesic regimens, morphine with ketamine (K group) or morphine with placebo (P group), for severe acute pain in trauma patients. At T30, morphine consumption was significantly lower in the K group vs. the P group. The VAS score at T30 did not differ significantly between the two groups. They declared that ketamine was able to provide a morphine-sparing effect.[11,28] The main problem with this study, nonetheless, seems to be possible ketamine-induced nystagmus, and a resultant bias pursued, which we tried to resolve it, as mentioned earlier.
Similarly, Babak Garaei and coworkers in Iran did a randomized, placebo-controlled clinical trial, in opioid abusers based on their daily opioid consumption. Lithotripsy was performed under moderate sedation with intermittent bolus doses of remifentanil (0.2 μg/kg) to alleviate pain. The incidences of bradypnea, apnea, nausea, vomiting, and hemodynamic changes were not statistically different between the ketamine and placebo groups. They concluded that preemptive low-dose ketamine (0.1 mg/kg) as a bolus has opioid-sparing effects in opioid abusers undergoing moderate sedation.[24,29]
What has not been studied in the above mentioned researches, however, is the comparison of morphine versus ketamine regarding ED pain control. They all used placebo in their studies but this intervention did not necessarily create an unethical issue in these studies, because all the patients received analgesic irrespective of taking ketamine.
Regarding midazolam, our goals were to decrease pain as Toshinobu Sumida et al followed in their study,[11] beside its already known effects on anxiety and ketamine induced agitation, known as ‘emergence phenomenon’. Of course, probable analgesic effects of midazolam are yet to be investigated by more precise, double-blind studies in the future.
Another issue that makes our sample more different from its predecessors is that it is a noninferiority trial. The term ‘noninferiority trial’ is commonly used to refer to a randomized clinical trial in which a new test treatment is compared with a standard active treatment rather than a placebo or untreated control group. One starting principle is that no patient is denied a known effective treatment by entering a clinical trial.[30,31]
In our study we noted no adverse pulmonary effects like hypoventilation as caused by morphine, in group ketamine-midazolam. The effect of ketamine on respiratory rate is in accordance with the findings of Presson et al.[5] They found that analgesic concentrations of ketamine antagonized alfentanil-induced hypoventilation. Alfentanil induced a decrease in respiratory rate, without affecting tidal volume and respiratory drive. They ascribe the effect of ketamine on ventilation to two possible mechanisms. Firstly, ketamine caused subjective side effects in all subjects (e.g., strange feeling, body feels tight, arms and legs strange, body feels heavy, etc) that might have caused general arousal, thereby stimulating respiration indirectly. Secondly, being an NMDA receptor antagonist, ketamine may antagonize the effect of opioids on ventilation as the effect of opioids on the control of breathing may be through inhibition of glutaminergic transmission.[5] No dreaming or hallucinations were reported. The ketamine dose was, therefore, high enough to have an analgesic effect, but lower than a dose that causes hallucinations.
Finally, we recommend more extensive, double-blinded studies with lower therapeutic range of ketamine, in the future which may clarify more exactly the real effect and safety of ketamine plus midazolam compared with morphine.
Limitations
We studied only adults between the ages of 18 and 60 years, and thus our data cannot apply to elderly patients who are perhaps more likely to experience the sympathomimetic effects of ketamine. Furthermore, according to the available data and medical textbooks, the incidence of the closed fractures of the extremities tends to be more at the extremes of ages (e.g., femoral head, intertrochanteric or distal radius fractures in the elderly and suprachondylar humeral fractures in children, both of them not involved in the study).
Closed limb fracture because of its low-energy nature (VAS of less than 6), most of the times does not need to be managed with analgesics; and if a patient who has been probably a drug seeker would apply for analgesic or declared that his pain score was above 6. We could not, unfortunately, trust his or her real intent, despite receiving analgesics and being assigned for this study. Another limitation was the ketamine induced nystagmus that made us perform this study as a single-blind trial, in fear for the probable physician bias while monitoring the patients.
CONCLUSIONS
We concluded that low-dose IV ketamine-midazolam has the same analgesic effects as IV morphine concerning pain control in adult patients sustaining closed limb fracture(s), with less pulmonary adverse events. Whether midazolam has significant analgesic effects, however, remains to be investigated.
AUTHOR'S CONTRIBUTIONS
Mehdi Nasr Isfahani, Awat Feizi: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. Mehdi Nasr Isfahani: Drafting the work or revising it critically for important intellectual content. Omid Ahmadi, Mehdi Nasr Isfahani: Final approval of the version to be published. Omid Ahmadi, Mehdi Nasr Isfahani: Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGMENTS
The authors wish to thank Mehrdad Esmaeilian, MD, Assistant Professor of emergency medicine, for his helpful advice. We appreciate the nursing staff of the Al-Zahra ED who were involved in the monitoring trend of the study. We are indebted to our wives and children for their many sacrifices. We also thank health research center of Isfahan University of Medical Sciences for providing financial support for this study with the research project number: 390284.
Footnotes
Source of Support: Isfahan university of medical sciences research center
Conflict of Interest: None declared.
REFERENCES
- 1.Green SM, Rothrock SG, Harris T. Intravenous ketamine for pediatric sedation in the emergency department: Safety profile with 156 cases. Acad Emerg Med. 1998;5:971–6. doi: 10.1111/j.1553-2712.1998.tb02773.x. [DOI] [PubMed] [Google Scholar]
- 2.Allerton J. Prehospital use of ketamine in mountain rescue. Emerg Med J. 2009;26:760–1. doi: 10.1136/emj.2008.072561. [DOI] [PubMed] [Google Scholar]
- 3.Harley JA, Sullivan AF, Dickenson AH. Evidence for N-methyl-D-aspartate receptor involvement in prolonged chemical nociception in the rat. Brain Res. 1990;518:218–22. doi: 10.1016/0006-8993(90)90975-h. [DOI] [PubMed] [Google Scholar]
- 4.Besson JM, Chaouch A. Peripheral and spinal mechanisms of nociception. Physiol Rev. 1987;67:67–186. doi: 10.1152/physrev.1987.67.1.67. [DOI] [PubMed] [Google Scholar]
- 5.Presson J, Scheinin H, Hellström G, Björkman S, Götharson E, Gustafsson LL. Ketamine antagonises alfentanil-induced hypoventilation in healthy male volunteers. Acta Anaesth Scand. 1999;43:744–52. doi: 10.1034/j.1399-6576.1999.430710.x. [DOI] [PubMed] [Google Scholar]
- 6.Pierrefiche O, Foutz AS, Denavit-Saubié M. Pneumotaxic mechanisms in the non-human primate: Effect of the N-methyl-D-aspartate (NMDA) antagonist ketamine. Neurosci Lett. 1990;119:90–3. doi: 10.1016/0304-3940(90)90763-y. [DOI] [PubMed] [Google Scholar]
- 7.Bell RF, Dahl JB, Moore RA, Kalso E. Perioperative ketamine for acute postoperative pain: A quantitative and qualitative review (Cochrane review) Acta Anaesthesiol Scand. 2005;49:1405–28. doi: 10.1111/j.1399-6576.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- 8.Geisslinger G, Hering W, Thomann P, Knoll R, Kamp HD, Brune K. Pharmacokinetics and pharmacodynamics of ketamine enantiomers in surgical patients using a stereo selective analytical method. Br J Anaesth. 1993;70:666–71. doi: 10.1093/bja/70.6.666. [DOI] [PubMed] [Google Scholar]
- 9.Snapinn SM. Noninferiority trials. Curr Cont Trials Cardiovasc Med. 2000;1:19–21. doi: 10.1186/cvm-1-1-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webb AR, Skinner BS, Leong S, Kolawole H, Crofts T, Taverner M, et al. The addition of a small-dose ketamine infusion to tramadol for postoperative analgesia: A double-blinded, placebo-controlled, randomized trial after abdominal surgery. Anesth Anal. 2007;104:912–17. doi: 10.1213/01.ane.0000256961.01813.da. [DOI] [PubMed] [Google Scholar]
- 11.Sumida T, Tagami M, Ide Y, Nagase M, Sekiyama H, Hanaoka K. The effects of intravenously (IV) administered midazolam on noxiously evoked activity of spinal wide dy- namic range (WDR) neurons were investigated in de- cerebrate, spinal-cord-transected cats. Anesth Analg. 1995;80:58–63. doi: 10.1097/00000539-199501000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Edwards M, Serrao JM, Gent JP, Goodchild CS. On the mechanism by which midazolam causes spinally mediated analgesia. Anesthesiology. 1990;73:273–7. doi: 10.1097/00000542-199008000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Lester L, Braude DA, Niles C, Crandall CS. Low-dose ketamine for analgesia in the ED, a retrospective case series. Am J Emerg Med. 2010;28:820–7. doi: 10.1016/j.ajem.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Mao J, Price DD, Mayer DJ. Experimental mononeuropathy reduces the antinociceptive effects of morphine: Implications for common intracellular mechanisms involved in morphine tolerance and hyperalgesia. Pain. 1995;61:353–64. doi: 10.1016/0304-3959(95)00022-K. [DOI] [PubMed] [Google Scholar]
- 15.Stubhaug A, Breivik H, Eide PK, Kreunen M, Foss A. Mapping of punctuate hyperalgesia around a surgical incision demonstrates that ketamine is a powerful suppressor of central sensitization to pain following surgery. Acta Anaesthsiol Scand. 1997;41:1124–32. doi: 10.1111/j.1399-6576.1997.tb04854.x. [DOI] [PubMed] [Google Scholar]
- 16.Javery KB, Ussery TW, Steger HG, Colclough GW. Comparison of morphine and morphine with ketamine for postoperative analgesia. Can J Anaesth. 1996;43:212–5. doi: 10.1007/BF03011736. [DOI] [PubMed] [Google Scholar]
- 17.Chia YY, Liu K, Liu YC, Chang HC, Wong CS. Adding ketamine in a multimodal patient-controlled epidural regimen reduces postoperative pain and analgesic concentration. Anesth Analg. 1998;86:1245–9. doi: 10.1097/00000539-199806000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Wong CS, Liaw WJ, Tung CS. Ketamine potentiates analgesic effect of morphine in postoperative epidural pain control. Reg Anesth. 1996;21:534–41. [PubMed] [Google Scholar]
- 19.Kochs E, Scharein E, Möllenberg O, Bromm B, Schulte am Esch J. Analgesic efficacy of low-dose ketamine somatosensory-evoked responses in relation to subjective pain rating. Anesthesiology. 1996;85:304–14. doi: 10.1097/00000542-199608000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Maurset A, Skoglund LA, Hustveit O, Øye L. Comparison of ketamine and pethidine in experimental and postoperative pain. Pain. 1989;36:37–41. doi: 10.1016/0304-3959(89)90109-7. [DOI] [PubMed] [Google Scholar]
- 21.Sener S, Eken C, Schultz CH, Serinken M, Ozsarac M. Ketamine With and Without Midazolam for Emergency Department Sedation in Adults: A Randomized Controlled Trial. Ann emerg Med. 2011;57:109–14. doi: 10.1016/j.annemergmed.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Dich-Nielsen JO, Svendsen LB, Berthelsen P. Intramuscular low-dose ketamine versus pethidine for postoperative pain treatment after thoracic surgery. Acta Anaesthesiol Scand. 1992;36:583–7. doi: 10.1111/j.1399-6576.1992.tb03523.x. [DOI] [PubMed] [Google Scholar]
- 23.Carstensen M, Møller AM. Adding ketamine to morphine for intravenous patient-controlled analgesia for acute postoperative pain: A qualitative review of randomized trials. Br J Anaest. 2010;104:401–6. doi: 10.1093/bja/aeq041. [DOI] [PubMed] [Google Scholar]
- 24.Gharaei B, Jafari A, Aghamohammadi H, Kamranmanesh M, Poorzamani M, Elyassi H, et al. Opioid-Sparing Effect of Preemptive Bolus Low-Dose Ketamine for Moderate Sedation in Opioid Abusers Undergoing Extracorporeal Shock Wave Lithotripsy: A Randomized Clinical Trial. Anesth Analg. 2013;116:75–80. doi: 10.1213/ANE.0b013e31826f0622. [DOI] [PubMed] [Google Scholar]
- 25.Galinski M, Dolveck F, Combes X, Limoges V, Smaïl N, Pommier V, et al. Management of severe acute pain in emergency settings: Ketamine reduce morphine consumption. Am J Emerg Med. 2007;25:385–90. doi: 10.1016/j.ajem.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Bounes V, Charpentier S, Houze-Cerfon CH, Bellard C, Ducassé JL. Is there an ideal morphine dose for prehospital treatment of severe acute pain? A randomized, double-blind comparison of 2 doses. Am J Emerg Med. 2008;26:148–54. doi: 10.1016/j.ajem.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 27.Sener S, Eken C, Schultz CH, Serinken M, Ozsarac M. Ketamine with and without midazolam for emergency department sedation in adults: A randomized controlled trial. Ann Emerg Med. 2011;57:109–14. doi: 10.1016/j.annemergmed.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Arroyo-Novoa CM, Figueroa-Ramos MI, Miaskowski C, Padilla G, Paul SM, Rodríguez-Ortiz P, et al. Efficacy of small doses of ketamine with morphine to decrease procedural pain responses during open wound care. Clin J Pain. 2011;27:561–6. doi: 10.1097/AJP.0b013e318211936a. [DOI] [PubMed] [Google Scholar]
- 29.Atangana R, Ngowe Ngowe M, Binam F, Sosso MA. Morphine versus morphine-ketamine association in the management of post operative pain in thoracic surgery. Acta Anaesth. 2007;58:125–7. [PubMed] [Google Scholar]
- 30.Suppa E, Valente A, Catarci S, Zanfini BA, Draisci G. A study of low-dose S-ketamine infusion as “preventive” pain treatment for cesarean section with spinal anesthesia: Benefits and side effects. Minerva Anestesiol. 2012;78:774–81. [PubMed] [Google Scholar]
- 31.Temple R, Ellenberg SS. Placebo-controlled trials and active control trials in the evaluation of new treatments. Ann Intern Med. 2000;133:455–70. doi: 10.7326/0003-4819-133-6-200009190-00014. [DOI] [PubMed] [Google Scholar]
- 32.Schmid RL, Sandler AN, Katz J. Use and efficacy of low-dose ketamine in the management of acute postoperative pain: A review of current techniques and outcomes. Pain. 1999;82:111–25. doi: 10.1016/S0304-3959(99)00044-5. [DOI] [PubMed] [Google Scholar]


