Abstract
Background:
The role of obesity in clinical curse of rheumatoid arthritis (RA) is not clear. We investigated the association of obesity and adiposity with disease activity and clinical response to combination therapy in RA patients.
Materials and Methods:
Active RA patients with the disease activity score using 28 joint counts (DAS28) > 2.6 were studied. Height, weight, and waist and hip circumferences were measured and body mass index (BMI) and waist to hip ratio were calculated. Patients were treated with methotrexate (7.5 to 10 mg/week) plus hydroxychloroquine (200 to 400 mg/day) and prednisolone (2.5 to 10 mg/day) and were followed by DAS28 for up to 24 weeks.
Results:
One hundred and six patients were studied; age = 48.5 ± 13.8 years, 87.7% female, disease duration = 4.4 years [SE = 0.48]. DAS28 was decreased from 4.5 ± 1.6 to 2.9 ± 1.4 (P < 0.001) after 24 weeks of treatment. Only in patients with disease duration of ≤2 years, BMI (r = –0.415, P = 0.005) and waist circumference (r = –0.296, P = 0.05) were correlated with baseline DAS28. Although BMI (r = –0.337, P = 0.025) and waist circumference (r = –0.315, P = 0.038) were correlated with change in DAS28 after therapy, these correlations were disappeared after controlling for baseline DAS28.
Conclusion:
Obesity and adiposity are associated with less severe disease activity in early stage of RA, but are not associated with response to combination therapy with methotrexate plus hydroxychloroquine in RA patients.
Keywords: Adiposity, adipose tissue, hydroxychloroquine, methotrexate, obesity, rheumatoid arthritis, treatment outcome
INTRODUCTION
Body weight change in patients with rheumatoid arthritis (RA) is a complex issue. On one hand, active disease could lead to weight loss and frank wasting, termed rheumatoid cachexia, which correlates with the intensity of systemic inflammation.[1] On the other hand, some studies showed that a higher body mass index (BMI) is associated with less severe radiographic joint damage in the early phases of the disease.[2] Even, some evidence showed that all-cause mortality and cardiovascular-related mortality rates decrease with increasing BMI in RA patients.[3,4] The subject becomes more complex when considering the adipose tissue as an active site that, by releasing adipocytokines, has some immunological effects.[5] Therefore, it seems to be a bi-directional relationship between body weight change and disease activity in patients with RA, which is not only affected by biological factors but also by patients’ behaviors and life style.[6]
The association of BMI and the amount of body fat with clinical course of RA is still a matter of debate, and clinical applications of current data are not clear. Some studies with long follow-up periods have showed that obesity is associated with worse outcomes,[7] while others have found lower BMI to be associated with decreased survival, and higher BMI as being protective.[8] Also, some data are available on the association between BMI and treatment response in RA patients. In this regard, recent studies reported that a higher BMI is associated with less well response to antitumor necrosis factor alpha (anti-TNFα) agents.[9,10] Considering the controversial and complex results of the previous studies and lack of data, the primary aim of this prospective study was to determine whether body fat composition affects response to combination therapy with disease-modifying antirheumatic drugs (DMARDs) including methotrexate plus hydroxychloroquine in RA patients. Moreover, RA patients may encounter a decrease in muscle mass that can mask the increase in fat mass and result in having a normal BMI. Therefore, BMI cannot distinguish between the tissues that comprise it and may not accurately reflect the body fat content. Hence, the secondary aim of this study was to evaluate, in addition to BMI, the association of other anthropometric measurements including waist and hip circumferences and waist to hip ratio with treatment response, because they can provide more accurate information about the body fat distribution.[11]
MATERIALS AND METHODS
Patients and settings
This observational cohort study was conducted on patients with RA (16 to 80 year old) referred to the rheumatology clinic of a University Hospital in Isfahan (central Iran) in 2012. Consecutive patients who met the American College of Rheumatology (ACR) criteria for the classification of RA[12] and had active disease based on the disease activity score using 28 joint counts (DAS28) >2.6 were included.[13] Those with previous toxicity to combination therapy with methotrexate plus hydroxychloroquine, and those with significant change of BMI (≥10%) in the preceding three months were not included. Also, we excluded patients whose treatment contained DMARDs or drugs other than methotrexate plus hydroxychloroquine (e.g. sulfasalazine). Considering type-I error = 0.05, study power = 0.8, and expecting correlation between BMI and ΔDAS28 as at least 0.3,[9] the sample size was calculated as 106 cases. The ethics committee of the Isfahan University of Medical Sciences approved the study and informed consent was obtained from all patients.
Assessments
An internist interviewed with all patients and gathered demographic data (age, gender) and clinical data (disease duration, previous therapies) from patients’ history and documents. Height, weight, and waist and hip circumferences were measured by an educated nurse. Body mass index (calculated as weight in kilograms divided by the square of height in meters) was categorized into underweight (<18.5 kg/m2), normal (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), and obese (≥30 kg/m2).[14] Patients were examined by a single rheumatologist who evaluated the number of swollen/tender joints, patient's global activity (general health) on a visual analog scale (VAS, 0 to 100), and disease functional class based on the American College of Rheumatology 1991 revised criteria.[15] All patients were referred to a reference laboratory for measurement of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Finally, DAS28 was calculated based on the formula of DAS28-ESR; 0.56 × √(TJC28) + 0.28 × √(SJC28) + 0.70 × lognat (ESR) + 0.014 × VAS, and classified as in remission (<2.6), low (≥2.6 to <3.2), moderate (≥3.2 to ≤5.1), and high (>5.1) disease activity.
Treatment strategy
Most of the patients have been under treatment with methotrexate monotherapy or combination therapy with methotrexate plus hydroxychloroquine when they were evaluated for entering to our study. Considering response to previous therapies, the clinician started combination therapy (or increased drugs’ dose) with methotrexate (7.5 to 10 mg/week) plus hydroxychloroquine (200 to 400 mg/day) and prednisolone (2.5 to 10 mg/day) based on the clinical course. Dosage of treatment and change of the treatment for patients was registered in data gathering form. Patients were followed for 24 weeks and DAS28 was calculated with the same rheumatologist again every 12 weeks. A decrease of >1.2 score in DAS28 was defined as response.[16]
Statistical analysis
Data were analyzed using the SPSS software for windows version 16.0. Correlation between variables was evaluated using the Pearson test if data were parametric and Spearman test if not normally distributed. Comparisons between those with and without response to treatment were done using Chi-Square test for qualitative variables and independent sample t-Test and Mann-Whitney test (if not normally distributed) for quantitative variables. Because the clinical response was defined based on the change in the DAS28 values, ANCOVA and regression analyses were applied to correct for the baseline DAS28 while analyzing the effect of BMI on treatment response (ΔDAS28). A P value of <0.05 was considered significant in all analyses.
RESULTS
Demographic data
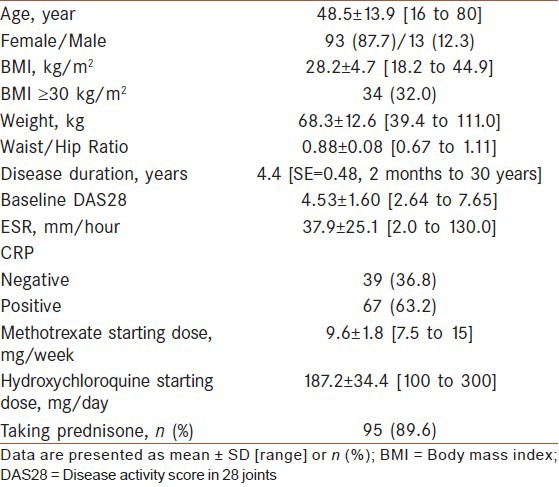
From a total of 140 patients investigated during the study period, 19 patients were under treatment with DMARDs or drugs other than methotrexate plus hydroxychloroquine including sulfasalazine, tacrolimus, and cyclosporine. Also, 15 patients were lost to follow or had missing data. These patients were excluded from our analyses. Finally, 106 patients were included to the analyses and completely studied; (87.7% female) with mean age of 48.5 ± 13.8 years and mean disease duration of 4.4 years (SE = 0.48). Demographic data and clinical characteristics of all participants are presented in [Table 1].
Table 1.
Demographic data and disease characteristics of the patients
Factors associated with baseline disease activity and treatment response
Including all patients, there was a negative correlation between BMI and baseline DAS28 (r = −0.217, P = 0.026). DAS28 score was decreased from 4.5 ± 1.6 to 3.3 ± 1.5 after 12 weeks of treatment (P = 0.005), and then was decreased to 2.9 ± 1.4 after 24 weeks from starting the treatment (P < 0.001). The association between BMI and ΔDAS28 after therapy was weak and not significant (r = −0.132, P = 0.177). Regarding other anthropometric measures, there was no association of waist circumference or waist to hip ratio with baseline disease activity or with ΔDAS28 after therapy [Table 2].
Table 2.
Correlation of demographic data with baseline and change in disease activity
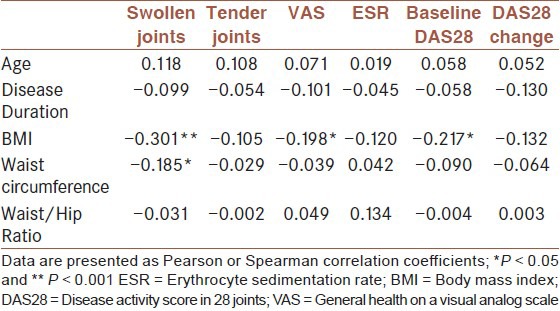
With regards to DAS28 components, BMI was associated with baseline VAS score (r = −0.198, P = 0.040). Also, BMI (r = −0.301, P = 0.001) and waist circumference (r = −0.185, P = 0.020) were associated with the baseline number of swollen joint [Table 2]. Regarding changes in DAS28 components after therapy, there was only a week association between BMI and changes in ESR (r = −0.196, P = 0.043).
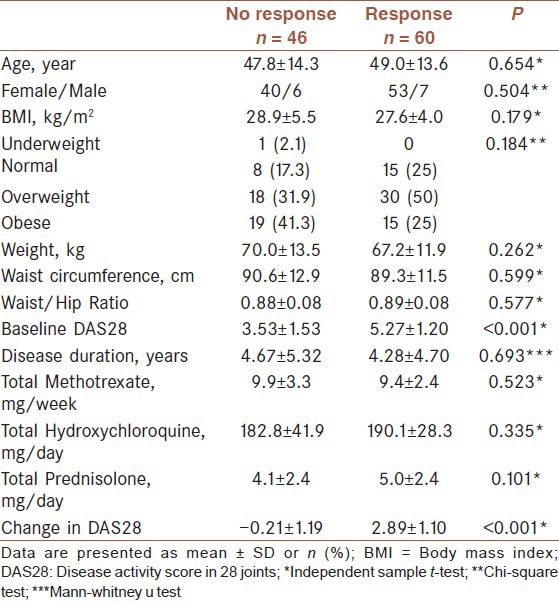
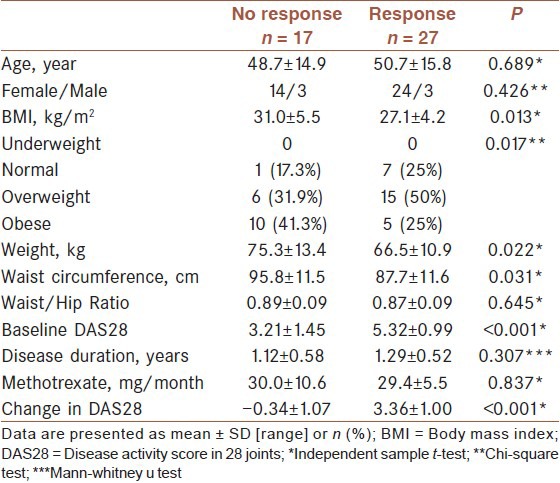
Defining response as a decrease of > 1.2 score in DAS28, comparison of responders and non-responders is presented in [Table 3]. Responders were not different from non-responders in terms of age, gender, disease duration, and anthropometric data. Although there was a trend for non-responders to be more obese (41.3% vs. 25%, P = 0.184) and having higher BMI (28.9 ± 5.5 vs. 27.6 ± 4.0, P = 0.179) than responders, this differences were not statistically significant.
Table 3.
Comparison of responders with nonresponders
Separate analysis of patients with disease duration of ≤2 years and >2 years
We categorized the patients to those with disease duration of ≤2 years indicating early disease and those with disease duration of >2 years. In those with early disease stage, analysis showed a significant and strong association of BMI (r = –0.415, P = 0.005) and waist circumference (r = –0.296, P = 0.05) with baseline DAS28. BMI was also associated with the number of swollen joints (r = –0.415, P = 0.005) and tender joints (r = –0.338, P = 0.025), and with VAS score (patient's global activity) (r = –0.447, P = 0.002). In this group of patients, BMI (r = –0.337, P = 0.025) and waist circumference (r = –0.315, P = 0.038) were also correlated with ΔDAS28 after therapy [Table 4]. Also, responders had higher BMI (31.0 ± 5.5 vs. 27.1 ± 4.2) and greater waist circumference (95.8 ± 11.5 vs. 87.7 ± 11.6), [Table 5]. None of the aforementioned associations or differences was present in patients with disease duration of >2 years.
Table 4.
Correlation of demographic data with baseline and change in disease activity in patients of early disease stage
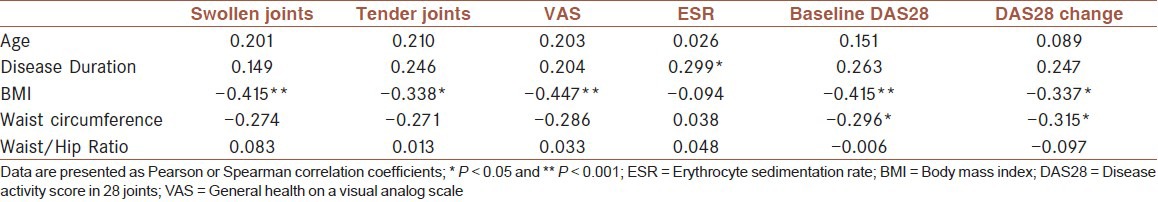
Table 5.
Comparison of responders with nonresponders in those with early disease stage
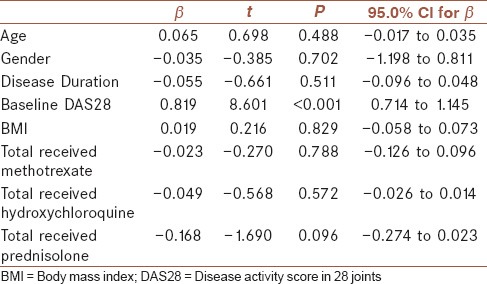
Considering the difference between responders and non-responders in baseline DAS28 and also considering correlation between BMI and baseline DAS28, ANCOVA was applied to correct for the baseline DAS28. This analysis showed no influence of BMI on ΔDAS28 (F = 1.523, P = 0.223). Also, a linear regression analysis controlling for age, gender, disease duration, baseline DAS28, and methotrexate, hydroxychloroquine, and prednisolone total received dose showed no association between BMI and the amount of change in DAS28 (β = 0.019, 95% CI: −0.058 to 0.073), but there was a significant association between baseline DAS28 with ΔDAS28 (β = 0.819, 95% CI: 0.714 to 1.145), [Table 6]. By separate insertion of waist circumference and waist to hip ratio instead of BMI into the linear regression model, there was no association between these anthropometric measures and ΔDAS28 after therapy. Also, separate linear regression analysis in those with disease duration of ≤ 2 years and > 2 years did not change these results.
Table 6.
Linear regression analysis of factors in association with change in DAS28
DISCUSSION
The purpose of this study was to determine if BMI or other anthropometric measures including waist circumference and waist to hip ratio are associated with disease activity and clinical response to combination therapy with methotrexate and hydroxychloroquine in RA patients. With regard to the association between obesity and disease activity at the study entry, we found an inverse correlation of BMI with baseline disease activity. After separating patients based on disease duration, this association was only present in patients with disease duration of ≤2 years. The association between waist circumference and baseline disease activity was also only present in these patients. These results were similar to some previous studies that have found a possible protective role for higher BMI in RA patients at early disease stages.[17,18] Also, we found an association of BMI with clinically swollen and tender joint counts and also with patient's global activity in early disease stage. This finding was similar to radiologic studies in patients with early RA, of up to 3-year duration, indicating the protective role of higher BMI against joint damage.[2,19,20,21] However, it must be noted that BMI was no longer associated with disease activity or separately with its components in patients with disease duration of more than 2 years. This finding indicates that the possible protective effects of obesity in early RA may be diminished later in the course of the disease.[17] Even, some studies have showed a direct association between obesity and disease activity in patients with long-time RA.[7,22] In advanced RA, both underweight and obese states are associated with worse disease activity; an active disease lead to loss of lean body tissue and better control of the disease is associated with weight gain.[23] Also, obesity increases the physical disability.[24] These findings highlight the complex relationship between adiposity and obesity and clinical course in RA patients. Adipose tissue produces adipocytokines with various pro-inflammatory and anti-inflammatory effects, but the exact mechanisms behind the immune-modulatory effects of adipose tissue in RA patients are yet unexplored.[5,17]
With regard to the effects of obesity on treatment response, we found an inverse correlation of BMI and waist circumference with the amount of change in DAS28 after therapy in patients at early disease stage. However, after controlling baseline disease activity, these correlations were no longer existed, indicating a large confounding effect of the baseline disease activity. In contrast to these results, previous studies showed a high BMI associated with less well response to anti-TNFα agents even after adjustment for the baseline disease activity. Klaasen et al. found that RA patients with a higher BMI have a more active disease at study entry and respond less well to infliximab.[9] The study by Gremese and colleagues in a larger sample of patients (n = 641) and longer treatment period (12 months) with anti-TNFα blockers (Adalimumab, Etanercept, and Infliximab) found no association between BMI and disease activity at baseline, but better response to infliximab in those with lower BMI and lower disease activity at baseline.[10] In another recent study by Heimans and colleagues,[25] 508 patients were allocated to initial monotherapy with methotrexate or combination therapy of methotrexate with prednisone or infliximab for 1 year. Authors found that higher BMI was associated with failure to achieve response (a DAS ≤2.4) on initial therapy with methotrexate. In this study, high BMI (≥25 kg/m2) was also associated with failure on delayed combination therapy with infliximab, and in the first year after starting the study, patients with a high BMI had higher DAS, worse functional ability, more tender joints and a higher VAS global health. But, high BMI in this study was not associated with more swollen joints or systemic inflammation.[25] Differences between these findings and ours may be related to different patients’ characteristics and different treatments. Patients in the mentioned studies had longer disease duration. Also, our patients had significantly higher BMI (28.2 kg/m2) compared with the mentioned studies (24.9 and 26), while disease activity score was lower in our study (4.5 vs. 5.6 and 5.9). Anyway, the exact role of BMI in response to various treatments in patients with various disease stages is yet to be clarified.
It has been suggested that the association of obesity with disease activity and response to treatment in RA patients may be due to high levels of proinflammatory cytokines produced by adipocytes.[5,26] Waist circumferences and waist to hip ratio provide more accurate information about the body fat distribution. Hence, we evaluated these parameters to better investigate if adipose tissue has a role in creating an inflammatory and therapy-resistant state in RA patients. However, compared with BMI, we found smaller correlation coefficient of waist circumference and no association of waist to hip ratio with disease activity. In our study as well as in previous studies, BMI and waist circumference were associated with baseline swollen joint count, rather than inflammatory markers. These findings are not in favor of a mechanism involving adipose tissue-derived mediators of inflammation.[9,25] It should be considered that clinical synovitis might be less easy to assess in RA patients with obesity and it is possible that our study as well as others underestimated joint swelling, associated with a local inflammation, in patients with a high BMI.[25] More investigations including advanced imaging and biomarker studies are needed to further elucidate the relation between BMI and disease activity as well as treatment response.
There are some limitations to our study. The association between obesity and treatment response in our study has been confounded by baseline disease activity. Indeed, a larger sample of patients was required for more precise evaluation of the role of obesity on various components of RA activity as well as in different disease stages, and consideration of possible confounders. Moreover, our study period was six months which, compared to other studies, was not long enough. Although, in addition to BMI, we investigated waist circumference and waist to hip ratio which provide more accurate information about the body fat distribution,[11] these are not the most accurate measures, and also we did not evaluate adipocytokines, the hypothesized underlying mediators.
CONCLUSIONS
Our study results showed that obesity is associated with less severe disease activity in early stage of RA, but is not associated with response to combination therapy with methotrexate plus hydroxychloroquine in RA patients. However, due to our study limitations, these results should be considered with cautious. Further cohorts with larger sample size and longer follow-ups, and with more accurate investigation of body adipose composition and possible immunological mechanisms, are warranted in this regards.
ACKNOWLEDGMENTS
This study was supported as a thesis for obtaining specialty degree in Internal Medicine by the Isfahan University of Medical Sciences (project number: 390387). Also we appreciate Dr. Ali Gholamrezaei who helped us in data analysis and editing this report.
Footnotes
Source of Support: Isfahan university of medical sciences (project number: 390387)
Conflict of Interest: None declared.
REFERENCES
- 1.Summers GD, Metsios GS, Stavropoulos-Kalinoglou A, Kitas GD. Rheumatoid cachexia and cardiovascular disease. Nat Rev Rheumatol. 2010;6:445–51. doi: 10.1038/nrrheum.2010.105. [DOI] [PubMed] [Google Scholar]
- 2.Westhoff G, Rau R, Zink A. Radiographic joint damage in early rheumatoid arthritis is highly dependent on body mass index. Arthritis Rheum. 2007;56:3575–82. doi: 10.1002/art.23033. [DOI] [PubMed] [Google Scholar]
- 3.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: A population-based study. Arthritis Rheum. 2005;52:722–32. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:1471–9. doi: 10.1002/acr.21627. [DOI] [PubMed] [Google Scholar]
- 5.Tilg H, Moschen AR. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 6.Rahnama N, Mazloum V. Effects of strengthening and aerobic exercises on pain severity and function in patients with knee rheumatoid arthritis. Int J Prev Med. 2012;3:493–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Ajeganova S, Andersson ML Hafström I; BARFOT Study Group. Association of obesity with worse disease severity in rheumatoid arthritis as well as with comorbidities: A long-term followup from disease onset. Arthritis Care Res (Hoboken) 2013;65:78–87. doi: 10.1002/acr.21710. [DOI] [PubMed] [Google Scholar]
- 8.Escalante A, Haas RW, del Rincón I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: Role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624–9. doi: 10.1001/archinte.165.14.1624. [DOI] [PubMed] [Google Scholar]
- 9.Klaasen R, Wijbrandts CA, Gerlag DM, Tak PP. Body mass index and clinical response to infliximab in rheumatoid arthritis. Arthritis Rheum. 2011;63:359–64. doi: 10.1002/art.30136. [DOI] [PubMed] [Google Scholar]
- 10.Gremese E, Carletto A, Padovan M, Atzeni F, Raffeiner B, Giardina AR, et al. Obesity reduces the response rate to anti TNFα in rheumatoid arthritis. An approach to a personalized medicine. Arthritis Care Res (Hoboken) 2013;65:94–100. doi: 10.1002/acr.21768. [DOI] [PubMed] [Google Scholar]
- 11.Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J. Body mass index, waist circumference and waist: Hip ratio as predictors of cardiovascular risk — a review of the literature. Eur J Clin Nutr. 2010;64:16–22. doi: 10.1038/ejcn.2009.68. [DOI] [PubMed] [Google Scholar]
- 12.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 13.Fransen J, Creemers MC, van Riel PL. Remission in rheumatoid arthritis: Agreement of the disease activity score (DAS28. with the ARA preliminary remission criteria. Rheumatology (Oxford) 2004;43:1252–5. doi: 10.1093/rheumatology/keh297. [DOI] [PubMed] [Google Scholar]
- 14.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 15.Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum. 1992;35:498–502. doi: 10.1002/art.1780350502. [DOI] [PubMed] [Google Scholar]
- 16.Van Gestel AM, Prevoo ML, van ’t Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996;39:34–40. doi: 10.1002/art.1780390105. [DOI] [PubMed] [Google Scholar]
- 17.Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y, Kitas GD. Obesity in rheumatoid arthritis. Rheumatology (Oxford) 2011;50:450–62. doi: 10.1093/rheumatology/keq266. [DOI] [PubMed] [Google Scholar]
- 18.Summers GD, Deighton CM, Rennie MJ, Booth AH. Rheumatoid cachexia: A clinical perspective. Rheumatology (Oxford) 2008;47:1124–31. doi: 10.1093/rheumatology/ken146. [DOI] [PubMed] [Google Scholar]
- 19.Baker JF, George M, Baker DG, Toedter G, Von Feldt JM, Leonard MB. Associations between body mass, radiographic joint damage, adipokines and risk factors for bone loss in rheumatoid arthritis. Rheumatology (Oxford) 2011;50:2100–7. doi: 10.1093/rheumatology/ker294. [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann J, Kielstein V, Kilian S, Stein G, Hein G. Relation between body mass index and radiological progression in patients with rheumatoid arthritis. J Rheumatol. 2003;30:2350–5. [PubMed] [Google Scholar]
- 21.van der Helm-van Mil AH, van der Kooij SM, Allaart CF, Toes RE, Huizinga TW. A high body mass index has a protective effect on the amount of joint destruction in small joints in early rheumatoid arthritis. Ann Rheum Dis. 2008;67:769–74. doi: 10.1136/ard.2007.078832. [DOI] [PubMed] [Google Scholar]
- 22.Ibn YY, Amine B, Laatiris A, Wafki F, Znat F, Hajjaj-Hassouni N. Prevalence of overweight in Moroccan patients with rheumatoid arthritis and its relationships with disease features. Clin Rheumatol. 2012;31:479–82. doi: 10.1007/s10067-011-1874-3. [DOI] [PubMed] [Google Scholar]
- 23.Jurgens MS, Jacobs JW, Geenen R, Bossema ER, Bakker MF, Bijlsma JW, et al. Increase of body mass index in a tight controlled methotrexate-based strategy with prednisone in early rheumatoid arthritis (CAMERA-II): Side-effect of the prednisone or better control of disease activity? Arthritis Care Res (Hoboken) 2013;65:88–93. doi: 10.1002/acr.21797. [DOI] [PubMed] [Google Scholar]
- 24.Stavropoulos-Kalinoglou A, Metsios GS, Panoulas VF, Nevill AM, Jamurtas AZ, Koutedakis Y, et al. Underweight and obese states both associate with worse disease activity and physical function in patients with established rheumatoid arthritis. Clin Rheumatol. 2009;28:439–44. doi: 10.1007/s10067-008-1073-z. [DOI] [PubMed] [Google Scholar]
- 25.Heimans L, van den Broek M, le Cessie S, Siegerink B, Riyazi N, Han KH, et al. Association of high body mass index with decreased treatment response to combination therapy in recent-onset rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2013;65:1235–42. doi: 10.1002/acr.21978. [DOI] [PubMed] [Google Scholar]
- 26.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–15. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]


