Abstract
Background:
Proper management of depression in elderly population would improve the outcome of the disease and reduce its related disability and mortality. Use of memantine with minimal side effects and drug interaction seems reasonable in the elderly but its antidepressant activity is controversial. The aim of the current research is to investigate the effects of add-on memantine during citalopram therapy in elderly patients with depression, in Isfahan.
Materials and Methods:
In this double-blind, placebo controlled trial study; elderly patients aged more than 60 years who were recently diagnosed with depression, were enrolled. The selected patients were randomlysplit into two groups, viz. intervention and placebo groups. The intervention was memantine (20 mg daily) or identical placebo plus citalopram for 8 weeks. The severity of depression and quality of life was evaluated using Geriatric Depression Scale (GDS-15), Hamilton Rating Scale for depression (HRSD) and World Health Organization Quality of Life WHOQOL-BREF respectively. The mentioned scores were evaluated at baseline, 4 weeks and 8 weeks, after initiating the trial in two studied groups and compared with each other.
Results:
28 and 29 patients were studied in the intervention and placebo groups, respectively. Score of GDS-15, HRSD and WHO-QOL-BREF scales at baseline, 4 weeks and 8 weeks, after initiating trial did not change significantly after use of memantine (P > 0.05). There was no significant difference in mean +/- SD of GDS-15, HRSD and WHO-QOL-BREF scales among intervention and placebo groups (P > 0.05).
Conclusion:
The outcome of this clinical trial did not support the antidepressant effect of add-on memantine in elderly patients with depression receiving citalopram. It is recommended to design further studies considering the limitations of the current study mentioned herein and the effect of memantine with other anti-depressant agents.
Keywords: Depression, geriatric, memantine, quality of life
INTRODUCTION
Major depressive disorder (MDD) is a common debilitating mental health problem worldwide. It is estimated to be the second main cause of overall disease burden in 2020.[1,2]
The disorder is more prevalent among older adults. The overall prevalence of MDD in geriatric population is estimated to be 10-20% in different cultural populations according to the report of World Health Organization and as many as 10% of adults aged 65 years or more who are seen in primary care setting have clinically significant depression.[3]
Late life depression is considered as a major health problem in many developed countries due to the higher proportion of elderly population. Though the population of Iran comprises largely of young people, but according to the estimation of WHO, the elder population in Iran would have increased significantly by the year 2030 (from 7% in 2000 to 15% and more in 2030).[4,5]
The disparity is associated with higher rates of disability, mortality and impaired quality of life.[6] The management cost of the disease is high due to increased health service use.[7] Evidences reported that compared to other chronic diseases including cardiovascular disease and diabetes, the disability related to depression is higher than the afore-mentioned diseases.[8]
In spite of effective pharmacological evolution of anti-depressive agents, management of depression especially in elderly patients is considered a challenge to health problem due to the common side effects of the drug in the elderly, delayed onset of action and non-responsiveness of the agents in 30-40% of cases.[9,10] Coexisting problems such as chronic medical disorders, pain, cognitive impairment (which can be associated with depression or dementia) and alcohol or substance misuse, complicate the diagnosis and treatment of depression in elderly patients. Some studies reports that late life depression may be associated with subsequent mild cognitive impairment and dementia but it is not clear whether depression represents a risk factor for or occurs in the prodromal stage of dementia.[11]
Better understanding of the pathophysiology of the disease would help us in its proper management. Evidence suggests that glutamate has a crucial role in the pathophysiology of mood disorders including depression.[12] Recent studies reported antidepressant response of glutamate neurotransmission modulation. Some studies reported higher level of glutamate in serum and CSF of patients with depression.[13] Others showed that there is a significant relationship between severity of the disease and the glutamate level.[12] N-methyl-D-aspartate (NMDA) is one of the postsynaptic ionotropic receptors that could modulate glutamate neurotransmission via its postsynaptic action. The antidepressant effect of NMDA antagonists have been reported in many animal models.[14,15] In clinical trials a single dose of NMDA receptor antagonist Ketamine has shown a rapid antidepressant effect in patients with treatment resistant depression and bipolar depression.[16] Memantine is a low to moderate-affinity noncompetitive NMDA receptor antagonist. It is approved for the treatment of Alzheimer's disease and recent studies have investigated its utility in the treatment of other psychiatric disorders. Antidepressant activity of memantine is indicated in many animal models but the results of its effectiveness on humans are controversial.[15,16,17,18,19,20] According to recent preclinical and clinical evidences, it is suggested that add-on memantine could have mood-stabilizing effects. Administration of memantine is more favorable than other NMDA antagonists since it does not have psychotomimetic effects at therapeutic doses and has fewer side effects.[21] Moreover, NMDA antagonists could mediate delayed onset of action of traditional antidepressant agents.[22] Since the elderly often use many drugs, the use of a drug with minimal side effects and drug interactions seems reasonable. In addition, proper management of depression in elderly population would improve the outcome of the disease and reduce its related disability and mortality.
The aim of current research was to investigate the effect of add-on memantine therapy in elderly patients with depression, who had received citalopram antidepressant in Isfahan.
MATERIALS AND METHODS
In this double-blind, placebo-controlled trial study elderly patients diagnosed recently with depression aged >60 years and who had met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition-text revision (DSM-IV-TR) criteria for major depression[8] and attended the geriatric clinics of Nour hospitals in Isfahan from December 2011 to October 2012, were enrolled. The patients selected by consecutive sampling method. The Medical Ethics Committee of the Isfahan University of Medical Sciences approved the study protocol (Research Project Number; 390231), and all subjects gave their written consent.
Inclusion criteria were; Depressive elderly patients (aged >60) selected by an interview based on DSM-IV-TR by a resident of psychiatry and were rated by the same psychiatric at first with three scales: Hamilton Depression Rating Scale (HDRS) >17, Geriatric Depression Scale (GDS-15) >5 and Mini Mental State Examination (MMSE) >24.
Exclusion criteria were: Patients with a history of previous psychiatric disorders such as bipolar disorder, psychosis, dementia or substance dependence (during last 6 months), previous severe and unstable medical disorders and history of taking other psychotropic medication.
Selected patients were randomized into two groups: Intervention and placebo groups using permuted block design sampling with size of two for each block. Two consecutive patients were randomly allocated to the treatment and control groups in each step till to complete the sample size.
Sample size was calculated as sixty patients (30 in each group). It was calculated base on confidence coefficient of 95% (1/96) and criterion deviations of Hamilton score (S = 16/7) and the sampling bias (d = 8/5).
The intervention was memantine or placebo (with the same shape and taste as memantine) for 8 weeks. Memantine is administrated in the intervention group with a 5 mg/daily dose for first week, second week dosage of 5 mg twice daily, third week 5 mg in the morning and 10 mg in the evening, forth week 10 mg twice daily and the target dose of 20 mg/daily continued till eighth week.
All patients received citalopram 10 mg/daily initiation dose for first week and continued with 20 mg/daily for the rest of the study. The 8-week follow-up started since the beginning of administrating the memantine + citalopram or placebo + citalopram in intervention and placebo groups, respectively. All the tablets of memantin and citalopram were manufactured in Sobhan factory in Iran. Baseline characteristics of patients including severity of depression and quality of life were evaluated using GDS-1 HRSD and WHO-QOL-(brief), respectively. Mentioned scores were re-evaluated by the resident of psychiatry during study, at week 4 and 8 after initiating trial. Results in the intervention and placebo groups were compared in baseline and during the follow-up period.
Both the examiner and the patients were unaware of the component of the drugs and they used the drugs in the name of A and B. A questionnaire was filled secretly and every patient received a code for trial.
Psychological tests
Hamilton depression rating scale (HDRS)
It is a multidimensional clinical assessment scale, which is used to determine the severity of depression based on mood, cognitive and physical symptoms of depression. It is a semi-structured interview questionnaire. It has 24 items with (0-4) or three (0-2) Likert spectrum scale and a cut-off point of 17. The validity and reliability of the score was confirmed in Iran and the Persian version of the scale was used in our study.[23]
Geriatric depression scale (GDS-15)
It is a 15 item self-report assessment, which was developed from the GDS-30. It is commonly used for screening depression among geriatric population. It consist of 15 yes/no question. Score >5 is suggestive of depression (GDS5-8 mild, GDS 9-11 moderate and GDS12-15 severe depression). In this study, we used the standardized Iranian version of the questionnaire.[24]
WHO-QOL-(brief)
The WHOQOL-BREF is a 26-item questionnaire for evaluating four domains including psychological, physical, environmental health and social relationships and two QOL and general health items. Scoring rate is ranging from 0-20 for each domain. In this study, we used the standardized Iranian version of the questionnaire.[25]
Mini mental state examination (MMSE)
MMSE is a 30-item questionnaire for screening and estimation of cognitive impairment as well as follows the course of cognitive changes overtime. It includes questions regarding the time and place of the test, repeating lists of words, arithmetic such as the serial sevens, language use and comprehension, and basic motor skills. MMSE score > = 25, 21-24,10-20 and < = 9 are indicator of normal cognition state, mild, moderate and severe cognitive impairment.[26]
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences software (SPSS, version 18.0, SPSS Inc., Chicago, IL, USA) and descriptive and repeated measure of ANCOVA tests. P value <0.05 was considered statistically significant.
RESULTS
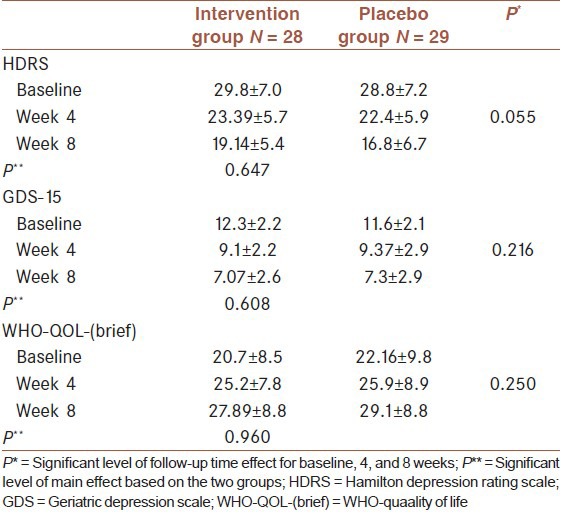
In this study, 100 elderly patients with major depression were enrolled and 60 patients were randomly allocated in two intervention (n = 30) and placebo (n = 30) groups [Figure 1]. Baseline characteristics of studied population in intervention and placebo groups are presented in [Table 1]. Scores of severity of depression and quality of life, which were evaluated using GDS-15, HRSD and WHO-QOL-(brief) scales at baseline, 4 weeks and 8 weeks, after initiation of trial are presented in [Table 2]. According to these result, as shown in table 2, there was not a significant difference between the results of the three scales and trends of changes throughout the study was not significant either.
Figure 1.
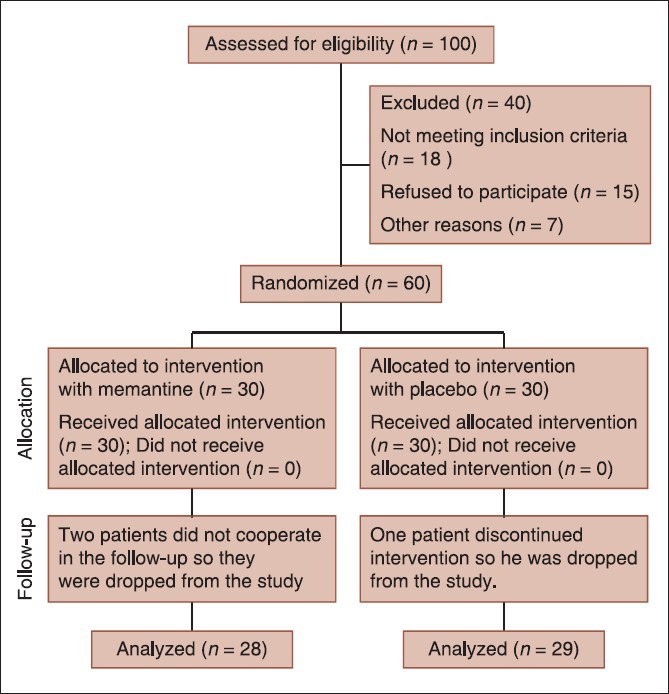
Consort diagram of the study
Table 1.
Baseline characteristics (Mean +/- SD) of studied population in intervention and placebo groups
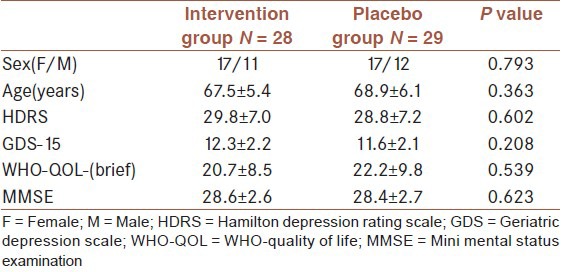
Table 2.
Scores of severity of depression and quality of life evaluated using GDS-15, HDRS and WHO-QOL-(brief) scales at baseline, 4 weeks and 8 weeks in intervention and placebo groups controlling of sex and age
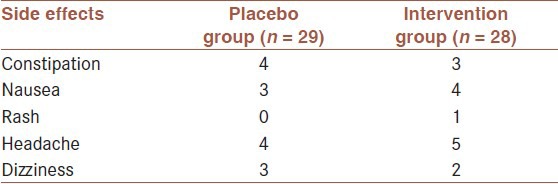
The side effects were recorded during the study. Reported side effects were mild and did not result in withdrawal. Frequency of side effects was not significantly different between two groups (P < 0.05) [Table 3].
Table 3.
Frequency of reported side effects during the study in intervention and placebo groups (P < 0.05)
DISCUSSION
In this study, we investigate the effect of add-on memantine versus placebo to citalopram for depressive symptoms and quality of life in older adults receiving 20 mg of citalopram. The results showed that memantine had no superior effect on the mentioned factors than placebo.
The role of memantine in improving cognitive impairment in elderly patients has been reported in many studies.[27] Considering the therapeutic effect of citalopram in depressive elderly patients,[28] the aim of current study was to determine the effect of memantine add-on therapy in depressed elderly patients without obvious cognitive impairment (MMSE >24). In current study, mean score of MMSE and the cognitive status of the studied depressed patients were similar in two studied groups, so the possible role of memantine as an add-on therapy on depressive symptoms of elderly patients was investigated.
Although, the role of glutamate and its related NMDA receptor antagonists in modulating mood disorders have recognized in several animal and human studies,[29] but results of different clinical trials in this regard is conflicting and inconclusive.
Zarate et al., indicated that a single intravenous dose of ketamin, an N-methyl-D-aspartate antagonist, has a rapid antidepressant effect.[30] The antidepressant effects of ketamine as well as its effects on reducing delayed therapeutic effects of other antidepressant agents have reported in some studies also.[31,32]
In this study, we examined the effect of memantine add-on therapy in elderly patients with depression in a double-blind placebo-controlled study. Memantine is another N-methyl-D-aspartate antagonist with partial trapping property and lower side effects than ketamin, with trapping the block property.[33] In addition, comparing with other NMDA receptor antagonists such as ketamine, it is well-tolerated NMDA receptor antagonists without any psychotomimetic effects.[34] The results would be helpful in planning new management strategies for elderly people affected with MDD and evaluating the mediating role of memantine in reducing delayed therapeutic effects of citalopram.
Our results are consistent with the results of Zarate et al. They conducted the first double-blind placebo-controlled study on the effectiveness of memantine among patients with major depression. They did not report any antidepressant effect for mematine among depressed patients.[17]
In a recent study in the USA, Lenze and colleagues examined the effect of memantine on the depressive symptoms of older adults after a disabling medical event. They showed that memantine has no significant effect in this regard.[35] Whereas in another study in Utah, Ferguson et al. have demonstrated an early-onset efficacy of memantine in patients with major depression.[18] In their study, which had been designed as open-label study, the efficacy as well as safety of flexible dose of memantine (i.e increasing titration of 20 mg/d memantine to 30 or 40 mg/day in nonresponsive patients) in 8 patients with MDD was evaluated. According to their findings, all efficacy measures were improved in studied patients within 1 week after memantine therapy. Improved status peaked at week 8 and remained during 12-week study period. They did not report any significant side effect regarding memantine and its different dosage. They concluded that memantine is a safe and effective drug in patients with MDD and recommended to evaluate the efficacy of memantine in this regard through a double-blind, placebo-controlled study.
Koukopoulos et al., have investigated the mood-stabilizing effect of memantine as augmenting agent in patients with treatment-resistant BMD. Their results indicated sustained mood-stabilizing effect of memantine.[19]
Though the methods of mentioned studies were not similar regarding evaluation of depressive symptoms and memantine administration, factors which could influence on the findings of our study are as follows; short duration of the trial, dose of memantine and long duration of its dose upward titration.
We used approved dose of memantine, 20 mg/daily, for treatment of dementia, but it seems that higher dose of the drug with short upward titration period would be more effective in elderly patients. Other studies with similar result have recommended to test the higher dose, also.[35,17] As justified by Zarete et al., selected dose of memantine in our study is sufficient for evaluating the validity of the concept of using memantine for depression treatment in elderly.
Another explanation for obtained negative results is that the different mechanism of action of memantine than ketamin regarding its lower affinity to NMDA receptor and faster open-channel blocking/unblocking kinetics.[33]
The limitation of current study was that the patients were not followed up for long term period to determine the long term effect of memantine. In addition, though our studied sample size was larger than other studies in this field, it seems that further studies with larger sample size could help us to obtain better conclusive results.
In sum, the outcome of this pilot clinical trial did not support the antidepressant effect of add-on memantine in elderly patients with depression receiving citalopram. It is recommended to design further studies considering mentioned limitations of current study and the effect on memantine with other antidepressant agents.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Michaud CM, Murray CJ, Bloom BR. Burden of disease — implications for future research. JAMA. 2001;285:535–9. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- 2.Murthy RS, editor. The World Health Report 2001. Mental Health: New Understanding, New Hope. Geneva, Switzerland: WHO; 2001. Burden of mental and behavioral disorders. 24Y29. [Google Scholar]
- 3.Rangaswamy SM. Geneva: Switzerland: World Health Report: Mental Health: New understanding New Hope; 2001. The World Health Organization. [Google Scholar]
- 4.Stordal E, Bjartveit Kruger M, Dahl NH, Kruger Q, Mykletun A, DahI AA. Depression in relation to age and gender in the general population: The Nord-Trondelag Health Study (HUNT) Acta Psychiatr Scand. 2001;104:210–6. doi: 10.1034/j.1600-0447.2001.00130.x. [DOI] [PubMed] [Google Scholar]
- 5.Namadian M, Ghobadi S, editors. Tehran: Ashena press; 2006. Evaluation of mental status old age s Zanjan on 2001. Persian. persian. [Google Scholar]
- 6.Barua A, Ghosh MK, Kar N, Basilio MA. Socio-demographic factors of geriatric depression. Indian J Psychol Med. 2010;32:87–92. doi: 10.4103/0253-7176.78503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katon WJ, Lin E, Russo J, Unützer J. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psychiatry. 2003;60:897–903. doi: 10.1001/archpsyc.60.9.897. [DOI] [PubMed] [Google Scholar]
- 8.Akiskal HS. Comprehensive textbook of psychiatry. In: Sadock BJ, Sadock VA, editors. Mood disorders: Historical introduction and conceptual overview. Philadelphia: Williams and Willkins; 2005. pp. 1559–27. [Google Scholar]
- 9.Delgado PL. Depression: The case for a monoamine deficiency. J Clin Psychiatry. 2000;61:7–11. [PubMed] [Google Scholar]
- 10.Joffe R, Sokolov S, Streiner D. Antidepressant treatment of depression: A metaanalysis. Can J Psychiatry. 1996;41:613–6. doi: 10.1177/070674379604101002. [DOI] [PubMed] [Google Scholar]
- 11.Bhalla RK, Butter MA, Becker JT, Houck PR, Snitz BE, Lopez OL, et al. Patterns of mild cognitive impairment after treatment of deoression in the elderly. AM J Geriatr psychiatry. 2009;17:308–16. doi: 10.1097/JGP.0b013e318190b8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev. 2009;61:105–23. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Maeng S, Zarate CA., Jr The role of glutamate in mood disorders: Results from the ketemin in Major depressive study and the presumed cellular mechanism underlying its antidepressant effects. Curr Psychiatry Rep. 2007;9:467–74. doi: 10.1007/s11920-007-0063-1. [DOI] [PubMed] [Google Scholar]
- 14.Kiss JP, Szasz BK, Fodor L, Mike A, Lenkey N, Kurkó D, et al. GluN2B-containing NMDA receptors as possible targets for the neuroprotective and antidepressant effects of fluoxetine. Neurochem Int. 2012;60:170–6. doi: 10.1016/j.neuint.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Ates-Alagoz Z, Adejare A. NMDA Receptor Antagonists for Treatment of Depression. Pharmaceuticals (Basel) 2013;6:480–99. doi: 10.3390/ph6040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hjerrild S, Bjerre J, Pedersen RH, Videbech P. Ketaminefor treatment of acute depression. Ugeskr Laeger. 2013;175:2090–3. [PubMed] [Google Scholar]
- 17.Zarate CA, Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006;163:153–5. doi: 10.1176/appi.ajp.163.1.153. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson JM, Shingleton RN. An open-label, flexible-dose study of memantine in major depressive disorder. Clin Neuropharmacol. 2007;30:136–44. doi: 10.1097/WNF.0b013e3180314ae7. [DOI] [PubMed] [Google Scholar]
- 19.Koukopoulos A, Serra G, Koukopoulos AE, Reginaldi D, Serra G. The sustained mood-stabilizing effect of memantine in the management of treatment resistant bipolar disorders: Findings from a 12-month naturalistic trial. J Affect Disord. 2012;136:163–6. doi: 10.1016/j.jad.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 20.Tsapakis EM, Travis MJ. Glutamate and psychiatric disorders. Adv Psychiatr Treat. 2002;8:189–97. [Google Scholar]
- 21.Sani G, Serra G, Kotzalidis GD, Romano S, Tamorri SM, Manfredi G, et al. The role of memantine in the treatment of psychiatric disorders other than the dementias: A review of current preclinical and clinical evidence. CNS Drugs. 2012;26:663–90. doi: 10.2165/11634390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA., Jr Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther. 2009;123:143–50. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sargolzaee MR, Fayyazi Bordbar MR, Samari AA, Shakiba M. The comparison of the efficacy of citrus fragrance and fluoxetine in the treatment of major depressive disorder. J Ofoghe-Danesh. 2004;10:43–8. [Google Scholar]
- 24.Malakouti SK, Fatollahi P, Mirabzadeh A, Salavati M, Zandi T. Reliability, validity and factor structure of the GDS-15 in Iranian elderly. Int J Geriatr Psychiatry. 2006;21:588–93. doi: 10.1002/gps.1533. [DOI] [PubMed] [Google Scholar]
- 25.Nedjat S, Montazeri A, Holakouie K, Mohammad K, Majdzadeh R. Psychometric properties of the Iranian interview-administered version of the World Health Organization's Quality of Life Questionnaire (WHOQOL-BREF): A population-based study. BMC Health Serv Res. 2008;8:61. doi: 10.1186/1472-6963-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Coria H, Green KN, Billings LM, Kitazawa M, Albrecht M, Rammes G, et al. Memantine improves cognition and reduces Alzheimer's-like neuropathology in transgenic mice. Am J Pathol. 2010;176:870–80. doi: 10.2353/ajpath.2010.090452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apler A. Citalopram for major depressive disorder in adults: A systematic review and meta-analysis of published placebo-controlled trials. BMJ Open. 2011;1:e000106. doi: 10.1136/bmjopen-2011-000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krystal JH. N-methyl-D-aspartate glutamate receptor antagonists and the promise of rapid-acting antidepressants. Arch Gen Psychiatry. 2010;67:1110–1. doi: 10.1001/archgenpsychiatry.2010.138. [DOI] [PubMed] [Google Scholar]
- 30.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 31.Yilmaz A, Schulz D, Aksoy A, Canbeyli R. Prolonged effect of an anesthetic dose of ketamine on behavioral despair. Pharmacol Biochem Behav. 2002;71:341–4. doi: 10.1016/s0091-3057(01)00693-1. [DOI] [PubMed] [Google Scholar]
- 32.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 33.Bolshakov KV, Gmiro VE, Tikhonov DB, Magazanik LG. Determinants of trap-ping block of N-methyl-D-aspartate receptor channels. J Neurochem. 2003;87:56–65. doi: 10.1046/j.1471-4159.2003.01956.x. [DOI] [PubMed] [Google Scholar]
- 34.Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist — a review of preclinical data. Neuropharmacology. 1999;38:735–67. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 35.Lenze EJ, Skidmore ER, Begley AE, Newcomer JW, Butters MA, Whyte EM. Memantine for late-life depression and apathy after a disabling medical event: A 12-week, double-blind placebo-controlled pilot study. Int J Geriatr Psychiatry. 2012;27:974–80. doi: 10.1002/gps.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]


