Abstract
Immunocompetent (nu/+) and athymic (nu/nu) BALB/c mice were infected intravenously with Wangiella dermatitidis and treated with posaconazole. Posaconazole reduced the counts in tissues and prolonged survival. Of particular interest, posaconazole reduced the counts of this neurotropic pathogen in the brain.
Phaeohyphomycosis is a collective term describing infection caused by a number of primarily mycelial fungi which produce a melanin pigment. This pigment gives the colonies a dark color (1). These dark molds are also called dematiaceous fungi. Many infections involve respiratory sites (sinusitis, pneumonia) or skin and subcutaneous lesions following local inoculation (8, 10, 12, 14, 15, 20, 22). While many localized infections occur in otherwise healthy individuals, there is a predilection for phaeohyphomycosis in patients with solid-organ transplants or corticosteroid therapy, and a few cases have been reported in patients with AIDS (20, 22, 25). Infection of immune-suppressed patients suggests that cell-mediated immune defenses may be important for the control of phaeohyphomycosis. Disseminated disease can involve multiple tissues. However, some species, including Wangiella dermatitidis, are neurotropic, in that they have a predilection to produce brain abscesses (2, 10, 15, 16, 20, 21, 24).
Treatment of phaeohyphomycosis commonly involves amphotericin B. The clinical responses to amphotericin B treatment vary, and amphotericin B treatment often fails (10, 20, 21). Some patients have been given azole antifungals as primary or salvage therapy. Of the antifungal triazoles, itraconazole is highly effective in vitro and is effective clinically in about two-thirds of patients, including some who have failed amphotericin B therapy (21). Fluconazole is less effective in vitro, is effective in mice, and is occasionally effective clinically (3, 6, 21). While debridement and resection of focal lesions may be effective for some patients, these are not always possible for patients with widespread dissemination or brain abscesses. For these patients, other alternatives are needed. Posaconazole (Schering-Plough Research Institute) is a broad-spectrum triazole antifungal drug with broad-spectrum activities, including activities against Aspergillus and many other fungal species (4, 5, 9, 11, 13, 23). In the present studies, we evaluated posaconazole as an alternative therapy for central nervous system infections caused by W. dermatitidis.
A clinical isolate of W. dermatitidis, isolate 8656, was obtained from Paul Szaniszlo, The University of Texas at Austin (7). W. dermatitidis yeast-like forms were used for the in vitro studies, in which NCCLS methodology M-38A (17) and 80% inhibition (19) were used. MICs were measured at 24 and 48 h of incubation. The MICs of posaconazole were <0.03 and 0.125 μg/ml. Prior to infection, W. dermatitidis was cultured for up to a week on potato dextrose agar at room temperature. Conidia were harvested with isotonic saline, filtered through glass wool to remove fragments of mycelia, counted with a hemacytometer, suspended in isotonic saline, and injected intravenously into unanesthetized mice in a 0.2-ml volume. The infecting inoculum size was confirmed by counting the colonies from serial dilutions.
Six-week-old BALB/c nu/+ and nu/nu mice were obtained from the South Texas Veterans Health Care System, Audie L. Murphy Division, Veterinary Medical Unit Breeding Colony. Responses were evaluated by using measurements of the prolongation of survival and reductions of the counts in brain and spleen tissues. One day after infection the mice were randomized to groups of 7 to 10 mice each. Mice received orally (p.o.) by gavage 0.5% Noble agar (controls) or posaconazole at 0.1, 0.625, 1, 2, 2.5, 5, 10, or 25 mg/kg of body weight suspended in 0.5% Noble agar p.o. once daily. For the survival studies, treatment was continued through day 7 and the mice were observed through day 30. For the tissue burden studies, the mice were treated from day 1 through day 7 and the survivors were killed 1 day later. The brains and spleens were removed and homogenized in 2 ml of saline. Aliquots of undiluted suspensions and serial 10-fold dilutions of suspensions were plated on Sabouraud dextrose agar, and quantitative counts were calculated. Care was given to using the same intensity and duration of homogenization for each specimen by using a mechanical stirrer. The log rank test was used to assess survival. The Mann-Whitney U test was used for comparisons of counts in tissue. A P value ≤0.05 was used for significance.
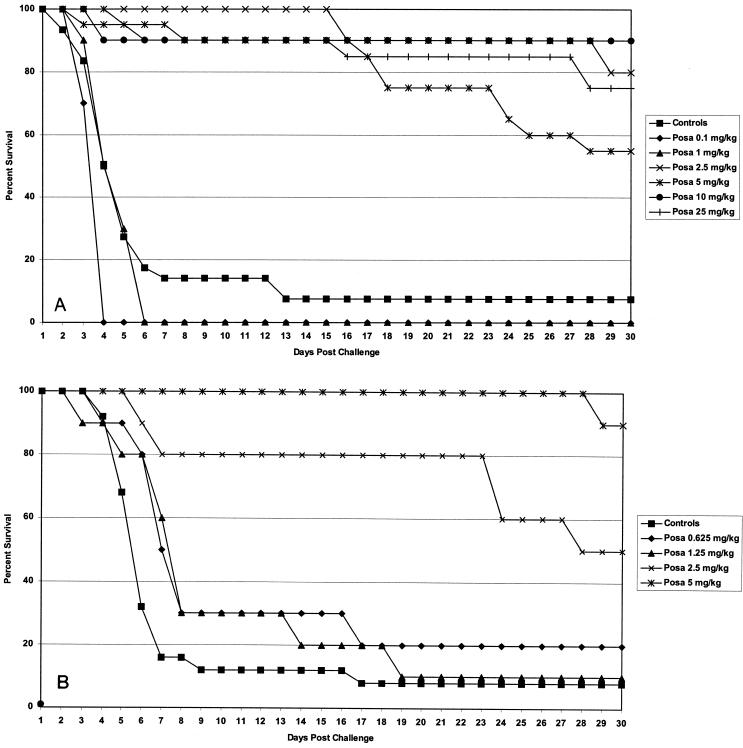
Figure 1A summarizes the survival data for nu/+ immunocompetent mice. The results of three studies are included. There were 30 control mice, 20 mice each in the groups treated with posaconazole at 5 and 25 mg/kg, and 10 mice each in the additional treatment groups. Posaconazole at ≥2.5 mg/kg/day significantly prolonged survival (P > 0.001). There was a sharp increase from no effect at 0.1 and 1 mg/kg/day to similarly strong levels of protection at 2.5 mg/kg/day and higher doses. Two additional studies with posaconazole were done with athymic mice. The pooled results are presented in Fig. 1B. There were 25 mice in the control group and 10 mice each in the additional posaconazole treatment groups. Posaconazole at ≥2.5 mg/kg prolonged survival (P < 0.0001). The results for the nu/+ and the athymic mice are remarkably similar.
FIG. 1.
Survival study results. (A) Results for nu/+ mice from three studies. The infecting doses ranged from 3.6 × 106 to 3.3 × 107 CFU/mouse. The mice were treated with posaconazole (Posa) at 0.1, 1, 2.5, 5, 10, or 25 mg/kg p.o. for 7 days and were observed for survival through day 30 postinfection. Controls received 0.5% Noble agar p.o. The mice treated with ≥2.5 mg/kg/day survived significantly longer than the control mice. (B) Results for athymic mice from three studies. The infecting doses ranged from 9 × 106 to 1.9 × 107 CFU/mouse. Mice were treated with posaconazole at 0.625, 1.25, 2.5, or 5 mg/kg p.o. for 7 days and were observed for survival through day 30 postinfection. Controls received 0.5% Noble agar p.o. The mice treated with ≥2.5 mg/kg/day survived significantly longer than the control mice.
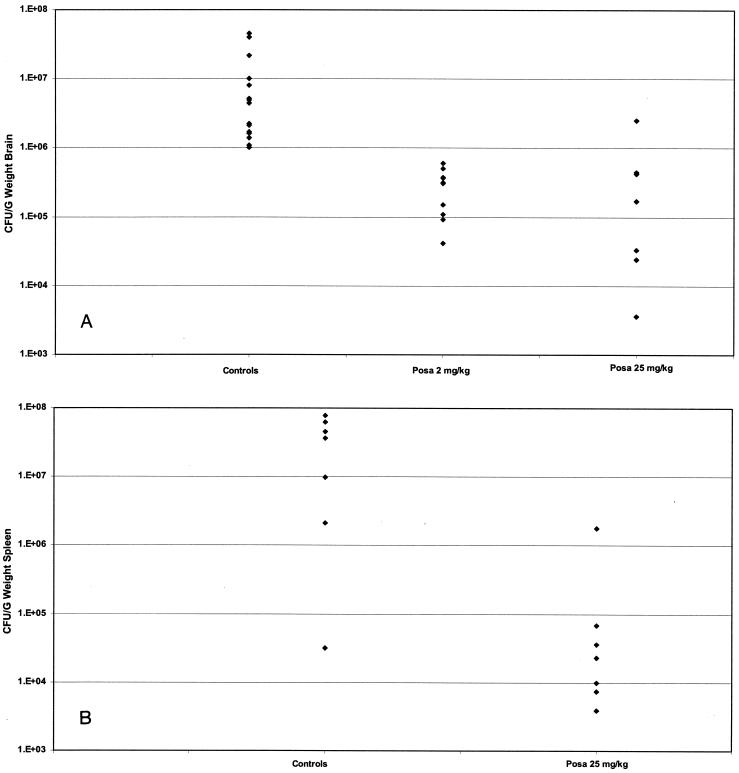
The burdens in the brain tissues of nu/+ mice are presented in Fig. 2A. The results of two studies in which similar inocula were used were pooled. The results for the mice receiving the two doses of posaconazole were compared with those for the untreated controls. Both regimens reduced the counts in brains significantly compared with those in the brains of the controls (P < 0.0015). The burdens in spleen tissues are presented in Fig. 2B. Only posaconazole at 25 mg/kg was tested in this study, and it showed a protective effect (P < 0.004).
FIG. 2.
Tissue W. dermatitidis counts. (A) Brain W. dermatitidis counts. Mice from two studies were infected with 3.6 × 106 to 1.9 × 107 CFU/mouse and were treated from days 1 to 7. When possible the brains were harvested from mice that succumbed during treatment, and the brains were harvested from all survivors on day 8. Regimens included posaconazole (Posa) at 2 and 25 mg/kg. Controls received 0.5% Noble agar. Treatment with posaconazole at both doses was superior to the control treatment. (B) Spleen W. dermatitidis counts. Groups of seven nu/+ mice were infected with 1.9 × 107 CFU/mouse and were treated from days 1 to 7. When possible the spleens were harvested from mice that succumbed during treatment, and the spleens were harvested from all survivors on day 8. Controls received 0.5% Noble agar. Treatment with posaconazole at 25 mg/kg was superior to the control treatment.
The present studies were undertaken to evaluate posaconazole as a possible alternative to amphotericin B for the treatment of Wangiella infections. Posaconazole was consistently effective in the most severe test, prolongation of survival after administration of a lethal inoculum. Posaconazole showed a dose-dependent effect, being highly efficacious at doses of ≥2.5 mg/kg/day. Posaconazole was also highly effective in athymic mice. This suggests that immune deficiency may not blunt the beneficial effect of posaconazole treatment. Because the worst site of fungal infection is usually the central nervous system, because W. dermatitidis is neurotropic in humans, and because we could readily detect infection of the brain in untreated control mice, we elected to measure the density of infection in brain tissue. Studies of the burden in brain tissue gave results similar to those obtained in the survival study. Increasing doses of posaconazole showed dose-associated reductions in the counts in brain tissue. The burden in spleen tissue was also reduced by use of a dose of 25 mg/kg.
Relatively little attention has been paid to preclinical studies of phaeohyphomycosis with animal models. There are essentially no data from preclinical studies with voriconazole, although Perfect et al. (18) have found clinical responses in all 11 patients treated with this drug. Several of these patients had central nervous system involvement. Preclinical studies with Ramichloridium mackenzii, another agent with a strong neurotropism, showed that the response to treatment with posaconazole was excellent (2). The present studies extend this observation to another pathogenic species. However, there are very few data from clinical studies with posaconazole for the treatment of phaeohyphomycosis. In a recent presentation at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy (P. Pittisuttithum, V. Gaono-Flores, R. Negroni, I. Sanne, J. R. Graybill, A. Bustamante, R. Hare, and C. Hardalo, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-978, p. 450, 2003), it was shown that patients with a variety of mycoses responded to posaconazole treatment. Two responders were patients with phaeohyphomycosis. The present studies suggest that posaconazole may be broadly effective against phaeohyphomycetes. Posaconazole prolonged survival and significantly reduced the brain W. dermatitidis counts. The use of posaconazole for the treatment of phaeohyphomycosis, including those patients with central nervous system lesions, is worth exploring in humans.
Acknowledgments
We thank the representatives of Schering-Plough Research Institute for providing posaconazole for these studies.
REFERENCES
- 1.Adam, R. D., M. L. Paquin, E. A. Petersen, M. A. Saubolle, M. G. Rinaldi, J. G. Corcoran, J. N. Galgiani, and R. E. Sobonya. 1986. Phaeohyphomycosis caused by the fungal genera Bipolaris and Exserohilum. Medicine 65:203-217. [DOI] [PubMed] [Google Scholar]
- 2.Al Abdely, H., L. Najvar, R. Bocanegra, A. Fothergill, and J. R. Graybill. 2000. SCH56592, amphotericin B, or itraconazole therapy of experimental murine cerebral phaeohyphomycosis due to Ramichloridium obovoidem (“Ramichloridium mackeniei”). Antimicrob. Agents Chemother. 44:1159-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colciron, B. M., E. L. Wiley, and M. G. Rinaldi. 1990. Cutaneous phaeohyphomycosis caused by a rare fungal pathogen Hormonema dematioides: successful treatment with fluconazole. J. Am. Acad. Dermatol. 23:363-367. [DOI] [PubMed] [Google Scholar]
- 4.Connolly, P., L. J. Wheat, C. Schnizlein-Bick, M. Durkin, S. Kohler, M. Smedema, J. Goldberg, E. Brizendine, and D. Loebenberg. 1999. Comparison of a new triazole, posaconazole, with itraconazole and amphotericin B for treatment of histoplasmosis in immunocompetent mice. Antimicrob. Agents Chemother. 43:322-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connolly, P., L. J. Wheat, C. Schnizlein-Bick, M. Durkin, S. Kohler, M. Smedema, J. Goldberg, E. Brizendine, and D. Loebenberg. 2000. Comparison of a new triazole, posaconazole, with itraconazole and amphotericin B for treatment of histoplasmosis following pulmonary challenge in immunocompromised mice. Antimicrob. Agents Chemother. 44:2604-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon, D. M., and A. Polak. 1987. In vitro and in vivo drug studies with three agents of central nervous system phaeohyphomycosis. Chemotherapy 33:129-140. [DOI] [PubMed] [Google Scholar]
- 7.Feng, B., X. Wang, M. Hauser, S. Kaufmann, S. Jentsch, G. Haase, J. M. Becker, and P. J. Szaniszlo. 2001. Molecular cloning and characterization of WdPKS1, a gene involved in dihydroxynaphthalene melanin biosynthesis and virulence in Wangiella (Exophiala) dermatitidis. Infect. Immun. 69:1781-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gené, J., A. Azón-Masoliver, J. Guarro, F. Ballester, I. Pujol, M. Llovera, and C. Ferrer. 1995. Cutaneous phaeohyphomycosis caused by Alternaria longipes in an immunosuppressed patient. J. Clin. Microbiol. 33:2774-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez, G. M., R. Tijerina, L. K. Najvar, R. Bocanegra, M. G. Rinaldi, D. Loebenberg, and J. R. Graybill. 2003. Activity of posaconazole against Pseudallescheria boydii: in vitro and in vivo assays. Antimicrob. Agents Chemother. 47:1436-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graybill, J. R. 1988. Zygomycosis and phaeohyphomycosis. In Current therapy in pediatric infectious diseases, vol. 2, p. 177-178. B. C. Deecker Inc., Philadelphia, Pa.
- 11.Graybill, J. R., R. Bocanegra, M. Luther, and D. Loebenberg. 1998. SCH56592 treatment of murine invasive aspergillosis. J. Antimicrob. Chemother. 42:539-542. [DOI] [PubMed] [Google Scholar]
- 12.Hsu, M. M. L., and J. Y. Y. Lee. 1993. Cutaneous and subcutaneous phaeohyphomycosis caused by Exserohilum rostratum. J. Am. Acad. Dermatol. 28(Suppl.):340-344. [DOI] [PubMed] [Google Scholar]
- 13.Kirkpatrick, W. R., R. K. McAtee, A. W. Fothergill, D. Loebenberg, M. G. Rinaldi, and T. F. Patterson. 2000. Efficacy of SCH56592 in a rabbit model of invasive aspergillosis. Antimicrob. Agents Chemother. 44:780-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotylo, P. K., K. S. Israel, J. S. Cohen, and M. S. Bartlett. 1993. Subcutaneous phaeohyphomycosis of the finger caused by Exophiala spinifera. Am. J. Clin. Pathol. 91:624-627. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto, T., T. Matsuda, M. R. McGinnis, and L. Ajello. 1993. Clinical and mycological spectra of Wangiella dermatitidis infections. Mycoses 36:145-155. [DOI] [PubMed] [Google Scholar]
- 16.Mukherji, S. K., and M. Castillo. 1995. Cerebral phaeohyphomycosis caused by Xylohypha bantiana: MR findings. Am. J. Roentgenol. 164:1304-1305. [DOI] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Perfect, J. R., K. A. Marr, T. J. Walsh, R. N. Greenberg, B. DuPont, J. de la Torre-Cisneros, G. Just-Nubling, H. T. Schlamm, I. Lutsar, A. Espinof-Ingroff, and E. Johnson. 2003. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin. Infect. Dis. 36:1122-1131. [DOI] [PubMed] [Google Scholar]
- 19.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, A. L. Barry, and National Committee for Clinical Laboratory Standards. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 20.Rinaldi, M. G. 1996. Phaeohyphomycosis. Dermatol. Clin. 14:147-153. [DOI] [PubMed] [Google Scholar]
- 21.Sharkey, P. K., J. R. Graybill, M. G. Rinaldi, D. A. Stevens, R. M. Tucker, J. D. Peterie, P. D. Hoeprich, D. L. Greer, L. Frenkel, G. W. Counts, J. Goodrich, S. Zellner, R. W. Bradsher, C. M. van der Horst, K. Israel, G. A. Pankey, and C. P. Barranco. 1990. Itraconazole treatment of phaeohyphomycosis. J. Am. Acad. Dermatol. 23:577-586. [DOI] [PubMed] [Google Scholar]
- 22.Shugar, M. A., W. W. Montgomery, and N. E. Hyslop. 1981. Alternaria sinusitis. Ann. Otol. Rhinol. Laryngol. 90:251-254. [DOI] [PubMed] [Google Scholar]
- 23.Sun, Q. N., L. Najvar, R. Bocanegra, D. Loebenberg, and J. R. Graybill. 2002. In vivo activity of posaconazole against Mucor spp. in an immunosuppressed mouse model. Antimicrob. Agents Chemother. 46:2310-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vukmir, R. B., S. Kusne, P. Linden, W. Pasculle, A. W. Fothergill, J. Sheaffer, J. Nieto, R. Segal, H. Merhav, A. J. Martinez, and M. G. Rinaldi. 1994. Successful therapy for cerebral phaeohyphomycosis due to Dactylaria gallopava in a liver transplant recipient. Clin. Infect. Dis. 19:714-719. [DOI] [PubMed] [Google Scholar]
- 25.Wiest, P. M., K. Wiese, M. R. Jacobs, A. B. Morrissey, T. I. Abelson, W. Witt, and M. M. Lederman. 1987. Alternaria infection in a patient with acquired immunodeficiency syndrome: case report and review of invasive alternaria infections. Rev. Infect. Dis. 9:799-803. [DOI] [PubMed] [Google Scholar]