Abstract
Decontamination of the radioactive cesium that is widely dispersed owing to a nuclear power station accident and concentrated in fly ash requires an effective elimination system. Radioactive fly ash contains large amounts of water-soluble cesium that can cause severe secondary contamination and represents a serious health risk, yet its complete removal is complicated and difficult. Here it is shown that a new fine-powder formulation can be magnetically guided to eliminate cesium after being mixed with the ash slurry. This formulation, termed MagCE, consists of a ferromagnetic porous structure and alkaline- and salt-resistant nickel ferrocyanide. It has potent cesium-adsorption- and magnetic-separation-properties. Because of its resistance against physical and chemical attack such as with ash particles, as well as with the high pH and salt concentration of the ash slurry, MagCE simplifies the decontamination process without the need of the continued presence of the hazardous water-soluble cesium in the treated ash.
We have recently devised a magnetically guidable rapid elimination system (MagCE: Magnetic Cesium Eliminator) for the radioactive cesium that is concentrated in the fly ash ash produced by the incineration of radioactive materials1,2. To the best of our knowledge, the present study is the first report of a cesium decontamination powder that may be added directly to fly ash slurry and that is also readily separable after mixing.
Cesium is the main radioactive contaminant in the fly ash that is currently present in a wide area of eastern Japan because of the severe damage to the Fukushima I Nuclear Power Plant3. According to information published by the National Cancer Institute of the U.S. National Institute of Health, the two radioactive isotopes released in a nuclear accident that generally pose the greatest cancer risks are iodine-131 (I-131) and cesium-137 (Cs-137) (http://www.cancer.gov/cancertopics/factsheet/Risk/nuclear-power-accidents#ques2). Both are known to diffuse easily over a wider area than other isotopes such as strontium, and we have actually confirmed that much lower level of radioactive strontium was contained in the fly ash compared with radioactive cesium (See Table S1, Supplementary Information). Moreover, Cs-137 still retains 80% of its radioactivity even 10 years after its release, while the radioactivity of I-131 decreases to 0.04% within 3 months. Should radioactive cesium be ingested, it becomes rapidly distributed throughout all of the muscle tissues, and severe arrhythmia, heart failure, or sudden death can result from the accumulation of radioactive cesium in cardiac muscle4.
The guidelines of the Ministry of the Environment of Japan indicate that incineration ash having <8,000 Bq/kg of radioactivity may be deposited in a controlled landfill site. As a result, by the end of December 2013, over 140,000 tons of such ash was classified and treated as radioactive waste (http://shiteihaiki.env.go.jp/02/02.html#amount). Moreover, combustible garbage collected through decontamination work contains radioactive cesium, so the incineration of this garbage will generate a large amount of highly radioactive fly ash in the near future. The cesium in fly ash is readily eluted into water and can spread extensively through the environment. Since most fly ash exists in a water-soluble form, a high degree of health risk is at stake1,2,5.
For a sharp reduction in the stored amount of fly ash through the decontamination of radioactive cesium, effective methods are urgently required. In this study, we demonstrate the advantages of magnetically guidable MagCE as a rapid and secure cesium eliminator from radioactive fly ash, with great emphasis placed on the merits of MagCE directly mixed with fly ash slurry.
Results & Discussion
The events leading up to the development of MagCE
Initially, to eliminate radioactive cesium from the fly ash slurry, we tested the applicability of the previously developed Prussian blue-coated iron oxide nanoparticles (PB-IO) that we had used to effectively remove cesium from seawater6. PB-IO (40 mg) was able to eliminate 95.4% of radioactive cesium from 400 mL of the solid-liquid-separated supernatant (828.45 Bq/kg) of the fly ash slurry after neutralization (pH 7.0); however, after direct mixing with fly ash slurry (fly ash: 40 g; DW: 400 mL), PB-IO (2 g) and the fly ash particles became strongly bonded with one another, and their magnetic separation was difficult. Both the PB-IO and fly ash clustered around a magnet when a strong magnetic field was present or when a large amount of PB-IO was mixed with the fly ash. In contrast, neither PB-IO nor the fly ash accumulated when weak magnetic fields or smaller amounts (40 mg) of PB-IO were used (See Fig. S10, Supplementary Information). We believe that one of the reasons for these phenomena might be the small size of the PB-IO particles (average size: 304.2 nm), and we therefore attempted to increase their magnetic components. Such enlargement seems to be effective for more rapid magnetic separation; however, the surface of the magnetic core could not be securely coated with the outer iron ferrocyanide layer of PB-IO, which was easily peeled off by collisions with the fly ash particles during vigorous stirring. Moreover, the degree of specific surface area available for the cesium adsorption was greatly reduced through enlargement of the size of the magnetic material (the surface area of a sphere is 4πr2; where r is the radius). Therefore, we attempted to increase its entire size and specific surface area using fine magnetic particles (Fig. 1c). The magnetic properties of the magnetic particles tended to decrease as a result of the loading of cesium-adsorbent substance on them (Table 1); hence, we used ferromagnetic and anticorrosive acicular iron nanomaterials to reduce this degradation. Moreover, for MagCE, we planned to have the potential to adsorb large amounts of cesium in as short a time as possible and to resist both high pH values and mechanical collisions. Thus, we provided MagCE with a porous structure composed of acicular iron nanoparticles covered with alkali-resistant nickel ferrocyanide, and a porous space that was partially occupied with nickel ferrocyanide (Fig. 1d). We believe that this design of MagCE is useful for the enhancement of the large internal and external specific surface area of the nickel ferrocyanide.
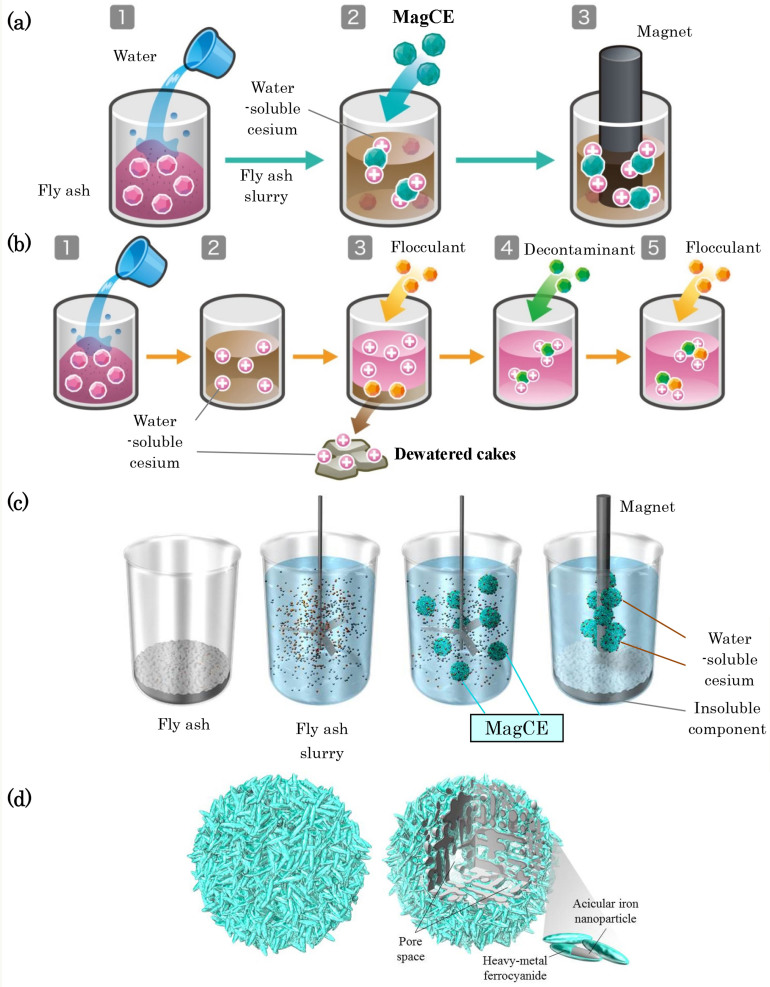
Figure 1. Design concept of MagCE.
The removal of radioactive cesium from the fly ash slurry using (a) MagCE, and (b) conventional decontaminants. Problems in the decontamination of radioactive cesium from fly ash slurry using PB-IO (See Fig. S10, Supplementary Information); and their resolution using (c) MagCE, (d), Schematic representation of the structure of MagCE.
Table 1. Magnetic and powder properties for each type of MagCE and for the source materials (Please also see Table S3 (detailed information for this Table), Supplementary file).
| Size | Pore distribution | Magnetic property | |||||
|---|---|---|---|---|---|---|---|
| Ave (μm) | Fine pore volume (μm3/g) | Specific surface area (μm2/g) | Porosity (%) | Hc (kA/m) | σs (A·m2/kg) | ||
| Acicular iron nanoparticle | - | - | - | - | 116.2 | 119 | |
| MagCEBAG | 1.68 | 0.04 | 0.62 | - | 115.4 | 62.1 | |
| MagCE (value of rotameter (mm)) | 35 | 10.28 | 0.71 | 1.97 | 62.7 | 113.5 | 60.8 |
| 40 | 8.3 | 0.55 | 1.85 | 63.8 | 114.4 | 59.7 | |
| 45 | 6.51 | 0.44 | 1.78 | 69.1 | 114.4 | 60.3 | |
| 50 | 5.19 | 0.36 | 1.64 | 71.2 | 114.8 | 60.3 | |
| 55 | 4.38 | 0.31 | 1.54 | 71.9 | 114.8 | 60.0 | |
| Cracked powder | 23.88 | 0.25 | 0.93 | - | 106.1 | 75.5 | |
| Spherical iron nanoparticle | - | - | - | - | 39.8 | 150.3 | |
| Spherical iron nanoparticle- based MagCE | 5.71 | 0.0011 | 1.72 | 65.1 | 39.3 | 59.6 | |
| Spherical iron oxide nanoparticle | - | - | - | - | 10.3 | 62.8 | |
| Spherical iron nanoparticle- based MagCE | 5.6 | 0.0022 | 1.65 | 65.3 | 12.5 | 22.7 | |
The design concept of MagCE
We here report the development concept and the details of the MagCE structure in relation to cesium adsorption. In view of the complicated operations and drawbacks that characterize the conventional method for removal of radioactive cesium from fly ash, we sought to resolve them by the direct addition of a decontaminant powder to the fly ash slurry, in the knowledge that powder-adsorbed cesium is readily separable after mixing (Fig. 1a). To achieve this, in other words, to reduce the number of stages for decontamination and to avoid any presence of water-soluble cesium in the dewatered cakes, we devised a magnetically guidable decontaminant powder, MagCE, using a drug delivery system for cancer we had previously developed7,8.
In the conventional procedure, it is necessary, first, to separate cesium-eluted water from the fly ash slurry, after which decontaminants are added to the liquid component thus obtained5,9,10,11,12,13,14,15. This separation process is dependent on particle size, so a decontaminant powder of smaller particle size possesses a greater degree of cesium adsorption because of its larger specific surface area; however, the rapid collection of such a fine powder is considerably more difficult. Thus, the powder-adsorbed cesium must be precipitated in the final step (Fig. 1b). As an added complication, the continued presence of water-soluble cesium in the dewatered fly ash cakes is also an important issue, because complete removal of the water that contains a significant amount of cesium from cakes is challenging, and water-soluble cesium can cause radioactive contamination of natural resources such as soil and groundwater.
More specifically, to perform the processes illustrated in Fig. 1a, MagCE must possess the following characteristics.
(1) Salt tolerance
Fly ash contains many types of salts (See Table S1, Supplementary Information) such as chlorides and hydrosulfates of potassium and sodium. Zeolite and Prussian blue (Fe4[Fe(CN)6]3) are well known to adsorb for radioactive cesium; however, zeolite cannot be used for our purpose because its absorbability is too low in water with a high concentration of salts1,2,5.
(2) Alkali resistance
An alkaline agent (e.g., slaked lime or sodium bicarbonate) is added to neutralize the acid gases produced during the incineration process, so the fly ash slurry becomes alkaline. Although Prussian blue is salt-tolerant, it starts to decompose (Fe4[Fe(CN)6]3) + 12 OH− → 4Fe(OH)3 + 3[Fe(CN)6]4−) and lose its ability to adsorb radioactive cesium in the alkaline region2,16. The addition of acid to the slurry to maintain the pH below 7 is possible; however, this is neither economical nor safe, because a large amount of acid would be needed and harmful heavy metals such as lead and cadmium would elute into the water from the ash particles.
(3) Solid-solid separability of fly ash and MagCE
To achieve maximum reduction of the concentration of radioactive cesium in fly ash, MagCE must be recovered from the slurry as efficiently as possible. The recovery process uses magnets, and the most desirable characteristics of this MagCE are as follows: large grain size, strong magnetism and low affinity for fly ash particles. On the other hand, to effectively adsorb water-soluble radioactive cesium using MagCE, it is desirable for the grain size of MagCE to be small, its surface area large and its dispersion high. It is critically important to have a proper balance of these characteristics.
(4) Physical strength
In our execution of the process, the fly ash decontamination is performed in the fly ash slurry. Both the processing speed and the shear stress increase with the force of the stirring. MagCE contains a material that adsorbs radioactive cesium, and its adsorption ability decreases if the adsorbent is exfoliated by an application of sufficient force (e.g., vigorous stirring). Presumably, this effect increases with the concentration of fly ash in the slurry because of the increased density. Thus, MagCE must have the capacity to withstand stirring.
The morphology and cesium adsorption of MagCE
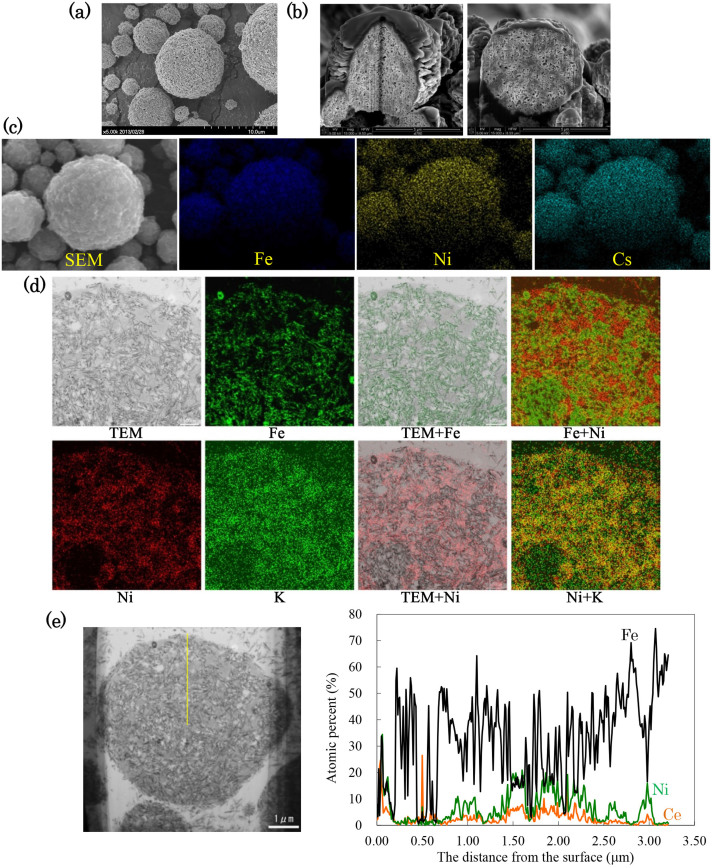
As the first assessment of MagCE, we evaluated its nanostructure with scanning electron microscopy (SEM) after processing with focused ion beam milling (FIB) (Fig. 2a, b). These analyses revealed that MagCE displayed a micron-sized (5 to 10 μm) porous sponge-like ball structure that resembles the decorative Japanese paper balls or spheres known as “Kusudama” (See Fig. S11, Supplementary Information). Subsequently, using SEM and energy dispersive X-ray spectroscopy (EDX), we observed the surface distribution of nickel ferrocyanide and adsorption of cesium by the MagCE after reaction with cesium sulfate solution. We detected both of them on the outer layer of MagCE (Fig. 2c and see Fig. S13, Supplementary Information). We then assessed the internal structure and distribution of the MagCE elements using transmission electron microscopy (TEM) and EDX (Fig. 2d). We found that each of the micron-size secondary particle groups consists of nickel ferrocyanide-coated acicular primary iron nanoparticles. The particles had a porous structure and was partially occupied nickel ferrocyanide. This architecture of MagCE may help to prevent the peeling off of nickel ferrocyanide that occurs due to vigorous stirring of the fly ash slurry for long periods. The fine spherical droplets, which were sprayed through a spray dryer's nozzle, contained nickel ferrocyanide, poly(diallyldimethylammonium chloride) (PDDA)-coated magnetic nanoparticles and water. The nickel ferrocyanide-containing water swells out and shrinks by evaporation of the water through the heating process just after spraying. We think that this shrinkage of nickel ferrocyanide, which strongly binds the PDDA-coated magnetic nanoparticles, is one of the reasons for the formation of the porous structure of MagCE. Spray dryers have been used to obtain not only dry powders but also hollow capsules17. In terms of avoiding decomposition and the loss of the ferromagnetism of the iron nanoparticles through the preparation of MagCE and the reaction in the alkaline slurry, we used iron nanoparticles on which the surface had been coated with a corrosion-resistant iron oxide layer18. As for the decomposition of the iron nanoparticles, we confirmed that strong corrosion and rusting occurred when we prepared MagCE using iron particles without the anti-oxidative layer (data not shown). EDX line analysis showed that MagCE had a lower relative density of iron and a higher porosity than the cracked powder version (Fig. 2e).
Figure 2. Electromicroscopic observation of MagCE.
SEM images taken (a) before and (b) after FIB processing. (SEM images of cracked powder and MagCEBAG are shown in Fig. S12, Supplementary Information). SEM–EDX of MagCE before (See Fig. S13, Supplementary Information) and (c) after adsorption of nonradioactive cesium. MagCE was mixed with 1% cesium phosphate aqueous solution for 30 min, and then washed with DW and filtrate using filter paper No. 4A. (d), TEM-EDX of MagCE. Color treatment was performed to reveal the distribution of each element. (e), TEM–EDX line analysis of MagCE. TEM–EDX and TEM–EDX line analyses of cracked powder are shown in Fig. S14, Supplementary Information.
Ferrocyanide-salt-type- and fly-ash-type-dependent cesium-decontamination degree of MagCE
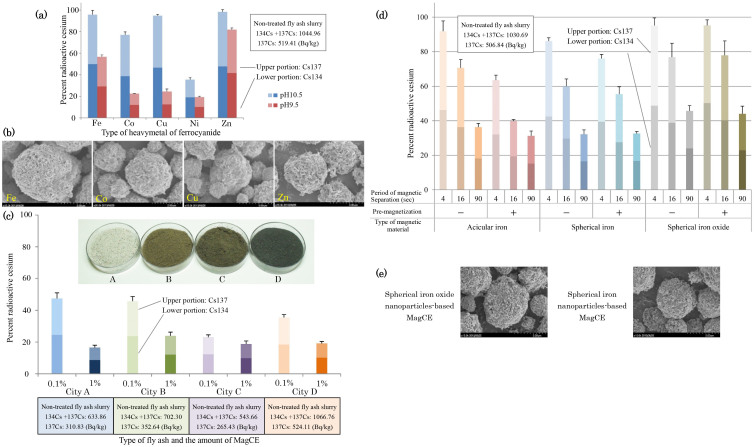
Moreover, we compared the degree of ferrocyanide salt type-dependent cesium elimination by MagCE (Fig. 3a). By mixing a solution of potassium ferrocyanide with one of the five types of metal ions (iron, cobalt, copper, nickel and zinc), representative insoluble ferrocyanide salts are formed which can adsorb a certain degree of cesium ions. Regarding the diffusion of heavy metals to the environment, we were able to prevent the elution of harmful lead from fly ash when the pH range of slurry was adjusted so as to remain between 9.5 and 10.5. Therefore, we assessed the ferrocyanide-type dependence at pH 9.5 and 10.5, and found that nickel ferrocyanide displayed the largest and the most stable adsorption of cesium among the five types of ferrocyanide. An electromicrograph of MagCE particles with these ferrocyanides revealed that only zinc ferrocyanide had a large cubic crystal structure, and this crystal size might be a disadvantage for the enlargement of the specific surface area available to adsorb cesium (Fig. 3b). We then evaluated the radioactivity of fly ash slurry after treatment with MagCE using the four types of fly ash obtained from the different refuse incineration plants (Fig. 3c and see Table S1, Supplementary Information). This evaluation revealed that 52.5–77.0% and 76.1–83.4% of the radioactive cesium (Cs-134 + Cs-137) was eliminated when 0.1 and 1 wt.% of MagCE per unit weight of fly ash was used, respectively. We confirmed that 84.3% (city A), 77.0% (city B), 80.6% (city C) and 81.7% (city D) of radioactive cesium (Cs-134 + Cs-137) was eluted in the liquid component which was obtained by the solid-liquid separation of fly ash slurry. These elution ratios were almost identical to the decontamination ratios of MagCE when a sufficient amount of MagCE (1 wt.% per fly ash) was used. In terms of the surface electric charges of MagCE and the fly ash particles, their electrical repulsion force was larger at a pH of 10.5 than at pH 6, at and below which is the value for iron ferrocyanide to maintain its chemical stability (See Table S2, Supplementary Information). Moreover, we confirmed that the structure of MagCE was maintained even at 24 h after the stirring in water at a pH of 10.5 (See Fig. S15, Supplementary Information). In terms of the alkaline-resistance, we have preliminarily confirmed that iron ion, which was released from MagCE, increased sharply in water at a pH of over 11.5 (data not shown). Therefore pH 10.5 may be preferable for good electrostatic repulsion and prevention of coagulation between these particles, and may thus be advantageous for the promotion of the solid-solid separation of MagCE from fly ash slurry.
Figure 3. Assessment of the MagCE performance in radioactive cesium elimination from fly ash slurry under various conditions.
Ferrocyanide-type-dependent (a) degree of removal of radioactive cesium and (b) each of the SEM images (See also Fig. S16, Supplementary Information). MagCE (40 mg) composed of each type of insoluble ferrocyanide salt and acicular iron was mixed for 24 h. As ferrocyanide salts, we used iron-, cobalt-, cupper-, nickel- and zinc-ferrocyanide. (c), The appearance of four types of fly ash (See Table S1, Supplementary Information) obtained from the different refuse incineration plants and fly-ash-type-dependent removal of radioactive cesium. MagCE (40 or 400 mg) composed of nickel ferrocyanide and acicular iron was mixed. (d), Degrees of the magnetic-material-type-dependent and magnetic-separation-time-dependent removal of the radioactive cesium, which are increased by the magnetization of MagCE before its addition to the fly ash slurry. MagCE (40 mg), composed of nickel ferrocyanide, and each type of magnetic nanoparticle was mixed. As magnetic materials, we used acicular iron, spherical iron and spherical iron oxide. (e), SEM images of spherical iron oxide nanoparticle- and spherical iron nanoparticle-based MagCE (See also Fig. S17, Supplementary Information). (a), (d), Fly ash obtained from city D was used. (a), (c), (d), MagCE, which was prepared when the floating ball-height of rotameter was 45 mm, which then was mixed with fly ash slurry (fly ash: 40 g; DW: 400 mL). After the removal of MagCE using a bar type magnetic separator (See Fig. S8, Supplementary Information), the radioactivity of the slurry was measured using an Na-I scintillator. Data are expressed as the mean ± SD, All samples for the assay were prepared in triplicate.
Magnetic-material-type- and pre-magnetization-dependent cesium-decontamination degree of MagCE
In addition, for the practical application of MagCE, rapid and complete magnetic separation is very important, especially when large amounts of ash are to be processed. Therefore, we tried to enhance the magnetic separation by pre-magnetization of the MagCE composed of ferromagnetic materials prior to mixing it with slurry. We tested the degree of cesium elimination affected by the pre-magnetization of MagCE comprising three different types of magnetic materials, namely, acicular iron (Hc: 116.2 kA/m; σs 119 A·m2/kg), spherical iron (Hc: 39.8 kA/m; σs 150 A·m2/kg) and conventional spherical iron oxide (Hc: 10.3 kA/m; σs 62.8 A·m2/kg) (Fig. 3d, e). σs and Hc were the largest in the acicular iron and spherical iron, respectively, and both of them were larger in both acicular and spherical iron than spherical iron oxide (Table 1). Spherical iron nanoparticles with an anticorrosive layer were synthesized by reducing spherical iron oxide nanoparticles. The MagCE comprising acicular iron, which possesses the largest coercivity, displayed the most marked enhancement of cesium elimination by pre-magnetization (0.1 wt.% of MagCE per unit weight of fly ash was used): 36.5–60.2% of the radioactive cesium (Cs-134 + Cs-137) was eliminated when the pre-magnetization of MagCE was performed (magnetic separation periods: 4 to 16 sec), by contrast, only 8.16–29.3% of the radioactive cesium was removed without the pre-magnetization.
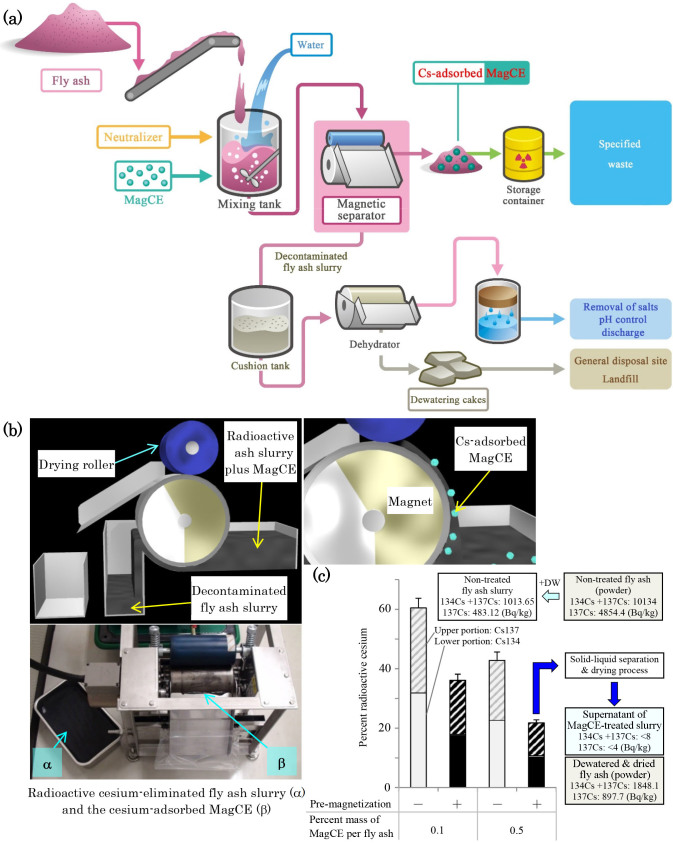
These results suggest that the pre-magnetization of MagCE comprising ferromagnetic acicular iron particles created sufficient remanence (i.e. residual magnetization) for rapid magnetic separation, even 24 h after mixing with the slurry. We consider that the magnetization-induced soft clustering of this type of ferromagnetic MagCE to have accelerated the magnetic separation by maintaining a large specific surface area for cesium adsorption. Finally, we tested a drum-type magnetic separator that is compact, low cost and provides continuous magnetic separation of cesium-adsorbed MagCE from large amounts of fly ash slurry (Fig. 4 and see Fig. S9, Supplementary Information). Also in this assessment, the pre-magnetization of MagCE appeared to be more effective, probably because the magnetic separation time is generally less when using a drum-type separator: 78.2% of the radioactive cesium (Cs-134 + Cs-137) was eliminated when the pre-magnetization of MagCE was performed, by contrast, 57.2% of the radioactive cesium was removed without the pre-magnetization (0.5 wt.% of MagCE per unit weight of fly ash was used). Moreover, the radioactivity of the supernatant after the solid-liquid separation of the slurry was <8.0 Bq/kg (under the detection limit) and 31.5 Bq/kg, when the percentage of the mass of MagCE per fly ash was 0.5 and 0.1, respectively. In terms of the magnetic separation efficiency of MagCE, we confirmed that MagCE removed over 99.0% of the radioactive cesium from the liquid component (before treatment: 821.64 Bq/kg; after treatment: <8.0 Bq/kg) of the solid-liquid separated fly ash slurry using a magnet bar equipped with a Z axis stage (separation period: 90 sec) and drum-type magnetic separator (separation speed: 2 L/min). Moreover, radioactive cesium was 1848.1 (≪8000) Bq/kg in the solid-liquid separated solid component, so it may be placed in a final disposal site according to the current Japanese Waste Management Law. We confirmed that fly ash contained 30 to 35 wt.% of the water soluble component, so the insoluble sludge was reduced to 65 to 70 wt.% after the fly ash had been mixed with water and the obtained slurry was solid-liquid separated (the water soluble components are removed in this process). As a result, the total amount of insoluble sludge (insoluble fly ash components + magnetically separated MagCE) is calculated as 65.5 to 70.5 wt.% per fly ash when 0.5 wt.% MagCE per fly ash was added to the ash slurry. In the present study, 2 L of the slurry, which contained 200 g of fly ash, could be treated per a minute (i.e., 144 kg of fly ash/day) using the compact type of magnetic separator. If the number and size of separator are increased, we can easily treat much larger amounts of fly ash (the magnetic separator of which treatment speed is 2000 L/min is commercially available).
Figure 4. Larger-scale study using a drum-type magnetic separator.
(a), Schematic representation of the concept of the compact decontamination system composed of a slurry-mixing tank, a magnetic separator to recover the MagCE and the dewatering equipment. This system is very simple and effective for processing highly radioactive fly ash. (b), Schematic representation of the decontamination involving removal of radioactive cesium using MagCE and a drum-type magnetic separator (See also Fig. S9, Supplementary Information). (c), MagCE composed of nickel ferrocyanide and acicular iron, which was prepared when the floating ball-height of rotameter was 45 mm, were magnetized and added to the fly ash slurry and stirred at 200 rpm for 24 h. Then, the MagCE-mixed slurry was circulated for 5 min and discharged to a magnetic separator from a slurry pump (2 L/min). Finally, the radioactive cesium of (1) fly ash slurry, (2) supernatant obtained after the centrifugation (9000 G, 15 min) of slurry and (3) dried powder, which was obtained after the treatment with a vacuum oven and a power mill (See Fig. S18, Supplementary Information), was measured by using a Ge detector. The percentage of the removal of the radioactive cesium was calculated. Fly ash obtained from city D (See Table S1, Supplementary Information) was used. Data are expressed as the mean ± SD. All samples for the assay were prepared in triplicate.
Conclusion
To the best of our knowledge, this report of the development of the cesium decontaminant powder described here, which can be directly mixed with fly ash slurry and rapidly achieves both magnetic separation and cesium adsorption even after mixing is the first such study in this area of research. These features not only simplify the decontamination process, but also enable the complete removal of water-soluble cesium from fly ash slurry. MagCE affords the following advantages. (1) Its porous particle structure is useful for the enlargement of both internal and external specific surface areas for cesium adsorption. (2) The placement of alkaline-resistant nickel ferrocyanide into the porous space is desirable for maintaining the stability (the prevention of both chemical decomposition and physical removal) of the cesium-adsorption sites. (3) The ferromagnetic material (large Hc and σs), with its anticorrosive layer, enhances the magnetic separation by pre-magnetization of MagCE. This new technique will be useful for the decontamination of the areas surrounding Fukushima and Chernobyl, and, in cases of emergency, for the decontamination of nuclear power stations and their surrounding areas in any country in which is needed. In terms of the possible use for other than fly ash, MagCE may be available for the removal of radioactive cesium from solid samples such as soil, plant biomass and so on, when water-soluble cesium can be effectively eluted in the washing fluid of these samples. Moreover, if new types of MagCE which contains specific sorbent for other isotopes are developed, various kinds of isotopes will be rapidly removed in a similar manner.
Methods
Preparation of MagCE and MagCEBAG
Each magnetic nanoparticle was supplied by Dowa Electronics Materials (Tokyo, Japan) and each reagent was purchased from Wako (Tokyo, Japan). Magnetic nanoparticles (10 wt.%) were mixed with a 1% PDDA aqueous solution for 1 h and centrifuged (3000 G, 15 min) to remove the supernatant. This PDDA-coated iron nanoparticles-precipitate was dispersed in DW (15 wt.%). This PDDA-coated magnetic nanoparticle slurries (50 mL) and heavy metal ferrocyanide slurries (50 mL; Supplementary Information) were then thoroughly mixed using a T-shaped micro-mixer (passage diameter: 600 μm; Nakamura Choukou, Osaka, Japan)19. This mixer was equipped with a high pressure syringe pump (Nexus 6000, Science Products GmbH, Hofheim, Germany) and stainless syringe (100 mL; Science Products GmbH). Both of these slurries were extruded from the outlet of the syringe and flowed into the inlet of the micro-mixer at the speed of 150 mL/min (movie file S1, Supplementary Information). The mixed slurry obtained was treated with a spray dryer (Fig. 3a; Model B-290, Buchi Laboratoriums Technik AG, Switzerland)20. The inlet and outlet temperatures of a dryer were set at 497 K and 368 K, respectively. The sprayed air volume and negative pressure suction force were controlled at 414–670 L/h and −50 mbar, respectively. Finally, the dried MagCE powder was recovered by a cyclone separator and any uncollected portion (MagCEBAG) was trapped with an outlet bag filter (Fig. S1, Supplementary Information). The optimal conditions of the sprayed air volume, the reaction time with MagCE and the concentration of MagCE, are shown in Fig. S2–S3 (Supplementary Information).
Fly ash and quantitative analysis of radioactive cesium
Each type of fly ash was obtained from 4 different incineration plants in eastern Japan. In all of the studies except for Fig. 3c, the fly ash from city D was used. The radioactivity of the fly ash slurry was measured in all of the decontamination tests except for a part of Fig. 4C. The radioactivity of cesium was measured using a multi-channel analyzer for γ-spectroscopy (Na-I scintillator: MUCHA, Raytest, Straubenhardt, Germany) or a germanium semiconductor detector (Ge detector: GC-2020-7500SL-2002CSL, Canberra. Meriden, Connecticut, US).
Magnetic separation of the cesium-adsorbed MagCE from fly ash slurry (small scale study)
Fly ash (40 g) was mixed with water (400 ml) at 200 rpm for 24 h in a storage bottle (500 ml; Corning International, Inc., NY, US). MagCE amounts ranging from 25 to 400 mg were added to samples of this slurry. A magnetic separation system consisting of a long bar magnet (1.0 tesla: Niroku Seisakusho Co., LTD, Kobe, Japan) and Z-axis electric stage (COMS, Amagasaki, Japan) was used for the magnetic separation of the MagCE mixed with this slurry. This type of separator is equipped with an incubator shaker (200 rpm, 25°C: New Brunswick INNOVA 4400, Eppendorf AG, Hamburg, Germany) (Figure S8, movie file S2, Supplementary Information). A magnet covered by a 50 mL tube was inserted into the mouth of the bottle, whose contents were shaken at 200 rpm with this shaker. After each designated period, the magnet was lifted up by a Z-axis stage and a tube on which the surface with the adsorbed MagCE was removed.
Magnetic separation of the cesium-adsorbed MagCE from fly ash slurry (large scale study)
Fly ash (100 g) was mixed with water (1 L) to obtain slurry at 200 rpm for 24 h in a storage bottles (1000 ml; Corning International, Inc.). MagCE (0.1 or 0.5 g) were added to samples of this slurry. Ten sets of this samples were prepared and all of them were mixed. Finally, the slurry which contained fly ash (1 kg), water (10 L) and MagCE (1 g or 5 g) were obtained. A magnetic separation system consisting of a slurry pump and a magnetic separator (0.5 tesla, 20 rpm, clearance between magnet drum and receiver: 3 mm; magnet drum: ϕ113 mm × L160 mm; Noritake Co., Ltd., Tokyo, Japan) was used for the magnetic separation of the MagCE mixed with this slurry (See Fig. S9, Supplementary Information). A rotary pump (Nanofilter Demi, Noritake) connected with a static mixer (Static mixer, Noritake Co., Ltd.) was used as the slurry pump. This large scale study was also conducted at 25°C.
Magnetization of MagCE
To magnetize MagCE, a neodymium magnet (0.5 tesla) was attached to the outer wall of an aluminum foil container of MagCE (See movie file. S3, Supplementary Information).
Author Contributions
Y.N. conceived and designed the experiments. Y.N., T.U., T.Y., R.W. and T.N. performed the experiments. Y.N. and T.Y. co-wrote the paper. S.K. and T.N. performed the statistical analysis.
Supplementary Material
Supplementary information
Microreactor
Magnetic separator (small scale study)
Magnetization
Magnetic separator (large scale study)
Acknowledgments
We dedicate this work to the late T. Terada, the late K.Nariai, and the many people who suffered in the March 11 disaster. We would like to acknowledge Satoshi Kawakami (DOWA ECO-PUBLIC "-//NPG//DTD XML Article//EN"), Hiroki Maekawa (DOWA Electronics Materials) and Yuki Nagase. This work was supported by a Funding Program for the Next Generation of World-Leading Researchers (LS114) from the JSPS.
Footnotes
Yes there is a potential Competing Interest. We have received a research grant (DOWA Techno Fund: JPY 1,7500,000) from DOWA Holdings Co., Ltd. for the initial phases of this study.
References
- National Institute for Environmental Studies, Center for Material Cycles and Waste Management Research. Proper waste disposal viewed form the behavior of radiological materials (technical data). Second edition. (2012).
- Nishizaki Y. et al. New technologies for decontamination of radioactive substances scattered by nuclear accident. Arch. Metall. Mater. 58, 283–289 (2013). [Google Scholar]
- Brumfiel G. & Cyranoski D. Quake sparks nuclear crisis. Nature 471, 273–275 (2011). [DOI] [PubMed] [Google Scholar]
- Bandazhevsky Y. I. Radioactive cesium and the heart: pathophysiological aspects. The Belrad Institute 2001. Minsk, pp.1–59. (ISBN 985-434-080-5).
- Parajuli D. et al. Dealing with the aftermath of Fukushima Daiichi nuclear accident: decontamination of radioactive cesium enriched ash. Environ. Sci. Technol. 47, 3800–3806 (2013). [DOI] [PubMed] [Google Scholar]
- Namiki Y. et al. Inorganic-organic magnetic nanocomposites for use in preventive medicine: A rapid and reliable elimination system for cesium. Pharm Res 29, 1404–1418 (2012). [DOI] [PubMed] [Google Scholar]
- Namiki Y. et al. A novel magnetic crystal-lipid nanostructure for magnetically guided in vivo gene delivery. Nat. Nanotechnol 4, 598–606 (2009). [DOI] [PubMed] [Google Scholar]
- Namiki Y. et al. Nanomedicine for cancer: lipid-based nanostructures for drug delivery and monitoring. Accounts Chem. Res. 44, 1080–1093 (2011). [DOI] [PubMed] [Google Scholar]
- Han F., Zhang G. H. & Gu P. Removal of cesium from simulated liquid waste with countercurrent two-stage adsorption followed by microfiltration. J. Hazard. Mater. 225–226, 107–113 (2012). [DOI] [PubMed] [Google Scholar]
- Kosaka K. et al. Removal of radioactive iodine and cesium in water purification processes after an explosion at a nuclear power plant due to the Great East Japan Earthquake. Water Res. 46, 4397–4404 (2012). [DOI] [PubMed] [Google Scholar]
- Avramenko V. et al. Colloid stable sorbents for cesium removal: preparation and application of latex particles functionalized with transition metals ferrocyanides. J. Hazard. Mater. 186, 1343–1350 (2011). [DOI] [PubMed] [Google Scholar]
- Xu C., Yuan L., Shen X. & Zhai M. Efficient removal of caesium ions from aqueous solution using a calix crown ether in ionic liquids: mechanism and radiation effect. Dalton Trans. 39, 3897–3902 (2010). [DOI] [PubMed] [Google Scholar]
- Valsala T. P. et al. Removal of radioactive caesium from low level radioactive waste (LLW) streams using cobalt ferrocyanide impregnated organic anion exchanger. J. Hazard. Mater. 166, 1148–1153 (2009). [DOI] [PubMed] [Google Scholar]
- Wang T. H., Li M. H., Yeh W. C., Wei Y. Y. & Teng S. P. Removal of cesium ions from aqueous solution by adsorption onto local Taiwan Laterite. J. Hazard. Mater. 160, 638–642 (2008). [DOI] [PubMed] [Google Scholar]
- Klika Z., Kraus L. & Vopalka D. Cesium uptake from aqueous solutions by bentonite: a comparison of multicomponent sorption with ion-exchange models. Langmuir 23, 1227–1233 (2007). [DOI] [PubMed] [Google Scholar]
- Johannes C. L. M., Meindert G. K. & Frans A. M. De H. Chemical stability and decomposition rate of iron cyanide complexes in soil solutions. Environ. Sci. Technol. 26, 511–516 (1992). [Google Scholar]
- Narayan P., Marchant D. & Wheatley M. A. Optimization of spray drying by factorial design for production of hollow microspheres for ultrasound imaging. J. Biomed. Mater. Res. 56, 333–341 (2001). [DOI] [PubMed] [Google Scholar]
- Hisano S. et al. Ferromagnetic metal powder and magnetic recording medium containing the powder. Japanese patent 5,130,456 (2012).
- Garcia-Eqido E., Spikmans V., Wong S. Y. & Warrington B. H. Synthesis and analysis of combinatorial libraries performed in an automated micro reactor system. Lab. Chip 3, 73–76 (2003). [DOI] [PubMed] [Google Scholar]
- Carné-Sánchez A., Imaz I., Cano-Sarabia M. & Maspoch D. A spray-drying strategy for synthesis of nanoscale metal-organic frameworks and their assembly into hollow superstructures. Nat. Chem. 5, 203–211 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Microreactor
Magnetic separator (small scale study)
Magnetization
Magnetic separator (large scale study)