Abstract
Purpose
Diffuse pontine gliomas are a pediatric brain tumor that is fatal in nearly all patients. Given the poor prognosis for patients with this tumor, their quality of life is very important. Radiation therapy provides some palliation, but can result in radiation necrosis and associated neurologic decline. The typical treatment for this necrosis is steroid therapy. While the steroids are effective, they have numerous side effects that can often significantly compromise quality of life. Bevacizumab, an antibody against vascular endothelial growth factor (VEGF), has been suggested as a treatment for radiation necrosis. We report on our initial experience with bevacizumab therapy for radiation necrosis in pediatric pontine gliomas.
Methods and Materials
Four children with pontine gliomas treated at the Children’s Hospital in Denver and the University of Colorado Denver developed evidence of radiation necrosis both clinically and on imaging. Those four children then received bevacizumab as a treatment for the radiation necrosis. We reviewed the clinical outcome and imaging findings.
Results
After bevacizumab therapy, three children had significant clinical improvement and were able to discontinue steroid use. One child continued to decline, and in retrospect, had disease progression, not radiation necrosis. In all cases, bevacizumab was well tolerated.
Conclusions
In children with pontine gliomas, bevacizumab may provide both therapeutic benefit and diagnostic information. More formal evaluation of bevacizumab in these children is needed.
Keywords: Diffuse pontine glioma, Pediatric, Radiation necrosis, Bevacizumab
Introduction
Diffuse pontine gliomas (DPGs) primarily occur in children and the outcomes for these children are dismal. There are very few, if any, long term survivors, with the median overall survival approximately 1 year (1–4). Given the very poor survival for these children, their quality of life during and after therapy is very important to consider. Standard therapy for newly diagnosed DPGs is conformal radiation therapy. A well described toxicity of this treatment is radiation necrosis, which can result in worsening neurologic symptoms. Treatment for radiation necrosis has typically been symptomatic management, including steroids. Recently, two groups reported on their experience with the use of bevacizumab (Avastin, Genentech, San Francisco, CA) as a treatment for radiation necrosis in the brain (5, 6). They showed that treatment with bevacizumab resulted in a reduction in steroid requirements and improvement on imaging.
Steroids are known to have numerous side effects including changes in behavior, difficulty with sleep and increased appetite. These symptoms often adversely affect the quality of life in children, something important to consider in patients with diffuse pontine gliomas, particularly those who subsequently develop radiation necrosis. We describe four children with pontine gliomas who appeared to have radiation necrosis and were treated with bevacizumab. Three children had typical DPGs and one child had an atypical presentation on imaging and underwent biopsy.
Methods and Materials
We reviewed the children with pontine gliomas who were treated at The Children’s Hospital, Denver and the University of Colorado Denver, Department of Radiation Oncology from 1995 to 2008 and received bevacizumab after initial treatment. Clinical information, chemotherapy and radiation therapy were reviewed in those children. The review of medical records was approved by our Institutional Review Board.
Results
Four children with pontine gliomas received bevacizumab (10 mg/kg IV every two weeks) for treatment of neurologic worsening due to presumed radiation necrosis. Three of the four received radiation therapy consisting of stereotactic radiotherapy to the tumor to a dose of 54 Gray (Gy) in 1.8 Gy fractions. One child received a short course of radiation therapy, 25 Gy in 5 Gy fractions, to allow the child to return home more quickly. Two children received an investigational agent on a phase I trial along with standard radiation therapy as initial therapy. The four children are described in detail below.
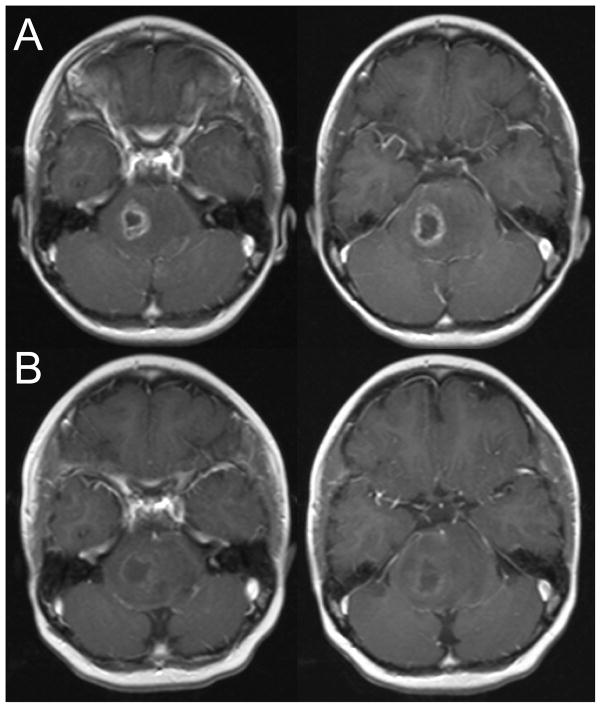
Patient 1 presented with a 2 week history of ataxia and speech difficulty. MRI revealed a pontine mass with areas concerning for necrosis. She received conventional radiation therapy (54 Gy in 1.8 Gy fractions). Within three weeks of starting therapy, her neurologic function had returned to baseline. MRI performed at the end of radiation showed a decrease in the size of the pontine mass with necrosis on the right. Three months following completion of radiation, she developed significant left sided weakness, slurred speech and facial droop. The MRI revealed increased in the right sided area of necrosis (Figure 1, row A). She responded to decadron treatment and was then started on bevacizumab therapy with significant improvement in weakness. She received a total of five courses of bevacizumab. Repeat imaging two months later showed decreased enhancement in the region of necrosis (Figure 1, row B). She has subsequently progressed, both clinically and radiographically. She was briefly enrolled on a phase 1 trial, but continued to have progressive symptoms and was placed on terminal care. She is alive with disease 10 months from initial diagnosis.
Figure 1.
Two consecutive axial slices from the post-gadolinium T1-weighted MRI performed three months after completion of therapy (row A) and after two months of bevacizumab therapy (row B).
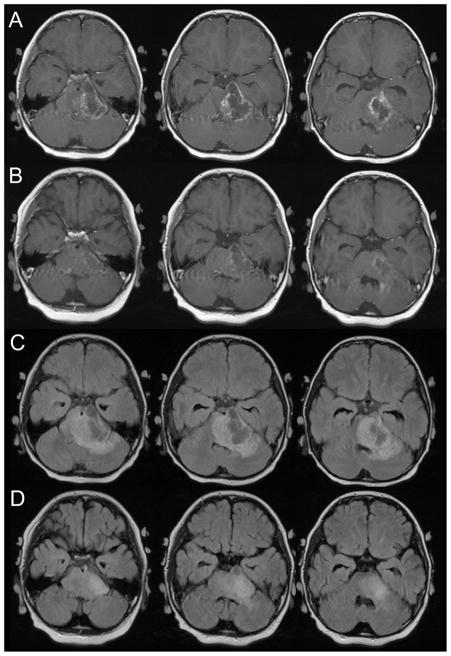
Patient 2 presented with a 3 week history of ataxia, slurred speech, right sided weakness and fatigue. MRI revealed an infiltrating pontine mass with a region of necrosis on the left. He received conventional radiation therapy and a concurrent phase I agent. Prior to starting radiation treatment, he developed a tachycardic/bradycardic rhythm, somnolence and worsening neurologic functioning. His radiation treatments were started urgently and he responded to mannitol and decadron. Within 1 week of starting radiation, he had improvement in his level of consciousness and neurologic function. By the end of radiation, he had minimal cranial nerve palsies but moderate residual right weakness.
Three weeks after completion of treatment, he developed ataxia, right sided weakness and significant nausea and vomiting. MRI at that time revealed a decrease in the size of the tumor with marked cystic radiation necrosis on the left (Figure 2, rows A and C). He was started on decadron and subsequently bevacizumab. His gait and weakness rapidly improved. Follow-up imaging three weeks later revealed significant improvement, with a decrease in enhancing necrotic region and edema seen on the FLAIR sequence (Figure 2, rows B and D). He has received five courses of bevacizumab and did well clinically for four months He now has progressed clinically and on imaging and is alive with disease seven months from initial diagnosis
Figure 2.
Three consecutive axial slices from the post-gadolinium T1-weighted and FLAIR MRI performed three weeks after completion of therapy (rows A and C) and after one month of bevacizumab therapy (rows B and D).
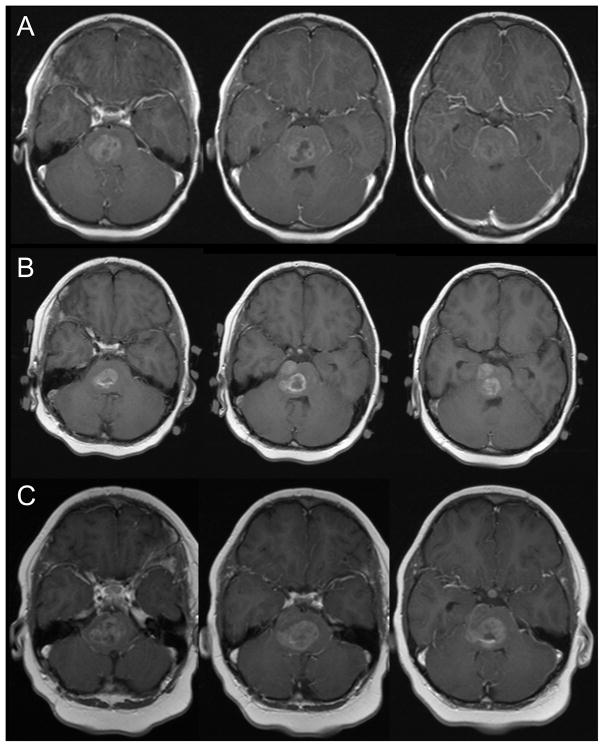
Patient 3 presented with ataxia, right facial weakness, left arm and left leg weakness. MRI revealed a pontine mass without evidence of necrosis. Initially, his gait and weakness improved with radiation treatments. However, during the last week of radiation, he deteriorated with recurrence of his neurologic deficits and MRI revealed a decreased tumor with new development of radiation necrosis on the right. He initially responded to decadron, but then one month later began to deteriorate again as he tapered off the steroids. The MRI performed at time demonstrated progression of the necrosis (Figure 3, rows A and C). He then received four courses of every 2 week bevacizumab with good improvement, both clinically and by imaging (Figure 3, rows B and D). He is doing well five months from initial diagnosis.
Figure 3.
Three consecutive axial slices from the post-gadolinium T1-weighted and FLAIR MRI performed one month after completion of therapy (rows A and C) and after two months of bevacizumab therapy (rows B and D).
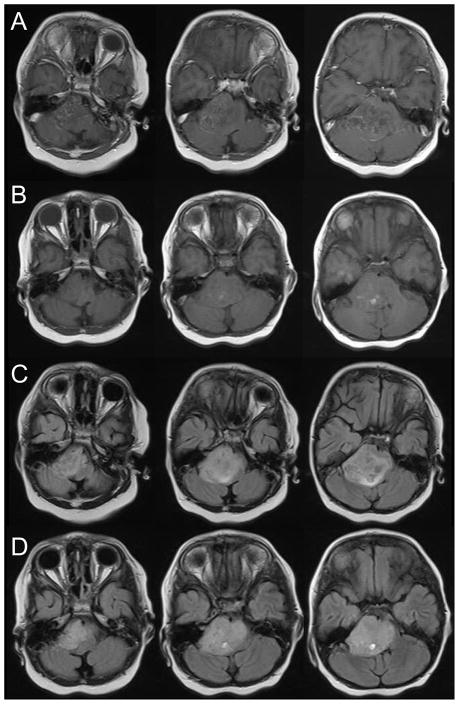
Patient 4 presented with ataxia, slurred speech, and left facial weakness. MRI revealed a right sided pontine lesion (Figure 4, row A). Given the atypical appearance of the mass, he underwent stereotactic biopsy and pathology revealed glioblastoma. After extensive discussions with the parents, they chose to undergo a short course of palliative radiation therapy to allow him to return home more quickly. He received 25 Gy in five fractions. He responded very well and within a few weeks had returned to baseline functioning. Three months after completion of radiation, he developed left sided weakness. MRI showed increased enhancement in the tumor and increasing edema, consistent with radiation necrosis (Figure 4, row B). He was started on decadron and improved dramatically over the next few weeks. However, as the steroid was tapered, his weakness returned. He received three courses of bevacizumab while attempting to decrease his decadron. However, his weakness progressively worsened and MRI revealed progressive disease (Figure 4, row C). He died six months after initial diagnosis.
Figure 4.
Three consecutive axial slices from the post-gadolinium T1-weighted MRI at initial presentation (row A), three months after completion of therapy (row B), and after three courses of bevacizumab (row C).
Discussion
Necrosis occurs frequently in children with DPGs that receive radiation therapy. In one series, 28 of 29 children developed necrosis after treatment (7). The necrosis likely results from a combination of radiation therapy and the biology of the tumor itself. Early tumor necrosis resulting in symptoms is more likely to be treatment-related and can improve over time in many cases (8). The treatment for radiation necrosis is typically steroids, which non-specifically reduces edema, but is associated with numerous significant side effects.
Endothelial abnormalities, including elevation in vascular endothelial growth factor (VEGF), play a significant role in the development of radiation necrosis (9–12). Agents that interfere with the VEGF signaling pathways have been shown to reduce vascular permeability and decrease edema in glioblastoma patients (13). It seems reasonable to hypothesize that vascular normalization would similarly improve radiation necrosis. This has been shown in a small group of eight adults with brain tumors treated at M.D. Anderson Cancer Center who received bevacizumab for radiation necrosis. Reductions in contrast enhancement and FLAIR abnormalities were seen on imaging and correlated with reductions in steroid requirements (5). Similarly, at Beth Israel Deaconess Medical Center in Boston, one patient with temporal lobe necrosis after treatment for nasopharyngeal carcinoma was treated with bevacizumab (6). She had reduction of enhancement on MRI along with neurocognitive improvement.
There is less experience with bevacizumab in the pediatric population, but it appears to be very well tolerated in early clinical trials (14, 15). These two trials treated a total of 36 children with refractory or recurrent solid tumors, including brain tumors. Side effects included hematologic toxicity, abnormal liver function tests, hypertension, epistaxis, nausea, weight loss, mucositis, cough, hematuria, proteinuria, poor wound healing, erythema and rash. There were no grade III or higher toxicities seen.
We similarly found bevacizumab was very well tolerated. Our patients reported no additional toxicity related to bevacizumab. Three of the four children were able to discontinue steroids and had significant clinical improvement in neurologic symptoms caused by radiation necrosis. The fourth child did not respond to bevacizumab and was unable to wean off steroids, but in retrospect appeared to have progressive disease.
It is difficult to distinguish between treatment related necrosis and disease progression with conventional imaging (16, 17). Our experience suggests that bevacizumab can be both therapeutic and diagnostic for radiation necrosis. Children who do not respond to bevacizumab therapy may have progressive disease rather than radiation necrosis. Even in patients with such a poor prognosis, this distinction is important for the family and allows consideration of additional therapy or enrollment on phase I trials.
With this very small number of children, it is not possible to make definitive treatment recommendations. However, the results are promising and suggest that bevacizumab provides symptom relief from radiation necrosis with minimal toxicity. For children with diffuse pontine gliomas, treatment is essentially palliative and quality of life is of paramount concern. Based on this initial cohort of children treated, we are planning on a more formal trial to study the safety and efficacy of bevacizumab in these children.
Footnotes
Conflicts of Interest Notification:
The authors have no conflict of interest.
References
- 1.Packer RJ, Boyett JM, Zimmerman RA, et al. Hyperfractionated radiation therapy (72 Gy) for children with brain stem gliomas. A Childrens Cancer Group Phase I/II Trial. Cancer. 1993;72:1414–1421. doi: 10.1002/1097-0142(19930815)72:4<1414::aid-cncr2820720442>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 2.Freeman CR, Krischer JP, Sanford RA, et al. Final results of a study of escalating doses of hyperfractionated radiotherapy in brain stem tumors in children: a Pediatric Oncology Group study. Int J Radiat Oncol Biol Phys. 1993;27:197–206. doi: 10.1016/0360-3016(93)90228-n. [DOI] [PubMed] [Google Scholar]
- 3.Mandell LR, Kadota R, Freeman C, et al. There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a Pediatric Oncology Group phase III trial comparing conventional vs. hyperfractionated radiotherapy. Int J Radiat Oncol Biol Phys. 1999;43:959–964. doi: 10.1016/s0360-3016(98)00501-x. [DOI] [PubMed] [Google Scholar]
- 4.Korones DN, Fisher PG, Kretschmar C, et al. Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: a Children’s Oncology Group phase II study. Pediatr Blood Cancer. 2008;50:227–230. doi: 10.1002/pbc.21154. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez J, Kumar AJ, Conrad CA, et al. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67:323–326. doi: 10.1016/j.ijrobp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Wong ET, Huberman M, Lu XQ, et al. Bevacizumab reverses cerebral radiation necrosis. J Clin Oncol. 2008;26 doi: 10.1200/JCO.2008.19.1866. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Nelson MD, Jr, Soni D, Baram TZ. Necrosis in pontine gliomas: radiation induced or natural history? Radiology. 1994;191:279–282. doi: 10.1148/radiology.191.1.8134588. [DOI] [PubMed] [Google Scholar]
- 8.Packer RJ, Zimmerman RA, Kaplan A, et al. Early cystic/necrotic changes after hyperfractionated radiation therapy in children with brain stem gliomas. Data from the Childrens Cancer Group. Cancer. 1993;71:2666–2674. doi: 10.1002/1097-0142(19930415)71:8<2666::aid-cncr2820710836>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 9.Bartholdi D, Rubin BP, Schwab ME. VEGF mRNA induction correlates with changes in the vascular architecture upon spinal cord damage in the rat. Eur J Neurosci. 1997;9:2549–2560. doi: 10.1111/j.1460-9568.1997.tb01684.x. [DOI] [PubMed] [Google Scholar]
- 10.Tsao MN, Li YQ, Lu G, et al. Upregulation of vascular endothelial growth factor is associated with radiation-induced blood-spinal cord barrier breakdown. J Neuropathol Exp Neurol. 1999;58:1051–1060. doi: 10.1097/00005072-199910000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Li YQ, Ballinger JR, Nordal RA, et al. Hypoxia in radiation-induced blood-spinal cord barrier breakdown. Cancer Res. 2001;61:3348–3354. [PubMed] [Google Scholar]
- 12.Nordal RA, Nagy A, Pintilie M, et al. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin Cancer Res. 2004;10:3342–3353. doi: 10.1158/1078-0432.CCR-03-0426. [DOI] [PubMed] [Google Scholar]
- 13.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bender JL, Adamson PC, Reid JM, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children’s Oncology Group Study. J Clin Oncol. 2008;26:399–405. doi: 10.1200/JCO.2007.11.9230. [DOI] [PubMed] [Google Scholar]
- 15.Benesch M, Windelberg M, Sauseng W, et al. Compassionate use of bevacizumab (Avastin) in children and young adults with refractory or recurrent solid tumors. Ann Oncol. 2008;19:807–813. doi: 10.1093/annonc/mdm510. [DOI] [PubMed] [Google Scholar]
- 16.Kumar AJ, Leeds NE, Fuller GN, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217:377–384. doi: 10.1148/radiology.217.2.r00nv36377. [DOI] [PubMed] [Google Scholar]
- 17.Mullins ME, Barest GD, Schaefer PW, et al. Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR Am J Neuroradiol. 2005;26:1967–1972. [PMC free article] [PubMed] [Google Scholar]