Introduction
Evidence garnered from genetic,(Hollingworth et al., 2007; Sweet et al., 2010; Sweet et al., 2002) longitudinal (below), and imaging (below) studies of Alzheimer’s disease (AD) complicated by a psychotic course (AD+P) support its syndromal distinction from its non-psychotic counterpart (AD−P); very recent work on the neurobiology of the disorder point towards frontal systems deficits and an overrepresentation of one of the pathological protein hallmarks of the disease as potentially etiologically related to the development of psychosis. The identification of a specific neuropathological etiology of psychosis could pave the way to new treatment approaches for a condition that currently relies on the use of antipsychotic medication that have been reported to contribute to an exacerbation of cognitive impairment,(Schneider et al., 2006; Vigen et al., 2011) and that carry a ‘black box warning’ for use in the elderly with AD. (FDA, 2005)
Converging evidence suggests that AD+P may represent a relative acceleration of frontal pathology, with tau pathology playing a primary role. AD+P can be distinguished from AD−P in its association with a greater burden of cognitive impairment and a more rapid cognitive decline, as concluded by a review of 55 studies of AD+P. (Ropacki and Jeste, 2005) This association has subsequently been confirmed in more recent studies (Sweet et al., 2010; Sweet et al., 2012; Wilkosz et al., 2010); the decline has consistently been found to predate the onset of psychosis, suggesting a pathological process that finds expression in both deterioration and psychosis. (Emanuel et al., 2011; Paulsen et al., 2000; Weamer et al., 2009) The contribution of neurodegenerative pathology to the psychotic course of AD is suggested by a hastened mortality. (Lopez et al., 2013; Scarmeas et al., 2005; Wilson et al., 2005) The constellation of aggravated cognitive deficits in AD+P suggests disruption of frontal systems (Jeste et al., 1992; Paulsen et al., 2000) with working memory tasks particularly affected. (Koppel et al., 2012; Koppel et al., 2013c; Murray et al., 2013a) Computer-assisted tomography; magnetic resonance imaging; single-photon emission computed tomography; and [18F]-Fluorodexyglucose Positron Emission Tomography studies of AD+P also consistently but not exclusively implicate frontal systems. (Murray et al., 2013b)
A number of reports suggest that tau may be a unique contributor to the putative neurodegenerative process in AD+P. Neurofibrillary tangles (NFTs) are one of the pathological hallmarks of AD whose distribution correlates with severity of disease. (Arriagada et al., 1992) Tau is abnormally phosphorylated in AD, a critical event leading to abnormal folding and cleavage with aggregation of the protein and the eventual development of NFT pathology. (Goedert, 1993; Mondragon-Rodriguez et al., 2008) Tau is hyperphosphorylated in AD at 19 specific amino acid sequences, with evidence indicating that phosphorylation at particular sites correlate with the maturity of pathology and that individual sites may be critical for conformational changes from pre-tangles leading to neuronal tangles to eventual extracellular tangles. (Augustinack et al., 2002; Kimura et al., 1996) Previous studies have reported an increased burden of NFT pathology in frontal cortex in AD+P, although these studies have relied on semi-quantitative transformation of histochemical detection of fibrillar tau. (Farber et al., 2000; Zubenko et al., 1991) A recent neuropathological correlation study of prefrontal cortical sections from a post-mortem AD+P sample used quantitative fluorescence microsopy and observed an increased burden of intraneuronal tau phosphorylated at Ser199/Ser202/Thr205. (Murray et al., 2013a) In a study conducted on a data sample drawn from the Alzheimer’s Disease Neuroimaging Initiative (ADNI), cerebrospinal fluid (CSF) biomarkers of AD (total tau, Thr181 phospho-tau, and Aβ) were explored as predictors of the development of psychosis. Elevation of total tau was associated with the future development of AD+P. (Koppel et al., 2013a) An association of CSF total and Thr181 phospho-tau with gender was also reported; when the genders were separated, females- but not males-with subsequent development of AD+P manifested baseline elevations of Thr181 phosho-tau and total tau.
No previous studies have used a biochemical approach to precisely quantify concentrations of pathogenic tau in brains of those with AD+P, or explored whether particular tau phosphorylation sites predominate in AD+P that could distinguish it from AD−P. In order to quantify tau abnormalities in the frontal cortex in AD+P across a range of phosphorylation sites, and to explore the impact of gender on those abnormalities, we employed a sensitive biochemical assay of 4 epitopes of phospho-tau (Ser396/404; Ser202;Thr231; Ser199/202/Thr205) utilizing monoclonal antibodies known to recognize a wide range of tau pathology in a post-mortem sample of AD+P and AD−P.
Materials and Methods
All subjects (Table 1) underwent neurologic, neuropsychologic, and psychiatric diagnostic evaluations as part of their participation in the Clinical Core of the Alzheimer Disease Research Center (ADRC) at the University of Pittsburgh. (Murray et al.; Sweet et al., 2001; Sweet et al., 2000) Psychiatrists with specialized training in geriatric psychiatry conducted semi-structured examinations, and the patient, primary caregiver, and all other available informants were interviewed. As part of the semi-structured examinations, the presence or absence of hallucinations and delusions was determined and rated on the Consortium to Establish a Registry for Alzheimer’s disease (CERAD) Behavioral Rating Scale. (Tariot et al., 1995) Hallucinations were defined as sensory perceptions with no basis in reality. Delusions were defined as a false belief based on incorrect inference about reality and not attributable to membership in a social or cultural group. Psychosis was the presence of hallucinations or delusions at any visit. Subjects with psychosis occurring solely during an episode of delirium were not rated as AD+P. No patient had a history of schizophrenia, schizoaffective disorder, or other idiopathic psychosis. Years of death ranged between 2000 to 2011.
Table 1.
Demographics of AD subjects with psychosis (AD+P, N=45) and without psychosis (AD−P, N=26). Means (standard deviation) shown.
| Total Sample | ||
|---|---|---|
| AD + P | AD − P | |
| Male | 22 | 13 |
| Female | 23 | 13 |
| Age | 82.9 (1.0) | 83.4 (1.8) |
| Braak | 4.9 (0.1) | 4.9 (0.2) |
|
| ||
| Females | ||
| Age | 83.5 (1.3) | 87 (2.4) |
| Braak | 5.1 (0.1) | 4.6 (0.3) |
|
| ||
| Males | ||
| Age | 82.2 (0.7) | 79.9 (2.4) |
| Braak | 4.7 (0.2) | 5.1 (0.2) |
A neuropathologic diagnosis of AD was made at postmortem examination. (Mirra et al., 1991) At the time of brain removal, the post mortem interval in hours was recorded, and the brain was removed intact, examined grossly, and divided in the midsagittal plane. Gray matter samples from the right superior frontal gyrus were dissected and frozen at −80 °C. The left hemibrain was immersion fixed in 10% buffered formalin for at least one week, sectioned into 1.0–2.0 cm coronal slabs, and sampled according to CERAD protocol for neuropathological diagnosis. (Mirra et al., 1991) Samples from the midfrontal cortex, cingulate gyrus and caudate nucleus, hippocampus at the level of the lateral geniculate nucleus, inferior parietal lobule, superior and middle temporal cortex, primary visual cortex, amygdala, and transentorhinal cortex were stained using the Bielschowsky technique according to the Yamamoto and Hirano modification. (Yamamoto and Hirano, 1986) Samples from amygdala, midbrain, pons and medulla were immunostained for ubiquitin and α-synuclein, and Lewy body pathology was rated. (McKeith et al., 1996) If any of these sections were positive, sections from cortex, hippocampus, and remaining brainstem were also stained for α-synuclein. Neuritic plaques were semiquantiatively scored according to CERAD criteria;(Mirra et al., 1991) plaque density per 100x microscopy field was rated as “none,” “sparse,” “moderate,” or “frequent,” with most subjects scored as “frequent”. Braak staging was done on Bielschowsky stained sections of mesial temporal lobe (hippocampus with entorhinal, transentorhinal and inferior temporal lobe cortex), mid-frontal, inferior parietal, superior temporal gyri and occipital lobe containing the primary visual cortex and adjacent cortex. (Braak and Braak, 1991)
Brain Sample Preparations/Tau Sandwich ELISA
Previous studies have suggested that accelerated frontal neurodegeneration may be a feature of the pathogenic process in psychotic AD. (Koppel et al., 2013c; Murray et al., 2013a) For this reason, the current study focused on frontal cortex. Brain samples of superior frontal gyrus drawn from 26 AD−P subjects and 45 AD+P subjects. There were 35 males and 36 females included in the study. AD+P and AD−P groups were well balanced for gender, age at death, and post-mortem Braak staging (Table 1). The Braak staging system (I–VI) is based on the gradual appearance of neurofibrillary tangle pathology in a hierarchical pattern beginning in the perirhinal cortex (Stage I) in preclinical disease and eventually appearing to all areas of association cortex in severe disease (Stage VI). (Braak and Braak, 1995) The approximate equivalence of Braak stage between AD+P and AD−P sample groups assures that the groups are well matched for global neuropathological severity and allows for an exploration of the regional specificity of tau protein pathology in AD+P.
In order to quantify levels of tau in the cortex across a broad spectrum of phospho-tau epitopes implicated in AD, a series of sandwich ELISAs employing monoclonal antibodies were performed in the AD+P and AD−P groups. A level of total tau was measured with DA31; Ser396/404 phospho-tau known to be well represented in early and late Braak stages and present in a spectrum from early to mature tau aggregates (Mondragon-Rodriguez et al., 2013) was quantified with PHF-1; Ser202 phospho-tau was quantified with CP13, an antibody that detects early neuritic pathology(Janocko et al., 2012); Thr231phospho-tau, a conformational epitope often phosphorylated even in pre-tangles (Augustinack et al., 2002) was quantified with RZ3; and Ser199/202/Thr205 phospho-tau, represented in more severe cases, (Augustinack et al., 2002) was quantified with AT8.
Preparation of brain samples and ELISA were performed according to previously published methods. (Acker et al., 2013)
Briefly, the brain regions were extracted and homogenized in a 10x volume of homogenizing buffer, a solution of Tris-buffered saline (TBS), pH 7.4 containing 10mM NaF, 1mM NaVO3, and 2mM EGTA, including a complete Mini protease inhibitor (Roche). Samples were stored in aliquots at −80°C until use. Prior to use, homogenates were thawed and spun at 14,000 g for 10 min at 4°C. Brain homogenate samples were diluted in 20% SuperBlock in 1X TBS (ThermoScientific) and processed as previously described. (Acker et al., 2013)
Quantification of tau levels were analyzed by sandwich ELISA measurements. DA31 (aa150–190), PHF1 (pSer396/404), CP13 (pSer202), and RZ3 (pThr231) and AT8 (pSer199/p202/pThr205) at a concentration of 6 μg/ml were used as the capture antibody for four separate ELISA measurements. DA9-HRP (aa102–140) at a concentration of 0.3 μg/ml was used as the detection antibody for all assays. Tau sandwich ELISA was performed as already published 96-well plates were respectively coated with DA31, CP13 at a final concentration of 6 mg/ml in coating buffer, for at least 48 h at 4uC. After washing 3X in wash buffer, the plates were blocked for 1 h at room temperature using StartingBlock (Thermoscientific) to avoid non-specific binding. Each plate was then washed 5X and 50 ml of the appropriate sample was added to the wells. Concurrently, 50 ml of DA9-HRP detection antibody was added to the samples and tapped to combine. Plates were incubated O/N shaking at 4uC and then washed 9X in wash buffer. 1-Step ULTRA TMB-ELISA (Thermoscientific) was added for 30 minutes at room temperature before stopping the reaction with 2 M H2SO4. Plates were read with an Infinite m200 plate reader (Tecan) at 450 nm
Statistics
The current study was designed around a convenience sample of well characterized pre-mortem and post-mortem pathology in subjects with AD+P and AD−P with available frontal cortical tissue for tau analysis. As such, a power analysis for sample size calculation was not conducted. Parametric (two-way ANOVA, Student t) and non-parametric (Chi-squared) tests of demographic independent and dependent outcome variables were employed, with an alpha risk set at 0.05. As total tau was not anticipated to differ between groups, while phospho-tau species were expected to differ, these analyses were separated. Differences in levels of total tau (DA31) between AD+P and AD−P groups were compared with a Student’s t-test. For levels of phosphotau, the entire panel of epitopes (PHF-1; CP13; RZ3; AT8) in AD+P and AD−P were first compared with a two-way ANOVA to evaluate differences between the groups, followed by individual two-tailed Student’s t-test within each epitope.
Results
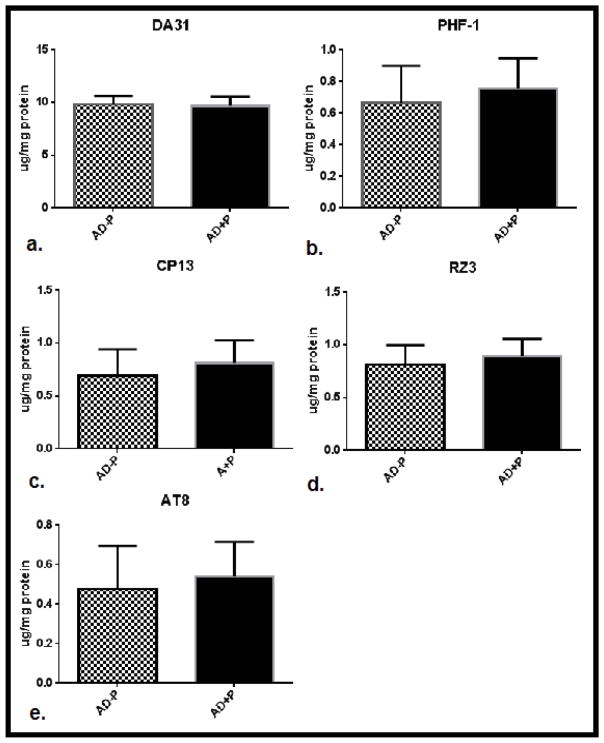
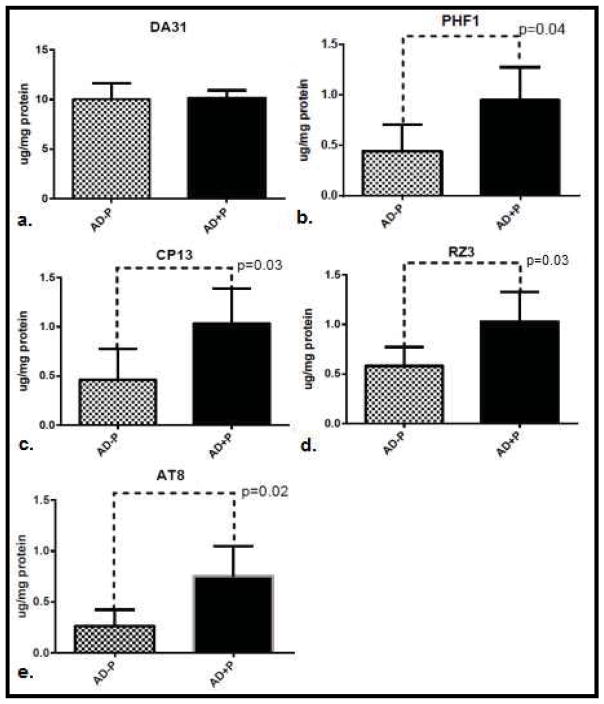
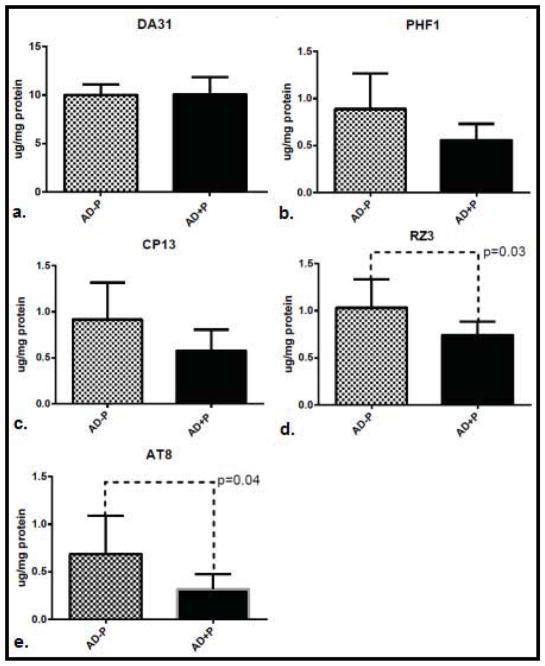
In the total sample, levels of phospho-tau did not differ between psychotic (AD+P) and non-psychotic (AD−P) groups (Figure 1.) A post-hoc analysis of evidence from a previously published report of CSF biomarkers in AD+P suggest that while total tau and Thr181 phosphotau are elevated in females with AD+P, males with AD+P may not manifest these differences. (Koppel et al., 2013b) For this reason, we divided our sample by gender. This analysis included 13 AD−P and 23 AD+P female subjects; 13 AD−P and 22 AD+P male subjects. Both gender groups were well balanced for age at death, with Braak staging reflecting slightly more extensive tangle pathology in females with AD+P, and slightly less tangle pathology in males with AD+P (Table 1). In females, levels of non-pathogenic total tau tau did not differ between AD+P and AD−P groups. However, pathogenic phospho-tau species did differ significantly between groups (F [1,136] = 20.56, p<0.0001). AD+P females had significantly higher levels of phosphorylated tau than AD−P females at every epitope interrogated: Ser396/404; Ser202; Thr231; and Ser199/202/Thr205 (Figure 2). This suggests a broad range of pre-tangle, neuritic, and mature tangle pathology in the frontal cortex of AD+P female. In males, as in females, levels of non-pathogenic total tau did not differ between groups. Levels of pathogenic phospho-tau species did differ between groups, although in an opposite direction from females (F [1,132] =14.94, p=0.0002). Unexpectedly, levels of Thr231 and Ser199/202/Thr205 were statistically significantly higher in AD−P males when compared with AD+P males, an trend of inversion of what was observed in females; levels of Ser202 and Ser396/404 followed the same trend, but were not statistically significantly different (Figure 3).
Figure 1.
Mean levels of total tau (a.) and hyperphosphorylated tau: PHF1 (pSer396/404), CP13 (pSer202), RZ3 (pThr231) and AT8 (pSer199/p202/pThr205) (b.–e.) in superior frontal gyri of subjects with AD without psychosis (AD−P, N=26) compared with AD and psychosis (AD+P, N=45), 95% confidence intervals are represented.
Figure 2.
Mean levels of total tau (a.) and hyperphosphorylated tau: PHF1 (pSer396/404), CP13 (pSer202), RZ3 (pThr231) and AT8 (pSer199/p202/pThr205) (b.–e.) in superior frontal gyri of females without psychosis (AD−P, N=13) compared with AD and psychosis (AD+P, N=23). 95% confidence intervals are represented and significant individual differences (p<0.05) are highlighted.
Figure 3.
Mean levels of total tau (a.) and hyperphosphorylated tau: PHF1 (pSer396/404), CP13 (pSer202), RZ3 (pThr231) and AT8 (pSer199/p202/pThr205 (b.–e.) in superior frontal gyri of males with AD without psychosis (AD−P, N=13) compared with AD and psychosis (AD+P, N=22). 95% confidence intervals are represented and significant individual differences (p<0.05) are highlighted.
The gender-specific inversion of risk modification was not predicted. In order to explore what was driving psychosis in males, the impact of α-synuclein pathology on psychosis risk was explored in males and females. Psychosis-canonically expressed in visual hallucinations but often including delusions- is a core diagnostic feature of dementia with Lewy bodies (DLB), a disease of α-synuclein pathology. (McKeith, 2006) In fact, the early presence of psychosis is more predictive of DLB than either of its other core features: parkinsonism or fluctuating cognition. (Tiraboschi et al., 2006) In order to explore the contribution of α-synuclein pathology to gender stratified psychosis risk, the sample was divided into a group with and without Lewy body pathology anywhere in the brain at post-mortem examination. 36 subjects had Lewy body pathology and 35 subjects did not. In females, 14/23 AD+P subjects had Lewy body pathology, compared with 6/13 AD−P subjects (Χ2=0.7, p=0.4). In males, 13/22 AD=P subjects had Lewy body pathology, compared with only 3/13 AD−P subjects (Χ2=4.3=, p=0.04). This suggests that while tau pathology is the critical molecular modifier of psychosis risk in females with AD, α-synuclein may play an analogous role in males.
Discussion
The identification of accelerated decline and early mortality in AD+P necessitates an explication of the mechanism responsible for the divergent path this syndrome takes. From the standpoint of brain topography, it seems clear from imaging studies that the frontal cortex plays a unique role in AD+P. However, the molecular biology of the frontal pathogenic process has yet to be elucidated. The starting point for any such exploration should begin with the known histological hallmarks of AD, amyloid-beta (Aβ) and tau protein pathology. Indeed, previous studies have explored both pathologies. While results of investigations correlating regional Aβ plaque deposition with AD+P have been largely negative, similar approaches focused on tau pathology have been generally positive. (Murray et al., 2013b) The first study to explore neuropathological relationships with psychosis in AD demonstrated increased neurofibrillary tangle pathology in the middle frontal cortex of subjects with AD+P. (Zubenko et al., 1991) Two subsequent studies of similar design were split in reporting an increased burden of tangle pathology in AD+P. (Farber et al., 2000; Sweet et al., 2000) Taken together, the preponderance of evidence from semi-quantitative tangle burden association studies does suggest a risk relationship between tau pathology and psychosis in AD.
Results of the current quantitative tau pathology report suggest that while alterations in tau pathology are a feature of AD+P, those alterations are gender-specific. Previous neuropathology studies have not divided samples by gender. However, female gender has been reported in several studies to be an independent risk factor for psychosis in AD. (Hirono et al., 1998; Leroi et al., 2003; Rockwell et al., 1994; Schneider and Dagerman, 2004) Recent imaging studies have explored the biologic correlates of gender-based psychosis risk in AD, and implicate frontal involvement in women only. A magnetic resonance imaging study in AD+P demonstrated reduced frontotemporal cortical thickness in women, but not men, with AD+P. (Whitehead et al., 2012) A voxel-based analysis of whole brain cerebral perfusion was undertaken in a large sample of AD+P (N=51) and AD−P (N=52) subjects. (Moran et al., 2008) In this study, perfusion patterns were vastly different between AD+P females and AD+P males. In female AD+P subjects, there was a marked hypoperfusion in right infero-lateral prefrontal cortex and right inferior temporal region compared with controls. In males, AD+P correlated with an increased perfusion in the right striatum compared with controls. Of note, these deficits were non-overlapping and represent what the authors describe as a “double dissociation of gender and cerebral function,” and suggest that localized pathology contributing to behavioral abnormalities may have gender specificity. (Moran et al., 2008)
The results of this report proved further evidence of a potential “double dissociation” of gender and pathophysiology in AD+P. In the current sample, females with AD+P had an increased burden of phosphotau evident in the superior frontal gyrus at all four epitopes interrogated compared with female AD−P subjects. Inversely, males with AD+P did not have of evidence of this burden, and appear to have a relative deficit of phosphotau. The results from the females are consistent with the concatenation of evidence pointing to a frontal deficit in AD+P. This may represent a focal frontal-superimposed on a necessary but cortically diffuse-neurodegenerative process. The elevation of concentrations of phospho-tau across a broad range of epitopes, together with the lack of specificity of tau kinases for any particular epitope (Augustinack et al., 2002) preclude a search for a responsible kinase. Whether accelerated tangle pathology is the pathogenic driver of psychosis in AD females, with a concomitant but accidental impact on frontal brain function, or whether frontal impairment is pathogenic, with tangle pathology as a convenient source, is an important question.
In the males, while a lack of hyper-phosphorylation in AD+P could be understood as an issue of regional, gender-based specificity in a study that only looked at one region, the inversion of the risk relationship with tau requires elucidation. Males with AD+P were more likely to have α-synuclein pathology, while females were not. This suggests that while tau driven neurodegeneration of frontal cortex may be a risk factor for psychosis in females, α-synuclein pathology, known to be associated with psychosis in DLB, may be risk factor in males. The relative deficit of phosphotau in the superior fontal gyrus males with AD+P suggests that preservation of integrity in this region is a risk factor for psychosis. In a previous study correlating delusional severity with regional brain metabolism in a sample of 24 men and 1 woman, increased metabolism in the right lateral orbitofrontal region (Brodmann’s area 47) was associated with a greater severity of delusions, while other regional associations with delusional severity were in the direction of deficient metabolism. (Sultzer et al., 2003) If true, this would suggest that tangle pathology itself is not the causative agent in psychotic AD, but that the complex relationship of frontal systems with other brain regions, altered in AD, may be the critical mediator of the emergence of psychosis.
In synthesizing the available data, we suggest a new model for exploring the neurobiology of AD+P. In this model, regional networks of altered brain functions that include areas of relative deficit and preservation driven by the topography of pathology- whether tau or α-synuclein- may be responsible for psychosis in AD; these may be gender-specific risk patterns. If so, future research in psychotic AD exploring the impact of regional brain dysfunction and its potential gender-specific correlation with tau or α-synuclein pathology would be of particular interest. The recently reported positive developments in tau imaging technology in PET scanning (Chien et al., 2014) may afford the opportunity for those studies to move forward. This model may also help to guide future studies seeking to identify genetic variants responsible for AD+P; indeed, the heritability of AD+P has been estimated at 61%,(Sweet et al., 2010) yet despite identifying a number of candidate genes, a meta-analysis of three combined genome-wide association datasets including over 7,500 subjects did not ascertain any genetic polymorphisms that achieved threshold statistical significance. (Hollingworth et al., 2012) One potential explanation for this may involve gender-differentiated effects that have not been explored in these datasets, in which the magnitude or even direction of genetic effect could differ based on gender. (Magi et al., 2010) Future analyses employing male-specific, female-specific, or gender-differentiated approaches (Magi et al., 2010) may help to bridge the gap between heritability estimates and gene identification.
Acknowledgments
This work was supported by the Veterans Health Administration [BX000452 to R.A.S] and the National Institute on Aging [AG005133 to O.L.L., AG027224 to R.A.S.].
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs, the National Institutes of Health, or the United States Government.
No authors have any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acker CM, et al. Sensitive quantitative assays for tau and phospho-tau in transgenic mouse models. Neurobiol Aging. 2013;34:338–50. doi: 10.1016/j.neurobiolaging.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada PV, et al. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–9. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Augustinack JC, et al. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 2002;103:26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropahological staging of Alzheimer-related changes. Acta Neuropath Appl Neurobiol. 1991;14:39–44. [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–8. doi: 10.1016/0197-4580(95)00021-6. discussion 278–84. [DOI] [PubMed] [Google Scholar]
- Chien DT, et al. Early Clinical PET Imaging Results with the Novel PHF-Tau Radioligand [F18]-T808. J Alzheimers Dis. 2014;38:171–84. doi: 10.3233/JAD-130098. [DOI] [PubMed] [Google Scholar]
- Emanuel JE, et al. Trajectory of cognitive decline as a predictor of psychosis in early Alzheimer disease in the cardiovascular health study. Am J Geriatr Psychiatry. 2011;19:160–8. doi: 10.1097/JGP.0b013e3181e446c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber NB, et al. Increased neocortical neurofibrillary tangle density in subjects with Alzheimer disease and psychosis. Arch Gen Psychiatry. 2000;57:1165–73. doi: 10.1001/archpsyc.57.12.1165. [DOI] [PubMed] [Google Scholar]
- FDA. Deaths with antipsychotics in elderly patients with behavioral disturbances. Public Health Advisory 2005 [Google Scholar]
- Goedert M. Tau protein and the neurofibrillary pathology of Alzheimer’s disease. Trends Neurosci. 1993;16:460–5. doi: 10.1016/0166-2236(93)90078-z. [DOI] [PubMed] [Google Scholar]
- Hirono N, et al. Factors associated with psychotic symptoms in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1998;64:648–52. doi: 10.1136/jnnp.64.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P, et al. Increased familial risk and genomewide significant linkage for Alzheimer’s disease with psychosis. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:841–8. doi: 10.1002/ajmg.b.30515. [DOI] [PubMed] [Google Scholar]
- Hollingworth P, et al. Genome-wide association study of Alzheimer’s disease with psychotic symptoms. Mol Psychiatry. 2012 doi: 10.1038/mp.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janocko NJ, et al. Neuropathologically defined subtypes of Alzheimer’s disease differ significantly from neurofibrillary tangle-predominant dementia. Acta Neuropathol. 2012;124:681–92. doi: 10.1007/s00401-012-1044-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, et al. Cognitive deficits of patients with Alzheimer’s disease with and without delusions. Am J Psychiatry. 1992;149:184–9. doi: 10.1176/ajp.149.2.184. [DOI] [PubMed] [Google Scholar]
- Kimura T, et al. Sequential changes of tau-site-specific phosphorylation during development of paired helical filaments. Dementia. 1996;7:177–81. doi: 10.1159/000106875. [DOI] [PubMed] [Google Scholar]
- Koppel J, et al. Relationships Between Behavioral Syndromes and Cognitive Domains in Alzheimer Disease: The Impact of Mood and Psychosis. Am J Geriatr Psychiatry. 2012;20:994–1000. doi: 10.1097/JGP.0b013e3182358921. [DOI] [PubMed] [Google Scholar]
- Koppel J, et al. Elevated CSF Tau is Associated with Psychosis In Alzheimer’s Disease. The American Journal of Psychiatry. 2013a doi: 10.1176/appi.ajp.2013.13040466. In press. [DOI] [PubMed] [Google Scholar]
- Koppel J, et al. Elevated CSF Tau is Associated With Psychosis in Alzheimer’s Disease. Am J Psychiatry. 2013b;170:1212–3. doi: 10.1176/appi.ajp.2013.13040466. [DOI] [PubMed] [Google Scholar]
- Koppel J, et al. Psychosis in Alzheimer’s Disease Is Associated with Frontal Metabolic Impairment and Accelerated Decline in Working Memory: Findings From the Alzheimer’s Disease Neuroimaging Initiative. The American Journal for Geriatric Psychiatry. 2013c doi: 10.1016/j.jagp.2012.10.028. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Leroi I, et al. The epidemiology of psychosis in dementia. Am J Geriatr Psychiatry. 2003;11:83–91. [PubMed] [Google Scholar]
- Lopez OL, et al. The long-term effects of conventional and atypical antipsychotics in patients with probable Alzheimer’s disease. Am J Psychiatry. 2013;170:1051–8. doi: 10.1176/appi.ajp.2013.12081046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magi R, et al. Meta-analysis of sex-specific genome-wide association studies. Genet Epidemiol. 2010;34:846–53. doi: 10.1002/gepi.20540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9:417–23. doi: 10.3233/jad-2006-9s347. [DOI] [PubMed] [Google Scholar]
- McKeith IG, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–24. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- Mirra SS, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mondragon-Rodriguez S, et al. Cleavage and conformational changes of tau protein follow phosphorylation during Alzheimer’s disease. Int J Exp Pathol. 2008;89:81–90. doi: 10.1111/j.1365-2613.2007.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondragon-Rodriguez S, et al. Phosphorylation of tau protein at sites Ser is one of the earliest events in Alzheimer’s disease and Down syndrome. Neuropathol Appl Neurobiol. 2013 doi: 10.1111/nan.12084. [DOI] [PubMed] [Google Scholar]
- Moran EK, et al. Psychosis of Alzheimer’s disease: Gender differences in regional perfusion. Neurobiol Aging. 2008;29:1218–25. doi: 10.1016/j.neurobiolaging.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Murray PS, et al. Hyperphosphorylated Tau is Elevated in Alzheimer’s Disease with Psychosis. J Alzheimers Dis. 2013a doi: 10.3233/JAD-131166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PS, et al. beta-Amyloid 42/40 ratio and kalirin expression in Alzheimer disease with psychosis. Neurobiol Aging. 33:2807–16. doi: 10.1016/j.neurobiolaging.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PS, et al. Psychosis in Alzheimer’s Disease. Biol Psychiatry. 2013b doi: 10.1016/j.biopsych.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, et al. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology. 2000;54:1965–71. doi: 10.1212/wnl.54.10.1965. [DOI] [PubMed] [Google Scholar]
- Rockwell E, et al. Late-onset psychosis with somatic delusions. Psychosomatics. 1994;35:66–72. doi: 10.1016/S0033-3182(94)71809-0. [DOI] [PubMed] [Google Scholar]
- Ropacki SA, Jeste DV. Epidemiology of and risk factors for psychosis of Alzheimer’s disease: a review of 55 studies published from 1990 to 2003. Am J Psychiatry. 2005;162:2022–30. doi: 10.1176/appi.ajp.162.11.2022. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62:1601–8. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LS, et al. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry. 2006;14:191–210. doi: 10.1097/01.JGP.0000200589.01396.6d. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Dagerman KS. Psychosis of Alzheimer’s disease: clinical characteristics and history. J Psychiatr Res. 2004;38:105–11. doi: 10.1016/s0022-3956(03)00092-x. [DOI] [PubMed] [Google Scholar]
- Sultzer DL, et al. Delusional thoughts and regional frontal/temporal cortex metabolism in Alzheimer’s disease. Am J Psychiatry. 2003;160:341–9. doi: 10.1176/appi.ajp.160.2.341. [DOI] [PubMed] [Google Scholar]
- Sweet RA, et al. Assessment and familial aggregation of psychosis in Alzheimer’s disease from the National Institute on Aging Late Onset Alzheimer’s Disease Family Study. Brain. 2010;133:1155–62. doi: 10.1093/brain/awq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, et al. Alterations of striatal dopamine receptor binding in Alzheimer disease are associated with Lewy body pathology and antemortem psychosis. Arch Neurol. 2001;58:466–72. doi: 10.1001/archneur.58.3.466. [DOI] [PubMed] [Google Scholar]
- Sweet RA, et al. Psychotic symptoms in Alzheimer’s disease are not associated with more severe neuropathologic features. Int Psychogeriatr. 2000;12:547–58. doi: 10.1017/s1041610200006657. [DOI] [PubMed] [Google Scholar]
- Sweet RA, et al. Increased familial risk of the psychotic phenotype of Alzheimer disease. Neurology. 2002;58:907–11. doi: 10.1212/wnl.58.6.907. [DOI] [PubMed] [Google Scholar]
- Sweet RA, et al. Effect of Alzheimer’s disease risk genes on trajectories of cognitive function in the Cardiovascular Health Study. Am J Psychiatry. 2012;169:954–62. doi: 10.1176/appi.ajp.2012.11121815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariot PN, et al. The Behavior Rating Scale for Dementia of the Consortium to Establish a Registry for Alzheimer’s Disease. The Behavioral Pathology Committee of the Consortium to Establish a Registry for Alzheimer’s Disease. Am J Psychiatry. 1995;152:1349–57. doi: 10.1176/ajp.152.9.1349. [DOI] [PubMed] [Google Scholar]
- Tiraboschi P, et al. What best differentiates Lewy body from Alzheimer’s disease in early-stage dementia? Brain. 2006;129:729–35. doi: 10.1093/brain/awh725. [DOI] [PubMed] [Google Scholar]
- Vigen CL, et al. Cognitive effects of atypical antipsychotic medications in patients with Alzheimer’s disease: outcomes from CATIE-AD. Am J Psychiatry. 2011;168:831–9. doi: 10.1176/appi.ajp.2011.08121844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weamer EA, et al. The relationship of excess cognitive impairment in MCI and early Alzheimer’s disease to the subsequent emergence of psychosis. Int Psychogeriatr. 2009;21:78–85. doi: 10.1017/S1041610208007734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead D, et al. Frontotemporal atrophy associated with paranoid delusions in women with Alzheimer’s disease. Int Psychogeriatr. 2012;24:99–107. doi: 10.1017/S1041610211000974. [DOI] [PubMed] [Google Scholar]
- Wilkosz PA, et al. Trajectories of cognitive decline in Alzheimer’s disease. Int Psychogeriatr. 2010;22:281–90. doi: 10.1017/S1041610209991001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, et al. Hallucinations and mortality in Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:984–90. doi: 10.1176/appi.ajgp.13.11.984. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Hirano A. A comparative study of modified Bielschowsky, Bodian and thioflavin S stains on Alzheimer’s neurofibrillary tangles. Neuropathol Appl Neurobiol. 1986;12:3–9. doi: 10.1111/j.1365-2990.1986.tb00677.x. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, et al. Neuropathologic and neurochemical correlates of psychosis in primary dementia. Arch Neurol. 1991;48:619–24. doi: 10.1001/archneur.1991.00530180075020. [DOI] [PubMed] [Google Scholar]