Abstract
Purpose
We prospectively evaluated the amino acid analogue positron emission tomography radiotracer anti-3-[18F]FACBC compared to ProstaScint® (111In-capromab pendetide) single photon emission computerized tomography-computerized tomography to detect recurrent prostate carcinoma.
Materials and Methods
A total of 93 patients met study inclusion criteria who underwent anti-3-[18F]FACBC positron emission tomography-computerized tomography plus 111In-capromab pendetide single photon emission computerized tomography-computerized tomography for suspected recurrent prostate carcinoma within 90 days. Reference standards were applied by a multidisciplinary board. We calculated diagnostic performance for detecting disease.
Results
In the 91 of 93 patients with sufficient data for a consensus on the presence or absence of prostate/bed disease anti-3-[18F]FACBC had 90.2% sensitivity, 40.0% specificity, 73.6% accuracy, 75.3% positive predictive value and 66.7% negative predictive value compared to 111In-capromab pendetide with 67.2%, 56.7%, 63.7%, 75.9% and 45.9%, respectively. In the 70 of 93 patients with a consensus on the presence or absence of extraprostatic disease anti-3-[18F]FACBC had 55.0% sensitivity, 96.7% specificity, 72.9% accuracy, 95.7% positive predictive value and 61.7% negative predictive value compared to 111In-capromabpendetide with10.0%, 86.7%, 42.9%, 50.0% and 41.9%, respectively. Of 77 index lesions used to prove positivity histological proof was obtained in 74 (96.1%). Anti-3-[18F]FACBC identified 14 more positive prostate bed recurrences (55 vs 41) and 18 more patients with extraprostatic involvement (22 vs 4). Anti-3-[18F]FACBC positron emission tomography-computerized tomography correctly up-staged 18 of 70 cases (25.7%) in which there was a consensus on the presence or absence of extraprostatic involvement.
Conclusions
Better diagnostic performance was noted for anti-3-[18F]FACBC positron emission tomography-computerized tomography than for 111In-capromab pendetide single photon emission computerized tomography-computerized tomography for prostate carcinoma recurrence. The former method detected significantly more prostatic and extraprostatic disease.
Keywords: prostatic neoplasms, tomography, emission-computed, photon, positron-emission tomography, capromab pendetide, 1-amino-3-fluorocyclobutane-1-carboxylic acid
Prostate cancer will develop in 1 of 6 men.1 Therapy may be performed via locally directed interventions, such as radical prostatectomy, brachytherapy, external beam radiotherapy or cryotherapy. However, 30% to 50% of patients experience recurrent disease after definitive local therapy.2
Differentiating local from extraprostatic recurrence is critical since salvage techniques can cure disease confined to the prostate bed. If pelvic nodal involvement is suspected, radiation fields can be extended to include pelvic nodes.3 Systemic disease is treated with hormonal manipulation and/or chemotherapy.
Imaging is central to the differentiation of prostatic from extraprostatic recurrence. Conventional methodology, including CT, MR, transrectal ultrasound and bone scan, have the disadvantage of less than optimal diagnostic performance.4–7 Thus, molecular techniques have been used, including imaging based on an antibody to prostate specific membrane antigen using 111In-capromab pendetide (ProstaScint).7,8
Anti-3-[18F]FACBC is an investigational PET radiotracer being studied for staging and restaging prostate carcinoma.9,10 Anti-3-[18F]FACBC is a synthetic amino acid analogue with little renal excretion. Transport is likely mediated by the sodium dependent and independent amino acid transporters ASCT2 and LAT1, respectively, which are associated with carcinoma signaling pathways, including mTOR.11,12
We recently completed a clinical trial with the primary aim of comparing anti-3-[18F]FACBC to 111In-capromab pendetide for detecting recurrent prostate carcinoma. We prospectively investigated diagnostic performance using similar reference standards, relying on histological verification and longitudinal multiyear followup.
MATERIALS AND METHODS
Patient Selection
This study was approved by the Emory University institutional review board. Studies were done from November 28, 2007 to July 10, 2012 after obtaining written informed consent. No adverse events were reported. Patients were enrolled according to certain inclusion criteria, including 1) an original diagnosis of localized (stage T1c, T2 or T3) prostate carcinoma with subsequent definitive therapy, 2) suspicion of recurrent prostate carcinoma, as defined by the previous ASTRO (American Society for Radiation Oncology) criteria of 3 consecutive PSA increases and/or the more recent ASTRO/Phoenix criteria of nadir PSA greater than 2.0 ng/ml after radiotherapy or cryotherapy and/or greater than 0.2 ng/ml after prostatectomy and 3) bone scan negative for metastatic disease.
A total of 128 scans were performed in 115 patients. If a patient underwent followup anti-3-[18F]FACBC, only the first study was used. Thus, 115 patients were eligible for analysis, of whom 5 did not have 111In-capromab pendetide studies available. Of these 110 remaining patients 93 met study criteria of 111In-capromab pendetide as well as anti-3-[18F]FACBC imaging acquired within 90 days.
Anti-3-[18F]FACBC PET-CT and 111In-Capromab Pendetide Imaging Protocols
The preparation of anti-3-[18F]FACBC under Investigational New Drug Application 72,437 and acquisition protocols were previously reported.10,13 Scanning was done on a Discovery DLS or 690 PET-CT scanner (GE Health-care, Milwaukee, Wisconsin) and interpreted on a MIM-Vista workstation (MIM Software™). Patients fasted for 4 to 6 hours before the anti-3-[18F]FACBC scan.
Anti-3-[18F]FACBC (161.7 to 484.7 MBq) was injected intravenously during 2 minutes. After a 3-minute delay for blood pool clearance abdominopelvic PET-CT was completed with 5 to 16-minute (early), 17 to 28-minute (delayed 1) and 29 to 40-minute (delayed 2) acquisitions. 111In-capromab pendetide imaging was performed via a standard protocol, including whole body planar and abdominopelvic SPECT-CT.14
Criteria for Positive Studies
The anti-3-[18F]FACBC scan was interpreted individually by a nuclear radiologist and a nuclear medicine physician blinded to other imaging and reference validations. Disagreement was resolved by consensus. Abnormal moderate (greater than marrow) focal uptake that deviated from the expected biodistribution and persisted from early to delayed images was interpreted as prospectively positive, as previously reported.10 111In-capromab pendetide was co-interpreted in blinded fashion using well established criteria.15
Clinical and Histological Followup Reference Standards
A multidisciplinary consensus panel composed of a nuclear radiologist, 2 urologists and 2 radiation oncologists met regularly and communicated via e-mail to adjudicate the reference standards for the presence or absence of disease.
The reference standard for the prostate/bed was histological sampling with transrectal ultrasound/biopsy. Absent tissue to biopsy was considered negative. Negative biopsy could be overridden by achieving durable PSA control after salvage therapy to the prostate/bed, thus, proving the presence of disease that was missed at biopsy, for example in patients treated with radical prostatectomy who had no evidence of extraprostatic disease and received prostate bed radiation therapy.
The reference standard for extraprostatic involvement per patient was histological sampling for lymph nodes via image guided needle biopsy and laparoscopic or open lymph node dissection. Inguinal nodes were evaluated by physical examination and biopsied only if suspicious due to the low prevalence of metastatic disease to inguinal nodes.16 For bone involvement, histological proof or a characteristic appearance on no fewer than 2 other imaging studies (MR, CT and/or bone scan) was accepted. For a study to be considered positive there had to be concordance between the reference lesion for disease proof and imaging findings. Similar to verification standard in the prostate/bed, durable PSA control after directed therapy to a lymph node group (with no prostate/bed involvement) was accepted as verification of extraprostatic disease in 1 patient in lieu of biopsy.
Absent extraprostatic disease was confirmed by achieving durable PSA control after prostate/bed salvage therapy with PSA less than 0.2 ng/ml after prostatectomy or less than a PSA nadir of greater than 2 ng/ml in non-prostatectomy cases. In cases of subsequent biochemical failure after salvage therapy and with biopsy negative disease in the prostate bed we conservatively assumed that undetected microscopic disease was present outside the prostate/bed and considered these cases extraprostatic false-negative. In 2 such patients findings on 111In-capromab pendetide were equivocal for extraprostatic disease, thus, were conservatively categorized as positive for 111In-capromab pendetide.
If a patient had decreasing PSA with time in the absence of therapy, it was considered that PSA had originally been increased due to a nonneoplastic cause. If there were yet insufficient data to establish the presence or absence of prostatic or extraprostatic disease at the last followup, the outcome was indeterminate. Similarly, if subsequent extraprostatic involvement may have been secondary to interim seeding from persistent disease in the prostate/bed on a study that was originally extraprostatic negative, these findings were considered indeterminate for extraprostatic diagnostic performance.
Statistical Analysis
We report measures of diagnostic performance for disease detection in the prostate/bed and in extraprostatic tissue, including sensitivity, specificity, PPV, NPV and overall accuracy. We calculated the corresponding exact 95% CI of each accuracy measure as a binomial proportion, shown as (95% CI x, y) after each accuracy estimate. Interobserver agreement was assessed and the κ statistic was calculated.
We determined the statistical significance of differences in sensitivity, specificity and overall accuracy between anti-3-[18F]FACBC PET-CT and 111In-capromab pendetide SPECT-CT using the McNemar chi-square test, which adjusts for correlations in the accuracy measures for each patient. The statistical significance of differences in PPV and NPV was assessed using approximate tests based on the difference between 2 proportions. A logistic regression model was constructed to determine the probability of positive scan interpretations at various PSA cutoffs. Statistical significance was determined using a type I error rate of α = 0.05. Statistical analysis was done using MatLab® (R2013a) version 8.1.0.604 and R (http://www.R-project.org).
RESULTS
Demographics
Table 1 lists select demographics. Median followup after anti-3-[18F]FACBC scanning was 41.0 months (mean ± SD 39.1 ± 14.1). Of 93 patients 24 (25.8%) were treated with prostatectomy alone or combined with other treatment. A total of 69 patients (74.2%) underwent nonprostatectomy therapy, including brachytherapy, cryotherapy, radiation therapy and/or ADT. Median PSA was 4.0 ng/ml obtained within a mean of 12.7 ± 33.9 days from scanning. Mean PSA was 9.8 ng/ml due to an outlying patient with an unexpected rapid PSA increase to 301.7 ng/ml between recruitment and scanning. In 1 patient ADT ceased at the time of anti-3-[18F]FACBC imaging but no other patient was treated with ADT. Mean ± SD time between anti-3-[18F]FACBC and 111In-capromab pendetide scans was 19.7 ± 29.8 days.
Table 1.
Demographic characteristics of 93 study participants and positive scan locations
| Age: | |
| Mean ± SD | 68.0 ± 7.6 |
| Median (range) | 68.0 (49–90) |
| Q1, Q3 | 63.0, 73.3 |
| PSA (ng/ml): | |
| Mean ± SD | 9.8 ± 31.5 |
| Median (range) | 4.0 (0.11–301.7) |
| Q1, Q3 | 1.8, 9.7 |
| No. original prostate Ca therapy (%): | |
| Prostatectomy with/without other treatments | 24 (25.8) |
| Nonprostatectomy alone or combined | 69 (74.2) |
| Original Gleason score:* | |
| Mean ± SD | 6.9 ± 0.8 |
| Median (range) | 7.0 (5–10) |
| Q1, Q3 | 6.0, 7.0 |
| No. Gleason score (%):* | |
| 3 + 4 or Less | 52 (60.5) |
| 4 + 3 or Greater | 34 (39.5) |
| No. anti-3-[18F]FACBC pos scan (%): | |
| Whole body | 77 (82.8) |
| Prostate only | 49 (63.6) |
| Prostatic + extraprostatic | 24 (31.2) |
| Extraprostatic only | 4 (5.2) |
| No. 111In-capromab pendetide pos scan (%): | |
| Whole body | 56 (60.2) |
| Prostate only | 46 (82.1) |
| Prostatic + extraprostatic | 9 (16.1) |
| Extraprostatic only | 1 (1.8) |
Unavailable in 7 patients.
Scan Interpretation
Before truth verification we interpreted 93 anti-3-[18F]FACBC and 111In-capromab pendetide scans (table 1). Of 93 anti-3-[18F]FACBC scans 77 (82.8%) were positive, including 49 (63.6%) in the prostate/bed only, 24 (31.2%) in the prostate/bed and extraprostatically, and 4 (5.2%) extraprostatically only. Of 93 111In-capromab pendetide scans 56 (60.2%) were positive, including 46 (82.1%) in the prostate/bed only, 9 (16.1%) in the prostate/bed and extraprostatically, and 1 (1.8%) extraprostatically only. Based on logistic regression a patient with a PSA of 1 ng/ml had a 71.8% probability of a positive anti-3-[18F]FACBC scan and a 49.5% probability of a positive 111In-capromab pendetide scan. Initial interobserver agreement for anti-3-[18F]FACBC PET-CT interpretation was 98.9% (92 of 93 scans) in the prostate/bed and 94.6% (88 of 93) for extraprostatic locations.
Truth Verification Reference Standard
In 91 of 93 patients there were sufficient data to determine disease presence or absence in the prostate/bed. All 55 cases (100%) with true positive anti-3-[18F]FACBC PET-CT findings in the prostate/bed showed histological proof. In 70 of 93 patients there were sufficient data to determine disease presence or absence at extraprostatic locations. Of the 70 patients 22 had true positive anti-3-[18F]FACBC PET-CT findings for extraprostatic disease, including node only in 19 and bone only in 3. There was histological proof in 19 cases (86.4%). In the other 3 patients skeletal disease was confirmed on other imaging (2) and or durable PSA control was achieved after directed therapy to a lymph node group (1). Thus, there was histological proof for 74 of the 77 index lesions (96.1%) used for positivity, including 55 in the prostate and 22 that were extraprostatic. A total of 13 patients had true positive index lesions in the prostate/bed and at extraprostatic sites. The size of detected lymph nodes was 0.5 × 0.5 to 2.3 × 2 cm.
Disease Detection Diagnostic Performance
Prostate/bed
In the 91 of 93 patients with a definitive consensus on the presence or absence of prostatic/bed disease anti-3-[18F]FACBC sensitivity was 90.2% (95% CI 79.8, 96.3), specificity was 40.0% (95% CI 22.7, 59.4), accuracy was 73.6% (95% CI 63.3, 82.3), PPV was 75.3% (95% CI 63.9, 84.7) and NPV was 66.7% (95% CI 41.0, 86.7). For 111In-capromab pendetide sensitivity was 67.2% (95% CI 54.0, 78.7), specificity was 56.7% (95% CI 37.4, 74.5), accuracy was 63.7% (95% CI 53.0, 73.6), PPV was 75.9% (95% CI 62.4, 86.5) and NPV was 45.9% (95% CI 29.5, 63.1). Sensitivity and accuracy significantly differed (table 2). There was agreement between anti-3-[18F]FACBC and 111In-capromab pendetide interpretations in 54 of 93 patients. Figure 1 shows an example of a biopsy confirmed lesion in the prostate/bed.
Table 2.
Anti-3-[18F]FACBC vs 111In-capromab pendetide diagnostic performance in prostate/bed and extraprostatic sites
| Anti-3-[18F]FACBC | 111In-Capromab Pendetide | p Value | |
|---|---|---|---|
| Prostate/bed (91 pts): | |||
| No. true pos | 55 | 41 | – |
| No. true neg | 12 | 17 | – |
| No. false-pos | 18 | 13 | – |
| No. false-neg | 6 | 20 | – |
| % Sensitivity (95% CI) | 90.2 (79.8, 96.3) | 67.2 (54.0, 78.7) | 0.002 |
| % Specificity (95% CI) | 40.0 (22.7, 59.4) | 56.7 (37.4, 74.5) | 0.182 |
| % Accuracy (95% CI) | 73.6 (63.3, 82.3) | 63.7 (53.0, 73.6) | <0.001 |
| % PPV (95% CI) | 75.3 (63.9, 84.7) | 75.9 (62.4, 86.5) | 0.530 |
| % NPV (95% CI) | 66.7 (41.0, 86.7) | 45.9 (29.5, 63.1) | 0.074 |
| Extraprostatic (70 pts): | |||
| No. true pos | 22 | 4 | – |
| No. true neg | 29 | 26 | – |
| No. false-pos | 1 | 4 | – |
| No. false-neg | 18 | 36 | – |
| % Sensitivity (95% CI) | 55.0 (38.5, 70.7) | 10.0 (2.8, 23.7) | <0.001 |
| % Specificity (95% CI) | 96.7 (82.8, 99.9) | 86.7 (69.3, 96.2) | 0.248 |
| % Accuracy (95% CI) | 72.9 (60.9, 82.8) | 42.9 (31.1, 55.3) | 0.003 |
| % PPV (95% CI) | 95.7 (78.1, 99.9) | 50.0 (15.7, 84.3) | 0.001 |
| % NPV (95% CI) | 61.7 (46.4, 75.5) | 41.9 (29.5, 55.2) | 0.021 |
Figure 1.
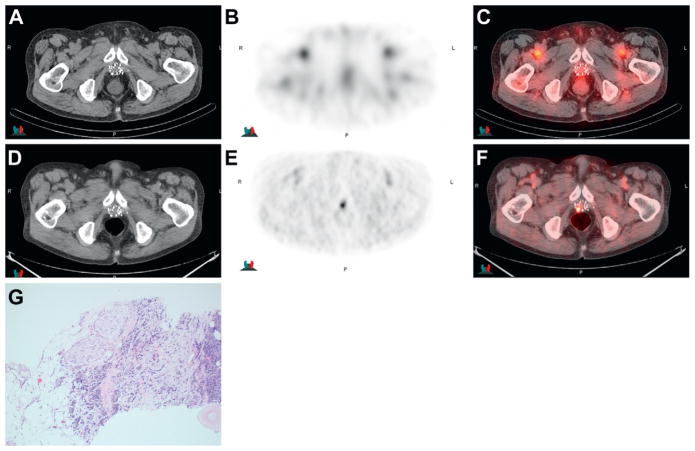
Imaging in 80-year-old patient after external beam radiotherapy, cryotherapy and brachytherapy with increasing PSA to 1.6 ng/ml and biopsy positive prostate bed. 111In-capromab pendetide CT (A), scintigraphy (B) and fused image (C ) show no significant uptake in prostate bed over background but note abnormal uptake in right posterior bed using anti-3-[18F]FACBC on CT (D), PET (E ) and fused PET-CT (F ). Biopsy specimen section shows Gleason score 4 + 5 = 9 prostatic adenocarcinoma invading adipose tissue with extraprostatic extension (G). H&E, reduced from ×20.
Extraprostatic sites
In the 70 of 93 patients with a definitive consensus for the presence or absence of extraprostatic disease anti-3-[18F]FACBC had 55.0% sensitivity (95% CI 38.5, 70.7), 96.7% specificity (95% CI 82.8, 99.9), 72.9% accuracy (95% CI 60.9, 82.8), 95.7% PPV (95% CI 78.1, 99.9) and 61.7% NPV (95% CI 46.4, 75.5). For 111In-capromab pendetide sensitivity was 10.0% (95% CI 2.8, 23.7), specificity was 86.7% (95% CI 69.3, 96.2), accuracy was 42.9% (95% CI 31.1, 55.3), PPV was 50.0% (95% CI 15.7, 84.3) and NPV was 41.9% (95% CI 29.5, 55.2). Sensitivity, accuracy, PPV and NPV significantly differed (table 2). There was agreement between anti-3-[18F]FACBC and 111In-capromab pendetide interpretations in 61 of 93 patients. Figures 2 and 3 show examples of biopsy proven extraprostatic disease.
Figure 2.
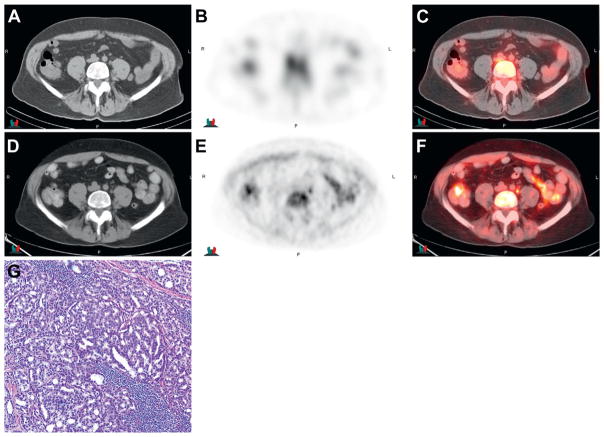
Imaging in 65-year-old patient after external beam radiation therapy and cryotherapy with increasing PSA to 13.8 ng/ml and biopsy negative prostate bed with metastasis confirmed by laparoscopic biopsy in small left common iliac node. 111In-capromab pendetide CT (A), scintigraphy (B) and fused image (C ) show no uptake in 0.7 × 1.1 cm left common iliac node but note abnormal uptake using anti-3-[18F]FACBC on CT (D), PET (E ) and fused PET-CT (F ). Stained lymph node section shows metastatic prostate adenocarcinoma (G). H&E, reduced from ×40.
Figure 3.
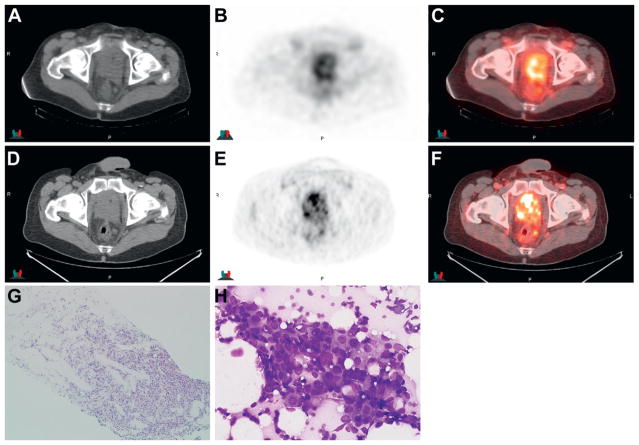
Imaging in 61-year-old patient after external beam radiation therapy and hormonal therapy with increasing PSA to 1.96 ng/ml reveals extensive biopsy proven recurrent disease in prostate and multiple pelvic nodes. 111In-capromab pendetide CT (A), scintigraphy (B) and fused image (C ) show abnormal uptake in prostate and left perirectal node. Anti-3-[18F]FACBC CT (D), PET (E ) and fused image (F ) at same level also show abnormal uptake in prostate and left perirectal node. Prostate core biopsy demonstrates prostatic Gleason 4 + 4 = 8 adenocarcinoma (G). H&E, reduced from × 10. Fine needle aspiration of perirectal node demonstrates malignant prostate adenocarcinoma cells with glandular formation and prominent nucleoli (H ). 111In-capromab pendetide findings were considered abnormal in node but there was better lesion contrast on anti-3-[18F]FACBC imaging with more nodes identified in pelvis. Diff-Quik stain, reduced from ×40.
Stage Change Based on Anti-3-[18F]FACBC PET-CT
Anti-3-[18F]FACBC correctly identified 14 more positive prostate bed recurrences (55 vs 41) and 18 more patients with extraprostatic involvement (22 vs 4). Thus, anti-3-[18F]FACBC correctly upstaged recurrence in 18 of 70 patients (25.7%) in whom there was a consensus on the presence or absence of extraprostatic disease.
DISCUSSION
We determined whether molecular imaging with the synthetic amino acid analogue anti-3-[18F]FACBC PET-CT would have diagnostic performance comparable to that of 111In-capromab pendetide for restaging prostate cancer. We found that anti-3-[18F]FACBC PET-CT had significantly higher accuracy, detecting more prostatic and extraprostatic disease, and effectively up-staging 25.7% of cases.
Our findings are important since the defining factor in therapy for recurrent prostate carcinoma is whether disease is confined in the prostate/bed or is extraprostatic.17 The presence or absence of extraprostatic disease changes the therapeutic approach. ADT for systemic disease is costly with significant morbidity.18
Routine CT or MR is limited for detecting recurrent prostate carcinoma.19 111In-capromab pendetide, which gained United States Food and Drug Administration (FDA) approval in 1996, has been promoted as an important adjunct in the evaluation of patients with recurrent prostate carcinoma, especially using SPECT-CT technology.7,20 However, the radiotracer has shown varying diagnostic performance with positive detection of metastatic disease in 1 of 6 patients compared to the histological standard and with low NPV for post-salvage radiotherapy PSA control.21,22
This broad range of reported diagnostic performance for 111In-capromab pendetide is due to a number of etiologies, including study population selection, reference standard veracity, followup duration and PSA distribution in the study population. Prostate cancer may take years to manifest clinically.23 Thus, we compared the 2 modalities in the same patients using the same reference standards. Overall our series showed 96.1% histological proof of positivity for anti-3-[18F]FACBC and had a median patient followup of 41 months.
On a whole body basis 82.8% of anti-3-[18F]FACBC PET-CTs vs 60.2% of 111In-capromab pendetide studies were positive with a 71.8% vs 49.5% probability of a positive test at PSA 1 ng/ml. However, determining diagnostic performance in the prostate/bed and for extraprostatic disease is more clinically relevant since the central issue is that of prostatic vs extraprostatic recurrence. Our study was designed and powered with these end points in mind.
In the prostate/bed anti-3-[18F]FACBC compared favorably to 111In-capromab pendetide, detecting 14 more patients (55 vs 41) with prostate bed recurrence than 111In-capromab pendetide with fewer false-negative findings. Although there were 5 more false-positive findings in the prostate/bed (18 vs 13) using anti-3-[18F]FACBC, specificity and PPV did not significantly differ. Diagnostic performance in the prostate/bed is similar to our published data on identifying primary prostate carcinoma.24 Because of the possibility of false-positive uptake using either radio-tracer, histological confirmation is recommended.
Anti-3-[18F]FACBC detected 18 more patients (22 vs 4) with extraprostatic spread than 111In-capromab pendetide. The overall accuracy of anti-3-[18F]FACBC was 72.9% vs 42.9% for 111In-capromab pendetide with 55.0% vs 10.0% sensitivity. While specificity was high for each radiotracer, anti-3-[18F]FACBC showed a significantly higher PPV of 95.7% vs 50.0% for 111In-capromab pendetide. Thus, anti-3-[18F]FACBC may prove valuable for restaging prostate carcinoma since more accurate restaging would result in the most appropriate therapy.
Our finding of a relatively low rate of extraprostatic disease detection using 111In-capromab pendetide, in line with that reported by others,21,22 was likely due to antibody targeting to the intracellular epitope of prostate specific membrane antigen and to our use of a more vigorous reference standard. While we used SPECT-CT for 111In-capromab pendetide, the even higher spatial resolution of PET-CT may also have partially improved disease detection using anti-3-[18F]FACBC.15
Many techniques are currently under investigation for optimal staging and restaging of prostate carcinoma, reflecting the clinical need for better imaging. These techniques include 18F-fluorocholine and the recently FDA approved 11C-choline radio-tracer.7,25 A preliminary study directly comparing anti-3-[18F]FACBC and 11C-choline PET-CT showed higher per patient and per lesion detection rates with better lesion conspicuity for anti-3-[18F] FACBC.26 Other promising new methods include prostate specific membrane antigen directed radio-tracers, multiparametric MR and intravenous, lymphotropic, ultrasmall superparamagnetic iron oxide particles.5,7,8,27,28 Modalities are best compared using the same or similar populations and well-defined, systematically applied reference standards with histological proof, when feasible.29 Our study showed a 96.1% histological verification rate for true positive index lesions. This was in contrast to most other studies, in which the histological verification rate was considerably lower.25,29
The limitations of this study are the standards used to establish positive and negative proof, especially for extraprostatic disease. We relied on histological proof per patient for positivity and yet it would be unethical and impractical to biopsy every positive lesion. In addition, this trial was not designed to evaluate diagnostic performance for skeletal metastasis since a negative bone scan was a study entry criterion. Establishing the absence of extraprostatic disease was also difficult since microscopic disease may not initially be detected clinically or by imaging.23 We applied commonly used criteria for PSA control after therapy.25,30 Although our median followup was 41 months, even longer followup may be required to optimally assess diagnostic performance and patient outcome.
For better or for worse we defaulted to tissue biopsy as the reference standard for truth in the prostate/bed despite the well-known prostate/bed biopsy sampling error.7 Thus, we may have underestimated true positivity in the prostate/bed. We conservatively assumed that if there was no proven disease in the prostate/bed to explain a PSA increase, there was then undetected extraprostatic disease, which would have decreased apparent extraprostatic disease detection. Anecdotally, the 55.0% overall sensitivity of anti-3-[18F]FACBC seems to be related to indolent or small volume extraprostatic disease especially in bone despite negative bone scanning. This will be the subject of more in-depth analysis. Lower sensitivity for such disease is a commonly reported shortcoming of imaging.5 An earlier analysis in which we noted higher sensitivity for detecting extraprostatic disease was based on fewer patients and limited followup.10 More study is also needed to determine whether apparently false-positive findings in the prostate/bed were indeed secondary to sampling error vs confounding uptake in post-therapy inflammatory prostate tissue.
Finally, since anti-3-[18F]FACBC was scanned below the diaphragm while planar imaging for 111In-capromab pendetide included the entire body, detection may have been biased in favor of 111In-capromab pendetide. However, isolated metastasis above the diaphragm is rare.
CONCLUSIONS
PET-CT with the amino acid analogue radiotracer anti-3-[18F]FACBC PET-CT showed higher accuracy than 111In-capromab pendetide SPECT-CT to detect recurrent prostate carcinoma. Significantly more prostatic and extraprostatic disease was detected with anti-3-[18F]FACBC, up-staging recurrence in 25.7% of patients. Studies in other populations are ongoing at our institution and elsewhere. Multi-center trials would be valuable to more definitively analyze the practical usefulness of this radiotracer.
Acknowledgments
Supported by National Institutes of Health Grant 5R01CA129356 and the Georgia Cancer Coalition.
Abbreviations and Acronyms
- ADT
androgen deprivation therapy
- anti-3-[18F]FACBC
radiotracer anti-1-amino-3-[18F] fluorocyclobutane-1-carboxylic acid
- CT
computerized tomography
- MR
magnetic resonance imaging
- NPV
negative predictive value
- PET
positron emission tomography
- PPV
positive predictive value
- PSA
prostate specific antigen
- SPECT
single photon emission CT
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Ward JF, Blute ML, Slezak J, et al. The long-term clinical impact of biochemical recurrence of prostate cancer 5 or more years after radical prostatectomy. J Urol. 2003;170:1872. doi: 10.1097/01.ju.0000091876.13656.2e. [DOI] [PubMed] [Google Scholar]
- 3.Lawton CA, Michalski J, El-Naqa I, et al. RTOG GU Radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:383. doi: 10.1016/j.ijrobp.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choo R. Salvage radiotherapy for patients with PSA relapse following radical prostatectomy: issues and challenges. Cancer Res Treat. 2010;42:1. doi: 10.4143/crt.2010.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouchelouche K, Turkbey B, Choyke P, et al. Imaging prostate cancer: an update on positron emission tomography and magnetic resonance imaging. Curr Urol Rep. 2010;11:180. doi: 10.1007/s11934-010-0105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choueiri TK, Dreicer R, Paciorek A, et al. A model that predicts the probability of positive imaging in prostate cancer cases with biochemical failure after initial definitive local therapy. J Urol. 2008;179:906. doi: 10.1016/j.juro.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 7.Beresford MJ, Gillatt D, Benson RJ, et al. A systematic review of the role of imaging before salvage radiotherapy for post-prostatectomy biochemical recurrence. Clin Oncol (R Coll Radiol) 2010;22:46. doi: 10.1016/j.clon.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Beer AJ, Eiber M, Souvatzoglou M, et al. Radionuclide and hybrid imaging of recurrent prostate cancer. Lancet Oncol. 2011;12:181. doi: 10.1016/S1470-2045(10)70103-0. [DOI] [PubMed] [Google Scholar]
- 9.Schuster D, Votaw J, Nieh P, et al. Initial experience with the radiotracer anti-1-amino-3-F-18-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med. 2007;48:56. [PubMed] [Google Scholar]
- 10.Schuster DM, Savir-Baruch B, Nieh PT, et al. Detection of recurrent prostate carcinoma with anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT and 111In-capromab pendetide SPECT/CT. Radiology. 2011;259:852. doi: 10.1148/radiol.11102023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McConathy J, Yu W, Jarkas N, et al. Radio-halogenated nonnatural amino acids as PET and SPECT tumor imaging agents. Med Res Rev. 2012;32:868. doi: 10.1002/med.20250. [DOI] [PubMed] [Google Scholar]
- 12.Oka S, Okudaira H, Yoshida Y, et al. Transport mechanisms of trans-1-amino-3-fluoro[1-(14)C] cyclobutanecarboxylic acid in prostate cancer cells. Nucl Med Biol. 2012;39:109. doi: 10.1016/j.nucmedbio.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 13.McConathy J, Voll RJ, Yu W, et al. Improved synthesis of anti-[18F]FACBC: improved preparation of labeling precursor and automated radio-synthesis. Appl Radiat Isot. 2003;58:657. doi: 10.1016/s0969-8043(03)00029-0. [DOI] [PubMed] [Google Scholar]
- 14.Sodee DB, Nelson AD, Faulhaber PF, et al. Update on fused capromab pendetide imaging of prostate cancer. Clin Prostate Cancer. 2005;3:230. doi: 10.3816/cgc.2005.n.004. [DOI] [PubMed] [Google Scholar]
- 15.Schettino CJ, Kramer EL, Noz ME, et al. Impact of fusion of indium-111 capromab pendetide volume data sets with those from MRI or CT in patients with recurrent prostate cancer. AJR Am J Roentgenol. 2004;183:519. doi: 10.2214/ajr.183.2.1830519. [DOI] [PubMed] [Google Scholar]
- 16.Mattei A, Fuechsel FG, Bhatta Dhar N, et al. The template of the primary lymphatic landing sites of the prostate should be revisited: results of a multimodality mapping study. Eur Urol. 2008;53:118. doi: 10.1016/j.eururo.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Tabatabaei S, Saylor PJ, Coen J, et al. Prostate cancer imaging: what surgeons, radiation oncologists, and medical oncologists want to know. AJR Am J Roentgenol. 2011;196:1263. doi: 10.2214/AJR.10.6263. [DOI] [PubMed] [Google Scholar]
- 18.Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. CA Cancer J Clin. 2010;60:194. doi: 10.3322/caac.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hovels AM, Heesakkers RA, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63:387. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Rieter WJ, Keane TE, Ahlman MA, et al. Diagnostic performance of In-111 capromab pendetide SPECT/CT in localized and metastatic prostate cancer. Clin Nucl Med. 2011;36:872. doi: 10.1097/RLU.0b013e318219ae29. [DOI] [PubMed] [Google Scholar]
- 21.Seltzer MA, Barbaric Z, Belldegrun A, et al. Comparison of helical computerized tomography, positron emission tomography and monoclonal antibody scans for evaluation of lymph node metastases in patients with prostate specific antigen relapse after treatment for localized prostate cancer. J Urol. 1999;162:1322. [PubMed] [Google Scholar]
- 22.Thomas CT, Bradshaw PT, Pollock BH, et al. Indium-111-capromab pendetide radio-immunoscintigraphy and prognosis for durable biochemical response to salvage radiation therapy in men after failed prostatectomy. J Clin Oncol. 2003;21:1715. doi: 10.1200/JCO.2003.05.138. [DOI] [PubMed] [Google Scholar]
- 23.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 24.Schuster DM, Taleghani PA, Nieh PT, et al. Characterization of primary prostate carcinoma by anti-1-amino-2-[(18)F]-fluorocyclobutane-1-carboxylic acid (anti-3-[(18)F] FACBC) uptake. Am J Nucl Med Mol Imaging. 2013;3:85. [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell CR, Lowe VJ, Rangel LJ, et al. Operational characteristics of (11)c-choline positron emission tomography/computerized tomography for prostate cancer with biochemical recurrence after initial treatment. J Urol. 2013;189:1308. doi: 10.1016/j.juro.2012.10.069. [DOI] [PubMed] [Google Scholar]
- 26.Nanni C, Schiavina R, Boschi S, et al. Comparison of (18)F-FACBC and (11)C-choline PET/CT in patients with radically treated prostate cancer and biochemical relapse: preliminary results. Eur J Nucl Med Mol Imaging, suppl. 2013;40:11. doi: 10.1007/s00259-013-2373-3. [DOI] [PubMed] [Google Scholar]
- 27.Barrett JA, Coleman RE, Goldsmith SJ, et al. First-in-man evaluation of 2 high-affinity PSMA-avid small molecules for imaging prostate cancer. J Nucl Med. 2013;54:380. doi: 10.2967/jnumed.112.111203. [DOI] [PubMed] [Google Scholar]
- 28.Cho SY, Gage KL, Mease RC, et al. Bio-distribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J Nucl Med. 2012;53:1883. doi: 10.2967/jnumed.112.104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Picchio M, Briganti A, Fanti S, et al. The role of choline positron emission tomography/computed tomography in the management of patients with prostate-specific antigen progression after radical treatment of prostate cancer. Eur Urol. 2011;59:51. doi: 10.1016/j.eururo.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 30. [Accessed July 7, 2013];NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. Version 2.2013. Available at: http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.