Abstract
Background
With conflicting evidence regarding the usefulness of intra-aortic balloon pump (IABP), reports of IABP use in the United States (US) have been inconsistent. Our objective was to examine trends in IABP usage in percutaneous coronary intervention (PCI) in the US, and to evaluate the association of IABP use with mortality.
Methods
Retrospective, observational study using patient data obtained from the Nationwide Inpatient Sample (NIS) database from 1998 to 2008. Patients undergoing any PCI (1,552,602 procedures) for a primary diagnosis of symptomatic coronary artery disease (CAD) and acute coronary syndrome (ACS), including non-ST elevation MI (NSTEMI) and ST elevation MI (STEMI), were evaluated.
Results
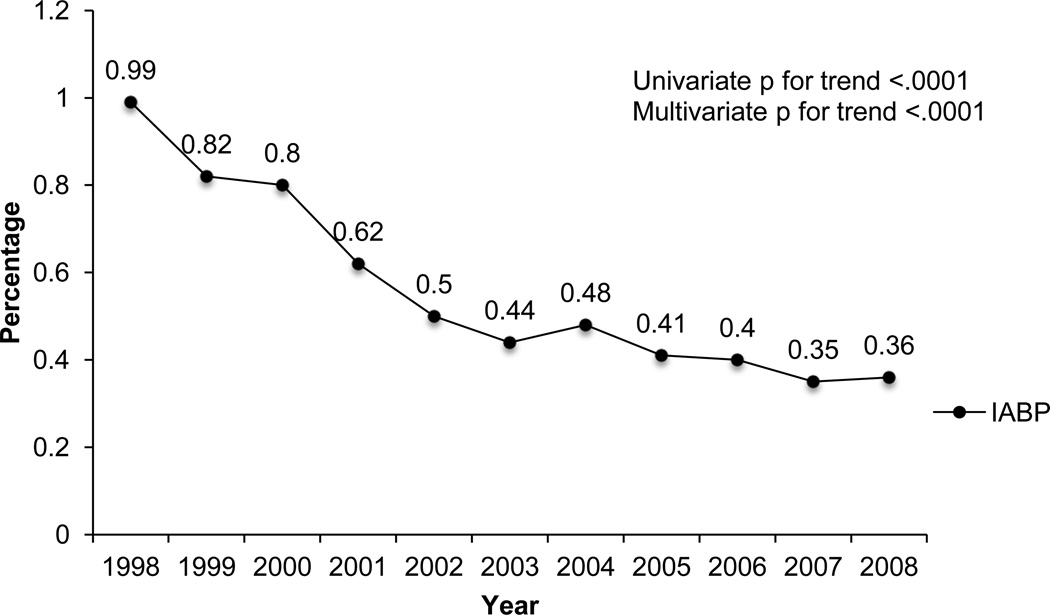
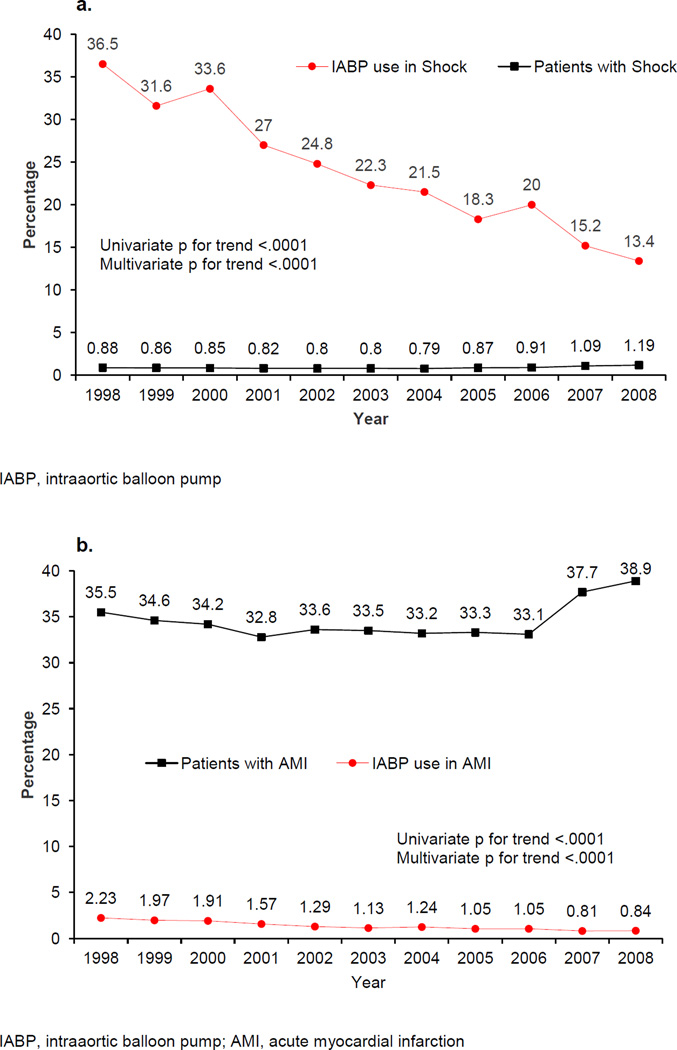
The overall use of IABP significantly decreased during the study period from 0.99% in 1998 to 0.36% in 2008 (univariate and multivariate p for trend <.0001). Patients who received IABP had substantially higher rates of shock compared to those who did not receive IABP (38.09% vs. 0.70%, p<.0001), which was associated with markedly higher in-hospital mortality rates (20.31% vs. 0.72%, p<.0001). However, IABP use significantly decreased in patients with shock (36.5% to 13.4%) and AMI (2.23% to 0.84%) (univariate and multivariate p for trend for both <.0001). A temporal reduction in all-cause PCI-associated mortality from 1.1% in 1998 to 0.86% in 2008 (univariate and multivariate p for trend <.0001) was also observed.
Conclusions
The utilization of IABP associated with PCI significantly decreased between 1998 and 2008 in the US, even amongst patients with acute myocardial infarction and shock.
INTRODUCTION
Intra-aortic balloon pump (IABP) first became available in the 1960s for hemodynamic support in patients with cardiogenic shock resulting from acute myocardial infarction (AMI).1 It increases coronary perfusion by inflating during diastole, and reduces afterload and decreases myocardial oxygen demand by deflating during systole. This unique mechanism enables IABP to serve as a viable hemodynamic support option in unstable patients. In the 1996 American College of Cardiology/American Heart Association (ACC/AHA) guidelines, IABP support was given a Class I recommendation for the management of shock in AMI.2 However, due to the emergence of conflicting evidence on the usefulness of IABP, the 2013 ACC/AHA Guidelines have given IABP a Class IIb recommendation (level of evidence C) in patients with unstable angina (UA)/non-ST elevation myocardial infarction (NSTEMI) complicated with hemodynamic instability,3 and a Class IIa (level of evidence B) for patients with cardiogenic shock after ST-elevation myocardial infarction (STEMI).4 The 2012 European Society of Cardiology AMI Guidelines do not recommend routine IABP use in absence of shock.5
Much has been investigated regarding its mechanism, technique of insertion, indications, complications, and mortality benefit. However, data on current patterns of IABP use in the United States (US) are lacking. Reports from multiple centers have suggested inconsistency in its utilization in the US6–9 and non-US centers.10 The purpose of our study is to determine the trend of IABP utilization in the US from 1998 to 2008.
METHODS
Data Source
We analyzed data provided by The Healthcare Cost and Utilization Project (HCUP),11 which is a family of health care databases that has been gathering a large collection of longitudinal hospital care data in the US beginning in 1988. The HCUP databases combine the data collection efforts of state data organizations, hospital associations, private data organizations, and Federal government to create a national pool of patient-level health care data, allowing researchers to investigate on a broad range of healthcare related issues.12
Study Patients
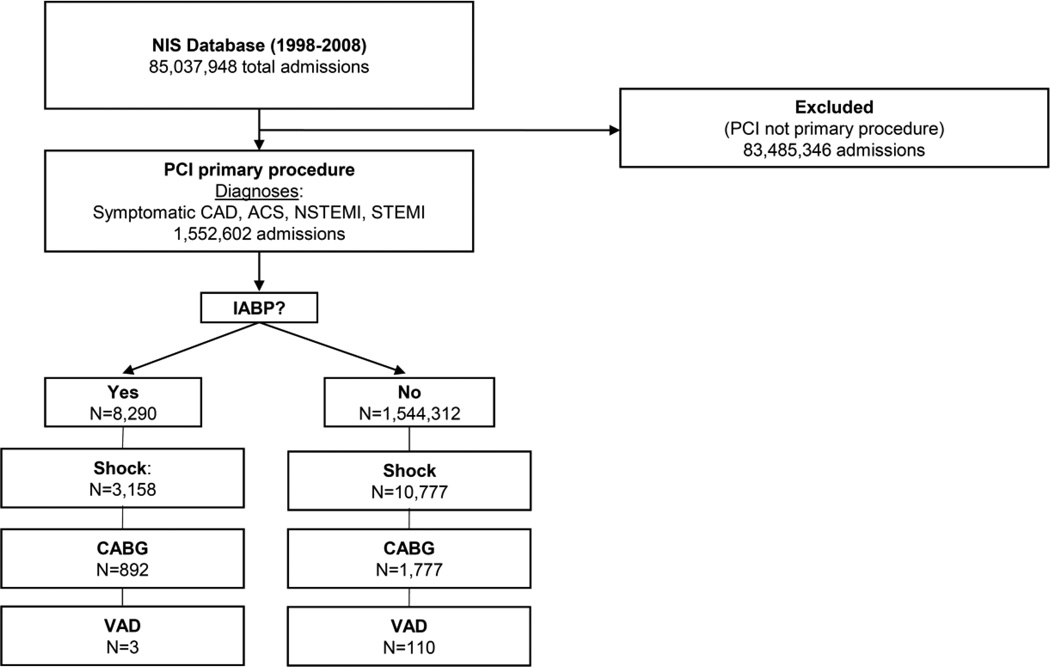
We examined a cohort of patients between 1998 and 2008. A total of 1,552,602 patients undergoing percutaneous coronary intervention (PCI) procedures for symptomatic coronary artery disease (CAD) and acute coronary syndrome (ACS), including NSTEMI and STEMI were identified (Figure 1). Additional patient data collected were procedure year, age, race, gender, in-hospital mortality, cost, length of stay, hypertension, AMI, CAD, congestive heart failure (CHF), transient cerebral ischemia, peripheral atherosclerosis (PVD), aortic/peripheral/visceral artery aneurysm/embolism/thrombosis, any malignancy, chronic obstructive pulmonary disease (COPD)/bronchiectasis, systemic lupus erythematosus/connective tissue disease, cardiac and circulatory congenital anomalies, shock, diabetes mellitus (DM), coagulation and hemorrhagic disorders, heart valve disorders, peri-/endo-/myocarditis, pulmonary heart disease, chronic renal failure (CRF), hemorrhagic cerebrovascular disease (CVD), ischemic CVD, acute CVD, other CVD, disorders of lipid metabolism, anemia, prior and current tobacco use, atrial fibrillation/flutter, placement of drug-eluting stent and bare-metal stent, coronary artery bypass grafting (CABG) and percutaneous and surgical ventricular assist device placement (VAD).
Figure 1.
Flow Diagram of study patients
NIS, Nationwide Inpatient Sample; PCI, percutaneous coronary intervention; CAD, coronary artery disease; ACS, acute coronary syndrome; NSTEMI, non-ST elevation myocardial infarction; STEMI, ST-elevation myocardial infarction; IABP, intraaortic balloon pump; CABG, coronary artery bypass graft; VAD, ventricular assist device
Primary analysis
The primary analysis was to assess the trend of IABP use in the US over the study period, and to evaluate the association of IABP use with mortality.
Secondary analysis
The secondary analysis was to examine the trend in mortality with PCI and to determine patient characteristics associated with both IABP placement and mortality.
Statistical analyses
The study population was separated into two groups - those with IABP and those without IABP. The summary statistics on the baseline patient characteristics were generated for the entire population separated into the aforementioned groups, as well as for the subpopulations stratified by the year.
Univariate analysis was initially conducted to summarize the data. Continuous data are presented as mean +/− SD, and the nonparametric Wilcoxon rank sum tests were used to test for all continuous variables. Categorical variables are presented as category percentages and the Pearson Chi-square tests were used for test for categorical variables. All tests were two-tailed, and a P-value of less than .05 was considered significant for all tests.
The multivariate logistic regressions were fit to the data to evaluate IABP trend over the study period. Wald test with a .05 level of significance was used to test the null hypothesis of no trend. The logistic regression model was then used to assess independent predictors of IABP after adjusting for the observed baseline demographic and clinical characteristics. The logistic regression model was also used to investigate the trends for incidence of in-hospital mortality with and without IABP, as well as to assess the trends for the adjusted and unadjusted OR for the association between death and IABP over the study period.
We used a two-step propensity score method to evaluate the effect of IABP use on the mortality rate – first by estimating propensity scores using a logistic regression model with IABP as the outcome, then estimating the effect of IABP on mortality rate using the method of regression adjustment. All of our variables were accounted for. Advantage of this two-step procedure is that it allows for fitting a complicated propensity score model with interactions and higher order terms for more accurate estimation of IABP probability.13 Fisher’s Z test was used to perform the correlation test between trends in IABP use and mortality rates.
The missing data were omitted as follows: in the “No IABP group” (n=1,544,312), age (n=36, 0.002%), death (n=330, 0.02%), female sex (n=137, 0.009%), length of stay (n=21, 0.001%), mean financial cost (n=20,729, 1.3%), and race (n=419,760, 27.2%); in the “IABP” group (n=8,290), death (n=5, 0.06%), female gender (n=1, 0.01%), mean financial cost (n=211, 1.6%), and race (n=3,672, 27.3%).
All analyses were performed using SAS statistical software version 9.2 (SAS Institute Inc. Cary, NC).
Sources of Funding
This project was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000050 and the Division of Cardiology, University of Illinois at Chicago.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
RESULTS
Trends and patterns in IABP use
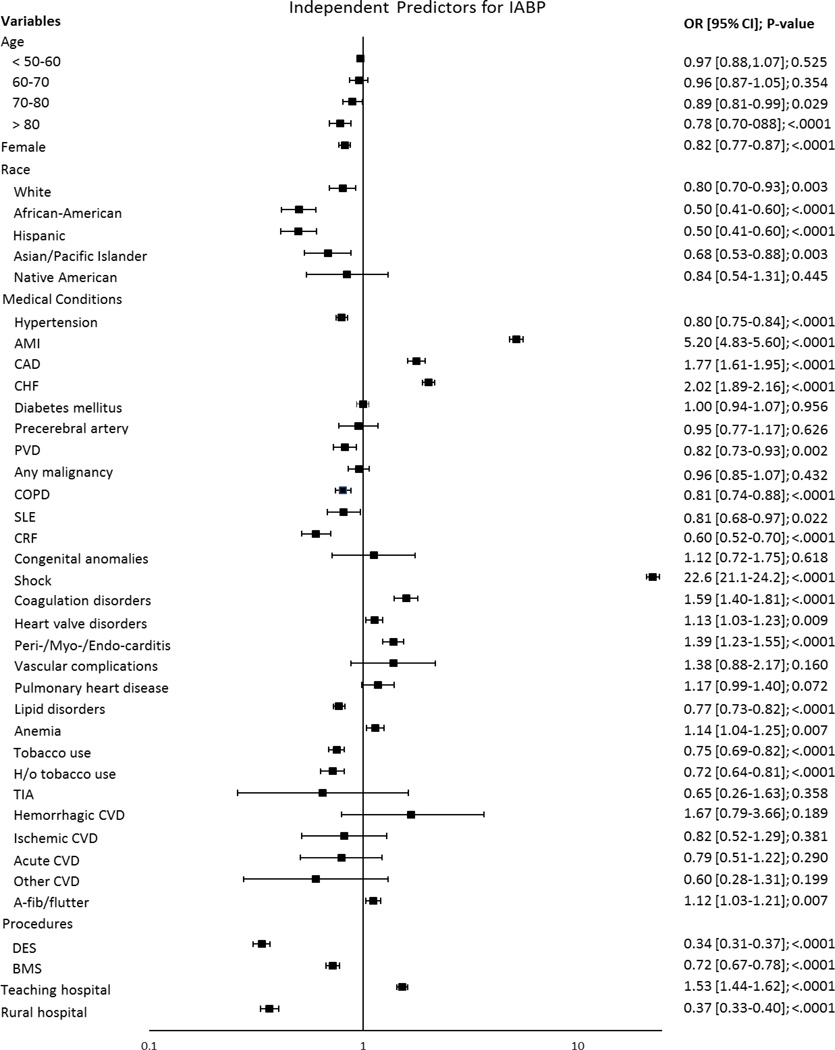
The overall IABP use in the US decreased significantly during the study period from 0.99% in 1998 to 0.36% in 2008 (univariate and multivariate p for trend <.0001; Figure 2). The patients’ baseline characteristics are presented in Table I. The patients who received an IABP did not differ in age or gender compared to the non-IABP group. Compared to the non-IABP group, the IABP group appeared acutely ill with substantially higher percentages of CHF (39.4% vs. 11.1%, p<.0001), AMI (84.6% vs. 34.2%, p<.0001), and shock (38.1% vs. 0.70%, p<.0001) (Table I). 10% were subsequently referred for CABG in the IABP group, compared to 0.1% in the other. The non-IABP group appeared to have a higher prevalence of chronic medical issues, such as DM, HTN, CAD, lipid disorders, and tobacco use. IABP placement was also associated with markedly higher mortality rates (20.3% vs. 0.72%, p<.0001), longer mean hospital stays (8.4 vs. 2.8 days, p<.0001), and higher mean financial hospital charges ($86,061 vs. $39,866 p<.0001) (Table I). Significant patient characteristics associated with IABP placement included shock (OR 22.6; 95% CI 21.1–24.2; p<.0001), AMI (OR 5.20; 95% CI 4.82–5.60; p<.0001), CHF (OR 2.02; 95% CI 1.89–2.16; p<.0001), and CAD (OR 1.77; 95% CI 1.61–1.95; p<.0001) (Figure 3).
Figure 2.
Trend in overall IABP use in PCI from 1998–2008.
IABP, intraaortic balloon pump
Table I.
Baseline patient characteristics
| No IABP [n=1,544,312] |
IABP [n=8290] |
P-value | |
|---|---|---|---|
| Age, mean ± SD | 64.2 ± 12.2 | 65. 1 ± 12.9 | <.0001 |
| Women, total (%) | 528159 (34.20) | 2809 (33.89) | 0.5465 |
| Race, total (%) | <.0001 | ||
| White | 921092 (81.91) | 5054 (83.91) | |
| Black | 75408 (6.71) | 290 (4.81) | |
| Hispanic | 68547 (6.10) | 279 (4.63) | |
| Asian | 19291 (1.72) | 121 (2.01) | |
| Native American | 4054 (0.36) | 27 (0.45) | |
| Other | 36160 (3.22) | 252 (4.18) | |
| Died during hospitalization, total (%) | 11054 (0.72) | 1683 (20.31) | <.0001 |
| Length of stay, mean ± SD | 2.77 ± 3.09 | 8.39 ± 8.46 | <.0001 |
| Financial cost, mean ($) ± SD | 39,866 ± 29,495 | 86,061 ± 89,133 | <.0001 |
| Hospital, total (%) | <.0001 | ||
| Non-teaching | 647992 (41.96) | 3118 (37.61) | |
| Teaching | 896320 (58.04) | 5172 (62.39) | |
| Location, total (%) | <.0001 | ||
| Rural | 67329 (4.36) | 657 (7.93) | |
| Urban | 1476983 (95.64) | 7633 (92.07) | |
| Medical history, total (%) | |||
| Diabetes mellitus | 446476 (28.91) | 2096 (25.28) | <.0001 |
| Hypertension | 977347 (63.29) | 3622 (43.69) | <.0001 |
| AMI | 528589 (34.23) | 7012 (84.58) | <.0001 |
| CAD | 1485068 (96.16) | 7082 (85.43) | <.0001 |
| CHF | 170801 (11.06) | 3266 (39.40) | <.0001 |
| Occlusion or stenosis of precerebral arteries | 23434 (1.52) | 82 (0.99) | <.0001 |
| Other and ill-defined cerebrovascular disease | 3280 (0.21) | <11* (0.11) | 0.0403 |
| TIA | 2877 (0.19) | 12 (0.14) | 0.3814 |
| PVD | 105166 (6.81) | 396 (4.78) | <.0001 |
| A/V/P artery aneurysm/embolism/thrombosis | 23369 (1.51) | 160 (1.93) | 0.0019 |
| Any malignancy | 95795 (6.20) | 499 (6.02) | 0.4890 |
| COPD/Bronchiectasis | 150720 (9.76) | 1079 (13.02) | <.0001 |
| SLE/Connective Tissue Disease | 47201 (3.06) | 205 (2.47) | 0.0021 |
| Cardiac and circulatory congenital anomalies | 4292 (0.28) | 36 (0.43) | 0.0071 |
| Shock | 10777 (0.70) | 3158 (38.09) | <.0001 |
| Coagulation and hemorrhagic disorders | 20893 (1.35) | 541 (6.53) | <.0001 |
| Heart valve disorders | 109804 (7.11) | 994 (11.99) | <.0001 |
| Peri- endo- and myocarditis, cardiomyopathy | 45014 (2.91) | 585 (7.06) | <.0001 |
| Pulmonary heart disease | 21571 (1.40) | 232 (2.80) | <.0001 |
| Chronic renal failure | 44368 (2.87) | 281 (3.39) | 0.0050 |
| Disorders of lipid metabolism | 840840 (54.45) | 2561 (30.89) | <.0001 |
| Anemia | 85427 (5.53) | 859 (10.36) | <.0001 |
| Tobacco use | 259393 (16.80) | 1144 (13.80) | <.0001 |
| History of tobacco use | 148834 (9.64) | 386 (4.66) | <.0001 |
| Ischemic CVD | 25702 (1.66) | 149 (1.80) | 0.3451 |
| Hemorrhagic CVD | 694 (0.04) | 21 (0.25) | <.0001 |
| Acute CVD | 8597 (0.56) | 147 (1.77) | <.0001 |
| Atrial fibrillation/flutter | 121408 (7.86) | 1436 (17.32) | <.0001 |
| Procedure | |||
| DES | 632,917 (40.98) | 1,713 (20.66) | <.0001 |
| BMS | 783,718 (50.75) | 5,143 (62.04) | <.0001 |
| CABG | 1,777 (0.12) | 892 (10.76) | <.0001 |
| VAD | 110 (0.01) | <11* (0.04) | 0.0020 |
AMI indicates acute myocardial infarction; CAD, coronary artery disease; CHF, congestive heart failure; TIA, transient ischemic attack; PVD, peripheral vascular disease; A/V/P, Aortic/peripheral/visceral; COPD, chronic obstructive pulmonary disease; SLE, systemic lupus erythematosus; CVD, cerebrovascular disease; CABG, coronary artery bypass graft; VAD, ventricular assist device
HCUP DUA prohibits the reporting of fewer than 11 observations
Figure 3.
Independent predictors for IABP placement in PCI.
AMI indicates acute myocardial infarction; CAD, coronary artery disease; CHF, congestive heart failure; TIA, transient ischemic attack; PVD, peripheral vascular disease; A/V/P, Aortic/peripheral/visceral; COPD, chronic obstructive pulmonary disease; SLE, systemic lupus erythematosus; CVD, cerebrovascular disease
Trends of IABP use in shock and AMI
There was a slight, but statically significant, increase in the percentage of patients with shock (0.88% in 1998 vs. 1.19% in 2008, univariate and multivariate p for trend <.0001) and AMI (35.5% in 1998 vs. 38.9% in 2008, univariate and multivariate p for trend <.0001) during the study period. However, the use of IABP in shock and AMI decreased. In 1998, 36.5% of patients presenting with shock were supported with IABP, which decreased to 13.4% in 2008 (univariate and multivariate p for trend <.0001; Figure 4a). The usage of IABP in patients with AMI declined from 2.23% in 1998 to 0.84% in 2008 (univariate and multivariate p for trend <.0001; Figure 4b).
Figure 4.
a. Trend in overall percentage of patients with shock by year from 1998–2008.
Trend in IABP use in shock by year from 1998–2008.
b. Trend in overall percentage of patients with AMI by year from 1998–2008.
Trend in IABP use in AMI by year from 1998–2008.
All-cause mortality in PCI
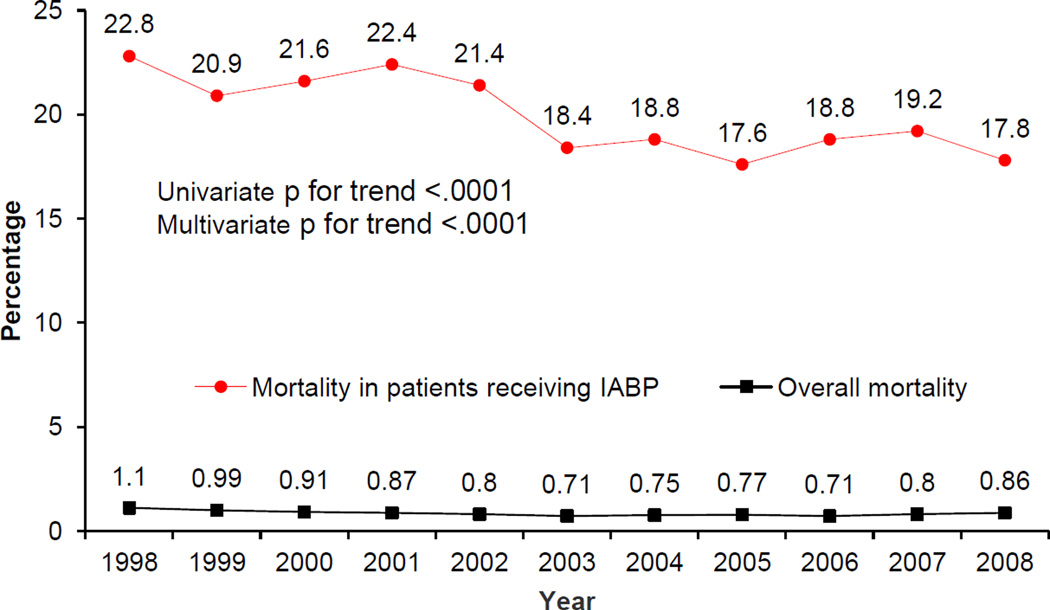
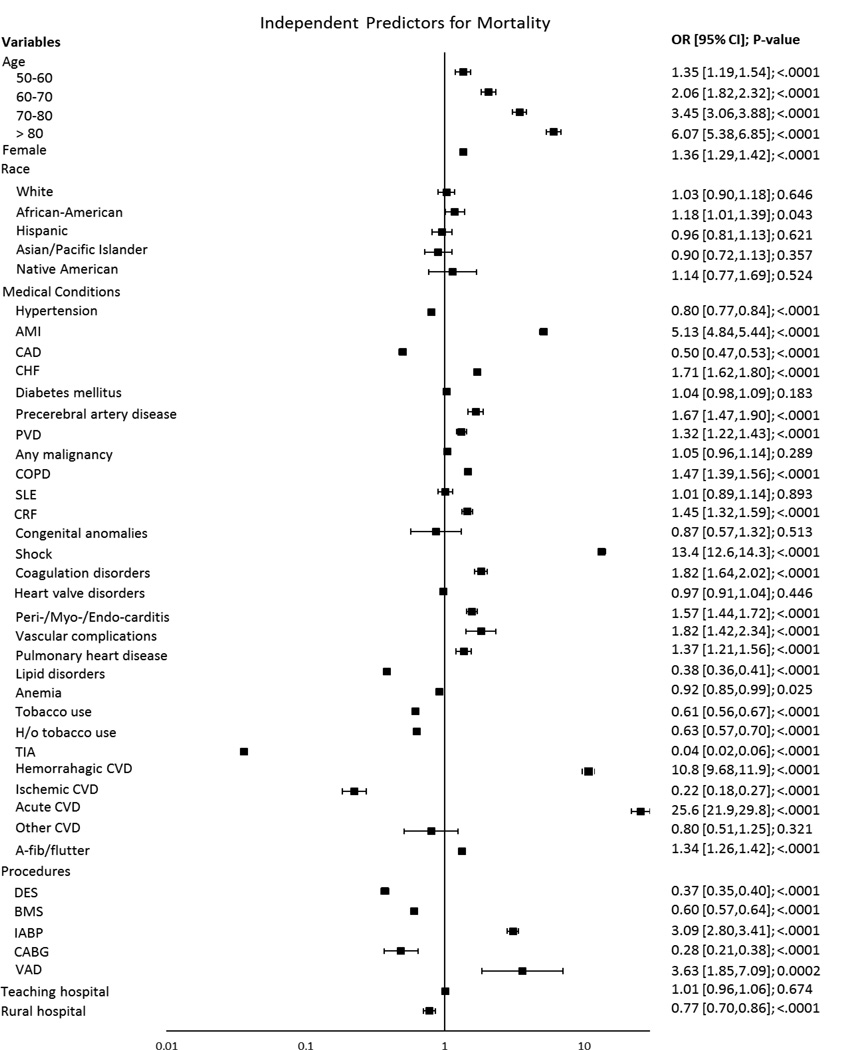
The all-cause trend in PCI-associated mortality decreased over the study period from 1.1% in 1998 to 0.86% in 2008 (univariate and multivariate p for trend <.0001; Figure 5.) Patient characteristics associated with mortality included advanced age (70–80 years old; OR 3.45; 95% CI 3.06–3.88; p<.0001; >80 years old; OR 6.07; 95% CI 5.38–6.85; p<.0001), diagnosis of AMI (OR 5.13; 95% CI 4.84–5.44; p<.0001), shock (OR 13.4; 95% CI 12.6–14.3; p<.0001), hemorrhagic CVD (OR 10.8; 95% CI 9.68–11.9; p<.0001) and acute CVD (OR 25.6; 95% CI 21.9–29.8; p<.0001; Figure 6).
Figure 5.
Trend in all-cause, in-hospital mortality in PCI from 1998–2008.
Trend in death with IABP use in PCI from 1998–2008.
IABP, intraaortic balloon pump
Figure 6.
Independent predictors for all-cause, in-hospital mortality in all patients undergoing PCI.
AMI indicates acute myocardial infarction; CAD, coronary artery disease; CHF, congestive heart failure; TIA, transient ischemic attack; PVD, peripheral vascular disease; A/V/P, Aortic/peripheral/visceral; COPD, chronic obstructive pulmonary disease; SLE, systemic lupus erythematosus; CVD, cerebrovascular disease; CABG, coronary artery bypass graft; VAD, ventricular assist device
Death and IABP use associated with PCI
Patients with IABP had a higher mortality rate (20.3% vs. 0.72; p <.0001) (Table I). Patients who received balloon pump intervention in 1998 had a mortality rate of 22.8%, which down-trended to 17.8% in 2008 (Figure 5). When testing the overall association between IABP and mortality, patients were nearly three times more likely to die with an IABP (adjusted p<.0001; Table II). In 1998, patients were five times more likely to die with IABP intervention after adjusting for variables. However, in 2008, odds ratio for death with IABP was 2.14 (p for trend <.0001). The association between IABP and death remained statistically significant for each individual year. (Table II). Using the Fischer Z test to examine a correlation between the decline in both IABP use and mortality demonstrated a significant correlation between the two trends (adjusted p<.0001). Patients who had an IABP placed and died were more likely to be female, and have acute CVD, shock, AMI, CHF, CRF, COPD, PVD, and atrial fibrillation.
Table II.
Association between death and IABP use by year with and without adjusting for covariates
| W/o adjusting for covariates | With adjusting for covariates | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Overall died | 35.35 (33.40,37.41) | <.0001 | 3.11 (2.82,3.43) | <.0001 |
| Individual years | ||||
| 1998 | 37.00 (31.38, 43.63) | <.0001 | 5.16 (3.92, 6.80) | <.0001 |
| 1999 | 31.71 (26.60, 37.81) | <.0001 | 2.94 (2.14, 4.03) | <.0001 |
| 2000 | 36.74 (31.25, 43.19) | <.0001 | 4.42 (3.32, 5.88) | <.0001 |
| 2001 | 39.31 (33.18, 46.58) | <.0001 | 5.27 (3.97, 6.99) | <.0001 |
| 2002 | 38.83 (32.20, 46.81) | <.0001 | 2.56 (1.83, 3.58) | <.0001 |
| 2003 | 35.73 (29.20, 43.71) | <.0001 | 1.98 (1.36, 2.88) | 0.0004 |
| 2004 | 34.69 (28.54, 42.17) | <.0001 | 3.04 (2.14, 4.30) | <.0001 |
| 2005 | 30.45 (24.60, 37.70) | <.0001 | 2.04 (1.40, 2.96) | 0.0002 |
| 2006 | 35.89 (29.46, 43.72) | <.0001 | 1.68 (1.21, 2.36) | 0.0023 |
| 2007 | 31.99 (25.31, 40.44) | <.0001 | 2.18 (1.44, 3.29) | 0.0002 |
| 2008 | 26.84 (21.34, 33.75) | <.0001 | 2.14 (1.47, 3.11) | <.0001 |
The adjusting covariates are year, age, race, gender, in-hospital mortality, cost, length of stay, hypertension, AMI, coronary atherosclerosis, congestive heart failure, occlusion or stenosis of precerebral arteries, cerebrovascular disease, transient cerebral ischemia, peripheral atherosclerosis, aortic/peripheral/visceral artery aneurysm/embolism/thrombosis, any malignancy, chronic obstructive pulmonary disease/bronchiectasis, SLE/connective tissue disease, cardiac and circulatory congenital anomalies, shock, diabetes mellitus, coagulation and hemorrhagic disorders, heart valve disorders, peri-/endo-/myocarditis, pulmonary heart disease, chronic renal failure, hemorrhagic cerebrovascular disease (CVD), ischemic CVD, acute CVD, other cerebrovascular disease, disorders of lipid metabolism, anemia, prior and current tobacco use, atrial fibrillation/flutter, aortic valve disorder, drug-eluding stent, bare-metal stent, coronary artery bypass graft, and ventricular assist device.
CABG and VAD in PCI
There were 1,777 patients with CABG in the no IABP group, and 892 patients in the IABP group (0.12% vs. 10.8%, Table I). Significant predictors for CABG included: AMI (OR 2.67; 95% CI 2.38–3.00; p<.0001), CAD (OR 2.94; 95% CI 2.31–3.74; p<.0001), and IABP use (OR 44.5; 95% CI 38.6–51.4 p<.0001) (See Figure 1 in supplemental files). Patients who underwent CABG were also less likely to die after adjusting for all variables (OR 0.28; 95% CI 0.21–0.38; p<.0001). The overall utilization of VAD’s in PCI was fairly low, 113 out of 1,556,602 patients undergoing PCI (Table I). The use of VAD in PCI was associated with higher mortality (OR: 3.63; 95% CI 1.85–7.09; p=0.0002, Figure 6).
DISCUSSION
Through time, with advances in device technology, insertion technique from surgical to percutaneous, and increasing operator experience, IABP has been successfully employed in a wide variety of clinical settings. Although still globally used in patients with shock, accumulating data from studies have demonstrated variable results in overall use and its impact on patient mortality. To date, there have been no consistent reports on IABP use in the US. In this large and representative dataset, we observed a marked reduction in IABP usage in the US between 1998 and 2008, even among patients with shock.
After its introduction, there appeared to be an increase in IABP usage based on literature supporting a mortality benefit with initial reports of it being used in patients undergoing coronary artery bypass grafting (CABG) pre-operatively.14,15 Additionally, the GUSTO-1 trial demonstrated that the use of IABP was also associated with improved mortality when introduced in patients with shock treated with thrombolysis.16 This mortality benefit was further validated by other studies, including the SHOCK and TACTICS trials.16–19 The results of these studies, along with the potential protective effects of balloon pumping in high-risk patients,20 a subsequent initial increase in IABP utilization at multiple centers in the United States and worldwide was noted.6,14,21,22 However, increased utilization rates were not consistent at every center.23 While some studies have indicated an increase in the use of IABP during our study period,24,25 we observed the contrary, which can in part be explained by smaller sample size and limited datasets previously reported in other studies. Also, patient selection based on procedure undertaken (high-risk PCI vs. all-comer PCI) and our study period spanning greater than a decade could have potentially contributed to the differences reported.
Shock is a well-known indication for IABP consideration. However, with rates of PCI increasing steadily from as early as mid-1980s based on evidence showing improved outcomes,26–28 there has been a decreasing trend in shock complicating AMI.25,29–31. Although our results demonstrated a numerically small, but significant, increase in the rates of shock and AMI, it is important to note that this was in relation to the proportion of patients who underwent PCI, and does not represent the overall incidence of shock and AMI in the US.
Examination of mortality benefit using IABP continues to be an area of investigation. Studies over the past 15 years have failed to prove a net mortality benefit when IABP is used in PCI.32–38 Based on these studies, it is apparent there is conflicting published evidence for IABP use, with some suggesting no mortality benefit while others supporting the contrary. The growing evidence demonstrating limited mortality benefit that IABP can offer, may have contributed, at least in part, to the observed decrease in IABP utilization.
Consistent with other studies,25,39 our study further confirms a temporal reduction in the rates in PCI-associated mortality. The mortality rates that were observed with balloon pump use are comparable to other studies,40,41 and independent predictors for mortality - AMI, shock, hemorrhagic and acute CVD and increasing age42,43 - are also congruous with previously published information.29 When tested for likelihood of mortality with IABP and predictors for mortality in patients presenting for PCI, patients with shock tend to have higher mortality rates, which is backed by prior literature demonstrating high mortality rates in shock with or without IABP support.29,31,42 A group of patients underwent CABG during the same hospitalization. A proportion of these patients who underwent CABG in the IABP group were likely referred for urgent/emergent CABG based on the higher rates of acute MI and shock observed in this group. It appears that in this select group of patients there appeared to be a mortality benefit. These findings need to be interpreted with great caution given likely significant selection bias and lack of detail about CABG procedures and indications in the NIS dataset.
There was a significant association between IABP use and mortality for each year during our study period. Although our results demonstrated a statistically significant association between the decline in mortality and decreasing IABP use, it is critical to stress the importance that our results do not infer any causation between mortality and IABP use. Being an observational study, we are unable to establish causation between the declining IABP and mortality trends. Unexplained procedures, unaddressed confounders, differences in practice patterns, and other elements likely confounded the correlation observed. Although there was a slight increase in the overall percentage of shock and AMI, IABP application in this cohort of patients declined, with a striking decline in patients with shock. Based on these observations, perhaps IABP is being reserved for critically ill patients who require any potentially life-saving measure and, as such, is associated with a patient population in whom high mortality rates may be inevitable.
STUDY LIMITATIONS
There are several important limitations to our analysis. This is a retrospective analysis of registry data with the limitations inherent to such analyses, which precludes us from confirming causation between the trends in the utilization of IABP to mortality. The NIS database does not allow for evaluation of practice patterns, which can affect observed IABP usage. Participation in the NIS registry is voluntary and data obtained are from selected centers that participated in the registry. The NIS registry may include hospitals with a varying likelihood of following evidence-based recommendations. Therefore, the results may not be generalized to other US hospitals that were not a part of the registry. Despite multivariable adjustment, we cannot exclude the possibility that residual measured and unmeasured confounding variables might account for the observed differences. Data quality is dependent upon the accuracy and completeness of documentation and abstraction. We cannot eliminate the potential confounding introduced by over- or under-coding. Although we adjusted for multiple baseline differences, selection bias affecting physician decision-making and IABP placement decisions may influence our findings. Our results only present IABP usage associated with PCI, and do not represent overall rates of IABP usage and mortality. This study only observed in-hospital mortality. Therefore, rates and trends of out-of-hospital mortality were not accounted for and not included in this study.
CONCLUSION
IABP has traditionally been the most commonly used mechanical assist device in AMI patients complicated by shock. However, clinicians appear to be using it less in this clinical setting. Aims toward early revascularization, improvements in management of AMI from the time of symptom onset to cardiac catheterization laboratory intervention, and better primary and secondary prevention may have contributed to the downward trend in in-hospital mortality rates in PCI we observed in our study. Perhaps the evolution in the treatment of ACS and the growing evidence of limited mortality benefit with balloon pumps have played some role in the decline of IABP utilization that was observed in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kantrowitz A, Tjonneland S, Freed PS, et al. Intraaortic balloon pumping. JAMA : the journal of the American Medical Association. 1968;203:988. [PubMed] [Google Scholar]
- 2.Ryan TJ, Anderson JL, Antman EM, et al. ACC/AHA guidelines for the management of patients with acute myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction) Journal of the American College of Cardiology. 1996;28:1328–1428. doi: 10.1016/s0735-1097(96)00392-0. [DOI] [PubMed] [Google Scholar]
- 3.Wright RS, Anderson JL, Adams CD, et al. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Academy of Family Physicians, Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. Journal of the American College of Cardiology. 2011;57:e215–e367. doi: 10.1016/j.jacc.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 4.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 6.Baskett RJ, O'Connor GT, Hirsch GM, et al. A multicenter comparison of intraaortic balloon pump utilization in isolated coronary artery bypass graft surgery. The Annals of thoracic surgery. 2003;76:1988–1992. doi: 10.1016/s0003-4975(03)01197-4. discussion 1992. [DOI] [PubMed] [Google Scholar]
- 7.Baskett RJ, O'Connor GT, Hirsch GM, et al. The preoperative intraaortic balloon pump in coronary bypass surgery: a lack of evidence of effectiveness. American heart journal. 2005;150:1122–1127. doi: 10.1016/j.ahj.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 8.Ghali WA, Ash AS, Hall RE, et al. Variation in hospital rates of intraaortic balloon pump use in coronary artery bypass operations. The Annals of thoracic surgery. 1999;67:441–445. doi: 10.1016/s0003-4975(98)01138-2. [DOI] [PubMed] [Google Scholar]
- 9.Sirbu H, Busch T, Aleksic I, et al. Ischaemic complications with intra-aortic balloon counter-pulsation: incidence and management. Cardiovasc Surg. 2000;8:66–71. doi: 10.1016/s0967-2109(99)00087-3. [DOI] [PubMed] [Google Scholar]
- 10.Cohen M, Urban P, Christenson JT, et al. Intra-aortic balloon counterpulsation in US and non-US centres: results of the Benchmark Registry. European heart journal. 2003;24:1763–1770. doi: 10.1016/j.ehj.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 11.HCUP Databases. Rockville, MD: Agency for Healthcare Research and Quality; 1998–2008. Healthcare Cost and Utilization Project (HCUP) [PubMed] [Google Scholar]
- 12.HCUP Overview. Healthcare Cost and Utilization Project (HCUP) 2009 Nov; http://www.hcup-us.ahrq.gov/overview.jsp.
- 13.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statistics in medicine. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Torchiana DF, Hirsch G, Buckley MJ, et al. Intraaortic balloon pumping for cardiac support: trends in practice and outcome, 1968 to 1995. The Journal of thoracic and cardiovascular surgery. 1997;113:758–764. doi: 10.1016/S0022-5223(97)70235-6. discussion 764-759. [DOI] [PubMed] [Google Scholar]
- 15.Christenson JT, Simonet F, Badel P, et al. Evaluation of preoperative intra-aortic balloon pump support in high risk coronary patients. European journal of cardiothoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 1997;11:1097–1103. doi: 10.1016/s1010-7940(97)00087-0. discussion 1104. [DOI] [PubMed] [Google Scholar]
- 16.Anderson RD, Ohman EM, Holmes DR, Jr, et al. Use of intraaortic balloon counterpulsation in patients presenting with cardiogenic shock: observations from the GUSTO-I Study. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. Journal of the American College of Cardiology. 1997;30:708–715. doi: 10.1016/s0735-1097(97)00227-1. [DOI] [PubMed] [Google Scholar]
- 17.Ohman EM, Nanas J, Stomel RJ, et al. Thrombolysis and counterpulsation to improve survival in myocardial infarction complicated by hypotension and suspected cardiogenic shock or heart failure: results of the TACTICS Trial. Journal of thrombosis and thrombolysis. 2005;19:33–39. doi: 10.1007/s11239-005-0938-0. [DOI] [PubMed] [Google Scholar]
- 18.Sanborn TA, Sleeper LA, Bates ER, et al. Impact of thrombolysis, intra-aortic balloon pump counterpulsation, and their combination in cardiogenic shock complicating acute myocardial infarction: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK? Journal of the American College of Cardiology. 2000;36:1123–1129. doi: 10.1016/s0735-1097(00)00875-5. [DOI] [PubMed] [Google Scholar]
- 19.Barron HV, Every NR, Parsons LS, et al. The use of intra-aortic balloon counterpulsation in patients with cardiogenic shock complicating acute myocardial infarction: data from the National Registry of Myocardial Infarction 2. American heart journal. 2001;141:933–939. doi: 10.1067/mhj.2001.115295. [DOI] [PubMed] [Google Scholar]
- 20.Christenson JT, Badel P, Simonet F, et al. Preoperative intraaortic balloon pump enhances cardiac performance and improves the outcome of redo CABG. The Annals of thoracic surgery. 1997;64:1237–1244. doi: 10.1016/S0003-4975(97)00898-9. [DOI] [PubMed] [Google Scholar]
- 21.Creswell LL, Rosenbloom M, Cox JL, et al. Intraaortic balloon counterpulsation: patterns of usage and outcome in cardiac surgery patients. The Annals of thoracic surgery. 1992;54:11–18. doi: 10.1016/0003-4975(92)91133-t. discussion 18–20. [DOI] [PubMed] [Google Scholar]
- 22.Cheng JM, Valk SD, den Uil CA, et al. Usefulness of intra-aortic balloon pump counterpulsation in patients with cardiogenic shock from acute myocardial infarction. The American journal of cardiology. 2009;104:327–332. doi: 10.1016/j.amjcard.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 23.Zaky SS, Hanna AH, Sakr Esa WA, et al. An 11-year, single-institution analysis of intra-aortic balloon pump use in cardiac surgery. Journal of cardiothoracic and vascular anesthesia. 2009;23:479–483. doi: 10.1053/j.jvca.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 24.Curtis JP, Rathore SS, Wang Y, et al. Use and effectiveness of intra-aortic balloon pumps among patients undergoing high risk percutaneous coronary intervention: insights from the National Cardiovascular Data Registry. Circulation. Cardiovascular quality and outcomes. 2012;5:21–30. doi: 10.1161/CIRCOUTCOMES.110.960385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang J, Mensah GA, Alderman MH, et al. Trends in acute myocardial infarction complicated by cardiogenic shock, 1979–2003, United States. American heart journal. 2006;152:1035–1041. doi: 10.1016/j.ahj.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Dzavik V, Sleeper LA, Cocke TP, et al. Early revascularization is associated with improved survival in elderly patients with acute myocardial infarction complicated by cardiogenic shock: a report from the SHOCK Trial Registry. European heart journal. 2003;24:828–837. doi: 10.1016/s0195-668x(02)00844-8. [DOI] [PubMed] [Google Scholar]
- 27.Hochman JS, Sleeper LA, White HD, et al. One-year survival following early revascularization for cardiogenic shock. JAMA : the journal of the American Medical Association. 2001;285:190–192. doi: 10.1001/jama.285.2.190. [DOI] [PubMed] [Google Scholar]
- 28.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. The New England journal of medicine. 1999;341:625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 29.Awad HH, Anderson FA, Jr, Gore JM, et al. Cardiogenic shock complicating acute coronary syndromes: insights from the Global Registry of Acute Coronary Events. American heart journal. 2012;163:963–971. doi: 10.1016/j.ahj.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Abdel-Qadir HM, Ivanov J, Austin PC, et al. Temporal trends in cardiogenic shock treatment and outcomes among ontario patients with myocardial infarction between 1992 and 2008. Circulation. Cardiovascular quality and outcomes. 2011;4:440–447. doi: 10.1161/CIRCOUTCOMES.110.959262. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg RJ, Spencer FA, Gore JM, et al. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation. 2009;119:1211–1219. doi: 10.1161/CIRCULATIONAHA.108.814947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van 't Hof AW, Liem AL, de Boer MJ, et al. A randomized comparison of intra-aortic balloon pumping after primary coronary angioplasty in high risk patients with acute myocardial infarction. European heart journal. 1999;20:659–665. doi: 10.1053/euhj.1998.1348. [DOI] [PubMed] [Google Scholar]
- 33.Stone GW, Marsalese D, Brodie BR, et al. A prospective, randomized evaluation of prophylactic intraaortic balloon counterpulsation in high risk patients with acute myocardial infarction treated with primary angioplasty. Second Primary Angioplasty in Myocardial Infarction (PAMI-II) Trial Investigators. Journal of the American College of Cardiology. 1997;29:1459–1467. doi: 10.1016/s0735-1097(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 34.Patel MR, Smalling RW, Thiele H, et al. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trial. JAMA : the journal of the American Medical Association. 2011;306:1329–1337. doi: 10.1001/jama.2011.1280. [DOI] [PubMed] [Google Scholar]
- 35.Sjauw KD, Engstrom AE, Vis MM, et al. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? European heart journal. 2009;30:459–468. doi: 10.1093/eurheartj/ehn602. [DOI] [PubMed] [Google Scholar]
- 36.Perera D, Stables R, Clayton T, et al. Long-term mortality data from the balloon pump-assisted coronary intervention study (BCIS-1): a randomized, controlled trial of elective balloon counterpulsation during high-risk percutaneous coronary intervention. Circulation. 2013;127:207–212. doi: 10.1161/CIRCULATIONAHA.112.132209. [DOI] [PubMed] [Google Scholar]
- 37.Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. The New England journal of medicine. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 38.Zeymer U, Hochadel M, Hauptmann KE, et al. Intra-aortic balloon pump in patients with acute myocardial infarction complicated by cardiogenic shock: results of the ALKK-PCI registry. Clinical research in cardiology : official journal of the German Cardiac Society. 2013;102:223–227. doi: 10.1007/s00392-012-0523-4. [DOI] [PubMed] [Google Scholar]
- 39.Fox KA, Steg PG, Eagle KA, et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA : the journal of the American Medical Association. 2007;297:1892–1900. doi: 10.1001/jama.297.17.1892. [DOI] [PubMed] [Google Scholar]
- 40.Ferguson JJ, 3rd, Cohen M, Freedman RJ, Jr, et al. The current practice of intra-aortic balloon counterpulsation: results from the Benchmark Registry. Journal of the American College of Cardiology. 2001;38:1456–1462. doi: 10.1016/s0735-1097(01)01553-4. [DOI] [PubMed] [Google Scholar]
- 41.Stone GW, Ohman EM, Miller MF, et al. Contemporary utilization and outcomes of intra-aortic balloon counterpulsation in acute myocardial infarction: the benchmark registry. Journal of the American College of Cardiology. 2003;41:1940–1945. doi: 10.1016/s0735-1097(03)00400-5. [DOI] [PubMed] [Google Scholar]
- 42.Berger PB, Tuttle RH, Holmes DR, Jr, et al. One-year survival among patients with acute myocardial infarction complicated by cardiogenic shock, and its relation to early revascularization: results from the GUSTO-I trial. Circulation. 1999;99:873–878. doi: 10.1161/01.cir.99.7.873. [DOI] [PubMed] [Google Scholar]
- 43.Hasdai D, Holmes DR, Jr, Califf RM, et al. Cardiogenic shock complicating acute myocardial infarction: predictors of death. GUSTO Investigators. Global Utilization of Streptokinase and Tissue-Plasminogen Activator for Occluded Coronary Arteries. American heart journal. 1999;138:21–31. doi: 10.1016/s0002-8703(99)70241-3. [DOI] [PubMed] [Google Scholar]