Abstract
Increased plasma levels of both leptin and C reactive protein (CRP) have been reported in a number of conditions, including obesity, and have been linked to cardiovascular pathophysiological processes and increased cardiovascular risk; interestingly these two biomarkers appear to be able to reciprocally regulate their bioavailability, through complex mechanisms that have not been completely clarified yet. Here we first review clinical evidence suggesting not only that the circulatory levels of CRP and leptin show an independent correlation, but also that assessing them in tandem may result in an increased ability to predict cardiovascular disease. We summarize also molecular studies showing that leptin is able to promote CRP production from hepatocytes and endothelial cells in vitro and discuss the studies addressing the possibility that in vivo leptin administration may be able to modulate plasma CRP levels. Furthermore, we describe two studies demonstrating that CRP directly binds leptin in extra-cellular settings, thus impairing its biological actions. Finally we report genetic evidence that common variations at the leptin receptor locus are associated with CRP blood levels. Overall, the data reviewed here show that the chronic elevation of CRP observed in obese subjects may worsen leptin resistance, contributing to the pathogenesis of cardiovascular disease, and highlight a potential link between conditions, such as leptin resistance and endothelial dysfunction, that may be amenable of pharmacological treatment targeted to the disruption of leptin-CRP interaction.
Keywords: Leptin, CRP, cardiovascular disease, leptin resistance.
INTRODUCTION
The role of the adipokine leptin in the regulation of energy balance, has been firmly established since its discovery almost twenty years ago [1]. Animal models lacking either the leptin gene (ob/ob mice) or the gene encoding for its membrane receptor (db/db mice) are extremely obese, hyperinsulinemic and insulin resistant. More importantly, it has been demonstrated that reestablishing leptin signaling in these mice normalizes body weight as well as all metabolic and endocrine alterations [2,3]. Soon after the discovery of this adipokine, it was therefore hypothesized that a reduction in leptin levels and/or an impairment in its secretion would be responsible also for human obesity. However, while leptin deficiency has indeed been shown to cause rare forms of severe human obesity [4], the majority of obese individuals show markedly increased plasma leptin concentrations, reflecting the greater amount of adipose tissue [5]. This excess of circulating leptin results in a state of resistance, characterized by a reduced response to the hormone action. In fact, hyperleptinemia could be considered a marker of leptin resistance and is not only commonly observed in obese subjects, but also independently associated with insulin resistance and cardiovascular disease (CVD) in humans [6]. Interestingly, several studies have shown an independent relationship between high leptin and atherosclerosis, myocardial infarction, stroke, and coronary artery intima-media thickness, suggesting that high leptin levels are associated with increased cardiovascular risk [7].
Mechanicistically, it has been demonstrated that leptin has a role in several processes relevant to cardiovascular disease, including the regulation of arterial pressure and vascular function and that there is a significant cross- talk between leptin and insulin signaling pathways [8]. These important mechanisms have been reviewed elsewhere, whereas in this Review we will focus on the multi-faceted cross talk between leptin and the pro-inflammatory molecule C reactive protein (CRP).
CRP is an acute-phase protein produced mainly by hepatocytes, which belongs to the family of pentraxins and as such consists of five identical non-covalently linked subunits. CRP concentration increases 4 to 6 hours after acute tissue injury or inflammation and declines rapidly with the resolution of the inflammatory process [9]. Conversely, low-grade chronic inflammation, a condition underlying insulin resistance and associated with cardiovascular disease and type 2 diabetes [10], produces minor elevations of CRP in the 3- to 10-mg/L range. Epidemiological evidence indicates that elevated CRP levels predict the development of type 2 diabetes and glucose disorders [11-13]. In addition, several cross-sectional studies in nondiabetic subjects, in the general population or in individuals with impaired glucose tolerance (IGT)/impaired fasting glucose (IFG) have confirmed that acute-phase reactants, such as CRP, are positively correlated with measures of insulin resistance, adiposity, and circulating triglyceride and negatively correlated with HDL cholesterol concentrations [14-18]. Furthermore in vitro studies have shown that, in addition to being a sensitive marker of inflammation, CRP has direct proinflammatory effects. In endothelial cells, CRP decreases nitric oxide and prostacyclin release and increases the expression levels of monocyte chemoattractant protein-1, interleukin-8, and plasminogen activator inhibitor-1. In monocyte-macrophages, CRP induces tissue factor secretion, increases reactive oxygen species and proinflammatory cytokine release, promotes monocyte chemotaxis and adhesion, and increases oxidized low-density lipoprotein uptake. Also, CRP has been shown in vascular smooth muscle cells to increase inducible nitric oxide production, increase NF-kB and mitogen-activated protein kinase activities, and, most importantly, up-regulate angiotensin type-1 receptor resulting in increased reactive oxygen species and vascular smooth muscle cell proliferation [19-21]. More recently, it has also been reported that CRP has a direct inhibitory effect on insulin signaling and action in a skeletal muscle cell model [22].
PLASMA LEPTIN AND CRP LEVELS SHOW A DIRECT CORRELATION
Increased plasma levels of both leptin and CRP have been reported in a number of conditions, including obesity and inflammation, and have been linked to cardiovascular pathophysiological processes and increased cardiovascular risk [9,11-18,23-26]. Several studies have demonstrated that a direct correlation exists between the concentrations of the two biomarkers (Table 1). A first, cross-sectional study on 179 apparently healthy Japanese male college students aged 18 to 22 reported that CRP serum levels had a positive correlation with leptin levels (R=0.28, P<0.0002), which was independent from body mass index (BMI) [27]. This finding was confirmed by a subsequent study, carried out in a cohort of 100 healthy volunteers (48 men, and 52 women). In this study, it has been observed that leptin was independently associated with CRP after adjustment for age, gender, BMI, waist-to-hip ratio, smoking, and alcohol consumption (P<0.0007). Furthermore, a significant relationship between leptin and CRP was reported in both sexes, when men (R=0.55, P<0.0001) and women (R=0.61, P<0.0001) were analyzed separately. Importantly, the association between leptin and CRP remained significant when the analysis was restricted to lean individuals (BMI <25 kg/m2) (R=0.55, P<0.0001) [28].
Table 1.
Clinical studies showing a direct association between CRP and leptin plasma levels.
| Subjects (N and description) | AgeMean±SD or range | CRP (mg/L)Mean±SD or median | Leptin (ng/ml)Mean±SD or median | Statistical Correlations |
|---|---|---|---|---|
| 179 healthy volunteersa | 18-22 | 0.51±1.45 | 2.3±2.9 | R =0.28, P<0.0002 |
| 100 healthy volunteersb | 36±2 | 3.07±0.046 W1.06±0.012 M | 16.9±2 W5.5±0.5 M | R=0.61, P<0.0001 WR=0.55, P<0.0001 M |
| 946 healthy community-dwelling, older adultsc | 65-102 | 2.8 (4.4) | 8.6 (12.3) | β = 0.20, P <0.0001 |
| 1862 healthy young adultsd | 24-39 | 0.75 (0.32-1.93) W0.56 (0.29-1.27) M | 12.5(7.8-19.5) W4.1(2.4-6.5) M | R=0.47, P<0.0001 WR=0.46, P<0.0001 M |
| 20 OB5 NO e | 47.8±13.3 OB56.8±7.6 NO | 5.7±3.4 OB3.8±1.6 NO | 17.6±8.7 OB6.2±3.3 NO | R=0.43,P<0.044 # |
| 63 (28 MO)f | 35±6.89 | 8.2 (0.13-56.6) | 52.7±19.8 MO7.6±4.8 | R=0.53,P<0.001# |
| 148 with T2Dg | 37-84 | 0.788±0.049 | 5.6±0.4 | R=0.330, P<0.001 |
| 150 with T2Dh | 52.8±11.1 W51.9±9.9 M | 2.34±1.651.72±2.1 | 9.82±6.784.76±2.44 | β= 0.326, P = 0.01 |
| 6251 (598 withT2D)i | 44.4±0.21 § | NA | NA | ρ = 0.32, P<0.0001 Wρ = 0.22, P<0.0001 M |
| 1460 (894 with T2D)l | 30-77 | 1.7(0.8-3.8) | 12.1(6.5-23.2) | R=0.41, P<0.001 WR=0.29, P<0.001 M |
[27]a ; [28]b; [29]c; [30]d; [31]e; [32]f; [33]g; [34]h; [35]i; [38]l
W=women; M=men; OB=obese; NO=non-obese; MO=morbidly obese; T2D= type 2 diabetes; NA=not available
The correlations were not longer significant after adjustment for BMI in obese individuals; § values are expressed as mean ±SE
An independent association between leptin and CRP levels (β coefficient=0.20, P<0.0001) was found in a study carried out in 946 community-dwelling older subjects (398 men, 548 women; age range 65 to 102 years) [29]. The independent association between leptin and CRP in healthy individuals was also confirmed by a larger population-based study conducted at five health centers in Finland comprising 1862 young adults (971 women; 891 men) aged 24-39 yr [30]. This study reported that CRP and leptin levels were significantly correlated (R=0.47, P<0.0001 for women; R=0.46, P<0.0001 for men). In multiple regression analyses including age, BMI, waist circumference, insulin, lipids, systolic and diastolic blood pressure, smoking status, and use of oral contraceptives in women, leptin proved to be the main determinant of CRP in men (P<0.0001) and the second most important determinant in women (P<0.0001).
Similar findings have been reported in obese individuals [31,32], even if the results from these studies indicate that, in this group of subjects, the relationship between leptin and CRP may be a reflection of fat mass, since the correlation between the two variables was abolished when the data were adjusted for BMI. The independent correlation between leptin and CRP levels has also been investigated in subjects with type 2 diabetes. In a study comprising 148 Japanese subjects with type 2 diabetes, serum CRP levels were positively correlated with leptin (R=0.330, P<0.001), and leptin was shown, by multiple regression analysis, to be an independent predictor of CRP concentration (R=0.330, P<0.001), along with interleukin-6 (IL-6) and triglycerides plasma levels [33]. Similar results were reported by a study carried out in 150 recently (≤3 years) diagnosed type 2 diabetic patients showing a significant association between serum CRP and leptin (β=0.326, P=0.01), after adjustment for age and gender [34].
Notably, a study that analyzed data from the Third National Health and Nutrition Examination Survey (NHANES III) with a total of 6,251 participants, including 598 patients with type 2 diabetes, not only confirmed a significant correlation between leptin and CRP levels (Spearman correlation ρ=0.22 in men and ρ=0.32 in women, both P<0.0001), but demonstrated also that individuals with high levels of both leptin and CRP were at higher risk of CVD than those with high levels of leptin or CRP alone [35]. Furthermore, while leptin concentration remained independently associated with CVD after adjustment for CRP, the reverse was not true, as elevated CRP levels were no longer associated with CVD after adjustment for leptin. This observation might explain the suboptimal performance of CRP as a biomarker of cardiovascular risk in some studies [36,37] and suggest that the two markers should be considered in tandem when estimating CVD risk [35]. This suggestion is supported by the results of a more recent study aimed to test the hypothesis that CRP levels may modify the relationship of leptin concentration with coronary artery calcium (CAC), a measure of coronary atherosclerosis in individuals with excess adiposity. The authors examined 1,460 asymptomatic individuals from two community-based cross-sectional studies, the Study of Inherited Risk of Coronary Atherosclerosis (SIRCA) and the Penn Diabetes Heart Study (PDHS), coordinated at a single, university-based research center at the University of Pennsylvania [38], and analyzed the interaction of log-transformed plasma leptin levels with higher CRP levels. The association of plasma leptin with CAC was modified by higher CRP regardless of the cut-point used (interaction term P values all <0.01 in fully adjusted models), while no interaction with CRP was observed in control analyses with adiponectin, BMI or waist circumference. The authors concluded that CRP itself, or the inflammatory pathways that it captures, affects the relationship of circulating leptin concentration to the burden of underlying atherosclerosis in overweight and obese individuals, clinical conditions where chronic inflammation and leptin resistance coexist.
MOLECULAR MECHANISMS LINKING LEPTIN AND CRP: LEPTIN IS ABLE TO REGULATE CRP EXPRESSION
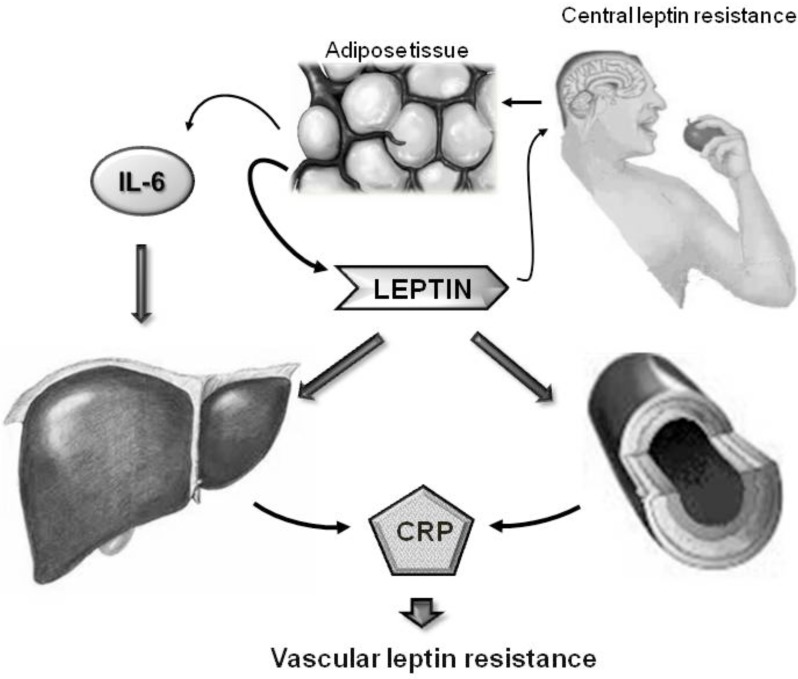
The physiological and molecular mechanisms that link leptin and CRP appear complex (Fig. 1). Leptin is produced by the adipose tissue, and adipocytes are also an important source of circulating inflammatory cytokines, such as IL-6, which in turn promote CRP synthesis [39]. However, leptin itself may be able to stimulate CRP synthesis from the liver, as suggested by experiments performed in primary human hepatocytes. In fact when human hepatocytes were incubated with human leptin at physiological concentrations (1-16 nM) for 24 h a dose-dependent accumulation of secreted CRP in the culture medium was observed; interestingly preincubation with a specific Phosphatidylinositol 3-kinases (PI3K) inhibitor completely blocked this leptin action, suggesting that leptin-induced hepatic production of CRP is a PI3K-dependent process. To exclude the possibility that they were observing a IL-6 mediated effect, the authors incubated primary hepatocytes in the presence of IL-6 at doses comparable to those observed in vivo in obese individuals and showed that, at these concentrations, IL-6 was unable to promote CRP synthesis [40]. Subsequently, following the accumulating evidence indicating that CRP can be produced not only from hepatocytes, but also from additional cell types [41], similar results have been obtained by two independent studies in endothelial cells [42,43]. A first study showed that in human artery coronary endothelial cells (HAECs) a dose dependent increase of CRP levels was observed with increasing concentration of leptin (0 to 400 ng/ml) [42]. Interestingly, the increase of CRP expression was attenuated in the presence of anti-leptin receptor antibodies, indicating that a classical leptin signaling was mediating this leptin effect. A subsequent study confirmed these findings, showing that lower, and more physiological, leptin concentrations (5-10 ng/ml) were able not only to induce CRP synthesis, but also to promote its release in the culture medium [43].
Fig. (1).
Schematic representation of the regulatory loop linking leptin and CRP. Leptin is produced by the adipose tissue, and adipocytes are also an important source of circulating inflammatory cytokines, such as IL-6, which in turn promote CRP synthesis In parallel, leptin itself is able to directly stimulate CRP synthesis from the liver and from the vasculature.
These in vitro data are supported by in vivo experiments showing that exogenous leptin administration is able to increase plasma CRP concentration. In a first single-blind, 22-day, placebo/drug/ placebo study, six subjects received recombinant methionyl human leptin (r-metHuLeptin) at the dose of 0.3 mg/kilogram subcutaneously for 6 days. No demonstrable effect of leptin administration on energy metabolism was evident in this small group of never-obese individuals; leptin treatment induced an elevation in CRP, IL-6, and haptoglobin, even if the small number of patients studied made impossible to determine whether these changes were related to leptin itself, to the interaction of leptin with a preexisting inflammatory process (e.g., dental abscess reported in one subjects), or to mild subcutaneous inflammation at the site of injection (e.g., observed in two subjects) [44]. In a proof-of-concept study aimed to sought evidence for a proinflammatory role of leptin, pegylated human recombinant leptin (PEG-OB 80 mg) was administered weekly by subcutaneous injections to 12 obese subjects undergoing diet-induced weight loss. The only proinflammatory molecule whose circulatory levels increased significantly upon PEG-OB administration was plasma CRP (p<0.05) [45]. In a subsequent randomized, placebo-controlled study, twenty healthy, young (18-35 yr old), normal-weight (BMI=20-26 kg/m2) female volunteers underwent a 4-d fasting during which they received r-metHuLeptin at a dose sufficient to prevent the fasting-induced decline in leptin, thus maintaining physiological concentrations of the hormone. Leptin administration increased CRP levels (6.3±2.4 vs. 0.7±0.3 mg/liter; P<0.04 in leptin-treated vs placebo-treated, respectively), and stimulated other inflammatory markers, including circulating P-selectin levels and platelet aggregation, as compared to placebo-treated fasting women. While the study was not specifically designed to discriminate between the direct and indirect effects of leptin, it is worth noting that changes in proinflammatory markers levels at the end of the study significantly correlated with the leptin levels achieved, but not with leptin-induced changes in endocrine and metabolic function [46]. These results were however not replicated from other research groups [47-49]; notably when r-metHuLeptin (20 mg/daily in two doses) was administered for 16-weeks to 117 obese subjects with type 2 diabetes, no significant changes in CRP levels nor in the levels of other cytokines (soluble Tumor Necrosis Factor-α receptors, interleukin-10, monocyte chemoattractant protein-1, IL-6) were observed as compared to placebo-treated matched obese diabetic subjects [49]. Taken together the results from these studies suggest that the ability of leptin to directly induce CRP in vivo is highly dependent from the leptin sensitivity state and indeed the more profound changes were observed in a state of enhanced leptin sensitivity [46], while no effect was reported in obese leptin resistant individuals [49]. It is also worth underlying that exogeneous administration of leptin may not provide an accurate picture of the actions of the adipokine in more physiological settings.
MOLECULAR MECHANISMS LINKING LEPTIN AND CRP: CRP MAY ABLE TO MODULATE LEPTIN ACTION
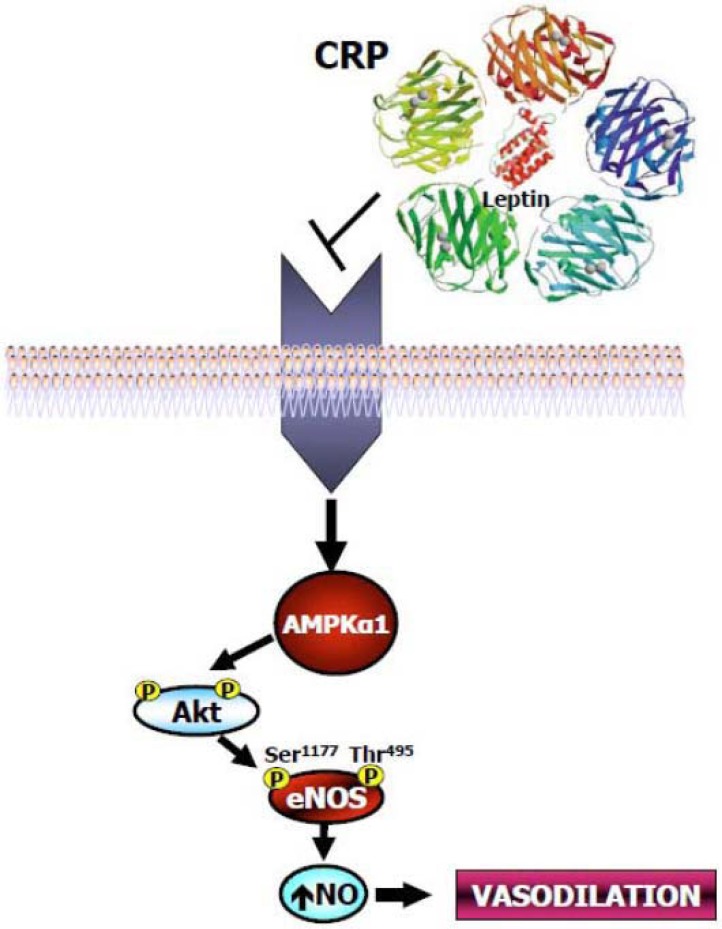
The data reviewed above clearly demonstrate that circulating leptin and CRP levels are linked by a regulatory loop in which leptin stimulates hepatic and vascular CRP expression (Fig. 1). Interestingly, there are experimental data suggesting that a feedback inhibitory mechanism may exist with CRP directly binding leptin and inhibiting its action (Fig. 2). Chen and colleagues reported the presence of five major serum leptin-interacting proteins (SLIPs) in human blood, isolated by leptin-affinity chromatography and identified by mass spectrometry and immunochemical analysis [40]. These five proteins were termed SLIP-1, SLIP-2, SLIP-3, SLIP-4 and SLIP-5, and had apparent molecular weights on silver-stained SDS-PAGE gels of ≅30, ≅42, ≅65, ≅70 and ≅85 kDa, respectively. All five human SLIPs had rat counterparts, as passage of rat serum through the mouse leptin affinity column yielded proteins of similar molecular weights. SLIP-1 has been identified as human CRP by excision from the SDS-PAGE gel and mass spectrometry analysis. The identity of both human and rat SLIP-1 was further confirmed by running the correspondent column eluates on SDS-PAGE, transferring the gels on nitrocellulose membrane and analyzing them with the appropriate human or rat anti-CRP antibodies. Direct interaction between CRP and leptin was further demonstrated by immunoprecipitation assays, where purified human and rat CRP proteins were premixed with recombinant human and mouse leptin, respectively, before addition of species-specific antibodies to leptin and immunoprecipitation. Protein precipitates were then subjected to western blot assays using specific antibodies to CRP and it was revealed that immunoprecipitation with antibodies to leptin pulled down CRP from the leptin-CRP mixtures. The results obtained from Chen et al. were unfortunately not confirmed by two other groups, despite the complementary well-designed experimental attempts to reproduce them, and were therefore judged to be experimental artifacts due to the known calcium-dependent binding of CRP to the agarose matrix used [48,50,51]. By contrast, in a sub-sequent study we were able to demonstrate a direct interaction of CRP and leptin and to show that CRP inhibits leptin action in both a cellular and an animal model [52]. In this study, leptin was preincubated for 30 min with 0.9 μg/ml human recombinant (hr)CRP followed by further incubation with either anti-CRP or anti-leptin antibody; the incubation mixture was then immuno-precipitated and immunoblotted with either anti-CRP or leptin antibody. It was observed that the anti-leptin antibody was able to precipitate hrCRP from the complex, and that the anti-CRP antibody coimmunoprecipitated leptin from the incubation mixture. These first experiments confirmed a direct, physical interaction between the two molecules. Next, to address whether the direct interaction between leptin and hrCRP might attenuate physiological functions of leptin, we preincubated leptin with increasing concen-trations of hrCRP and assessed the ability of leptin to stimulate AMP-activated protein kinase (AMPK) and its downstream target acetyl-coenzyme A carboxylase (ACC) phosphorylation in HAECs. Pre-incubation of hrCRP with leptin impaired in a dose-dependent manner both AMPK Thr172 and ACC Ser79 phosphorylation induced by leptin alone. In addition, preincubation of hrCRP with leptin blocked both Akt Ser473 and endothelial NO synthase (eNOS) Ser1177 phosphorylation induced by leptin alone (Fig. 2). Pre-incubation of hrCRP with leptin resulted also in a marked reduction in both NO production and intracellular cGMP accumulation in response to leptin alone.
Fig. (2).
Schematic representation of the physical interaction between CRP pentameric molecule and leptin. Pre-incubation of hrCRP with leptin impaired AMPK phosphorylation and blocked both Akt Ser473 and endothelial NO synthase (eNOS) Ser1177 phosphorylation induced by leptin alone.
Similar results were obtained in vivo. When C57BL6J mice were treated with leptin alone, we observed a significant increase in AMPK Thr172, ACC Ser79, Akt Ser473, and eNOS Ser1177 phospho-rylation in aorta lysates from these animals; by contrast, prein-cubation with hrCRP significantly reduced this in vivo physio-logical function of leptin.
Notably, when a calcium chelant (EGTA 10 mM) was added to the mixture, the inhibitory effect of hrCRP on leptin signaling was blocked, indicating that leptin and CRP interaction requires calcium.
Taken together, these data suggest that during the preincubation of hrCRP with leptin, the two molecules bind each other and are thus unavailable to further bind their respective membrane receptors. The concentration of hrCRP (0.9 μg/ml) required to inhibit leptin effect on HAECs was within the ranges observed in individuals with cardiovascular disease or obesity, thus suggesting that these findings may be clinically meaningful.
MOLECULAR MECHANISMS LINKING LEPTIN AND CRP: GENETIC VARIANTS AT THE LEPTIN RECEPTOR LOCUS ARE ASSOCIATED WITH CRP PLASMA LEVELS
An additional level to the complexity of the interactions between CRP and leptin is represented by the observation that genetic variants at the leptin receptor locus (LEPR) are independently related to circulating CRP levels. A linkage disequilibrium analysis of 71 single-nucleotide polymorphisms (SNPs) spanning the LEPR locus in a cohort of 630 healthy Caucasian individuals, revealed four haplotype blocks; of these, the fourth block was significantly associated with CRP levels (r2=0.022, P=0.049). The strongest effect was observed for the SNP rs1805096, with homozygous carriers of the major allele showing 32% higher CRP levels than carriers of the minor allele (P=0.011) [53]. The association of the LEPR locus with CRP circulating levels was confirmed by a genome-wide study in which 336,108 SNPs have been evaluated among 6,345 apparently healthy women as potential determinants of plasma CRP concentration. Overall, seven loci associated with plasma CRP at levels achieving genome-wide statistical significance have been identified, including loci in or near the CRP gene itself, and the leptin receptor protein gene. Notably, virtually identical results were observed when the analysis was restricted to the study participants with CRP levels < 10 mg/l, characteristic of chronic inflammation [54].
CONCLUSIONS
The data reviewed here demonstrate that a complex interplay links plasma leptin and plasma CRP levels. Clinical data underlie the importance of both markers in estimating CVD risk and strongly suggest that an additional value will be attained by assessing them in tandem, especially in clinical states, such as obesity, where chronically elevated CRP levels and leptin resistance coexist [27-38]. Molecular studies indicate that leptin is able to modulate CRP expression levels, both indirectly, throughout its action on other proinflammatory molecules, such as IL-6, and directly promoting its hepatic and vascular production [40,42,43]. In turn, CRP may be able to regulate circulatory leptin bioavailability, as it has been demonstrated that in extracellular settings the two molecules coprecipitate [40, 52] and that this interaction impairs leptin ability to bind its receptor and activate intracellular signaling [52]. If confirmed by future in vivo studies in humans, these findings may highlight a potential link between conditions, such as leptin resistance and endothelial dysfunction, that may be amenable of pharmacological treatment targeted to the disruption of leptin-CRP interaction. Notably, as reported above, leptin induced-vascular NO production is impaired in obesity/metabolic syndrome, conditions characterized by hyperleptinemia, higher plasma CRP levels and leptin resistance [6-8]. Therapies aimed to improve the beneficial effects of leptin by modulating its intracellular signal transduction might thus be helpful in prevention and treatment of leptin resistance-related cardiovascular pathologies. On the other hand, CRP, in addition to being a marker of inflammation, has been shown to exert proatherogenic effects on endothelial and vascular smooth muscle cells [19-21]. Pharmacological agents able to reduce CRP production may therefore not only be useful to prevent the direct negative effects of CRP, but also to increase leptin bioavailability. Finally, several studies have demonstrated that weight loss and physical aerobic activity are associated with a decrease in CRP and leptin levels as well as with improved endothelial function and decreased CVD risk [55-58]. These findings, in addition to confirming the link between leptin, CRP levels and CVD, point to weight loss and physical activity as therapeutic approaches able to break the vicious circle feeding CVD pathogenesis.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Pelleymounter MA, Cullen MJ, Baker MB , et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540 –3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Charlat O, Tartaglia LA , et al. Evidence that the diabetes gene encodes the leptin receptor: Identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–5. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 4.Considine RV, Considine EL, Williams CJ , et al. Evidence against either a premature stop codon or the absence of obese gene mRNA in human obesity. J Clin Invest. 1995;95:2986–8. doi: 10.1172/JCI118007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiegelman BM, Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell. 1996;87:377–89. doi: 10.1016/s0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- 6.Zimmet P, Boyko EJ, Collier GR, de Courten M. Etiology of the metabolic syndrome potential role of insulin resistance, leptin resistance, and other players. Ann N Y Acad Sci. 1999;892:25–44. doi: 10.1111/j.1749-6632.1999.tb07783.x. [DOI] [PubMed] [Google Scholar]
- 7.Martin SS, Qasim A, Reilly MP. Leptin resistance a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52:1201–10. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beltowski J. Leptin and the regulation of endothelial function in physiological and pathological conditions. Clin Exp Pharmacol Physiol. 2012;39:168–78. doi: 10.1111/j.1440-1681.2011.05623.x. [DOI] [PubMed] [Google Scholar]
- 9.Blake GJ, Ridker PM. Novel Clinical Markers of Vascular Wall Inflammation. Circ Res. 2001;89:763–71. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- 10.Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herder C, Baumert J, Zierer A , et al. Immunological and cardiometabolic risk factors in the prediction of type 2 diabetes and coronary events: MONICA/KORA Augsburg case-cohort study. LoS One. 2011;6:e19852. doi: 10.1371/journal.pone.0019852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 13.Perticone F, Maio R, Sciacqua A , et al. Endothelial dysfunction and C-reactive protein are risk factors for diabetes in essential hypertension. Diabetes. 2008;57:167–71. doi: 10.2337/db07-1189. [DOI] [PubMed] [Google Scholar]
- 14.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects association with obesity insulin resistance and endothelial dysfunction a potential role for cytokines originating from the adipose tissue?. Arterioscler Thromb Vasc Biol. 1999;19:972–8. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 15.Yoon SS, Dillon CF, Carroll M, Illoh K, Ostchega Y. Effects of statins on serum inflammatory markers the U.. National Health and Nutrition Examination Survey 1999-2004. J Atheroscler Thromb. 2010;17:1176–82. doi: 10.5551/jat.5652. [DOI] [PubMed] [Google Scholar]
- 16.Cardellini M, Andreozzi F, Laratta Eetal. Plasma interleukin-6 levels are increased in subjects with impaired glucose tolerance but not in those with impaired fasting glucose in a cohort of Italian Caucasians. Diabetes Metab Res Rev. 2007;23:141–5. doi: 10.1002/dmrr.679. [DOI] [PubMed] [Google Scholar]
- 17.Knudsen EC, Seljeflot I, Michael A , et al. Increased levels of CRP and MCP-1 are associated with previously unknown abnormal glucose regulation in patients with acute STEMI a cohort study. Cardiovasc Diabetol. 2010;9:47. doi: 10.1186/1475-2840-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Temelkova-Kurktschiev T, Henkel E, Koehler C, Karrei K, Hanefeld M. Subclinical inflammation in newly detected type II diabetes and impaired glucose tolerance. Diabetologia. 2002;45:151. doi: 10.1007/s125-002-8256-1. [DOI] [PubMed] [Google Scholar]
- 19.Verma S, Wang CH, Li SH , et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–9. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 20.Montero I, Orbe J, Varo N , et al. C-reactive protein induces matrix metalloproteinase-1 and -10 in human endothelial cells: implications for clinical and subclinical atherosclerosis. J Am Coll Cardiol. 2006;47:1369–78. doi: 10.1016/j.jacc.2005.10.070. [DOI] [PubMed] [Google Scholar]
- 21.Hattori Y, Matsumura M, Kasai K. Vascular smooth muscle cell activation by C-reactive protein. Cardiovasc Res. 2003;58:186–95. doi: 10.1016/s0008-6363(02)00855-6. [DOI] [PubMed] [Google Scholar]
- 22.D’Alessandris C, Lauro R, Presta I, Sesti G. C-reactive protein induces phosphorylation of insulin receptor substrate-1 on Ser307 and Ser 612 in L6 myocytes, thereby impairing the insulin signalling pathway that promotes glucose transport. Diabetologia. 2007;50:840–9. doi: 10.1007/s00125-006-0522-y. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 24.Singh M, Bedi US, Singh PP, Arora R, Khosla S. Leptin and the clinical cardiovascular risk. Int J Cardiol. 2010;140:266–71. doi: 10.1016/j.ijcard.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Reilly MP, Iqbal N, Schutta M , et al. Plasma Leptin Levels Are Associated with Coronary Atherosclerosis in Type 2 Diabetes. J Clin Endocrinol Metab. 2004;89:3872–8. doi: 10.1210/jc.2003-031676. [DOI] [PubMed] [Google Scholar]
- 26.Wallace AM, McMahon AD, Packard CJetal. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation. 2001;104:3052–6. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 27.Kazumi T, Kawaguchi A, Hirano T, Yoshino G. C-reactive protein in young apparently healthy men associations with serum leptin QTc interval and high-density lipoprotein-cholesterol. Metabolism. 2003;52:1113–6. doi: 10.1016/s0026-0495(03)00184-7. [DOI] [PubMed] [Google Scholar]
- 28.Shamsuzzaman AS, Winnicki M, Wolk Retal. Independent association between plasma leptin and C-reactive protein in healthy humans. Circulation. 2004;109:2181–5. doi: 10.1161/01.CIR.0000127960.28627.75. [DOI] [PubMed] [Google Scholar]
- 29.Ble A, Windham BG, Bandinelli S , et al. Relation of plasma leptin to C-reactive protein in older adults (from the Invecchiare nel Chianti study). Am J Cardiol. 2005;96:991–5. doi: 10.1016/j.amjcard.2005.05.058. [DOI] [PubMed] [Google Scholar]
- 30.Viikari LA, Huupponen RK, Viikari JSA , et al. Relationship between Leptin and C-Reactive Protein in Young Finnish Adults. J Clin Endocrinol Metab. 2007;92:4753–8. doi: 10.1210/jc.2007-0103. [DOI] [PubMed] [Google Scholar]
- 31.Gómez-Ambrosi J, Salvador J, Páramo JAetal. Involvement of leptin in the association between percentage of body fat and cardiovascular risk factors. Clin Biochem. 2002;35:315–20. doi: 10.1016/s0009-9120(02)00320-x. [DOI] [PubMed] [Google Scholar]
- 32.van Dielen FMH, van't Veer C, Schols AM, Soeters PB, Buurman WA, Greve JWM. Increased leptin concentrations correlate with increased concentrations of inflammatory markers in morbidly obese individuals. Int J Obesity. 2001;25:1759–66. doi: 10.1038/sj.ijo.0801825. [DOI] [PubMed] [Google Scholar]
- 33.Yanagawa T, Taniguchi A, Fukushima M , et al. Leptin triglycerides and interleukin 6 are independently associated with C-reactive protein in Japanese type 2 diabetic. patients. 2007;75:2–6. doi: 10.1016/j.diabres.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 34.Park JS, Cho MH, Nam JS , et al. Visceral adiposity and leptin are independently associated with C-reactive protein in Korean type 2 diabetic patients. Acta Diabetol. 2010;47:113–8. doi: 10.1007/s00592-009-0125-4. [DOI] [PubMed] [Google Scholar]
- 35.Romero-Corral A, Sierra-Johnson J, Lopez-Jimenez F , et al. Relationships between leptin and C-reactive protein with cardiovascular disease in the adult general population. Nat Clin Pract Cardiovasc Med. 2008;5:418–25. doi: 10.1038/ncpcardio1218. [DOI] [PubMed] [Google Scholar]
- 36.Wolk R Berger P, Lennon RJ, Brilakis ES, Johnson BD, Somers VK. Plasma leptin and prognosis in patients with established coronary atherosclerosis. J Am Coll Cardiol. 2004;44:1819–24. doi: 10.1016/j.jacc.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 37.Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, Sattar N. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation. 2001;104:3052–3056. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 38.Martin SS, Qasim AN, Rader DJ, Reilly MP. C-Reactive Protein Modifies the Association of Plasma Leptin With Coronary Calcium in Asymptomatic Overweight Individuals. Obesity. 2011 doi: 10.1038/oby.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinhold B, Rüther U. Interleukin-6-dependent and -independent regulation of the human C-reactive protein gene. Biochem J. 1997;327:425–9. doi: 10.1042/bj3270425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen K, Li F, Li J , et al. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat Med. 2006;4:426–32. doi: 10.1038/nm1372. [DOI] [PubMed] [Google Scholar]
- 41.Calabro P, Willerson JT, Yeh ETH. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. 2003;108:1930–2. doi: 10.1161/01.CIR.0000096055.62724.C5. [DOI] [PubMed] [Google Scholar]
- 42.Singh P, Hoffmann M, Wolk R, Shamsuzzaman ASM, Somers K. Leptin Induces C-Reactive Protein Expression in Vascular Endothelial Cells. Arterioscler Thromb Vasc Biol. 2007;27:e302–7. doi: 10.1161/ATVBAHA.107.148353. [DOI] [PubMed] [Google Scholar]
- 43.De Rosa S, Cirillo P, Pacileo M, Di Palma V, Paglia A, Chiariello M. Leptin stimulated C-reactive protein production by human coronary artery endothelial cells. J Vasc Res. 2009;46:609–17. doi: 10.1159/000226229. [DOI] [PubMed] [Google Scholar]
- 44.Mackintosh RM, Hirsch J. The effects of leptin administration in non-obese human subjects. Ob Res. 2001;9:462–9. doi: 10.1038/oby.2001.60. [DOI] [PubMed] [Google Scholar]
- 45.Hukshorn CJ, Lindeman JHN, Toet KH , et al. Leptin and the Proinflammatory State Associated with Human Obesity. J Clin Endocrinol Metab. 2004;89:1773–8. doi: 10.1210/jc.2003-030803. [DOI] [PubMed] [Google Scholar]
- 46.Canavan B, Salem RO, Schurgin S , et al. Effects of physiological leptin administration on markers of inflammation, platelet activation, and platelet aggregation during caloric deprivation. J Clin Endocrinol Metab. 2005;90:5779–85. doi: 10.1210/jc.2005-0780. [DOI] [PubMed] [Google Scholar]
- 47.Hukshorn CJ, van Dielen FM, Buurman WA, Westerterp-Plantenga MS, Campfield LA, Saris WH. The effect of pegylated recombinant human leptin (PEG-OB) on weight loss and inflammatory status in obese subjects. Int J Obes Relat Metab Disord. 2002;26:504–509. doi: 10.1038/sj.ijo.0801952. [DOI] [PubMed] [Google Scholar]
- 48.Farooqi SI, O'Rahilly S. Is leptin an important physiological regulator of CRP?. Nat Med. 2007;13:16–7. doi: 10.1038/nm0107-16. [DOI] [PubMed] [Google Scholar]
- 49.Chan JL, Bullen J, Stoyneva V, Depaoli AM, Addy C, Mantzoros CS. Recombinant methionyl human leptin administration to achieve high physiologic or pharmacologic leptin levels does not alter circulating inflammatory marker levels in humans with leptin sufficiency or excess. J Clin Endocrinol Metab. 2005;90:1618–24. doi: 10.1210/jc.2004-1921. [DOI] [PubMed] [Google Scholar]
- 50.Hutchinson WL, Coll AP, Gallimore JR, Tennent GA, Pepys MB. Is leptin an important physiological regulator of CRP?. Nat Med. 2007;13:17–8. doi: 10.1038/nm0107-17. [DOI] [PubMed] [Google Scholar]
- 51.Gertler A, Niv-Spector L, Reicher S. Is leptin an important physiological regulator of CRP?. Nat Med. 2007;13:18–9. doi: 10.1038/nm0107-18. [DOI] [PubMed] [Google Scholar]
- 52.Procopio C, Andreozzi F, Laratta E , et al. Leptin-Stimulated Endothelial Nitric-Oxide Synthase via an Adenosine 5’-Monophosphate-Activated Protein Kinase/Akt Signaling Pathway Is Attenuated by Interaction with C-Reactive Protein. Endocrinology. 2009;150:3584–93. doi: 10.1210/en.2008-0921. [DOI] [PubMed] [Google Scholar]
- 53.Zhang YY, Gottardo L, Mlynarski W , et al. Genetic variability at the leptin receptor (LEPR) locus is a determinant of plasma fibrinogen and C-reactive protein levels. Atherosclerosis. 2007;191:121–7. doi: 10.1016/j.atherosclerosis.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 54.Ridker PM, Pare G, Parker A , et al. Loci related to metabolic-syndrome pathways including LEPR, HNF1A, IL6R, and GCKR associate with plasma C-reactive protein: the Women’s Genome Health Study. Am J Hum Gen. 2008;82:1185–92. doi: 10.1016/j.ajhg.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balducci S, Zanuso S, Nicolucci A , et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis. 2010;20:608–17. doi: 10.1016/j.numecd.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 56.Rider OJ, Tayal U, Francis JM , et al. The effect of obesity and weight loss on aortic pulse wave velocity as assessed by magnetic resonance imaging. Obesity(Silver Spring) 2010;18:2311–6. doi: 10.1038/oby.2010.64. [DOI] [PubMed] [Google Scholar]
- 57.Pardina E, Ferrer R, Baena-Fustegueras JA , et al. The relationships between IGF-1 and CRP, NO, leptin, and adiponectin during weight loss in the morbidly obese. Obes Surg. 2010;20:623–32. doi: 10.1007/s11695-010-0103-5. [DOI] [PubMed] [Google Scholar]
- 58.Brethauer SA, Heneghan HM, Eldar Setal. Early effects of gastric bypass on endothelial function inflammation and cardiovascular risk in obese patients. Surg Endosc. 2011;25:2650–9. doi: 10.1007/s00464-011-1620-6. [DOI] [PubMed] [Google Scholar]