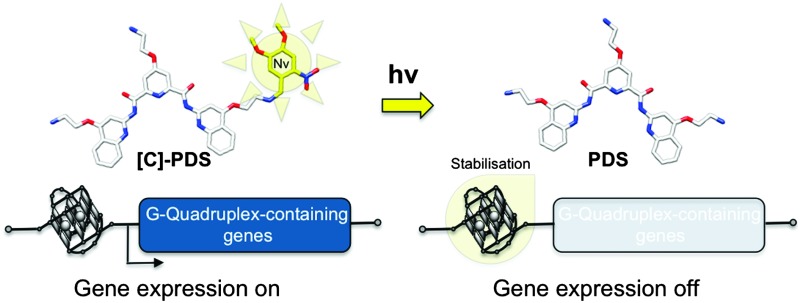
 The use of a caged G-quadruplex ligand allows for transcriptional control of quadruplex-containing genes using UV light as an external trigger.
The use of a caged G-quadruplex ligand allows for transcriptional control of quadruplex-containing genes using UV light as an external trigger.
Abstract
The use of a caged G-quadruplex ligand allows for transcriptional control of quadruplex-containing genes using UV light as an external trigger. An important oncogene, SRC, involved in the initiation and proliferation of epithelial tumours is shown to be significantly downregulated in cells treated by the caged ligand in synergy with UV light treatment.
Control over the expression of one or a subgroup of genes with targeted small molecules is a major goal of chemical biologists. One approach towards this goal has involved targeting DNA. Two main challenges include target specificity of many DNA binding ligands as well as the need to target such ligand treatments in a tissue-specific manner to mitigate undesirable secondary effects.1 Non-double-stranded structural elements within genomic DNA provide enticing targets for recognition and alteration at specific regions of the genome thus controlling transcription at single gene resolution. G-quadruplexes represent a promising target given their implication in the control of key steps of cellular life from replication to transcription to translation.2 Molecules that stabilise DNA G-quadruplex motifs in promoter regions or gene bodies can inhibit mRNA synthesis and downstream protein expression.3 We, and others, have developed small molecules able to specifically recognise and stabilise G-quadruplex motifs.4 We recently reported that a pyridine-2,6-bis-quinilodicarboxamide derivative, pyridostatin5 (PDS, Scheme 1), promotes growth arrest in human MRC5-SV40 cancer cells.6 Furthermore, we were able to demonstrate explicit sites within certain genes where PDS binds to G-quadruplex motifs and causes a functional response. PDS was also shown to trap quadruplex structures in U2OS cells.7 We then wished to enable future chemical biological studies by increasing its specificity for diseased tissues and control its action with spatial and temporal control.
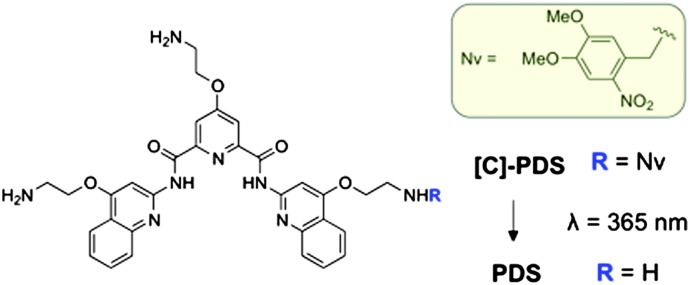
Scheme 1. Structure of [C]-PDS and PDS. The structure of the photolabile protecting (caging) group nitroveratryl (Nv) is highlighted in the yellow box.
Caging technology via the use of photolabile protecting groups has been proposed to develop pro-drugs that can be activated in a temporally and spatially controlled manner using light as an external trigger.8 We therefore set out to use a caged derivative of PDS as a photo-activated pro-drug for the selective release of PDS in a spatially controlled manner. Caged molecules have been previously employed to control cell replication, proliferation and growth. For example, caged hormones or protein substrates have been used to control gene activation and recombination,9 but to our knowledge no caged nucleic acid ligands have been studied for the control of gene expression using light as an external trigger. Herein we show that the use of the caged version of a small molecule known to stabilise G-quadruplex DNA allows the transcriptional regulation of G-quadruplex-containing genes in cellulo using light as an external trigger.
Previous reports have shown that the primary amine moieties of the side chains along the PDS aromatic scaffold are essential for the recognition and the stabilisation of G-quadruplex motifs by creating crucial electrostatic interactions.10 Therefore, we postulated that the introduction of a bulky, photolabile aromatic group on one of the side chains would create a steric clash with G-quadruplex targets and significantly reduce the compound's ability to stabilise such motifs. In order to cage the quadruplex ligandPDS we chose a simple one step approach from the active molecule that can be applied generally to nucleic acid ligands possessing reactive nucleophilic moieties. The caged compound, [C]-PDS (Scheme 1), was obtained by the nucleophilic attack of one primary amino group on one equivalent of the commercially available 4,5-dimethoxy-2-nitrobenzyl bromide (6-nitroveratryl bromide). Nitroveratryl protecting groups and their derivatives have been successfully used to cage a G-quadruplex ligand telomerase inhibitor11 and DNA binders.12 The major compound [C]-PDS was obtained as a unique regioisomer in 85.1% yield after purification (protocol and characterisation are reported in the ESI†).
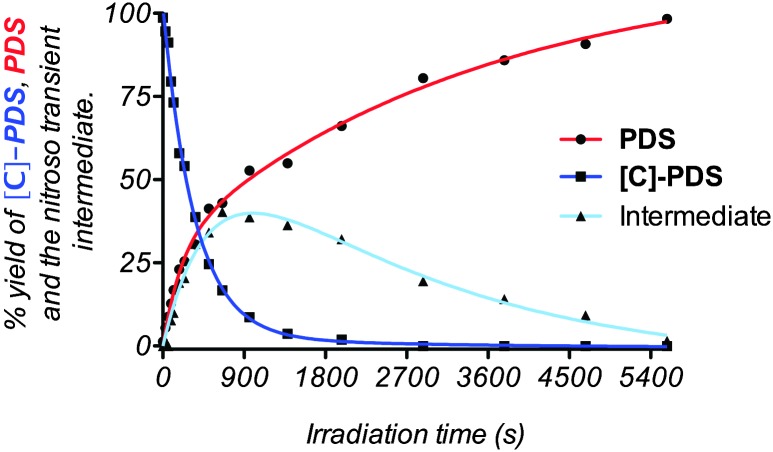
The caged moiety is stable (no degradation was observed by MS after 72 h) in buffered solution; however photodeprotection may be easily achieved via UV irradiation. UV exposure at 365 nm (see the complete protocol in the ESI†) leads to the regeneration of the original quadruplex ligandPDS through the formation of an intermediate attributed to a 2-nitrosobenzyl hemiaminal derivative (see Fig. S1, ESI†).13 To investigate the uncaging process, the time course of uncaging was first analyzed by tandem HPLC-MS analysis. A buffered solution containing [C]-PDS was irradiated at 365 nm and the activation of the quadruplex ligand was quantified by comparing the HPLC peak areas of the different species in solution. As shown in Fig. 1, the caged derivative disappeared in an irradiation dose-dependent manner and was completely converted within 30 min of exposure.
Fig. 1. Time course of uncaging of [C]-PDS monitored by tandem HPLC-MS analysis. The experiment was conducted using a 100 μM solution of the caged compound in a 10 mM PBS buffer pH 7.0 supplemented with 70 mM KCl.
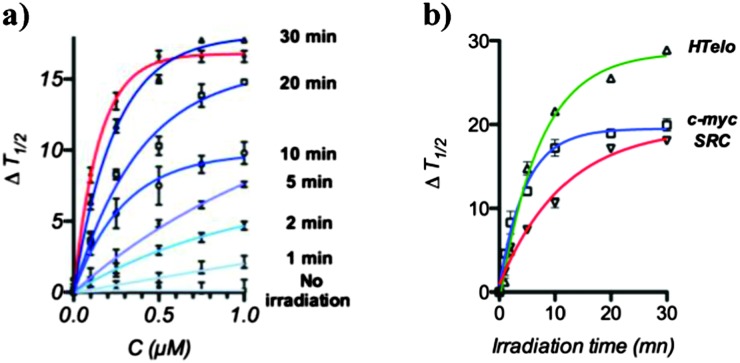
During our previous investigations we identified the proto-oncogene SRC as a gene target affected by PDS treatment in MRC5-SV40 cells. SRC is involved in the proliferation and progression of malignant cancer14 and is characterised by the presence of multiple quadruplex-forming sequences organised in clusters throughout the gene body. We therefore examined the ability of [C]-PDS to stabilise one of the quadruplex motifs found in SRC, d(GGGCGGCGGGCTGGGCGGGG),6 for different irradiation times using a FRET melting assay.15 The caged compound, [C]-PDS, exhibited poor ability to stabilise a dual-labelled SRC quadruplex (Fig. 2a). At 1 μM [C]-PDS, no significant stabilisation of the quadruplex motif was observed suggesting that the Nv cage effectively prevents quadruplex stabilisation. Upon UV irradiation in presence of the ODN, the thermal stabilisation of SRC dramatically increased and we observed increasing restoration of the quadruplex stabilising properties of the ligand over 30 min of irradiation. Indeed, after 30 min irradiation [C]-PDS treatment stabilised the SRC motif to the same extent as unmodified PDS confirming that uncaging had proceeded to completion with recovery of quadruplex stabilisation activity. The same trend has been observed using other reported quadruplex motifs such as HTelo or c-myc (Fig. 2b). These results confirm that complete uncaging of the derivative is achieved in 30 min in the presence of ODNs and occurs with restoration of quadruplex stabilisation properties.
Fig. 2. UV-light mediated restoration of quadruplex stabilisation property of [C]-PDS. (a) FRET melting profiles showing the stabilization of a SRC quadruplex motif (200 nM) by an increasing amount of [C]-PDS after different irradiation times (blue curves) and by PDS (red line), (b) stabilisation of HTelo, c-myc and SRC quadruplex motifs (200 nM) with 1 μM of [C]-PDS as a function of irradiation time.
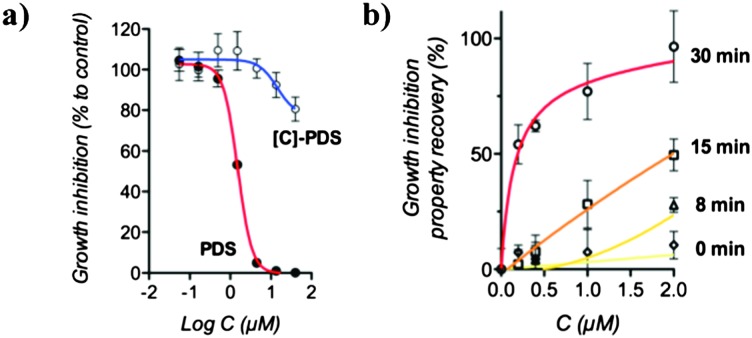
We then examined the growth inhibition (GI 50) properties of the caged compound to investigate its potential use in cellulo. We found that [C]-PDS exhibits a high GI 50 of above 40 μM in MRC5-SV40 cells whereas PDS has a potent GI 50 of 1.5 μM after 72 h incubation (see Fig. 3a and Fig. S2, ESI†). This result shows clearly that the introduction of the photolabile protecting group efficiently suppresses the biological activity of the quadruplex ligand and suggests that light-mediated restoration of PDS potency can be used to control gene expression.
Fig. 3. UV-light mediated restoration of growth inhibition properties of [C]-PDS. The experiments were conducted using MRC5-SV40 cells. (a) Growth inhibition properties of PDS and [C]-PDS compared to an untreated sample. (b) Comparison of the effect of [C]-PDS, at different concentrations and for different irradiation times, with the effect of a 2 μM treatment with PDS. These results are the combination of triplicate and error bars represent the standard deviation.
We first carried out control experiments to confirm that the cells were not affected by 365 nm UV irradiation alone. We found that a 30 min UV light treatment had no growth inhibitory effect on untreated cells (see Table S1, ESI†). We then investigated the potential of UV light to restore the biological activity of the caged compound. For this purpose, the growth inhibition properties of increasing concentrations of [C]-PDS in combination with different doses of irradiation were compared to the effect of a 2 μM dose of the uncaged, active ligand. Fig. 3b shows the light-mediated recovery of the growth inhibitory effect of the caged compound. We found that UV light restored the ability of the caged compound to inhibit the growth of MRC5-SV40 cells in an irradiation-time dependent manner. Importantly, as expected from the FRET melting assay, after 30 min of UV irradiation the caged compound displayed potency comparable to that of the free, active quadruplex ligand. This latter result shows that the biological properties of PDS can be fully recovered from [C]-PDS using light as an external trigger.
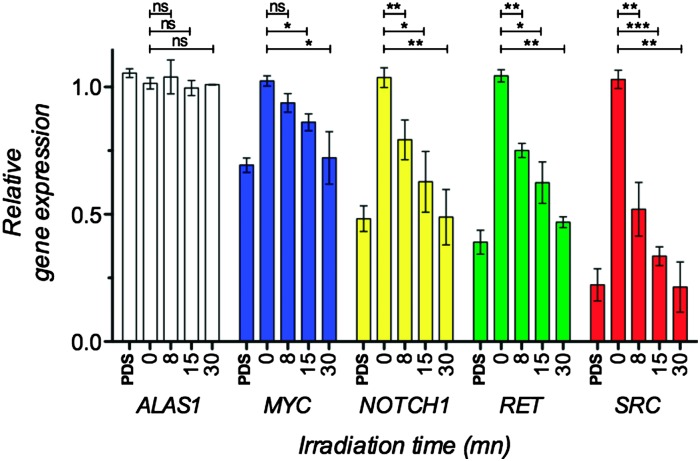
Finally, we evaluated the effect of combined UV light and [C]-PDS treatment on the expression of G-quadruplex-containing genes previously identified as targets in cellulo. The light-mediated changes in gene expression were measured by quantification of mRNAs using quantitative reverse transcription polymerase chain reaction (qRT-PCR) (see the ESI† for details). MRC5-SV40 cells were treated with a 2 μM dose of the caged derivative and irradiated with 365 nm UV-light for 0, 8, 15 or 30 min. Total RNA was extracted, purified, reverse transcribed and analysed by qRT-PCR for 6 different genes. A house keeping gene lacking the putative quadruplex (PQS) in its promoter or body and thus expected to be unchanged by PDS treatment, B2M, was used to normalize the expression data. We then quantified the expression of ALAS1—another house-keeping gene containing a low density of PQS and not affected by PDS treatment—along with MYC, NOTCH1, RET and SRC, four genes that possess high PQS density either in their promoter regions or gene bodies.6 We first checked that the treatment with UV light had no effect on the expression of these genes. After 30 min of irradiation and in the absence of the caged derivative the expression of the studied genes were not affected (see Fig. S3, ESI†). As expected, in the presence of 2 μM of [C]-PDS the expression of quadruplex containing genes was found to be downregulated in an irradiation time dependent manner. Fig. 4 and Fig. S4 (ESI†) show the relative gene expression of the five studied genes normalized to the expression of the house-keeping gene B2M for different irradiation times. As expected, the expression of the house-keeping gene ALAS1 was not affected by the combination of the caged compound and UV treatment. Conversely, transcript levels of the quadruplex containing genes were significantly affected in an irradiation time-dependent manner. The proto-oncogene SRC was most strongly affected, with its mRNA levels being reduced by over 80% after 30 min of irradiation (p value < 0.01). The expression of the tumor suppressor NOTCH1 and the oncogene RET was found to be reduced by over 50% after 30 min of irradiation (p value < 0.01). Finally the proto-oncogene MYC was found to be downregulated by over 30% after 30 min of irradiation (p value < 0.05). Importantly, the observed downregulation effects are similar in magnitude to those originally reported for PDS, showing that the caged compound is efficiently uncaged in a biological context, restoring the activity of the original ligand. This latter experiment shows that the combination of UV irradiation and [C]-PDS allows selective and efficient control of the expression of quadruplex-containing genes.
Fig. 4. Light-mediated downregulation of mRNAs of quadruplex containing genes. MRC5-SV40 cells have been treated with 2 μM [C]-PDS and irradiated at 365 nm for 0, 8, 15 or 30 min or with 2 μM PDS. These results are the combination of biological triplicate and error bars represent the standard deviation. ns: not statically relevant, *p value < 0.05, **p value < 0.01, ***p value < 0.001.
In conclusion, we have shown that light can modulate the expression of G-quadruplex-containing genes in cells growing in media containing [C]-PDS. Light-modulated gene expression mediated by a photocaged G-quadruplex stabilising ligand offers a unique tool for regulating genes in vivo in a tissue specific manner. Our results highlight the potential of [C]-PDS to selectively and efficiently downregulate SRC and other important proto-oncogenes in a spatiotemporal fashion. Given that SRC has been associated with the initiation and proliferation of different epithelial tumours (overexpressed in melanoma or colon cancer for example),16 this work may serve as a proof of concept to inspire future consideration of G-quadruplex-specific photodynamic therapies.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c3cc44737e
References
- Demeunynck M., Bailly C. and Wilson W. D., DNA and RNA Binders, From Small Molecules to Drugs, Wiley-VCH, 2003. [Google Scholar]
- Balasubramanian S., Hurley L. H., Neidle S., Kumari S., Bugaut A., Huppert J. L., Balasubramanian S., Zhao J., Bacolla A., Wang G., Vasquez K. M. Nat. Rev. Drug Discovery. Nat. Chem. Biol. Cell Mol. Life Sci. 2011;2007;2010;10367:261. 218, 43. doi: 10.1038/nrd3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui-Jain A., Grand C. L., Bearss D. J., Hurley L. H., Bejugam M., Sewitz S., Shirude P. S., Rodriguez R., Shahid R., Balasubramanian S. Proc. Natl. Acad. Sci. U. S. A. J. Am. Chem. Soc. 2002;2007;99129:11593. 12926. doi: 10.1073/pnas.182256799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchaud D., Teulade-Fichou M.-P., Ou T.-M., Lu Y.-J., Tan J.-H., Huang Z.-S., Wong K.-Y., Gu L.-Q. Org. Biomol. Chem. ChemMedChem. 2008;2008;63:627. 690. doi: 10.1039/b714772b. [DOI] [PubMed] [Google Scholar]
- Rodriguez R., Müller S., Yeoman J. A., Trenteseaux C., Riou J.-F., Balasubramanian S. J. Am. Chem. Soc. 2008;130:15758. doi: 10.1021/ja805615w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R., Miller K., Forment J. V., Bradshaw C. R., Nikan M., Britton S., Oelschlaegel T., Xhemlace B., Balasubramanian S., Jackson S. P. Nat. Chem. Biol. 2012;8:301. doi: 10.1038/nchembio.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi G., Tannahill D., McCafferty J., Balasubramanian S. Nat. Chem. 2013;5:182. doi: 10.1038/nchem.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer G., Heckel A., Deiters A., Ellis-Davies G. C. R. Angew. Chem., Int. Ed. ChemBioChem. Nat. Methods. 2006;2010;2007;45114:4900. 47, 619. [Google Scholar]
- Cruz F. G., Koh J. T., Link K. H., Link K. H., Shi Y., Koh J. T. J. Am. Chem. Soc. J. Am. Chem. Soc. 2000;2005;122127:8777. 13088. [Google Scholar]
- Müller S., Sanders D. A., Di Antonio M., Matsis S., Riou J.-F., Rodriguez R., Balasubramanian S. Org. Biomol. Chem. 2012;10:6537. doi: 10.1039/c2ob25830g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Iida K., Tera M., Shin-ya K., Seimiya H., Nagasawa K. ChemBioChem. 2012;13:774. doi: 10.1002/cbic.201200013. [DOI] [PubMed] [Google Scholar]
- Sánchez M. I., Vázquez O., Vázquez M. E., Mascareñas J. L., Sánchez M. I., Martinez-Costas J., Gonzalez F., Bermudez M. A., Vázquez M. E., Mascareñas J. L. Chem. Commun. ACS Chem. Biol. 2011;2012;477:11107. 1276. [Google Scholar]
- Il'ichev Y. V., Schwörer M. A., Wirz J. J. Am. Chem. Soc. 2004;126:4581. doi: 10.1021/ja039071z. [DOI] [PubMed] [Google Scholar]
- Ishizawar R., Parsons S. J. Cancer Cell. 2004;6:209. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Mergny J.-L., Lacroix L., Teulade-Fichou M.-P., Hounsou C., Guittat L., Hoarau M., Arimondo P. B., Vigneron J.-P., Lehn J.-M., Riou J.-F., Garestier T., Hélène C., Murat P., Singh Y., Defrancq E. Proc. Natl. Acad. Sci. U. S. A. Chem. Soc. Rev. 2001;2011;9840:3062. 5293. doi: 10.1073/pnas.051620698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu G., Bowman T., Huang M., Shivers S., Reintgen D., Daud A., Chang A., Kraker A., Jove R., Yu H., Irby R. B. Oncogene. Oncogene. 2002;2000;2119:7001. 5636. doi: 10.1038/sj.onc.1205859. [DOI] [PubMed] [Google Scholar]