Abstract
Diffuse panbronchiolitis (DPB) is a chronic lower respiratory tract infection commonly associated with persistent late-stage Pseudomonas aeruginosa infection. However, low-dose long-term therapy with certain macrolides is effective in most patients with DPB. The present study was designed to examine the effects of long-term erythromycin (ERY) therapy by using our established murine model of chronic respiratory P. aeruginosa infection. ERY or saline was administered from day 80 after intubation with a P. aeruginosa-precoated tube for the subsequent 10, 20, 40, and 80 days. Bacteriologic and histologic analyses of the murine lungs and electron microscopy of the intubated tube were performed. In the murine model, treatment with ERY for 80 days significantly reduced the number of viable P. aeruginosa organisms in the lungs (P < 0.05). The biofilm formed in situ by P. aeruginosa on the inner wall of the inoculation tube placed into the murine bronchus became significantly thinner after 80 days of ERY treatment. We conclude that the clinical efficacy of macrolides in DPB may be due at least in part to the reduction in P. aeruginosa biofilm formation.
Diffuse panbronchiolitis (DPB) is a chronic infection of the lower respiratory tract first reported in Japan in 1969 by Yamanaka et al. (30) and is more common in Japan than in western countries (4). The main features of DPB include chronic cough, copious sputum, shortness of breath, wheezing, and hypoxemia, and about 75% of patients have chronic sinusitis. Histopathologically, the disease is characterized by thickening of the walls of the respiratory bronchioles and infiltration of inflammatory cells (5). The isolation of Haemophilus influenzae and Streptococcus pneumoniae from the sputum in the early course of DPB can change to the isolation of Pseudomonas aeruginosa as the disease progresses. The clinical features of persistent P. aeruginosa infection and neutrophil retention in the airways in DPB are similar to those of cystic fibrosis (CF), the most common lethal inherited condition among Caucasians. Bacteria growing in biofilm, such as P. aeruginosa, are a major concern for clinicians in the treatment of infectious diseases because of their resistance to a wide range of antibiotics. Chronic infections caused by P. aeruginosa lead to a serious deterioration of lung function in DPB and CF patients (12, 15).
Several Japanese studies have demonstrated the therapeutic benefits of long-term therapy with macrolides, such as erythromycin (ERY), clarithromycin (CAM), roxithromycin (RXM), and azithromycin (AZM), in DPB patients (3, 6, 13, 17, 19, 20, 22). Recently, two studies from western countries demonstrated similar effects of AZM therapy in CF patients (1, 29). Macrolides are common antibiotics used in patients with respiratory infections but are regarded to have weak or no activity against P. aeruginosa, since the maximum concentrations of macrolide antibiotics in serum and sputum are below the MICs for this organism and never inhibit the proliferation of P. aeruginosa (23).
Several mechanisms for the therapeutic benefits of macrolides, both in vitro and in vivo, have been proposed; these include the effect of ERY on neutrophil function (7); the effects of ERY, CAM, RXM, and AZM on interleukin-8 production (9, 10, 21, 24); the effect of ERY on tracheal secretions (26); the effects of ERY, CAM, and AZM on the biofilm produced by P. aeruginosa (14); the inhibition of quorum-sensing P. aeruginosa by AZM (27); and even direct P. aeruginosa reduction by ERY, CAM, and AZM (28). Although all of these mechanisms have been discussed, the precise mechanism is not yet clear.
Recently, several studies with experimental animal models demonstrated the potential of CAM in combination with an antipseudomonal agent for the treatment of acute (11) and chronic (31) respiratory infections caused by mucoid-producing P. aeruginosa. However, to our knowledge, no studies with experimental animal models have demonstrated the clearance of organisms by ERY alone, especially in chronic respiratory infections caused by P. aeruginosa.
In the present study, we investigated the clinical effects of long-term ERY therapy in our established murine model of chronic bronchial P. aeruginosa infection.
MATERIALS AND METHODS
Animals.
Male, 7-week-old, 30- to 35-g, specific-pathogen-free ddY mice were purchased from Shizuoka Agricultural Cooperative Association Laboratory Animals (Shizuoka, Japan). All mice were housed in a pathogen-free environment and received sterile food and water in the Laboratory Animal Center for Biomedical Science at Nagasaki University. The experimental protocol was approved by the Animal Care and Use Committee, Nagasaki University.
Preparation of tubes precoated with bacteria.
P. aeruginosa NUS10, a clinical mucoid isolate from the sputum of a DPB patient at Nagasaki University Hospital, was cultured on a Mueller-Hinton II agar (Becton Dickinson Microbiology Systems, Cockeysville, Md.) plate for 24 h. The bacteria were suspended in saline, harvested by centrifugation (3,000 × g, 4°C, 10 min), resuspended in sterile saline, and adjusted to 109 CFU/ml, as estimated by turbidimetry. The intubation tubes, disposable sterile plastic cutdown intravenous catheters (3 French, 1.0-mm diameter; Atom Co., Tokyo, Japan) cut to 3.0-mm lengths and with a few slits made at the proximal end to prevent clogging by airway secretions, were immersed in the bacterium-saline suspensions for 3 days at 37°C. To count bacterial numbers, the bacteria were detached from the tubes by using a concussion machine for 5 min. Only viable bacteria were counted. The number of bacteria at 3 days after incubation and before intubation was 6.32 ± 0.57 log10 CFU/tube (mean and standard deviation [SD]; n = 10).
Experimental model of chronic airway infection.
Chronic airway infection was induced in mice by using the method described previously by Yanagihara et al. (32, 33). Briefly, the blunted end of the inner needle of an intravenous catheter (Angiocath; Becton Dickinson Vascular Access, Sandy, Utah) was inserted through the oral cavity of fully anesthetized mice, with the outer sheath and the attached tube at the tip. The tube was advanced through the vocal cords into the trachea. The inner needle was pulled out, and the outer sheath was gently pushed to place the precoated tube into the main bronchus. After intubation, the infected mice were allowed to recover, eat, and drink spontaneously. The infection was restricted to the lungs.
Drug administration.
ERY (Sigma Chemical Co., St. Louis, Mo.) was dissolved in sterile water immediately before use. The MIC of the agent was determined by the agar dilution technique with Mueller-Hinton II agar plates and an inoculum size of 104 CFU per spot. The MIC of ERY for P. aeruginosa mucoid isolate NUS10 was >400 μg/ml. Treatment commenced from day 80 after intubation with a tube carrying P. aeruginosa or a sterile tube. After this 80-day interval, 84 mice were allocated into two groups. The first group was treated with ERY (10 mg/kg of body weight/day), and the other group was treated with saline as the control. Both groups of mice were sacrificed and examined after 10, 20, 40, and 80 days of administration of ERY or saline once a day by intraperitoneal injection. For this study, the treatment drug and its dosage were based on the DPB clinical practice guidelines of the Japanese Ministry of Health and Welfare. According to those details, ERY is the first-choice drug for low-dose macrolide therapy because of its low rate of side effects, and the usual therapeutic dose of ERY (400 to 600 mg/person/day) is almost equal to those used in this study (10 mg/kg/day).
Bacteriologic and histopathologic examinations.
After treatment, the animals were sacrificed by severing the axillary artery under general anesthesia, and the lungs were excised under aseptic conditions. For bacteriologic analysis, both lungs were homogenized, including the implanted tube, to avoid the detachment of biofilm from the outer surface of the tube by picking it up from the murine bronchus, and cultured quantitatively. The infected tube and the infected lung tissue were separately cultured in a previous study (33). In that study, Yanagihara et al. showed that the counts of viable bacteria isolated from the lung tissue and the tube removed from the murine bronchus were approximately similar, at 105 CFU per specimen. Bacterial enumeration was performed for four mice in each group by serially diluting samples on Mueller-Hinton II agar plates, incubating the plates at 37°C in air overnight, and then counting colonies on the plates to estimate the CFU in the lungs of the mice.
For histopathologic examination, lung specimens from four mice in each group were fixed in 10% formalin buffer. For scanning electron microscopy, the tube was removed from two mice in each group and cut longitudinally. These specimens were fixed for 2 h at 4°C with 2% glutaraldehyde in 0.1 M phosphate buffer, followed by refixation for 2 h at 4°C in 1% osmium acid in the same buffer, dehydration in a series of aqueous ethanol solutions (50 to 100%), and freeze-drying. The specimens were coated with platinum-palladium by using an ion sputter and observed by using a JSM-35C scanning electron microscope (JEOL, Tokyo, Japan).
Statistical analysis.
Data were expressed as the mean and standard deviation. Differences between groups were examined for statistical significance by using the unpaired Student's t test. A P value of less than 0.05 denoted the presence of a statistically significant difference.
RESULTS
Therapeutic effects of ERY in mice with chronic respiratory P. aeruginosa infection.
In the present study, we first examined the serial changes in the numbers of viable mucoid P. aeruginosa organisms isolated from the lungs, including the tube, following the administration of ERY. As shown in Table 1, similar numbers of viable bacteria were found in the lungs of saline-treated mice throughout the examination period. On the other hand, in mice treated with ERY, the numbers of viable bacteria were similar to those in control mice from days 10 to 40 of ERY treatment, but the numbers significantly decreased after 80 days of treatment (7.03 ± 0.49 and 3.28 ± 0.92 log10 CFU/lungs, respectively; P < 0.01) (Table 1). Only P. aeruginosa exposed to ERY for 80 days could not form apparent colonies after 24 h of culturing and required 48 h of culturing, twice as long as bacteria in nontreated mice, to form colonies (data not shown).
TABLE 1.
Counts of viable P. aeruginosa NUS10 in lungs after treatment with ERY
| Day | Mean ± SD (n = 4) log10 CFU of P. aeruginosa/lung after treatment with:
|
|
|---|---|---|
| Saline | ERY | |
| 10 | 6.91 ± 0.39 | 6.37 ± 0.13 |
| 20 | 6.03 ± 0.80 | 6.55 ± 1.09 |
| 40 | 6.51 ± 0.37 | 6.69 ± 0.28 |
| 80 | 7.03 ± 0.49 | 3.28 ± 0.92a |
The P value for the ERY-treated group compared to the saline-treated group was <0.01.
Histopathologic examination.
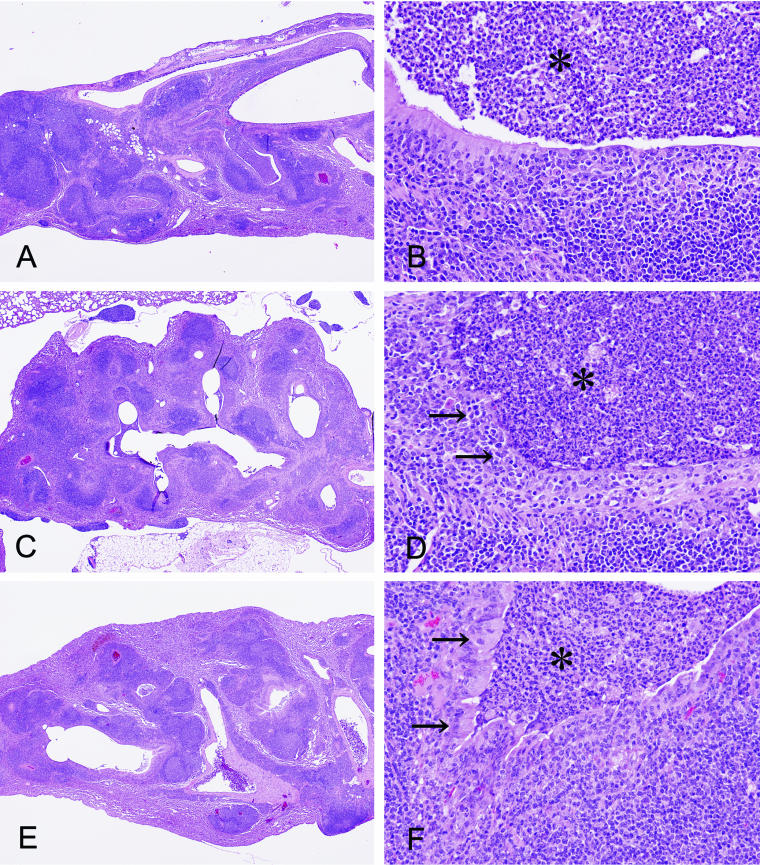
The lungs of animals intubated with a sterile tube (four animals per group) showed localized inflammatory changes throughout the examination period, regardless of whether they were ERY treated or not. On the other hand, mice treated for 80 days after infection with P. aeruginosa NUS10 showed bronchopneumonia, with the accumulation of neutrophils inside the airways. These airways were surrounded by dense lymphocytic infiltration and lymphoid follicle formation (Fig. 1A). The lining epithelium showed areas of destruction by infiltrating mononuclear cells, and some areas of the epithelium showed reactive hyperplasia of reserve cells (Fig. 1B). On the other hand, control mice treated with saline for 80 days after infection showed chronic inflammation around the airways similar to that seen before treatment (Fig. 1C). High-power microscopic examination showed that the airways were filled with neutrophils and foam cells, and more destruction of the epithelial layer was apparent (Fig. 1D). Mice treated with ERY for 80 days after infection showed a similar pattern when examined with the scanning microscope (Fig. 1E). However, the degree of epithelial destruction was mild, and the airways were covered with highly columnar ciliated bronchial cells (Fig. 1F).
FIG. 1.
Pulmonary changes in mice just 80 days after inoculation of P. aeruginosa (A and B), after 80 days of saline treatment (C and D), and after 80 days of ERY treatment (E and F) (hematoxylin-eosin stain; magnifications: A, C, and E, ×17.4, and B, D, and F, ×174). The airways (asterisks) are filled with neutrophils. Mice treated with saline showed the progression of disease, including the further destruction of the epithelium (D, arrows). ERY-treated mice showed the recovery of columnar ciliated cells (F, arrows).
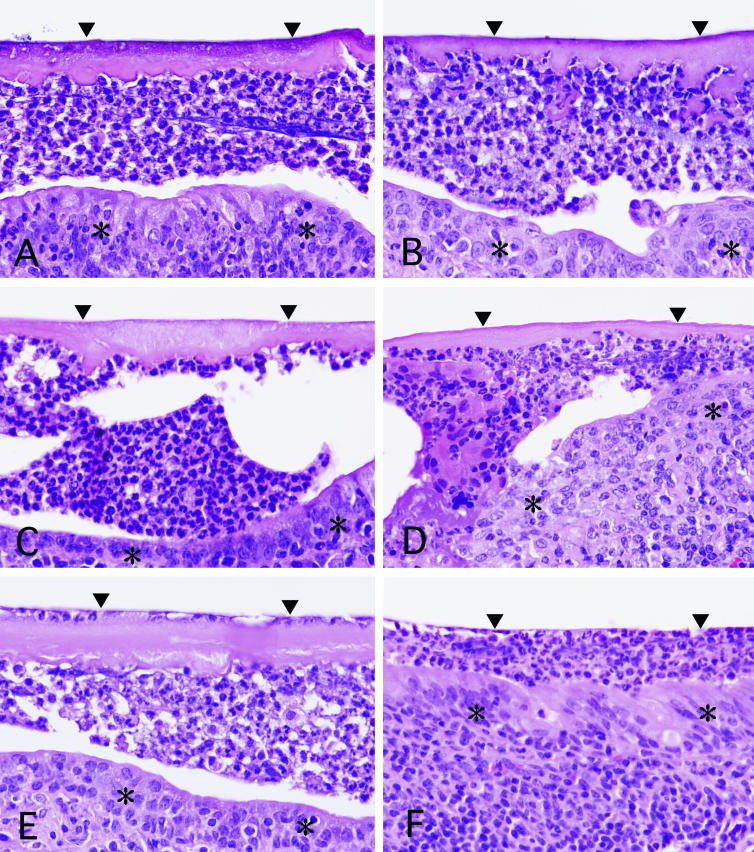
At 80 days, mice with an infected tube (four animals per group) showed one-layer homogeneous eosinophilic membranes attached to the inner and outer walls of the tube. Between the membrane of the outer side of the tube and the airway epithelium, a thick band-like infiltration of neutrophils was noted. The neutrophil accumulation did not infiltrate the membrane (Fig. 2A). During 80 days of treatment with saline, mice in the control group tended to show thickening of the membrane (Fig. 2A, C, and E). On the other hand, those treated with ERY showed gradual improvement, including thinning and disappearance of the membrane, a thinner neutrophil layer, and recovery of epithelial cells (Fig. 2B, D, and F). After 80 days of treatment with ERY, the membranes almost disappeared, and only a thin neutrophil zone remained (Fig. 2F). The differences between the ERY- and saline-treated groups were similar for almost all of the animals.
FIG. 2.
Interface between the inoculation tube (arrowheads) and the lining epithelia (asterisks) in saline- and ERY-treated mice (hematoxylin-eosin stain; magnification, ca. ×392). (A and B) Mice treated with saline (A) and with ERY (B) showed similar features after 20 days. (C and D) After 40 days of treatment, mice treated with saline (C) and with ERY (D). (E and F) After 80 days of treatment, mice treated with saline (E) and with ERY (F).
Scanning electron microscopy of the intubated tube.
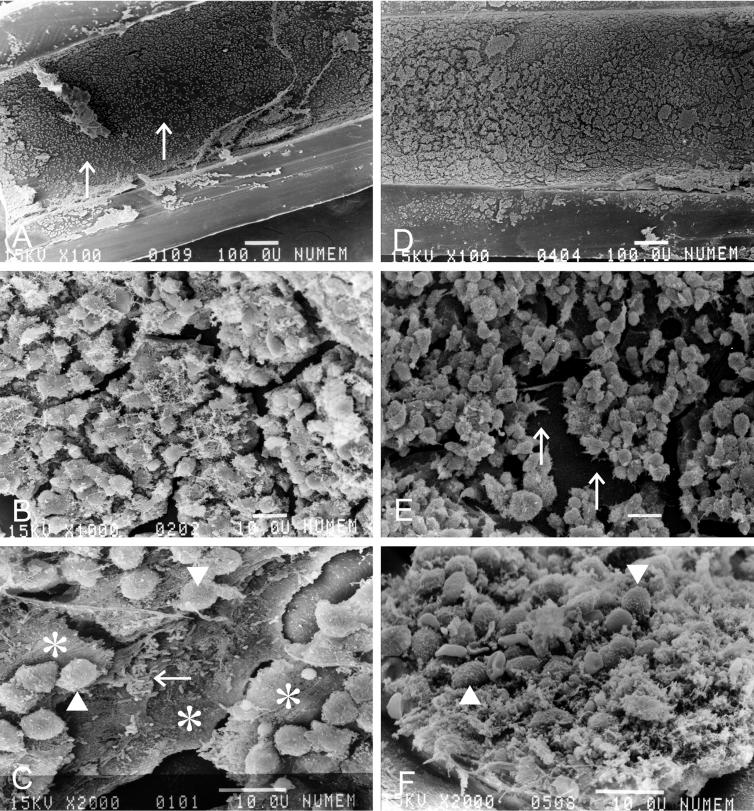
Scanning electron microscopy of the inner wall of the tube (longitudinal section) (two animals per group) revealed in situ formation of a biofilm structure. In control mice treated with saline for 40 days, the inner wall of the tube was covered with a smooth membrane revealed at low magnification to have granular particles on its surface (Fig. 3A). These particles, forming a cracked tile-like pattern, were composed of a paste of interbacterial materials containing fibrous structures (Fig. 3B). At higher magnification, these particles were revealed to form a multilayer biofilm, and the inflammatory cells were separated from the bacteria embedded deeply in the biofilm by a multilayer coat of amorphous material (Fig. 3C). On the other hand, the biofilm in mice treated with ERY was revealed at low magnification to form irregularly cracked skin-like pieces with a rougher surface (Fig. 3D). The inflammatory cells, forming clusters, attached to the surface of the basal layer (Fig. 3E). At higher magnification, the surface of the biofilm was revealed not to form smooth plates like those seen in control mice, and the inflammatory cells were mixed with the biofilm and were in direct contact with the bacteria (Fig. 3F).
FIG. 3.
Scanning electron microscopy of the biofilm on the inner space of the inoculation tube (longitudinal section) in mice that received 40 days of saline treatment after inoculation with P. aeruginosa (A, B, and C) and 40 days of ERY treatment (D, E, and F). Bars, 100 μm (A and D) and 10 μm (B, C, E, and F). The arrows in panel A show the smooth surface of the biofilm covering the inner wall of the tube. The inflammatory cells (arrowheads) are separated from the bacteria (arrow) embedded deeply in the multilayer biofilm (asterisks) (C). The arrows in panel E show the surface of the base layer of the biofilm. In mice treated with ERY, the inflammatory cells (arrowheads) were mixed with the biofilm (F).
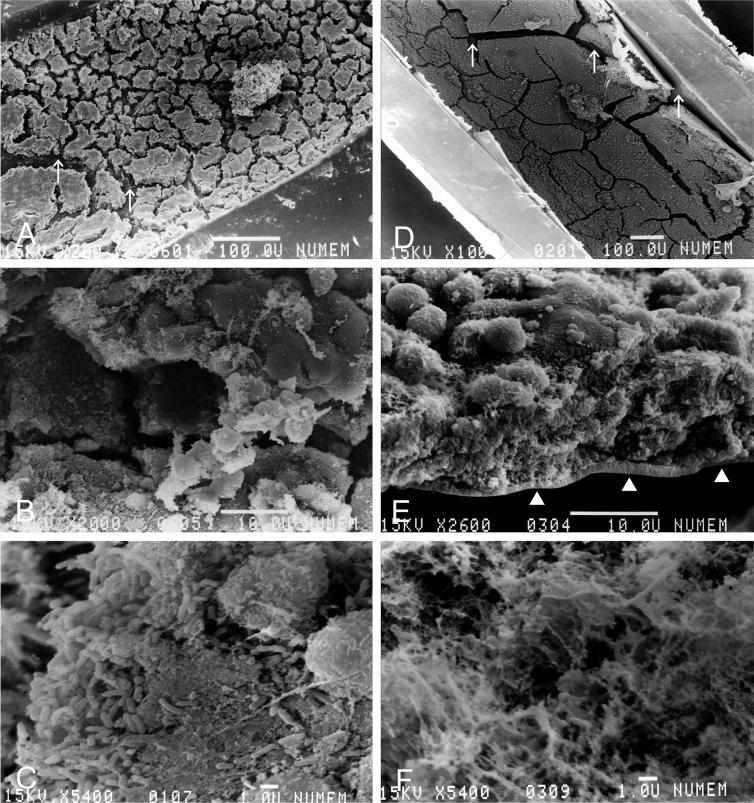
Additionally, in mice treated with saline for 80 days, the inner wall of the tube was encrusted with a thick membrane revealed at low magnification to contain irregularly cracked tile-like pieces (Fig. 4A). The biofilm was thicker than that seen in mice treated with saline for 40 days (Fig. 4B). At higher magnification, each of the biofilms appeared as a dense and tightly formed structure composed of innumerable bacterial materials containing fibrous structures (Fig. 4C). On the other hand, in mice treated with ERY for 80 days, examination at low magnification revealed that the biofilm formed a thin single layer, and the inner wall of the tube appeared from a chap in the membrane (Fig. 4D). The biofilm did not form a multilayer structure with the inflammatory cells attached to its surface (Fig. 4E). At higher magnification, the very thin remnant of the biofilm comprised a debris-like granular and fibrous spongy-like meshwork material (Fig. 4F). A few bacteria were observed at the bottom of the thin biofilm.
FIG. 4.
Scanning electron microscopy of the biofilm on the inner space of the inoculation tube (longitudinal section) in mice that received 80 days of saline treatment after inoculation with P. aeruginosa (A, B, and C) and 80 days of ERY treatment (D, E, and F). Bars, 100 μm (A and D), 10 μm (B and E), and 1 μm (C and F). The arrows show the smooth surface of the biofilm, not the inner wall of the tube (A). Mice treated with ERY showed a very thin, single-layer biofilm, and the inner wall of the tube can be seen (arrows in D and arrowheads in E).
DISCUSSION
In 1987, Kudoh et al. (18) first reported that “low-dose and long-term” treatment with ERY was effective in chronic lower respiratory tract infections, including DPB. Recently, the efficacies of 14-membered ring macrolides (e.g., ERY, CAM, and RXM) and 15-membered ring macrolides (e.g., AZM) in the treatment of both DPB (3, 6, 13, 17, 19, 20, 22) and CF (1, 29) were reported. These reports showed that macrolide therapy had little impact on the bacterial flora in the sputum, except in a few cases, but that these drugs may be effective even if the patient is already colonized with P. aeruginosa and that the prognosis is good regardless of P. aeruginosa infection (22). Therefore, the effect of macrolide treatment in DPB patients with chronic bacterial infections of the lower respiratory tract seems to be mainly related to suppression of the tissue response, i.e., an anti-inflammatory effect rather than an antimicrobial effect.
In addition to previous reports showing the anti-inflammatory effects of certain macrolides, Tateda et al. reported that AZM inhibited quorum-sensing P. aeruginosa (27) and that AZM, ERY, and CAM at clinically achievable concentrations resulted in direct P. aeruginosa reduction (28). Fujii et al. also reported the disappearance of P. aeruginosa in cultures of sputum samples from DPB patients after 12 months of ERY therapy (3). Therefore, we speculate that certain macrolides have some antipseudomonal activities.
P. aeruginosa is one of the most important bacterial pathogens in patients with chronic pulmonary diseases such as CF (15) and DPB (5). It is well known that P. aeruginosa forms a bacterial biofilm, and biofilms have been detected not only on various biomaterial objects in the body, such as catheters, pacemakers, and the surface of artificial organs, but also on a variety of living and inert surfaces within the human body (12, 14, 16); biofilms are often observed on the airway surfaces of patients with DPB, CF, and bronchiectasis (14). Morphologically, the biofilm produced by P. aeruginosa tended to form a large mass on the affected portion of the airway surface, and there the bacteria were associated with secreted mucus and host cell debris (as shown in Fig. 4A, B, and C). The dense and tightly structured biofilm was characterized by resistance to attack by a wide range of antibacterial agents and humoral or cellular host defense mechanisms (14). Consequently, despite treatment with potent antibiotics, P. aeruginosa infection in the lungs of such patients typically leads to death by respiratory failure or other complications. Biofilm bacteria are a major concern for clinicians in the treatment of chronic infectious diseases.
As in a previous study (34) with the same murine model of chronic infection with mucoid-producing P. aeruginosa NUS10, in the present study we started treatment with ERY or saline 80 days after infection. Yanagihara et al. (34) demonstrated serial changes in the concentrations of proinflammatory cytokines measured up to 60 days after the induction of respiratory infection. They showed that significant increases in the tumor necrosis factor alpha and interleukin 1β concentrations at 7 days after inoculation of the tube with P. aeruginosa were still seen even at 60 days postinfection. Although increases in the concentrations of gamma interferon and interleukin 2 up to 30 days after inoculation were moderate, the concentrations showed a significant increase 60 days later. Based on these results, we speculate that stabilization of these cytokines needs at least 60 days. Therefore, we started ERY treatment 80 days after inoculation to avoid any effects of the above changes in proinflammatory cytokines.
Using the same animal model as that described in the present study, Yanagihara et al. previously reported that the mean number of viable NUS10 bacteria recovered from the lungs was 105 to 106 CFU/specimen throughout the entire year (32). In the present study, we demonstrated that long-term administration of ERY alone could reduce the number of viable P. aeruginosa without the use of other antipseudomonal agents. To our knowledge, this is the first report to show the time course of morphologic changes in the biofilm structure of P. aeruginosa in situ in association with the administration of ERY alone. Moreover, we noted that P. aeruginosa exposed to ERY for 80 days required 48 h, twice as long as nontreated bacteria, to form apparent colonies. This result suggests that prolonged exposure to ERY may inhibit the replication of P. aeruginosa. Thus, we speculate that the antipseudomonal effects of prolonged exposure to ERY (reductions in biofilm formation and number of viable P. aeruginosa) lead to improved intrabronchial clearing by phagocytic cells and by the mucociliary transportation system after thinning of the biofilm in vivo (Fig. 1F). Although the ERY therapy did not change the presence of peribronchial dense lymphocytic infiltration and atelectatic changes, the recovery of ciliated bronchial cells may have a positive effect on mucociliary transportation and the clearance of debris. These results support the use of long-term macrolide therapy in the treatment of DPB.
With respect to bacterial biofilm, Yasuda et al. (35), using a rat model of experimentally induced subcutaneous infection with the presence of biofilm formed by P. aeruginosa, reported that the quantities of alginate and hexose in which bacterial biofilm had been formed clearly decreased in a dose-dependent manner after treatment with CAM. Tateda et al. (27, 28) also reported that the abilities of certain macrolides (AZM, ERY, and CAM) to reduce the viability of P. aeruginosa following prolonged incubation might be associated with the inhibition of protein synthesis by P. aeruginosa (28). Furthermore, they demonstrated that AZM inhibited quorum-sensing P. aeruginosa and might reduce the production of quorum-sensing-dependent extracellular virulence factors, elastase and rhamnolipids, by reducing the production of both the 3-oxo-C12-homoserine lactone (3-oxo-C12-HSL) and C4-HSL autoinducers by P. aeruginosa (27). Recently, Favre-Bonté et al. (2) showed that the inhibition of biofilm formation by P. aeruginosa observed with AZM could be complemented with the exogenous autoinducers 3-oxo-C12-HSL and C4-HSL. They suggested that AZM, because of its ability to block cell-to-cell signaling by reducing both 3-oxo-C12-HSL and C4-HSL formation, not only leads to a reduction in the total amount of biofilm formed but also affects its differentiation (2).
Certain macrolides, such as ERY, CAM, RXM, and AZM, are known to exhibit good penetration and distribution into organs and tissues not only in animals but also in humans (8, 25, 36). Tateda et al. (28) also demonstrated AZM accumulation in strain PAO-1 in a time-dependent manner, and such intracellular accumulation of this macrolide was responsible for bactericidal activity through inhibition of the protein synthesis of P. aeruginosa. They suggested that macrolide exposure induces changes in the cell surface structures of bacteria, which may in turn facilitate macrolide entry and allow the antibiotic to accumulate within the bacterial cell. We therefore speculate that both the antibacterial and the anti-quorum-sensing effects of ERY in the present study are due to the high intracellular concentrations of ERY within P. aeruginosa following long-term administration.
In conclusion, we report the antipseudomonal effect of long-term ERY therapy in a murine model of chronic infection with mucoid-producing P. aeruginosa. In the present study, prolonged treatment with ERY suppressed biofilm formation as part of the virulence of P. aeruginosa and led to a significant decrease in the number of viable bacteria. We speculate that prolonged exposure to this macrolide is one of the critical factors for its antipseudomonal effect. Based on the results presented here, we speculate that the efficacy of this macrolide therefore is the result not only of its suggested anti-inflammatory effect but also, at least in part, its antipseudomonal activity.
Acknowledgments
We thank A. Yokoyama (Second Department of Internal Medicine, School of Medicine, Nagasaki University) for technical assistance and T. Suematsu (Central Electron Microscopy Laboratory, School of Medicine, Nagasaki University) for assistance with scanning electron microscopy.
This study was supported in part by grants-in-aid for scientific research on priority areas (14021089) and scientific research (13670605) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Equi, A., I. M. Balfour-Lynn, A. Bush, and M. Rosenthal. 2002. Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. Lancet 360:978-984. [DOI] [PubMed] [Google Scholar]
- 2.Favre-Bonté, S., T. Köhler, and C. V. Delden. 2003. Biofilm formation by Pseudomonas aeruginosa: role of the C4-HSL cell-to-cell signal and inhibition by azithromycin. J. Antimicrob. Chemother. 52:598-604. [DOI] [PubMed] [Google Scholar]
- 3.Fujii, T., J. Kadota, K. Kawakami, K. Iida, R. Shirai, M. Kaseda, S. Kawamoto, and S. Kohno. 1995. Long term effect of erythromycin therapy in patients with chronic Pseudomonas aeruginosa infection. Thorax 50:1246-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Høiby, N. 1994. Diffuse panbronchiolitis and cystic fibrosis: East meets West. Thorax 49:531-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Homma, H., A. Yamanaka, S. Tanimoto, M. Tamura, Y. Chijimatsu, S. Kira, and T. Izumi. 1983. Diffuse panbronchiolitis. A disease of the transitional zone of the lung. Chest 83:63-69. [DOI] [PubMed] [Google Scholar]
- 6.Kadota, J., O. Sakito, S. Kohno, K. Abe, R. Shirai, K. Kawakami, K. Iida, T. Morikawa, S. Kusano, and K. Hara. 1994. Roxithromycin treatment in patients with chronic lower respiratory tract disease—its clinical efficacy and effect on cytokine. J. Jpn. Assoc. Infect. Dis. 68:27-33. [DOI] [PubMed] [Google Scholar]
- 7.Kadota, J., O. Sakito, S. Kohno, H. Sawa, H. Mukae, H. Oda, K. Kawakami, K. Fukushima, K. Hiratani, and K. Hara. 1993. A mechanism of erythromycin treatment in patients with diffuse panbronchiolitis. Am. Rev. Respir. Dis. 147:153-159. [DOI] [PubMed] [Google Scholar]
- 8.Kaneko, A., and M. Kouno. 2001. The characteristics of new macrolide, azithromycin. Dental Diamond 26:78-81. [Google Scholar]
- 9.Khair, O. A., J. L. Devalia, M. M. Abdelaziz, R. J. Sapsford, and R. J. Davies. 1995. Effect of erythromycin on Haemophilus influenzae endotoxin-induced release of il-6, il-8 and sICAM-1 by cultured human bronchial epithelial cells. Eur. Respir. J. 8:1451-1457. [PubMed] [Google Scholar]
- 10.Khan, A. A., T. R. Slifer, F. G. Araujo, and J. S. Remington. 1999. Effect of clarithromycin and azithromycin on production of cytokines by human monocytes. Int. J. Antimicrob. Agents 11:121-132. [DOI] [PubMed] [Google Scholar]
- 11.Khanh, Q. B., M. A. Banevicius, C. H. Nightingale, R. Quintiliani, and D. P. Nicolau. 2000. In vitro and in vivo influence of adjunct clarithromycin on the treatment of mucoid Pseudomonas aeruginosa. J. Antimicrob. Chemother. 45:57-62. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi, H. 1995. Biofilm disease: its clinical manifestation and therapeutic possibilities of macrolides. Am. J. Med. 99:26S-30S. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi, H., H. Takeda, S. Sakayori, Y. Kawakami, Y. Otsuka, M. Tamura, K. Konishi, S. Tanimoto, M. Fukakusa, K. Shimada, Y. Sano, Y. Arai, H. Shihido, H. Watanabe, E. Sakamoto, K. Nagata, T. Nakatani, N. Tsuboi, S. Odagiri, K. Suzuki, Y. Yoshiike, Y. Hirai, T. Okubo, H. Ikeda, M. Arakawa, K. Wada, H. Tsukada, A. Sato, K. Chida, N. Narita, M. Sawaki, K. Mikasa, R. Soejima, Y. Niki, N. Okimoto, T. Sasaki, Y. Matsumoto, Y. Sugimoto, M. Kido, Y. Nikaido, K. Arakawa, K. Kohno, T. Ishibashi, M. Takamoto, Y. Kitahara, K. Hara, S. Kohno, J. Kadora, M. Nasu, Y. Goto, T. Yamasaki, A. Saito, H. Fukuhara, and J. Inadome. 1995. Study on azithromycin in treatment of diffuse panbronchiolitis. J. Jpn. Assoc. Infect. Dis. 69:711-722. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi, H. 2001. Airway biofilm disease. Int. J. Antimicrob. Agents 17:351-356. [DOI] [PubMed] [Google Scholar]
- 15.Koch, C., and N. Høiby. 1993. Pathogenesis of cystic fibrosis. Lancet 341:1065-1069. [DOI] [PubMed] [Google Scholar]
- 16.Kondoh, K., and M. Hashiba. 1998. Inhibitory effect of macrolide antibiotics on biofilm formation by Pseudomonas aeruginosa. Nippon Jibiinkoka Gakkai Kaiho 101:25-36. [DOI] [PubMed] [Google Scholar]
- 17.Kudoh, S., A. Azuma, M. Yamamoto, T. Izumi, and M. Ando. 1998. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am. J. Respir. Crit. Care Med. 157:1829-1832. [DOI] [PubMed] [Google Scholar]
- 18.Kudoh, S., T. Uetake, K. Hagiwara, M. Hirayama, L. Hus, H. Kimura, and Y. Sugiyama. 1987. Clinical effect of low-dose long-term erythromycin chemotherapy on diffuse panbronchiolitis. Jpn. Thorac. Dis. 25:632-642. [PubMed] [Google Scholar]
- 19.Mimoto, T., K. Uchida, S. Sakuraba, Y. Doi, T. Nukiwa, T. Uekusa, N. Kuwabara, K. Inatomi, and S. Kira. 1991. A case of diffuse panbronchiolitis, performed an open lung biopsy after improvement with 6 years medication. Nihon Kyobu Shikkan Gakkai Zasshi 29:893-899. [PubMed] [Google Scholar]
- 20.Mukae, H., J. Kadota, S. Kohno, S. Kusano, T. Morikawa, S. Matsukura, and K. Hara. 1995. Increase of activated CD8+ cells in bronchoalveolar lavage fluid in patients with diffuse panbronchiolitis. Am. J. Respir. Crit. Care Med. 152:613-618. [DOI] [PubMed] [Google Scholar]
- 21.Mukae, H., J. Kadota, J. Ashitani, H. Taniguchi, H. Mashimoto, S. Kohno, and S. Matsukura. 1997. Elevated levels of soluble adhesion molecules in serum of patients with diffuse panbronchiolitis. Chest 112:1615-1621. [DOI] [PubMed] [Google Scholar]
- 22.Nagai, H., H. Shishido, R. Yoneda, E. Yamaguchi, A. Tamura, and A. Kurashima. 1991. Long-term low-dose administration of erythromycin to patients with diffuse panbronchiolitis. Respiration 58:145-149. [DOI] [PubMed] [Google Scholar]
- 23.Sakata, K., H. Yajima, K. Tanaka, Y. Sakamoto, K. Yamamoto, A. Yoshida, and Y. Dohi. 1993. Erythromycin inhibits the production of elastase by Pseudomonas aeruginosa without affecting its proliferation in vitro. Am. Rev. Respir. Dis. 148:1061-1065. [DOI] [PubMed] [Google Scholar]
- 24.Sakito, O., J. Kadota, S. Kohno, K. Abe, R. Shirai, and K. Hara. 1996. Interleukin 1 beta, tumor necrosis factor alpha, and interleukin 8 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis: a potential mechanism of macrolide therapy. Respiration 63:42-48. [DOI] [PubMed] [Google Scholar]
- 25.Suwa, T., H. Yoshida, K. Fukushima, and T. Nagate. 1988. Metabolic fate of TE-031 (A-56268). I. Comparative pharmacokinetics of TE-031 and erythromycin stearate in rats and mice. Chemotherapy 36:198-204. [Google Scholar]
- 26.Tamaoki, J., K. Isono, N. Sakai, T. Kanemura, and K. Konno. 1992. Erythromycin inhibits CI secretion across canine tracheal epithelial cells. Eur. Respir. J. 5:234-238. [PubMed] [Google Scholar]
- 27.Tateda, K., R. Comte, J. C. Pechere, T. Kohler, K. Yamaguchi, and C. Van Delden. 2001. Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 45:1930-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tateda, K., Y. Ishii, T. Matsumoto, N. Furuya, M. Nagashima, T. Matsunaga, A. Ohno, S. Miyazaki, and K. Yamaguchi. 1996. Direct evidence for antipseudomonal activity of macrolides: exposure-dependent bactericidal activity and inhibition of protein synthesis by erythromycin, clarithromycin, and azithromycin. Antimicrob. Agents Chemother. 40:2271-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolter, J., S. Seeney, S. Bell, S. Bowler, P. Masel, and J. McCormack. 2002. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 57:212-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamanaka, A., S. Saiki, S. Tamura, and K. Saito. 1969. Problems in chronic obstructive bronchial diseases, with special reference to diffuse panbronchiolitis. Naika 23:442-451. [PubMed] [Google Scholar]
- 31.Yanagihara, K., K. Tomono, T. Sawai, M. Kuroki, Y. Kaneko, H. Ohno, Y. Higashiyama, Y. Miyazaki, Y. Hirakata, S. Maesaki, J. Kadota, T. Tashiro, S. Kohno. 2000. Combination therapy for chronic Pseudomonas aeruginosa respiratory infection associated with biofilm formation. J. Antimicrob. Chemother. 46:69-72. [DOI] [PubMed] [Google Scholar]
- 32.Yanagihara, K., K. Tomono, T. Sawai, Y. Hirakata, J. Kadota, H. Koga, T. Tashiro, and S. Kohno. 1997. Effect of clarithromycin on lymphocytes in chronic respiratory Pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med. 155:337-342. [DOI] [PubMed] [Google Scholar]
- 33.Yanagihara, K., K. Tomono, T. Sawai, E. Sasaki, H. Kakeya, Y. Yamamoto, H. Ohno, K. Ogawa, J. Kadota, H. Koga, S. Kohno, Y. Hirakata, and T. Tashiro. 1997. Efficacy of erythromycin inhalation in chronic respiratory infection caused by Pseudomonas aeruginosa. Kansenshogaku Zasshi 71:337-341. [DOI] [PubMed] [Google Scholar]
- 34.Yanagihara, K., K. Tomono, M. Kuroki, Y. Kaneko, T. Sawai, H. Ohno, Y. Miyazaki, Y. Higashiyama, S. Maesaki, J. Kadota, and S. Kohno. 2000. Intrapulmonary concentrations of inflammatory cytokines in a mouse model of chronic respiratory infection caused by Pseudomonas aeruginosa. Clin. Exp. Immunol. 122:67-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yasuda, H., Y. Ajiki, T. Koga, H. Kawada, and T. Yokota. 1993. Interaction between biofilms formed by Pseudomonas aeruginosa and clarithromycin. Antimicrob. Agents Chemother. 37:1749-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida, H., and T. Furuta. 1999. Tissue penetration properties of macrolide antibiotics—comparative tissue distribution of erythromycin-stearate, clarithromycin, roxithromycin, and azithromycin in rats. Jpn. J. Antibiot. 503:52-57. [PubMed] [Google Scholar]