Abstract
Heme oxygenase-1 (HO-1) is a highly inducible and ubiquitous cellular enzyme that subserves cytoprotective responses to toxic insults, including inflammation and oxidative stress. In neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease and multiple sclerosis, HO-1 expression is increased, presumably reflecting an endogenous neuroprotective response against ongoing cellular injury. In contrast, we have found that in human immunodeficiency virus (HIV) infection of the brain, which is also associated with inflammation, oxidative stress and neurodegeneration, HO-1 expression is decreased, likely reflecting a unique role for HO-1 deficiency in neurodegeneration pathways activated by HIV infection. We have also shown that HO-1 expression is significantly suppressed by HIV replication in cultured macrophages which represent the primary cellular reservoir for HIV in the brain. HO-1 deficiency is associated with release of neurotoxic levels of glutamate from both HIV-infected and immune-activated macrophages; this glutamate-mediated neurotoxicity is suppressed by pharmacological induction of HO-1 expression in the macrophages. Thus, HO-1 induction could be a therapeutic strategy for neuroprotection against HIV infection and other neuroinflammatory brain diseases. Here, we review various stimuli and signaling pathways regulating HO-1 expression in macrophages, which could promote neuronal survival through HO-1-modulation of endogenous antioxidant and immune modulatory pathways, thus limiting the oxidative stress that can promote HIV disease progression in the CNS. The use of pharmacological inducers of endogenous HO-1 expression as potential adjunctive neuroprotective therapeutics in HIV infection is also discussed.
Keywords: Dimethyl fumarate, heme oxygenase, HIV associated neurocognitive disorders, HO-1, oxidative stress, neuroinflammation.
OXIDATIVE STRESS IN HIV INFECTION
Evidence for a role of oxidative stress in the pathogenesis of HIV infection in both systemic and central nervous system (CNS) compartments has consistently been demonstrated in HIV infected individuals. HIV infection can induce systemic oxidative stress [1-4] through both chronic immune activation [5-7] and direct effects of HIV proteins [8-10]. Individuals infected with HIV express diminished levels of glutathione – a potent endogenous antioxidant – in plasma and peripheral blood mononuclear cells (PBMCs) generally, and specifically in lymphocytes and monocytes [11-13]. Infected individuals also express elevated serum levels of the lipid peroxidation products malondialdehyde and hydroperoxide [14-16]. HIV infected cells demonstrate reduced levels of thioredoxin (a thiol antioxidant) [17], and the HIV proteins gp120 [8, 18-22], Vpr [10, 23], and Tat [9, 18] can directly induce cellular oxidative stress. Oxidative stress within infected cells can promote HIV replication through nuclear factor-kappa B (NF-κB)-driven transcriptional regulation [24-26] and inflammatory cytokine release [24, 27-31], thereby perpetuating systemic immune activation and disease progression. In clinical studies, markers for increased oxidative stress in plasma and circulating CD4+ T-lymphocytes correlate with disease progression in HIV infected individuals [32-34].
HIV infection of the brain, which likely occurs through infiltration of infected immune cells such as monocytes and T lymphocytes, also induces oxidative stress within the CNS [5, 6, 27, 35-37]. HIV proteins such as gp120 [8, 18, 38], Vpr [10, 39], and Tat [9, 18, 30] have been implicated in the direct induction of oxidative stress in several CNS cell types. In macrophages, the primary CNS reservoir for HIV replication, oxidative stress drives neurotoxin production [28, 37, 40-44], and markers of oxidative stress correlate with neurocognitive impairment [27, 43, 45] in HIV-infected individuals. Thus, the mechanisms and pathways by which HIV infection drives immune activation and its associated oxidative stress could be the targets for attenuating disease progression in both systemic and CNS compartments.
Although HIV does not infect neurons, HIV infection can result in a clinical syndrome of neurological and behavioral deficits known as HIV-associated neurocognitive disorders (HAND), which are pathologically associated with neuronal injury and neuronal loss. Chronic HIV infection in the CNS drives immune activation of resident macrophages and microglia, pervasive reactive astrocytosis, perivascular inflammation and infiltration of monocytic cells [46], which can result in HIV-encephalitis (HIVE), especially in those not receiving combination antiretroviral therapy (cART)[47]. Although the morbidity and mortality of HIV infection and the severity of neurocognitive impairment and associated neuroinflammation in HAND have significantly decreased since the introduction of cART in 1996 [48-53], neuroinflammation persists at or above that observed in the pre-ART era [48, 50, 51, 53, 54], and systemic and CNS markers of activation of macrophages correlate with neurocognitive impairment in chronic HIV infection [55-63], even when HIV levels are controlled with cART [64-68]. Interestingly, the clinical severity of HAND correlates more with monocyte infiltration and monocyte-derived macrophages (MDM) and microglia activation than with CNS viral antigen load or number of HIV-infected cells in the brain [68-72].
MACROPHAGES AND MICROGLIA AS MEDIA-TORS OF HAND NEUROPATHOGENESIS
While systemic effects of HIV infection are likely driven by HIV infection of CD4+ T-lymphocytes, the CNS effects of infection are predominantly driven by HIV infection of macrophages and microglia. Within the CNS productive HIV infection, i.e. infection resulting in the production and release of new infectious virions, occurs in perivascular macrophages and microglia, while HIV infection in astrocytes is non-productive [73-76]. Monocytes originate from the bone marrow, travel through the vasculature, and can differentiate into dendritic cells (DCs) in the periphery, or given the appropriate chemotaxis signal can transverse across the endothelial cell barrier into underlying end-organs; DCs do not cross this vascular barrier as readily as monocytes. After or during transmigration, monocytes differentiate into perivascular macrophages, often referred to as monocyte-derived macrophages (MDM). Monocytes express the receptor CD14, and all cells of the monocyte-lineage – e.g. MDM and DC – continue to express CD14 after differentiation. Migration of HIV-infected monocytes across the blood-brain-barrier (BBB) is believed to be the primary source of HIV entry into the CNS. However, it should be noted that other possible routes of HIV entry into the brain include transmigration of HIV-infected T lymphocytes from the blood into CNS tissue or parenchyma [77-79], and infection of or transcytosis through brain microvascular endothelial cells that comprise the BBB [80-86], although there is limited pathological evidence for these routes of entry. See Fig. (1) for a schematic model of HIV neuropathogenesis.
Fig. (1).
Model of HIV Neuropathogenesis. HIV infected immune cells, including CD4+ T cells and CD14+ monocytes, circulate through the blood and produce reactive oxygen species (ROS), which can further enhance viral replication and release. Infected or activated immune cells can also release the pro-inflammatory cytokines, TNF-α and IL-1β, which ROS can exacerbate. HIV proteins – gp120, Tat, and Vpr – may also be released and induce toxicity independently of infectious virions. ROS contribute to increased blood brain barrier (BBB) permeability, which can allow for increased entry of infected immune cells (e.g. monocytes) from the blood into central nervous tissue; infected T lymphocytes may also be a source of virus entry into the CNS. As monocytes enter the nervous tissue they differentiate into macrophages, and release IFN-γ in addition to TNF-α. HIV may replicate in macrophages and may be transmitted to microglia, which in turn can further replicate and transmit HIV. Infected or immune-activated microglia or macrophages can produce IFN-γ and TNF-α in a positive feedback loop, and can release a host of neurotoxic factors. Most neurotoxicity is initially limited to synaptic loss and decrease in dendritic density that is dependent on the NMDA-type glutamate receptor. This leads to eventual loss of neuronal function and finally neuronal death. HIV can infect but not productively replicate in astrocytes. Activated astrocytes have abnormal glutamate metabolism, leading to excess glutamate release and excitotoxicity. TNF-α and IL-1β stimulation of astrocytes can further increase glutamate release. Astrocytes, microglia and macrophages are potent inducers of heme oxygenase-1 (HO-1), which is downregulated in infected macrophages and in HIV-infected brains, and thus may also contribute to neuropathogenesis of HIV infection; neurons demonstrate very limited expression of HO-1. Red arrows indicate potential neurotoxins or direct neurotoxic pathways.
It is still uncertain how and when HIV enters the CNS, and two different models have been proposed: the “Trojan horse” and “late invasion” models. In the “Trojan horse” model, the virus is carried across the BBB early during the course of infection by infiltrating macrophages, and only later during infection, the virus is activated to replicate, recruiting additional monocyte/macrophages into the CNS via cytokine and chemokine signaling, and increased BBB permeability. In the “late invasion” model, chronic immune dysregulation in the periphery leads to an expansion of activated monocyte population from bone marrow that is highly invasive into the CNS and other end-organs [87].
MACROPHAGE NEUROTOXIN PRODUCTION
The neuropathology of HAND is described as decreased CNS neuronal synaptic and dendritic density [70, 88, 89], neuronal cell death [72, 90-92], and altered neuronal cell functioning [93-101], each of which has been linked to persistent inflammation and oxidative stress. These neurotoxic effects likely result from effects of soluble factors released from HIV-infected monocyte-derived macrophages (HIV-MDM) and immune-activated MDM, microglia and astrocytes [102-105]. Many studies have implicated excitotoxic activity through N-methyl-D-aspartate-receptor (NMDAR) over-activation [106-108]. The other neurotoxins released from HIV-MDM or microglia are glutamate [109], quinolinic acid [110, 111], NTox [112], platelet activating factor [113], TNFα [114], and the HIV proteins gp120 [115, 116] and Tat [108, 117] and others. Analysis of cerebrospinal fluid (CSF) from HIV-infected individuals demonstrated increased levels of glutamate [118-120], quinolinic acid [111, 121], platelet activation factor [113], TNFα [108, 114], and other excitotoxins, which correlate with increased severity of HAND and with neuroinflammation. Several of these neurotoxins also alter astrocyte function, in particular glutamate homeostasis.
HEME OXYGENASE-1 AND HIV CNS INFECTION
Despite the multi-faceted nature of CNS damage by HIV, there may be an innate host response to combat the pathological effects of neuroinflammation and oxidative stress. Heme oxygenase-1 (HO-1, alternatively referred to as heat shock protein-32, HSP32), initially identified as a phase II detoxifying enzyme that degrades free heme, has been shown to have potent anti-oxidant qualities and to be highly inducible in response to numerous toxic insults, including heat-shock [122-127], ultraviolet radiation [128], ischemia and/or hypoxia [129-131] or hyperoxia [132], heavy metals [130, 133, 134] and exposure to reactive oxygen species (ROS) [126, 128, 135-141]. To counter the neuroinflammation and oxidative stress, a normal cellular response is acute induction of HO-1 expression, which generates the potent antioxidants bilirubin and biliverdin, as well as carbon monoxide, which has also pro-survival effects [142, 143]. However, whether this response occurs within the CNS during HIV infection has not been reported.
HO-1 is highly expressed in CNS cells, primarily astrocytes, macrophages and microglia, particularly during brain injury in several disease states, including Alzheimer’s disease [144-146], Parkinson’s disease [147-149], Pick disease [150], Huntington’s disease [151] and multiple sclerosis [152, 153] (for more reviews on neurodegenerative disorders: [154-157]). Recent work from our lab has demonstrated a deficiency of HO-1 expression in the brains of individuals with HAND with and without encephalitis (Gill et al. in revision), and in HIV-MDM in vitro [158], which suggests a possible role for HO-1 deficiency in the neuropathogenesis of HAND.
HEME, HEMOGLOBIN SCAVENGING AND HEME OXYGENASE-1 INDUCTION
As the name suggests, a primary function of heme oxygenase is to metabolize heme. Heme is a porphyrin molecule with a bound iron (Fe++) ion, and is a prosthetic group used by hemoglobin and myoglobin proteins to transport oxygen (O2) by the binding of O2 to Fe++. Erythrocytes also known as red blood cells (RBCs) are abundant with heme due to their abundance in hemoglobin, as the primary carriers of oxygen to tissues not capable of direct gas exchange with the external environment. Macrophage scavenging and phagocytosis of hemoglobin-containing senescent erythrocytes (but not erythrocytes without hemoglobin) induce HO-1 expression [136, 159]. Indeed, monocytes/macrophages appear to function as the major scavenger of free hemoglobin in the vasculature. Monocytes/macrophages express the membrane protein CD163, which binds to free hemoglobin [160-162] and induces HO-1 expression [163-165]. HO-1 expression is essential for monocytes/macrophages viability during erythr-ophagocytosis as such cells deficient in HO-1 (HO-1 -/-) die due to this activity [166].
Free heme or hemin also induces HO-1 in monocytes/macrophages [167-169]. Macrophages also express CD91/lipoprotein receptor related protein (LRP), which scavenges free hemoglobin and induces HO-1 transcription upon binding [170]. Interestingly, CD91/LRP is expressed in several other cell types, such as neurons, although it is unclear if it also functions to scavenge hemoglobin or induce HO-1 in neurons [170]. This induction of HO-1 in monocytes/macrophages through hemoglobin/heme binding, however, can be abrogated by other cellular cues. In particular, binding of the platelet derived CXCL4 inhibits CD163 expression and thus also HO-1 induction [171]. Exposure to lipopolysaccharide (LPS) can also inhibit CD163 expression and HO-1 induction in monocytes [162]. Moreover, oxidized hemoglobin induces a structural alteration that does not allow binding of CD163 to signal downstream to increase HO-1 expression [172]. Thus HIV may indirectly reduce HO-1 in monocytes/macrophages due to the increased levels of ROS and oxidative damage in HIV infection.
HO-1 enzymatic breakdown of heme yields bilirubin, which is further converted to biliverdin, both of which are potent free radical scavengers. Thus, macrophages can induce HO-1 to counteract the high levels of ROS they produce during phagocytosis, including phagocytosis of parasites, which are released in order to better destroy the engulfed material [173-175]. Indeed, exposure to external ROS can induce HO-1 in monocytes/macrophages [139, 176, 177]. HO-1 may also induce the production of glutathione, another potent endogenous antioxidant [173, 178], and depletion of glutathione may itself trigger HO-1 induction [179].
INFECTIOUS AGENTS AND HEME OXYGENASE-1: BENEFICIAL AND INJURIOUS RESPONSES
In addition to heme metabolism and oxidative stress, monocytes/macrophages increase HO-1 expression in response to viral and bacterial agents as well. Several studies have shown robust induction of HO-1 in monocytes/macrophages following exposure to LPS, typically found in Gram-negative bacteria [180-185]. Also, in at least one study, HO-1 is found to be induced by and protective against non-tuberculous mycobacterial infection [186]. HO-1 is also induced during tuberculosis infection, however it is unclear whether this promotes or reduces infection [187]. Herpes simplex virus-1 (HSV-1) encephalitis induces a strong HO-1 response in macrophages in the brain [188, 189]; and infection of neurotropic Borna disease virus (BSV) in the brain leads to sustained HO-1 induction in activated microglia, reactive astrocytes and mononuclear infiltrates for up to 4 weeks post-infection, presumably as a neuroprotective response [190].
The macrophage production of HO-1 during other parasitic infections might subserve both beneficial and injurious responses. As monocytes/macrophages express HO-1 to protect against the ROS they release to kill engulfed pathogens, induction of HO-1 helps to promote monocytes/macrophages survival and maintain degradative function. However, certain parasites have found ways to exploit the protective effects of HO-1 expression in monocytes/macrophages. For example, Leishmania chagasi promastigote infection in macrophages, persists longer in macrophages that express HO-1 [191], and the ability of HO-1 to reduce superoxide production is thought to aid in Leishmania Mexicana pifano amastigote infection [174]. However, drugs that are successful in controlling Leishmania donovani appear to do so by increasing HO-1 expression in THP-1 monocytic lines [192].
In contrast, higher HO-1 expression is associated with increased susceptibility to malaria (Plasmodium falciparum) infection [193-196]. This may be due to the heme overload from malaria-induced hemolysis, from which increased HO-1 activity would lead to an overload of free iron [197]. HO-1 expression is high in the malaria-infected brain, monocytic cells and microglia that surround the classic malaria granulomas (Durck’s granulomas), [198]; however, whether this is a beneficial or injurious response to bleeding and free hemoglobin rather than a direct response to the malaria pathogen is unknown, as other reports have found that HO-1 expression can be protective against the disease [199, 200].
INJURY-INDUCED HEME OXYGENASE-1 EXPRESSION IN THE BRAIN
Although neurons appear to have little to no HO-1 inducibility within the brain, some neurons in the spinal cord peripheral nervous system (PNS) show increased HO-1 expression in response to injury [201-203]. Some reports indicate that cortical neurons can express HO-1, if only for a few hours [204-207]. In contrast, HO-1 is robustly expressed in astrocytes, macrophages, microglia, and oligodendrocytes in response to injury [201, 208, 209], including hypoxia/ischemia [204, 207, 210].
Perivascular macrophages within the brain can express high levels of immunoreactive HO-1 during injury. This is especially true where there is hemorrhage [201, 205, 211, 212]. In particular, CD163+ macrophages appear to be primary inducers of HO-1, at least in response to traumatic brain injury (TBI) [213, 214]. Increased permeability of the BBB is also sufficient to induce HO-1 expression in astrocytes and macrophages/microglia [215]. There is prolonged expression of HO-1 in microglia and macrophages, with reports of up to 42 days [203], 5 months [206], or 6 months after the initial injury [216].
FUNCTIONAL CONSEQUENCES OF INDUCED BRAIN HO-1 EXPRESSION
In most reports, the increased expression of HO-1 is thought to be neuroprotective given the many beneficial cellular effects of HO-1 [157]. However, there is evidence that in some cases HO-1 induction may exacerbate injury, and therapeutic interventions that reduce HO-1 in glia have been proposed for certain neurodegenerative diseases [156]. For example, Wang & Doré [212] reported that HO-1 knock-out mice had less brain injury due to intracerebral hemorrhage than their wild-type counterparts, along with overall reduction in leukocyte infiltration, microglial/macrophage activation, and free radical levels. Also, macrophage cell lines (RAW 264.7) that express the Apo-ε4 allele – a known genetic risk factor for Alzheimer’s disease – express higher levels of HO-1 than cells expressing the Apo-ε3 allele [217].
There are some interesting reports of brain injury surprisingly causing damage in other organ systems. For example, increased macrophage HO-1 expression in either the kidney or lung is seen in brain death during kidney transplantation [218], and acute lung injury following TBI [219]. This suggests that either there is a humoral signal released within the brain (e.g. monocytes/macrophages, astrocytes, microglia, neurons) that activates macrophages in the periphery, or that macrophages activated in the injured brain are able to migrate out of the CNS into the periphery and into other organs. These findings may suggest that constitutive HO-1 expression in non-injured or healthy tissue may cause tissue damage, and may be the reason HO-1 is expressed as an inducible rather than a constitutive protein.
There are numerous examples of beneficial effects of HO-1 induction in the CNS. Induction of HO-1 in astrocytes is reported to reduce microglial activation, ROS levels and leukocyte infiltration in a mouse model of HSV-1 encephalitis [189], and HO-1 is briefly induced in astrocytes following excitotoxicity [220]. In addition, some metabolites of the kynurenine pathway expressed in activated macrophages and microglia - hydroxyanthranilic acid (3-HAA) and 3-hydroxykynurenine (3-HK) – inhibit cytokine release and cytokine-induced neuronal death, which may be due to the ability of 3-HAA to induce HO-1 in astroyctes [221]. HO-1 expression in macrophages/microglia is particularly beneficial in the spinal cord, as shown in experimental autoimmune encephalitis mouse models [222] and spinal cord injury [223]. Furthermore, HO-2 may be neuroprotective, as neuronal expression of HO-2 is increased in response to hypoxia-ischemia [207], and HO-2 knock-out mice show increased brain swelling, neuronal death, leukocyte infiltration, free radicals and activation of astrocytes and microglia/macrophages due to intracerebral hemorrhage in comparison with wild-type mice with intracerebral hemorrhage [224].
SIGNALING PATHWAYS OF HEME OXYGENASE-1 EXPRESSION
The canonical mechanism of HO-1 induction is related to the antioxidant response (AR) pathway mediated by the master transcriptional regulator, nuclear factor erythrocyte-2 related factor 2 (Nrf2). Nrf2 is normally sequestered in the cytosol by its binding partner, Keap1, which helps target Nrf2 for degradation [225]. However, upon exposure to oxidative stress, Keap1 is degraded, which allows Nrf2 to translocate to the nucleus and activate transcription of many genes that function to protect and repair damage due to oxidative stress [226]. However, the specific signaling pathway(s) of Nrf2 activation are still unclear, as several regulatory kinases have been implicated, which may vary due to the type of stimulus the cell detects.
There appear to be several signaling pathways that can lead to HO-1 expression. LPS is commonly used to induce HO-1 expression; and as the major detector of LPS, Toll-like receptor-4 (TLR4) would be predicted to mediate HO-1 induction. Indeed, several studies have shown TLR4-mediated HO-1 induction, particularly in macrophages [182, 184, 227] and MyD88-dependent signaling of HO-1 expression [228]. There appears to be a negative feedback loop whereby HO-1 induction leads to decreased TLR4 expression [229, 230], or inhibits the signaling properties of TLR4 [231, 232]. The alpha7 nicotinic acetylcholine receptor [233] and the beta adrenergic receptor in RAW 264.7 macrophage cell lines [234] are also reported to initiate HO-1 expression, either through protein kinase C (PKC) or protein kinase A (PKA), respectively. Some reports state the HO-1 expression is signaled via PKC activity [183, 235], while others report extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK)-mediated induction [236, 237], and perhaps activation of both PKC and ERK [238]. ERK may also regulate HO-1 production at the translational level rather than at the transcriptional level [239]. Other major kinases have also been shown to function in HO-1 induction, including p38 MAPK [231, 240-246] and c-Jun N-terminal kinase (JNK) 1/2 [247, 248].
While many reports show or suggest Nrf2-dependent binding to the antioxidant response element (ARE) in the HO-1 promoter as the major inducer of HO-1 expression [249], there is evidence for Nrf2 independent HO-1 induction. The transcription factor activator protein-1 (AP-1) binds to at least 2 different enhancer elements (4kb and 6kb upstream) in the HO-1 promoter, and such binding is necessary for LPS and hyperoxia-mediated induction of HO-1 [180, 250, 251]. In ethanol induction of HO-1, Nrf2 was explicitly determined to be nonfunctional in HO-1 expression while AP-1 was necessary [248]. The HO-1 promoter also contains a cAMP-response element (CRE) approximately 650 bp upstream of the transcription start site, which is necessary for HO-1 induction [252]. AP-1 can bind this region, as well as the transcription factor CREB (cAMP-response element binding) protein, known to be regulated by PKA [253, 254]. The HO-1 promoter also contains binding sites for nuclear factor-kappa B (NF-κB) 156 bp and 370 bp upstream from the transcription start site [181, 255-257]. It is also reported that HO-1 leads to reduced NF-κB p65/Rel A levels [258, 259], which may suggest a negative feedback mechanism of NF-κB.
CYTOKINE AND HEME OXYGENASE-1 SIGNALING AND FEEDBACK
Apart from the primary enzymatic function of heme catabolism, and bilirubin and carbon monoxide production, all of which have cytoprotective effects, increased HO-1 expression also correlates with altered expression of several cytokines. In many reports, HO-1 appears to promote the expression of anti-inflammatory cytokines and dampen the expression of pro-inflammatory cytokines. Interleukin-10 (IL-10) is a classic anti-inflammatory cytokine, and several reports have shown that induction of HO-1 directly leads to increased IL-10 production [260-265]. Furthermore, HO-1 expression inhibits expression of the prototypical pro-inflammatory cytokine tumor necrosis factor-alpha (TNFα) [230, 263, 264, 266, 267]. Induction of HO-1 also reduces expression of other pro-inflammatory cytokines such as IL-1β [267, 268], IL-6 [175, 261, 269], IL-33 [230] and monocyte chemoattractant protein-1 (MCP-1) [175, 259, 267, 270, 271].
At the same time, these HO-1 regulated cytokines may feedback to regulate HO-1 expression itself. In macrophages, IL-6 appears to increase HO-1 [272], while TNFα treatment in CD14+ cells leads to decreased HO-1 expression [273]. Several reports show that exposure to IL-10 directly induces HO-1 [163, 272, 274-276]. Furthermore, some evidence suggests that IL-10-induced inhibition of TNFα expression is mediated by HO-1 expression [241]. In microglia, IL-10 and HO-1 appear to be co-induced by the anti-inflammatory cytokine, IL-34 [277], further implicating a synergistic association between IL-10 and HO-1.
Although most reports point to the anti-inflammatory properties of HO-1, there are a few studies to the contrary. In RAW 264.7 macrophages, HO-1 inhibition is associated with decreased TNFα expression [268]; however the reverse experiment of HO-1 induction has not been performed to confirm this conclusion. It is also reported that HO-1 induces MCP-1 expression, although contradictory evidence has been published. In one study, genetic deficiency of HO-1 in macrophages (and other myeloid cells) resulted in reduced MCP-1 expression upon viral infection or TLR3 and TLR4 stimulation [222]; however given the range of other cytokines and downstream transcription processes HO-1 regulates, it is difficult to state a direct effect of HO-1 expression leading to increased MCP-1 production. Another study with epithelial cells also reported increased MCP-1 with increased HO-1, using hemin as an inducer of HO-1 [278]; while hemin is a strong inducer of HO-1, it may also trigger other signaling pathways that regulate MCP-1 expression independently of HO-1. Indeed, the authors state that there is a delayed phase of MCP-1 in these conditions that is HO-1 independent, which may suggest that hemin co-induces HO-1 with MCP-1 to function as a negative regulator of MCP-1. HO-1 also decreases interferon-beta (IFN-β) [222] and interferon-gamma expression (IFN-γ) [266], which may serve either pro- or anti-inflammatory functions depending on the cell-type and surrounding cytokine milieu. Fig. (2) illustrates some of the signaling interactions described here.
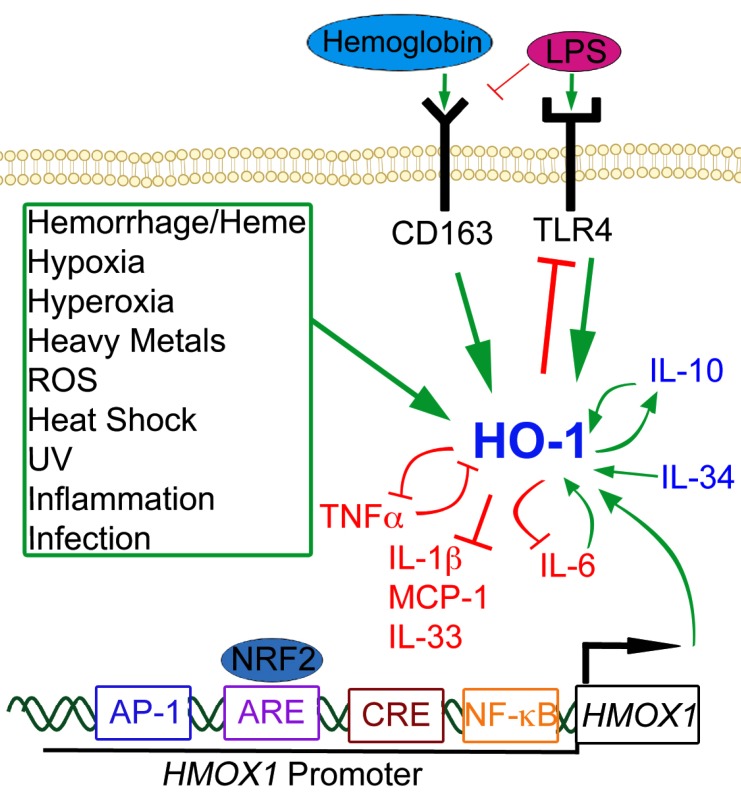
Fig. (2).
Signaling Pathways of HO-1. Green arrows represent increased HO-1 expression or activity. Red blocked lines represent inhibition or decreased expression. Several noxious stimuli, highlighted in the green box, are known inducers of HO-1, as well as hemoglobin/heme through the CD163 receptor and LPS through the TLR4 receptor. Anti-inflammatory cytokines are represented in blue; pro-inflammatory cytokines are depicted in red. There are many cytokine signaling loops that involve HO-1 activity or expression, including a positive feedback loop between HO-1 and IL-10 (anti-inflammatory), and a negative feedback loop between HO-1 and TNF-α (pro-inflammatory). Several transcription factors can also bind to the HO-1 promoter (HMOX1 is the gene symbol for HO-1), notably NRF2 at the ARE site, but also AP-1, CREB, and NF-kB can bind to the promoter at independent binding sites and induce HO-1 expression.
THERAPEUTIC POTENTIAL OF EXOGENOUS INDUCERS OF HEME OXYGENASE-1 EXPRESSION
Since HO-1 initiates potent endogenous antioxidant responses, induction of HO-1 expression has therapeutic potential for several diseases in which oxidative stress is symptomatic, including HIV infection. Treatment of mice with cobalt IX protoporphyrin (CoPP), a powerful HO-1 inducer, was shown to promote graft survival in a model of kidney transplantation [266]. However, HO-1 inducers used in vitro, including LPS, hemin, or proto- or mesoporphyrins, have limited clinical relevance given the range of negative side effects and/or limited tolerability or bioavailability. Gene-therapy models have also been developed to more permanently induce HO-1 in target cell types [260, 279]. While promising, this type of approach may be beneficial only in certain conditions, and such therapies have not yet been fully validated in the clinical setting. A more immediate and simpler approach is the use of orally available compounds that can induce HO-1 in vivo.
Many studies have shown that a number of plant-based extracts (phytopharmaceuticals) induce HO-1, leading to ameliorated oxidative stress and inflammation. The sources of these compounds are several different plant species and plant organs, ranging from seeds to fruits to leaves to roots [238, 245, 247, 280-293]. Some of these compounds can be found in commonly-used plants, such as brown rice [294], Chinese cinnamon [295] or Ginkgo biloba [243, 296]. Curcumin and omega-3 fatty acids are well-studied natural compounds with antioxidant and anti-inflammatory properties that might be related to their potent induction of HO-1 [270, 297-300], although it is not clear if their effects are directly due to their induction of HO-1. As dietary compounds that can be administered orally, such phytopharmaceuticals offer the promise of easily available therapy with potentially high tolerability. However, caution must be prioritized in directly translating these in vitro studies to the clinical setting, as no rigorous clinical trials have been performed for many of these compounds. The question of dosage, metabolism and bioavailability will need further and extensive study to determine the efficacy and safety of such compounds as a form of treatment.
Currently, there are two classes of FDA-approved drugs that have the immediate potential for testing HO-1 induction in clinical trials: statins and fumaric acid esters (FAEs). Statins have been widely prescribed to reduce cholesterol levels by inhibiting HMG-CoA reductase [301]. However, in vitro and in vivo rodent models have shown that statins can robustly induce HO-1 in monocytes/macrophages, leading to reduction of inflammation [302-305]. BBB-permeable statins therefore have the potential to induce HO-1 in cells of the CNS, including macrophages, microglia, and astrocytes, although such studies have yet to be reported.
Fumaric acid esters, on the other hand, have a demonstrated effect in the CNS. In particular, a formulation of dimethyl fumarate (DMF) trade-marked as Tecfidera, was approved by the FDA on March 27, 2013 for the treatment of multiple sclerosis (MS). DMF is orally delivered, and is quickly metabolized to monomethyl fumarate (MMF), and both compounds exert similar, though not equivalent, biological activities (for review see Gill & Kolson 2013 [306]). DMF functions as an immune modulator, and it was initially approved to treat psoriasis in Germany [307, 308]. Work from our lab has demonstrated that DMF/MMF can robustly induce HO-1 in macrophages [158], and others have shown the same effect in type II dendritic cells [309]. Furthermore, we have shown that DMF/MMF treatment of HIV-infected macrophages significantly attenuates the release of soluble neurotoxins. Pre-treatment of macrophages with DMF/MMF before HIV inoculation reduces HIV infection [158]. The reduction of neurotoxin release is clearly mediated by the increased HO-1 elicited by DMF/MMF, while the suppression of HIV infection might be related to inhibition of NFκ-B translocation [158]. The inhibition of HIV replication by HO-1 induction has also been previously observed in monocytes, using hemin as a stimulant [310], and in MDM using LPS to induce HO-1 [311]. The correlation with increased HO-1 and decreased HIV replication has been recently demonstrated in clinical studies in which HIV+ patients with lower HO-1 expression were found to have higher levels of viremia (when removed from cART) [312]. The effects of HO-1 induction in the CNS of HIV+ patients and its relation to HAND remain to be examined; however, given the proven CNS efficacy of DMF in suppressing inflammation and immune activity in MS patients, DMF stands as a compelling candidate for HAND and other HIV-associated disorders in other tissues (kidney, liver, heart, bone) not derived from opportunistic infections.
CONCLUSION
As this review highlights, there are several pathways through which HO-1 may be induced or activated, in monocytes/macrophages (the primary HIV reservoir in the CNS) and in CNS cells such as astrocytes and microglia, and even neurons in certain circumstances. Increased HO-1 expression in these cell types has the potential to reduce several salient features of HAND and other HIV associated end-organ diseases that have oxidative stress and chronic immune activation as common symptoms. Several classes of pharmaceuticals, from fumaric acid esters (e.g. DMF or MMF) to statins to phytochemicals, have the potential to target these pathways to be tested or developed for HIV adjunctive therapy in addition to current anti-retroviral therapy, and may also aid in the treatment for other neurodegenerative diseases such as Alzheimer’s or Parkinson’s disease.
ACKNOWLEDGEMENTS
We would like to thank A.J. Gill for his review of the manuscript and figure, and we thank the editors, including J. Rappaport, for their support of this submission. We acknowledge our funding sources, NIH-MH097671 and NIH-NS-07180.
LIST OF ABBREVIATIONS
- AP-1
= Activator protein 1
- ARE
= Antioxidant response element
- BBB
= Blood-brain barrier
- cART
= Combined anti-retroviral therapy
- CNS
= Central nervous system
- DC
= Dendritic cell
- DMF
= Dimethyl fumarate
- HAND
= HIV-Associated Neurocognitive Disorders
- HIV
= Human immunodeficiency virus
- HIVE
= HIV Encephalitis
- HIV-MDM
= HIV infected monocyte-derived macrophage
- HO-1
= Heme oxygenase-1
- HSV-1
= Herpes simplex virus-1
- IFNβ
= Interferon-beta
- IFNγ
= Interferon-gamma
- IL-10
= Interleukin 10
- IL-1β
= Interleukin 1 beta
- LPS
= Lipopolysaccharide
- MCP
= Monocyte chemoattractant protein 1
- MDM
= Monocyte-derived macrophage
- MMF
= Monomethyl fumarate
- MS
= Multiple sclerosis
- NF-κB
= Nuclear factor kappa B
- Nrf2
= Nuclear factor erythrocyte-2 related factor 2
- PBMC
= Peripheral blood mononuclear cells
- PNS
= Peripheral nervous system
- ROS
= Reactive oxygen species
- TBI
= Traumatic brain injury
- TLR
= Toll-like receptor
- TNFα
= Tumor necrosis factor alpha
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
PATIENT CONSENT
Declared none.
HUMAN/ANIMAL RIGHTS
Declared none.
REFERENCES
- 1.Perl A, Banki K. Genetic and metabolic control of the mitochondrial transmembrane potential and reactive oxygen intermediate production in HIV disease. Antioxidants and Redox Signaling. 2000;2(3): 551–73. doi: 10.1089/15230860050192323. [DOI] [PubMed] [Google Scholar]
- 2.Elbim C, Pillet S, Prevost MH, Preira A, Girard PM. The role of phagocytes in HIV-related oxidative stress. Journal of Clinical Virology. 2001;20(3): 99–109. doi: 10.1016/s1386-6532(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 3.Allard JP, Aghdassi E, Chau J, Salit I. Oxidative stress and plasma antioxidant micronutrients in humans with HIV infection. American Journal of Clinical Nutrition. 1998;67(1): 143–7. doi: 10.1093/ajcn/67.1.143. [DOI] [PubMed] [Google Scholar]
- 4.Repetto M, Reides C, Carretero MG, Costa M. Oxidative stress in blood of HIV infected patients. Clinica Chimica Acta. 1996;255(2): 107–17. doi: 10.1016/0009-8981(96)06394-2. [DOI] [PubMed] [Google Scholar]
- 5.Valcour V, Shiramizu B. HIV-associated dementia, mitochondrial dysfunction, and oxidative stress. Mitochondrion. 2004;4(2-3): 119–29. doi: 10.1016/j.mito.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Mollace V, Nottet HS, Clayette P , et al. Oxidative stress and neuroAIDS triggers, modulators and novel antioxidants. Trends in Neurosciences. 2001;24(7): 411–6. doi: 10.1016/s0166-2236(00)01819-1. [DOI] [PubMed] [Google Scholar]
- 7.Aukrust P, Svardal AM, Müller F, Lunden B, Berge RK, Frøland SS. Decreased levels of total and reduced glutathione in CD4+ lymphocytes in common variable immunodeficiency are associated with activation of the tumor necrosis factor system possible immunopathogenic role of oxidative stress. Blood. 1995;86(4): 1383–91. [PubMed] [Google Scholar]
- 8.Ronaldson PT, Bendayan R. HIV-1viral envelope glycoprotein gp120 produces oxidative stress and regulates the functional expression of multidrug resistance protein-1 (Mrp1):in glial cells. Journal of Neurochemistry. 2008;106(3): 1298–313. doi: 10.1111/j.1471-4159.2008.05479.x. [DOI] [PubMed] [Google Scholar]
- 9.Turchan-Cholewo J, Dimayuga FO, Gupta S , et al. Morphine and HIV-Tat increase microglial-free radical production and oxidative stress possible role in cytokine regulation. Journal of Neurochemistry. 2009;108(1): 202–15. doi: 10.1111/j.1471-4159.2008.05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshmane SL, Mukerjee R, Fan S , et al. Activation of the oxidative stress pathway by HIV-1 Vpr leads to induction of hypoxia-inducible factor 1alpha expression. Journal of Biological Chemistry. 2009;284(17): 11364–73. doi: 10.1074/jbc.M809266200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eck HP, Gmünder H, Hartmann M, Petzoldt D, Daniel V, Dröge W. Low concentrations of acid-soluble thiol (cysteine) in the blood plasma of HIV-1-infected patients. Biological Chemistry. 1989;370(2): 101–8. doi: 10.1515/bchm3.1989.370.1.101. [DOI] [PubMed] [Google Scholar]
- 12.de Quay B, Malinverni R, Lauterburg BH. Glutathione depletion in HIV-infected patients role of cysteine deficiency and effect of oral N-acetylcysteine. AIDS. 1992;6(8): 815–9. [PubMed] [Google Scholar]
- 13.Malvy DJ, Richard MJ, Arnaud J, Favier A, Amedee-Manesme O. Relationship of plasma malondialdehyde, vitamin E and antioxidant micronutrients to human immunodeficiency virus-1 seropositivity. Clinica Chimica Acta. 1994;224(1): 89–94. doi: 10.1016/0009-8981(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 14.Sönnerborg A, Carlin G, Akerlund B, Jarstrand C. Increased production of malondialdehyde in patients with HIV infection. Scandinavian Journal of Infectious Diseases. 1988;20(3): 287–90. doi: 10.3109/00365548809032453. [DOI] [PubMed] [Google Scholar]
- 15.Revillard JP, Vincent CM, Favier AE, Richard MJ, Zittoun M, Kazatchkine MD. Lipid peroxidation in human immunodeficiency virus infection. Journal of Acquired Immune Deficiency Syndromes. 1992;5(6): 637–8. [PubMed] [Google Scholar]
- 16.McLemore JL, Beeley P, Thorton K, Morrisroe K, Blackwell W, Dasgupta A. Rapid automated determination of lipid hydroperoxide concentrations and total antioxidant status of serum samples from patients infected with HIV elevated lipid hydroperoxide concentrations and depleted total antioxidant capacity of serum samples. American Journal of Clinical Pathology. 1998;109(3): 268–73. doi: 10.1093/ajcp/109.3.268. [DOI] [PubMed] [Google Scholar]
- 17.Masutani H, Naito M, Takahashi K , et al. Dysregulation of adult T-cell leukemia-derived factor (ADF)/thioredoxin in HIV infection loss of ADF high-producer cells in lymphoid tissues of AIDS patients. AIDS Research and Human Retroviruses. 1992;8(9): 1707–15. doi: 10.1089/aid.1992.8.1707. [DOI] [PubMed] [Google Scholar]
- 18.Price TO, Ercal N, Nakaoke R, Banks WA. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Research. 2005;1045(1-2): 57–63. doi: 10.1016/j.brainres.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 19.Foga IO, Nath A, Hasinoff BB, Geiger JD. Antioxidants and dipyridamole inhibit HIV-1 gp120-induced free radical-based oxidative damage to human monocytoid cells. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1997;16(4): 223–9. doi: 10.1097/00042560-199712010-00001. [DOI] [PubMed] [Google Scholar]
- 20.Otis JS, Ashikhmin YI, Brown LAS, Guidot DM. Effect of HIV-1-related protein expression on cardiac and skeletal muscles from transgenic rats. AIDS Research and Therapy. 2008;5: 8. doi: 10.1186/1742-6405-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Q-R, Zhang Z-P, Zhang H , et al. Inducible nitric oxide synthase is involved in the oxidation stress induced by HIV-1 gp120 in human retina pigment epithelial cells. Chinese Medical Journal. 2008;121(24): 2578–83. [PubMed] [Google Scholar]
- 22.Radrizzani M, Accornero P, Delia D, Kurrle R, Colombo MP. Apoptosis induced by HIV-gp120 in a Th1 clone involves the generation of reactive oxygen intermediates downstream CD95 triggering. FEBS Letters. 1997;411(1): 87–92. doi: 10.1016/s0014-5793(97)00672-8. [DOI] [PubMed] [Google Scholar]
- 23.Stromájer-Rácz T, Gazdag Z, Belágyi J, Vágvölgyi C, Zhao RY, Pesti M. Oxidative stress induced by HIV-1 F34IVpr in Schizosaccharomyces pombe is one of its multiple functions. Experimental and Molecular Pathology. 2010;88(1): 38–44. doi: 10.1016/j.yexmp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Poli G, Fauci AS. The effect of cytokines and pharmacologic agents on chronic HIV infection. AIDS Research and Human Retroviruses. 1992;8(2): 191–7. doi: 10.1089/aid.1992.8.191. [DOI] [PubMed] [Google Scholar]
- 25.Palamara AT, Perno CF, Aquaro S, Buè MC, Dini L, Garaci E. Glutathione inhibits HIV replication by acting at late stages of the virus life cycle. AIDS Research and Human Retroviruses. 1996;12(16): 1537–41. doi: 10.1089/aid.1996.12.1537. [DOI] [PubMed] [Google Scholar]
- 26.Israel N, Gougerot-Pocidalo MA. Oxidative stress in human immunodeficiency virus infection. Cellular and Molecular Life Sciences. 1997;53(11-12): 864–70. doi: 10.1007/s000180050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roc AC, Ances BM, Chawla S , et al. Detection of human immunodeficiency virus induced inflammation and oxidative stress in lenticular nuclei with magnetic resonance spectroscopy despite antiretroviral therapy. Archives of Neurology. 2007;64(9): 1249–57. doi: 10.1001/archneur.64.9.noc60125. [DOI] [PubMed] [Google Scholar]
- 28.Reddy PVB Agudelo, M. Atluri VSR, Nair MP. Inhibition of nuclear factor erythroid 2-related factor 2 exacerbates HIV-1 gp120-induced oxidative and inflammatory response role in HIV associated neurocognitive disorder. Neurochemical Research. 2012;37(8): 1697–706. doi: 10.1007/s11064-012-0779-0. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs J, Oelke N, Imhof M , et al. Multiparameter analysis of clastogenic factors, pro-oxidant cytokines, and inflammatory markers in HIV-1-infected patients with asymptomatic disease, opportunistic infections, and malignancies. Molecular Medicine. 1998;4(5): 333–43. [PMC free article] [PubMed] [Google Scholar]
- 30.Toborek M, Lee YW, Pu H , et al. HIV-Tat protein induces oxidative and inflammatory pathways in brain endothelium. Journal of Neurochemistry. 2003;84(1): 169–79. doi: 10.1046/j.1471-4159.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 31.Driscoll KE. TNFαlpha and MIP-2: role in particle-induced inflammation and regulation by oxidative stress. Toxicology Letters. 2000;112-113: 177–83. doi: 10.1016/s0378-4274(99)00282-9. [DOI] [PubMed] [Google Scholar]
- 32.Wanchu A, Rana SV, Pallikkuth S, Sachdeva RK. Short communication oxidative stress in HIV-infected individuals a cross-sectional study. AIDS Research and Human Retroviruses. 2009;25(12): 1307–11. doi: 10.1089/aid.2009.0062. [DOI] [PubMed] [Google Scholar]
- 33.Ibeh BO, Emeka-Nwabunnia IK. Increased oxidative stress condition found in different stages of HIV disease in patients undergoing antiretroviral therapy in Umuahia (Nigeria). Immunopharmacology and Immunotoxicology. 2012;34(6): 1060–6. doi: 10.3109/08923973.2012.681327. [DOI] [PubMed] [Google Scholar]
- 34.Suresh DR, Annam V, Pratibha K, Prasad BV. Total antioxidant capacity--a novel early bio-chemical marker of oxidative stress in HIV infected individuals. Journal of Biomedical Science. 2009;16: 61. doi: 10.1186/1423-0127-16-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haughey NJ, Cutler RG, Tamara A , et al. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Annals of Neurology. 2004;55(2): 257–67. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- 36.Bandaru VV, McArthur JC, Sacktor N , et al. Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology. 2007;68(18): 1481–7. doi: 10.1212/01.wnl.0000260610.79853.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turchan J, Pocernich CB, Gairola C , et al. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology. 2003;60(2): 307–14. doi: 10.1212/01.wnl.0000042048.85204.3d. [DOI] [PubMed] [Google Scholar]
- 38.Viviani B, Corsini E, Binaglia M, Galli CL, Marinovich M. Reactive oxygen species generated by glia are responsible for neuron death induced by human immunodeficiency virus-glycoprotein 120 in vitro. Neuroscience. 2001;107(1): 51–8. doi: 10.1016/s0306-4522(01)00332-3. [DOI] [PubMed] [Google Scholar]
- 39.Ferrucci A, Nonnemacher MR, Cohen EA, Wigdahl B. Extracellular human immunodeficiency virus type 1 viral protein R causes reductions in astrocytic ATP and glutathione levels compromising the antioxidant reservoir. Virus Research. 2012;167(2): 358–69. doi: 10.1016/j.virusres.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi B, Raina J, Lorenzo A, Busciglio J, Gabuzda D. Neuronal apoptosis induced by HIV-1 Tat protein and TNF-alpha potentiation of neurotoxicity mediated by oxidative stress and implications for HIV-1 dementia. Journal of Neurovirology. 1998;4(3): 281–90. doi: 10.3109/13550289809114529. [DOI] [PubMed] [Google Scholar]
- 41.Tian C, Sun L, Jia B , et al. Mitochondrial glutaminase release contributes to glutamate-mediated neurotoxicity during human immunodeficiency virus-1 infection. Journal of Neuroimmune Pharmacology. 2012;7(3): 619–28. doi: 10.1007/s11481-012-9364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Velazquez I, Plaud M, Wojna V, Skolasky R, Laspiur JP, Melendez LM. Antioxidant enzyme dysfunction in monocytes and CSF of Hispanic women with HIV-associated cognitive impairment. Journal of Neuroimmunology. 2009;206(1-2): 106–11. doi: 10.1016/j.jneuroim.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Wang M, Li H , et al. Accumulation of nuclear and mitochondrial DNA damage in the frontal cortex cells of patients with HIV-associated neurocognitive disorders. Brain Research. 2012;1458: 1–11. doi: 10.1016/j.brainres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death and Differentiation. 2005;12(suppl 1 ):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- 45.Sacktor N, Haughey N, Cutler R , et al. Novel markers of oxidative stress in actively progressive HIV dementia. Journal of Neuroimmunology. 2004;157(1-2): 176–84. doi: 10.1016/j.jneuroim.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 46.Gendelman HEGI, Everall IP, Fox HS, Gelbard HA, Lipton SA, Swindells S, Gelman BB. HIV-1 Neuropathology. The Neurology of AIDS. 2012 [Google Scholar]
- 47.Wiestler OD, Leib SL, Brustle O, Spiegel H, Kleihues P. Neuropathology and pathogenesis of HIV encephalopathies. Acta Histochem Suppl. 1992;42: 107–14. [PubMed] [Google Scholar]
- 48.Bell JE. An update on the neuropathology of HIV in the HAART era. Histopathology. 2004;45(6): 549–59. doi: 10.1111/j.1365-2559.2004.02004.x. [DOI] [PubMed] [Google Scholar]
- 49.Morgello S, Mahboob R, Yakoushina T, Khan S, Hague K. Autopsy findings in a human immunodeficiency virus-infected population over 2 decades influences of gender, ethnicity, risk factors, and time. Archives of Pathology and Laboratory Medicine. 2002;126(2): 182–90. doi: 10.5858/2002-126-0182-AFIAHI. [DOI] [PubMed] [Google Scholar]
- 50.Langford TD, Letendre SL, Larrea GJ, Masliah E. Changing patterns in the neuropathogenesis of HIV during the HAART era. Brain Pathology. 2003;13(2): 195–210. doi: 10.1111/j.1750-3639.2003.tb00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. Journal of Neuropathology and Experimental Neurology. 2005;64(6): 529–36. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- 52.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathologica. 2006;111(6): 529–38. doi: 10.1007/s00401-006-0037-0. [DOI] [PubMed] [Google Scholar]
- 53.Anthony IC, Bell P. The neuropathology of HIV/AIDS. International Review of Psychiatry. 2008;20(1): 15–24. doi: 10.1080/09540260701862037. [DOI] [PubMed] [Google Scholar]
- 54.Morgello S, Estanislao L, Simpson D , et al. HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy the Manhattan HIV Brain Bank. Archives of Neurology. 2004;61(4): 546–51. doi: 10.1001/archneur.61.4.546. [DOI] [PubMed] [Google Scholar]
- 55.Backe E, Schwarting R, Gerdes J, Ernst M, Stein H. Ber-MAC3: new monoclonal antibody that defines human monocyte/macrophage differentiation antigen. Journal of Clinical Pathology. 1991;44(11): 936–45. doi: 10.1136/jcp.44.11.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buechler C, Ritter M, Orsó E. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro-and antiinflammatory stimuli. Journal of Leukocyte Biology. 2000;67(1): 97–103. [PubMed] [Google Scholar]
- 57.Hintz KA, Rassias AJ, Wardwell K , et al. Endotoxin induces rapid metalloproteinase-mediated shedding followed by up-regulation of the monocyte hemoglobin scavenger receptor CD163. Journal of Leukocyte Biology. 2002;72(4): 711–7. [PubMed] [Google Scholar]
- 58.Weaver LK, Hintz-Goldstein KA. Pivotal advance activation of cell surface Toll-like receptors causes shedding of the hemoglobin scavenger receptor CD163. Journal of Leukocyte Biology. 2006;80(1): 26–35. doi: 10.1189/jlb.1205756. [DOI] [PubMed] [Google Scholar]
- 59.Weaver LK, Pioli PA, Wardwell K, Vogel SN, Guyre PM. Up-regulation of human monocyte CD163 upon activation of cell-surface Toll-like receptors. Journal of Leukocyte Biology. 2007;81(3): 663–71. doi: 10.1189/jlb.0706428. [DOI] [PubMed] [Google Scholar]
- 60.Timmermann M, Högger P. Oxidative stress and 8-iso-prostaglandin F(2alpha) induce ectodomain shedding of CD163 and release of tumor necrosis factor-alpha from human monocytes. Free Radical Biology and Medicine. 2005;39(1): 98–107. doi: 10.1016/j.freeradbiomed.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 61.Neaton JD, Neuhaus J, Emery S. Soluble biomarkers and morbidity and mortality among people infected with HIV summary of published reports from 1997,-2010,. Current Opinion in HIV and AIDS. 2010;5(6): 480–90. doi: 10.1097/COH.0b013e32833ed75d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nixon DE, Landay AL. Biomarkers of immune dysfunction in HIV. Current Opinion in HIV and AIDS. 2010;5(6): 498–503. doi: 10.1097/COH.0b013e32833ed6f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lyons JL, Uno H, Ancuta P , et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. Journal of Acquired Immune Deficiency Syndromes. 2011;57(5): 371–9. doi: 10.1097/QAI.0b013e3182237e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burdo TH, Lentz MR, Autissier P , et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. Journal of Infectious Diseases. 2011;204(1): 154–63. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burdo TH, Lo J, Abbara S , et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. Journal of Infectious Diseases. 2011;204(8): 1227–36. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burdo TH, Weiffenbach A, Woods SP, Letendre S. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS. 2013;7(9):1387–95. doi: 10.1097/QAD.0b013e32836010bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kamat A, Lyons JL, Misra V , et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. Journal of Acquired Immune Deficiency Syndromes. 2012;60(3): 234–43. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nolting T, Lindecke A, Hartung H-P , et al. Cytokine levels in CSF and neuropsychological performance in HIV patients. Journal of Neurovirology. 2012;18(3): 157–61. doi: 10.1007/s13365-012-0091-4. [DOI] [PubMed] [Google Scholar]
- 69.Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain correlations with dementia. Annals of Neurology. 1995;38(5): 755–62. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 70.Masliah E, Heaton RK, Marcotte TD , et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders.HNRC Group. The HIV Neurobehavioral Research Center. Annals of Neurology. 1997;42(6): 963–72. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- 71.Wesselingh SL, Takahashi K, Glass JD, McArthur JC, Griffin JW, Griffin DE. Cellular localization of tumor necrosis factor mRNA in neurological tissue from HIV-infected patients by combined reverse transcriptase/polymerase chain reaction in situ hybridization and immunohistochemistry. Journal of Neuroimmunology. 1997;74(1-2): 1–8. doi: 10.1016/s0165-5728(96)00160-9. [DOI] [PubMed] [Google Scholar]
- 72.Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS consequences for the central nervous system. Cell Death and Differentiation. 2005;2 (Suppl 1 ):878–92. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- 73.Williams KC, Corey S, Westmoreland SV , et al. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques implications for the neuropathogenesis of AIDS. Journal of Experimental Medicine. 2001;193(8): 905–15. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.González-Scarano F, Martín-García J. The neuropathogenesis of AIDS. Nature Reviews Immunology. 2005;5(1): 69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 75.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831): 988–94. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 76.Mörner A, Thomas JA, Björling E, Munson PJ, Lucas SB, McKnight A. Productive HIV-2 infection in the brain is restricted to macrophages/microglia. AIDS. 2003;17(10): 1451–5. doi: 10.1097/00002030-200307040-00005. [DOI] [PubMed] [Google Scholar]
- 77.Schnell G, Spudich S, Harrington P, Price RW, Swanstrom R. Compartmentalized human immunodeficiency virus type 1 originates from long-lived cells in some subjects with HIV-1-associated dementia. PLoS Pathogens. 2009;(4):e1000395. doi: 10.1371/journal.ppat.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schnell G, Joseph S, Spudich S, Price RW, Swanstrom R. HIV-1 replication in the central nervous system occurs in two distinct cell types. PLoS Pathogens. 2011;7(10): e1002286. doi: 10.1371/journal.ppat.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Petito CK. Human immunodeficiency virus type 1 compartmentalization in the central nervous system. Journal of Neurovirology. 2004;10(suppl 1 ):21–4. doi: 10.1080/753312748. [DOI] [PubMed] [Google Scholar]
- 80.Nottet HS, Persidsky Y, Sasseville VG , et al. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. Journal of Immunology. 1996;156(3): 1284–95. [PubMed] [Google Scholar]
- 81.Bagasra O, Lavi E, Bobroski L , et al. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10(6): 573–85. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 82.Mukhtar M, Pomerantz RJ. Development of an in vitro blood-brain barrier model to study molecular neuropathogenesis and neurovirologic disorders induced by human immunodeficiency virus type 1 infection. Journal of Human Virology. 2000;3(6): 324–34. [PubMed] [Google Scholar]
- 83.Gujuluva C, Burns AR, Pushkarsky T , et al. HIV-1 penetrates coronary artery endothelial cells by transcytosis. Molecular Medicine. 2001;7(3): 169–76. [PMC free article] [PubMed] [Google Scholar]
- 84.Liu NQ, Lossinsky AS, Popik W , et al. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. Journal of Virology. 2002;6(13):6689–700. doi: 10.1128/JVI.76.13.6689-6700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Argyris EG, Acheampong E, Nunnari G, Mukhtar M, Williams KJ, Pomerantz RJ. Human immunodeficiency virus type 1 enters primary human brain microvascular endothelial cells by a mechanism involving cell surface proteoglycans independent of lipid rafts. Journal of Virology. 2003;77(22): 12140–51. doi: 10.1128/JVI.77.22.12140-12151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Willey SJ, Reeves JD, Hudson R , et al. Identification of a subset of human immunodeficiency virus type 1 (HIV-1), HIV-2, and simian immunodeficiency virus strains able to exploit an alternative coreceptor on untransformed human brain and lymphoid cells. Journal of Virology. 2003;77(11): 6138–52. doi: 10.1128/JVI.77.11.6138-6152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fischer-Smith T, Rappaport J. Evolving paradigms in the pathogenesis of HIV-1-associated dementia. Expert Reviews in Molecular Medicine. 2005;7(27): 1–26. doi: 10.1017/S1462399405010239. [DOI] [PubMed] [Google Scholar]
- 88.Masliah E, Ge N, Morey M, DeTeresa R, Terry RD, Wiley CA. Cortical dendritic pathology in human immunodeficiency virus encephalitis. Laboratory Investigation. 1992;66(3): 285–91. [PubMed] [Google Scholar]
- 89.Wiley CA, Masliah E, Morey M , et al. Neocortical damage during HIV infection. Annals of Neurology. 1991;29(6): 651–7. doi: 10.1002/ana.410290613. [DOI] [PubMed] [Google Scholar]
- 90.Gelbard HA, James HJ, Sharer LR , et al. Apoptotic neurons in brains from paediatric patients with HIV-1 encephalitis and progressive encephalopathy. Neuropathology and Applied Neurobiology. 1995;21(3): 208–17. doi: 10.1111/j.1365-2990.1995.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 91.Petito CK, Roberts B. Evidence of apoptotic cell death in HIV encephalitis. American Journal of Pathology. 1995;146(5): 1121–30. [PMC free article] [PubMed] [Google Scholar]
- 92.Adamson DC, Dawson TM, Zink MC, Clements JE, Dawson VL. Neurovirulent simian immunodeficiency virus infection induces neuronal, endothelial, and glial apoptosis. Molecular Medicine. 1996;2(4): 417–28. [PMC free article] [PubMed] [Google Scholar]
- 93.Gelman BB, Soukup VM, Schuenke KW , et al. Acquired neuronal channelopathies in HIV-associated dementia. Journal of Neuroimmunology. 2004;157(1-2): 111–9. doi: 10.1016/j.jneuroim.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 94.Hu D, Liu J, Xiong H. Enhancement of neuronal outward delayed rectifier K+ current by human monocyte-derived macrophages. Glia. 2009;57(14): 1492–500. doi: 10.1002/glia.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu D, Liu J, Keblesh J, Xiong H. Involvement of the 4-aminopyridine-sensitive transient A-type K+ current in macrophage-induced neuronal injury. European Journal of Neuroscience. 2010;31(2): 214–22. doi: 10.1111/j.1460-9568.2009.07063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xiong H, Zeng YC, Zheng J, Thylin M, Gendelman HE. Soluble HIV-1 infected macrophage secretory products mediate blockade of long-term potentiation a mechanism for cognitive dysfunction in HIV-1-associated dementia. Journal of Neurovirology. 1999;5(5): 519–28. doi: 10.3109/13550289909045381. [DOI] [PubMed] [Google Scholar]
- 97.Dong J, Xiong H. Human immunodeficiency virus type 1 gp120 inhibits long-term potentiation via chemokine receptor CXCR4 in rat hippocampal slices. Journal of Neuroscience Research. 2006;83(3): 489–96. doi: 10.1002/jnr.20745. [DOI] [PubMed] [Google Scholar]
- 98.Behnisch T, Francesconi W, Sanna PP. HIV secreted protein Tat prevents long-term potentiation in the hippocampal CA1 region. Brain Research. 2004;1012(1-2): 187–9. doi: 10.1016/j.brainres.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 99.Anderson ER, Boyle J, Zink WE, Persidsky Y, Gendelman HE, Xiong H. Hippocampal synaptic dysfunction in a murine model of human immunodeficiency virus type 1 encephalitis. Neuroscience. 2003;118(2): 359–69. doi: 10.1016/s0306-4522(02)00925-9. [DOI] [PubMed] [Google Scholar]
- 100.Gelman BB, Nguyen TP. Synaptic proteins linked to HIV-1 infection and immunoproteasome induction proteomic analysis of human synaptosomes. Journal of Neuroimmune Pharmacology. 2010;5(1): 92–102. doi: 10.1007/s11481-009-9168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain neuronal injury and repair. Nature Reviews Neuroscience. 2007;8(1): 33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- 102.Deshpande M, Zheng J, Borgmann K , et al. Role of activated astrocytes in neuronal damage potential links to HIV-1-associated dementia. Neurotoxicity Research. 2005;7(3): 183–92. doi: 10.1007/BF03036448. [DOI] [PubMed] [Google Scholar]
- 103.Li W, Galey D, Mattson MP, Nath A. Molecular and cellular mechanisms of neuronal cell death in HIV dementia. Neurotoxicity Research. 2005;8(1-2): 119–34. doi: 10.1007/BF03033824. [DOI] [PubMed] [Google Scholar]
- 104.Cisneros IE, Ghorpade A. HIV-1, methamphetamine and astrocyte glutamate regulation combined excitotoxic implications for neuro-AIDS. Current HIV Research. 2012;10(5): 392–406. doi: 10.2174/157016212802138832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fan Y, Zou W, Green LA, Kim BO, He JJ. Activation of Egr-1 expression in astrocytes by HIV-1 Tat new insights into astrocyte-mediated Tat neurotoxicity. Journal of Neuroimmune Pharmacology. 2011;6(1): 121–9. doi: 10.1007/s11481-010-9217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.O'Donnell LA, Agrawal A, Jordan-Sciutto KL, Dichter MA, Lynch DR, Kolson DL. Human immunodeficiency virus (HIV)-induced neurotoxicity roles for the NMDA receptor subtypes. Journal of Neuroscience. 2006;26(3): 981–90. doi: 10.1523/JNEUROSCI.4617-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anderson ER, Gendelman HE, Xiong H. Memantine protects hippocampal neuronal function in murine human immunodeficiency virus type 1 encephalitis. Journal of Neuroscience. 2004;24(32): 7194–8. doi: 10.1523/JNEUROSCI.1933-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meisner F, Scheller C, Kneitz S , et al. Memantine upregulates BDNF and prevents dopamine deficits in SIV-infected macaques a novel pharmacological action of memantine. Neuropsychopharmacology. 2008;33(9): 2228–36. doi: 10.1038/sj.npp.1301615. [DOI] [PubMed] [Google Scholar]
- 109.Jiang ZG, Piggee C, Heyes MP , et al. Glutamate is a mediator of neurotoxicity in secretions of activated HIV-1-infected macrophages. Journal of Neuroimmunology. 2001;117(1-2): 97–107. doi: 10.1016/s0165-5728(01)00315-0. [DOI] [PubMed] [Google Scholar]
- 110.Heyes MP, Brew BJ, Martin A , et al. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection relationship to clinical and neurological status. Annals of Neurology. 1991;29(2): 202–9. doi: 10.1002/ana.410290215. [DOI] [PubMed] [Google Scholar]
- 111.Heyes MP, Brew BJ, Saito K , et al. Inter-relationships between quinolinic acid, neuroactive kynurenines, neopterin and beta 2-microglobulin in cerebrospinal fluid and serum of HIV-1-infected patients. Journal of Neuroimmunology. 1992;40(1): 71–80. doi: 10.1016/0165-5728(92)90214-6. [DOI] [PubMed] [Google Scholar]
- 112.Giulian D, Yu J, Li X , et al. Study of receptor-mediated neurotoxins released by HIV-1-infected mononuclear phagocytes found in human brain. Journal of Neuroscience. 1996;16(10): 3139–53. doi: 10.1523/JNEUROSCI.16-10-03139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gelbard HA, Nottet HS, Swindells S , et al. Platelet-activating factor a candidate human immunodeficiency virus type 1-induced neurotoxin. Journal of Virology. 1994;68(7): 4628–35. doi: 10.1128/jvi.68.7.4628-4635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Achim CL, Heyes MP, Wiley CA. Quantitation of human immunodeficiency virus, immune activation factors, and quinolinic acid in AIDS brains. Journal of Clinical Investigation. 1993;91(6): 2769–75. doi: 10.1172/JCI116518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pulliam L, West D, Haigwood N, Swanson RA. HIV-1 envelope gp120 alters astrocytes in human brain cultures. AIDS Research and Human Retroviruses. 1993;9(5): 439–44. doi: 10.1089/aid.1993.9.439. [DOI] [PubMed] [Google Scholar]
- 116.Lipton SA. Requirement for macrophages in neuronal injury induced by HIV envelope protein gp120. Neuroreport. 1992;3(10): 913–5. doi: 10.1097/00001756-199210000-00023. [DOI] [PubMed] [Google Scholar]
- 117.Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. Journal of Infectious Diseases. 2002;186(suppl 2 ):S193–8. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- 118.Ferrarese C, Aliprandi A, Tremolizzo L , et al. Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology. 2001;57(4): 671–5. doi: 10.1212/wnl.57.4.671. [DOI] [PubMed] [Google Scholar]
- 119.Espey MG, Ellis RJ, Heaton RK, Basile AS. Relevance of glutamate levels in the CSF of patients with HIV-1-associated dementia complex. Neurology. 1999;53(5): 1144–5. doi: 10.1212/wnl.53.5.1144. [DOI] [PubMed] [Google Scholar]
- 120.Espey MG, Basile AS, Heaton RK, Ellis RJ. Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology. 2002;8(9):1439 author reply –40. doi: 10.1212/wnl.58.9.1439. [DOI] [PubMed] [Google Scholar]
- 121.Martin A, Heyes MP, Salazar AM , et al. Progressive slowing of reaction time and increasing cerebrospinal fluid concentrations of quinolinic acid in HIV-infected individuals. Journal of Neuropsychiatry and Clinical Neurosciences. 1992;4(3): 270–9. doi: 10.1176/jnp.4.3.270. [DOI] [PubMed] [Google Scholar]
- 122.Shibahara S, Müller RM, Taguchi H. Transcriptional control of rat heme oxygenase by heat shock. Journal of Biological Chemistry. 1987;262(27): 12889–92. [PubMed] [Google Scholar]
- 123.Taketani S, Kohno H, Yoshinaga T, Tokunaga R. Induction of heme oxygenase in rat hepatoma cells by exposure to heavy metals and hyperthermia. Biochemistry International. 1988;17(4): 665–72. [PubMed] [Google Scholar]
- 124.Ewing JF, Haber SN, Maines MD. Normal and heat-induced patterns of expression of heme oxygenase-1 (HSP32):in rat brain hyperthermia causes rapid induction of mRNA and protein. Journal of Neurochemistry. 1992;58(3): 1140–9. doi: 10.1111/j.1471-4159.1992.tb09373.x. [DOI] [PubMed] [Google Scholar]
- 125.Ewing JF, Maines MD. Rapid induction of heme oxygenase 1 mRNA and protein by hyperthermia in rat brain heme oxygenase 2 is not a heat shock protein. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(12): 5364–8. doi: 10.1073/pnas.88.12.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dwyer BE, Nishimura RN, De Vellis J, Yoshida T. Heme oxygenase is a heat shock protein and PEST protein in rat astroglial cells. Glia. 1992;5(4): 300–5. doi: 10.1002/glia.440050407. [DOI] [PubMed] [Google Scholar]
- 127.Maines MD, Ewing JF. Stress response of the rat testis in situ hydridization and immunohistochemical analysis of heme oxygenase-1 (HSP32):induction by hyperthermia. Biology of Reproduction. 1996;54(5): 1070–9. doi: 10.1095/biolreprod54.5.1070. [DOI] [PubMed] [Google Scholar]
- 128.Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proceedings of the National Academy of Sciences. 1989;86(1): 99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Paller MS, Nath KA, Rosenberg ME. Heme oxygenase is not expressed as a stress protein after renal ischemia. Journal of Laboratory and Clinical Medicine. 1993;122(3): 341–5. [PubMed] [Google Scholar]
- 130.Eyssen-Hernandez R, Ladoux A, Frelin C. Differential regulation of cardiac heme oxygenase-1 and vascular endothelial growth factor mRNA expressions by hemin, heavy metals, heat shock and anoxia. FEBS Letters. 1996;382(3): 229–33. doi: 10.1016/0014-5793(96)00127-5. [DOI] [PubMed] [Google Scholar]
- 131.Nimura T, Weinstein PR, Massa SM, Panter S. Heme oxygenase-1 (HO-1):protein induction in rat brain following focal ischemia. Molecular Brain. 1996;37(1-2): 201–8. doi: 10.1016/0169-328x(95)00315-j. [DOI] [PubMed] [Google Scholar]
- 132.Visner GA, Fogg S, Nick HS. Hyperoxia-responsive proteins in rat pulmonary microvascular endothelial cells. American Journal of Physiology. 1996;270(4): L517–L25. doi: 10.1152/ajplung.1996.270.4.L517. [DOI] [PubMed] [Google Scholar]
- 133.Sunderman FW. Metal induction of heme oxygenase. Annals of the New York Academy of Sciences. 1987;514: 65–80. doi: 10.1111/j.1749-6632.1987.tb48762.x. [DOI] [PubMed] [Google Scholar]
- 134.Opanashuk LA, Finkelstein JN. Relationship of lead-induced proteins to stress response proteins in astroglial cells. Journal of Neuroscience Research. 1995;42(5): 623–32. doi: 10.1002/jnr.490420504. [DOI] [PubMed] [Google Scholar]
- 135.Stocker R. Induction of haem oxygenase as a defence against oxidative stress. Free Radical Research Communications. 1990;9(2): 101–12. doi: 10.3109/10715769009148577. [DOI] [PubMed] [Google Scholar]
- 136.Clerget M, Polla BS. Erythrophagocytosis induces heat shock protein synthesis by human monocytes-macrophages. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(3): 1081–5. doi: 10.1073/pnas.87.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Saunders EL, Maines MD, Meredith MJ, Freeman ML. Enhancement of heme oxygenase-1 synthesis by glutathione depletion in Chinese hamster ovary cells. Archives of Biochemistry and Biophysics. 1991;288(2): 368–73. doi: 10.1016/0003-9861(91)90208-z. [DOI] [PubMed] [Google Scholar]
- 138.Katoh T, Ohmori H, Murakami T, Karasaki Y, Higashi K, Muramatsu M. Induction of glutathione-S-transferase and heat-shock proteins in rat liver after ethylene oxide exposure. Biochemical Pharmacology. 1991;42(6): 1247–54. doi: 10.1016/0006-2952(91)90261-3. [DOI] [PubMed] [Google Scholar]
- 139.Sato H, Ishii T, Sugita Y, Tateishi N, Bannai S. Induction of a 23 kDa stress protein by oxidative and sulfhydryl-reactive agents in mouse peritoneal macrophages. Biochimica et Biophysica Acta. 1993;1148(1): 127–32. doi: 10.1016/0005-2736(93)90169-z. [DOI] [PubMed] [Google Scholar]
- 140.Yamaguchi M, Sato H, Bannai S. Induction of stress proteins in mouse peritoneal macrophages by oxidized low-density lipoprotein. Biochemical and Biophysical Research Communications. 1993;193(3): 1198–201. doi: 10.1006/bbrc.1993.1752. [DOI] [PubMed] [Google Scholar]
- 141.Hoshida S, Nishida M, Yamashita N , et al. Heme oxygenase-1 expression and its relation to oxidative stress during primary culture of cardiomyocytes. Journal of Molecular and Cellular Cardiology. 1996;28(9): 1845–55. doi: 10.1006/jmcc.1996.0177. [DOI] [PubMed] [Google Scholar]
- 142.McDonagh AF. Is bilirubin good for you?. Clinics in Perinatology. 1990;17(2): 359–69. [PubMed] [Google Scholar]
- 143.Ryter SW, Choi AMK. Heme Oxygenase-1/Carbon Monoxide. American Journal of Respiratory Cell and Molecular Biology. 2009;41(3): 251–60. doi: 10.1165/rcmb.2009-0170TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Smith MA, Kutty RK, Richey PL , et al. Heme oxygenase-1 is associated with the neurofibrillary pathology of Alzheimer's disease. American Journal of Pathology. 1994;145(1): 42. [PMC free article] [PubMed] [Google Scholar]
- 145.Schipper HM, Cissé S, Stopa EG. Expression of heme oxygenase-1 in the senescent and Alzheimer-diseased brain. Annals of Neurology. 1995;37(6): 758–68. doi: 10.1002/ana.410370609. [DOI] [PubMed] [Google Scholar]
- 146.Takeda A, Perry G, Abraham N , et al. Overexpression of Heme Oxygenase in Neuronal Cells, the Possible Interaction with Tau. Journal of Biological Chemistry. 2000;275(8): 5395–9. doi: 10.1074/jbc.275.8.5395. [DOI] [PubMed] [Google Scholar]
- 147.Schipper HM, Liberman A, Stopa MD EG. Neural heme oxygenase-1 expression in idiopathic Parkinson's disease. Experimental Neurology. 1998;150(1): 60–8. doi: 10.1006/exnr.1997.6752. [DOI] [PubMed] [Google Scholar]
- 148.Castellani R, Smith MA, Richey PL, Perry G. Glycoxidation and oxidative stress in Parkinson disease and diffuse Lewy body disease. Brain Research. 1996;737(1-2): 195–200. doi: 10.1016/0006-8993(96)00729-9. [DOI] [PubMed] [Google Scholar]
- 149.Mateo I, Infante J, Sanchez-Juan P , et al. Serum heme oxygenase-1 levels are increased in Parkinson's disease but not in Alzheimer's disease. Acta Neurologica Scandinavica. 2010;121(2): 136–8. doi: 10.1111/j.1600-0404.2009.01261.x. [DOI] [PubMed] [Google Scholar]
- 150.Castellani R, Smith MA, Richey PL, Kalaria R, Gambetti P, Perry G. Evidence for oxidative stress in Pick disease and corticobasal degeneration. Brain Research. 1995;696(1-2): 268–71. doi: 10.1016/0006-8993(95)00535-x. [DOI] [PubMed] [Google Scholar]
- 151.Browne SE, Ferrante RJ, Beal MF. Oxidative stress in Huntington's disease. Brain Pathology. 1999;9(1): 147–63. doi: 10.1111/j.1750-3639.1999.tb00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mehindate K, Sahlas DJ, Frankel D , et al. Proinflammatory cytokines promote glial heme oxygenase-1 expression and mitochondrial iron deposition implications for multiple sclerosis. Journal of Neurochemistry. 2001;77(5): 1386–95. doi: 10.1046/j.1471-4159.2001.00354.x. [DOI] [PubMed] [Google Scholar]
- 153.Fagone P, Patti F, Mangano K , et al. Heme oxygenase-1 expression in peripheral blood mononuclear cells correlates with disease activity in multiple sclerosis. Journal of Neuroimmunology. 2013;261(1-2): 82–6. doi: 10.1016/j.jneuroim.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 154.Schipper HM. Heme oxygenase-1: role in brain aging and neurodegeneration. Experimental Gerontology. 2000;35(6-7): 821–30. doi: 10.1016/s0531-5565(00)00148-0. [DOI] [PubMed] [Google Scholar]
- 155.Schipper HM. Heme oxygenase expression in human central nervous system disorders. Free Radical Biology and Medicine. 2004;37(12): 1995,–2011,. doi: 10.1016/j.freeradbiomed.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 156.Schipper HM, Gupta A, Szarek WA. Suppression of glial HO-1 activity as a potential neurotherapeutic intervention in, AD. Current Alzheimer Research. 2009;6(5): 424–30. doi: 10.2174/156720509789207985. [DOI] [PubMed] [Google Scholar]
- 157.Jazwa A, Cuadrado A. Targeting heme oxygenase-1 for neuroprotection and neuroinflammation in neurodegenerative diseases. Current Drug Targets. 2010;11(12): 1517–31. doi: 10.2174/1389450111009011517. [DOI] [PubMed] [Google Scholar]
- 158.Cross SA, Cook DR, Chi AWS , et al. Dimethyl Fumarate, an Immune Modulator and Inducer of the Antioxidant Response, Suppresses HIV Replication and Macrophage-Mediated Neurotoxicity A Novel Candidate for HIV Neuroprotection. Journal of Immunology. 2011;187(10): 5015–25. doi: 10.4049/jimmunol.1101868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bornman L, Baladi S, Richard MJ, Tyrrell RM, Polla BS. Differential regulation and expression of stress proteins and ferritin in human monocytes. Journal of Cellular Physiology. 1999;178(1): 1–8. doi: 10.1002/(SICI)1097-4652(199901)178:1<1::AID-JCP1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 160.Kristiansen M, Graversen JH, Jacobsen C , et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409(6817): 198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 161.Schaer DJ, Schaer CA, Buehler PW , et al. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood. 2006;107(1): 373–80. doi: 10.1182/blood-2005-03-1014. [DOI] [PubMed] [Google Scholar]
- 162.Schaer CA, Vallelian F, Imhof A, Schoedon G, Schaer DJ. CD163-expressing monocytes constitute an endotoxin-sensitive Hb clearance compartment within the vascular system. Journal of Leukocyte Biology. 2007;82(1): 106–10. doi: 10.1189/jlb.0706453. [DOI] [PubMed] [Google Scholar]
- 163.Philippidis P, Mason JC, Evans BJ , et al. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circulation Research. 2004;94(1): 119–26. doi: 10.1161/01.RES.0000109414.78907.F9. [DOI] [PubMed] [Google Scholar]
- 164.Delaby C, Pilard N, Hetet G , et al. A physiological model to study iron recycling in macrophages. Experimental Cell Research. 2005;310(1): 43–53. doi: 10.1016/j.yexcr.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 165.Schaer CA, Schoedon G, Imhof A, Kurrer MO, Schaer DJ. Constitutive endocytosis of CD163 mediates hemoglobin-heme uptake and determines the noninflammatory and protective transcriptional response of macrophages to hemoglobin. Circulation Research. 2006;99(9): 943–50. doi: 10.1161/01.RES.0000247067.34173.1b. [DOI] [PubMed] [Google Scholar]
- 166.Kovtunovych G, Eckhaus MA, Ghosh MC, Ollivierre-Wilson H, Rouault TA. Dysfunction of the heme recycling system in heme oxygenase 1-deficient mice effects on macrophage viability and tissue iron distribution. Blood. 2010;116(26): 6054–62. doi: 10.1182/blood-2010-03-272138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Turcanu V, Dhouib M, Poindron P. Nitric oxide synthase inhibition by haem oxygenase decreases macrophage nitric-oxide-dependent cytotoxicity a negative feedback mechanism for the regulation of nitric oxide production. Research in Immunology. 1998;149(7-8): 741–4. doi: 10.1016/s0923-2494(99)80050-9. [DOI] [PubMed] [Google Scholar]
- 168.Wagener FADTG, van Beurden HE, von den Hoff JW, Adema GJ, Figdor CG. The heme-heme oxygenase system a molecular switch in wound healing. Blood. 2003;102(2): 521–8. doi: 10.1182/blood-2002-07-2248. [DOI] [PubMed] [Google Scholar]
- 169.Lang D, Reuter S, Buzescu T, August C, Heidenreich S. Heme-induced heme oxygenase-1 (HO-1):in human monocytes inhibits apoptosis despite caspase-3 up-regulation. International Immunology. 2005;17(2): 155–65. doi: 10.1093/intimm/dxh196. [DOI] [PubMed] [Google Scholar]
- 170.Hvidberg V, Maniecki MB, Jacobsen C, Højrup P, Møller HJ, Moestrup SK. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106(7): 2572–9. doi: 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- 171.Gleissner CA, Shaked I, Erbel C, Böckler D, Katus HA, Ley K. CXCL4 downregulates the atheroprotective hemoglobin receptor CD163 in human macrophages. Circulation Research. 2010;106(1): 203–11. doi: 10.1161/CIRCRESAHA.109.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vallelian F, Pimenova T, Pereira CP , et al. The reaction of hydrogen peroxide with hemoglobin induces extensive alpha-globin crosslinking and impairs the interaction of hemoglobin with endogenous scavenger pathways. Free Radical Biology and Medicine. 2008;45(8): 1150–8. doi: 10.1016/j.freeradbiomed.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 173.Srisook K, Cha YN. Super-induction of HO-1 in macrophages stimulated with lipopolysaccharide by prior depletion of glutathione decreases iNOS expression and NO production. Nitric Oxide. 2005;12(2): 70–9. doi: 10.1016/j.niox.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 174.Pham N-K, Mouriz J, Kima PE. Leishmania pifanoi amastigotes avoid macrophage production of superoxide by inducing heme degradation. Infection and Immunity. 2005;73(12): 8322–33. doi: 10.1128/IAI.73.12.8322-8333.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Orozco LD, Kapturczak MH, Barajas B , et al. Heme oxygenase-1 expression in macrophages plays a beneficial role in atherosclerosis. Circulation Research. 2007;100(12): 1703–11. doi: 10.1161/CIRCRESAHA.107.151720. [DOI] [PubMed] [Google Scholar]
- 176.Taketani S, Sato H, Yoshinaga T, Tokunaga R, Ishii T, Bannai S. Induction in mouse peritoneal macrophages of 34 kDa stress protein and heme oxygenase by sulfhydryl-reactive agents. Journal of Biochemistry. 1990;108(1): 28–32. doi: 10.1093/oxfordjournals.jbchem.a123156. [DOI] [PubMed] [Google Scholar]
- 177.Paine A, Eiz-Vesper B, Blasczyk R. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochemical Pharmacology. 2010;80(12): 1895–903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 178.Srisook K, Kim C, Cha YN. Cytotoxic and cytoprotective actions of O2- and NO (ONOO-) are determined both by cellular GSH level and HO activity in macrophages. Methods in Enzymology. 2005;396: 414–24. doi: 10.1016/S0076-6879(05)96035-7. [DOI] [PubMed] [Google Scholar]
- 179.Ryter S, Choi A. Heme oxygenase-1: molecular mechanisms of gene expression in oxygen-related stress. Antioxidants and Redox Signaling. 2002;4(4): 625–32. doi: 10.1089/15230860260220120. [DOI] [PubMed] [Google Scholar]
- 180.Camhi SL, Alam J, Otterbein L, Sylvester SL, Choi AM. Induction of heme oxygenase-1 gene expression by lipopolysaccharide is mediated by AP-1 activation. American Journal of Respiratory Cell and Molecular Biology. 1995;13(4): 387–98. doi: 10.1165/ajrcmb.13.4.7546768. [DOI] [PubMed] [Google Scholar]
- 181.Kurata S, Matsumoto M, Tsuji Y, Nakajima H. Lipopolysaccharide activates transcription of the heme oxygenase gene in mouse M1 cells through oxidative activation of nuclear factor kappa, B. European Journal of Biochemistry. 1996;239(3): 566–71. doi: 10.1111/j.1432-1033.1996.0566u.x. [DOI] [PubMed] [Google Scholar]
- 182.Song Y, Shi Y, Ao L-H, Harken AH, Meng X-Z. TLR4 mediates LPS-induced HO-1 expression in mouse liver role of TNF-alpha and IL-1βeta. World Journal of Gastroenterology. 2003;9(8): 1799–803. doi: 10.3748/wjg.v9.i8.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Devadas K, Hewlett IK, Dhawan S. Lipopolysaccharide suppresses HIV-1 replication in human monocytes by protein kinase C-dependent heme oxygenase-1 induction. Journal of Leukocyte Biology. 2010;87(5): 915–24. doi: 10.1189/jlb.0307172. [DOI] [PubMed] [Google Scholar]
- 184.Connor AJ, Chen LC, Joseph LB, Laskin JD, Laskin DL. Distinct responses of lung and liver macrophages to acute endotoxemia. Experimental and Molecular Pathology. 2013;94(1): 216–27. doi: 10.1016/j.yexmp.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yachie A, Toma T, Mizuno K , et al. Heme oxygenase-1 production by peripheral blood monocytes during acute inflammatory illnesses of children. Experimental Biology and Medicine. 2003;228(5): 550–6. doi: 10.1177/15353702-0322805-26. [DOI] [PubMed] [Google Scholar]
- 186.Regev D, Surolia R, Karki S , et al. Heme oxygenase-1 promotes granuloma development and protects against dissemination of mycobacteria. Laboratory Investigation. 2012;92(11): 1541–52. doi: 10.1038/labinvest.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zacharia VM, Shiloh MU. Effect of carbon monoxide on Mycobacterium tuberculosis pathogenesis. Medical Gas Research. 2012;2(1): 30. doi: 10.1186/2045-9912-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fujii S, Akaike T, Maeda H. Role of nitric oxide in pathogenesis of herpes simplex virus encephalitis in rats. Virology. 1999;256(2): 203–12. doi: 10.1006/viro.1999.9610. [DOI] [PubMed] [Google Scholar]
- 189.Schachtele SJ, Hu S, Lokensgard Jr. Modulation of experimental herpes encephalitis-associated neurotoxicity through sulforaphane treatment. PLoS One. 2012;7(4): e36216. doi: 10.1371/journal.pone.0036216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Herden C, Schluesener HJ, Richt JA. Expression of allograft inflammatory factor-1 and haeme oxygenase-1 in brains of rats infected with the neurotropic Borna disease virus. Neuropathology and Applied Neurobiology. 2005;31(5): 512–21. doi: 10.1111/j.1365-2990.2005.00668.x. [DOI] [PubMed] [Google Scholar]
- 191.Luz NF, Andrade BB, Feijo DF , et al. Heme Oxygenase-1 Promotes the Persistence of Leishmania chagasi Infection. Journal of Immunology. 2012;188(9): 4460–7. doi: 10.4049/jimmunol.1103072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.El Fadili K, Imbeault M, Messier N , et al. Modulation of gene expression in human macrophages treated with the anti-leishmania pentavalent antimonial drug sodium stibogluconate. Antimicrobial Agents and Chemotherapy. 2008;52(2): 526–33. doi: 10.1128/AAC.01183-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Epiphanio S, Mikolajczak SA, Gonçalves LA , et al. Heme oxygenase-1 is an anti-inflammatory host factor that promotes murine plasmodium liver infection. Cell Host and Microbe. 2008;3(5): 331–8. doi: 10.1016/j.chom.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 194.Mendonça VRR, Luz NF., Santos NJG , et al. Association between the haptoglobin and heme oxygenase 1 genetic profiles and soluble CD163 in susceptibility to and severity of human malaria. Infection and Immunity. 2012;80(4): 1445–54. doi: 10.1128/IAI.05933-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cunnington AJ, Njie M, Correa S, Takem EN, Riley EM, Walther M. Prolonged Neutrophil Dysfunction after Plasmodium falciparum Malaria Is Related to Hemolysis and Heme Oxygenase-1 Induction. Journal of Immunology. 2012;189(11): 5336–46. doi: 10.4049/jimmunol.1201028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Walther M, De Caul A, Aka P , et al. HMOX1 Gene Promoter Alleles and High HO-1 Levels Are Associated with Severe Malaria in Gambian Children. PLoS Pathogens. 2012;8(3): e1002579. doi: 10.1371/journal.ppat.1002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dey S, Bindu S, Goyal M, Pal C, Alam A, Iqbal MS , et al. Impact of intravascular hemolysis in malaria on liver dysfunction involvement of hepatic free heme overload, NF-?B activation, and neutrophil infiltration. Journal of Biological Chemistry. 2012;287(32): 26630–46. doi: 10.1074/jbc.M112.341255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Schluesener H, Kremsner P, Meyermann R. Heme oxygenase-1 in lesions of human cerebral malaria. Acta Neuropathologica. 2001;101(1): 65–8. doi: 10.1007/s004010000250. [DOI] [PubMed] [Google Scholar]
- 199.Pamplona A, Ferreira A, Balla J , et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nature Medicine. 2007;13(6): 703–10. doi: 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- 200.Seixas E, Gozzelino R, Chora A , et al. Heme oxygenase-1 affords protection against noncerebral forms of severe malaria. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(37): 15837–42. doi: 10.1073/pnas.0903419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mautes AE, Kim DH, Sharp FR , et al. Induction of heme oxygenase-1 (HO-1):in the contused spinal cord of the rat. Brain Research. 1998;795(1-2): 17–24. doi: 10.1016/s0006-8993(98)00230-3. [DOI] [PubMed] [Google Scholar]
- 202.Magnusson S, Ekström TJ, Elmér E, Kanje M, Ny L, Alm P. Heme oxygenase-1, heme oxygenase-2 and biliverdin reductase in peripheral ganglia from rat, expression and plasticity. Neuroscience. 2000;95(3): 821–9. doi: 10.1016/s0306-4522(99)00466-2. [DOI] [PubMed] [Google Scholar]
- 203.Mautes AE, Bergeron M, Sharp FR , et al. Sustained induction of heme oxygenase-1 in the traumatized spinal cord. Experimental Neurology. 2000;166(2): 254–65. doi: 10.1006/exnr.2000.7520. [DOI] [PubMed] [Google Scholar]
- 204.Koistinaho J, Miettinen S, Keinänen R, Vartiainen N, Roivainen R, Laitinen JT. Long-term induction of haem oxygenase-1 (HSP-32):in astrocytes and microglia following transient focal brain ischaemia in the rat. European Journal of Neuroscience. 1996;8(11): 2265–72. doi: 10.1111/j.1460-9568.1996.tb01190.x. [DOI] [PubMed] [Google Scholar]
- 205.Dwyer BE, Nishimura RN, Lu SY, Alcaraz A. Transient induction of heme oxygenase after cortical stab wound injury. Brain Research Molecular Brain Research. 1996;38(2): 251–9. doi: 10.1016/0169-328x(95)00341-o. [DOI] [PubMed] [Google Scholar]
- 206.Orihara Y, Tsuda R, Ikematsu K, Nakasono I, Ogata M. Immunohistochemical study on the induction of heme oxygenase-1 by traumatic brain injury. Legal Medicine. 2003;5(Suppl 1 ):S278–9. doi: 10.1016/s1344-6223(02)00149-9. [DOI] [PubMed] [Google Scholar]
- 207.Sutherland BA, Rahman RMA, Clarkson AN, Shaw OM, Nair SM, Appleton I. Cerebral heme oxygenase 1 and 2 spatial distribution is modulated following injury from hypoxia-ischemia and middle cerebral artery occlusion in rats. Neuroscience Research. 2009;65(4): 326–34. doi: 10.1016/j.neures.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 208.Fukuda K, Richmon JD, Sato M, Sharp FR, Panter SS, Noble LJ. Induction of heme oxygenase-1 (HO-1):in glia after traumatic brain injury. Brain Research. 1996;736(1-2): 68–75. doi: 10.1016/0006-8993(96)00680-4. [DOI] [PubMed] [Google Scholar]
- 209.Hirata K, He JW, Kuraoka A , et al. Heme oxygenase1 (HSP-32):is induced in myelin-phagocytosing Schwann cells of injured sciatic nerves in the rat. European Journal of Neuroscience. 2000;12(11): 4147–52. doi: 10.1046/j.1460-9568.2000.00307.x. [DOI] [PubMed] [Google Scholar]
- 210.Jelinski SE, Yager JY, Juurlink BH. Preferential injury of oligodendroblasts by a short hypoxic-ischemic insult. Brain Research. 1999;815(1): 150–3. doi: 10.1016/s0006-8993(98)01053-1. [DOI] [PubMed] [Google Scholar]
- 211.Fukuda K, Scott Panter S, Sharp FR, Noble LJ. Induction of heme oxygenase-1 (HO-1):after traumatic brain injury in the rat. Neuroscience Letters. 1995;199(2): 127–30. doi: 10.1016/0304-3940(95)12042-3. [DOI] [PubMed] [Google Scholar]
- 212.Wang J, Doré S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007;130(Pt 6): 1643–52. doi: 10.1093/brain/awm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhang Z, Zhang Z-Y, Wu Y, Schluesener HJ. Lesional accumulation of CD163+ macrophages/microglia in rat traumatic brain injury. Brain Research. 2012;1461: 102–10. doi: 10.1016/j.brainres.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 214.Liu Y, Zhang Z, Luo B, Schluesener HJ, Zhang Z. Lesional accumulation of heme oxygenase-1+ microglia/macrophages in rat traumatic brain injury. Neuroreport. 2013;24(6): 281–6. doi: 10.1097/WNR.0b013e32835f2810. [DOI] [PubMed] [Google Scholar]
- 215.Richmon JD, Fukuda K, Maida N , et al. Induction of heme oxygenase-1 after hyperosmotic opening of the blood-brain barrier. Brain Research. 1998;780(1): 108–18. doi: 10.1016/s0006-8993(97)01314-0. [DOI] [PubMed] [Google Scholar]
- 216.Beschorner R, Adjodah D, Schwab JM , et al. Long-term expression of heme oxygenase-1 (HO-1, HSP-32):following focal cerebral infarctions and traumatic brain injury in humans. Acta Neuropathologica. 2000;100(4): 377–84. doi: 10.1007/s004010000202. [DOI] [PubMed] [Google Scholar]
- 217.Jofre-Monseny L, Loboda A, Wagner AE , et al. Effects of apoE genotype on macrophage inflammation and heme oxygenase-1 expression. Biochemical and Biophysical Research Communications. 2007;357(1): 319–24. doi: 10.1016/j.bbrc.2007.03.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nijboer WN, Schuurs TA, van der Hoeven JAB , et al. Effect of brain death on gene expression and tissue activation in human donor kidneys. Transplantation. 2004;78(7): 978–86. doi: 10.1097/01.tp.0000135565.49535.60. [DOI] [PubMed] [Google Scholar]
- 219.Yan W, Wang H-D, Zhu L , et al. Traumatic brain injury induces the activation of the Nrf2-ARE pathway in the lung in rats. Brain Injury. 2008;22(10): 802–10. doi: 10.1080/02699050802372174. [DOI] [PubMed] [Google Scholar]
- 220.Acarin L, Paris J, González B, Castellano B. Glial expression of small heat shock proteins following an excitotoxic lesion in the immature rat brain. Glia. 2002;38(1): 1–14. doi: 10.1002/glia.10040. [DOI] [PubMed] [Google Scholar]
- 221.Krause D, Suh H-S, Tarassishin L , et al. The tryptophan metabolite 3-hydroxyanthranilic acid plays anti-inflammatory and neuroprotective roles during inflammation role of hemeoxygenase-1. American Journal of Pathology. 2011;179(3): 1360–72. doi: 10.1016/j.ajpath.2011.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tzima S, Victoratos P, Kranidioti K, Alexiou M, Kollias G. Myeloid heme oxygenase-1 regulates innate immunity and autoimmunity by modulating IFN-beta production. Journal of Experimental Medicine. 2009;206(5): 1167–79. doi: 10.1084/jem.20081582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yamada K, Tanaka N, Nakanishi K , et al. Modulation of the secondary injury process after spinal cord injury in Bach1-deficient mice by heme oxygenase-1. Journal of Neurosurgery Spine. 2008;9(6): 611–20. doi: 10.3171/SPI.2008.10.08488. [DOI] [PubMed] [Google Scholar]
- 224.Wang J, Doré S. Heme oxygenase 2 deficiency increases brain swelling and inflammation after intracerebral hemorrhage. Neuroscience. 2008;155(4): 1133–41. doi: 10.1016/j.neuroscience.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Grochot-Przeczek A, Dulak J, Jozkowicz A. Haem oxygenase-1: non-canonical roles in physiology and pathology. Clinical Science. 2012;122(3): 93–103. doi: 10.1042/CS20110147. [DOI] [PubMed] [Google Scholar]
- 226.Ishii T, Itoh K, Takahashi S , et al. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. Journal of Biological Chemistry. 2000;275(21): 16023–9. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 227.Vijayan V, Baumgart-Vogt E, Naidu S, Qian G, Immenschuh S. Bruton's tyrosine kinase is required for TLR-dependent heme oxygenase-1 gene activation via Nrf2 in macrophages. Journal of Immunology. 2011;187(2): 817–27. doi: 10.4049/jimmunol.1003631. [DOI] [PubMed] [Google Scholar]
- 228.Kim KH, Lyu JH, Koo ST , et al. MyD88 is a mediator for the activation of Nrf2. Biochemical and Biophysical Research Communications. 2011;404(1): 46–51. doi: 10.1016/j.bbrc.2010.11.051. [DOI] [PubMed] [Google Scholar]
- 229.Mandal P, Roychowdhury S, Park PH. Adiponectin and heme oxygenase-1 suppress TLR4/MyD88-independent signaling in rat Kupffer cells and in mice after chronic ethanol exposure. Journal of Immunology. 2010;185(8): 4928–37. doi: 10.4049/jimmunol.1002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yin H, Li X, Yuan B , et al. Heme oxygenase-1 ameliorates LPS-induced acute lung injury correlated with downregulation of interleukin-33. International Immunopharmacology. 2011;11(12): 2112–7. doi: 10.1016/j.intimp.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 231.Wang XM, Kim HP, Nakahira K, Ryter SW, Choi AMK. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. Journal of Immunology. 2009;182(6): 3809–18. doi: 10.4049/jimmunol.0712437. [DOI] [PubMed] [Google Scholar]
- 232.Ke B, Shen X-D, Ji H , et al. HO-1-STAT3 axis in mouse liver ischemia/reperfusion injury regulation of TLR4 innate responses through PI3K/PTEN signaling. Journal of Hepatology. 2012;56(2): 359–66. doi: 10.1016/j.jhep.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tsoyi K, Jang HJ, Kim JW , et al. Stimulation of alpha7 nicotinic acetylcholine receptor by nicotine attenuates inflammatory response in macrophages and improves survival in experimental model of sepsis through heme oxygenase-1 induction. Antioxidants and Redox Signaling. 2011;14(11): 2057–70. doi: 10.1089/ars.2010.3555. [DOI] [PubMed] [Google Scholar]
- 234.Sun J, Kim SJ, Park MK , et al. Selective activation of adrenergic beta1 receptors induces heme oxygenase 1 production in RAW264. cells. FEBS Letters. 2005;579(25): 5494–500. doi: 10.1016/j.febslet.2005.08.080. [DOI] [PubMed] [Google Scholar]
- 235.Rushworth SA, Chen X-L, Mackman N, Ogborne RM, O'Connell MA. Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C. Journal of Immunology. 2005;175(7): 4408–15. doi: 10.4049/jimmunol.175.7.4408. [DOI] [PubMed] [Google Scholar]
- 236.Park SY, Kim YH, Kim E-K, Ryu EY, Lee SJ. Heme oxygenase-1 signals are involved in preferential inhibition of pro-inflammatory cytokine release by surfactin in cells activated with Porphyromonas gingivalis lipopolysaccharide. Chemico-Biological Interactions. 2010;188(3): 437–45. doi: 10.1016/j.cbi.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 237.Oh G-S, Pae H-O, Lee B-S , et al. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW2647 macrophages stimulated with lipopolysaccharide. Free Radical Biology and Medicine. 2006;41(1): 106–19. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 238.Lee SE, Jeong SI, Yang H , et al. Extract of Salvia miltiorrhiza (Danshen) induces Nrf2-mediated heme oxygenase-1 expression as a cytoprotective action in RAW 264. macrophages. Journal of Ethnopharmacology. 2012;139(2): 541–8. doi: 10.1016/j.jep.2011.11.046. [DOI] [PubMed] [Google Scholar]
- 239.Iles KE, Dickinson DA, Wigley AF, Welty NE, Blank V, Forman HJ. HNE increases HO-1 through activation of the ERK pathway in pulmonary epithelial cells. Free Radical Biology and Medicine. 2005;39(3): 355–64. doi: 10.1016/j.freeradbiomed.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Pischke SE, Zhou Z, Song R , et al. Phosphatidylinositol 3-kinase/Akt pathway mediates heme oxygenase-1 regulation by lipopolysaccharide. Cellular and Molecular Biology. 2005;51(5): 461–70. [PubMed] [Google Scholar]
- 241.Lee T-S, Chau L-Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nature Medicine. 2002;8(3): 240–6. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 242.Wu W-T, Chi K-H, Ho F-M, Tsao W-C, Lin W-W. Proteasome inhibitors up-regulate haem oxygenase-1 gene expression requirement of p38 MAPK (mitogen-activated protein kinase) activation but not of NF-kappaB (nuclear factor kappaB) inhibition. Biochemical Journal. 2004;379(Pt 3): 587. doi: 10.1042/BJ20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chen JS, Huang PH, Wang CH, Lin FY, Tsai HY. Nrf-2 mediated heme oxygenase-1 expression, an antioxidant-independent mechanism, contributes to anti-atherogenesis and vascular protective effects Ginkgo biloba. Atherosclerosis. 2011;214(2): 301–9. doi: 10.1016/j.atherosclerosis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 244.Jang HJ, Kim YM, Tsoyi K , et al. Ethyl pyruvate induces heme oxygenase-1 through p38 mitogen-activated protein kinase activation by depletion of glutathione in RAW 264. cells and improves survival in septic animals. Antioxidants and Redox Signaling. 2012;17(6): 878–89. doi: 10.1089/ars.2011.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kurauchi Y, Hisatsune A, Isohama Y, Mishima S, Katsuki H. Caffeic acid phenethyl ester protects nigral dopaminergic neurons via dual mechanisms involving haem oxygenase-1 and brain-derived neurotrophic factor. British Journal of Pharmacology. 2012;166(3): 1151–68. doi: 10.1111/j.1476-5381.2012.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ogborne R, Rushworth S, Charalambos C, O'Connell M. Haem oxygenase-1: a target for dietary antioxidants. Biochemical Society Transactions. 2004;32(6): 1003–5. doi: 10.1042/BST0321003. [DOI] [PubMed] [Google Scholar]
- 247.Jeon WK, Hong HY, Kim BC. Genipin up-regulates heme oxygenase-1 via PI3-kinase-JNK1/2-Nrf2 signaling pathway to enhance the anti-inflammatory capacity in RAW264.7 macrophages. Archives of Biochemistry and Biophysics. 2011;512(2): 119–25. doi: 10.1016/j.abb.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 248.Yeligar SM, Machida K, Kalra VK. Ethanol-induced HO-1 and NQO1 are differentially regulated by HIF-1alpha and Nrf2 to attenuate inflammatory cytokine expression. Journal of Biological Chemistry. 2010;285(46): 35359–73. doi: 10.1074/jbc.M110.138636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Alam J, Cook JL. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Current Pharmaceutical Design. 2003;9(30): 2499–511. doi: 10.2174/1381612033453730. [DOI] [PubMed] [Google Scholar]
- 250.Lee PJ, Alam J, Sylvester SL, Inamdar N, Otterbein L, Choi AM. Regulation of heme oxygenase-1 expression in vivo and in vitro in hyperoxic lung injury. American Journal of Respiratory Cell and Molecular Biology. 1996;14(6): 556–68. doi: 10.1165/ajrcmb.14.6.8652184. [DOI] [PubMed] [Google Scholar]
- 251.Camhi SL, Alam J, Wiegand GW, Chin BY, Choi AM. Transcriptional activation of the HO-1 gene by lipopolysaccharide is mediated by 5' distal enhancers role of reactive oxygen intermediates and AP-1. American Journal of Respiratory Cell and Molecular Biology. 1998;18(2): 226–34. doi: 10.1165/ajrcmb.18.2.2910. [DOI] [PubMed] [Google Scholar]
- 252.Immenschuh S, Hinke V, Katz N, Kietzmann T. Transcriptional induction of heme oxygenase-1 gene expression by okadaic acid in primary rat hepatocyte cultures. Molecular Pharmacology. 2000;57(3): 610–8. doi: 10.1124/mol.57.3.610. [DOI] [PubMed] [Google Scholar]
- 253.Sassone-Corsi P. Coupling gene expression to cAMP signalling role of CREB and CREM. International Journal of Biochemistry and Cell Biology. 1998;30(1): 27–38. doi: 10.1016/s1357-2725(97)00093-9. [DOI] [PubMed] [Google Scholar]
- 254.Waltereit R, Weller M. Signaling from cAMP/PKA to MAPK and synaptic plasticity. Molecular Neurobiology. 2003;27(1): 99–106. doi: 10.1385/MN:27:1:99. [DOI] [PubMed] [Google Scholar]
- 255.Lavrovsky Y, Schwartzman ML, Abraham NG. Novel regulatory sites of the human heme oxygenase-1 promoter region. Biochemical and Biophysical Research Communications. 1993;196(1): 336–41. doi: 10.1006/bbrc.1993.2253. [DOI] [PubMed] [Google Scholar]
- 256.Wijayanti N, Kietzmann T, Immenschuh S. Heme oxygenase-1 gene activation by the NAD(P)H oxidase inhibitor 4-(2-aminoethyl) benzenesulfonyl fluoride via a protein kinase, B. p38-dependent signaling pathway in monocytes. Journal of Biological Chemistry. 2005;280(23): 21820–9. doi: 10.1074/jbc.M502943200. [DOI] [PubMed] [Google Scholar]
- 257.Bauer I, Rensing H, Florax A , et al. Expression pattern and regulation of heme oxygenase-1/heat shock protein 32 in human liver cells. Shock. 2003;20(2): 116–22. doi: 10.1097/01.shk.0000075568.93053.fa. [DOI] [PubMed] [Google Scholar]
- 258.Roach JP, Moore EE, Partrick DA , et al. Heme oxygenase-1 induction in macrophages by a hemoglobin-based oxygen carrier reduces endotoxin-stimulated cytokine secretion. Shock. 2009;31(3): 251–7. doi: 10.1097/SHK.0b013e3181834115. [DOI] [PubMed] [Google Scholar]
- 259.Pittock ST, Norby SM, Grande JP , et al. MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice Pathophysiologic correlates. Kidney International. 2005;68(2): 611–22. doi: 10.1111/j.1523-1755.2005.00439.x. [DOI] [PubMed] [Google Scholar]
- 260.Inoue S, Suzuki M, Nagashima Y , et al. Transfer of heme oxygenase 1 cDNA by a replication-deficient adenovirus enhances interleukin 10 production from alveolar macrophages that attenuates lipopolysaccharide-induced acute lung injury in mice. Human Gene Therapy. 2001;12(8): 967–79. doi: 10.1089/104303401750195926. [DOI] [PubMed] [Google Scholar]
- 261.Kamimoto M, Mizuno S, Nakamura T. Reciprocal regulation of IL-6 and IL-10 balance by HGF via recruitment of heme oxygenase-1 in macrophages for attenuation of liver injury in a mouse model of endotoxemia. International Journal of Molecular Medicine. 2009;24(2): 161–70. doi: 10.3892/ijmm_00000219. [DOI] [PubMed] [Google Scholar]
- 262.Sierra-Filardi E, Vega MA, Sánchez-Mateos P, Corbí AL, Puig-Kröger A. Heme Oxygenase-1 expression in M-CSF-polarized M2 macrophages contributes to LPS-induced IL-10 release. Immunobiology. 2010;215(9-10): 788–95. doi: 10.1016/j.imbio.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 263.Piantadosi CA, Withers CM, Bartz RR , et al. Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. Journal of Biological Chemistry. 2011;286(18): 16374–85. doi: 10.1074/jbc.M110.207738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Drechsler Y, Dolganiuc A, Norkina O , et al. Heme oxygenase-1 mediates the anti-inflammatory effects of acute alcohol on IL-10 induction involving p38 MAPK activation in monocytes. Journal of Immunology. 2006;177(4): 2592–600. doi: 10.4049/jimmunol.177.4.2592. [DOI] [PubMed] [Google Scholar]
- 265.Chauveau C, Rémy S, Royer PJ , et al. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106(5): 1694–702. doi: 10.1182/blood-2005-02-0494. [DOI] [PubMed] [Google Scholar]
- 266.Tullius SG, Nieminen-Kelhä M, Buelow R , et al. Inhibition of ischemia/reperfusion injury and chronic graft deterioration by a single-donor treatment with cobalt-protoporphyrin for the induction of heme oxygenase-1. Transplantation. 2002;74(5): 591–8. doi: 10.1097/00007890-200209150-00001. [DOI] [PubMed] [Google Scholar]
- 267.Shen X-D, Ke B, Uchida Y , et al. Native macrophages genetically modified to express heme oxygenase 1 protect rat liver transplants from ischemia/reperfusion injury. Liver Transplantation. 2011;17(2): 201–10. doi: 10.1002/lt.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kämpfer H, Kolb N, Manderscheid M, Wetzler C. Macrophage-derived heme-oxygenase-1: expression, regulation, and possible functions in skin repair. Molecular Medicine. 2001;7(7): 488–98. [PMC free article] [PubMed] [Google Scholar]
- 269.Morse D, Pischke SE, Zhou Z , et al. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. Journal of Biological Chemistry. 2003;278(39): 36993–8. doi: 10.1074/jbc.M302942200. [DOI] [PubMed] [Google Scholar]
- 270.Zhong Y, Liu T, Lai W, Tan Y, Tian D, Guo Z. Heme oxygenase-1-mediated reactive oxygen species reduction is involved in the inhibitory effect of curcumin on lipopolysaccharide-induced monocyte chemoattractant protein-1 production in RAW264. macrophages. . Molecular Medicine Reports. 2013;7(1): 242–6. doi: 10.3892/mmr.2012.1138. [DOI] [PubMed] [Google Scholar]
- 271.Sacerdoti D, Colombrita C, Ghattas MH , et al. Heme oxygenase-1 transduction in endothelial cells causes downregulation of monocyte chemoattractant protein-1 and of genes involved in inflammation and growth. Cellular and Molecular Biology. 2005;51(4): 363–70. [PubMed] [Google Scholar]
- 272.Ricchetti GA, Williams LM, Foxwell BMJ. Heme oxygenase 1 expression induced by IL-10 requires STAT-3 and phosphoinositol-3 kinase and is inhibited by lipopolysaccharide. Journal of Leukocyte Biology. 2004;76(3): 719–26. doi: 10.1189/jlb.0104046. [DOI] [PubMed] [Google Scholar]
- 273.Kirino Y, Takeno M, Murakami S , et al. Tumor necrosis factor ? acceleration of inflammatory responses by down-regulating heme oxygenase 1 in human peripheral monocytes. Arthritis and Rheumatism. 2007;56(2): 464–75. doi: 10.1002/art.22370. [DOI] [PubMed] [Google Scholar]
- 274.Ke B, Shen X-D, Tsuchihashi S-I , et al. Viral interleukin-10 gene transfer prevents liver ischemia-reperfusion injury Toll-like receptor-4 and heme oxygenase-1 signaling in innate and adaptive immunity. Human Gene Therapy. 2007;18(4): 355–66. doi: 10.1089/hum.2007.181. [DOI] [PubMed] [Google Scholar]
- 275.Koch N, Jung M, Sabat R , et al. IL-10 protects monocytes and macrophages from complement-mediated lysis. Journal of Leukocyte Biology. 2009;86(1): 155–66. doi: 10.1189/jlb.0708443. [DOI] [PubMed] [Google Scholar]
- 276.Petit-Bertron A-F, Fitting C, Cavaillon J-M, Adib-Conquy M. Adherence influences monocyte responsiveness to interleukin-10. Journal of Leukocyte Biology. 2003;73(1): 145–54. doi: 10.1189/jlb.0802388. [DOI] [PubMed] [Google Scholar]
- 277.Mizuno T, Doi Y, Mizoguchi H , et al. Interleukin-34 selectively enhances the neuroprotective effects of microglia to attenuate oligomeric amyloid-? neurotoxicity. American Journal of Pathology. 2011;179(4): 2016,–27. doi: 10.1016/j.ajpath.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kanakiriya SKR, Croatt AJ, Haggard JJ , et al. Heme a novel inducer of MCP-1 through HO-dependent and HO-independent mechanisms. American Journal of Physiology Renal Physiology. 2003;284(3): F546–54. doi: 10.1152/ajprenal.00298.2002. [DOI] [PubMed] [Google Scholar]
- 279.Cao J, Sodhi K, Inoue K , et al. Lentiviral-human heme oxygenase targeting endothelium improved vascular function in angiotensin II animal model of hypertension. Human Gene Therapy. 2011;22(3): 271–82. doi: 10.1089/hum.2010.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Park JH, Oh S-M, Lim SS , et al. Induction of heme oxygenase-1 mediates the anti-inflammatory effects of the ethanol extract of Rubus coreanus in murine macrophages. Biochemical and Biophysical Research Communications. 2006;351(1): 146–52. doi: 10.1016/j.bbrc.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 281.Kim B-C, Choi J-W, Hong H-Y , et al. Heme oxygenase-1 mediates the anti-inflammatory effect of mushroom Phellinus linteus in LPS-stimulated RAW264. macrophages. . Journal of Ethnopharmacology. 2006;106(3): 364–71. doi: 10.1016/j.jep.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 282.Oh HM, Kang YJ, Lee YS , et al. Protein kinase G-dependent heme oxygenase-1 induction by Agastache rugosa leaf extract protects RAW264. cells from hydrogen peroxide-induced injury. Journal of Ethnopharmacology. 2006;103(2): 229–35. doi: 10.1016/j.jep.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 283.Shyur L-F, Huang C-C, Hsu Y-Y, Cheng Y-W, Yang S-D. A sesquiterpenol extract potently suppresses inflammation in macrophages and mice skin and prevents chronic liver damage in mice through JNK-dependent HO-1 expression. Phytochemistry. 2011;72(4-5): 391–9. doi: 10.1016/j.phytochem.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 284.Jun MS, Kim HS, Kim YM , et al. Ethanol extract of Prunella vulgaris var.lilacina inhibits HMGB1 release by induction of heme oxygenase-1 in LPS-activated RAW 264.7 cells and CLP-induced septic mice. Phytotherapy Research. 2012;26(4): 605–12. doi: 10.1002/ptr.3613. [DOI] [PubMed] [Google Scholar]
- 285.Li B, Lee D-S, Choi H-G , et al. Sauchinone suppresses pro-inflammatory mediators by inducing heme oxygenase-1 in RAW264. macrophages. Biological and Pharmaceutical Bulletin. 2011;34(10): 1566–71. doi: 10.1248/bpb.34.1566. [DOI] [PubMed] [Google Scholar]
- 286.Tsoyi K, Jang HJ, Lee YS, Kim YM, Kim HJ. (+)-Nootkatone and (+)-valencene from rhizomes of Cyperus rotundus increase survival rates in septic mice due to heme oxygenase-1 induction. Journal of Ethnopharmacology. 2011;37(3): 1311–7. doi: 10.1016/j.jep.2011.07.062. [DOI] [PubMed] [Google Scholar]
- 287.Park SY, Park DJ, Kim YH, Kim Y, Choi Y-W, Lee SJ. Schisandra chinensis ?-iso-cubebenol induces heme oxygenase-1 expression through PI3K/Akt and Nrf2 signaling and has anti-inflammatory activity in Porphyromonas gingivalis lipopolysaccharide-stimulated macrophages. International Immunopharmacology. 2011;11(11): 1907–15. doi: 10.1016/j.intimp.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 288.Lee M-Y, Seo C-S, Lee J-A , et al. Alpinia katsumadai H(AYATA) seed extract inhibit LPS-induced inflammation by induction of heme oxygenase-1 in RAW264. cells. Inflammation. 2012;5(2):746–57. doi: 10.1007/s10753-011-9370-0. [DOI] [PubMed] [Google Scholar]
- 289.Cheng Y-W, Cheah K-P, Lin C-W , et al. Myrrh mediates haem oxygenase-1 expression to suppress the lipopolysaccharide-induced inflammatory response in RAW264. macrophages. Journal of Pharmacy and Pharmacology. 2011;63(9): 1211–8. doi: 10.1111/j.2042-7158.2011.01329.x. [DOI] [PubMed] [Google Scholar]
- 290.Lee J-A, Lee M-Y, Seo C-S , et al. Asiasari sieboldii suppresses inflammatory mediators through the induction of hemeoxygenase-1 expression in RAW264. cells. Immunopharmacology and Immunotoxicology. 2012;34(1): 15–20. doi: 10.3109/08923973.2011.572261. [DOI] [PubMed] [Google Scholar]
- 291.Bhaskaran N, Shukla S, Kanwal R, Srivastava JK, Gupta S. Induction of heme oxygenase-1 by chamomile protects murine macrophages against oxidative stress. Life Sciences. 2012;90(25-26): 1027–33. doi: 10.1016/j.lfs.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Seo WY, Ju SM, Song HY , et al. Celastrol suppresses IFN-gamma-induced ICAM-1 expression and subsequent monocyte adhesiveness via the induction of heme oxygenase-1 in the HaCaT cells. Biochemical and Biophysical Research Communications. 2010;398(1): 140–5. doi: 10.1016/j.bbrc.2010.06.053. [DOI] [PubMed] [Google Scholar]
- 293.Liby K, Hock T, Yore MM , et al. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Research. 2005;65(11): 4789–98. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 294.Park H-J, Han ES, Park DK, Lee C, Lee KW. An extract of Phellinus linteus grown on germinated brown rice inhibits inflammation markers in RAW264. macrophages by suppressing inflammatory cytoineschemokines, and mediators and up-regulating antioxidant activity. Journal of Medicinal Food . 2010;13(6): 1468–77. doi: 10.1089/jmf.2010.1131. [DOI] [PubMed] [Google Scholar]
- 295.Hwa JS, Jin YC, Lee YS , et al. 2-methoxycinnamaldehyde from Cinnamomum cassia reduces rat myocardial ischemia and reperfusion injury in vivo due to HO-1 induction. Journal of Ethnopharmacology. 2012;139(2): 605–15. doi: 10.1016/j.jep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 296.Ryu EY, Park AJ, Park SY , et al. Inhibitory effects of Ginkgo biloba extract on inflammatory mediator production by Porphyromonas gingivalis lipopolysaccharide in murine macrophages via Nrf-2 mediated heme oxygenase-1 signaling pathways. Inflammation. 2012;35(4): 1477–86. doi: 10.1007/s10753-012-9461-6. [DOI] [PubMed] [Google Scholar]
- 297.Lee JC, Bhora F, Sun J , et al. Dietary flaxseed enhances antioxidant defenses and is protective in a mouse model of lung ischemia-reperfusion injury. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2008;294(2): L255–65. doi: 10.1152/ajplung.00138.2007. [DOI] [PubMed] [Google Scholar]
- 298.Jeong S-O, Oh G-S, Ha H-Y , et al. Dimethoxycurcumin, a Synthetic Curcumin Analogue, Induces Heme Oxygenase-1 Expression through Nrf2 Activation in RAW264. Macrophages. Journal of Clinical Biochemistry and Nutrition. 2009;44(1): 79–84. doi: 10.3164/jcbn.08-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Kim AN, Jeon WK, Lee JJ, Kim BC. Up-regulation of heme oxygenase-1 expression through CaMKII-ERK1/2-Nrf2 signaling mediates the anti-inflammatory effect of bisdemethoxycurcumin in LPS-stimulated macrophages. Free Radical Biology and Medicine. 2010;49(3): 323–31. doi: 10.1016/j.freeradbiomed.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 300.Hsu H-Y, Chu L-C, Hua K-F, Chao LK. Heme oxygenase-1 mediates the anti-inflammatory effect of Curcumin within LPS-stimulated human monocytes. Journal of Cellular Physiology. 2008;215(3): 603–12. doi: 10.1002/jcp.21206. [DOI] [PubMed] [Google Scholar]
- 301.Slater EE, MacDonald JS. Mechanism of action and biological profile of HMG CoA reductase inhibitors. A new therapeutic alternative. Drugs. 1988;36(suppl 3 ): 72–82. doi: 10.2165/00003495-198800363-00016. [DOI] [PubMed] [Google Scholar]
- 302.Chen J-C, Huang K-C, Lin W-W. HMG-CoA reductase inhibitors upregulate heme oxygenase-1 expression in murine RAW264. macrophages vi ERKp38 MAPK and protein kinase G pathways. . Cellular Signalling . 2006;18(1): 32–9. doi: 10.1016/j.cellsig.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 303.Gueler F, Park J-K, Rong S , et al. Statins attenuate ischemia-reperfusion injury by inducing heme oxygenase-1 in infiltrating macrophages. American Journal of Pathology. 2007;170(4): 1192–9. doi: 10.2353/ajpath.2007.060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Fujita E, Shimizu A, Masuda Y , et al. Statin attenuates experimental anti-glomerular basement membrane glomerulonephritis together with the augmentation of alternatively activated macrophages. American Journal of Pathology. 2010;177(3): 1143–54. doi: 10.2353/ajpath.2010.090608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Leung P-O, Wang S-H, Lu S-H, Chou W-H, Shiau C-Y, Chou T-C. Simvastatin inhibits pro-inflammatory mediators through induction of heme oxygenase-1 expression in lipopolysaccharide-stimulated RAW264. macrophages. Toxicology Letters. 2011;207(2): 159–66. doi: 10.1016/j.toxlet.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 306.Gill AJ, Kolson DL. Dimethyl Fumarate Modulation of Immune and Antioxidant Responses Application to HIV Therapy. Critical Reviews in Immunology. 2013;33(4): 307–59. doi: 10.1615/critrevimmunol.2013007247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Nugteren-Huying WM, van der Schroeff JG, Hermans J, Suurmond D. Fumaric acid therapy for psoriasis a randomized, double-blind, placebo-controlled study. Journal of the American Academy of Dermatology. 1990;22(2 Pt 1): 311–2. doi: 10.1016/s0190-9622(08)80766-9. [DOI] [PubMed] [Google Scholar]
- 308.Kolbach DN, Nieboer C. Fumaric acid therapy in psoriasis results and side effects of 2 years of treatment. Journal of the American Academy of Dermatology. 1992;27(5 Pt 1): 769–71. doi: 10.1016/s0190-9622(08)80228-9. [DOI] [PubMed] [Google Scholar]
- 309.Ghoreschi K, Brück J, Kellerer C , et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. Journal of Experimental Medicine. 2011;208(11): 2291–303. doi: 10.1084/jem.20100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Devadas K, Dhawan S. Hemin activation ameliorates HIV-1 infection via heme oxygenase-1 induction. Journal of Immunology. 2006;176(7): 4252–7. doi: 10.4049/jimmunol.176.7.4252. [DOI] [PubMed] [Google Scholar]
- 311.Zhou ZH, Kumari N, Nekhai S , et al. Heme oxygenase-1 induction alters chemokine regulation and ameliorates human immunodeficiency virus-type-1 infection in lipopolysaccharide-stimulated macrophages. Biochemical and Biophysical Research Communications. 2013;435(3): 373–7. doi: 10.1016/j.bbrc.2013.04.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Seu L, Burt TD, Witte JS, Martin JN, Deeks SG, McCune JM. Variations in the heme oxygenase-1 microsatellite polymorphism are associated with plasma CD14 and viral load in HIV-infected African-Americans. Genes and Immunity. 2011;13(3): 258–67. doi: 10.1038/gene.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]