Abstract
Upon activation by extracellular matrix components or soluble agonists, platelets release in excess of 300 active molecules from intracellular granules. Those factors can both activate further platelets and mediate a range of responses in other cells. The complex microenvironment of a growing thrombus, as well as platelets' roles in both physiological and pathological processes, require platelet secretion to be highly spatially and temporally regulated to ensure appropriate responses to a range of stimuli. However, how this regulation is achieved remains incompletely understood. In this review we outline the importance of regulated secretion in thrombosis as well as in ‘novel’ scenarios beyond haemostasis and give a detailed summary of what is known about the molecular mechanisms of platelet exocytosis. We also discuss a number of theories of how different cargoes could be released in a tightly orchestrated manner, allowing complex interactions between platelets and their environment.
Keywords: familial haemophagocytic lymphohistiocytosis, mouse models, platelet secretion, platelet signalling, SNAREs
Why study platelet secretion?
Platelets are important in a range of diseases, most notably thrombosis, and many of their functions are mediated by secretion. They contain three types of intracellular secretory granules: dense (δ) granules (so called because of their appearance under the electron microscope), alpha (α) granules and lysosomes. The most numerous are α-granules (50–80/platelet), followed by 3–5 δ-granules and only a few lysosomes. In addition, platelets contain a complex system of internal membrane invaginations (open canalicular system) which provides a reservoir of membrane for platelet shape change and spreading and which also acts as a conduit for some of the platelet releasates (Escolar & White, 1991).
The function of each of the platelet granules is defined by their contents. Whereas δ-granules contain mainly small molecule platelet activators (Meyers et al, 1982) and lysosomes contain proteolytic enzymes thought to play a role in clot remodelling (Bentfeld & Bainton, 1975), it is quite striking that platelet α-granules are the most complex granule subtype, containing a number of factors with often opposing effects on their target cell (Blair & Flaumenhaft, 2009) (see Fig 1). With over 300 factors released upon activation, it is likely that platelet granule secretion is a tightly regulated process, rather than an uncontrollable ‘all-or-none’ response (White & Rompietti, 2007). Hypotheses for how this regulation is achieved are discussed later in this review.
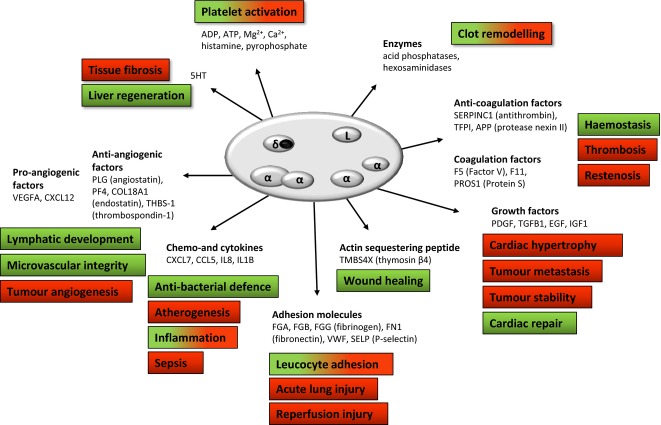
Fig 1.
An illustration of platelet granule contents and their important physiological (green) and pathological (red) correlates. Alpha granules contain cargoes with often opposing actions (e.g., angiogenesis and coagulation-related factors), hence a mechanism(s) ensuring tight spatial and temporal regulation of secretion is likely to be in place to allow platelets to exert their many functions. It should be noted that although functions are assigned to each cargo, many cargoes may contribute to multiple physiological/pathological processes, while the contribution of others still has not been fully elucidated.
The many functions of platelets – mediated by secretion?
Abnormal platelet function most notably leads to pathological thrombosis, and thereby plays a critical role in cardiovascular diseases, such as coronary artery disease and stroke. The role of platelets in thrombosis and haemostasis is well established (Coller, 2011), but a large body of research over the last 50 years now attributes many more roles to platelets. In addition to the initial ‘stemming’ of bleeding, they contribute to the later stages of haemostasis, including inflammation, immune responses, wound healing and the control of infection (Smyth et al, 2009). It is now known that the plethora of cytokines and chemokines released from platelet α-granules can propagate leucocyte recruitment in the inflammatory milieu (Kerrigan et al, 2012), and expressed platelet surface receptors can mediate adhesion to both endothelial cells and other circulatory cells, which is of importance in atherosclerotic plaque development and vascular restenosis (Lievens & von Hundelshausen, 2011).
In addition, tumour progression and metastasis is thought to be influenced by platelets, most notably via platelet-derived growth factor secretion (Labelle et al, 2011). Most recently, activation of the novel platelet CLEC1B (also known as CLEC2) receptor by tumour-derived PDPN (podoplanin) was also found to lead to release of bioactive molecules that provide a favourable environment for cancer cell survival, adhesion and extravasation; in addition, clustering of PDPN with platelet CLEC1B activates a number of migration and invasion pathways in the tumour (Lowe et al, 2012; Takagi et al, 2013). The same CLEC1B/PDPN interaction was also shown to be essential for maintaining integrity of lymphatic vessels by causing release of bioactive sphingosine-1 phosphate from platelets (Herzog et al, 2013). Further examples of novel roles for platelets include tissue fibrosis (Dees et al, 2011), liver regeneration (Papadimas et al, 2012) and wound healing (Kaur & Mutus, 2012). These and others (Blair & Flaumenhaft, 2009) are summarized in Figure 1.
Granule biogenesis and trafficking
At the other end of pathological thrombosis spectrum lie the much rarer platelet storage disorders, which can result in serious bleeding complications for affected patients. Dense granule synthesis defects include Hermansky Pudlak and Chediak-Higashi Syndromes, while α-granule synthesis defects are associated with ARC (Arthrogryposis-Renal dysfunction-Cholestasis syndrome) or GPS (Grey Platelet Syndrome) (Nurden & Nurden, 2011). Platelets are produced from megakaryocytes in bone marrow and limited evidence suggests that, unlike most other secretory organelles that bud from Golgi, platelet α- and δ-granules instead originate from multivesicular bodies (MVBs) or late endosomes in megakaryocytes (Heijnen et al, 1998; Ambrosio et al, 2012). For more detail on molecular determinants of platelet granule synthesis see (King & Reed, 2002) and (Masliah-Planchon et al, 2013).
What do we know about the regulation of secretion?
All secretion events require a ‘machinery’
Any vesicle fusion event is energetically unfavourable and must involve a series of coordinated steps to overcome the repulsive ionic forces and dissipate hydration between the lipid bilayers (Aunis & Bader, 1988). This requires NSF (N-ethylmaleimide sensitive fusion protein) and SNAP (Soluble NSF Attachment Protein), as first observed in the Golgi transport in yeast (Clary et al, 1990). Söllner et al (1993) first identified the SNARE (SNAp REceptor) family of proteins as the critical machinery for membrane fusion. Using affinity purification methods they isolated four proteins that bound NSF and SNAP and, most importantly, they found those novel SNAP receptors, or SNAREs, to also be associated with the synapse. SNAP25, the syntaxins STX1A and STX1B and synaptobrevin-1 (VAMP1) were the first identified SNARE complex. Since that seminal work 20 years ago, more than 60 SNARE proteins have been described in mammalian and yeast cells. They have since been implicated in mediating a range of membrane fusion events in all cell types, be it secretion, endocytosis or early-to-late endosome transport.
In the simplest terms, SNARE-mediated fusion involves transport of the vesicle to the target membrane and ‘priming’ it for release, followed by calcium-mediated conformational change in the complex that leads to the completion of membrane fusion leading to release of granule contents (Fig 2).
Fig 2.
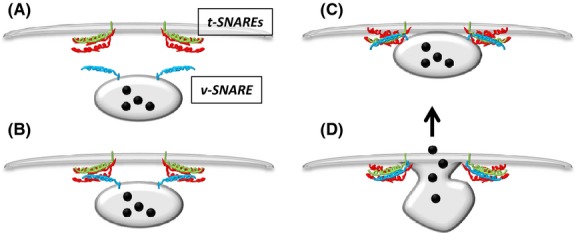
Vesicle (v) and target (t) SNAREs reside on opposing membranes. (A) In response to stimulus the vesicle translocates near to the target membrane and the four SNARE domains associate. (B) A conformational change in the complex brings the membranes close together and (C) eventually leads to overcoming the energy barrier enabling (D) fusion of the membranes and release of granular contents. This model and the core SNARE machinery is ubiquitously expressed throughout eukaryotic cells.
Classification of SNAREs
Initially SNAREs were classified according to their membrane location – vesicle or target SNAREs (see Fig 2A). It was not until 1998 that an extensive sequence analysis of all known SNAREs revealed that, in fact, the specificity of the complex is conferred by a segment in their cytosolic domains called a SNARE motif that consists of 60–70 amino acids, and specifically by a highly conserved 16 amino acids which lie up- and down-stream of an ionic ‘zero’ layer (Fig 3A). The ‘zero’ layer invariably consists of three glutamines (Q) and an arginine (R) residue (Fasshauer et al, 1998). This discovery led to a reclassification of SNAREs according to the amino acid in the zero layer: Qa, Qb, Qc and R (Fig 3B).
Fig 3.
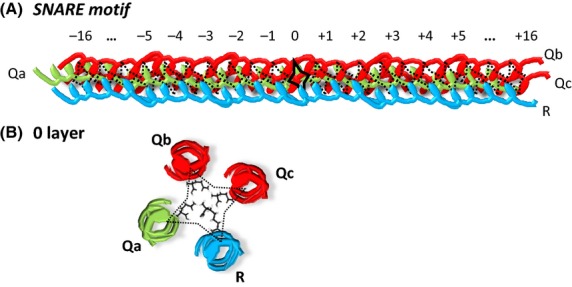
SNARE complex specificity is conferred by the 50–60 amino acid SNARE motif. (A) Four SNARE motifs from each of the Qa, Qb, Qc and R families form a functional four-helical bundle, which drives membrane fusion. (B) The QabcR configuration of the zero layer itself is completely conserved between different cell types and species, with the structure of the 16 amino acids up- and down-stream considerably less well conserved.
Along with the QabcR classification, SNAREs can be grouped into three broad subfamilies: synaptosomal-associated protein (SNAPs) type, vesicle-associated membrane proteins (VAMPs) and syntaxins. All SNAP members (SNAP23, SNAP25, SNAP29 and SNAP47) contain two SNARE motifs (contributing Qb and Qc to the SNARE complex) and lack transmembrane domains. They are instead anchored to the membrane by thioester-linked acyl groups (Hong, 2005). Concerning R-SNAREs, two subfamilies can be distinguished: short VAMPs or brevins, and long VAMPs or longins. The latter share a N-terminus extension, the so-called Longin Domain, LD (Filippini et al, 2001). Syntaxins are evolutionarily less-well conserved, but all mammalian syntaxins, with the exception of STX11, are transmembrane proteins anchored by their carboxy-terminal tails with the amino terminus and with the bulk of the polypeptide facing the cytosol (Hong, 2005).
SNARE regulators
The solving of the crystal structure of the endosomal SNARE complex (Antonin et al, 2002) further confirmed the high level of structural and sequence conservation in what is now believed to be the functional SNARE domain. In light of this conservation, a suggestion of accessory proteins mediating biological specificity of the complex was also made. For example, STXBP (Sec/Munc18-like) proteins are thought to bind directly to their cognate syntaxins and ‘prime’ them for assembly into the fusogenic SNARE complex (Zilly et al, 2006), as well as regulate the dynamics of secretion (Rickman & Duncan, 2010). UNC13 family members were also shown to be essential for the priming of synaptic vesicles and lytic granules (Dudenhoffer-Pfeifer et al, 2013). Small GTPases, RABs and RHOs have also been implicated in regulation of secretion. The role of those and other regulators, as well as signalling pathways regulating SNARE complex formation in platelets will now be discussed.
What do we know about secretion in platelets?
The identity of SNAREs in platelets
The first evidence for SNAREs in platelets was reported by Lemons et al (1997) when they detected both the general secretory proteins NSF and SNAP, and specific SNAREs STX2 and STX4, in platelet lysates. Since then, technological advances in platelet research have allowed us to characterize further elements of the platelet secretory machinery.
The R SNARE
A number of VAMPs (VAMP2, 3, 4, 5, 7, 8) are present in platelets, but recent evidence suggests that only some of them are involved in secretion. The initial studies, including inhibition of secretion with antibodies directed against SNAREs in permeabilized cell systems (Feng et al, 2002) and treatment with whole tetanus toxin (which cleaves VAMPs 2 and 3) (Flaumenhaft et al, 1999), suggested a role for VAMP3 in platelets. But the results remained equivocal until the generation of a range of Vamp knockout mice. Schraw et al (2003a) showed that VAMP3 is in fact redundant in secretion (in mouse platelets), and in 2007 the same group followed up with a report that knockout of Vamp8 results in a defect in platelet secretion (Ren et al, 2007). The secretion could be further attenuated by tetanus toxin light chain (TeNT LC) treatment, suggesting a secondary role for the tetanus-sensitive VAMPs, VAMP2 and/or VAMP3 in mouse platelets. This ranked redundancy of R-SNAREs is a common theme in other cells, for example in chromaffin cells, where VAMP2 deletion results in profound, but not absolute reduction in secretion, with residual secretion accounted for by VAMP3 (Borisovska et al, 2005). Vamp8−/− mice were later shown to have deficient thrombus formation in vivo (Graham et al, 2009). Moreover, VAMP8 was shown to be overexpressed in hyperactive human platelets (Kondkar et al, 2010), thus further confirming the importance of VAMP8 in human platelets.
The Qa SNARE
The story of discovery of the major platelet syntaxin followed a similar course to that of the VAMPs. Several SNAREs had been initially detected in platelets by Western blotting, including STX2, STX4 and STX7. Initial studies from the Whiteheart group introduced neutralizing antibodies into streptolysin-permeabilized platelets to suggest that both STX2 and STX4 play a role in secretion from lysosomes (Chen et al, 2000a) and α-granules (Lemons et al, 2000), whereas only STX2 was involved in δ-granule secretion (Chen et al, 2000b). More recently however, both Stx2−/−and Stx4−/−, as well as double Stx2−/−/Stx4−/− mice were shown by the same group to have intact secretion from all platelet granules (Ye et al, 2012).
The breakthrough came from studying a patient suffering from a subtype of Familial Haemophagocytic Lymphohistiocytosis (FHL or HLH) associated with a missense mutation in the STX11 gene, causing FHL4 (zur Stadt et al, 2005). FHL is a family of rare genetic autosomal auto-immune diseases that also manifest in reduced numbers of red blood cells and platelets (thrombocytopenia) and is lethal if left untreated. The Whiteheart group obtained blood samples from a FHL4 patient and found that, in addition to thrombocytopenia, the STX11-devoid platelets had an almost completely abolished δ- and α-granule secretion, while cargo levels and other elements of the secretory machinery were found to be normal (Ye et al, 2012). This study not only provided us with evidence for the role of STX11 in platelet secretion, but also stressed the importance of using appropriate models for studying platelet secretion.
The Qb and Qc SNAREs
The Qb and Qc domains can either derive from two different SNAREs or from two chains of the same protein. In the endosomal SNARE complex the Qb and Qc residues are contributed by VTI1B and STX8, respectively, whereas in the neuronal complex both come from a single protein (SNAP25). In platelets the initial evidence suggests that the latter is happening – with both Qb and Qc contributed by another SNAP family member, SNAP23. However, again, the evidence is not unequivocal. Snap23−/− global knockout is prenatally lethal (Suh et al, 2011) and no platelet-specific conditional knockouts have been reported to date. The initial evidence for SNAP23 came from a permeabilized platelet system, in the same study as the STX2 and STX4 results described above (Chen et al, 2000a,b2000b; Lemons et al, 2000). There is also evidence for protein kinase C (PKC)-mediated SNAP23 phosphorylation occurring simultaneously with secretion in platelets (Polgar et al, 2003; Konopatskaya et al, 2009), but the same was previously shown for STX4, which was subsequently proven to be redundant in secretion (Chung et al, 2000). More recently, the protein kinase IKK (IκB kinase) was shown to mediate phosphorylation of SNAP23, which in turn was shown to be important for SNARE complex formation, but the effects of an IKK inhibitor on other SNAREs were not evaluated, so it cannot be concluded that SNAP23 is the only SNARE undergoing this modification (Karim et al, 2013). Whereas it is perfectly plausible that SNAP23 plays a role in platelet secretion, we cannot rule out that secondary Qbc SNARE or SNAREs exist. It is possible that, as in the case of VAMPs, there is a ranked redundancy of the Qb/Qc SNAREs in platelets and that under different conditions other proteins could contribute to the complex formation. At present we lack the tools to answer this question conclusively.
SNARE chaperones
STXBP family (MUNC/SEC family)
An early study using inhibitory peptides in permeabilized platelets (Schraw et al, 2003b) suggested that different STXBP family members bind different SNAREs and that they all contribute to the regulation of secretion. STXBP1 was found in complexes with STX2, and STXBP3 with both STX2 and STX4. In this report, STXBP2 (also known as MUNC18B) was not analysed in the permeabilized system but described broadly as ‘interacting with the SNARE complex’. More recently however, a different FHL subtype – FHL5 was identified (Jain et al, 2012). In the patients studied, a disruptive mutation even in only one allele of the STXBP2 gene resulted in a significant defect in platelet secretion (Al Hawas et al, 2012). The levels of STX11 were also significantly altered in bi-allelic mutant patients – confirming the previously reported observation that STXBP2 preferentially interacts with STX11 in other cells (zur Stadt et al, 2009). This recent evidence is in line with observation that STX11 is likely to be the main syntaxin in human platelets.
UNC13D
MUNC13D is the most abundantly expressed UNC13 in platelets and was initially identified as a RAB27B binding protein, which suggested a role in secretion in platelets (see section below on Small GTPases) (Shirakawa et al, 2004). It is now known that it is mutated in FHL3 (Feldmann et al, 2003) which like other FHL types causes systemic defects in secretory organelles. A chemically mutagenized mouse model, Unc13dJinx, has been generated that incorporates a premature stop codon in the gene encoding UNC13D, causing loss of the second C-terminal C2 domain and part of the second UNC homology domain. This inactivates UNC13D, leading to complete ablation of δ-granule secretion in mouse platelets (Ren et al, 2010). Reduced α-granule secretion was also observed in this model, although it was not ablated. The reduced granule secretion leads to a reduction in thrombosis formation both in vitro and in vivo (Savage et al, 2013). Interestingly, a recent report has suggested that the defect in ATP secretion in Unc13dJinx mice reduces tumour metastasis in those animals (Schumacher et al, 2013). This was shown to be mediated principally by the released ATP leading to an increased opening of the endothelial barrier, allowing tumour cell extravasation, mediated through endothelial P2Y2 receptors. It is possible however that there is a role also for the reduced α-granule secretion in these mice, and given that many vasoactive cargoes are released from α-granules, this could also play a role in protection from metastasis in these animals (Harper et al, 2013a).
Small GTPases
RABs
The RAB family consists of more than 60 members and is associated with all vesicular transport events in many cells (as reviewed in (Hutagalung & Novick, 2011)). Several RAB family members were identified in platelets and megakaryocytes, and found to regulate both platelet granule biogenesis (RAB38/32) (Ambrosio et al, 2012) and exocytosis. The most important RAB regulating platelet secretion is RAB27B, deletion of which results in a defect in both δ-granule number and secretion (Tolmachova et al, 2007). RABs are in turn regulated by a number of other small accessory proteins, guanine exchange factors (GEFs) and GTPase-activating protein (GAPs), adding another level of regulation to an already complex system. The identity of many of those in platelets remains incompletely understood.
RHO family
RHO GTPases are another family of small GTPase regulators implicated in signalling in platelets (as reviewed in (Aslan & McCarty, 2013)), and the most prevalent CDC42/RHOA/RAC complex is also the most studied to date. Pleines et al (2010) reported the generation of Floxed platelet and megakaryocyte-specific Cdc42-deficient mice (Cdc42 global knockouts are prenatally lethal). Cdc42Floxed platelets showed increased aggregation, and most notably increased levels of ADP/ATP stored in δ-granules and increased secretion from both δ- and α-granules. This was contrary to previous observations where inhibition of platelet CDC42 by secramine A caused a decrease in aggregation and spreading on collagen (Pula & Poole, 2008). Whereas that effect could potentially be explained by poor specificity of the inhibitor or a difference between acute inhibition versus chronic genetic deletion, the decreased secretion in the absence of CDC42 was also reported in endothelial (Klarenbach et al, 2003) and Rat Basophilic Leukaemia (RBL) cells (Hong-Geller & Cerione, 2000). This may also emphasize the significant differences between platelets and other secretory cells. Rhoa-specific deletion led to reduced α- and δ-granule secretion in response to some agonists (Pleines et al, 2012), suggesting divergent signalling downstream from different receptors and complex regulation of the RHO GTPases.
RALA/B and the exocyst complex
Yet another small GTPase family implicated in platelet exocytosis are RALs, previously described as interactors of the ‘exocyst complex’ (SEC proteins) (Heider & Munson, 2012) and found to be activated upon platelet stimulation (Wolthuis et al, 1998). RALs A and B were found to play a role in neuronal and epithelial cell polarity and in tight junction establishment and function (as reviewed in Heider & Munson, 2012). Very little is known about the exocyst complex's role in platelets, but recently inhibition of RAL with a SEC5-RBD mutant that lacks the GTP-RAL binding activity was found to impair δ-granule secretion (Kawato et al, 2008). The SEC5-RBD does not distinguish between RALA and RALB so until specific knockout models are developed there is no way of further elucidating this pathway. The exocyst complex in other cells was proposed to form an initial connection between vesicle and target membrane through interactions with proteins and lipids on both surfaces and also to bring vesicles close enough to promote SNARE complex formation and vesicle fusion (Heider & Munson, 2012). The identity of exocyst proteins in platelets remains unknown.
Other potential regulators of secretion
Motor proteins and cytoskeleton
The unconventional, non-muscle myosins are thought to regulate a number of stages of exocytosis in different cell types. MYO5A (myosinVa) transcripts are highly expressed in human platelets (Rowley et al, 2011), and it was also shown to interact with an important regulator of platelet secretion RAB27 (Tolmachova et al, 2007), making it an attractive candidate as a platelet secretion regulator. However we recently analysed Myo5a−/− mouse platelets and found that secretion was not affected, nor were other platelet functions (Harper et al, 2013b). Other myosin mRNAs found in the genome-wide transcript analysis were MYO1C, MYO1F and Myo1g, and Myo6 in mouse platelets, and it is possible that they could play a role in secretion (Rowley et al, 2011). However, it is also possible that because of the small size of platelets, the ‘machinery’ that facilitates transport of granules across the intracellular space is not necessary.
In resting platelets, the actin cytoskeleton acts as a barrier to secretion as shown by Flaumenhaft et al (2005), and this effect is more pronounced in case of α- than δ-granules. In a more recent paper, the same group showed that upon activation, the actin cytoskeleton undergoes reorganization and binds some of the SNARE proteins, thus facilitating α-granule secretion (Woronowicz et al, 2010). The role of the platelet cytoskeleton is well evidenced in platelet development and granule trafficking in megakaryocytes, (Thon et al, 2010), but its role in platelet secretion is still uncertain.
Tomosyn (STXBP5/5L)
Tomosyn was first identified as a syntaxin-binding protein in rat cerebral tissue (and named accordingly: from Japanese –tomo (friend) of syntaxin (syn) (Fujita et al, 1998)). Two genes encoding STXBP5 were identified – STXBP5 and STXBP5L, but alternative slicing leads to 7 different isoforms. It is thought that tomosyn plays a role in anchoring the vesicles to the target membrane before release but the mechanism remains elusive. In platelets, there have been reports of tomosyn expression (Ren et al, 2008) and it has also been found in the transcriptome analysis of human and mouse platelets (Rowley et al, 2011). It is a promising candidate for regulating secretion in platelets, but its role remains to be elucidated.
Signalling in secretion
Platelet activation occurs through numerous signalling cascades that originate from diverse surface receptors being activated by different agonists. Many of these converge on common intracellular signalling events that ultimately elevate cytosolic calcium and release diacylglycerol. These and other signals also lead to activation of PKC. Platelet degranulation is ultimately regulated by many of these signalling cascades, but the exact nature of the pathways and how secretion is regulated remains unclear.
Phosphorylation
PKCs
Several PKC isotypes are expressed in human platelets. Classical PKCs α (PRKCA) and β (PRKCB), and the closely related novel PKCs δ (PRKCD) and θ (PRKCQ) all have been detected in both mouse and human platelets, whereas PKCε (PRKCE) is only expressed in mouse platelets. Several SNAREs have been shown to be phosphorylated in a PKC-dependent manner (Chung et al, 2000; Barclay et al, 2003; Polgar et al, 2003) and broad-spectrum PKC inhibitors can ablate secretion in intact platelets (reviewed in (Harper & Poole, 2010)). However, targeting of specific isoforms via pharmacological approaches is difficult and therefore to establish the roles of each isoform several groups have turned to knockout mouse models. We have previously shown that Prkca−/− mouse platelets have a very substantial reduction in α- and δ-granule secretion in response to agonists (Konopatskaya et al, 2009), and that it plays a role in platelet biogenesis, because δ-granule numbers were reduced. PKCα is therefore a major positive regulator of secretion in platelets.
The roles for other PKC isotypes are less clearly defined. Pharmacological inhibition of PRKCD enhanced secretion downstream of GPVI receptor but diminished secretion downstream of PAR (thrombin) receptors (Murugappan et al, 2004). Prkcd−/− platelets also showed a partially reduced granule secretion in response to stimulation of PAR4 (Chari et al, 2009), however no difference was found in these mice in response to stimulation of the collagen receptor GPVI (Pula et al, 2006). The role of PRKCD in regulating secretion may therefore be agonist-specific. Similarly, the role for PRKCQ is debatable (Harper & Poole, 2009). Nagy et al (2009) showed that platelet activation and secretion is impaired in Prkcq−/−animals, whereas another study suggested an enhancement of alpha granule secretion, but not dense granule secretion, at low levels of GPVI stimulation (Harper & Poole, 2009). These discrepancies may hint at the complex nature of control of secretion in platelets, with responses varying depending on agonist and degree of stimulation.
Other kinases
Protein kinase D2 (PRKD2) was shown to be an essential regulator of δ-, but not α-granule secretion downstream of classical PKC isoforms (Konopatskaya et al, 2011). IKK was recently shown to lie downstream from classical PKC isoforms (PRKCA and PRKCB) as well as PRKCD, and directly phosphorylate SNAP23, possibly providing a mechanistic explanation for how PKC regulates secretion (Karim et al, 2013). A family of cAMP dependent kinases (PRKACA, B and G) were shown to disrupt SNARE interactions in vitro (Foster et al, 1998) and, together with cGMP-dependent protein kinase isoforms, were implicated in the negative regulation of platelet activation by inhibition of RAS and RHO family GTPases, inhibition of the release of Ca2+ from intracellular stores and modulation of actin cytoskeleton dynamics, although no direct role in regulating secretion was shown (Smolenski, 2012).
Calcium in secretion
A common effector for agonists in platelets is an increase in cytosolic calcium. A rise in cytosolic calcium alone is able to stimulate and cause secretion from permeabilized platelets in the presence of ATP (Morimoto & Ogihara, 1996). It is possible that calcium directly removes inhibitory proteins or activates SNAREs but the link has not been made yet. UNC13D, with its two calcium-sensing domains C2A and C2B – is hinted to play a role as the secretory calcium sensor in platelets. In an artificial liposome fusion study, Ca2+-stimulated UNC13D interactions with SNAREs were mediated by the C2A domain and Ca2+-dependent membrane binding by the C2B domain (Boswell et al, 2012). In the apparent coupling of membrane and SNARE binding, UNC13D is the first priming factor shown to promote Ca2+-dependent SNARE complex formation and SNARE-mediated liposome fusion. Boswell et al (2012) suggested that these properties of UNC13D make it a possible Ca2+ sensor at rate-limiting priming steps in granule exocytosis.
Synaptotagmin-like proteins
Synaptotagmin-like proteins (SYTL) are another family of proteins with calcium-sensing properties that could play a role in signal integration in platelets. They share tandem calcium-sensing domains (C1 and C2) and a SYTL-homology domain (SHD), which is believed to function as a specific effector domain for RAB27 (Fukuda, 2007). SYTLs are thought to play a role in granule docking either by direct interaction with the cell membrane (via binding of the C2 domain to plasma phosphatidylserine) or via interaction with vesicular RAB27 (Fukuda, 2007). Of the five mammalian SYTLs, only SYTL1 and SYTL4 are expressed in platelets, and SYTL1 is thought to be a negative regulator of platelet δ-granule secretion, via interaction with RAB27 and RAP1GAP2, as shown in a permeabilized platelet model using purified SYTL1 inhibitory domain (Neumuller et al, 2009). On the other hand, SYTL4 is believed to interact with RAB8 to enhance δ-granule secretion in a permeabilized platelet model (Hampson et al, 2013). The mechanism and dynamics of those interactions remain largely unknown.
CalDAG-GEFI/RAP1/RAC
CAlDAG-GEFI (RASGRP2) is a member of the RASGRP family of intracellular signalling molecules that is expressed in platelets, megakaryocytes and neutrophils. Its calcium-sensing properties, as well as its diacylglycerol (DAG) binding site, make it an attractive candidate for signal integration downstream of platelet receptors (Crittenden et al, 2004). It activates RAP1B, which in turn activates the RAC1 pool responsible for secretion (Stefanini et al, 2012). Both Rap1b−/−and Rac1−/−platelets have significant α- and δ-granule secretion defects, further supporting the role for this complex in secretion (Akbar et al, 2007; Zhang et al, 2011). Furthermore, a recent study showed that RASGRP2 phosphorylation by PKA is the main mechanism responsible for keeping platelets in an inactive state by preventing RAC1 activation (Subramanian et al, 2013). Rasgrp2−/− platelets show a number of defects, including integrin activation, adhesion and aggregation, as well as α-granule secretion; similar defects were observed in canine platelets with mutated RASGRP2 (Boudreaux et al, 2007). It is apparent that the RASGRP2/RAP1B/RAC1 complex plays an important role in regulating secretion.
In summary, our current understanding of the secretion machinery in platelets has advanced hugely since the initial discoveries by Lemons et al. The proteins we now believe to be involved in platelet secretion are summarized in Table I.
Table I.
Summary of selected secretion-related genes discussed in this review and the experimental approaches used to validate the function identified. Pharmacological studies or evidence from permeabilized platelet system models are not included (nd, indicates no data available)
| Effect on granules | ||||||
|---|---|---|---|---|---|---|
| Protein | Alpha | Dense | Lysosome | Model used | Reference | |
| SNAREs | VAMP8 | Markedly reduced | Markedly reduced | Markedly reduced | Vamp8−/− (global) | Ren et al (2007) |
| VAMP2 | Secondary to VAMP8 | Secondary to VAMP8 | Secondary to VAMP8 | TeNT LC + Vamp8−/− (global) | ||
| VAMP3 | Secondary to VAMP8 | Secondary to VAMP8 | Secondary to VAMP8 | Vamp3−/− (global), TeNT LC + Vamp8−/− (global) | ||
| STX11 | Markedly reduced | Markedly reduced | Reduced | FHL4 patient | Ye et al (2012) | |
| STX2 | No change | No change | No change | Stx2−/− (global) | ||
| STX4 | No change | No change | No change | Stx4−/− (global) | ||
| STX2/STX4 | No change | No change | No change | Stx2−/−/Stx4−/− (global) | ||
| SNARE chaperones | STXBP2 | Completely abolished | Completely abolished | Markedly reduced | FHL5 patient | Al Hawas et al (2012) |
| UNC13D | Markedly reduced, rescued with ADP | Completely abolished | Markedly reduced | Unc13dJinx (global) | Ren et al (2010) | |
| Small GTPases | RAB27B | No effect | 50% reduced number, reduced serotonin content, secretion defect | nd | Rab27b−/− (global) | Tolmachova et al (2007) |
| CDC42 | Increased secretion | Increased ADP loading, increased secretion | nd | Cdc42−/− (megakaryocyte- and platelet-specific) | Pleines et al (2010) | |
| RHOA | Reduced secretion, not to GPIV | Reduced secretion, to some agonists | nd | Rhoa−/−(megakaryocyte- and platelet-specific) | Pleines et al (2012) | |
| RAC1 | Reduced secretion | Reduced secretion | nd | Rac1−/−(haematopoietic) | Akbar et al (2007) | |
| Motor protein | MYO5A | Normal | Normal | nd | Myo5a−/− (global) | Harper et al (2013b) |
| Kinases | PRKCA | Reduced secretion | Reduced number and secretion | nd | Prkca−/−(global) | Konopatskaya et al (2009) |
| PRKCD | Debatable | Debatable | nd | Prkcd−/−(global) | Chari et al (2009), Harper and Poole (2010) | |
| PRKCQ | Debatable | Debatable | nd | Prkcq−/−(global) | Harper and Poole (2009), Nagy et al (2009) | |
| PRKD2 | Normal | Reduced | nd | Pkd2−/−(global) | Konopatskaya et al (2011) | |
| Signalling molecule | RASGRP2 | Reduced secretion | Reduced secretion | nd | Rasgrp2−/−(global) | Crittenden et al (2004) |
What we don't know about platelet secretion
At this point it needs to be reiterated how tightly platelet secretion is regulated. Under physiological conditions, activated healthy platelets release enough cargo to activate sufficient number of their counterparts and promote stabilization of the haemostatic plug that will prevent excessive bleeding. If all the contents of the intracellular stores, including potent mitogenic, pro-angiogenic, anti-angiogenic, pro-inflammatory and adhesive factors were released in an uncoordinated manner, the thrombus growth would be similarly uncoordinated. Additionally, to exert all their newer non-haemostatic roles as described earlier, platelet secretion has to be tightly spatially and temporally regulated: pro-angiogenic and anti-angiogenic factors' interplay regulates vessel growth when required, mitogenic factors promote wound healing and metastasis is mediated by CLEC1B activation but not other receptors. Despite our extensive knowledge of the secretory machinery present in platelets, we are still relatively ignorant of HOW platelets regulate their secretion.
Differential packaging?
One of the hypotheses put forward is that platelet granules are not uniform, and that certain factors, especially α-granule cargoes, may be differentially packaged and thus released ‘thematically’. Ma et al (2005) observed that platelet stimulation with specific protease-activated receptor (PAR) 1 or PAR4 agonist resulted in preferential release of VEGF or endostatin, (anti- and pro-angiogenic factors, respectively). In addition PAR1 antagonist-treated rats showed retarded ulcer healing in vivo, which corresponded with inhibition of the pro-angiogenic action of endostatin. Differential packaging of granules was a very attractive explanation for that observation, and indeed, spinning-disc confocal microscope images of resting platelets, clearly showing distinct localization of two of the main α-granule cargoes – fibrinogen and von Wiillerbrand Factor (VWF) were published (Sehgal & Storrie, 2007). Subsequently, Italiano et al (2008) group revealed in both platelets and megakaryocytes that VEGF and endostatin preferentially colocalized with fibrinogen or VWF, respectively. Immunogold labelling from this study also showed α-granule populations containing either endostatin or VEGF. More recently, a super-resolution analysis of 15 platelet α-granule proteins using 3D structured illumination microscopy (3D-SIM) however failed to show any functional co-clustering (Kamykowski et al, 2011). These discrepancies illustrate the challenging nature of studies on platelet granule content and secretion. Differential packaging of α-granule cargo remains an attractive explanation for the observed separation of pro- and anti-angiogenic releasate, but is a concept that requires more investigation for definitive proof.
An intriguing additional hypothesis was put forward suggesting that, in fact, different cargoes are all packaged into the same granules, but differentially localized within the α-granule. van Nispen tot Pannerden et al (2010) took electron micrograph ‘snapshots’ of resting human platelets and identified different classes of α-granules, most notably a distinct population of approximately 50-nm-wide ‘tubes’ that they designated ‘tubular α-granules’. They also noted that those tubular granules contained fibrinogen but not VWF, as opposed to ‘spherical’ α-granules, which contained both. There is a possibility that if such granules were to preferentially fuse with plasma membrane or OCS at one or other end, this could result in the gradual release of their contents, with the physically furthest released last. Maybe if polarized packaging within such tubular α-granules existed, it could account both for the observed selective release and for different release kinetics (discussed below). But even if differential packaging exists, either within or between granules, it is still unclear how platelets ‘know’ which granules to release.
Differential kinetics?
It is thought that there is likely to be a hierarchy of kinetics of release of platelet granules, with δ-granule release being a rapid event, followed by α-granule release and finally, by much slower lysosome release. Until recently however, little quantification existed. Jonnalagadda et al (2012) performed a systematic quantification of granule secretion using micro-enzyme-linked immunosorbent assay arrays for 28 distinct α-granule cargo molecules in response to four different agonists. They then classified releasate as ‘fast’, ‘medium’ and ‘slow’ release, and surprisingly, found that not only did the release kinetic vary widely for each cargo depending on the agonist, but also that the kinetics of release did not correlate with the traditional ‘fast’ δ-granule, ‘slower’ α-granule and ‘slowest’ lysosome secretion model. They also found that cargoes with opposing functions had similar release profiles, suggesting limited thematic response to specific agonists. In conclusion, they proposed that kinetics of release are more complex than previously believed, and suggest that they may be regulated by a number of factors, such as granule shape, proximity to the membrane or cargo solubility (Jonnalagadda et al, 2012).
Interestingly a recent report has suggested that different SNAREs in the fusion complex are responsible for different steps of membrane fusion, at least in neurons. The proposal derives from in vitro work on nanodiscs, demonstrating that one SNARE is responsible for ‘fusion’ itself whereas the other three work together to keep the pore open (Shi et al, 2012). In line with this observation, in their comprehensive review of factors determining dynamics of secretion in various cell types, Kasai et al (2012) proposed a number of different fusion modes that could account for the range of fusion dynamics, from ultra-fast synaptic transmission, to slow (within a range of 10–100 s) exocrine release achieved by pancreatic islet cells or mast cells. Some of the mechanisms proposed also involve compound or multivesicular fusion, whereas vesicles fuse sequentially with a pre-docked granule, or several granules fuse together before reaching the membrane. It has previously been proposed that platelet granules may fuse with each other or with the open canalicular system, which could account for the slower release observed with certain stimulation conditions (Morgenstern, 1998). It is possible that fusion in platelets could occur therefore by more than one mechanism and that the aforementioned model could account for long, slow release, but maybe a ‘kiss and run’-style fusion could play a major role in ‘fast’ release. In this regard, dynamin-related protein-1 (DRP1) was recently shown to play a role in fusion pore stability, and could potentially provide more insight into this differential regulation (Koseoglu et al, 2013). This study showed that blocking DRP1 led to impaired secretion and that the defect was due to preventing complete granule collapse rather than the initial fusion pore formation (Koseoglu et al, 2013). Hence, DRP1 may be an important regulator of platelet secretion dynamics and kinetics.
Whereas in other cell types, most notably neuroendocrine cells, exocytosis is thought to be a multistep pathway that culminates in the (high) Ca2+-triggered fusion of secretory vesicles with the plasma membrane, little is known about those ‘pre-fusion’ steps in platelets. Synaptic vesicles first translocate and become physically docked, or tethered, at the membrane. Then the ATP-dependent ‘priming’ occurs, preparing the vesicle for fusion (Weimer & Jorgensen, 2003). SNARE proteins are thought to also associate relatively loosely before the high calcium trigger causes the conformational change in the SNARE complex that results in the fusion of membranes. In neuroendocrine cells, SNARE chaperones, such as MUNC18 family, small GTPases like RAB3 and calcium-sensing family of synaptotagmins, as well as SNAREs themselves are thought to be involved in those docking and ‘priming’ steps (Weimer & Jorgensen, 2003; Wu et al, 2012). Given that similar proteins are present in platelets, it is tempting to assume analogous functions. But whereas translocation, docking and priming steps could account for differential kinetics of platelet secretion as discussed above, there is no unequivocal evidence supporting this hypothesis. In fact, the recent study from Karim et al (2013) showed that platelet activation and SNAP23 phosphorylation by IKK are required for the SNARE complex association, and that no SNARE complexes were detected in resting platelets. Similarly, whereas a role for the cytoskeleton in granule translocation has been considered, it has not been shown conclusively whether granules translocate or tether to the membrane before the calcium trigger or not. While the idea of ‘pre-docked’ granules or granules differentially transported to the membrane could help explain the highly controlled secretion in platelets, the concept remains to be definitively demonstrated.
Do different SNAREs/SNARE regulators play differential roles in platelet secretion?
It is possible that different granules are controlled by different SNAREs, SNARE chaperones, small GTPases or other families of regulators involved in each type of secretion. Whole-genome transcript analysis revealed that hundreds of genes implicated in secretion in other cells are also expressed in platelets (Rowley et al, 2011) and only a fraction has been investigated to date (see Table I). In addition, novel roles for proteins we thought had no role in platelets emerge all the time. For example, Peters et al (2012) recently showed that spreading platelets require fusion of VAMP7-containing granules with the membrane, although VAMP7 is redundant as a regulator of granule secretion in platelets (Ren et al, 2007). Similarly, the recent report that STX11 is crucial for secretion overturned the previously accepted model implicating STX2 and/or STX4 (Ye et al, 2012). It is possible that tight spatial and temporal regulation of secretion is achieved by a number of different SNARE complexes interacting and mediating fusion at different rates and/or residing on granules located in different parts of the cell. The lack of overt secretion defect in many of the SNARE knockout models may not mean that they do not play a role in secretion at all – rather thatthey may be responsible for ‘fine-tuning’ of the reaction? As mentioned earlier, the evidence for SNAP23 role in secretion remains incomplete. Is it possible that other SNAREs can substitute for its Qbc domains in the SNARE complex to provide more versatility for the membrane fusion complex?
An interesting further possible development to consider is the existence of inhibitory, or ‘i-SNARE's. These were first investigated in liposome reconstitution experiments (Varlamov et al, 2004) and were proposed to act as specificity regulators of Golgi-derived SNARE fusion. If SNAREs with similar function exist in mammalian cells beyond Golgi, they could add another layer of complexity to the regulation of secretion.
Do different downstream signalling/sensors couple differentially to granules?
We are still unsure of the pathways from agonist stimulation that could lead to the differential release of cargoes. The accepted model of platelet signalling principally converges on the increase in cytosolic calcium to drive the cellular machinery upon activation. The signalling downstream of receptors however is likely to diverge at some point to differentially drive different secretory events. PKC isoforms have been proposed as the intermediate step between activation and secretion, but could they mediate differential signalling? Are the SNAREs themselves somehow playing the role of sensors downstream from receptors, similar to UNC13D? Do they undergo modifications that result in different affinity for the membrane? These questions remain open at the present time and it may be anticipated that, with the advent of ever higher resolution imaging and ever more sensitive assays for secretion, the field of platelet secretion research will evolve rapidly.
Conclusions
Platelet secretion is a tightly regulated process and, despite implication of a large number of genes in the process, we are still a long way from understanding the details of how that tight regulation is achieved. It is possible that different cargoes are packaged differently, either between or within granules, and further detailed imaging studies are required to resolve that issue fully. The differential kinetics of release are another possible explanation for control of cargo release. It is also possible that the number of SNAREs and SNARE-interacting proteins identified is just the tip of the iceberg, and that even more intricate regulation and fine-tuning can occur at the level of secretion itself. High-throughput analyses of genes and cargoes are going to be needed to understand the details of all possible combinations of regulators and effectors of secretion. It is also possible that a complex interplay between agonists is required to specifically release one cargo but not another. It is surely also the case that the complex environment that pertains in vivo, which is omitted from most in vitro studies, will provide an additional key to unravelling the intricacies of the control of platelet secretion and its wide-ranging roles in physiology and pathology.
Acknowledgments
This work was supported by the British Heart Foundation (Programme Grant RG/10/006/28299, FS/09/009/26444). EMG and AWP contributed equally to production of this paper. We would like to acknowledge also Dr Matthew T. Harper for his help with revision of the draft.
Competing interests
The authors have no competing interests.
References
- Akbar H, Kim J, Funk K, Cancelas JA, Shang X, Chen L, Johnson JF, Williams DA. Zheng Y. Genetic and pharmacologic evidence that Rac1 GTPase is involved in regulation of platelet secretion and aggregation. Journal of Thrombosis and Haemostasis: JTH. 2007;5:1747–1755. doi: 10.1111/j.1538-7836.2007.02646.x. [DOI] [PubMed] [Google Scholar]
- Al Hawas R, Ren Q, Ye S, Karim ZA, Filipovich AH. Whiteheart SW. Munc18b/STXBP2 is required for platelet secretion. Blood. 2012;120:2493–2500. doi: 10.1182/blood-2012-05-430629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio AL, Boyle JA. Di Pietro SM. Mechanism of platelet dense granule biogenesis: Study of cargo transport and function of Rab32 and Rab38 in a model system. Blood. 2012;120:4072–4081. doi: 10.1182/blood-2012-04-420745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonin W, Fasshauer D, Becker S, Jahn R. Schneider TR. Crystal structure of the endosomal SNARE complex reveals common structural principles of all SNAREs. Nature Structural Biology. 2002;9:107–111. doi: 10.1038/nsb746. [DOI] [PubMed] [Google Scholar]
- Aslan JE. McCarty OJT. Rho GTPases in platelet function. Journal of Thrombosis and Haemostasis: JTH. 2013;11:35–46. doi: 10.1111/jth.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aunis D. Bader MF. The cytoskeleton as a barrier to exocytosis in secretory cells. The Journal of Experimental Biology. 1988;139:253–266. doi: 10.1242/jeb.139.1.253. [DOI] [PubMed] [Google Scholar]
- Barclay JW, Craig TJ, Fisher RJ, Ciufo LF, Evans GJO, Morgan A. Burgoyne RD. Phosphorylation of Munc18 by protein kinase C regulates the kinetics of exocytosis. The Journal of Biological Chemistry. 2003;278:10538–10545. doi: 10.1074/jbc.M211114200. [DOI] [PubMed] [Google Scholar]
- Bentfeld ME. Bainton DF. Cytochemical localization of lysosomal enzymes in rat megakaryocytes and platelets. The Journal of Clinical Investigation. 1975;56:1635–1649. doi: 10.1172/JCI108246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair P. Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Reviews. 2009;23:177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisovska M, Zhao Y, Tsytsyura Y, Glyvuk N, Takamori S, Matti U, Rettig J, Sudhof T. Bruns D. v-SNAREs control exocytosis of vesicles from priming to fusion. The EMBO Journal. 2005;24:2114–2126. doi: 10.1038/sj.emboj.7600696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell KL, James DJ, Esquibel JM, Bruinsma S, Shirakawa R, Horiuchi H. Martin TFJ. Munc13-4 reconstitutes calcium-dependent SNARE-mediated membrane fusion. The Journal of Cell Biology. 2012;197:301–312. doi: 10.1083/jcb.201109132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreaux MK, Catalfamo JL. Klok M. Calcium-diacylglycerol guanine nucleotide exchange factor I gene mutations associated with loss of function in canine platelets. Translational Research: the Journal of Laboratory and Clinical Medicine. 2007;150:81–92. doi: 10.1016/j.trsl.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Chari R, Getz T, Nagy B, Jr, Bhavaraju K, Mao Y, Bynagari YS, Murugappan S, Nakayama K. Kunapuli SP. Protein kinase C[delta] differentially regulates platelet functional responses. Arteriosclerosis, Thrombosis & Vascular Biology. 2009;29:699–705. doi: 10.1161/ATVBAHA.109.184010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Lemons PP, Schraw T. Whiteheart SW. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 and 4 in lysosome release. Blood. 2000a;96:1782–1788. [PubMed] [Google Scholar]
- Chen D, Bernstein AM, Lemons PP. Whiteheart SW. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 in dense core granule release. Blood. 2000b;95:921–929. [PubMed] [Google Scholar]
- Chung SH, Polgar J. Reed GL. Protein kinase C phosphorylation of syntaxin 4 in thrombin-activated human platelets. The Journal of Biological Chemistry. 2000;275:25286–25291. doi: 10.1074/jbc.M004204200. [DOI] [PubMed] [Google Scholar]
- Clary DO, Griff IC. Rothman JE. SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell. 1990;61:709–721. doi: 10.1016/0092-8674(90)90482-t. [DOI] [PubMed] [Google Scholar]
- Coller BS. Historical perspective and future directions in platelet research. Journal of Thrombosis and Haemostasis: JTH. 2011;9(Suppl 1):374–395. doi: 10.1111/j.1538-7836.2011.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden JR, Bergmeier W, Zhang Y, Piffath CL, Liang Y, Wagner DD, Housman DE. Graybiel AM. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nature Medicine. 2004;10:982–986. doi: 10.1038/nm1098. [DOI] [PubMed] [Google Scholar]
- Dees C, Akhmetshina A, Zerr P, Reich N, Palumbo K, Horn A, Jungel A, Beyer C, Kronke G, Zwerina J, Reiter R, Alenina N, Maroteaux L, Gay S, Schett G, Distler O. Distler JHW. Platelet-derived serotonin links vascular disease and tissue fibrosis. The Journal of Experimental Medicine. 2011;208:961–972. doi: 10.1084/jem.20101629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudenhoffer-Pfeifer M, Schirra C, Pattu V, Halimani M, Maier-Peuschel M, Marshall MR, Matti U, Becherer U, Dirks J, Jung M, Lipp P, Hoth M, Sester M, Krause E. Rettig J. Different Munc13 isoforms function as priming factors in lytic granule release from murine cytotoxic T lymphocytes. Traffic (Copenhagen, Denmark) 2013;14:798–809. doi: 10.1111/tra.12074. [DOI] [PubMed] [Google Scholar]
- Escolar G. White JG. The platelet open canalicular system: a final common pathway. Blood Cells. 1991;17:467–485. discussion 486–495. [PubMed] [Google Scholar]
- Fasshauer D, Sutton RB, Brunger AT. Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, Lambert N, Ouachee-Chardin M, Chedeville G, Tamary H, Minard-Colin V, Vilmer E, Blanche S, Le Deist F, Fischer A. de Saint Basile G. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- Feng D, Crane K, Rozenvayn N, Dvorak AM. Flaumenhaft R. Subcellular distribution of 3 functional platelet SNARE proteins: human cellubrevin, SNAP-23, and syntaxin 2. Blood. 2002;99:4006–4014. doi: 10.1182/blood.v99.11.4006. [DOI] [PubMed] [Google Scholar]
- Filippini F, Rossi V, Galli T, Budillon A, D'Urso M. D'Esposito M. Longins: a new evolutionary conserved VAMP family sharing a novel SNARE domain. Trends in Biochemical Sciences. 2001;26:407–409. doi: 10.1016/s0968-0004(01)01861-8. [DOI] [PubMed] [Google Scholar]
- Flaumenhaft R, Croce K, Chen E, Furie B. Furie BC. Proteins of the exocytotic core complex mediate platelet alpha-granule secretion. Roles of vesicle-associated membrane protein, SNAP-23, and syntaxin 4. The Journal of Biological Chemistry. 1999;274:2492–2501. doi: 10.1074/jbc.274.4.2492. [DOI] [PubMed] [Google Scholar]
- Flaumenhaft R, Dilks JR, Rozenvayn N, Monahan-Earley RA, Feng D. Dvorak AM. The actin cytoskeleton differentially regulates platelet alpha-granule and dense-granule secretion. Blood. 2005;105:3879–3887. doi: 10.1182/blood-2004-04-1392. [DOI] [PubMed] [Google Scholar]
- Foster LJ, Yeung B, Mohtashami M, Ross K, Trimble WS. Klip A. Binary interactions of the SNARE proteins syntaxin-4, SNAP23, and VAMP-2 and their regulation by phosphorylation. Biochemistry. 1998;37:11089–11096. doi: 10.1021/bi980253t. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Shirataki H, Sakisaka T, Asakura T, Ohya T, Kotani H, Yokoyama S, Nishioka H, Matsuura Y, Mizoguchi A, Scheller RH. Takai Y. Tomosyn: a syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron. 1998;20:905–915. doi: 10.1016/s0896-6273(00)80472-9. [DOI] [PubMed] [Google Scholar]
- Fukuda M. The Role of Synaptotagmin and Synaptotagmin-Like Protein (Slp) in Regulated Exocytosis. In: Ragazzi R, editor. Molecular Mechanisms of Exocytosis. New York, NY: Springer New York; 2007. pp. 42–61. [Google Scholar]
- Graham GJ, Ren Q, Dilks JR, Blair P, Whiteheart SW. Flaumenhaft R. Endobrevin/VAMP-8-dependent dense granule release mediates thrombus formation in vivo. Blood. 2009;114:1083–1090. doi: 10.1182/blood-2009-03-210211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson A, O'Connor A. Smolenski A. Synaptotagmin-like protein 4 and Rab8 interact and increase dense granule release in platelets. Journal of Thrombosis and Haemostasis: JTH. 2013;11:161–168. doi: 10.1111/jth.12068. [DOI] [PubMed] [Google Scholar]
- Harper MT. Poole AW. PKCtheta in platelet activation. Blood. 2009;114:489–491. doi: 10.1182/blood-2009-03-208454. author reply 491-482. [DOI] [PubMed] [Google Scholar]
- Harper MT. Poole AW. Diverse functions of protein kinase C isoforms in platelet activation and thrombus formation. Journal of Thrombosis and Haemostasis: JTH. 2010;8:454–462. doi: 10.1111/j.1538-7836.2009.03722.x. [DOI] [PubMed] [Google Scholar]
- Harper MT, Savage JS. Poole AW. Comment on “platelet-derived nucleotides promote tumor cell transendothelial migration and metastasis via P2Y2 receptor” by Schumacher et al. Cancer Cell. 2013a;24:287. doi: 10.1016/j.ccr.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Harper MT, van den Bosch MTJ, Hers I. Poole AW. Absence of platelet phenotype in mice lacking the motor protein myosin Va. PLoS ONE. 2013b;8:e53239. doi: 10.1371/journal.pone.0053239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider MR. Munson M. Exorcising the exocyst complex. Traffic (Copenhagen, Denmark) 2012;13:898–907. doi: 10.1111/j.1600-0854.2012.01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijnen HF, Debili N, Vainchencker W, Breton-Gorius J, Geuze HJ. Sixma JJ. Multivesicular bodies are an intermediate stage in the formation of platelet alpha-granules. Blood. 1998;91:2313–2325. [PubMed] [Google Scholar]
- Herzog BH, Fu J, Wilson SJ, Hess PR, Sen A, McDaniel JM, Pan Y, Sheng M, Yago T, Silasi-Mansat R, McGee S, May F, Nieswandt B, Morris AJ, Lupu F, Coughlin SR, McEver RP, Chen H, Kahn ML. Xia L. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature. 2013;502:105–109. doi: 10.1038/nature12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W. SNAREs and traffic. Biochimica et biophysica acta. 2005;1744:493–517. [PubMed] [Google Scholar]
- Hong-Geller E. Cerione RA. Cdc42 and Rac stimulate exocytosis of secretory granules by activating the IP(3)/calcium pathway in RBL-2H3 mast cells. The Journal of Cell Biology. 2000;148:481–494. doi: 10.1083/jcb.148.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutagalung AH. Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiological Reviews. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italiano JE, Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J. Klement GL. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R, Puliyel M, Moses PD. Sieni E. Novel STXBP2 mutation causing familial hemophagocytic lymphohistiocytosis. Indian Pediatrics. 2012;49:488–490. doi: 10.1007/s13312-012-0094-5. [DOI] [PubMed] [Google Scholar]
- Jonnalagadda D, Izu LT. Whiteheart SW. Platelet secretion is kinetically heterogeneous in an agonist-responsive manner. Blood. 2012;120:5209–5216. doi: 10.1182/blood-2012-07-445080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamykowski J, Carlton P, Sehgal S. Storrie B. Quantitative immunofluorescence mapping reveals little functional coclustering of proteins within platelet alpha-granules. Blood. 2011;118:1370–1373. doi: 10.1182/blood-2011-01-330910. [DOI] [PubMed] [Google Scholar]
- Karim ZA, Zhang J, Banerjee M, Chicka MC, Al Hawas R, Hamilton TR, Roche PA. Whiteheart SW. IkappaB kinase phosphorylation of SNAP-23 controls platelet secretion. Blood. 2013;121:4567–4574. doi: 10.1182/blood-2012-11-470468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Takahashi N. Tokumaru H. Distinct initial SNARE configurations underlying the diversity of exocytosis. Physiological Reviews. 2012;92:1915–1964. doi: 10.1152/physrev.00007.2012. [DOI] [PubMed] [Google Scholar]
- Kaur H. Mutus B. Platelet function and thymosin beta4. Biological Chemistry. 2012;393:595–598. doi: 10.1515/hsz-2012-0131. [DOI] [PubMed] [Google Scholar]
- Kawato M, Shirakawa R, Kondo H, Higashi T, Ikeda T, Okawa K, Fukai S, Nureki O, Kita T. Horiuchi H. Regulation of platelet dense granule secretion by the Ral GTPase-exocyst pathway. The Journal of Biological Chemistry. 2008;283:166–174. doi: 10.1074/jbc.M705340200. [DOI] [PubMed] [Google Scholar]
- Kerrigan AM, Navarro-Nunez L, Pyz E, Finney BA, Willment JA, Watson SP. Brown GD. Podoplanin-expressing inflammatory macrophages activate murine platelets via CLEC-2. Journal of Thrombosis and Haemostasis: JTH. 2012;10:484–486. doi: 10.1111/j.1538-7836.2011.04614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM. Reed GL. Development of platelet secretory granules. Seminars in Cell & Developmental Biology. 2002;13:293–302. doi: 10.1016/s1084952102000599. [DOI] [PubMed] [Google Scholar]
- Klarenbach SW, Chipiuk A, Nelson RC, Hollenberg MD. Murray AG. Differential actions of PAR2 and PAR1 in stimulating human endothelial cell exocytosis and permeability: the role of Rho-GTPases. Circulation Research. 2003;92:272–278. doi: 10.1161/01.res.0000057386.15390.a3. [DOI] [PubMed] [Google Scholar]
- Kondkar AA, Bray MS, Leal SM, Nagalla S, Liu DJ, Jin Y, Dong JF, Ren Q, Whiteheart SW, Shaw C. Bray PF. VAMP8/endobrevin is overexpressed in hyperreactive human platelets: suggested role for platelet microRNA. Journal of Thrombosis and Haemostasis. 2010;8:369–378. doi: 10.1111/j.1538-7836.2009.03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopatskaya O, Gilio K, Harper MT, Zhao Y, Cosemans JMEM, Karim ZA, Whiteheart SW, Molkentin JD, Verkade P, Watson SP, Heemskerk JWM. Poole AW. PKCalpha regulates platelet granule secretion and thrombus formation in mice. The Journal of Clinical Investigation. 2009;119:399–407. doi: 10.1172/JCI34665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopatskaya O, Matthews SA, Harper MT, Gilio K, Cosemans JMEM, Williams CM, Navarro MN, Carter DA, Heemskerk JWM, Leitges M, Cantrell D. Poole AW. Protein kinase C mediates platelet secretion and thrombus formation through protein kinase D2. Blood. 2011;118:416–424. doi: 10.1182/blood-2010-10-312199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koseoglu S, Dilks JR, Peters CG, Fitch-Tewfik JL, Fadel NA, Jasuja R, Italiano JE, Jr, Haynes CL. Flaumenhaft R. Dynamin-related protein-1 controls fusion pore dynamics during platelet granule exocytosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33:481–488. doi: 10.1161/ATVBAHA.112.255737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle M, Begum S. Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemons PP, Chen D, Bernstein AM, Bennett MK. Whiteheart SW. Regulated secretion in platelets: identification of elements of the platelet exocytosis machinery. Blood. 1997;90:1490–1500. [PubMed] [Google Scholar]
- Lemons PP, Chen D. Whiteheart SW. Molecular mechanisms of platelet exocytosis: requirements for alpha-granule release. Biochemical and Biophysical Research Communications. 2000;267:875–880. doi: 10.1006/bbrc.1999.2039. [DOI] [PubMed] [Google Scholar]
- Lievens D. von Hundelshausen P. Platelets in atherosclerosis. Thrombosis and Haemostasis. 2011;106:827–838. doi: 10.1160/TH11-08-0592. [DOI] [PubMed] [Google Scholar]
- Lowe KL, Navarro-Nunez L. Watson SP. Platelet CLEC-2 and podoplanin in cancer metastasis. Thrombosis Research. 2012;129(Suppl 1):S30–S37. doi: 10.1016/S0049-3848(12)70013-0. [DOI] [PubMed] [Google Scholar]
- Ma L, Perini R, McKnight W, Dicay M, Klein A, Hollenberg MD. Wallace JL. Proteinase-activated receptors 1 and 4 counter-regulate endostatin and VEGF release from human platelets. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:216–220. doi: 10.1073/pnas.0406682102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah-Planchon J, Darnige L. Bellucci S. Molecular determinants of platelet delta storage pool deficiencies: an update. British Journal of Haematology. 2013;160:5–11. doi: 10.1111/bjh.12064. [DOI] [PubMed] [Google Scholar]
- Meyers KM, Holmsen H. Seachord CL. Comparative study of platelet dense granule constituents. The American Journal of Physiology. 1982;243:R454–R461. doi: 10.1152/ajpregu.1982.243.3.R454. [DOI] [PubMed] [Google Scholar]
- Morgenstern E. The pathway of exocytosis in human platelets. Blood. 1998;92:2191–2192. [PubMed] [Google Scholar]
- Morimoto T. Ogihara S. ATP is required in platelet serotonin exocytosis for protein phosphorylation and priming of secretory vesicles docked on the plasma membrane. Journal of Cell Science. 1996;109:113–118. doi: 10.1242/jcs.109.1.113. [DOI] [PubMed] [Google Scholar]
- Murugappan S, Tuluc F, Dorsam RT, Shankar H. Kunapuli SP. Differential role of protein kinase C delta isoform in agonist-induced dense granule secretion in human platelets. Journal of Biological Chemistry. 2004;279:2360–2367. doi: 10.1074/jbc.M306960200. [DOI] [PubMed] [Google Scholar]
- Nagy B, Jr, Bhavaraju K, Getz T, Bynagari YS, Kim S. Kunapuli SP. Impaired activation of platelets lacking protein kinase C-theta isoform. Blood. 2009;113:2557–2567. doi: 10.1182/blood-2008-07-169268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumuller O, Hoffmeister M, Babica J, Prelle C, Gegenbauer K. Smolenski AP. Synaptotagmin-like protein 1 interacts with the GTPase-activating protein Rap1GAP2 and regulates dense granule secretion in platelets. Blood. 2009;114:1396–1404. doi: 10.1182/blood-2008-05-155234. [DOI] [PubMed] [Google Scholar]
- van Nispen tot Pannerden H, de Haas F, Geerts W, Posthuma G, van Dijk S. Heijnen HFG. The platelet interior revisited: electron tomography reveals tubular alpha-granule subtypes. Blood. 2010;116:1147–1156. doi: 10.1182/blood-2010-02-268680. [DOI] [PubMed] [Google Scholar]
- Nurden A. Nurden P. Advances in our understanding of the molecular basis of disorders of platelet function. Journal of Thrombosis and Haemostasis: JTH. 2011;9(Suppl 1):76–91. doi: 10.1111/j.1538-7836.2011.04274.x. [DOI] [PubMed] [Google Scholar]
- Papadimas GK, Tzirogiannis KN, Mykoniatis MG, Grypioti AD, Manta GA. Panoutsopoulos GI. The emerging role of serotonin in liver regeneration. Swiss Medical Weekly. 2012;142:w13548. doi: 10.4414/smw.2012.13548. [DOI] [PubMed] [Google Scholar]
- Peters CG, Michelson AD. Flaumenhaft R. Granule exocytosis is required for platelet spreading: differential sorting of alpha-granules expressing VAMP-7. Blood. 2012;120:199–206. doi: 10.1182/blood-2011-10-389247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleines I, Eckly A, Elvers M, Hagedorn I, Eliautou S, Bender M, Wu X, Lanza F, Gachet C, Brakebusch C. Nieswandt B. Multiple alterations of platelet functions dominated by increased secretion in mice lacking Cdc42 in platelets. Blood. 2010;115:3364–3373. doi: 10.1182/blood-2009-09-242271. [DOI] [PubMed] [Google Scholar]
- Pleines I, Hagedorn I, Gupta S, May F, Chakarova L, van Hengel J, Offermanns S, Krohne G, Kleinschnitz C, Brakebusch C. Nieswandt B. Megakaryocyte-specific RhoA deficiency causes macrothrombocytopenia and defective platelet activation in hemostasis and thrombosis. Blood. 2012;119:1054–1063. doi: 10.1182/blood-2011-08-372193. [DOI] [PubMed] [Google Scholar]
- Polgar J, Lane WS, Chung S-H, Houng AK. Reed GL. Phosphorylation of SNAP-23 in activated human platelets. The Journal of Biological Chemistry. 2003;278:44369–44376. doi: 10.1074/jbc.M307864200. [DOI] [PubMed] [Google Scholar]
- Pula G. Poole AW. Critical roles for the actin cytoskeleton and cdc42 in regulating platelet integrin alpha2beta1. Platelets. 2008;19:199–210. doi: 10.1080/09537100701777303. [DOI] [PubMed] [Google Scholar]
- Pula G, Schuh K, Nakayama K, Nakayama KI, Walter U. Poole AW. PKCdelta regulates collagen-induced platelet aggregation through inhibition of VASP-mediated filopodia formation. Blood. 2006;108:4035–4044. doi: 10.1182/blood-2006-05-023739. [DOI] [PubMed] [Google Scholar]
- Ren Q, Barber HK, Crawford GL, Karim ZA, Zhao C, Choi W, Wang C-C, Hong W. Whiteheart SW. Endobrevin/VAMP-8 is the primary v-SNARE for the platelet release reaction. Molecular Biology of the Cell. 2007;18:24–33. doi: 10.1091/mbc.E06-09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q, Ye S. Whiteheart SW. The platelet release reaction: just when you thought platelet secretion was simple. Current Opinion in Hematology. 2008;15:537–541. doi: 10.1097/MOH.0b013e328309ec74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q, Wimmer C, Chicka MC, Ye S, Ren Y, Hughson FM. Whiteheart SW. Munc13-4 is a limiting factor in the pathway required for platelet granule release and hemostasis. Blood. 2010;116:869–877. doi: 10.1182/blood-2010-02-270934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman C. Duncan RR. Munc18/Syntaxin interaction kinetics control secretory vesicle dynamics. Journal of Biological Chemistry. 2010;285:3965–3972. doi: 10.1074/jbc.M109.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley JW, Oler AJ, Tolley ND, Hunter BN, Low EN, Nix DA, Yost CC, Zimmerman GA. Weyrich AS. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood. 2011;118:e101–e111. doi: 10.1182/blood-2011-03-339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage JS, Williams CM, Konopatskaya O, Hers I, Harper MT. Poole AW. Munc13-4 is critical for thrombosis through regulating release of ADP from platelets. Journal of Thrombosis and Haemostasis: JTH. 2013;11:771–775. doi: 10.1111/jth.12138. [DOI] [PubMed] [Google Scholar]
- Schraw TD, Rutledge TW, Crawford GL, Bernstein AM, Kalen AL, Pessin JE. Whiteheart SW. Granule stores from cellubrevin/VAMP-3 null mouse platelets exhibit normal stimulus-induced release. Blood. 2003a;102:1716–1722. doi: 10.1182/blood-2003-01-0331. [DOI] [PubMed] [Google Scholar]
- Schraw TD, Lemons PP, Dean WL. Whiteheart SW. A role for Sec1/Munc18 proteins in platelet exocytosis. The Biochemical Journal. 2003b;374:207–217. doi: 10.1042/BJ20030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher D, Strilic B, Sivaraj KK, Wettschureck N. Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell. 2013;24:130–137. doi: 10.1016/j.ccr.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Sehgal S. Storrie B. Evidence that differential packaging of the major platelet granule proteins von Willebrand factor and fibrinogen can support their differential release. Journal of Thrombosis and Haemostasis: JTH. 2007;5:2009–2016. doi: 10.1111/j.1538-7836.2007.02698.x. [DOI] [PubMed] [Google Scholar]
- Shi L, Shen Q-T, Kiel A, Wang J, Wang H-W, Melia TJ, Rothman JE. Pincet F. SNARE proteins: one to fuse and three to keep the nascent fusion pore open. Science (New York, NY) 2012;335:1355–1359. doi: 10.1126/science.1214984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa R, Higashi T, Tabuchi A, Yoshioka A, Nishioka H, Fukuda M, Kita T. Horiuchi H. Munc13-4 is a GTP-Rab27-binding protein regulating dense core granule secretion in platelets. The Journal of Biological Chemistry. 2004;279:10730–10737. doi: 10.1074/jbc.M309426200. [DOI] [PubMed] [Google Scholar]
- Smolenski A. Novel roles of cAMP/cGMP-dependent signaling in platelets. Journal of Thrombosis and Haemostasis: JTH. 2012;10:167–176. doi: 10.1111/j.1538-7836.2011.04576.x. [DOI] [PubMed] [Google Scholar]
- Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, Arepally GM, French PA, Dauerman HL, Becker RC. Platelet Colloquium Participants Platelet functions beyond hemostasis. Journal of Thrombosis and Haemostasis: JTH. 2009;7:1759–1766. doi: 10.1111/j.1538-7836.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P. Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- zur Stadt U, Schmidt S, Kasper B, Beutel K, Diler AS, Henter J-I, Kabisch H, Schneppenheim R, Nurnberg P, Janka G. Hennies HC. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Human Molecular Genetics. 2005;14:827–834. doi: 10.1093/hmg/ddi076. [DOI] [PubMed] [Google Scholar]
- zur Stadt U, Rohr J, Seifert W, Koch F, Grieve S, Pagel J, Strauss J, Kasper B, Nurnberg G, Becker C, Maul-Pavicic A, Beutel K, Janka G, Griffiths G, Ehl S. Hennies HC. Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18-2 and impaired binding to syntaxin 11. American Journal of Human Genetics. 2009;85:482–492. doi: 10.1016/j.ajhg.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanini L, Boulaftali Y, Ouellette TD, Holinstat M, Desire L, Leblond B, Andre P, Conley PB. Bergmeier W. Rap1-Rac1 circuits potentiate platelet activation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32:434–441. doi: 10.1161/ATVBAHA.111.239194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian H, Zahedi RP, Sickmann A, Walter U. Gambaryan S. Phosphorylation of CalDAG-GEFI by protein kinase A prevents Rap1b activation. Journal of Thrombosis and Haemostasis: JTH. 2013;11:1574–1582. doi: 10.1111/jth.12271. [DOI] [PubMed] [Google Scholar]
- Suh YH, Yoshimoto-Furusawa A, Weih KA, Tessarollo L, Roche KW, Mackem S. Roche PA. Deletion of SNAP-23 results in pre-implantation embryonic lethality in mice. PLoS ONE. 2011;6:e18444. doi: 10.1371/journal.pone.0018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S, Sato S, Oh-Hara T, Takami M, Koike S, Mishima Y, Hatake K. Fujita N. Platelets promote tumor growth and metastasis via direct interaction between Aggrus/Podoplanin and CLEC-2. PLoS ONE. 2013;8:e73609. doi: 10.1371/journal.pone.0073609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon JN, Montalvo A, Patel-Hett S, Devine MT, Richardson JL, Ehrlicher A, Larson MK, Hoffmeister K, Hartwig JH. Italiano JE., Jr Cytoskeletal mechanics of proplatelet maturation and platelet release. The Journal of Cell Biology. 2010;191:861–874. doi: 10.1083/jcb.201006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmachova T, Abrink M, Futter CE, Authi KS. Seabra MC. Rab27b regulates number and secretion of platelet dense granules. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5872–5877. doi: 10.1073/pnas.0609879104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlamov O, Volchuk A, Rahimian V, Doege CA, Paumet F, Eng WS, Arango N, Parlati F, Ravazzola M, Orci L, Söllner TH. Rothman JE. i-SNAREs: inhibitory SNAREs that fine-tune the specificity of membrane fusion. The Journal of Cell Biology. 2004;164:79–88. doi: 10.1083/jcb.200307066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer RM. Jorgensen EM. Controversies in synaptic vesicle exocytosis. Journal of Cell Science. 2003;116:3661–3666. doi: 10.1242/jcs.00687. [DOI] [PubMed] [Google Scholar]
- White GC., II Rompietti R. Platelet secretion: indiscriminately spewed forth or highly orchestrated? Journal of Thrombosis and Haemostasis. 2007;5:2006–2008. doi: 10.1111/j.1538-7836.2007.02731.x. [DOI] [PubMed] [Google Scholar]
- Wolthuis RM, Franke B, van Triest M, Bauer B, Cool RH, Camonis JH, Akkerman JW. Bos JL. Activation of the small GTPase Ral in platelets. Molecular and Cellular Biology. 1998;18:2486–2491. doi: 10.1128/mcb.18.5.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woronowicz K, Dilks JR, Rozenvayn N, Dowal L, Blair PS, Peters CG, Woronowicz L. Flaumenhaft R. The platelet actin cytoskeleton associates with SNAREs and participates in alpha-granule secretion. Biochemistry. 2010;49:4533–4542. doi: 10.1021/bi100541t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Gu Y, Morphew MK, Yao J, Yeh FL, Dong M. Chapman ER. All three components of the neuronal SNARE complex contribute to secretory vesicle docking. Journal of Cell Biology. 2012;198:323–330. doi: 10.1083/jcb.201106158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Karim ZA, Al Hawas R, Pessin JE, Filipovich AH. Whiteheart SW. Syntaxin-11, but not syntaxin-2 or syntaxin-4, is required for platelet secretion. Blood. 2012;120:2484–2492. doi: 10.1182/blood-2012-05-430603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Xiang B, Ye S, Chrzanowska-Wodnicka M, Morris AJ, Gartner TK, Whiteheart SW, White GC, II, Smyth SS. Li Z. Distinct roles for Rap1b protein in platelet secretion and integrin alphaIIbbeta3 outside-in signaling. The Journal of Biological Chemistry. 2011;286:39466–39477. doi: 10.1074/jbc.M111.239608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilly FE, Sorensen JB, Jahn R. Lang T. Munc18-bound syntaxin readily forms SNARE complexes with synaptobrevin in native plasma membranes. PLoS Biology. 2006;4:e330. doi: 10.1371/journal.pbio.0040330. [DOI] [PMC free article] [PubMed] [Google Scholar]